Abstract
Titanium dioxide photocatalysis, using 200 mgl−1 of TiO2, and photo-Fenton, using 20 mg l−1 of iron, were applied to the treatment of dimethoate dissolved in water at 50 mg l−1. A heterogeneous photocatalysis test was performed in a 35-l solar pilot plant with Compound Parabolic Collectors (CPCs) under natural illumination. A homogeneous photocatalysis test was performed in a different solar pilot plant with four CPC units and a total volume of 75 l. In this work total disappearance of dimethoate and 90% of mineralization were attained in both solar treatments. Treatment time, hydrogen peroxide consumption and ferric phosphate precipitation during photo-Fenton treatment were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Protection of natural resources against the environmental impact of chemicals such as pesticides, herbicides, insecticides, etc., widely applied in agrochemical industry, today constitute a major social concern. EU legislation is continually adapted to protect and improve the quality of Europe's fresh water resources (Directive 2000/60/EC).
Because of the environmental longevity and toxicity of organochlorines, organophosphorous pesticides have been used as an alternative for pest control. Nevertheless, given suitable environmental conditions organophosphates can persist in many environmental compartments for long periods of time (Ragnarsdottir 2000). Moreover, because of their widespread application, they are the chemicals most frecuently associated with toxicity in domestic animals and wildlife as well as humans (Gallo et al. 1988).
Dimethoate (O,O-dimethyl S-methylcarbamoylmethyl phosphorodithioate), one of the most commonly used contact insecticides, is an organophosphorous compound used for systemic extermination of mites and insects. This highly water-soluble (25 g/l at 21°C) chemical interferes with the activities of cholinesterase, an enzyme that is essential for proper functioning of the nervous systems of both humans and insects, it is highly toxic by all routes of exposure (Occupational Health Services, Inc. 1991). In this context, effective purification methods for removing these contaminants from water are required.
Advanced Oxidation Processes (AOPs) are oxidative chemical processes, which can be applied as alternative water treatment technologies for organic pollutants not treatable by conventional techniques due to their high chemical stability and/or low biodegradability (Gogate and Pandit 2004; Konstantinou and Albanis 2003). AOPs involve generation and subsequent reaction of hydroxyl radicals (•OH), which are the most powerful oxidizing species after fluorine (2.8 V vs. standard hydrogen electrode). Many AOPs, such as TiO2/UV, H2O2/UV, photo-Fenton and ozone (O3, O3/UV, O3/H2O2) are currently employed for this purpose. Their non-selective attack, which is a useful attribute for use in pollution treatment. The versatility of AOPs is also enhanced by the fact that there are several different ways in which •OH radical can be produced, so they can be adapted to specific treatment requirements. Their main disadvantage is their high cost. Therefore, research is focusing more and more on those AOPs which can be driven by solar radiation, i.e., light with a wavelength longer than 300 nm: photo-Fenton and heterogeneous catalysis with UV/TiO2 (Bockelmann et al. 2004).
Previous photocatalytic degradation studies of Dimethoate have been performed using several catalysts as TiO2 (Evgenidou et al. 2005), but no research have been focused specifically in photo-Fenton degradation of this pesticide. This work evaluates and compare the degradation of Dimethoate with photo-Fenton and UV/TiO2 in two different CPC solar pilot plants.
Experimental
Chemicals
Dimethoate (98.2% technical grade C5H12NO3PS2, Aragonesas Agro S.A.) was used as received. Analytical standards for chromatography analyses were purchased from Sigma-Aldrich. Distilled water used in the pilot plant was obtained from the Plataforma Solar de Almería (PSA) distillation plant (conductivity <10 μS/cm, Cl−= 0.7–0.8 mg/l, NO3 −= 0.5 mg/l, organic carbon < 0.5 mg/l). The heterogeneous photocatalytic degradation test was carried out using a slurry suspension (200 mg/l of TiO2) of Degussa (Frankfurt, Germany) P-25 titanium dioxide (surface area 51–55 m2/g). For the photo-Fenton experiment, the following chemicals were used: iron sulphate (FeSO4.7H2O), reagent-grade hydrogen peroxide (30% w/v) and sulphuric acid for pH adjustment (around 2.7–2.9).
