Abstract
Arsenic and the compounds thereof can be carcinogens or therapeutic agents for different cancer types. However, for breast cancer (BC), studies have yielded conflicted results on the role of arsenic. A previous study by the present authors indicated a potential relationship between circDHX34 and sodium arsenite-treated BC cells. As such, the expression, function, and potential mechanism of circDHX34 in sodium arsenite-treated MDA-MB-231 cells were further detected. In the present study, findings were made that sodium arsenite upregulated circDHX34 expression in MDA-MB-231 cells in a dose-dependent manner, and knockdown of circDHX34 could promote cell proliferation and inhibit apoptosis. Further investigations revealed that knockdown of circDHX34 upregulated the expression levels of antiapoptotic genes BCL2 and BCL2L1 and downregulated the expression levels of proapoptotic genes CASP8 and CASP9. To conclude, by regulating apoptotic genes, sodium arsenite-mediated upregulation of circDHX34 promotes apoptosis in hormone-independent breast cancer cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic and the compounds thereof are considered to be paradoxical in terms of their role as both carcinogens and therapeutic agents [Prajapati et al. 2011]. Arsenic has been a global environmental pollutant since ancient times. In humans, acute or chronic damage can occur through the intake of water or food, or exposure in occupational or industrial settings. Acute arsenic poisoning can induce vomit and abdominal pain, but in severe cases, numbness, tingling in the extremities, muscle cramps, and death can occur [Khanjani et al. 2017]. Chronic exposure to arsenic is associated with immune disorders, peripheral neuropathy, liver damage, renal failure, nonmelanoma skin cancer, lung, bladder, and liver cancers [Reyes-Vázquez et al. 2020; Khairul et al. 2017]. Notably, in all human organs, arsenic may cause damage or dysfunction. As such, the International Agency for Research on Cancer (IARC) has classified arsenic and the compounds thereof as a group 1 carcinogen to humans [Prajapati et al. 2011]. Alternatively, arsenic and the compounds thereof can be used as a drug, with arsenic trioxide being a traditional drug used in ancient China and Greece [Swindell et al. 2013] and being the standard treatment for acute promyelocytic leukemia (APL) patients at present. Furthermore, for the treatment of cancers, there are currently over 100 clinical trials involving inorganic arsenic or organoarsenic compounds registered with the FDA [Swindell et al. 2013]. Despite the numerous clinical trials, for the relationship between arsenic compounds and breast cancer, epidemiological studies and cell studies have yielded conflicting results for different populations or different cell lines.
According to the 2018 GLOBOCAN statistics, breast cancer (BC) was the most commonly diagnosed cancer and the most frequent cause of death in women, with 2088.8 new cases and 626.7 deaths per 100,000 worldwide [Ferlay et al. 2019]. Epidemiological studies in different populations have investigated the relationship between exposure to arsenic and BC. In Poland, Marciniak et al. [2020] observed that the risk of BC increased more significantly in women with high blood arsenic levels. In Mexico, López-Carrillo et al. [2014] identified that women with a lower capacity to methylate monomethylarsonous acid (MMA) to dimethylarsinious acid (DMA) and/or higher capacity to methylate inorganic arsenic to MMA were at higher risk of BC. However, in Argentina, Aballay et al. [2012] did not report any significant association between arsenic concentrations in water and BC, even though the concentrations of arsenic in water were higher (0–1.8 mg/L) than WHO guidelines (0.01 mg/L). The aforementioned epidemiological studies seem to have different conclusions in respect of different populations. In cell studies, conclusions also vary in respect of different cell lines. Nakareangrit et al. [2016] demonstrated that sodium arsenite dose-dependently increased the viability of the hormone-dependent BC cell lines of MCF-7 and T47D but not the hormone-independent MDA-MB-231 cells. As reported by Selmin et al. [2019], long-term exposure to sodium arsenite promoted cells proliferation and compromised the response of MCF7 cells to tamoxifen. Ruiz-Ramos et al. [2009] found that sodium arsenite induced cells proliferation in the estrogen responsive BC cell line MCF-7 by activating several pathways. Most researchers are in agreement that sodium arsenite could mimic the effects of estradiol and induce the proliferation of the hormone-dependent BC cell lines through the ER pathway or the induction of aromatase activity [Nakareangrit et al. 2016; Selmin et al. 2019; Ruiz-Ramos et al. 2009]. However, there is no current consensus on an effect on the hormone-independent BC cell line, such as MDA-MB-231. Accordingly, in the present study, the focus was on the effect of sodium arsenite on MDA-MB-231 cells.
