Abstract
Some pesticides such as organochlorines are of critical environmental concern because they are highly persistent due to their stable chemical nature. As a consequence, even after banning, dichlorodiphenyltrichloroethane and endosulfan can be detected at concentrations above permissible limits. Moreover, classical pesticide degradation of these compounds using physiochemical processes is limited. Alternatively, biodegradation using microorganisms isolated in contaminated sites appears promising. For instance, the bacterium Pseudomonas fluorescens degrades aldrin by 94.8%, and the fungus Ganoderma lucidum can bring down the levels of lindane by 75.5%. In addition, the toxicity is reduced by enzymes that perform oxidation, reduction, hydrolysis, dehydrogenation, dehalogenation and decarboxylation. Then, the metabolites are further degraded by mineralisation and cometabolism. The biodegradation process can be manipulated by applying techniques such as bioattenuation, bioaugmentation and biostimulation. This article discusses the latest advances in microbial degradation of recalcitrant pesticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the era of modern agriculture, there has been an extensive usage of pesticides in order to meet the increasing demand of crop production. The economic importance of pesticide is also huge and has a market of billions of dollars. In 2019, a revenue of 84.5 billion has been generated and by 2023 it is expected to attain the mark of 130.7 billion dollars (The Business Research Company 2020). In the last 50 years, with the rise of intensive agriculture, there has been a need for controlling pests and disease vectors with the usage of pesticides (Gad 2020). About 2 million tons of pesticide are used worldwide annually which is estimated to increase up to 3.5 million tonnes by 2021 (Sharma et al. 2019; Riah et al. 2014). According to World Health Organisation (WHO), pesticide has been defined as a chemical compound which is being used to control the population of insects, pests, rodents and to keep plant diseases under check. Different kinds of pesticides like herbicides, insecticides, and fungicides are being used for different reasons in crop production and each of them targets a variety of pests or weeds. It has also been utilised in public health to curb mosquito-borne diseases like malaria, dengue (WHO 2018). Prolonged usage of such synthetic chemicals has led to the discovery that most of the pests have become resistant to pesticides which has led to higher usage of the same (Aktar et al. 2009; Sánchez Salinas and Ortiz Hernández 2011). Also, higher quantities are harmful for the beneficial non-target organisms that can otherwise retain the pest population under check (Rani and Dhania 2014).
Pesticide usage leads to enhanced agricultural production, but indiscriminate usage can lead to pollution and toxicity (WHO 2018). Excessive pesticides sometime lead to leaching into groundwater and can eventually contaminate surface water bodies like lakes, ponds, rivers (Hui et al. 2020; Dsikowitzky and Schwarzbauer 2014). The contamination mainly arises as a result of run-off from agricultural fields or via spray drifting (Clyde 2013; Ben Chekroun et al. 2014). Apart from degrading water quality, pesticide contamination also affects aquatic plants and animals (lower phytoplankton and zooplankton population) creating a biological imbalance (Sánchez Salinas and Ortiz Hernández 2011).
The intake of pesticide polluted water can lead to various health problems ranging from cancer, neurological diseases, cardiovascular disease, diarrhoea, respiratory ailments, and reproductive problems (Gad 2020). Certain pesticides are acetylcholinesterase (AChE) inhibitors leading to nervous disorders. These chemicals are highly fat-soluble which results to low excretion levels leading to bioaccumulation (Agrawal et al. 2010).
The chemical composition of the pesticide determines its persistence or natural level of degradation. According to their chemical composition, they can be grouped under two categories- chlorinated and non-chlorinated pesticides (Aresta et al. 2015). Under chlorinated pesticides, organochlorine pesticides are the ones that are strongly persistent in nature (Gad 2020; Kaur et al. 2008) and result in bioaccumulation in biotic compounds leading to food contamination (Briz et al. 2011). They are biologically very stable and are lipophilic resulting in slower natural degradation (Rani and Dhania 2014). Under organochlorine pesticides, there are different categories which also have different levels of toxicity, e.g. eldrin, dieldrin, endosulfan (Das and Chandran 2011; Bhalerao and Puranik 2007).
Previously, various physicochemical methods have been implied to reduce the pesticide burden in water but none of them yielded satisfactory results in the long term. Among the chemical treatments, advanced oxidation has been in use which utilises hydroxyl ion while in case of physical treatment percolation filter and adsorption are used. Alongside these, photocatalytic treatment with titanium dioxide (TiO2) has been performed (Ferrusquía-García et al. 2008). Recently, incineration at high temperatures is being frequently opted for. The estimated cost of these processes according to FAO is within 3000–4000/ton (Laura et al. 2013). In case of removing certain pesticides, alkaline hydrolysis is being used which needs to be operated under strict laboratory conditions in presence of complex metal ions. This sometimes leads to the formation of secondary pollutants (Laura et al. 2013). Most of the conventional treatment options are highly expensive, complex or toxic chemical substances are involved in the process (Meleiro Porto et al. 2011; Brião et al. 2020). In view of the above-mentioned problems related to conventional treatment, scientists have come up with an innovative solution in the recent past for reducing the pesticide load which has been termed as bioremediation/biodegradation. Biodegradation is a process through which the chemical compounds are rendered benign or less toxic than their parent compounds with the help of microorganisms like fungi, bacteria and their metabolic mechanisms (Sun et al. 2020). In comparison to the traditional techniques, bioremediation does not need disposal of the chemical pesticides and can be used for in situ treatment of the residues. The microorganisms present naturally in the area of contamination gets adapted over a period of time after continuous exposure to the chemical. Thus, the process can take place organically in soil or aquatic environment. Most of the times, external stimulation is not required as the pesticide serves as a source of nutrient or carbon for the degradation process (Laura et al. 2013). Thus, this method has been supported by scientists due to its economic and environmentally friendly nature (Aresta et al. 2015).
This article serves as a summarised review of the current advances in the sphere of biodegradation. There has been a wealth of information available in the research community on the detection of toxic or banned pesticide in water bodies throughout the world. The focus will be on the available literature on fungal and bacterial degradation of persistent organochlorine pesticides (OCP) residues discussing the toxic effects of the same. It includes the various mechanisms, strategies and pathways adopted by the microorganisms in order to degrade the pesticide residues. This article will extensively cover literature on identifying relevant microbial strains efficient for pesticide degradation.
Types of pesticides and their chemical nature
Pesticides are of different composition based on which there are different varieties of pesticides available in the market comprising a varied range of targets. Table 1 lists the various pesticides and their classification.
Organochlorine pesticides: Organochlorine pesticides are prepared synthetically and are mainly composed of carbon, chlorine and hydrogen. They might include solvents like organochlorine/chloride, chlorinated hydrocarbons, and chlorocarbon (Satish et al. 2017). This kind of pesticides is lipophilic and hence deposits in the fatty tissue of animals and is toxic due to their persistent nature (Laura et al. 2013; Ricking and Schwarzbauer 2012). Few types of highly utilised organochlorine pesticides like dichlorodiphenyltrichloroethane (DDT), lindane, aldrin, heptachlor, and endosulfan exhibit high levels of toxicity (Pal et al. 2005; Rani and Dhania 2014; Benimeli et al. 2008). They have widespread applications in the agricultural sector (Ghaffar et al. 2013). Table 2 shows the chlorinated derivatives of certain compounds and their chemical structures.
Organophosphorus pesticides: Organophosphorus pesticides (OPP) are soluble in organic solvents and hence, they can percolate through soil and reach groundwater (Laura et al. 2013). It is used as an insecticide on a wide range of fruits and vegetables and even on ornamentals (Romeh and Hendawi 2013) Methyl parathion (O,O-dimethyl-O-(p-nitro-phenylphosphorothioate) is being applied on a regular basis on a wide variety of agricultural products including rice, avocados, onion, spinach, peach, strawberries (Fernández-López et al. 2017). To increase the efficiency of insecticide, a mixture of two or more pesticides is also used (Meleiro Porto et al. 2011).
Carbamate pesticides: This kind of pesticides is of relatively low persistence. They are mainly carbamic acid derivative and can operate as insecticides, herbicides or fungicides (Laura et al. 2013; Meleiro Porto et al. 2011). Hence, they are used extensively in the agricultural sector. Carbamates are soluble in water and thermally quite unstable (García et al. 2006). It is also considered to be highly toxic to vertebrates (Mustapha et al. 2019; Laura et al. 2013).
Pyrethroids: It is a widely used pesticide and is derived from pyrethrin. Pyrethrins which consists of two functional groups (acid and alcohol) are originally extracted from chrysanthemum flower (Urkude et al. 2019; Pfeil 2014). They are divided into two classes depending on their chemical structure and mode of action on pests (Ullah et al. 2019). Type I is known as permethrin lacks the cyano group while the type II alternatively named as deltamethrin possesses the cyano group at the phenyl benzyl alcohol position (Khambay and Jewess 2005). Pyrethroids are relatively less persistent and are susceptible to photodegradation (Urkude et al. 2019). It affects the nervous system and delays opening of the sodium channels of insects leading to muscular paralysis and mortality (Laura et al. 2013; Suppiramaniam et al. 2010).
Biopesticides: These are extracted from naturally occurring substances having the ability to act as pesticides. They are highly biodegradable and are not detrimental to non-target organisms. They can be classified into three categories—microbial, plant and biochemical (Meleiro Porto et al. 2011).
