Abstract
Weed control in maize (Zea mays L.) crops is usually undertaken using the postemergence herbicide nicosulfuron. The toxicity of nicosulfuron on maize, especially sweet maize, has been widely reported. In order to examine the effect of nicosulfuron on seedling photosynthetic characteristics, chlorophyll fluorescence, reactive oxygen species production, antioxidant enzyme activities, and gene expressions on sweet maize, nicosulfuron-tolerant “HK310” and nicosulfuron-sensitive “HK320” were studied. All experiment samples were subjected to a water or 80 mg kg−1 of nicosulfuron treatment when sweet maize seedlings grow to the stage of four leaves. After treatment with nicosulfuron, results for HK301 were significantly higher than those for HK320 for net photosynthetic rate, transpiration rate, stomatal conductance, leaf maximum photochemical efficiency of PSII, photochemical quenching of chlorophyll fluorescence, and the electron transport rate. These results were contrary to nonphotochemical quenching and intercellular CO2 concentration. As exposure time increased, associated effects also increased. Both O2·− and H2O2 detoxification is modulated by antioxidant enzymes. Compared to HK301, SOD, POD, and CAT activities of HK320 were significantly reduced as exposure time increase. Compared to HK320, the gene expression for the majority of SOD genes, except for SOD2, increased due to inducement by nicosulfuron, and it significantly upregulated the gene expression of CAT in HK301. Results from this study indicate that plants can improve photosynthesis, scavenging capabilities of ROS, and protective mechanisms to alleviate phytotoxic effect of nicosulfuron. Future research is needed to further elucidate the important role antioxidant systems and gene regulation play in herbicide detoxification in sweet maize.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nicosulfuron, a sulfonylurea herbicide that is active at low doses, has a low propensity to contaminate groundwater and it has a low toxicity to mammals (Corbett et al. 2005). This herbicide not only has a strong control over a variety of annual and perennial grasses, but also has a level of control over a number of broadleaf weeds. Therefore, the combination of nicosulfuron with broadleaf herbicides provides effective weed control over a broad spectrum (Stall and Bewick 1992; Dobbels and Kapusta 1993; Williams and Harvey 2000).
Nicosulfuron acts to reduce branched-chain amino acid synthesis, inhibiting acetolactate synthase (ALS) enzyme activity in sensitive plants (Rey-Caballero et al. 2016). Crops have different tolerances to sulfonylurea herbicides as their rates of metabolism to herbicides differ; plants sensitive to these herbicides detoxify the herbicides slower than plants with a tolerance. Studies have shown that the sensitivity or tolerance of sweet corn to several herbicides metabolized by cytochrome P450 is a simple genetic trait. Previous studies have highlighted that sensitivity of maize to various P450 herbicides is mainly regulated by a single CYP locus or a group of tightly linked CYP locus on the short arm of chromosome 5S (Pataky et al. 2008, 2009). A single gene sensitive to nicosulfuron was identified from the field maize inbred line W703A and named nsf1 (Kang 1993). A single-gene cross-sensitivity to nicosulfuron and bentazon was identified in the field corn inbred GA209 and named ben1 (Barrett et al. 1997; Bradshaw et al. 1994; Fleming et al. 1988). The allele of nsf1 locus on chromosome 5S of nicosulfuron-resistant field maize inbred line B73 contains a highly conserved heme-binding sequence found in most cytochrome P450 genes (Williams et al. 2006). The alleles in field maize inbred lines GA209 and W703a contained the same 392 bp insertion relative to B73 in the gene sequence. Therefore, this insertion mutation seems to lead to a nonfunctional P450 allele, leading to herbicide sensitivity, and nsf1 and ben1 genes are the same mutants of CYP gene. In the sweet corn population produced by the hybridization of Cr1 (herbicide sensitive inbred line) and Cr2 (herbicide tolerant inbred line), the chromosomal positions responsible for herbicide cross-sensitivity, including nicosulfuron, are closely related to nsf1 (Nordby et al. 2008). In addition, 29 sweet corn inbred lines and 45 sweet corn hybrids have the same or closely linked genes with Cr1, resulting in cross sensitivity to a variety of postemergence herbicides including nicosulfuron (Pataky et al. 2009). nsf1 plays an important role in herbicide resistance, and its functional relationship with herbicide resistance is being continuously established (Williams et al. 2006; Liu et al. 2019; Choe and Williams 2020). However, we still lack knowledge whether it may be associated to the complex nature of CYP450 genes, especially as these genes are difficult to isolate and functionally identify due to their sequence complexity and their low abundance of transcripts (Battett 2000; Nelson et al. 2004).
Nicosulfuron is not rapidly metabolized in plants with a sensitivity, and it will exist as a toxic substance. Herbicide residues in plants (as an abiotic stress) induce oxidative stress through the electron transport chain of the PSI and PSII systems (Hussain et al. 2010; Wang et al. 2018). Stress that destroys electron transport and other metabolic processes in photosynthetic organs can lead to an increase in O2− production. Previous studies have demonstrated that abiotic stresses (cold, drought, heavy metals, and salinity) induces stomatal closure, reduces the utilization efficiency of carbon dioxide in photosynthesis, and increases electron transfer to O2, thereby increasing the rate of O2·− production in chloroplasts (Vaahtera et al. 2014; Zarepour et al. 2010). Although O2·− is very active, it cannot diffuse into adjacent organelles far from its production site, and it can be rapidly converted into H2O2 by SOD; H2O2 is another slightly stable reactive oxygen species (ROS) (Bienert and Chaumont 2014). Plants have evolved complex antioxidant defense mechanisms to scavenge ROS, including enzymes and non-enzymatic substances. Biotic and abiotic stresses have been recorded to induce changes in plant antioxidant enzyme activity on the physiological and biochemical levels (Hossain et al. 2015). However, data relating to isozyme encoding due to the impact of nicosulfuron on gene expression in higher plants involved in the antioxidant enzyme system are rare.