As a newly recognized class of noncoding RNA, circular RNA (circRNA) is often but not always derived from exons in the coding region of a gene. In contrast to linear RNA, circRNA forms a special circular covalently bonded structure without the 5′-terminal cap structure and 3′-terminal poly A [Lei et al. 2019], which makes circRNA well expressed and consequently stable in the cell. CircRNAs have abundant biological functions and are involved in various physiological and pathological processes of tumor cells, including proliferation, apoptosis, invasion, and migration [Geng et al. 2018]. Through next-generation sequencing and circRNA microarray, a number of circRNAs were discovered to be involved in the pathology of BC. A previous study by the present authors indicated a potential relationship between circular RNA DHX34 (circDHX34) and sodium arsenite-treated BC cells. Thus, in the present study, the expression of circDHX34 was detected in sodium arsenite-treated MDA-MB-231 cells. Furthermore, the function and potential mechanism of circDHX34 in said cells were determined, thereby providing more information about circDHX34 as a biomarker in exposure to sodium arsenite.
Material and methods
Chemical reagents
Sodium arsenite was obtained from Xiya Chemical Industry Co., Ltd (Linyi, Shandong, China). Monomethylarsonous acid (MMA) and dimethylarsinious acid (DMA) were purchased from Accustandard Inc (New Haven, Connecticut, USA).
Cell culture and treatment
Human triple negative BC cell line MDA-MB-231 was obtained from the Kunming Institute of Zoology, Chinese Academy of Sciences (Kunming, Yunnan, China). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) and 1% penicillin/streptomycin (Solarbio, Beijing, China). Cells were incubated in an incubator containing 5% CO2 at 37°C.
MDA-MB-231 cells in logarithmic growth period were seeded into 6-well plates at a density of 1.5×105 cells/well. After 21 h, the cells were treated with 0, 3, 6μM sodium arsenite solution (sodium arsenite was dissolved in deionized water and added into complete medium to form different concentration) for 72 h. Experiments were repeated for three times. For arsenic metabolites treatment, cells were treated with 0, 3, 6μM MMA/DMA solution (MMA/DMA was dissolved in deionized water and added into complete medium to form different concentration) for 72 h [Gomes et al. 2020]. Experiments were repeated for three times.
Cell transfection
Small interfering RNA (siRNA) against circDHX34 (siRNA1 and siRNA2) and its control (si-NC) was synthesized and purchased from GenePharma Co., Ltd (Shanghai, China). The sequences of siRNAs were shown in Table 1. Fluorescent tag was added on the siRNAs when they were produced.
MDA-MB-231 cells in logarithmic growth period were seeded into 6-well plates at a density of 1.2×105 cells/well. After 18 h, cells were transfected with siRNA using Lipo8000 (Beyotime, Shanghai, China) according to the manufacturers’ instructions for 6 h and then replaced by complete medium and cultured for 66 h. The transfection efficiency was measured by observing with fluorescence microscope and detecting the expression level of circDHX34 with qRT-PCR. Experiments were repeated for eight times.
RNA isolation and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and transcribed into complementary DNA (cDNA) using the HiFiScript cDNA Synthesis Kit (CoWin Biotech, Beijing, China) according to the manufacturer’s instructions. The qRT-PCR was performed using SYBR Green PCR Master Mix (CoWin Biotech) on the LightCycler ® 96 instrument real-time PCR system (Roche Molecular Systems, Basel, Switzerland). Primers were synthesized and purchased from GenePharma Co., Ltd (Shanghai, China); β-actin was used as an internal reference. Primer sequences were shown in Table 2. PCR cycling reaction conditions were as follows: performed at 95°C for 8 min, followed by 40 cycles at the conditions of 95°C for 10s, 60°C for 10s, and 72°C for 15s. The relative expression levels of each target genes were determined using the formula 2-△△Ct. Experiments were repeated for eight times.