Sources of pesticide entry into water
The indiscriminate level of pesticide usage leads to serious environmental problems and is currently considered as a global issue. It has been studied that only 10% of the used pesticide reaches the target while the rest of it ends up depositing in soil and water bodies (Oliveira-Silva et al. 2001; Satish et al. 2017). The amount of pesticide that gets deposited in soil without being utilised by the target depends on many factors such as type and chemical composition of pesticide, soil fertility (Joko et al. 2017; Karthik et al. 2020). The residual pesticide ends up in nearby water bodies through diffuse sources like percolation, spray drifting, surface run-off, drain flow or through flow (Ben Chekroun et al. 2014; van Grinsven et al. 2012). Drain flow is the artificial drainage of the excess water present in the soil or land surface which consists of substantial quantities of pesticide. On the other hand, the quantity of pesticide present in through flow is less as the water passes naturally through soil matrices resulting in the degradation or sorption of the pesticide residues. This kind of contamination not only affects the quality of surface water but also degrades the quality of underground water reserves (Srivastava et al. 2018). High concentrations of pesticides are detected in the washed water from the batch preparation of synthetic pesticides in pesticide industries (Babu et al. 2011). The seasonal pollution of pesticides varies as the concentration of pesticide is found to be high post-spring and summer seasons and the curve decreases comparatively during the winter season (Konstantinou et al. 2006).
Status of pesticide usage in India
In the year of 2019, India has invested over 70 billion INR (Indian rupee) on pesticide production and in 2020, the amount of pesticide produced is 93 thousand metric tonnes. India ranks fourth as pesticide supplier after USA, China and Japan while Indian agrochemical industry is the leading one in Asia but ranks 12th in the world. According to a study, on average, 600 gm/hectare of pesticides are used in India (“India—pesticides production volume 2020 Statista” 2020) and within a span of 5 years (from 2010 to 2015), pesticide consumption increased by about 50% (The Business Research Company 2020). Out of that, the presence of pesticides like lindane, endosulfan, aldrin, metolachlor, and alachlor are the highest (Konstantinou et al. 2006). Amongst the Indian states, Punjab is leading in terms of pesticide consumption followed by Maharashtra, and Andhra Pradesh while the consumption is much lower in North-eastern states like Sikkim, Assam, Manipur, and Nagaland. It must be noted that in many states, though the total consumption is on a medium side but their rate for intensity of pesticide application is high like in Jammu Kashmir and West Bengal (Subash et al. 2017). According to a survey conducted in 2014, organochlorine pesticides was the highest utilised pesticide in India because of its high efficiency levels in controlling tropical pest populations. Insecticides were used at the highest amount when compared to herbicides or fungicides and crops like cotton, wheat and paddy demand the high usage of these chemicals (Subash et al. 2017; Baxter and Cummings 2006).
Previous studies on pesticide detection in water
According to a study conducted in Lake Tashk, Iran by Kafilzadeh (2015), the level of organochlorine pesticides was detected in the following manner: sediments > fish > water. This variation is mainly attributed to the reason that organochlorine pesticides are lipophilic and less soluble in water (Petrović et al. 2011). The concentration of dichlorodiphenyldichloroethylene (DDE) is higher than dichlorodiphenyltrichloroethane. The concentration of dichlorodiphenyltrichloroethane in water, sediment and fish tissue samples was 0.028, 5.220 and 4.218 ppb, respectively, while in case of dichlorodiphenyldichloroethylene, it was 0.075, 8.750 and 4.446 ppb, respectively. It has been deduced that dichlorodiphenyltrichloroethane has been applied in the past and over time, it has been degraded to its metabolite dichlorodiphenyldichloroethylene. Most of the other organochlorine pesticides like chlordane, lindane, and heptachlor were not detected in water but in fishes. It mostly gets accumulated in the fatty tissues of the fishes (Kafilzadeh 2015). Endosulfan concentration was found to be very high in lake sediments, i.e. 15.263 ppb, which can imply that if the sediment remixes with the lake water then the chemicals can be released back and hence, the cycle of toxicity continues (Kafilzadeh 2015). Bioaccumulation has been occurring at the site of contamination can be understood by the fact that the concentration of endosulfan in fish muscle tissue is 11 times higher than in water samples.
In line with the above findings, organochlorine pesticides like heptachlor, hexachlorocyclohexane, aldrin, eldrin, dichlorodiphenyldichloroethylene, endosulfan sulphate and endosulfan were detected in the sediments of Densu river basin in Ghana. The concentration was higher in the sediments than the river water. Endosulfan sulphate was of the highest concentration, i.e. 0.185 μg/L and some of the pesticides were higher than the prescribed levels by World Health Organisation (WHO) (Kuranchie-Mensah et al. 2012).
Hashmi et al. (2020) conducted a study in Tapi river of Gujarat (2019) where high levels of banned organochlorine pesticides such as endosulfan, methyl parathion, and dichlorodiphenyltrichloroethane were detected. This indicated the current usage of such pesticides in the surrounding areas and the lack of stringent law enforcement. The distribution of pesticides is described in Table 3.
Kaushik et al. (2012) conducted a study for the presence of organochlorine pesticides in the drinking water of rural areas of three districts in Haryana, India namely, Ambala, Gurgaon and Hisar during the pre- and post-monsoon seasons. The source of drinking water in Ambala and Gurgaon districts is groundwater while that of Hisar is surface water. They were tested for the presence of endosulfan, dichlorodiphenyldichloroethylene, hexachlorocyclohexane (HCH), and dieldrin. It was deduced that approximately, 37% of the samples was contaminated with total pesticide residues of above 500 ng/L which is much above the permissible limit. Additionally, seasonal variations were noticed during the observation period. Table 4 describes the various levels of detected pesticides.
Mutiyar et al. (2011) conducted a study in the Yamuna flood plain of Delhi. The presence of 17 major organochlorine pesticides was tested in 21 borewells and 5 ranney wells during post- and pre-monsoon seasons. It was detected that the water quality of borewells is better than the ranney wells as the concentration of organochlorine pesticides in Ranney wells was above the Bureau of Indian Standards (BIS), i.e. 1 μg/L. On the other hand, the levels are lower than Bureau of Indian Standards in borewells but it must be noted that the results are still quite close to the standard values set by the World Health Organisation (WHO) and European Union (EU), i.e. 0.5 μg/L. Thus, this can lead to human body impairments if water from such sources is consumed over an extended period.
Effects of pesticide pollution
Pesticide not only degrades water quality but also harms the biotic community. According to World Health Organisation, most of the diseases in developing countries are water borne and unhygienic water is related to almost 3.1% death (Pawari and Gawande 2015; Khan et al. 2013). In most of the developing countries, water bodies are the source of drinking water which implies that toxic water can directly hamper human health. It might cause neurological problems, cancer, tumours, reproductive defects, and immune system suppression (Srivastava et al. 2018). Few kinds of persistent pesticide can even lead to sodium, and potassium neurological imbalance. From an ecological point of view, biomagnification and bioconcentration are the most common effects. Pollinating insects like bees which fall under the category of non-target species are also affected by such chemical compounds (Srivastava et al. 2018). Alongside this, when chemicals like nitrogen and phosphorus get deposited in water, it leads to eutrophication resulting in dead zones in water bodies. Dissolved oxygen (DO) level shrinks down when the algal bloom decomposes at the bottom of the seafloor (Srivastava et al. 2018).
Organochlorine pesticides are hydrophobic and lipophilic in nature; hence, it is mostly reflected in the biotic community of the water body. Thus, if fishes of these biotic communities are ingested by humans then the chemical/toxic element enters into their body getting deposited in their fatty tissue (Srivastava et al. 2018). In comparison to organochlorine pesticides, organophosphorus pesticides and carbamates are less persistent, but they can affect the central nervous system by inhibiting the enzyme acetylcholinesterase (AChE) (Rani and Dhania 2014).
Issues with organochlorine pesticides
Organochlorine pesticides are the most persistent of all types of pesticide because of its stability and lipophilic nature. They are recalcitrant as they are resistant to degradation and persist in the environment for a very long time (Ajiboye et al. 2020; Chaussonnerie et al. 2016). The toxic properties of this pesticide vary according to its position of chlorine molecule. For example, if the chlorine atom in the dichlorodiphenyltrichloroethane ring is being substituted with a methoxide group then the level of toxicity decreases (Patnaik 2006). This kind of pesticide loses its efficacy over time leading to application in higher doses, thus scientists are intending to develop formulas which can be more efficient but easily biodegradable (Patnaik 2006). Due to lack of proper laws and regulation, overuse of organochlorine pesticides has led to accumulation of pesticides in soil and water (Chaussonnerie et al. 2016). Even banned/ obsolete pesticides like dichlorodiphenyltrichloroethane can be still detected in places almost 20 years after their application (Laura et al. 2013; Chaussonnerie et al. 2016; Valenzuela et al. 2020). The level of persistence of all kinds of organochlorine pesticides is not similar, it is mainly based on their chemical structure. Once this class of pesticides enters into the human system, it affects the central nervous system and the toxicity level is higher when it is directly inhaled than absorption through skin (Mnif et al. 2011). They are also responsible for the disruption of the endocrine system when consumed in higher levels (Mnif et al. 2011).
Biodegradation
Biodegradation has been defined as a process which involves degrading the organic components completely into simpler inorganic products (Laura et al. 2013). This process is carried out by microorganisms which derives their energy or nutrient requirements from the process and in turn degrade the xenobiotic compound (Laura et al. 2013). The microorganisms utilise the pesticides as a nutrient media for their growth while degrading the same into inorganic compounds like carbon dioxide and water (Huang et al. 2018). It is considered that in the microbial community, there is a diversity of microorganisms which are sufficient to deal with the complex and wide range of chemical compounds awaiting degradation (Mohammed and Bartakke 2015). Microbial populations are capable of interacting with the target compound both physically and chemically, ultimately either absolutely degrading, eliminating certain toxic properties or rendering it non-toxic by incorporating some structural changes (Laura et al. 2013). Bacteria, fungi and actinomycetes are the primary pesticide degraders. The degradation process sometimes produces by-products which are further degraded by mechanisms like cometabolism and mineralisation (Ye et al. 2018; Arora et al. 2012). Endosulfan, a toxic organochlorine pesticide when degraded by relevant bacterial species produces metabolites like endosulfan ether, endosulfan diol and endosulfan lactone which are of much lower toxicity than their parent compound (Kafilzadeh et al. 2015; Kamei et al. 2011).
