Abstract
Organophosphorus pesticides (OPPs) are used worldwide and pose great risks to human health. However, information on their presence in agricultural soils at regional scale and the associated risks is limited. In this study, an extensive investigation on agricultural soils was conducted throughout the Yangtze River Delta (YRD) of China to reveal the status of OPP pollution. The total concentrations of the nine OPPs ranged from <3.0 to 521 ng g−1 dry weight, with a mean of 64.7 ng g−1 dry weight and a detection rate of 93 %. Dimethoate was found to be the primary compound, followed by methyl parathion and parathion. The highest concentrations of OPPs were found in Jiangsu province due to the intensive agricultural activities. The pollution of OPPs is also highly associated with the land use types. The lower concentrations of OPPs found in vegetable fields could be attributed to their easy photodegradation and hydrolysis in aerobic soils. There was no significant difference in microbial communities among the sample sites, indicating that OPPs in agricultural soils of the YRD region cause negligible effects on microbiota. The risks of OPPs in the soils to human health were further evaluated. The hazard indexes in all the soil samples were below 1, suggesting absence of non-cancer risks. This study provides valuable information for a better understanding of the pollution status of OPPs in agricultural soils and a scientific basis for soil quality assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus pesticides (OPPs) have been extensively used throughout the world for crop protection and orchard treatment due to their high efficiency of pest control and low cost (Zhang et al. 2002). These so-called new generation insecticides are gradually replacing organochlorine pesticides (OCPs), because they are readily degradable in the environment and have brought significant economic benefits during the last five decades (Baker et al. 1996; Subhani and Liao 2001; Toan et al. 2007). However, it has been reported that OPPs have a moderate persistence (for weeks) and can accumulate in the environment for a long time due to their frequent application in intensively cultivated areas (Velasco et al. 2016; Pedersen et al. 2006). Qiu et al. (2016) recently found that OPPs were less accumulative and persistent than OCPs in roots, and their accumulation and persistence in leaves and stems were similar. Generally, OPPs are used for various vegetables, grain crops, and fruits to improve quality and yield. The residues of OPPs in the environment can be toxic to humans, microorganisms, and other organisms (Asselborn et al. 2015; Fakhri-Bafghi et al. 2016; Songa and Okonkwo 2016). For instance, many kinds of OPPs, especially parathion, phorate, and dimethoate, may cause health effects, including neurological damage, endocrine disruption, and genotoxic effects (Beach et al. 1996; Cecchi et al. 2012; Timoroglu et al. 2014). Recent studies reported that organophosphorus insecticide intoxication is a worldwide health problem with around three million poisonings every year and contributes to the largest proportion to human acute toxicity (Babu et al. 2011; Sogorb et al. 2004). Therefore, there is an increasing human health concern regarding OPP residues in the environment and food (Sanagi et al. 2013; Wang et al. 2008).
Agricultural activities are the major sources of OPP pollution in the environment (Babu et al. 2011). Continuous and excessive use of OPPs has led to the pollution of water and sediment in different regions of the world (Babu et al. 2011; Pedersen et al. 2006). The contamination of grains, vegetables, and fruits with OPPs was also studied (Chen et al. 2009; Sapbamrer and Hongsibsong 2014; Xiao et al. 2006). However, the knowledge of the pollution status of OPPs in soils is limited. Agricultural soil could receive OPPs from both direct inputs and atmospheric deposition (Cabrerizo et al. 2011). Soil may also be polluted by agricultural irrigation waters with OPPs (Calderon-Preciado et al. 2011). On the contrary, OPPs in soil could be discharged to surface water and leached to groundwater (Hantush et al. 2000). Emission from soil is also an important source for air pollution (Sweetman et al. 2005). Thus, soil is a major reservoir and a secondary emission source of organic pollutants including OPPs (Tao et al. 2008; Zhong and Zhu 2013). Many studies suggested that the residues of absorbed pesticides in soil could enter agricultural foods via plant uptake and then posed risks to mammals (Fantke and Jolliet 2015; Liu et al. 2016). Non-target microorganisms in soil may also be affected by pesticides, which can lead to the suppression of microorganisms and deterioration of soil fertility (Johnsen et al. 2001; Niemi et al. 2009). Thus, the current status of OPPs in soils needs to be investigated to evaluate possible human and ecological risks.