Widening our understanding of gene expression involved in antioxidant enzyme activities will advance our knowledge of plant mechanisms to molecular adaptation and detoxification against various biotic and abiotic stresses. Expression of genes associated with antioxidants and other stresses can modulate the overproduction of ROS in plant cells. For example, stress related to drought, salinity, oxidative, and cold results in upregulation in the transcript level of antioxidative enzyme genes (Li et al. 2017; He et al. 2017; Zhang et al. 2017). Reports on ROS generation, elevated transcript levels of antioxidant enzymes, and an increase in antioxidant enzyme activity have also been recorded in response to various abiotic stresses (Zhao et al. 2019; AbdElgawad et al. 2020; Han et al. 2021). However, after treatment with nicosulfuron, regulatory mechanisms associated to antioxidant system genes family in sweet maize have not been reported. Our previous study have indicated that in different resistant sweet maize, C4 photosynthetic enzymes activity and key gene expression play a critical role in enhancing the adaptability of plants to nicosulfuron stress at a photosynthetic physiological level (Wang et al. 2021). In this study, therefore, we continued using a pair of sister lines differing in nicosulfuron tolerance to (1) understand related photosynthetic physiological mechanisms, (2) investigate ROS accumulation and the change of antioxidant enzyme activity in plants after spraying with nicosulfuron, and (3) investigate the expression level of antioxidant enzyme–related genes induced by nicosulfuron. Our results will increase our understanding of oxidative stress responses of sweet maize, and how gene expression and enzyme activity systems of sweet maize adapt to nicosulfuron stress.
Materials and methods
Plant materials
In this study, a pair of sister lines (nicosulfuron-tolerant HK301 and nicosulfuron-sensitive HK320) was used as plant material. After spraying with nicosulfuron, preliminary results indicated that HK301 recorded normal growth patterns, exhibiting a better level of tolerance; HK320 recorded high mortality rates and its growth was found to be inhibited.
Experimental design and treatments
Field experiment
Our experiment was conducted at Changli Farm of Hebei Normal University of Science and Technology (39°25′N, 118°45′E), and Dongyang experimental station of Zhejiang Academy of Agricultural Sciences (28°63′N, 120°31′E). The Changli Farm is located in a warm temperate zone, having a continental, monsoon-affected semi-humid climate, while the Dongyang experimental station is located in a subtropical monsoon climate zone with enough light and rainfall. Experimental analysis was undertaken from 2018 to 2019 using a herbicide concentration screening test. In order to screen HK301 and HK320, a pilot study was initially undertaken in Changli and Dongyang. A split-plot experimental design was used with nicosulfuron treatment defining the main plots and inbred lines within subplots, with three replicates. Each inbred line was sown in fifteen rows measuring 5 m in length, with 60 cm between rows and 14.9 cm between plants. Two seeds were planted in each hole to ensure 500 seedlings per plot were grown. Maize seedlings at the four-leaf stage were sprayed with nicosulfuron at effective concentrations of 0 (control), 20, 40, 80, 120, 160, 200, 240, 280, and 320 mg kg−1 using an electric backpack sprayer with a nozzle. The survival rate of seedlings was recorded after spraying (Table 1).
In 2019–2020, we designed a field experiment in Changli, Hebei Province, China. The experiment was designed as a randomized complete block design with three replicates. Each inbred line was sown in four rows measuring 5 m in length, with 60 cm between rows and 14.9 cm between plants. Two seeds were planted in each hole. When maize seedlings reached the four-leaf stage, an electric backpack sprayer with a nozzle was used to spray nicosulfuron, with water used as a control. We sprayed seedling plots at an effective concentration of 80 mg kg−1. When the effective concentration of the nicosulfuron was 80 mg kg−1, HK301 plants were able to attain normal growth, while HK320 plants either wilted or completely died. Field investigation and sampling were conducted 1, 3, 5, and 7 days after nicosulfuron treatment (DAT).
Pot experiment
Maize seeds were soaked for 24 h at 25 °C before being germinated for 24 h at 27 °C until white. In order to avoid the impact of the environment on growth, all samples were placed in an artificial climate chamber with a photoperiod of 24 h, an illumination intensity of 12,000 lx, a culture temperature of 25 °C during the day and 22 °C at night, and a relative humidity of 70%. The experiment utilized a randomized complete block design with three replicates of five pots each. Three seeds were planted in each pot (20 cm in diameter, 18 cm in depth) filled with soil. The soil was obtained from the top 0–15-cm soil layer of the Changli experimental station. All samples were watered daily during the culture period. At the four-leaf stage, the maize seedlings were sprayed with nicosulfuron; a water treatment was used as a control. As the best response time for maize to nicosulfuron herbicide is 24 h (Liu et al. 2015), 24 h after the samples had been sprayed they were frozen in liquid nitrogen and stored at − 80 °C for gene expression determination.
Gas exchange properties
Gas exchange properties for all leaf samples were analyzed for net photosynthetic rate (Pn), intercellular CO2 concentrations (Ci), stomatal conductance (Gs), and transpiration rate (E) using a LI-6400 portable optical instrument (LI-COR Biosciences Inc., Lincoln, NE, USA). Measurements were undertaken from 9:00 to 11:00 a.m. under ambient temperatures between 23 and 27 °C, and relative air humidity between 60 and 70%. No differences between the treatments were recorded during the experiment.
Chlorophyll fluorescence parameters
A PAM-2500 pulse modulated fluorometer (Walz, Germany) was used to measure chlorophyll fluorescence parameters, using PAMwin3 as the data acquisition software. By using the equations of Maxwell (2002), maximal quantum yield of PSII photochemistry (Fv/Fm), the effective quantum yield of PSII photochemistry (ΦPSII), and electron transport rate (ETR) were calculated. Calculations were also undertaken to determine the photochemical quenching coefficient (qP) and nonphotochemical quenching (NPQ), respectively: qP = (Fm − Fs)/(Fm − F0); NPQ = (Fm − Fm′)/Fm′.
O2 − production rate, H2O2, and lipid peroxidation assay
The method of Jiang and Zhang (2002) was used to calculate O2·− production rate. The methods of Jena and Choudhuri (1981) were used to determine H2O2 content. The methods of Heath and Packer (1968) were used to calculate malondialdehyde (MDA) content in the samples.
Proline content
Determination of proline content by Wang’s method (Wang et al. 2018).