Types of biodegradation
Biodegradation process depends on the capability of the microorganisms. In case of pesticide degradation, primarily three types of biodegradation come into the scenario which have been listed down:
Bacterial degradation: Bacterial population is highly efficient in pesticide degradation. The important groups are from the genus Bacillus, Klebsiella, Flavobacterium, Acinetobacter, Aerobacter, Alcaligenes, Micrococcus, Neisseria, Sphingomonas, Burkholderia, Pseudomonas, Micrococcus and Arthrobacter (Mohammed and Bartakke 2015; Mamta et al. 2015; Van Herwijnen et al. 2003). A number of studies have been carried out previously which intended to identify and isolate microorganisms which are capable of degrading persistent organochlorine pesticides (Mohammed and Bartakke 2015). They are isolated from contaminated sites and the techniques that are followed traditionally include enrichment culture, plating and screening (Huang et al. 2018). It has been studied that bacteria species like Bacillus polymyxa and Pseudomonas fluorescens can degrade aldrin efficiently in a short span of time, i.e. they can degrade aldrin by 48.2% and 43.2%, respectively, at a concentration of 0.2 mg. Table 5 enlists the bacteria that have the potential to degrade organochlorine pesticides.
It is normally observed that degradation is faster when mixed culture (two or more types of bacteria) is used instead of utilising a single strain (Sariwati et al. 2017). A study conducted by Doolotkeldieva et al. 2018 has stated that bacteria of genus Flavobacteria and Micrococcus are capable of degrading only 27.0% and 24.2%, respectively, of aldrin at the concentration level of 0.2 mg. But when both are present together, they can degrade aldrin rapidly within an incubation period of 12 days (Doolotkeldieva et al. 2018). In case of co-cultivation of bacteria, at certain times, the metabolites of one bacterium are utilised by another bacteria as nutrient media for their growth (Meleiro Porto et al. 2011).
Fungal degradation- Fungal population acts on pesticide residues converting into non-toxic products releasing them back into the soil and then they are further degraded by the bacterial population through processes such as cometabolism and mineralisation (Coelho et al. 2015; Zhang et al. 2011). The scientific community has been working to isolate specific fungal species having the capacity to degrade organochlorine pesticides. In the recent past, a lot of studies have attempted to understand the degrading capacity of endosulfan with the help of Aspergillus niger (Mukhtar et al. 2015; Grizca Boelan and Setyo Purnomo 2018; Kumar et al. 2008). It was deduced that A. niger can tolerate 400 mg/L of endosulfan and it got totally degraded after an incubation period of 12 days. This was confirmed as there were the change of pH and evolution of carbon dioxide. Table 6 shows various species of fungi that have exhibited their capacity of degrading organochlorine pesticides.
Enzymatic degradation- The potential of microbial degradation of xenobiotic compounds is derived from the enzymes present in microorganisms (Tang et al. 2017; Chen et al. 2011). According to a study conducted by Bazhanov et al. (2016), the pesticide Atrazine was intended to be degraded by Pseudomonas sp strain ADP. It utilised Atrazine as the nutrient source and with the help of 3 enzymes (viz., AtzA, AtzB, and AtzC), it finally got degraded into carbon dioxide and ammonia after producing intermediates like hydroxyl atrazine and N-isopropyl cyanuric amide (Ortega et al. 2011; Rebelo et al. 2007). Enzymes are having much higher efficiency than the microorganisms from which it can be isolated. They eliminate the functional group of the parent compound to reduce the toxicity levels (Satish et al. 2017; Besse-Hoggan et al. 2009). In case of degradation of dieldrin, it has been noticed that photodieldrin is a metabolite formed and it is assumed that epoxide hydrolase is the enzyme responsible for this conversion, but there is a lack of literature to support this view (Purnomo 2017; Singh et al. 2018).
Enzymes like hydrolases, transferases, ligases and isomerases are the ones that mostly degrade pesticide residues (Verma et al. 2014a, b). Most of the literature available for enzymes mainly focuses on organophosphorus pesticides. Dehydrogenase, hydrolytic and dehydrochlorination are the most important types of enzymes proposed for organochlorine pesticide degradation but still research needs to be conducted extensively on it (Javaid et al. 2016).
Mechanism and steps of degradation
The microbial degradation of pesticide residues progresses in various steps and it is shown in Fig. 1. The main processes through which the degradation takes place are oxidation, reduction, hydrolysis, dehydrogenation, dehalogenation, decarboxylation, rearrangements, conjugations, isomerisation (Hugo et al. 2016). Table 7 shows the different kinds of oxidation, reduction and hydrolysis processes that takes place through which the complex molecules are broken down into simpler inorganic compounds (Hugo et al. 2016).
The main enzymes responsible for biotransformation of pesticide molecules belong to four classes namely hydrolases, translocases, oxidoreductases and transferases (Fernández-López et al. 2017). The classification of these enzymes are listed down below:
-
1.
Hydrolases: A-esterases is an important class of enzymes which includes phosphodiesterase, phosphotriesterase and monophosphates (De la Peña Mattozzi et al. 2006). They are useful in degradation of carbamate and organophosphate compounds (Pointing 2001).
-
2.
Oxidoreductases: They can be classified into peroxidases, monooxygenases (e.g. cytochromeP450), dioxygenase, oxidases. Cytochrome P450 participates in oxidation, reduction and oxidative breakdown of pesticides (Lamb et al. 2009). This enzyme is present in the endoplasmic reticulum of eukaryotes (Sevrioukova and Poulos 2011).
Peroxidases and oxidases can execute redox reactions. This enzyme is present in fungi, bacteria, plants but the degradation of xenobiotics are reported from enzymes secreted by fungi (Lamb et al. 2009). It has been observed in the past that the peroxidases isolated from ligninolytic fungi can degrade lignin (Sevrioukova and Poulos 2011).
-
3.
Transferases: There are many kinds of transferases, but it primarily depends on the group they conjugate to the pesticide molecule such as acetyl transferases, methyl transferases and glutathione S transferases (De la Peña Mattozzi et al. 2006). Glutathione S transferases is mainly responsible for biodegradation activities. They exhibit peroxidase and hydrolytic degradation. It can also participate in de-halogenation of ring ((Hervé et al. 2008).
-
4.
Translocases: They serve as pumps and only limited variety of them have been identified for biodegradation purposes. This kind of enzymes can catalyse the transfer mechanism of a molecule from one cell compartment to another (Copley 2009).
Oxidation process: It forms an important mechanism for pesticide degradation. It increases the water solubility of the compound and in most of the cases alters the bioactivity of the parent compound. The reactive forms of oxygen involved in the process are hydrogen peroxide, superoxide ion and hydroxyl ion (Patil et al. 2014). This process can be inhibited by high levels of pH (alkalinity) (Chen et al. 2019).
Xiao et al. (2011) studied the degradation pathway of dieldrin by fungal Phlebia sp. Three chosen strains were Phlebia acanthocystis, Phlebia aurea and Phlebia brevispora and they were incubated in a low nitrogen media for 42 days. It was seen that they could degrade dieldrin by 50% and the main by-products of the degradation process were 9-hydroxyaldrin and two compounds of the carboxylic acid group. Thus, the degradation of the primary substrate mainly took place by hydroxylation reaction.
The degradation pathway of dichlorodiphenyltrichloroethane by Stenotrophomonas sp. has been studied by Pan et al. (2016) which revealed the various intermediate compounds formed before the compound gets mineralised. The primary mechanisms involved are hydroxylation and carboxylation.
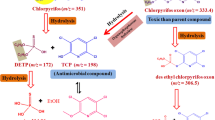
Hydrolysis: Hydrolysis (cleavage) of esters result in two fragments which are nontoxic compared to its parent compound. Mild alkaline solutions can induce hydraulic activity leading to rapid degradation while for acid induced hydrolysis strong acidic (pH 3–4) environment is required (Katagi 2002). An example of this phenomenon is that the degradation of endosulfan mainly takes place through oxidation and hydrolysis and produces metabolites like endosulfan diol and endosulfan sulphate (Katagi 2002).
Reduction: These reactions of pesticide undergoes under certain factors such as- low pH, anaerobic conditions as well as in the presence of anaerobic microorganisms who are capable of carrying out the degradation under the above-mentioned conditions (Katagi 2018). Such reductions of pesticide occur under conditions like stagnant water, bogs, eutrophic lakes (Cycoń et al. 2017).
Hexachlorocyclohexane degradation involves dehalogenation through which the toxicity is reduced. This process replaces chlorine (which imparts the toxic nature to the compound) by hydrogen atom or hydroxyl group. After the removal of chlorine, the less toxic metabolites can be further degraded. Thus, it can be deduced that the primary pathway of hexachlorocyclohexane degradation involves reductive and nonreductive elimination, as well as substitution (Chen et al. 2015).
Rearrangements: In these reactions, structural modifications of the pesticide molecule takes place which reduces the toxic properties of the compound considerably (Dyguda-Kazimierowicz et al. 2014).
Cyclisation: In these many acyclic molecules sometimes form a cycling ring and the reaction is catalysed by factors like light, pH and enzymes. The formation of photodieldrin from dieldrin is a classic example of the same (Cycoń et al. 2017).
Dimerisation: This step takes place after the molecule is initially degraded by conjugation or similar processes. Under extreme conditions of light and heat, the pesticide molecules can form dimers, trimers or even polymers but under moderate conditions mainly dimers are formed (Burrows et al. 2002).
Biosorption: The biosorption process takes place on the cell surface and a physicochemical interaction takes place between the xenobiotic and the functional group of the microbial cell. This process undergoes passively in the absence of living biomass and is much faster than bioaccumulation. It is also inexpensive than the latter (Jin et al. 2018).