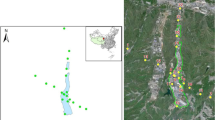
In order to characterize the regional-scale spatial distribution of OPPs in agricultural soils from rapidly developing regions in China, we conducted an extensive survey in the Yangtze River Delta (YRD), which consists of Shanghai, northern Zhejiang, and southern Jiangsu, with a population of more than 110 million. The sampling network composed of 241 sites was schemed to cover an area of nearly 45,800 km2. The objectives of this study are (a) to provide information about the levels and spatial distribution of OPPs in agricultural soils of the YRD region, (b) to reveal the influence of OPPs on the soil microbial community, and (c) to evaluate the possible health risk of OPPs in soils. In China, soil quality criteria have not been established for OPPs; thus, the present study could provide basic data for risk management and control of OPPs in China.
Materials and methods
Reagents and materials
The analytical standards of nine OPPs, including O,O,O-triethylphosphorothioate, thionazin, sulfotep, phorate, dimethoate, disulfoton, methyl parathion, parathion, and famphur, were purchased from Supelco (Bellefonte, PA, USA). These pesticides are the most widely used OPPs in the study area and have been detected in various environmental media in China and worldwide (Wei et al. 2009). The compound properties are provided in Supporting Information (Table SI-1). 2D-labeled chrysene was used as surrogate standard and purchased from AccuStandard (New Haven, CT, USA). Acetone, hexane, and petroleum ether were of pesticide grade and purchased from Fisher Scientific (Pittsburgh, PA, USA). All other chemicals and reagents used in this study were of analytical reagent grade or higher purity. Anhydrous sodium sulfate and Florisil were activated in advance.
Sample collection
The YRD region is mainly located in a low-lying alluvial plain and is situated in a subtropical monsoon climatic zone with a temperate and humid climate throughout the year and four distinct seasons. The study area was located between 29° 34′ 43″ N and 32° 09′ 10″ N and between 118° 41′ 55″ E and 122° 02′ 24″ E. The coordinates of all the sampling sites were recorded with a Garmin® GPS unit. In total, 241 soil samples were collected from the surface layer (0–15 cm depth) of agricultural soils in the YRD region (approximately 45,800 km2) during June 2014. Figure 1 shows the sampling zones and their distribution in the study area. The collection procedures were detailed in the previous study (Sun et al. 2016b). Briefly, soils from five cores were collected using a stainless scoop and composited to form a single sample. All the soil samples were wrapped in aluminum foil and sealed in Kraft bags. The samples were transported back to the laboratory after collection and stored at −20 °C before analysis. According to the World Reference Base of Soil Resources, the soils include Anthrosols and Fluvisols (IUSS Working Group WRB 2014). The land use types include rice paddy field and vegetable field, among others.
Sample preparation
After freeze-drying, each soil sample was homogenized and sieved through a stainless steel 75-mesh sieve. The analysis of OPPs was adapted from previous method (Wan and Chen 2006). An aliquot of 5 g soil sample was spiked with surrogate standard 2D-labeled chrysene (20 ng) and then extracted with 20 mL of acetone/petroleum ether (4:1, v/v) in an ultrasonic bath for 60 min. The extract was transferred to a flask, and the procedure was repeated two more times. The extract was concentrated and solvent exchanged into hexane with the use of a rotary evaporator (Heidolph 4000, Germany). Finally, the extract was eluted through a multi-layered column (25 × 1.0 cm) containing (from bottom to top) 2 cm of Na2SO4, 6 cm of activated Florisil, and 2 cm of Na2SO4 to remove the interferences. The target analytes were recovered in 60 mL of hexane/acetone (4:1, v/v). The final elution with OPPs was concentrated, exchanged into hexane, and reduced to 0.5 mL prior to instrumental analysis.