Measurement of antioxidant enzyme activities
Enzymes were extracted by grinding of 0.5 g leaf sample in 5 ml phosphate buffer (pH 7.5) that contained 1 mm EDTA, 1% PVP, 1 mm DTT, and 1 mm PMSF. The homogenate was centrifuged at 15,000 × g at 4 °C for 30 min and the supernatant was collected to determine the enzyme activity. The method of Giannopolitis and Ries (1977) was used for the estimation of SOD activity. The amount of enzyme required to inhibit the photoreduction of NBT to purple formazan by 50% was defined as one unit of SOD activity. The method of Cakmak and Marschner (1992) was used to measure guaiacol peroxidase (POD, EC 1.11.1.7) activity by observing the increase in absorbance at 470 nm. Catalase (CAT, EC 1.11.1.6) activity levels were assayed by observing the decrease in absorbance at 240 nm (Aebi 1984).
RNA isolation and RT-PCR
Sample RNA was extracted by using total RNA extraction reagents (TaKaRa, Japan), and the experimental procedures were performed according to the product specifications. RT-PCR was used to detect the target gene primers. The following primers were synthesized in Beijing Invitrogen Company (Table 2). In this experiment, only primers with high amplification efficiency (more than 90%) were used. Reverse transcription of cDNA was performed using PrimeScript™ RT reagent Kit with gDNA Eraser, and experimental procedure was performed according to the product manual. According to the manufacturer’s instructions (CFX Connect Optics Module, Singapore), the RT-PCR reaction was performed on the target gene and the internal reference (actin) of each sample in the real-time PCR detection system, and each sample was tested for 3 replicates. Data were analyzed using 2−ΔΔct method.
Statistical analysis
Data processing and mapping was undertaken using Microsoft Excel and SigmaPlot 12.5. Analysis of variance (ANOVA) and mean values were compared using the least significant difference (LSD) test in SPSS (V. 12.0; SPSS Inc., Chicago, IL, USA). Significant differences were identified at the P < 0.05 threshold.
Results and discussion
Gas exchange properties
Apart from results at 5 DAT, nicosulfuron treatment resulted in no significant changes in Pn and Gs in HK301 (Fig. 1). Compared to HK301-CK, Pn in HK301 decreased by 17.64% at 5 DAT. Results for HK320 recorded a significant decrease in Pn and Gs in maize seedling leaves after 3 DAT due to the addition of nicosulfuron. Compared to HK320-CK, the addition of nicosulfuron significantly reduced Pn in HK320 at 3, 5, and 7 DAT by 35.41%, 79.05%, and 82.15%, respectively; nicosulfuron treatment significantly decreased Gs in HK320 at 3, 5, and 7 DAT by 36.18%, 71.92%, and 67.21%, respectively. In addition to 3 DAT, treatment using nicosulfuron resulted in no significant effect on Ci and E in HK301, a result contrary to those in HK320 (Fig. 2). Compared to HK320-CK, Ci in HK320 significantly increased by 65.62% (5 DAT) and 121.80% (7 DAT); E in HK320 significantly declined by 22.39% (3 DAT), 48.75% (5 DAT), and 75.57% (7 DAT).
Effects of nicosulfuron on the net photosynthetic rate (Pn) (A, B) and stomatal conductance (Gs) (C, D) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
Effects of nicosulfuron on the intercellular CO2 concentrations (Ci) (A, B), and transpiration rate (E) (C, D) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
The phytotoxic effect of nicosulfuron on maize, especially on sweet maize, has previously been reported (Stall and Bewick 1992; Grey et al. 2000). Our results indicated some phenotypic changes were observed on the leaves of sweet maize sprayed with nicosulfuron. Herbicides affect plant cell metabolism, chloroplast disintegration, and leaf color change in plants, ultimately affecting the photosynthetic mechanism of plants (Hess 2000; Xu et al. 2019). In our study, compared to HK301, Pn, Gs, and E in HK320 were significantly reduced after 3 DAT. In contrast, Ci in HK320 significantly increased after 5 DAT, suggesting that stomatal limitation was the main cause of Pn decrease. Although nicosulfuron targets specific areas in plants, evidence suggests that Pn, photosynthetic pigments, and photosynthesis-related protein activity of plants are also significantly reduced by nicosulfuron (Hess 2000). In the sensitive sweet inbred line HK320, Ci significantly increased and Gs significantly decreased after nicosulfuron treatment. Our results suggest that the action of nicosulfuron destroyed the chloroplast structure of sweet maize seedling leaves, resulting in a decrease in their photosynthetic carbon assimilation ability, an increase in the risk of photo oxidation damage, and a reduction in light absorption, transmission, and distribution between PS II and PSI (Murata et al. 2007), thereby affecting ATP and NADPH synthesis.
Chlorophyll fluorescence measurements
In HK320, nicosulfuron treatment caused no significant changes in Fv/Fm (Fig. 3(a)). After 3 DAT, nicosulfuron treatment significantly decreased Fv/Fm in HK320 (Fig. 3(b)). Compared to HK320-CK, Fv/Fm in HK320 significantly declined by 19.47%, 23.35%, and 23.99% at 3, 5, and 7 DAT, respectively. The actual photochemical reactivity of the PSII system is reflected using ΦPSII (Govindjee 2002). After nicosulfuron treatment, ΦPSII in HK301 only increased at 1 DAT while in HK320 ΦPSII significantly reduced at 3 and 7 DAT (Fig. 3(c, d)). After 3 DAT, ETR in HK301 recorded its minimum value before increasing. ETR results in HK320 recorded a continuous decrease (Fig. 3(e, f)); average ETR in HK301 was 37.03% higher than that of HK320. The decline of Pn in HK320 was linked to the reduction of ETR.
Effects of nicosulfuron on the PSII photochemistry (Fv/Fm) (A, B), PSII photochemistry (ΦPSII) (C, D), and electron transport rate (ETR) (E, F) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
In addition to 1 DAT, the addition of nicosulfuron in HK301 resulted in no significant changes in qP and a significant reduction in qP in HK320; qP in HK320 was significantly lower than that in HK301 (Fig. 4). After herbicide treatment, Fv/Fm, ΦPSII, ETR, and qP in HK320 leaves were reduced. In contrast, NPQ in HK320 increased. These results indicate that higher non-radiative energy dissipation reduced the openness of the PSII system.