Bioaccumulation: This process takes place actively where the presence of living biomass forms an important criteria. The xenobiotic gets attracted to the charged functional groups of the microbial cell leading to bioaccumulation. Some of the charged functional groups involved are amide, amine, ketone, carboxyl, and aldehyde (Zabochnicka-Świątek and Krzywonos 2014). This occurs mostly intracellular and the process is much more efficient than biosorption in terms of the degradation of pesticide residues. Since this process involves living biomass, it demands high energy (Zabochnicka-Świątek and Krzywonos 2014).
Criteria for biodegradation
Biodegradation process needs to meet certain criteria in order to be carried out in an efficient manner.
-
1.
Cost efficiency: This technique should be more economical than the other available techniques for biodegradation (Fernández-López et al. 2017).
-
2.
Correct strains: The chosen strain must be capable of degrading the xenobiotic at a considerable speed in order to bring down the concentration (Satish et al. 2017). The adaptability of the microorganisms should be considerably high for the process to operate without any hindrance (Hugo et al. 2016). The microorganism should be able to adapt with the environmental conditions or adapt their enzymatic pathway in order to break down newly introduced species (Zhang et al. 2015; Hussain et al. 2016).
It must be understood that enzymes present in certain microorganisms can be efficient in degrading a particular pesticide without producing toxic metabolite but the same might not be suitable to degrade another kind of pesticide residue. Mycobacterium tuberculosis ESD is capable of degrading toxic beta endosulfan to metabolites like hydroxyether and monoaldehyde which are of lower toxicity. On the other hand, it converts alpha endosulfan to more toxic endosulfan sulphate (Weir et al. 2006). Bacterial species like Burkholderia cepaeiahydrolysis and Pseudomonas aeruginosa produce lower toxic metabolite like endosulfan diol when the enzyme present in it degrades endosulfan by hydrolysis (Kumar et al. 2007).
-
3.
Optimum environmental conditions: Environmental conditions like pH, temperature, salinity, availability of water needs to be at an optimum level to carry out proper degradation (Jin et al. 2018). In a study conducted by Pan et al. (2016), it was observed that the optimum degradation of dichlorodiphenyltrichloroethane by Stenotrophomonas sp. took place at pH 7.0, temperature 30 °C with an initial concentration of 10 mg/L. But when the temperature is increased further, the degradation rate and the growth of microorganism slow down considerably. In line with the above study, the degradation of dichlorodiphenyltrichloroethane by Sphingobacterium sp. is the best at neutral pH conditions and at a temperature of 30 °C (Purnomo 2017).
Bacteria or fungi that can degrade pesticides over a wide range of pH are mainly preferred. Kataoka et al. (2010) studied the degradation of organochlorine pesticide dieldrin by Mucor racemosus strain DDF at different pH levels of 4.0, 6.0 and 8.0. It was observed that this species of fungi can degrade the pesticide under a wide range of pH. Some of the bacterial strains like Bacillus subtilis and Bacillus cereus can degrade aldrin to its metabolite exo-dieldrin only under pH range of 5–9.5 (Purnomo 2017). Thus, maintaining optimum pH conditions forms an important aspect of biodegradation.
-
4.
Presence of microorganisms: The number of microorganism present must be high enough to degrade the pesticide residues and the microorganisms should be in contact with the target compounds.
-
5.
Availability of nutrients: The nutrients should be enough to support the growth of microorganisms. In case of cometabolism, there should be an alternative source for carbon. It has been studied that PAH (Polycyclic Aromatic Hydrocarbon) compounds can be degraded faster if C:N:P ratios are adjusted. Thus, under certain conditions, ammonia and phosphate need to be added in order to rectify the nutrient ratios (Huang et al. 2018).
In a study, the effect of nutrient amendments was tested to understand its effect on the rate of degradation of aldrin and dieldrin. It was observed that aldrin was degraded by 84-96% while dieldrin degraded by 50-79% in the presence of inositol and xylose after an incubation period of 60 days. These rates were quite high when compared to the control samples (without the introduction of any nutrient amendments). Thus, it was assumed that there is a positive impact of these amendments on the degradation rate of microorganisms as it might act as an excellent nutrient media for accelerated growth (Purnomo 2017). Another study on the degradation of dichlorodiphenyltrichloroethane by Sphingobacterium sp. revealed that the additional source of nutrients like glucose, yeast extract, fructose and sucrose reduced the degradation time. The addition of glucose seems to have the best impact on degradation (Fang et al. 2010).
-
6.
Toxicity: The toxicity of the target compound is extremely important criteria to access the efficiency of microbial degradation. In many cases, when the concentration of the substrate is high, the level of degradation reduces (Satish et al. 2017). A particular microorganism can degrade a pesticide efficiently only up to a certain amount present and above that can be toxic for the microorganism. According to a study, it was noticed that Sphingobacterium sp. was incapable of degrading dichlorodiphenyltrichloroethane when the concentration was as high as 50 mg/L (Meleiro Porto et al. 2011). It is stated that for a certain genus of bacteria like Bacillus polymyxa, Micrococcus sp., Flavobacterium sp., and Pseudomonas fluorescens, the maximum dose of aldrin above which the growth of bacteria will be inhibited is 0.01 mg/kg. However, this value can differ depending on the microorganism intending to degrade the pesticide residue.
Jesitha et al. (2015) conducted an experiment where they studied the rate of degradation of endosulfan by Pseudomonas fluorescens. The initial concentration of the substrate was varied between 350 and 550 μg/L. The degradation was consistently high but once the concentration was above 550 μg/L, the degradation rate steadily decreased. It was assumed that this phenomenon occurred due to the accumulation of beta endosulfan, a metabolite produced during endosulfan degradation (El et al. 2014).
-
7.
Pesticides structure: Pesticides structure forms an important aspect for faster degradation. The molecular weight, chemical and spatial structure, composition of the pesticides are the important factors. Polymers are comparatively more resistant to degradation than low molecular weight compounds (Ye et al. 2018). Pesticides are chemically synthesised which is introduced into the environment much faster than the rate at which the degrading microbial species are evolving.
Strategies for enhancing biodegradation
The rate of biodegradation is manipulated by certain methods in order to degrade the xenobiotic faster. Mostly three methods are applied which have been discussed here. (a) Bioattenuation: It refers to the natural degrading potential of the microorganisms to degrade the xenobiotics/pesticide residues. The time consumed depends on the complexity of the compounds. This process converts the target compounds into less toxic ones and the processes undertaken are, namely, biodegradation, sorption, and volatilisation (Vásquez-Murrieta et al. 2016; Perelo 2010). (b) Bioaugmentation:- Microbial species will be introduced in the site of contamination to enhance the catabolic activity. This process is termed as bioaugmentation (Nzila et al. 2016; Head and Oleszkiewicz 2004). (c) Biostimulation: In order to enhance microbial activity, nutrient supply to the site of contamination is increased to enhance the enzymatic activity. This process is called biostimulation (Kanissery and Sims 2011; Ortíz et al. 2013). According to the research conducted by Sun et al. (2020), when the concentration of atrazine is increased by 20–200 folds, it is seen that the degradation rate also increases which signifies the importance of biostimulation.
Mechanism of pesticide transformation
Pesticides are naturally degraded by the biotic mass in soil. Most of the time, the by-products are further degraded by microorganisms. On the contrary, pesticides like dichlorodiphenyltrichloroethane, endosulfan are persistent in nature and get accumulated ultimately leading to biomagnification (Lushchak et al. 2018). Pesticide either gets transformed through biotic and abiotic components or it remains unaltered as it does not undergo any transformation (Srivastava et al. 2018).
Abiotic transformation: In abiotic transformation the main two kinds of transformation are physical and chemical transformation (Cycon and Piotrowska-Seget 2006). The mechanisms involved in physical transformation are aggregation, diffusion, dispersion, deposition, and adsorption while those involved in chemical transformation are hydrolysis, oxidation and reduction (Coelho et al. 2015).
Biotic transformation: The two mechanisms for biotic transformation are cometabolism and mineralisation (Purnomo 2017).
Cometabolism: It mainly refers to the process where the substance that is to be degraded does not act as the source of food for the microorganism. Thus, they get attached to another substrate to fulfil their nutrient demands which eventually also gets degraded (Coelho et al. 2015). The by-products are comparatively less toxic and complex than their parent compounds. It has been observed through studies that cometabolism creates multiple reactions which are more efficient in pesticide degradation when compared to a single set of reactions (Huang et al. 2018). It has been studied that in order to degrade dichlorodiphenyltrichloroethane, cometabolism plays a very important role. The growth of the microorganism depends on an alternative source of carbon while they degrade the target compounds. Consequently, the process is much faster and efficient (Hussain et al. 2016; Wanguyun and Geraldi 2019).
Mineralisation: The term implies the breakdown of complex organic substances into much simpler inorganic products like carbon dioxide, and water (Eissa et al. 2014).
No transformation: Organochlorine pesticides are persistent or recalcitrant in nature; thus, it does not undergo enough transformation over time. Hence, the parent compound gets accumulated on either soil or water components of nature (Lushchak et al. 2018).
The mechanism of pesticide metabolism takes place in three distinct steps. The parent xenobiotic compound is ultimately transformed into non-toxic products and thus harmless to the environment (Purnomo 2017). The enzymes present in the microorganisms are solely responsible for this breakdown of organic compounds into simple inorganic products. The reactions are discussed sequentially in Fig. 2.
Enzymatic attack of the xenobiotic compound takes place through certain steps and ultimately degrades it into final metabolites which are either of lower toxicity or nontoxic in nature. There are certain intermediate compounds which are formed in this degradation process (Mathieu et al. 2015). It must be noted that degradation of the same xenobiotic by different microorganisms can produce different/ similar final and intermediate metabolites of varying quantity (Kafilzadeh et al. 2015). Table 8 shows different metabolites produced in the microbial degradation pathway of certain organochlorine pesticide residues and their corresponding quantities. Figure 3 depicts the pathways that are followed during the various transformation processes.