CCK-8 proliferation assay
Cell viability was measured using the cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) [Zhou et al. 2020]. Cells were seeded in 96-well plates at a density of 4×103 cells/well with serum-free DMEM medium and cultured for 18 h then treated with sodium arsenite for 72h or transfected with siRNA for 6h. When finishing cell treatment by sodium arsenite or siRNAs, 10μL of CCK-8 reagent was added to each well and incubated for 2h. The absorbance was measured at 450nm using a spectrophotometer (Bio-Rad, Hercules, CA, USA). Experiments were repeated for three times.
Cell apoptosis assay
Apoptosis was assessed using an Apoptosis and Necrosis Assay Kit (Beyotime) according to the manufacturer’s instructions. Cells were seeded into 6-well plates at a density of 2×105 cells/well. After treatment by sodium arsenite or siRNAs, cells were stained using Hoechst 33342 (HO) and Propidium Iodide (PI) and incubated in the dark at room temperature for 20 min then observed by fluorescence microscope (BD Biosciences, USA) at the maximum emission wavelength of 352 and 488nm, respectively [Velozo-Sá et al. 2019]. For normal cells, they can’t stain by both stains. However, due to the presence of the gaps in cell membranes, they can show no/weak blue fluorescence. For apoptotic cells, they can stain by HO but not PI, showing strong blue fluorescence and no/weak red fluorescence. For necrotic cells, they can stain by HO and PI, showing strong blue and red fluorescence. Cells in the images were counted by the ImageJ software and calculated the percentage of apoptotic cells. Experiments were repeated for four times.
Knockdown efficiency assay
Knockdown efficiency was assessed using fluorescence microscope according to the manufacturer’s instructions [Tanaka et al. 2013]. Cells were seeded into 6-well plates at a density of 1.2×105 cells/well. After 18h, siRNAs with transfection reagent Lipo8000 were added to DMEM for incubating at room temperature. After 20min, complexes were added and the cells and further cultured for 6h at 37°C, followed by observing on fluorescence microscope at the maximum emission wavelength of 480nm. Transfected cells with FAM could show green fluorescence under 480-nm wave. Cells in the image were counted by the ImageJ software and calculated the percentage of transfected cells. Experiments were repeated for three times.
Statistical analysis
All experiments were repeated at least three times. Data were presented as mean ± SEM. Statistical analysis was performed using SPSS 20.0 software (IBM, Armonk, NY, USA), GraphPad Prism 5.0 and ImageJ software (NIH, Bethesda, MD, USA). Student’s t-test and one-way ANOVA were used. P value <0.05 was considered as significant difference. For all data, * means P<0.05, ** means P<0.01, *** means P<0.001.
Results
Sodium arsenite repressed cell proliferation and facilitated apoptosis in MDA-MB-231 cells
To detect the sodium arsenite function on the hormone-independent BC cell line, the cell proliferation and apoptosis of MDA-MB-231 cells under different doses of sodium arsenite treatment were firstly determined by CCK-8 proliferation assay and HO-PI stained apoptosis assay, respectively. In pre-experiments, the effects of different sodium arsenite concentrations (1, 2, 3, 4, 6, 8, and 9μM) were investigated by CCK-8 proliferation assay, which demonstrated that sodium arsenite further repressed cell proliferation as the concentration increased. In order to keep a certain number of adherent cells for subsequent assays, 3 and 6μM were chosen as the final sodium arsenite solution concentrations. As shown in Figure 1, sodium arsenite repressed BC cells proliferation and facilitated apoptosis in a dose-dependent manner.