Analytical determinations
Mineralization was followed by measuring the Total Organic Carbon (TOC) by direct injection of filtered samples into a Shimadzu-5050A TOC analyser. Pesticide concentration was analysed using reverse-phase liquid chromatography (flow 0.5 ml/min) with UV detector in an HPLC (Agilent Technololgies, series 1100) with C-18 column (LUNA 5 μm, 3 mm × 150 mm from Phenomenex), the composition of mobile phase is H2O/Acetonitrile (80/20) at a wavelength of 210 nm. Ultra pure distilled-deionized water obtained from a Milli-Q (Millipore Co.) system and HPLC-graded organic solvents were used to prepare all the solutions. Cation concentrations were determined with a Dionex DX-120 ion chromatograph equipped with a Dionex Ionpac CS12A 4 mm 250 mm column. Isocratic elution was done with H2SO4 (10 mM) at a flow rate of 1.2 ml min−1. Anion concentrations were measured with a Dionex DX-600 ion chromatograph using a Dionex Ionpac AS11-HC 4 mm × 250 mm column. The gradient programme was pre-run 5min with 20 mM of NaOH, an 8-min injection of 20 mM of NaOH, and 7-min with 35 mM of NaOH, at a flow rate of 1.5 ml min−1. H2O2 concentration was determined by iodometric tritation. Determination of total iron concentration follows ISO 6332.
Experiment setup
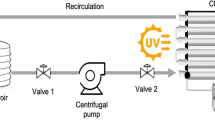
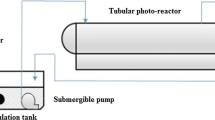
The heterogeneous photocatalytic experiment was carried out under sunlight in a pilot plant at the Plataforma Solar of Almería (PSA) (latitude 37°N, longitude 2.4°W). The pilot plant, which has three compound parabolic collectors (CPCs), one tank and one recirculation pump, operates in batch mode. Each collector (1.03 m2 each) is mounted on a fixed platform tilted 37° (local latitude). The total volume (V T) of 35 l is made up of 22 l (transparent glass tubes in the CPC) of total irradiated volume (V i) and the dead reactor volume (tank + high density polyethylene tubes), which is not illuminated, as recently described in detail elsewhere (Kositzi et al. 2004).
The photo-Fenton experiment was also carried out under sunlight, but in a different pilot plant at the PSA specially developed for photo-Fenton applications (Malato et al. 2004). This solar reactor is composed of four 1.04 m2 compound parabolic collector units, supported by an aluminium profile frame and mounted on a fixed, south-facing platform tilted 37° (local latitude). Each unit has five glass tubes with an optical diameter of 50 mm. The total volume of the reactor (75 l) consists of 44.6 l (glass tubes) total irradiated volume (V i) and the dead reactor volume (tank + tubes) Fig. 1.
Solar ultraviolet radiation (UV) was measured by a global UV radiometer (KIPP&ZONEN, model CUV 3), mounted on a platform tilted 37° (the same as the CPCs), which provides data in terms of incident W UV m−2. In this way, the energy reaching any surface in the same position with regard to the sun can be measured. With Eq. (1), combination of the data from several days experiments and their comparison with other photocatalytic experiments is possible.
where t n is the experimental time for each sample, UV is the average solar ultraviolet radiation measured during Δt n, and t 30 W is a “normalized illumination time”. In this case, time refers to a constant solar UV power of 30 W m−2 (typical solar UV power on a perfectly sunny day around noon). The system used for heterogeneous photocatalytic experiment is not thermally insulated, so the temperature achieved inside the reactor is continuously recorded by a PT-100 inserted in the tubing. However, the pilot plant used for photo-Fenton experiment is equipped with a temperature control system which maintains this parameter at a set point of 30°C. pH was adjusted to 2.7–2.9, with sulphuric acid in order to avoid iron hydroxide precipitation. The initial dose of hydrogen peroxide was always added after pH adjustment and iron addition, according to 200% of the calculated chemical oxygen demand for complete mineralization to CO2. The concentration of peroxide in the reactor was determined by frequent analysis and controlled to avoid complete disappearance by adding small amounts as consumed.
Dimethoate was previously dissolved in a 5-l flask and added directly into the pilot plant. After 15 min of homogenisation, with the collectors covered to avoid illumination, a sample was taken (initial concentration) and sulphuric acid was added (Point 1, Fig. 2). After 15 min, a sample was taken to confirm that pH was around 2.7–2.9. Then iron salt (20 mg/l of Fe) was added, homogenised and another sample taken (Point 2, Fig. 2). Finally initial dose of hydrogen peroxide was added (Point 3, Fig. 2) and a sample was taken 15 min later (“dark Fenton” reaction occurred). At that moment collectors were uncovered and so photo-Fenton began.
The titanium dioxide procedure was shorter. The compound was added to the pilot plant, and homogenised during 15 min and a sample was taken (initial concentration), then TiO2 (200 mg/l) was added. After 15 min, a sample was taken and the collectors were uncovered.