Challenges
The microbial degradation of pesticides is proposed as one of the effective methods to reduce the pesticide pollution load. Bacteria and fungi are considered as the most effective ones for the degradation process. The enzymes present in the microorganisms are the responsible factor for carrying out the process and it is comparatively much more efficient and faster than the microorganisms in which they are present. Thus, scientists are emphasising on isolating relevant enzymes in order to degrade pesticide residues (Verma et al. 2014a, b; Urlacher et al. 2004). Nevertheless, the enzymes cannot withstand adverse environmental conditions and they are comparatively immobile posing a problem for utilising it in long time research activities (Huang et al. 2018). It is also important to understand the degradation pathway of the microorganisms which helps in predicting the by-products of microbial degradation (Satish et al. 2017). Sometimes, the by-products produced after degradation of the parent compound are toxic which can accumulate in the environment and can also reduce the degradation rate of parent compound (Verma et al. 2014a, b). Alongside these, the process of biodegradation has its own disadvantages which have been listed down below.
Time: In comparison to the physicochemical processes, biological degradation requires a much longer time (Verma et al. 2014a, b).
Effectiveness: Screening of relevant and effective microorganisms still remains a problem because of the varying complexity of pesticide (Vikrant et al. 2018; Verma et al. 2014a, b). Also, it must be considered that certain strains can degrade only specific pesticides which makes it more important to build a wide database for microbial strains through extensive research (Bernardino et al. 2012).
Operating conditions: In the laboratory, there have been various efforts to conduct microbial degradation of pesticide residues under controlled conditions but conducting this process directly on the contaminated areas still remains a problem because of the unstable and wide range of environmental conditions (Sun et al. 2020). The concentration of pesticides which are considered for laboratory experiments is considerably high or maximum (0.5 mg to 50 mg/kg) which can only occur under accidental spillages or immediately after application (Fenner et al. 2013). Also, pesticides are sometimes adsorbed onto the soil particles limiting its bioavailability (Girardi et al. 2013). It has been demonstrated in previous studies that the degradation rate might not necessarily be the same under lower and higher concentrations. Thus, the value of mineralisation can differ a lot under both conditions (laboratory and real time) (Dechesne et al. 2010).
Bioavailability: Microorganisms might be present in the contaminated areas but the presence of xenobiotic in a diffused manner might limit the degradation process (Dechesne et al. 2010). Also, sometimes different compositions of pesticides are mixed and applied which might limit the degrading capability of the microorganism (Rosenbom et al. 2014).
Conclusion
In the current scenario, the usage of pesticides has increased indiscriminately leading to environmental pollution specifically soil and water pollution. There are a variety of pesticides available but the most persistent is organochlorine pesticides which is resistant to degradation. To reduce the pollution load, there have been continuous efforts from the scientific community to come up with innovative efforts like biological degradation. The process takes place via microorganisms like bacteria and fungi while enzymes form an important aspect as the detoxification of xenobiotics takes place through it. Though this process is an eco-friendly, feasible and promising approach but there are some limitations related to it. The biochemical pathways followed by the microorganisms are greatly dependent on the external environmental conditions. Thus, there are still certain areas where further research needs to be conducted to claim the success of this approach.
The future scope of research is huge in this area. Since enzymatic degradation poses as a promising approach, it becomes increasingly important to do extensive research in order to identify enzymes that are capable of degrading organochlorine pesticides. Since microbial degradation takes place at a much slower rate and is sometimes not as efficient or easy to conduct as conventional bioremediation technologies; hence, it is advisable to take a mutualistic approach. This approach suggests involving both microbial degradation process and conventional physiochemical approaches at the same time to speed up the breakdown process (Arya et al. 2017). Developing relevant and robust microbial strains via genetical engineering and biotechnology is very important. Moreover, appropriate genes responsible for the degradation process need to be targeted and isolated for further studies (Sun et al. 2020). Thus, it can be expected that with a collaborative technical approach, the downside of this environmentally friendly technique can be negated.
Abbreviations
- AChE:
-
Acetylcholinesterase
- BIS:
-
Bureau of Indian Standards
- DBP:
-
Dibutyl phthalate
- DDA:
-
2,2-Bis(p-chlorophenyl) acetate
- DDD:
-
Dichlorodiphenyldichloroethane
- DDE:
-
Dichlorodiphenyldichloroethylene
- DDMU:
-
1-Chloro-2–2-bis-(4ʹ-chlorophenyl) ethylene
- DDNS:
-
2,2-Bis(p-chlorophenyl) ethane
- DDT:
-
Dichlorodiphenyltrichloroethane
- DO:
-
Dissolved Oxygen
- EU:
-
European Union
- HCH:
-
Hexachlorocyclohexane
- OCP:
-
Organochlorine pesticides
- OPP:
-
Organophosphorus pesticides
- PAH:
-
Polycyclic Aromatic Hydrocarbon
- WHO:
-
World Health Organisation
References
Abdul Salam J, Lakshmi V, Das D, Das N (2013) Biodegradation of lindane using a novel yeast strain, Rhodotorula sp. VITJzN03 isolated from agricultural soil. World J Microbiol Biotechnol 29:475–487. https://doi.org/10.1007/s11274-012-1201-4
Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Resour Prot 2(5):432–448
Ajiboye TO, Kuvarega AT, Onwudiwe DC (2020) Recent strategies for environmental remediation of organochlorine pesticides. Appl Sci 10:6286. https://doi.org/10.3390/app10186286
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12. https://doi.org/10.2478/v10102-009-0001-7
Alvarez A, Benimeli C, Saez J, Fuentes M, Cuozzo S, Polti M, Amoroso M (2012) Bacterial bio-resources for remediation of Hexachlorocyclohexane. Int J Mol Sci 13(12):15086–15106
Aresta A, Marzano CN, Lopane C, Corriero G, Longo C, Zambonin C, Stabili L (2015) Analytical investigations on the lindane bioremediation capability of the demosponge Hymeniacidon perlevis. Marine Pollut Bul 90(1–2):143–149
Arora PK, Sasikala C, Ramana CV (2012) Degradation of chlorinated nitroaromatic compounds. Appl Microbiol Biotechnol 93:2265–2277. https://doi.org/10.1007/s00253-012-3927-1
Arya R, Kumar R, Mishra NK, Sharma AK (2017) Microbial flora and biodegradation of pesticides: trends, scope, and relevance. Advances in Environmental Biotechnology. Springer, Singapore, pp 243–263
Asemoloye MD, Ahmad R, Jonathan SG (2017) Synergistic rhizosphere degradation of γ-hexachlorocyclohexane (lindane) through the combinatorial plant-fungal action. PLoS ONE. https://doi.org/10.1371/journal.pone.0183373
Babu BR, Meera KMS, Venkatesan P (2011) Removal of pesticides from wastewater by electrochemical methods—a comparative approach. Sustain Environ Res 21:401–406
Baxter J, Cummings SP (2006) The application of the herbicide bromoxynil to a model soil-derived bacterial community: impact on degradation and community structure. Lett Appl Microbiol 43:659–665. https://doi.org/10.1111/j.1472-765X.2006.02003
Bazhanov DP, Li C, Li H, Li J, Zhang X, Chen X, Yang H (2016) Occurrence, diversity and community structure of culturable atrazine degraders in industrial and agricultural soils exposed to the herbicide in Shandong Province. PR China BMC Microbiol 16(1):265
Ben Chekroun K, Sánchez E, Baghour M (2014) The role of algae in bioremediation of organic pollutants. Int Res J Public Environ Heal 1:19–32
Benimeli CS, Fuentes MS, Abate CM, Amoroso MJ (2008) Bioremediation of lindane contaminated soil by Streptomyces sp. M7 and its effects on zea mays growth. Int Biodeterior Biodegradation 61:233–239. https://doi.org/10.1016/j.ibiod.2007.09.001
Bernardino J, Bernardette A, Ramrez-Sandoval M, Domnguez-Oje D (2012) Biodegradation and bioremediation of organic pesticides. In: Pesticides—Recent Trends in Pesticide Residue Assay
Besse-Hoggan P, Alekseeva T, Sancelme M et al (2009) Atrazine biodegradation modulated by clays and clay/humic acid complexes. Environ Pollut 157:2837–2844. https://doi.org/10.1016/j.envpol.2009.04.005
Bhalerao TS, Puranik PR (2007) Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate. Aspergillus niger. Int BiodeteriorationBiodegradation 59(4):315–321
Birolli WG, Yamamoto KY, de Oliveira JR et al (2015) Biotransformation of dieldrin by the marine fungus Penicillium miczynskii CBMAI 930. Biocatal Agric Biotechnol 4:39–43. https://doi.org/10.1016/j.bcab.2014.06.002