Sodium arsenite repressed cell proliferation and facilitated apoptosis in MDA-MB-231 cells. After 72-h treatment by 0, 3, 6μM sodium arsenite, a cell proliferation rate of MDA-MB-231 was assessed by CCK-8 proliferation assay. b Cell apoptosis image of MDA-MB-231 was evaluated by fluorescence microscope after staining by HO-PI, apoptotic cells showed weak red fluorescence and strong blue fluorescence. c Cell apoptotic rate by ImageJ software counting the number of cells with weak red fluorescence and strong blue fluorescence in Figure 1B. *P < 0.05, ***P < 0.001
Sodium arsenite upregulated circDHX34 expression in MDA-MB-231 cells in a dose-dependent manner
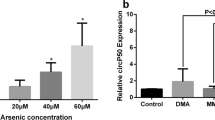
To detect the potential role of circDHX34 in sodium arsenite-treated BC cells, the expression level of circDHX34 under different doses of sodium arsenite treatment was evaluated by qRT-PCR. As shown in Figure 2A, after 72-h treatment, sodium arsenite was able to significantly elevate the expression level of circDHX34 in a dose-dependent manner in MDA-MB-231 cells.
The effects of sodium arsenite and arsenic metabolites (MMA and DMA) on the expression of circDHX34 and DHX34-pre in MDA-MB-231 cells. a Relative expression level of circDHX34 was assessed by qRT-PCR after 72-h treatment by 0, 3, 6, 9μM sodium arsenite. b Relative expression level of circDHX34 was assessed by qRT-PCR after 72-h treatment by 0, 9μM MMA/DMA. c Relative expression level of DHX34-pre was assessed by qRT-PCR after 72-h treatment by 0, 3, 6, 9μM sodium arsenite. **P < 0.01, ***P < 0.001
MMA and DMA are the metabolites of sodium arsenite. To further explore whether the elevated expression level of circDHX34 was the result of the treatment of sodium arsenite or the metabolites thereof, MDA-MB-231 was treated with MMA and DMA under the same conditions. As shown in Figure 2B, MMA and DMA had no effect on the expression of circDHX34 in MDA-MB-231 cells, indicating that the elevated expression of circDHX34 was the result of the treatment of sodium arsenite rather than the metabolites thereof.
To investigate whether sodium arsenite acts on the transcriptional process of circDHX34 or the posttranscriptional process, the expression level of DHX34-pre was assessed after treatment, which presented a negative result. DHX34-pre was the precursor of circDHX34, and sodium arsenite had no effect on DHX34-pre, which indicated effect on circDHX34 was on the posttranscriptional process.
Knockdown of circDHX34 was observed in MDA-MB-231 cells
siRNAs against circDHX34 (siRNA1 and siRNA2) were transfected into cells to knock down the expression level of circDHX34, so as to investigate the functions of circDHX34 in MDA-MB-231 cells. The transfection efficiency was detected by fluorescence microscope, while the knockdown efficiency was measured by qRT-PCR. Over 85% of cells were transfected by siRNAs, and the expression of circDHX34 was significantly decreased in MDA-MB-231 cells after transfection with siRNA1/siRNA2 compared with the si-NC group (Figure 3).
Knockdown of circDHX34 was observed in MDA-MB-231 cells. a Cells transfected with siRNA was stained by FAM-siRNA and showed green fluorescence under fluorescence microscopy. b Use ImageJ software to count the number of green cells in Figure 3A to measure knockdown efficiency. c Relative circDHX34 mRNA expression level was assessed by qRT-PCR. *P < 0.05
Knockdown of circDHX34 in MDA-MB-231 cells promoted cell proliferation and inhibited apoptosis
To assess whether knockdown of circDHX34 may affect MDA-MB-231 cells, CCK-8 proliferation assay was conducted to measure the cell proliferation of MDA-MB-231 cells upon transfection with siRNAs for 72h. Additionally, HO-PI stained apoptotic assay was conducted to assess cell apoptosis upon transfection with siRNAs for 72h. As shown in Figure 4, findings were made that the number of proliferating cells in siRNA1/2 groups was significantly higher than that in the si-NC group (Figure 4A). Conversely, apoptotic cells, which exhibited weak red and strong blue fluorescence under microscope (Figure 4B), were significantly less in siRNA1/2 groups. Quantitative analysis by ImageJ software revealed that the number of apoptotic cells accounts for 17.0%, 12.6%, and 13.1% in the si-NC group, the siRNA1 group and the siRNA2 group, respectively (Figure 4C). Thus, the present study revealed that knockdown of circDHX34 in MDA-MB-231 cells could promote cell proliferation and inhibit apoptosis.