Instrumental analysis
The quantitative analysis of OPPs was performed on Agilent 7890B gas chromatograph (GC) coupled with a 5977A mass spectrometer (MS) detector using an electron impact (EI) ion source. DB-5 MS (J&W Scientific, Folsom, CA, USA) fused silica capillary column (30 m, 0.25 mm i.d., 0.25 μm film thickness) was employed for the separation. The selective ion monitoring mode was employed. The carrier helium gas was kept at a constant flow of 1.0 mL min−1. The gas chromatography temperature program was as follows: initial temperature 90 °C for 1.0 min and increased to 150 °C at a rate of 10 °C min−1, then ramp at 5 °C min−1 to 250 °C. The post-run was set at 290 °C, held for 1 min. Quantification ions of OPPs are described in Table SI-2.
Quality assurance and quality control
A procedural blank, a spiked blank, and a sample duplicate were included for every 10 samples. The procedural blanks used anhydrous sodium sulfate to monitor possible system contamination. The spiked blanks were analyzed to determine the surrogate recoveries, using blank soils spiked with the OPP standard mixture. No targeted compound was detected in any of the procedural blanks. The recovery rates of the standards in spiked samples ranged from 76.6 to 97.2 %. The variations of the detected concentrations in duplicate were lower than 15 %. Five-point calibration curves with concentrations of 10, 20, 50, 200, and 500 ng mL−1 were employed for individual OPPs to quantify the concentrations of analytes in the samples. The limits of detection (LODs), which were determined as the quantity of analytes yielding a peak three times of the noise, were from 3.0 to 5.5 ng g−1 dry weight for different OPPs.
Microbiological analysis
To reveal the influence of OPPs on the soil microbial community structure, the microbial phospholipid fatty acid (PLFAs) from 60 soil samples were analyzed after the chemical analysis. Among these selected sampling sites, 13 sites were located in Shanghai municipality, 17 sites were located in Jiangsu province, and 30 sites were located in Zhejiang province (Fig. 1). These sampling sites are evenly distributed in the YRD region, and the concentrations of OPPs in the selected soil samples ranged from <LOD to 521 ng g−1.
The PLFAs were extracted from the soil samples with a single-phase mixture of chloroform–methanol–citrate buffer system (1:2:0.8, v/v/v; 0.15 M, pH 4.0) (He et al. 2013). Then, the phospholipids were separated from neutral lipids and glycolipids using solid-phase extraction column. Briefly, lipids were separated into phospholipids, glycolipids, and neutral lipids using C18 filled gel chromatography. Polar lipids were subjected to mild alkaline methanolysis to form fatty acid methyl esters. The resulting fatty acid methyl esters were analyzed by GC (Agilent 6890N) fitted with MIDI Sherlock microbial identification system (Version 4.5, MIDI). The biomass of bacteria, including gram-positive bacteria (GP) and gram-negative bacteria (GN), actinomycete, and fungi was identified based on the detected 42 PLFAs (He et al. 2009; Pratt et al. 2012; Wang et al. 2016).
Human health risk assessment
The exposure non-cancer risk was calculated with the methods recommended by the US EPA (2013) to evaluate the health risk of OPPs to inhabitants. Two primary routes, including soil ingestion and dermal contact, were applied to assess the human health risk. The average daily doses (ADD, mg kg−1 day−1) of soil ingestion and dermal contact exposure routes were calculated as follows in the assessment.
where C soil means the concentration of OPPs in agricultural soil (mg kg−1), IRS is the agricultural soil ingestion rate (mg day−1), EF is the exposure frequency (days year−1), ED is the exposure duration (years), BW is the body weight (kg), AT is the average lifetime exposure (days), SA is the dermal surface area (cm2 day−1), AF is the soil adherence factor (mg cm−2), ABS is the fraction absorbed dermally from the soil (unitless), and CF is the conversion factor (kg mg−1).