Briz V, Molina-Molina J-M, Sánchez-Redondo S et al (2011) Differential estrogenic effects of the persistent organochlorine pesticides dieldrin, endosulfan, and lindane in primary neuronal cultures. Toxicol Sci 120:413–427. https://doi.org/10.1093/toxsci/kfr019
Burrows HD, Canle LM, Santaballa JA, Steenken S (2002) Reaction pathways and mechanisms of photodegradation of pesticides. J Photochem Photobiol B Biol 67:71–108. https://doi.org/10.1016/S1011-1344(02)00277-4
Chaussonnerie S, Saaidi PL, Ugarte E et al (2016) Microbial degradation of a recalcitrant pesticide: chlordecone. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.02025
Chen S, Hu Q, Hu M et al (2011) Isolation and characterization of a fungus able to degrade pyrethroids and 3-phenoxybenzaldehyde. Bioresour Technol 102:8110–8116. https://doi.org/10.1016/j.biortech.2011.06.055
Chen H, Gao B, Wang S, Fang J (2015) Microbial Degradation of hexachlorocyclohexane (HCH) Pesticides. In: Advances in Biodegradation and Bioremediation of Industrial Waste. CRC Press, Boca Raton, pp 181–209
Chen S, Deng J, Deng Y, Gao N (2019) Influencing factors and kinetic studies of imidacloprid degradation by ozonation. Environ Technol 40:2127–2134. https://doi.org/10.1080/09593330.2018.1439105
Clyde LO (2013) Spray drift of pesticide. Neb guide. Retrieved from https://en.wikipedia.org/wiki/Pesticide_drift
Coelho LMM, Rezende HC, Coelho LMM et al (2015) Bioremediation of polluted waters using microorganisms. in: advances in bioremediation of wastewater and polluted soil. InTech, pp 1–23
Copley SD (2009) Evolution of efficient pathways for degradation of anthropogenic chemicals. Nat Chem Biol 5:559–566. https://doi.org/10.1038/nchembio.197
Cuozzo SA, Fuentes MS, Bourguignon N et al (2012) Chlordane biodegradation under aerobic conditions by indigenous Streptomyces strains. Int Biodeterior Biodegradation 66:19–24. https://doi.org/10.1016/j.ibiod.2011.09.011
Cycoń M, Mrozik A, Piotrowska-Seget Z (2017) Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: a review. Chemosphere 172:52–71. https://doi.org/10.1016/j.chemosphere.2016.12.129
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011:1–13. https://doi.org/10.4061/2011/941810
de Brião GV, de Andrade JR, da Silva MGC, Vieira MGA, (2020) Removal of toxic metals from water using chitosan-based magnetic adsorbents. A review. Environ Chem Lett 18:1145–1168. https://doi.org/10.1007/s10311-020-01003-y
de la Peña Mattozzi M, Tehara SK, Hong T, Keasling JD (2006) Mineralization of paraoxon and its use as a sole C and P source by a rationally designed catabolic pathway in pseudomonas putida. Appl Environ Microbiol 72(10):6699–6706
Dechesne A, Owsianiak M, Bazire A et al (2010) Biodegradation in a partially saturated sand matrix: compounding effects of water content, bacterial spatial distribution, and motility. Environ Sci Technol 44:2386–2392. https://doi.org/10.1021/es902760y
Doolotkeldieva T, Konurbaeva M, Bobusheva S (2018) Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bioremediation possibilities. Environ Sci Pollut Res 25:31848–31862. https://doi.org/10.1007/s11356-017-0048-5
Dsikowitzky L, Schwarzbauer J (2014) Industrial organic contaminants: identification, toxicity and fate in the environment. Environ Chem Lett 12:371–386. https://doi.org/10.1007/s10311-014-0467-1
Dyguda-Kazimierowicz E, Roszak S, Sokalski WA (2014) Alkaline hydrolysis of organophosphorus pesticides: the dependence of the reaction mechanism on the incoming group conformation. J Phys Chem B 118:7277–7289. https://doi.org/10.1021/jp503382j
Eissa FI, Mahmoud HA, Massoud ON, et al (2014) Biodegradation of chlorpyrifos by microbial strains isolated from agricultural wastewater. J Am Sci 10:98–108
El O, Elsaid G, Abdelbagi AO et al (2014) Microbial degradation of high endosulfan concentrations in carbon free media. International Conference on Biological, Civil and Environmental Engineering (BCEE-2014) March 17–18, 2014 Dubai (UAE). International Institute of Chemical, Biological & Environmental Engineering, pp 17–18
Fang H, Dong B, Yan H et al (2010) Characterization of a bacterial strain capable of degrading DDT congeners and its use in bioremediation of contaminated soil. J Hazard Mater 184:281–289. https://doi.org/10.1016/j.jhazmat.2010.08.034
Fenner K, Canonica S, Wackett LP, Elsner M (2013) Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 341:752–758. https://doi.org/10.1126/science.1236281
Fernández-López MG, Popoca-Ursino C, Sánchez-Salinas E et al (2017) Enhancing methyl parathion degradation by the immobilization of Burkholderia sp. isolated from agricultural soils. Microbiologyopen 6:e00507. https://doi.org/10.1002/mbo3.507
Ferrusquía-García CJ, Amaya-Chávez A, Pavón-Silva TB, García-Fabila MM (2008) Evaluación de la degradación de metil paratión en solución usando fotocatálisis heterogénea. Rev Latinoam Recur Nat 4:285–290
Gad WA (2020) Biodegradation of organochlorine pesticides by means of Pectobacterıum wasabiae. Int J Oceanogr Aquac 4. https://doi.org/10.23880/ijoac-16000183
García A, Amat AM, Arques A et al (2006) Detoxification of aqueous solutions of the pesticide “Sevnol” by solar photocatalysis. Environ Chem Lett 3:169–172. https://doi.org/10.1007/s10311-005-0026-x
Ghaffar A, Tabata M, Mashiatullah A, Alaamer AS (2013) Efficient DDT and trichlorophenol detoxification using NaBH4 and Devarda alloy. Environ Chem Lett 11:197–202. https://doi.org/10.1007/s10311-012-0397-8
Girardi C, Nowak KM, Carranza-Diaz O et al (2013) Microbial degradation of the pharmaceutical ibuprofen and the herbicide 2,4-D in water and soil — Use and limits of data obtained from aqueous systems for predicting their fate in soil. Sci Total Environ 444:32–42. https://doi.org/10.1016/j.scitotenv.2012.11.051
Grizca Boelan E, Setyo Purnomo A (2018) Abilities of co-cultures of white-rot fungus Ganoderma lingzhi and bacteria bacillus subtilis on biodegradation DDT. J Phys Conf Ser 1095:012015. https://doi.org/10.1088/1742-6596/1095/1/012015
Hashmi TA, Qureshi R, Tipre D, Menon S (2020) Investigation of pesticide residues in water, sediments and fish samples from Tapi River, India as a case study and its forensic significance. Environ Forensics 21:1–10. https://doi.org/10.1080/15275922.2019.1693441
Head MA, Oleszkiewicz JA (2004) Bioaugmentation for nitrification at cold temperatures. Water Res 38:523–530. https://doi.org/10.1016/j.watres.2003.11.003
Hervé C, de Franco P-O, Groisillier A et al (2008) New members of the glutathione transferase family discovered in red and brown algae. Biochem J 412:535–544. https://doi.org/10.1042/BJ20071464
Huang Y, Xiao L, Li F et al (2018) Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A Review. Molecules 23:2313. https://doi.org/10.3390/molecules23092313
Hugo HJ, Mouton C, Malan AP (2016) Accelerated microbial degradation of nematicides in vineyard and orchard soils. South African J Enol Vitic 35:157–167. https://doi.org/10.21548/35-1-998
Hui T, Xiujuan L, Qifa S et al (2020) Evaluation of drinking water quality using the water quality index (WQI), the synthetic pollution index (SPI) and geospatial tools in Lianhuashan district, China. Polish J Environ Stud 30:141–153. https://doi.org/10.15244/pjoes/120765
Hussain S, Hartley CJ, Shettigar M, Pandey G, Heipieper H (2016) Bacterial biodegradation of neonicotinoid pesticides in soil and water systems. FEMS Microbiology Lett 363(23):fnw252
India - pesticides production volume 2020 | Statista (2020) Statista. https://www.statista.com/statistics/726938/india-pesticides-production-volume/#:~:text=In%20fiscal%20year%202020%2C%20the,the%20country%20is%20highly%20diversified
Javaid MK, Ashiq M, Tahir M (2016) Potential of biological agents in decontamination of agricultural soil. Scientifica (Cairo) 2016:1–9. https://doi.org/10.1155/2016/1598325
Jayashree R, Vasudevan N (2007) Effect of tween 80 added to the soil on the degradation of endosulfan by Pseudomonas aeruginosa. Int J Environ Sci Technol 4:203–210. https://doi.org/10.1007/BF03326275
Jesitha K, Nimisha KM, Manjusha CM, Harikumar PS (2015) Biodegradation of endosulfan by Pseudomonas fluorescens. Environ Process 2:225–240. https://doi.org/10.1007/s40710-015-0059-5
Jin Y, Luan Y, Ning Y, Wang L (2018) Effects and mechanisms of microbial remediation of heavy metals in soil: a critical review. Appl Sci 8:1336. https://doi.org/10.3390/app8081336
Joko T, Anggoro S, Sunoko HR, Rachmawati S (2017) Pesticides usage in the soil quality degradation potential in Wanasari subdistrict, Brebes, Indonesia. Appl Environ Soil Sci 2017:1–7. https://doi.org/10.1155/2017/5896191
Kafilzadeh F (2015) Assessment of organochlorine pesticide residues in water, sediments and fish from lake Tashk. Iran Achiev Life Sci 9:107–111. https://doi.org/10.1016/j.als.2015.12.003
Kafilzadeh F, Ebrahimnezhad M, Tahery Y (2015) Isolation and identification of endosulfan-degrading bacteria and evaluation of their bioremediation in Kor river. Iran Osong Public Heal Res Perspect 6:39–46. https://doi.org/10.1016/j.phrp.2014.12.003