Effects of nicosulfuron on the photochemical quenching coefficient (qP) (A, B), and nonphotochemical quenching (NPQ) (C, D) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; Vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
Light emitted by chlorophyll molecules from the excited state to the non-excited state is termed chlorophyll fluorescence. Photosynthetic energy conversion in higher plants, algae, and bacteria is typically calculated using chlorophyll fluorescence as an indicator (Chen et al. 2016). Thus, information about the potential enantioselectivity of nicosulfuron to PSII in sweet maize was gathered using chlorophyll fluorescence measurements. In the control samples, the Fv/Fm ratio of the two sweet inbred lines were close to 0.80, results which were in accordance with findings related to a wide range of stress-free higher plants (Johnson et al. 1993; Li et al. 2018). After nicosulfuron treatment, the Fv/Fm was significantly reduced in HK320 compared to the controls, indicating a remarkable enantioselective effect. In addition, qP, ETR, and ΦPSII of HK320 were significantly reduced and NPQ significantly increased. About 50% of commercially available herbicides have been widely reported to inhibit the action of chloroplast electron transport chains (Flores et al. 2013). As their mode of action is related to disturbance of the photosynthetic electron flow, thereby affecting carbon fixation, they therefore affect plant photosynthesis and inhibit normal plant growth and development (Chen et al. 2015). In our study, the reduction of qP may lead to a decrease in openness of the PSII system, and ultimately to the decline of the utilization ratio of excitation energy for photochemistry reaction. At the same time, the increase of NPQ indicates that a reduction of PSII system activity in HK320 was related to non-energy dissipation.
Nicosulfuron-induced oxidative stress
After 3 DAT, the production rate of O2·− in HK301 attained its maximum value before decreasing. Compared to HK301-CK, the production rate of O2·− in HK301 significantly reduced by 30.06% at 5 DAT (Fig. 5(a, b)). In contrast, after 1 DAT, the production rate of O2·− in HK320 continued to increase. After 3 DAT, the H2O2 content in HK301 reached its maximum value before decreasing; H2O2 content in HK320 increased with exposure time (Fig. 5(c, d)). Results indicate that the addition of nicosulfuron significantly increased H2O2 content in HK320 by 70.32%, 70.35%, 141.91%, and 203.67% at 1, 3, 5, and 7 DAT, respectively, compared to the control.
Effects of nicosulfuron on the O2− production rate (A, B), and H2O2 (C, D) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
After 1 DAT, MDA content in HK301 attained its maximum value before decreasing. MDA content in HK301 significantly increased at 1, 3, and 5 DAT by up to 16.59%, 28.47%, and 42.59%, respectively, compared with HK301-CK (Fig. 6(a, b)). After 3 DAT, MDA reached its maximum value in HK320, after which it declined. Compared to HK320-CK, MDA content in HK320 significantly increased at 1, 3, 5, and 7 DAT by up to 45.19%, 206.54% 181.67%, and 250.38%, respectively. MDA content in HK320 was therefore higher than that of HK301.
Effects of nicosulfuron on the malondialdehyde (MDA) (A, B), and proline (C, D) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
After treatment with herbicide, proline content of the two sweet inbred lines significantly increased. Compared to HK301-CK, proline content in HK301 increased by 17.19%, 74.38%, 95.31%, and 91.27% at 1, 3, 5, and 7 DAT, respectively. However, average proline content of HK301 was 22.04% higher than that of HK320 (Fig. 6(c, d)), indicating that the increase in proline content was associated with sweet maize tolerance to herbicides.
Many studies have shown that the difference of maize sensitivity to nicosulfuron is related to the activity of CYP450 monooxygenase (Barrett 1995; Kreuz et al. 1996; Siminszky 2006). Tolerant plants can rapidly degrade nicosulfuron through CYP450 monooxygenase activity, resulting in the reduction of nicosulfuron in plants. In contrast, sensitive plants cannot rapidly degrade nicosulfuron. The residual nicosulfuron in plants will increase the production of ROS and may eventually damage plants (Wang et al. 2018). The effect of nicosulfuron-induced damage to the photosynthetic system and photosynthetic electron transport chain resulted in an excess of electrons to be transferred from O2 to O2·−. SOD scavenges O2·− by catalyzing its dismutation, where one O2·− molecule is decreased to H2O2 and another is oxidized to O2 (Prasad et al. 2016). In our experiment, nicosulfuron resulted in a significant increase in O2·− and H2O2 accumulation in all HK320 samples, indicating that nicosulfuron residue in plants induced the production of ROS as a toxic factor. This finding is in accordance with findings by Alla and Hassan (2007) who recorded isoproturon to significantly accelerate O2·− and H2O2 production in maize seedlings. An accumulation of excess H2O2 will ultimately result in membrane lipid peroxidation. Previous investigations have shown that, for phytotoxic and light-related herbicides, a key component of the development of plant phytotoxicity symptoms is the destruction of cell membrane integrity, which in turn results in severe tissue damage and eventually death (Hess 2000). Our results showed that MDA content in HK320 increased with exposure time, and MDA content in HK301 was significantly lower than that in HK320. H2O2 is potentially capable of inducing lipid peroxidation expressed as MDA content in plant (Wang et al. 2018). The present observations are supported by Wu et al. (2005) who found that drought stress reduced chlorophyll content and Fv/Fm which coordinately promoted the overproduction of H2O2 and MDA contents in cauliflower plants. Chen et al. (2018) showed that lead stress markedly altered photosynthetic efficiency of cauliflower which correlated with pronounced H2O2 and MDA content accumulation. It is clear from the above results that abiotic stress significantly impaired ROS homeostasis and hampered photosynthetic machinery and membrane stability. Our results suggest that nicosulfuron residue in HK320 plants induced oxidative stress on the leaves through photosynthetic electron transport pathways, destroyed plant membrane lipids, and eventually resulted in plant wilting and death.