The non-cancer risks of OPPs via soil ingestion and dermal contact were represented as HI, which was calculated with the following equations:
where HQ i is the hazard quotient of exposure pathway i and RfD (mg kg−1 day−1) represents the daily maximum permissible level of OPPs, including the reference dose for ingestion (RfDo) and the reference dose for dermal contact (RfDABS = RfDo × ABSGI). ABSGI is the fraction of OPPs absorbed in gastrointestinal tract (unitless).
The local inhabitants are considered unlikely to experience obvious adverse effect if HI < 1 (Niu et al. 2013). All parameters used in the human exposure risk assessment are listed in Tables SI-4 and SI-5.
Statistical analysis
Statistical analyses including the Pearson’s correlation analysis and spatial distribution of the concentrations of OPPs were performed on SPSS 18.0 and Origin 8.0, and the universal kriging for spatial interpolation was performed on ArcGIS 10.2 (ESRI, Redlands, CA, USA). Before the statistical analyses, the concentrations of OPPs were log-transformed to approximate normal distributions, where half of the LOD was considered as the concentrations below LOD. Statistical significance was considered as p < 0.05.
Results and discussion
Contamination status of OPPs
The concentrations of individual and total OPPs in the YRD agricultural soils are summarized in Table 1. All the concentrations were reported on a dry weight (dw) basis. Total OPPs were detected in 93 % of the 241 soil samples. The high detection rates indicate the ubiquitous pollution by OPPs in the YRD agricultural soils. Dimethoate exhibited the highest detection frequency of 80.9 %, followed by methyl parathion (53.5 %), parathion (24.5 %), disulfoton (22.0 %), and thionazin (22.0 %). The detection frequencies of the other OPPs were all below 20.0 %. The total concentrations of 9 OPPs ranged from <3.0 to 521 ng g−1 with a mean of 64.7 ng g−1. Dimethoate was the dominant compound with an average concentration of 50.8 ng g−1, which might be related to its intensive usage in this region. The levels of OPPs in this study were higher than those in Shenyang, China (below detection limit) (Shi et al. 2011) and Jiquilisco Bay, El Salvador (below detection limit) (Nomen et al. 2012), while lower than those in Tlaltizapan, Mexico (from below detection limit to 925 ng g−1 for dimethoate, from 23.4 to 856.2 ng g−1 for methyl parathion, and from below detection limit to 487 ng g−1 for disulfoton) (Velasco et al. 2016).
Geographic information system (GIS) was applied to map the spatial distribution of total OPPs in the agricultural soils across the YRD region. As shown in Fig. 1, relatively high concentrations of OPPs were found in Jiangsu province as well as the border between Zhejiang province and Shanghai municipality. In large areas of Ningshao plain and Shanghai municipality, OPPs were detected at relatively low concentrations in the agricultural soils, possibly reflecting little application or rapid degradation of these pesticides. High concentrations of OPPs were found in most parts of southern Jiangsu province, where many pesticide plants were built. Several OPPs, such as dimethoate, methyl parathion, and parathion, were the major products of these pesticide plants. OPPs might release to the surrounding environment via many routes such as waste disposal, atmospheric transmission, and deposition (Coscolla et al. 2013), resulting in regional agricultural soil pollution in the southern Jiangsu province. Besides, there were many agricultural bases around the Taihu Lake. Intensive agricultural activities might be another source of OPPs in this area.
Soil physicochemical properties such as total organic carbon (TOC), pH, total phosphorus (TP), and total nitrogen (TN) may affect the occurrence and behavior of OPPs. The soil TOC, pH, TP, and TN were reported in our previous studies (Sun et al. 2016a, 2016b). The contents of soil TOC ranged from 0.15 to 3.98 %, with a mean value of 1.48 %. The contents of pH ranged from 4.24 to 8.48, with a mean value of 6.14. The contents of TN varied from 0.62 to 4.81 g kg−1, with an average value of 2.06 g kg−1. The content of TP varied from 0.14 to 2.27 g kg−1, with an average value of 0.77 g kg−1. In this study, the concentrations of ΣOPPs were positively correlated with TOC (r = 0.338, p < 0.01, n = 241; r, correlation coefficient; p, significance level; n, sample size), indicating that organic matter content affected the level of OPPs. The results also show that the concentration of ΣOPPs was positively correlated with TN (r = 0.322, p < 0.01, n = 241), while there was no significant correlation between the concentration of ΣOPPs and pH (p > 0.05) and not significant between the concentration of ΣOPPs and TP (p > 0.05) neither.