Kamei I, Takagi K, Kondo R (2011) Degradation of endosulfan and endosulfan sulfate by white-rot fungus Trametes hirsuta. J Wood Sci 57:317–322. https://doi.org/10.1007/s10086-011-1176-z
Kanissery RG, Sims GK (2011) Biostimulation for the enhanced degradation of herbicides in soil. Appl Environ Soil Sci 2011:1–10. https://doi.org/10.1155/2011/843450
Karthik V, Kumar PS, Vo D-VN et al (2020) Hydrothermal production of algal biochar for environmental and fertilizer applications: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01139-x
Katagi T (2002) Abiotic hydrolysis of pesticides in the aquatic environment. Rev Environ Contam Toxicol 175:79–261
Katagi T (2018) Direct photolysis mechanism of pesticides in water. J Pestic Sci 43:57–72. https://doi.org/10.1584/jpestics.D17-081
Kataoka R, Takagi K, Kamei I et al (2010) Biodegradation of dieldrin by a soil fungus isolated from a soil with annual endosulfan Applications. Environ Sci Technol 44:6343–6349. https://doi.org/10.1021/es1000227
Kaur M, Sharma JK, Gill JP et al (2008) Determination of organochlorine pesticide residues in freshwater fish species in Punjab, India. Bull Environ Contam Toxicol 80:154–157. https://doi.org/10.1007/s00128-007-9335-z
Kaur H, Kapoor S, Kaur G (2016) Application of ligninolytic potentials of a white-rot fungus Ganoderma lucidum for degradation of lindane. Environ Monit Assess 188:588. https://doi.org/10.1007/s10661-016-5606-7
Kaushik CP, Sharma HR, Kaushik A (2012) Organochlorine pesticide residues in drinking water in the rural areas of Haryana, India. Environ Monit Assess 184:103–112. https://doi.org/10.1007/s10661-011-1950-9
Khambay BPS, Jewess PJ (2005) Pyrethroids. in: comprehensive molecular insect science. Elsevier, Amsterdam, pp 1–29
Khan N, Hussain ST, Saboor A et al (2013) Chemical investigation of the drinking water sources from Mardan, Khyber Pakhtunkhwa, Pakistan. World Appl Sci J 27:112–122. https://doi.org/10.5829/idosi.wasj.2013.27.01.1582
Konstantinou IK, Hela DG, Albanis TA (2006) The status of pesticide pollution in surface waters (rivers and lakes) of Greece. Part I. Review on occurrence and levels. Environ Pollut 141:555–570. https://doi.org/10.1016/j.envpol.2005.07.024
Kumar D, Pannu R (2018) Perspectives of lindane (γ-hexachlorocyclohexane) biodegradation from the environment: a review. Bioresour Bioprocess 5:29. https://doi.org/10.1186/s40643-018-0213-9
Kumar K, Devi SS, Krishnamurthi K et al (2007) Enrichment and isolation of endosulfan degrading and detoxifying bacteria. Chemosphere 68:317–322. https://doi.org/10.1016/j.chemosphere.2006.12.076
Kumar M, Lakshmi CV, Khanna S (2008) Biodegradation and bioremediation of endosulfan contaminated soil. Bioresour Technol 99:3116–3122. https://doi.org/10.1016/j.biortech.2007.05.057
Kuranchie-Mensah H, Atiemo SM, Palm LMN-D et al (2012) Determination of organochlorine pesticide residue in sediment and water from the Densu river basin, Ghana. Chemosphere 86:286–292. https://doi.org/10.1016/j.chemosphere.2011.10.031
Lamb DC, Lei L, Warrilow AGS et al (2009) The first virally encoded cytochrome p450. J Virol 83:8266–8269. https://doi.org/10.1128/JVI.00289-09
Laura M, Snchez-Salinas E, Dantn Gonzlez E, Luisa M (2013) Pesticide biodegradation: mechanisms, genetics and strategies to enhance the process. In: Biodegradation - Life of Science. InTech, pp 251–287
Li W, Dai Y, Xue B et al (2009) Biodegradation and detoxification of endosulfan in aqueous medium and soil by Achromobacter xylosoxidans strain CS5. J Hazard Mater 167:209–216. https://doi.org/10.1016/j.jhazmat.2008.12.111
Lushchak VI, Matviishyn TM, Husak V V., et al (2018) Pesticide toxicity: a mechanistic approach. EXCLI J 17:1101–1136. https://doi.org/10.17179/excli2018-1710
Mamta, Rao RJ, Wani KA (2015) Bioremediation of pesticides under the influence of bacteria and fungi. In: handbook of research on uncovering new methods for ecosystem management through bioremediation. pp 51–72
Mathieu C, Duval R, Xu X et al (2015) Effects of pesticide chemicals on the activity of metabolic enzymes: focus on thiocarbamates. Expert Opin Drug Metab Toxicol 11:81–94. https://doi.org/10.1517/17425255.2015.975691
Meleiro Porto AL, Zelayaran G, Consiglio M, Nitschke M (2011) Biodegradation of pesticides. In: pesticides in the modern world—pesticides use and management. InTech, pp 1–22
Mnif W, Hassine AIH, Bouaziz A et al (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8:2265–2303. https://doi.org/10.3390/ijerph8062265
Mohammed AI, K Bartakke (2015) Isolation of pesticide degrading microorganisms from soil. Adv Biores 5:164–168. https://doi.org/10.15515/abr.0976-4585.5.4.164168
Mukhtar H, Khizer I, Nawaz A et al (2015) Biodegradation of endosulfan by Aspergillus niger isolated from cotton fields of Punjab, Pakistan. Pakistan J Bot 47:333–336
Mustapha MU, Halimoon N, Johar WLW, Shukor MYA (2019) An overview on biodegradation of carbamate pesticides by soil bacteria. Pertanika J Sci Technol 27:547–563
Mutiyar PK, Mittal AK, Pekdeger A (2011) Status of organochlorine pesticides in the drinking water well-field located in the Delhi region of the flood plains of river Yamuna. Drink Water Eng Sci 4:51–60. https://doi.org/10.5194/dwes-4-51-2011
Nzila A, Razzak S, Zhu J (2016) Bioaugmentation: an emerging strategy of industrial wastewater treatment for reuse and discharge. Int J Environ Res Public Health 13:846. https://doi.org/10.3390/ijerph13090846
Oliveira-Silva JJ, Alves SR, Meyer A et al (2001) Influência de fatores socioeconômicos na contaminação por agrotóxicos, Brasil. Rev Saude Publica 35:130–135. https://doi.org/10.1590/S0034-89102001000200005
Ortega SN, Nitschke M, Mouad AM et al (2011) Isolation of brazilian marine fungi capable of growing on DDD pesticide. Biodegradation 22:43–50. https://doi.org/10.1007/s10532-010-9374-8
Ortíz I, Velasco A, Le Borgne S, Revah S (2013) Biodegradation of DDT by stimulation of indigenous microbial populations in soil with cosubstrates. Biodegradation 24:215–225. https://doi.org/10.1007/s10532-012-9578-1
Ozdal M, Ozdal OG, Algur OF (2016) Isolation and characterization of α-endosulfan degrading bacteria from the microflora of cockroaches. Polish J Microbiol 65:63–68. https://doi.org/10.5604/17331331.1197325
Pal R, Bala S, Dadhwal M et al (2005) Hexachlorocyclohexane-degrading bacterial strains Sphingomonas paucimobilis B90A, UT26 and Sp+, having similar lin genes, represent three distinct species, Sphingobium indicum sp. nov., Sphingobium japonicum sp. nov. and Sphingobium francense sp. nov., an. Int J Syst Evol Microbiol 55:1965–1972. https://doi.org/10.1099/ijs.0.63201-0
Pan X, Lin D, Zheng Y et al (2016) Biodegradation of DDT by Stenotrophomonas sp. DDT-1: characterization and genome functional analysis. Sci Rep 6:21332. https://doi.org/10.1038/srep21332
Pant G, Mistry SK, Sibi G (2013) Isolation, identification and characterization of p, p’-DDT degrading bacteria from soil. J Environ Sci Technol 6:130–137. https://doi.org/10.3923/jest.2013.130.137
Parte Satish G, Mohekar Ashokrao D, Kharat Arun S (2017) Microbial degradation of pesticide: A review. Afr J Microbiol Res 11(24):992–1012
Patil PN, Bote SD, Gogate PR (2014) Degradation of imidacloprid using combined advanced oxidation processes based on hydrodynamic cavitation. Ultrason Sonochem 21:1770–1777. https://doi.org/10.1016/j.ultsonch.2014.02.024
Patnaik P (2006) A comprehensive guide to the hazardous properties of chemical substances, 3rd edn
Pawari MJ, Gawande S (2015) Ground water pollution & its consequences. Int J Eng Res Gen Sci 3:773–776
Perelo LW (2010) Review: in situ and bioremediation of organic pollutants in aquatic sediments. J Hazard Mater 177:81–89. https://doi.org/10.1016/j.jhazmat.2009.12.090
Petrović G, Stojanović G, Palić R (2011) Modified β-cyclodextrins as prospective agents for improving water solubility of organic pesticides. Environ Chem Lett. https://doi.org/10.1007/s10311-010-0296-9
Pfeil R (2014) Pesticide residues: pyrethroids. in: encyclopedia of food safety. Elsevier, Amsterdam, pp 31–34
Pointing S (2001) Feasibility of bioremediation by white-rot fungi. Appl Microbiol Biotechnol 57:20–33. https://doi.org/10.1007/s002530100745
Purnomo AS (2017) Microbe-assisted degradation of aldrin and dieldrin. Environ Sci Eng (Subseries Environ Sci) 1–22. https://doi.org/10.1007/978-3-319-45156-5_1
Qiu L, Wang H, Wang X (2018) Conversion mechanism of heptachlor by a novel bacterial strain. RSC Adv 8:5828–5839. https://doi.org/10.1039/C7RA10097C
Rani K, Dhania G (2014) Bioremediation and biodegradation of pesticide from contaminated soil and water—a noval approach. Int J Curr Microbiol Appl Sci 3:23–33
Rebelo SLH, Melo A, Coimbra R et al (2007) Photodegradation of atrazine and ametryn with visible light using water soluble porphyrins as sensitizers. Environ Chem Lett 5:29–33. https://doi.org/10.1007/s10311-006-0072-z
Riah W, Laval K, Laroche-Ajzenberg E et al (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12:257–273. https://doi.org/10.1007/s10311-014-0458-2