Antioxidant enzyme activity response to oxidative stress
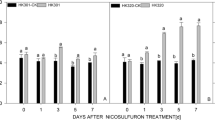
Significant increases in SOD enzyme activity in HK301 were induced at 1, 3, and 5 DAT due to the addition of nicosulfuron, while treatment significantly decreased SOD enzyme activity in HK320 at 3, 5, and 7 DAT (Fig. 7(a, b)). POD enzyme activity over time in the sweet inbred lines recorded different responses to the addition of nicosulfuron. Compared to the control, the addition of nicosulfuron promoted POD activity in HK301 leaves at 1, 3, 5, and 7 DAT. In HK320, POD activity initially increased before declining over time (Fig. 7(c, d)). CAT activity results for both sweet maize inbred lines attained maximum values after 1 DAT, after which they both declined. However, CAT results at 1 (58.46%), 3 (53.21%), 5 (180.49%), and 7 (191.89%) DAT in HK301 were significantly higher than the corresponding results in HK320 (Fig. 7(e, f)).
Effects of nicosulfuron on the SOD (A, B), POD (C, D) and CAT (E, F) in leaves of sweet maize seedlings. HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320; vertical bars represent the SE (n = 3). Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
Previous studies have indicated that SOD enzymes are the first barrier to alleviate oxidative stress in plants in the intracellular antioxidant system (Foyer 2018). However, SOD enzyme activity results in HK320 after treatment were significantly lower than results in the control; SOD activity results in HK301 were significantly higher than results in HK320. This may be because the SOD activity in HK301 was increased as a result of the formation of ROS by nicosulfuron exposure. Our findings indicate that CAT activity in HK301 significantly increased after 1 DAT, and CAT activity in HK320 increased at 1 and 3 DAT before declining compared to the control. CAT enzymes eliminate H2O2 by breaking it down directly to form water and oxygen (Hu et al. 2012). It is likely that excess production of ROS by nicosulfuron stress can inactivate CAT activity in HK320, probably by inactivating the enzyme-bound to heme group (Chaparzadeh et al. 2004). Amor et al. (2007) reported that lower CAT activity was related to a higher H2O2 accumulation in the leaves of C. maritima under salinity stress. POD is also among the major enzymes that scavenge H2O2 in chloroplasts (Hu et al. 2012). However, like SOD and CAT, the POD activity was higher in HK301 versus HK320, suggesting that tolerant genotype HK301 had a greater protection against the oxidative stress. Lower level of the H2O2 for HK301 compared with HK320 under nicosulfuron stress might be attributed to the relatively more efficient detoxifying enzymes CAT and POD. Our findings therefore suggest that increased CAT activity combined with SOD and POD activity changes in nicosulfuron-tolerant maizes play a vital protective role in the ROS-scavenging process. Oxidative stress tolerance in sweet maize due to nicosulfuron is thus partially related to the involvement of these enzymes. Our results also confirm changes in the antioxidant status and accumulation of antioxidants in response to the application of nicosulfuron.
Nicosulfuron-induced alterations in antioxidant enzyme-related genes
Transcript levels of antioxidant enzymes were measured in leaves treated with nicosulfuron and control samples from maize seedlings. Compared to the control, our results demonstrated that the Fe SOD gene of HK301 was significantly upregulated. Compared with the control, no significant differences in the intensity of Fe SOD2 transcript level were recorded. However, Fe SOD and Fe SOD2 genes in HK320 were significantly downregulated compared to the control. After the addition of nicosulfuron, compared to the control, Cu/Zn SOD1 and Cu/Zn SOD9 transcripts in HK301 significantly increased, contrary to HK320. The addition of nicosulfuron significantly increased the transcript levels of Mn SOD3 in HK301, while nicosulfuron treatment did not alter the transcription level of Mn SOD3 in HK320 (Figs. 8 and 9(a)).
Transcriptional levels of different antioxidant enzymes, expressed relative to the control, in leaves of sweet maize seedlings exposed to nicosulfuron ((A) SOD, (B) SOD1, (C) SOD2, (D) SOD3). HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320. Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
Transcriptional levels of different antioxidant enzymes, expressed relative to the control, in leaves of sweet maize seedlings exposed to nicosulfuron ((A) SOD9, (B) CAT1, (C) CAT2). HK301-CK: water treatment in HK301; HK301: nicosulfuron 80 mg·kg−1 treatment in HK301; HK320-CK: water treatment in HK320; HK320: nicosulfuron 80 mg·kg−1 treatment in HK320. Small letters (a, b) indicate differences between values obtained on different days after nicosulfuron treatment (P < 0.05) according to a least significant difference (LSD) test
The addition of nicosulfuron had a significant effect on the transcript level of CAT genes (Fig. 9(b, c)). CAT1 and CAT2 in HK301 recorded similar responses to the addition of nicosulfuron, having strong transcript inductions. Changes in the transcript level in CAT1 were not significant in HK320, although a slight increase was recorded in HK320. The transcript level of CAT2 in HK320 was significantly reduced after the addition of nicosulfuron.
SOD has been previously shown to be closely related to the quenching of superoxide to H2O2. Three types of SODs (Cu/Zn, Fe, and Mn SODs) have been previously identified in plants, and their metal cofactors in active sites differ. Exposure to environmental stress, such as drought, salinity, heavy metals, and heat, has resulted in significant changes in SOD activity (Rasoulnia et al. 2011; Kayihan et al. 2012; Rady and Osman 2012; Tian et al. 2012). Although total SOD activity was highest in HK301 after exposure to nicosulfuron, the transcript level of these three isoforms showed quite different responses. Compared to the control, Fe SOD, Cu/Zn SOD1, Cu/Zn SOD9, and Mn SOD3 had the strongest responses to nicosulfuron in HK301, with a 1.5–3.6-fold transcript increase 24 h after treatment. In contrast, the expression of Fe SOD, Fe SOD2, Cu/Zn SOD1, and Cu/Zn SOD9 in HK320 was significantly downregulated. However, Fe SOD2 transcript levels recorded a slight reduction in HK301, and the expression of Mn SOD remained unaltered in HK320. Plants have multiple genes encoding SOD. The isoenzymes of SOD are located in chloroplasts, mitochondria, peroxisomes, and cytoplasm. Mn SOD is most commonly found in mitochondria and peroxisomes, while Fe SOD is found in chloroplasts. When positioned in the chloroplast, Cu/Zn SODs provide less protection than Fe SODs (Bueno et al. 1995). Thus, our results suggested that chloroplast compartment might be more critical in scavenging O2·− in the stressed leaves of nicosulfuron-tolerant HK301. These results are very consistent with the study of perennial ryegrass (Hu et al. 2012); that is, under abiotic stress, the relative contribution of Fe SOD and Cu/Zn SOD to total SOD activity is higher than other SOD isoenzymes. Wang and Li (2008) studied the effects of water stress on the activities of total leaf SOD, Fe SOD, and Cu/Zn SOD in Trifolium repens L. and reported that the activity of SOD increased significantly under water stress. Eyidogan and Oz (2005) noted three SOD active bands (MnSOD, FeSOD, and Cu/ZnSOD) in C. arietinum under salt stress. In addition, the activities of Cu/ZnSOD and MnSOD isozymes increased significantly under salt stress. Our research proves that Cu/Zn SOD and Fe SOD strongly responds to nicosulfuron-induced oxidative stress compared with Mn SOD.