The concentrations of OPPs were highly associated with the land use types. In this study, higher concentrations of OPPs were found in paddy fields (mean = 139 ng g−1; n = 44). Lower concentrations of OPPs were found in vegetable fields (mean = 45.7 ng g−1; n = 48). Rotations of rice and wheat or corn are the main agricultural cropping systems for paddy fields in the YRD region. Thus, the soils in paddy fields are deprived of oxygen and are different from those in vegetable fields. As reported, these pesticides could be degraded by insecticide-degrading microbes, illumination as well hydrolysis (Konstantinou et al. 2001; Singh and Walker 2006). Therefore, it was likely that OPPs were readily to be photodegraded or hydrolyzed in aerobic soil in vegetable fields.
Relationship between OPPs and soil microbial communities
Changes in microbial biomass and community structure, which are sensitive to environmental stress, are useful indicators for assessing the impact of pollutants on soil microbes (Frey et al. 2006; Louati et al. 2013). The changes in soil microbial communities, such as microbial number, activity, and diversity, are considered to be important indices to monitor soil quality. In this study, the most abundant microbes were bacteria, with relative abundances of 32.0 to 90.7 %. The Spearman correlation coefficients between the concentrations of OPPs and the relative abundance of microorganisms were calculated in this study (Table 2). Positive correlation was observed between the concentrations of OPPs and the relative abundance of bacteria (r = 0.258, p < 0.05, n = 60).
OPPs might change the biomass of microbial by interacting with bacteria in agricultural soil. On one hand, OPPs could be toxic to soil bacteria, resulting in degraded activities and lower abundance (Wang et al. 2010). On the other hand, some insecticide-degrading bacteria can assimilate the OPPs and become more abundant in agricultural soils (Itoh et al. 2014). However, the correlation between OPPs and the biomass of bacteria was weak, suggesting that OPPs unlikely cause significant effect on soil bacteria (Table 3).
No clear correlation was found between OPP residues and the biomass of other microbes. It suggested that other factors such as land management practices, soil types, or other pollutants pose greater effect upon the microbial communities in agricultural soils. Overall, the results show that the OPPs in agricultural soils of the YRD region cause no apparent influence on microbial communities.
Human health risk assessments
The non-cancer risks of the OPPs to local villagers via soil ingestion and dermal contact exposure routes are outlined in Fig. 2. The HI values of the total OPPs were all below 1 for both children and adults, indicating negligible non-cancer risks in YRD agricultural soils. The average non-cancer risk of disulfoton to residents was the highest (HI = 0.0163 for children and 0.00175 for adults), followed by dimethoate (HI = 0.00352 for children and 0.000351 for adults) and methyl parathion (HI = 0.00260 for children and 0.000281 for adults). Overall, the non-cancer risks to children were relatively higher than those to adults. The possible reason is that children are more susceptible to given dose of OPPs and likely to inadvertently ingest significant amount of contaminated soil by virtue of pica behavior and hand or finger sucking (Rasmussen et al. 2001). The risks of different pathways were compared in this study (Fig. 2c). The result shows that soil ingestion was the primary pathway of OPP exposure and contributed to 70–80 % of the total risks.
The risk via dietary pathway was not considered in this method because data regarding intake of agricultural products harvested from the soils were not available. Moreover, metabolism and excretion of OPPs in human bodies were not considered in the assessment. Many soil samples in this study were collected from farmlands around farmers’ houses. People may frequently contact these soils. Further studies are required to measure the actual contents of OPPs in human bodies and evaluate the associated risks.