Knockdown of circDHX34 in MDA-MB-231 cells promoted cell proliferation and inhibited apoptosis. a Cell proliferation rate of MDA-MB-231 was assessed by CCK-8 proliferation assay after circDHX34 knockdown. b Cell apoptosis image of MDA-MB-231 after circDHX34 knockdown was evaluated by fluorescence microscope after staining by HO-PI, apoptotic cells showed weak red fluorescence and strong blue fluorescence. c Use ImageJ software to count the number of blue cells in Figure 4B to measure cell apoptotic rate. **P < 0.01
Knockdown of circDHX34 regulated the expression levels of apoptotic genes in MDA-MB-231 cells
For investigating whether the possible mechanism underlying the knockdown of circDHX34 could inhibit the apoptosis of MDA-MB-231 cells, qRT-PCR was performed to detect the expression level changes of apoptotic related genes. The results are shown in Figure 5. Compared with the si-NC group, the expression levels of BCL2 and BCL2L1 were elevated in the siRNA1/2 group. Conversely, the expression levels of CASP8 and CASP9 were decreased in the siRNA1/2 group. No significant changes were observed in the expression levels of CASP3, CASP7, BAD, BAK, BAX, and FAS.
Discussion
For breast cancer, arsenic and the compounds thereof are mainly considered as carcinogen. In epidemiological studies, arsenic has been shown to increase the risk of BC in women with specific features, such as higher capacity to methylate inorganic arsenic to MMA. In cell studies, sodium arsenite is considered as a mimic of estrogen and can induce the proliferation of the hormone-dependent BC cells lines through the ER pathway or the induction of aromatase activity. However, there is a scarcity of studies that have focused on the relationship between arsenic compounds and the hormone-independent BC cell lines. Based on previous studies on circRNAs by the present authors, circDHX34 was chosen, a rarely mentioned circRNA in BC, as the target RNA and the expression and function of circDHX34 in sodium arsenite-treated hormone-independent BC cell line MDA-MB-231 were investigated in the present study.
Firstly, the direct effects of sodium arsenite on MDA-MB-231 cells were investigated, which revealed a dose-dependent inhibiting effect on cell proliferation and a promoting effect on cell apoptosis. Previous research by Nakareangrit et al. [2016] demonstrated that <10 μM sodium arsenite had a limited effect on the viability of MDA-MB-231 cells. After comparing the experiments of the two articles, the exposure time to sodium arsenite in the present experiment was found to be 72 h, which was much longer than the 24 h in the experiment of Nakareangrit et al. Results obtained in a study by Reyes-Vázquez et al. [2020] suggested that 0.01, 0.1, and 1μM sodium arsenite could induce the promotion and progression of BC cells (MDA-MB-231, MDA-MB-453, MCF-7). However, in the said study, the effects of higher sodium arsenite concentrations on cells were not tested. In the present study, studies on MDA-MB-231 cells exposed to other arsenic compounds were taken as a reference. Studies on arsenic trioxide revealed that arsenic trioxide could inhibit the growth of MDA-MB-231 cells and induce apoptosis in a dosage-effect manner [Baj et al. 2002; Wei et al. 2005]. Arsenic disulfide could also significantly inhibit cell viabilities, induced apoptosis, and led to cell cycle arrest in MDA-MB-231 and MCF-7 cells [Zhao et al. 2018, 2019]. Hence, the present results on the effect of sodium arsenite on MDA-MB-231 cells are consistent with most existing studies.