Catalase, present in peroxisome, is capable of independently decomposing H2O2 under abiotic stress conditions (Halliwell 1981). Upregulated expression of the CAT gene was found in maize seedlings under drought stress (Hong et al. 2017). In our study, the addition of nicosulfuron significantly increased the transcript levels of CAT1 and CAT2 in HK301, compared to the control, and the expression of CAT1 slightly increase in HK320; the transcript levels of CAT2 was markedly reduced in HK320. This finding suggests that CAT2 may have a more important protective role than CAT1 in plants against nicosulfuron-induced oxidative stress.
Conclusion
After nicosulfuron treatment, compared with HK301, Pn, E, Fv/Fm, qP, ETR, and ΦPSII of HK320 were significantly reduced, while Ci, Gs, and NPQ were significantly increased. The destruction of the PSII system in HK 320 led to higher accumulation of O2−· and H2O2, which promotes membrane lipid peroxidation. Our study showed that SOD, POD, and CAT activities of HK301 were significantly higher than those of HK320. Compared to HK320, the gene expression for the majority of SOD genes, except for SOD2, increased due to inducement by nicosulfuron, and it significantly upregulated the gene expression of CAT in HK301. Results from this study indicated that plants can improve photosynthesis, scavenging capabilities of ROS, and protective mechanisms to alleviate phytotoxic effect of nicosulfuron.
Data availability
All data generated or analyzed during this study are included in this published article.
References
AbdElgawad H, Zinta G, Hamed BA, Selim S, Beemster G, Hozzein WN, Wadaan MAM, Asard H, Abuelsoud W (2020) Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ Pollut 258:113705. https://doi.org/10.1016/j.envpol.2019.113705
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Alla MMN, Hassan NM (2007) Changes of antioxidants and GSH-associated enzymes in isoproturon-treated maize. Acta Physiol Plant 29:247–258. https://doi.org/10.1007/s11738-007-0031-8
Amor NB, Jimenez A, Megdiche W, Lundqvist M, Sevilla F, Abdelly C (2007) Kinetics of the anti-oxidant response to salinity in the halophyte Cakile maritima. J Integr Plant Biol 49:1–11. https://doi.org/10.1111/j.1672-9072.2007.00491.x
Barrett M (1995) Metabolism of herbicides by cytochrome P450 in corn. Drug Metab Drug Interact 12:299–316. https://doi.org/10.1515/dmdi.1995.12.3-4.299
Barrett M, Polge N, Baerg R, Bradshaw R, Poneleit C (1997) Role of cytochrome P450 in herbicide metabolism and selectivity and multiple herbicides metabolizing cytochrome P450 activities in maize. In: Hatzios K (ed) Regulation of enzymatic systems detoxifying xenobiotics in plants. Kluwer Academic Publishers, Dordrecht, p 35–50. https://doi.org/10.1007/978-94-015-8927-7_4
Battett M (2000) The role of cytochrome P450 enzymes in herbicide metabolism. HortSci 51:357–366. https://doi.org/10.1007/s11101-006-9011-7
Bienert GP, Chaumont F (2014) Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. BBA-Gen Subjects 1840:1596–1604. https://doi.org/10.1016/j.bbagen.2013.09.017
Bradshaw LD, Barrett M, Poneleit CG (1994) Inheritance of bentazon susceptibility in a corn (Zea mays) line. Weed Sci 42:641–647. https://doi.org/10.1017/s0043174500077080
Bueno P, Varela J, Gimenez GG, DelRio LA (1995) Peroxisomal copper, zinc superoxide dismutase. Characterization of the isoenzyme from watermelon cotyledons. Plant Physiol 108:1151–1160. https://doi.org/10.1104/pp.108.3.1151
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Chaparzadeh N, Amico ML, Nejad RK, Izzo R, Izzo FN (2004) Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol Biochem 42:695–701. https://doi.org/10.1016/j.plaphy.2004.07.001
Chen Y, Nara K, Wen Z, Shi L, Xia Y, Shen Z, Lian C (2015) Growth and photosynthetic responses of ectomycorrhizal pine seedlings exposed to elevated Cu in soils. Mycorrhiza 25:561–571. https://doi.org/10.1007/s00572-015-0629-4
Chen ZW, Zou YQ, Wang J, Li MC, Wen YZ (2016) Phytotoxicity of chiral herbicide bromacil: enantioselectivity of photosynthesis in Arabidopsis thaliana. Sci Total Environ 548:139–147. https://doi.org/10.1016/j.scitotenv.2016.01.046
Chen HY, Yu XM, Zhang XD, Yang L, Huang X, Zhang J, Pritchard HW, Li WQ (2018) Phospholipase Dalpha1 mediated phosphatidic acid change is a key determinant of desiccation induced viability loss in seeds. Plant Cell Environ 41:50–63. https://doi.org/10.1111/pce.12925
Choe E, Williams MM II (2020) Expression and comparison of sweet corn CYP81A9s in relation to nicosulfuron sensitivity. Pest Manag Sci 76:3012–3019. https://doi.org/10.1002/ps.5848
Corbett CL, Soltani N, Hamill AS, Sikkema PH, Bowley S, Robinson DE (2005) Tolerance of three sweet corn hybrid to a postemergence tankmix of nicosulfuron plus bromoxymil. Hortsci 40(3):616–619. https://doi.org/10.21273/HORTSCI.40.3.616
Dobbels AF, Kapusta G (1993) Postemergence weeds control in corn (Zea mays) with nicosulfuron combinations. Weed Technol 7:844–850. http://www.jstor.org/stable/3987861
Eyidogan F, Oz MT (2005) Effect of salinity on antioxidant responses of chickpea seedlings. Acta Physiol Plant 29:485–493. https://doi.org/10.1007/s11738-007-0059-9