Conclusions
OPPs were widespread pesticides in agricultural soils of the Yangtze River Delta region of China. The mean level of dimethoate was the highest among the residual OPPs, followed by methyl parathion, parathion, disulfoton, and thionazin. The higher residue levels of total OPPs found in Jiangsu province might be attributed to the intensive agricultural activities and emissions from pesticide factories. The concentrations of OPPs in vegetable fields were lower than those in paddy fields. The microbial community compositions were not significantly correlated with the pollution levels of OPPs. The non-cancer risks of OPPs in the YRD agricultural soils were negligible (HI < 1).
References
Asselborn V, Fernandez C, Zalocar Y, Parodi EP (2015) Effects of chlorpyrifos on the growth and ultrastructure of green algae, Ankistrodesmus gracilis. Ecotox Environ Safe 120:334–341
Babu V, Unnikrishnan P, Anu G, Nair SM (2011) Distribution of organophosphorus pesticides in the bed sediments of a backwater system located in an agricultural watershed: influence of seasonal intrusion of seawater. Arch Environ Con Tox 60:597–609
Baker LW, Fitzell DL, Seiber JN, Parker TR, Shibamoto T, Poore MW, Longley KE, Tomlin RP, Propper R, Duncan DW (1996) Ambient air concentration of pesticides in California. Environ Sci Technol 10:1365–1368
Beach JR, Spurgeon A, Stephens R, Heafield T, Calvert IA, Levy LS, Harrington JM (1996) Abnormalities on neurological examination among sheep farmers exposed to organophosphorus pesticides. Occup Environ Med 53:520–525
Cabrerizo A, Dachs J, Moeckel C, Ojeda MJ, Caballero G, Barcelo D, Jones KC (2011) Factors influencing the soil-air partitioning and the strength of soils as a secondary source of polychlorinated biphenyls to the atmosphere. Environ Sci Technol 45:4785–4792
Calderon-Preciado D, Jimenez-Cartagena C, Matamoros V, Bayona JM (2011) Screening of 47 organic microcontaminants in agricultural irrigation waters and their soil loading. Water Res 45:221–231
Cecchi A, Rovedatti MG, Sabino G, Magnarelli GG (2012) Environmental exposure to organophosphate pesticides: assessment of endocrine disruption and hepatotoxicity in pregnant women. Ecotox Environ Safe 80:280–287
Chen C, Li Y, Chen MX, Chen ZJ, Qian YZ (2009) Organophosphorus pesticide residues in milled rice (Oryza sativa) on the Chinese market and dietary risk assessment. Food Addit Contam A 26:340–347
Coscolla C, Hart E, Pastor A, Yusa V (2013) LC-MS characterization of contemporary pesticides in PM10 of Valencia Region, Spain. Atmos Environ 77:394–403
Fakhri-Bafghi MS, Ghasemi-Niri SF, Mostafalou S, Navaei-Nigjeh M, Baeeri M, Mohammadirad A, Abdollahi M (2016) Protective effect of selenium-based medicines on toxicity of three common organophosphorus compounds in human erythrocytes in vitro. Cell J 17:740–747
Fantke P, Jolliet O (2015) Life cycle human health impacts of 875 pesticides. Int J Life 21:722–733
Frey B, Stemmer M, Widmer F, Luster J, Sperisen C (2006) Microbial activity and community structure of a soil after heavy metal contamination in a model forest ecosystem. Soil Biol and Biochem 38:1745–1756
Hantush MM, Marino MA, Islam MR (2000) Models for leaching of pesticides in soils and groundwater. J Hydrol 227:66–83
He Y, Ding N, Shi JC, Wu M, Liao H, Xu JM (2013) Profiling of microbial PLFAs: implications for interspecific interactions due to intercropping which increase phosphorus uptake in phosphorus limited acidic soils. Soil Biol Biochem 57:625–634
He Y, Xu JM, Lv XF, Ma ZH, Wu JJ, Shi JC (2009) Does the depletion of pentachlorophenol in root–soil interface follow a simple linear dependence on the distance to root surfaces? Soil Biol Biochem 41:1807–1813
Itoh H, Navarro R, Takeshita K, Tago K, Hayatsu M, Hori T, Kikuchi Y (2014) Bacterial population succession and adaptation affected by insecticide application and soil spraying history. Front Microbiol 5:457