Subsequently, the expression of circDHX34 in sodium arsenite-treated MDA-MB-231 cells was tested, which exhibited significant upregulation. However, no effect was observed when treated with MMA or DMA, which indicated that the elevating expression of circDHX34 was the result of sodium arsenite rather than the metabolites thereof.
In consideration of the aforementioned results, the inhibition of cell proliferation and the upregulation of circDHX34 expression could be attributed to sodium arsenite. In order to determine what would happen to the cell proliferation if circDHX34 was knocked down in MDA-MB-231 cells, circDHX34 was knocked down by transfecting with siRNAs, and then the cells proliferation and apoptosis were assessed. As shown in the “Results,” knockdown of circDHX34 in MDA-MB-231 cells promoted cell proliferation and inhibited cell apoptosis. However, the manner through which circDHX34 regulates the proliferation and apoptosis of MDA-MB-231 cells remains unclear.
To further investigate the possible mechanism, the expression of eight proliferation regulatory genes and ten apoptotic regulatory genes in circDHX34 knockdown cells were assessed by qRT-PCR. Findings were made that there were limited changes in the expression of proliferation regulatory genes compared with the control group. Conversely, knockdown of circDHX34 regulated several important cell apoptotic genes, including upregulating the expression level of the antiapoptotic genes of BCL2 and BCL2L1 and downregulating the expression level of the proapoptotic genes of CASP8 and CASP9. BCL2 and BCL2L1 are the key genes in the BCL2 pathway, which regulates programmed cell death in pathological conditions [Gao et al. 2018]. BCL2 and BCL2L1 are antiapoptotic genes, and the level of BCL2 affects cellular fates, death, or survival [Lim et al. 2010]. The expression of BCL2 and BCL2L1 are increased in knockdown cells, indicating that circDHX34 has as a proapoptotic function. In addition, CASP8 and CASP9 are key genes in the caspase cascade; downregulating of these genes in circDHX34 knockdown cells indicated circDHX34 could promote cell apoptosis, which is consistent with the phenomenon mentioned in the aforementioned experiments. In addition, the expression of Bax and Fas were reported to have an increased expression in a previous study [He et al. 2018] by the present authors in sodium arsenite-treated cells. However, compared to control group, there are no significant difference in the expression of BAX and Fas in circDHX34 knockdown cells, which are shown in Figure 5.
Although there is no existing research on circDHX34 with BC cells, the results of the present study are rational in terms of the function and mechanism. Several other articles used similar methods on the investigation of circRNAs in BC cells and obtained similar results [Reyes-Vázquez et al. 2020; Hou et al. 2019; Geng et al. 2019; Kaur et al. 2020, 2021]. However, further information on the mechanism is required. Since the miRNA sponge is the most common function of circRNAs, the aim of the present authors is to demonstrate if circDHX34 also acts as a miRNA sponge using RIP-qPCR and bioinformatics analysis.
In conclusion, the present results demonstrated that sodium arsenite upregulated circDHX34 expression in hormone-independent breast cancer MDA-MB-231 cells. At the same time, knockdown of circDHX34 could promote the proliferation and inhibit the apoptosis by regulating CASP8, CASP9, BCL2, and BCL2L1.
Data availability
The datasets generated and analyzed during the current study are not publicly available because of the follow-up studies is conducting but are available from the corresponding author on reasonable request.