Fleming AA, Banks PA, Legg JG (1988) Differential responses of maize inbreds to bentazon and other herbicides. Can J Plant Sci 68:501–507. https://doi.org/10.4141/cjps88-060
Flores F, Collier CJ, Mercurio P, Negri AP (2013) Phytotoxicity of four photosystem II herbicides to tropical seagrasses. PLoS ONE 8:e75798. https://doi.org/10.1371/journal.pone.0075798
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142. https://doi.org/10.1016/j.envexpbot.2018.05.003
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Govindjee (2002) A role for a light-harvesting antenna complex of photosystem II in photoprotection. Plant Cell 14:1663–1668. https://www.jstor.org/stable/3871666
Grey TL, Bridges DC, Raymer P, Day D, NeSmith DS (2000) Differential tolerance of fresh market sweet corn cultivars to the herbicides nicosulfuron and primisulfuron. Hortsci 35(6):1070–1073
Halliwell B (1981) Chloroplast metabolism: the structure and function of chloroplasts in green leaf cells. Bioscience 7:194–195. https://doi.org/10.1016/0014-5793(85)80685-2
Han T, Yan JW, Xiang Y, Zhang A (2021) Phosphorylation of ZmNAC84 at Ser-113 enhances the drought tolerance by directly modulating ZmSOD2 expression in maize Biochem Biophys Res Commun 567:86–91. https://doi.org/10.1016/j.bbrc.2021.06.026.
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. kinetics and stoic hiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hess FD (2000) Light-dependent herbicides: an overview. Weed Sci 48:160–170. https://www.jstor.org/stable/4046249
He F, Sheng M, Tang M (2017) Effects of Rhizophagus irregularis on photosynthesisand antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00183
Hong C, Cheng D, Zhang G, Zhu D, Chen Y, Tan M (2017) The role of ZmWRKY4 in regulating maize antioxidant defense under cadmium stress. Biochem Biophys Res Commun 482:1504–1510. https://doi.org/10.1016/j.bbrc.2016.12.064
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LSP (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Hussain MI, GonzáLez L, Reigosa M (2010) Phytotoxic effects of allelochemicals and herbicides on photosynthesis, growth and carbon isotope discrimination in Lactuca sativa. Allelopath J 26:157–174
Hu LX, Li HY, Pang HC, Fu JM (2012) Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. J Plant Physiol 169:146–156. https://doi.org/10.1016/j.jplph.2011.08.020
Jena S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354. https://doi.org/10.1016/0304-3770(82)90026-2
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and upregulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410. https://doi.org/10.1093/jxb/erf090
Johnson G, Young A, Scholes J, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679. https://doi.org/10.1111/j.1365-3040.1993.tb00485.x
Kang MS (1993) Inheritance of susceptibility to nicosulfuron herbicide in maize. J Hered 84:216–217. https://doi.org/10.1093/oxfordjournals.jhered.a111321
Kayihan C, Eyidogan F, Afsar N, Oktem HA, Yucel M (2012) Cu/Zn superoxide dismutase activity and respective gene expression during cold acclimation and freezing stress in barley cultivars. Biol Plant 56:693–698. https://doi.org/10.1007/s10535-012-0143-x
Kreuz K, Roberto T, Martinoia E (1996) Old enzymes for a new job. Plant Physiol 111:349–353. https://doi.org/10.1104/pp.111.2.349
Li Y, Chen Q, Nan H, Li X, Lu S, Zhao X, Liu B, Guo C, Kong F, Cao D (2017) Overexpression of GmFDL19 enhances tolerance to drought and salt stresses in soybean. PLoS ONE 12(6):e0179554. https://doi.org/10.1371/journal.pone.0179554
Li XX, Ke MJ, Zhang M, Peijnenburg WJGM, Fan XJ, Xu JH, Zhang ZY, Lu T, Fu ZW, Qian HF (2018) The interactive effects of diclofop-methyl and silver nanoparticles on Arabidopsis thaliana: Growth, photosynthesis and antioxidant system. Environ Pollut 232:212–219. https://doi.org/10.1016/j.envpol.2017.09.034
Liu XM, Xian X, Li BH, Wang XQ, Wang GQ, Li MR (2015) RNA-Seq transcriptome analysis of maize inbred carrying nicosulfuron-tolerant and nicosulfuron-susceptible alleles. Int J Mol Sci 16:5975–5989. https://doi.org/10.3390/ijms16035975
Liu XM, Bi B, Xu X, Li BH, Tian SM, Wang JP, Zhang H, Wang GQ, Han YJ, McElroy JS (2019) Rapid identification of a candidate nicosulfuron sensitivity gene (Nss) in maize (Zea mays L.) via combining bulked segregant analysis and RNA-seq. Theor Appl Genet 132:1351–1361. https://doi.org/10.1007/s00122-019-03282-8
Maxwell K (2002) Resistance is useful: diurnal patterns of photosynthesis in C3 and crassulacean acid metabolism epiphytic bromeliads. Funct Plant Biol 29:679–687. https://doi.org/10.1071/PP01193
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421. https://doi.org/10.1016/j.bbabio.2006.11.019
Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S (2004) Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol 135:756–772. https://doi.org/10.1104/pp.104.039826
Nordby JN, Williams MM II, Pataky JK, Riechers DE (2008) A common genetic basis in sweet corn inbred Cr1 for cross-sensitivity to multiple cytochrome P450-metabolized herbicides. Weed Sci 56:376–382. https://doi.org/10.2307/25148533
Pataky JK, Meyer JD, Meyer M, Bollman JD, Boerboom CM, Williams MM (2008) Genetic basis for varied levels of injury to sweet corn hybrids from three cytochrome P450-metabolized herbicides. J Am Soc Hortic Sci 133:438–447. https://doi.org/10.21273/JASHS.133.3.438