Results and discussion
The main parameters used to follow the degradation and mineralization of Dimethoate were the TOC, the iron concentration, H2O2 consumption throughout all the process, the concentration of inorganic species present in the wastewater and concentration of the parent compound.
Figure 2 shows the disappearance of dimethoate at 50 mg/l, and the mineralization (i.e., disappearance of TOC), attained during both photocatalytic experiments. Photo-Fenton is observed to be quicker than TiO2, as disappearance of dimethoate was almost complete during “dark Fenton”, whereas no degradation was detected with TiO2 in the dark. Total mineralization was attained in both experiments (90% of the initial TOC), however the photo-Fenton treatment was 1.5 times faster than with TiO2. Slight mineralization was also detected before illumination in the homogeneous photocatalytic test.
Concerning inorganic species appearance, it is worth mentioning that while organic phosphorous and organic sulphur were recovered completely as dissolved phosphate and sulphate anions during TiO2 experiment. In the photo-Fenton experiment, due to the precipitation of phosphate with ferric ions (from ferrous ions oxidation in photo-Fenton reactions), disappearance of released phosphorous was detected as observed in Fig. 3b. This required an extra addition of iron sulphate (Fig. 2b), in order to ensure that there was enough iron to continue photo-Fenton treatment. Figure 2b and c shows the decrease in the iron concentration as well as the increase in the hydrogen peroxide consumption during the photo-Fenton test.
Ammonium and nitrate were detected in different relative concentrations during both photocatalytic experiments, but the nitrogen mass balance was always incomplete (Fig. 3a and b). An unquantified methylamine peak was detected next to the ammonium peak on the ionic chromatogram. Consequently, this methylamine could close the incomplete N mass balance.
Conclusions
It has been demonstrated that wastewater containing Dimethoate can be treated successfully by both photo-Fenton and TiO2 photocatalytic processes. Photo-Fenton treatment is more efficient than TiO2 one. Treatment time for 90% of mineralization using 20 mg/l of Fe was 1.5 times lower than with 200 mg/l of TiO2. Iron precipitation as ferric phosphate is a key point. Iron must be measured and added as necessary in order to avoid stopping the photo-Fenton reactions. This should be taken into account when organophosphate contaminants are treated by photo-Fenton.
References
Bockelmann D, Dillert R, Dzengel J, Goslich R, Grob E, Higendorff M, Hufschmidt D, Memming R, Sagawe G, Schober M, Schuhmacher H-W, Selzer V, Siemon U, Vollmer S, Theurich J, Bahnemann D (2004) Photocatalysis. Solar Energy 77:445
Evgenidou E, Fytianos K, Poulios I (2005) Photocatalytic oxidation of dimethoate in aqueous solution. J Photochem Photobiol A: Chem 175:29–38
Gallo MA, Lawryk NJ (1988) In: Hayes WJ Jr, Laws ER Jr (eds), Handbook of pesticide toxicology. Academic Press, New York, p. 917
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment I: oxidations technologies at ambient conditions. Adv Environ Res 8:501–551
Konstantinou IK, Albanis TA (2003) Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways. Appl Catal B: Environ 42:319–335
Kositzi M, Poulios I, Malato S, Cáceres J, Campos A (2004) Solar photocatalytic treatment of synthetic municipal wastewater. Water Res 38:1147–1154
Malato Rodríguez S, Blanco Gálvez J, Maldonado Rubio MI, Fernández Ibáñez P, Alarcón Padilla D, Collares Pereira M, Farinha Mendes J, Correia de Oliveira J (2004) Engineering of solar photocatalytic collectors. Solar Energy 77:513–524
Occupational Health Services, Inc. (1991, Sept. 16) MSDS for dimethoate. OHS Inc., Secaucus, NJ
Ragnarsdottir KV (2000) Environmental fate and toxicology of organophosphate pesticides. J Geol Soc 157(18):859–876
The Water Framework Directive. Directive 2000/60/EC of the European Parliament and of the Council
Acknowledgements
The authors thank the Spanish Ministry of Education and Science for its financial assistance under the “Fotodetox” Project (PPQ 2003-07596-C03-01). They also thank Mrs. Deborah Fuldauer for correcting the English. Isabel Oller thanks the Ministry of Education and Science for her Ph.D research grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s10311-006-0075-9
Rights and permissions
About this article
Cite this article
Oller, I., Gernjak, W., Maldonado, M.I. et al. Photocatalytic treatment of dimethoate by solar photocatalysis at pilot plant scale. Environ Chem Lett 3, 118–121 (2005). https://doi.org/10.1007/s10311-005-0013-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-005-0013-2