References
Aballay LR, Díaz Mdel P, Francisca FM, Muñoz SE (2012) Cancer incidence and pattern of arsenic concentration in drinking water wells in Cordoba, Argentina. Int J Environ Health Res 22(3):220–231
Baj G, Arnulfo A, Deaglio S, Mallone R, Vigone A, de Cesaris MG, Surico N, Malavasi F, Ferrero E (2002) Arsenic trioxide and breast cancer: analysis of the apoptotic, differentiative and immunomodulatory effects. Breast Cancer Res Treat 73(1):61–73
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953
Gao XL, Yang JJ, Wang SJ, Chen Y, Wang B, Cheng EJ, Gong JN, Dong YT, Liu D, Wang XL, Huang YQ, An DD (2018) Effects of RNA interference-mediated silencing of toll-like receptor 4 gene on proliferation and apoptosis of human breast cancer MCF-7 and MDA-MB-231 cells: an in vitro study. J Cell Physiol 234(1):433–442
Geng Y, Jiang J, Wu C (2018) Function and clinical significance of circRNAs in solid tumors. J Hematol Oncol 11(1):98
Geng Z, Wang W, Chen H, Mao J, Li Z, Zhou J (2019) Circ_0001667 promotes breast cancer cell proliferation and survival via Hippo signal pathway by regulating TAZ. Cell Biosci 9:104
Gomes AP, Ilter D, Low V, Endress JE, Fernández-García J, Rosenzweig A, Schild T, Broekaert D, Ahmed A, Planque M, Elia I, Han J, Kinzig C, Mullarky E, Mutvei AP, Asara J, de Cabo R, Cantley LC, Dephoure N et al (2020) Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 585(7824):283–287
He Y, Zhang R, Song X, Shang L, Wu X, Huang D (2018) Inorganic arsenic exposure increased expression of Fas and Bax gene in vivo and vitro. Gene 671:135–141
Hou JC, Xu Z, Zhong SL, Zhang HD, Jiang LH, Chen X, Zhu LP, Li J, Zhou SY, Yang SJ, He YJ, Wang DD, Deng F, Zhang Q, Wang JY, Hu JH, Zhang W, Wu Y, Ding L et al (2019) Circular RNA circASS1 is downregulated in breast cancer cells MDA-MB-231 and suppressed invasion and migration. Epigenomics 11(2):199–213
Kaur S, Kumar A, Thakur S et al (2020) Antioxidant, antiproliferative and apoptosis-inducing efficacy of fractions from Cassia fistula L. Leaves. Antioxidants (Basel) 9(2):173
Kaur S, Kumar A, Pandit K, Kaur S (2021) Modulation of mutagenicity in Salmonella typhimurium and antioxidant properties and antiproliferative effects of fractions from Cassia fistula L. on human cervical HeLa and breast MCF-7 cancer cells. Environ Sci Pollut Res Int 28(6):6619–6634
Khairul I, Wang QQ, Jiang YH, Wang C, Naranmandura H (2017) Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 8(14):23905–23926
Khanjani N, Jafarnejad AB, Tavakkoli L (2017) Arsenic and breast cancer: a systematic review of epidemiologic studies. Rev Environ Health 32(3):267–277
Lei B, Tian Z, Fan W, Ni B (2019) Circular RNA: a novel biomarker and therapeutic target for human cancers. Int J Med Sci 16(2):292–301
Lim MH, Lee DH, Jung SE, Youn DY, Park CS, Lee JH (2010) Effect of modulation of hnRNP L levels on the decay of bcl-2 mRNA in MCF-7 cells. Korean J Physiol Pharmacol 14(1):15–20
López-Carrillo L, Hernández-Ramírez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sánchez L, Cebrian ME (2014) Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol 280(1):53–59
Marciniak W, Derkacz R, Muszyńska M, Baszuk P, Gronwald J, Huzarski T, Cybulski C, Jakubowska A, Falco M, Dębniak T, Lener M, Oszurek O, Pullella K, Kotsopoulos J, Sun P, Narod SA, Lubiński J (2020) Blood arsenic levels and the risk of familial breast cancer in Poland. Int J Cancer 146(10):2721–2727
Nakareangrit W, Thiantanawat A, Visitnonthachai D, Watcharasit P, Satayavivad J (2016) Sodium arsenite inhibited genomic estrogen signaling but induced pERalpha (Ser118) via MAPK pathway in breast cancer cells. Environ Toxicol 31(9):1133–1146