Ricking M, Schwarzbauer J (2012) DDT isomers and metabolites in the environment: an overview. Environ Chem Lett 10:317–323. https://doi.org/10.1007/s10311-012-0358-2
Rigas F, Dritsa V, Marchant R et al (2005) Biodegradation of lindane by Pleurotus ostreatus via central composite design. Environ Int 31:191–196. https://doi.org/10.1016/j.envint.2004.09.024
Romeh AA, Hendawi MY (2013) Chlorpyrifos insecticide uptake by plantain from polluted water and soil. Environ Chem Lett 11:163–170. https://doi.org/10.1007/s10311-012-0392-0
Romero-Aguilar M, Tovar-Sánchez E, Sánchez-Salinas E et al (2014) Penicillium sp. as an organism that degrades endosulfan and reduces its genotoxic effects. Springerplus 3:536. https://doi.org/10.1186/2193-1801-3-536
Rosenbom AE, Binning PJ, Aamand J et al (2014) Does microbial centimeter-scale heterogeneity impact MCPA degradation in and leaching from a loamy agricultural soil? Sci Total Environ 472:90–98. https://doi.org/10.1016/j.scitotenv.2013.11.009
Russo F, Ceci A, Pinzari F et al (2019) Bioremediation of dichlorodiphenyltrichloroethane (DDT)-contaminated agricultural soils: potential of two autochthonous saprotrophic fungal strains. Appl Environ Microbiol 85:1–18. https://doi.org/10.1128/AEM.01720-19
Sahoo B, Ningthoujam R, Chaudhuri S (2019) Isolation and characterization of a lindane degrading bacteria Paracoccus sp. NITDBR1 and evaluation of its plant growth promoting traits. Int Microbiol 22:155–167. https://doi.org/10.1007/s10123-018-00037-1
Sakakibara F, Takagi K, Kataoka R et al (2011) Isolation and identification of dieldrin-degrading Pseudonocardia sp. strain KSF27 using a soil–charcoal perfusion method with aldrin trans-diol as a structural analog of dieldrin. Biochem Biophys Res Commun 411:76–81. https://doi.org/10.1016/j.bbrc.2011.06.096
Sánchez Salinas E, Ortiz Hernández L (2011) Riesgos y estrategias en el uso de plaguicidas. Inven la génesis la Cult Univ en Morelos
Sariwati A, Purnomo AS, Kamei I (2017) Abilities of co-cultures of brown-rot fungus Fomitopsis pinicola and Bacillus subtilis on biodegradation of DDT. Curr Microbiol 74:1068–1075. https://doi.org/10.1007/s00284-017-1286-y
Satish GP, Ashokrao DM, Arun SK (2017) Microbial degradation of pesticide: a review. African J Microbiol Res 11:992–1012. https://doi.org/10.5897/AJMR2016.8402
Sevrioukova IF, Poulos TL (2011) Structural biology of redox partner interactions in P450cam monooxygenase: a fresh look at an old system. Arch Biochem Biophys 507:66–74. https://doi.org/10.1016/j.abb.2010.08.022
Sharma A, Kumar V, Shahzad B et al (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci. https://doi.org/10.1007/s42452-019-1485-1
Siddique T, Okeke BC, Arshad M, Frankenberger WT (2003) Enrichment and Isolation of Endosulfan-Degrading Microorganisms. J Environ Qual 32(1):47–54
Singh T, Singh DK (2019) Rhizospheric Microbacterium sp. P27 showing potential of lindane degradation and plant growth promoting traits. Curr Microbiol 76:888–895. https://doi.org/10.1007/s00284-019-01703-x
Singh S, Kumar V, Chauhan A et al (2018) Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 16:211–237. https://doi.org/10.1007/s10311-017-0665-8
Sonkong K, Prasertsan P, Sobhon V (2008) Screening and identification of p, p′-DDT degrading soil isolates. Songklanakarin J Sci Technol 30:103–110
Srivastava A, Jangid NK, Srivastava M, Rawat V (2018) Pesticides as water pollutants. in: groundwater for sustainable development: problems, perspectives and challenges. pp 1–19
Subash SP, Chand P, Balaji SJ, Pal S (2017) Pesticide use in indian agriculture: trends, market structure and policy issues
Sun Y, Kumar M, Wang L et al (2020) Biotechnology for soil decontamination: opportunity, challenges, and prospects for pesticide biodegradation. In: Bio-Based Materials and Biotechnologies for Eco-Efficient Construction. Elsevier, Amsterdam, pp 261–283
Suppiramaniam V, Abdel-Rahman EA, Buabeid MA, Parameshwaran K (2010) Ion channels. In: Comprehensive Toxicology, 2nd edn. Elsevier Inc., Amsredam, pp 129–171
Tang W, Ji H, Hou X (2017) Research progress of microbial degradation of organophosphorus pesticides. Prog Appl Microbiol 1:29–35
Taş N, van Eekert MHA, Wagner A, Schraa G, de Vos WM, Smidt H (2011) Role of “Dehalococcoides” spp. in the anaerobic transformation of hexachlorobenzene in European rivers. Appl Environ Microbiol 77(13):4437–4445
The Business Research Company (2020) Pesticides market - by type (herbicides, fungicides and insecticides), by region, opportunities and strategies – global forecast to 2023. TBRC Business Research Pvt Ltd., London, UK
Ullah S, Li Z, Zuberi A et al (2019) Biomarkers of pyrethroid toxicity in fish. Environ Chem Lett 17:945–973. https://doi.org/10.1007/s10311-018-00852-y
Urkude R, Dhurvey V, Kochhar S (2019) Pesticide residues in beverages. In: Quality Control in the Beverage Industry, vol 17: The Science of Beverages. Elsevier, Amsterdm, pp 529–560
Urlacher VB, Lutz-Wahl S, Schmid RD (2004) Microbial P450 enzymes in biotechnology. Appl Microbiol Biotechnol 64:317–325. https://doi.org/10.1007/s00253-003-1514-1
Valenzuela EF, Menezes HC, Cardeal ZL (2020) Passive and grab sampling methods to assess pesticide residues in water. A review Environ Chem Lett 18:1019–1048. https://doi.org/10.1007/s10311-020-00998-8
van Grinsven HJM, ten Berge HFM, Dalgaard T, Fraters B, Durand P, Hart A, Hofman G, Jacobsen BH, Lalor STJ, Lesschen JP, Osterburg B, Richards KG, Techen A-K, Vertès F, Webb J, Willems WJ (2012) Management, regulation and environmental impacts of nitrogen fertilization in northwestern Europe under the Nitrates Directive; a benchmark study. Biogeosciences 9(12):5143–5160
Van Herwijnen R, Van De Sande BF, Van Der Wielen FWM et al (2003) Influence of phenanthrene and fluoranthene on the degradation of fluorene and glucose by Sphingomonas sp. strain LB126 in chemostat cultures. FEMS Microbiol Ecol 46:105–111. https://doi.org/10.1016/S0168-6496(03)00202-2
Vásquez-Murrieta MS, Hernández-Hernández OJ, Cruz-Maya JA, et al (2016) Approaches for removal of pahs in soils: bioaugmentation, biostimulation and bioattenuation. In: Soil Contamination—Current Consequences and Further Solutions. InTech, pp 329–342
Verma H, Kumar R, Oldach P et al (2014a) Comparative genomic analysis of nine Sphingobium strains: insights into their evolution and hexachlorocyclohexane (HCH) degradation pathways. BMC Genomics 15:1014. https://doi.org/10.1186/1471-2164-15-1014
Verma JP, Jaiswal DK, Sagar R (2014b) Pesticide relevance and their microbial degradation: a-state-of-art. Rev Environ Sci Biotechnol 13:429–466. https://doi.org/10.1007/s11157-014-9341-7
Vikrant K, Giri BS, Raza N et al (2018) Recent advancements in bioremediation of dye: current status and challenges. Bioresour Technol 253:355–367. https://doi.org/10.1016/j.biortech.2018.01.029
Wanguyun AP, Geraldi A (2019) Understanding pesticide degrading-microbe community using molecular approaches. Pollut Res 38:118–122
Weir KM, Sutherland TD, Horne I et al (2006) A single monooxygenase, ese, is involved in the metabolism of the organochlorides endosulfan and endosulfate in an Arthrobacter sp. Appl Environ Microbiol 72:3524–3530. https://doi.org/10.1128/AEM.72.5.3524-3530.2006
WHO (2018) Pesticides. Retrieved October 05, 2020, from https://www.who.int/topics/pesticides/en/
Xiao P, Kondo R (2020) Potency of Phlebia species of white rot fungi for the aerobic degradation, transformation and mineralization of lindane. J Microbiol 58:395–404. https://doi.org/10.1007/s12275-020-9492-x
Xiao P, Mori T, Kamei I, Kondo R (2011) Metabolism of organochlorine pesticide heptachlor and its metabolite heptachlor epoxide by white rot fungi, belonging to genus Phlebia. FEMS Microbiol Lett 314:140–146. https://doi.org/10.1111/j.1574-6968.2010.02152.x
Xie H, Zhu L, Wang J et al (2017) Biodegradation of DDE and DDT by bacterial strain Stenotrophomonas sp. DXZ9. J Environ Anal Toxicol 07: https://doi.org/10.4172/2161-0525.1000496
Ye X, Dong F, Lei X (2018) Microbial resources and ecology - microbial degradation of pesticides. Nat Resour Conserv Res 1:. https://doi.org/10.24294/nrcr.v1i1.242
Zabochnicka-Światek M, Krzywonos M (2014) Potentials of biosorption and bioaccumulation processes for heavy metal removal. Polish J Environ Stud 23:551–561
Zhang S, Yin L, Liu Y et al (2011) Cometabolic biotransformation of fenpropathrin by Clostridium species strain ZP3. Biodegradation 22:869–875. https://doi.org/10.1007/s10532-010-9444-y
Zhang Z, Zheng P, Li W et al (2015) Effect of organic toxicants on the activity of denitrifying granular sludge. Environ Technol 36:699–705. https://doi.org/10.1080/09593330.2014.959065
Zhang W, Lin Z, Pang S et al (2020) Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system. Front Microbiol 11:1–12. https://doi.org/10.3389/fmicb.2020.00522
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bose, S., Kumar, P.S., Vo, DV.N. et al. Microbial degradation of recalcitrant pesticides: a review. Environ Chem Lett 19, 3209–3228 (2021). https://doi.org/10.1007/s10311-021-01236-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01236-5