IUSS Working Group WRB (2014) World reference base for soil resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps
Johnsen K, Jacobsen CS, Torsvik V, Sorensen J (2001) Pesticide effects on bacterial diversity in agricultural soils—a review. Biol Fert Soils 33:443–453
Konstantinou IK, Sakellarides TM, Sakkas VA, Albanis TA (2001) Photocatalytic degradation of selected s-triazine herbicides and organophosphorus insecticides over aqueous TiO2 suspensions. Environ Sci Technol 35:398–405
Liu YH, Li SL, Ni ZL, Qu MH, Zhong DL, Ye CF, Tang FB (2016) Pesticides in persimmons, jujubes and soil from China: residue levels, risk assessment and relationship between fruits and soils. Sci Total Environ 542:620–628
Louati H, Ben Said O, Soltani A, Got P, Mahmoudi E, Cravo-Laureau C, Duran R, Aissa P, Pringault O (2013) The roles of biological interactions and pollutant contamination in shaping microbial benthic community structure. Chemosphere 93:2535–2546
Niemi RM, Heiskanen I, Ahtiainen JH, Rahkonen A, Mantykoski K, Welling L, Laitinen P, Ruuttunen P (2009) Microbial toxicity and impacts on soil enzyme activities of pesticides used in potato cultivation. Appl Soil Ecol 41:293–304
Niu LL, Xu C, Yao YJ, Liu K, Yang FX, Tang ML, Liu WP (2013) Status, influences and risk assessment of hexachlorocyclohexanes in agricultural soils across China. Environ Sci Technol 47:12140–12147
Nomen R, Sempere J, Chavez F, de Lopez NA, Rovira MD (2012) Measurement of pollution levels of organochlorine and organophosphorus pesticides in water, soil, sediment, and shrimp to identify possible impacts on shrimp production at Jiquilisco Bay. Environ Sci Pollut R 19:3547–3555
Pedersen JA, Yeager MA, Suffet IH (2006) Organophosphorus insecticides in agricultural and residential runoff: field observations and implications for total maximum daily load development. Environ Sci Technol 40:2120–2127
Pratt B, Riesen R, Johnston CG (2012) PLFA analyses of microbial communities associated with PAH-contaminated riverbank sediment. Microb Ecol 64:680–691
Qiu JL, Chen GS, Xu JQ, Luo EL, Liu Y, Wang FX, Zhou H, Liu Y, Zhu F, Ouyang GF (2016) In vivo tracing of organochloride and organophosphorus pesticides in different of organs of hydroponically grown Malabar spinach (Basella alba L.). J Hazard Mater 316:52–59
Rasmussen PE, Subramanian KS, Jessiman BJ (2001) A multi-element profile of housedust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci Total Environ 267:125–140
Sanagi MM, Salleh S, Ibrahim WAW, Abu Naim A, Hermawan D, Miskam M, Hussain I, Aboul-Enein HY (2013) Molecularly imprinted polymer solid-phase extraction for the analysis of organophosphorus pesticides in fruit samples. J Food Compos Anal 32:155–161
Sapbamrer R, Hongsibsong S (2014) Organophosphorus pesticide residues in vegetables from farms, markets, and a supermarket around Kwan Phayao Lake of northern Thailand. Arch Environ Con Tox 67:60–67
Shi RG, Lv JG, Feng JM (2011) Assessment of pesticide pollution in suburban soil in South Shenyang, China. B Environ Contam Tox 87:567–573
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30:428–471
Sogorb MA, Vilanova E, Carrera V (2004) Future applications of phosphotriesterases in the prophylaxis and treatment of organophosporus insecticide and nerve agent poisonings. Toxicol Lett 151:219–233
Songa EA, Okonkwo JO (2016) Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: a review. Talanta 155:289–304
Subhani A, Liao M (2001) Impact of some agronomic practices on paddy field soil health under varied ecological conditions: influence of soil moisture. Pedosphere 11:39–48