Prajapati V, Kale RK, Singh RP (2011) Arsenic and its combinations in cancer therapeutics. Ther Deliv 2(6):793–806
Reyes-Vázquez L, Hernández AJA, Calderón-Aranda ES (2020) Role of aromatase activation on sodium arsenite-induced proliferation, migration, and invasion of MDA-MB-231 and MDA-MB-453 breast cancer cell lines. Toxicology 437:152440
Ruiz-Ramos R, Lopez-Carrillo L, Rios-Perez AD, De Vizcaya-Ruíz A, Cebrian ME (2009) Sodium arsenite induces ROS generation, DNA oxidative damage, HO-1 and c-Myc proteins, NF-kappaB activation and cell proliferation in human breast cancer MCF-7 cells. Mutat Res 674(1-2):109–115
Selmin OI, Donovan MG, Skovan B, Paine-Murieta GD, Romagnolo DF (2019) Arsenic-induced BRCA1 CpG promoter methylation is associated with the downregulation of ERalpha and resistance to tamoxifen in MCF7 breast cancer cells and mouse mammary tumor xenografts. Int J Oncol 54(3):869–878
Swindell EP, Hankins PL, Chen H, Miodragović DU, O'Halloran TV (2013) Anticancer activity of small-molecule and nanoparticulate arsenic(III) complexes. Inorg Chem 52(21):12292–12304
Tanaka K, Kanazawa T, Horiuchi S, Ando T, Sugawara K, Takashima Y, Seta Y, Okada H (2013) Cytoplasm-responsive nanocarriers conjugated with a functional cell-penetrating peptide for systemic siRNA delivery. Int J Pharm 455(1-2):40–47
Velozo-Sá VS, Pereira LR, Lima AP, Mello-Andrade F, Rezende MRM, Goveia RM, Pires WC, Silva MM, Oliveira KM, Ferreira AG, Ellena J, Deflon VM, Grisolia CK, Batista AA, Silveira-Lacerda EP (2019) In vitro cytotoxicity and in vivo zebrafish toxicity evaluation of Ru(ii)/2-mercaptopyrimidine complexes. Dalton Trans 48(18):6026–6039
Wei L, Xingwu W, Wenshu Z, Xianrang S (2005) Primary research on arsenic trioxide inhibiting human breast cancer cells growth and its mechanisms. Zhonghua Yi Xue Za Zhi 85(17):1209–1213
Zhao Y, Yuan B, Onda K, Sugiyama K, Tanaka S, Takagi N, Hirano T (2018) Anticancer efficacies of arsenic disulfide through apoptosis induction, cell cycle arrest, and pro-survival signal inhibition in human breast cancer cells. Am J Cancer Res 8(3):366–386
Zhao Y, Onda K, Sugiyama K, Yuan B, Tanaka S, Takagi N, Hirano T (2019) Antitumor effects of arsenic disulfide on the viability, migratory ability, apoptosis and autophagy of breast cancer cells. Oncol Rep 41(1):27–42
Zhou J, Jiang YY, Chen H, Wu YC, Zhang L (2020) Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif 53(2):e12739
Funding
This work was funded by Yunnan Applied Basic Research Projects-Union Foundation by Yunnan Provincial Department of Science and Technology and Kunming Medical University (Grant No. 202001AY070001-135), the National Natural Science Foundation of China (Grant No. 81860572).
Author information
Authors and Affiliations
Contributions
L.S.T. conducted most experiments and analyzed the data and was the major contributor in the manuscript writing. J.C.L. conducted cell apoptosis assays and was the writing assistance. T.J.W. contributes to data analyzing. Z.Q. and Y.J.Y. made the tables and figures in the manuscript. H.Y.F. designed the study concept, helped to do publication decision, and revised the first version of the manuscript. All authors have contributed to, read, and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Mohamed M. Abdel-Daim
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Jiang, C., Tan, J. et al. Sodium arsenite-mediated upregulation of circDHX34 promotes apoptosis in hormone-independent breast cancer cells by regulating apoptotic genes. Environ Sci Pollut Res 29, 2728–2736 (2022). https://doi.org/10.1007/s11356-021-15891-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15891-2