Pataky JK, Williams MM, Williams DE, Riechers MD, Meyer (2009) A common genetic basis for cross-sensitivity to mesotrione and nicosulfuron in sweet corn hybrid cultivars and inbreds grown throughout north America. J Am Soc Hortic Sci 134:252–260. https://doi.org/10.21273/JASHS.134.2.252
Prasad SM, Kumar S, Parihar P, Singh R (2016) Interactive effects of herbicide and enhanced UV-B on growth, oxidative damage and the ascorbate-glutathione cycle in two Azolla species. Ecotoxicol Environ Saf 133:341–349. https://doi.org/10.1016/j.ecoenv.2016.07.036
Rady MM, Osman ASH (2012) Response of growth and antioxidant system of heavy metal-contaminated tomato plants to 24-epibrassinolide. Afr J Agric Res 7:3249–3254. https://doi.org/10.5897/AJAR12.079
Rasoulnia A, Bihamta MR, Peyghambari SA, Alizadeh H, Rahnama A (2011) Proteomic response of barley leaves to salinity. Mol Biol Rep 38:5055–5063. https://doi.org/10.1007/s11033-010-0651-8
Rey-Caballero J, Menéndez J, Giné-Bordonaba J, Salas M, Alcántara R, Torra J (2016) Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic Biochem Physiol 133:67–72. https://doi.org/10.1016/j.pestbp.2016.03.002
Siminszky B (2006) Plant cytochrome P450-mediated herbicide metabolism. Phytochem Rev 5:445–458. https://doi.org/10.1007/s11101-006-9011-7
Stall WM, Bewick TA (1992) Sweet corn cultivars respond differentially to the herbicide nicosulfuron. Hort Sci 27:131–133. https://doi.org/10.21273/HORTSCI.27.2.131
Tian Z, Wang F, Zhang W, Liu C, Zhao X (2012) Antioxidant mechanism and lipid peroxidation patterns in leaves and petals of marigold in response to drought stress. Hort Environ Biotechnol 53:183–192. https://doi.org/10.1007/s13580-012-0069-4
Vaahtera L, Brosché M, Wrzaczek M, Kangasjärvi J (2014) Specificity in ROS signaling and transcript signatures. Antioxid Redox Signal 21:1422–1441
Wang CQ, Li RC (2008) Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol Plant 30:841–847. https://doi.org/10.1007/s11738-008-0189-8
Wang J, Zhong XM, Li FH, Shi ZS (2018) Effects of nicosulfuron on growth, oxidative damage, and the ascorbate-glutathione pathway in paired nearly isogenic lines of waxy maize (Zea mays L.). Pestic Biochem Physiol 145:108–117. https://doi.org/10.1016/j.pestbp.2018.01.015
Wang J, Gao H, Guo ZQ, Meng YY, Yang M, Li XL, Yang Q (2021) Adaptation responses in C4 photosynthesis of sweet maize (Zea mays L.) exposed to nicosulfuron. Ecotoxicol Environ Saf 214:1–12. https://doi.org/10.1016/j.ecoenv.2021.112096
Williams BJ, Harvey RG (2000) Effect of nicosulfuron timing on wild-Proso millet (Panicum miliaceum) control in sweet corn (Zea mays). Weed Technol 14:377–382. https://doi.org/10.1614/0890-037X(2000)014[0377:EONTOW]2.0.CO;2
Williams M, Sowinski S, Dam T, Li BL (2006) Map-based cloning of the nsf1 gene of maize. Program and Abstracts of the 48th Maize Genetics Conference, Pacific Grove, CA
Wu C, Wang ZQ, Sun HL, Guo SL (2005) Effects of different concentrations of nitrogen and phosphorus on chlorophyll biosynthesis, chlorophyll a fluorescence, and photosynthesis in Larix olgensis seedlings. Scientia Silvae Sinicae 4:31–36. https://doi.org/10.1360/biodiv.050121
Xu K, Racine F, He Z, Juneau P (2019) Impacts of hydroxyphenylpyruvate dioxygenase (HPPD) inhibitor (mesotrione) on photosynthetic processes in Chlamydomonas reinhardtii. Environ Pollut 244:295–303. https://doi.org/10.1016/j.envpol.2018.09.121
Zarepour M, Kaspari K, Stagge S, Rethmeier R, Mendel R, Bittner F (2010) Xanthine dehydrogenase atxdh1 from Arabidopsis thaliana is a potent producer of superoxide anions via its nadh oxidase activity. Plant Mol Biol 72:301–310. https://doi.org/10.1007/s11103-009-9570-2
Zhang L, Sun L, Zhang L, Qiu H, Liu C, Wang A, Deng F, Zhu J (2017) A Cu/Zn superoxide dismutase gene from Saussurea involucrata Kar. et Kir., SiCSD, enhances drought, cold and oxidative stress in transgenic tobacco. Can J Plant Sci 97:816–826. https://doi.org/10.1139/cjps-2016-0180
Zhao DQ, Li TT, Hao ZJ, Cheng ML, Tao J (2019) Exogenous trehalose confers high temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperones 24:247–257
Acknowledgements
The authors thank Hebei Key Laboratory of Crop Stress Biology (Hebei Normal University of Science and Technology) for the use of related instruments and consumables.
Funding
This work received financial support from the Natural Science Foundation of Hebei Province of China (No. C2019407095 and E2019407076).
Author information
Authors and Affiliations
Contributions
Zhen-Xing Wu: conceptualization, data curation, writing—original draft. Ning-Wei Xu: investigation, validation. Min Yang: investigation. Xiang-Ling Li: software, validation. Jin-Ling Han: visualization. Xiao-Hu Lin: formal analysis. Qing Yang: formal analysis. Gui-Hua Lv: conceptualization, supervision. Jian Wang: conceptualization, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, ZX., Xu, NW., Yang, M. et al. Responses of photosynthesis, antioxidant enzymes, and related gene expression to nicosulfuron stress in sweet maize (Zea mays L.). Environ Sci Pollut Res 29, 37248–37265 (2022). https://doi.org/10.1007/s11356-022-18641-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18641-0