Sun JT, Pan LL, Daniel CT, Zhan Y, Liu WX, Wang XL, Zhu LZ, Li XD (2016a) Polychlorinated biphenyls in agricultural soils from the Yangtze River Delta of China: regional contamination characteristics, combined ecological effects and human health risks. Chemosphere. doi:10.1016/j.chemosphere.2016.08.038
Sun JT, Pan LL, Zhan Y, Lu HN, Tsang DCW, Liu WX, Wang XL, Li XD, Zhu LZ (2016b) Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Sci Total Environ 544:670–676
Sweetman AJ, Dalla Valle M, Prevedouros K, Jone KC (2005) The role of soil organic carbon in the global cycling of persistent organic pollutants (POPs): interpreting and modelling field data. Chemosphere 60:959–972
Tao S, Liu WX, Li Y, Yang Y, Zuo Q, Li BG, Cao J (2008) Organochlorine pesticides contaminated surface soil as reemission source in the Haihe Plain, China. Environ Sci Technol 42:8395–8400
Timoroglu I, Yuzbasioglu D, Unal F, Yılmaz S, Aksoy H, Celik M (2014) Assessment of the genotoxic effects of organophosphorus insecticides phorate and trichlorfon in human lymphocytes. Environ Toxicol 29:577–587
Toan VD, Thao VD, Walder J, Schmutz HR, Ha CT (2007) Contamination by selected organochlorine pesticides (OCPs) in surface soils in Hanoi, Vietnam. Bull Environ Contam Toxicol 78:195–200
US EPA (United States Environmental Protection Agency) (2013) Mid Atlantic risk assessment. Regional Screening Level (RSL) Summary Table. Washington DC
Velasco A, Rodriguez J, Castillo R, Ortiz I (2016) Residues of organochlorine and organophosphorus pesticides in sugarcane crop soils and river water. J Environ Sci Heal B 47:833–841
Wan YQ, Chen ZB (2006) Determination of organophosphorus and carbamate pesticide residues in soil by gas chromatography-mass spectrometry. J Anal Sci 22:551–554 in Chinese
Wang F, Yao J, Chen HL, Chen K, Trebse P, Zaray G (2010) Comparative toxicity of chlorpyrifos and its oxon derivatives to soil microbial activity by combined methods. Chemosphere 78:319–326
Wang LG, Liang YC, Jiang X (2008) Analysis of eight organophosphorus pesticide residues in fresh vegetables retailed in agricultural product markets of Nanjing, China. B Environ Contam Tox 81:377–382
Wang LW, Li F, Zhan Y, Zhu LZ (2016) Shifts in microbial community structure during in situ surfactant enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ Sci Pollut R 23:14451–14461
Wei SH, Sun HX, Shen J (2009) Study on the residues of organophosphorus pesticides in the soil of Chinese wolfberry produce district in Ningxia. Chin Agric Sci Bull 25:488–490 in Chinese
Xiao Q, Hu B, Yu CH, Xia LB, Jiang ZC (2006) Optimization of a single-drop microextraction procedure for the determination of organophosphorus pesticides in water and fruit juice with gas chromatography-flame photometric detection. Talanta 69:848–855
Zhang ZL, Hong HS, Zhou JL, Yu G (2002) Occurrence and behavior of organophosphorus insecticides in the River Wuchuan, Southeast China. J Environ Monit 4:498–504
Zhong YC, Zhu LZ (2013) Distribution, input pathway and soil–air exchange of polycyclic aromatic hydrocarbons in Banshan Industry Park, China. Sci Total Environ 444:177–182
Acknowledgments
This work was jointly supported by the National Basic Research Program of China (973 Program, 2014CB441101), the National Natural Science Foundations of China (21137003), and the Fundamental Research Funds for the Central Universities (2016FZA6007). The authors would like to thank Ms. Zi Wei from the Analysis and Measurement Center of Zhejiang University for assistance in sample analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
ESM 1
(DOCX 31 kb)
Rights and permissions
About this article
Cite this article
Pan, L., Sun, J., Li, Z. et al. Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: concentration, distribution, and risk assessment. Environ Sci Pollut Res 25, 4–11 (2018). https://doi.org/10.1007/s11356-016-7664-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7664-3