Abstract
The recommended field dose (RFD) of isoproturon induced significant accumulations of H2O2 in the leaves of 10-d-old maize seedlings throughout the following 20 d; the accumulation increased with time and also with herbicide dose. Meanwhile, low doses significantly increased ascorbic acid, glutathione and thiols while high doses caused diminutions. Superoxide dismutase (SOD; EC 1.15.1.1) activity was significantly enhanced up to the 12th d whereas ascorbate peroxidase (APX; EC 1.11.1.7) activity was significantly reduced after the fourth d onwards. Catalase (CAT; EC 1.11.1.6) and guaiacol peroxidase (GPX; EC 1.11.1.7) activities were similarly increased during the first 4 d but decreased from the 12th and the eighth d, respectively. Low doses increased SOD and GPX activities but high doses led to diminutions whereas CAT and APX were reduced by all doses. The activities of γ-glutamyl-cysteine synthethase (γ-GCS; EC 6.3.2.2) and glutathione synthethase (GSS; EC 6.3.2.3) were enhanced for 4 d; high doses caused general reductions. Isoproturon significantly reduced activities of glutathione S-transferase (GST; EC 2.5.1.18) isoforms [GST(CDNB), GST(ALA), or GST(MET)] after the fourth d, however, it had no effect on GST(ATR). Similar reductions in activities of glutathione peroxidase (GSPX; EC 1.15.1.1) and glutathione reductase (GR; EC 1.6.4.2) were detected up to the 16th and the 12th d, respectively. The activities of GST isoforms, GSPX and GR were reduced by high doses. These changes seemed to be related and might point to an oxidative stress state that exacerbated with prolonged time and/or increased isoproturon dose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
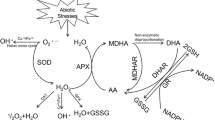
Isoproturon [arelon, 3-(4-isopropylphenyl)-1,1-dimethylurea] is widely used in Egypt for pre- and post-emergence control of weeds from maize. However, the herbicide may have unwanted side effects on the non-target crops. It is a photosynthetic inhibitor exerting its toxic effect by generating toxic ROS (Pyon et al. 2004). ROS typically result from the excitation of O2 to form singlet oxygen (1O2) or from the transfer of one, two or three electrons to O2 to form, respectively, superoxide radical (O −•2 ), hydrogen peroxide (H2O2) or a hydroxyl radical (HO•). ROS are capable of unrestricted oxidation of various cellular components and can lead to oxidative destruction of the cell (Mittler 2002; Hassan and Nemat Alla 2005; Nemat Alla and Hassan 2006). Plants develop defense antioxidants (Foyer et al. 2001; Aravind and Prasad 2005; Nemat Alla and Hassan 2006). Antioxidants are crucial for plant defense against oxidative stress. AsA and GSH are the most abundant non-enzymatic antioxidants in plant cells participating in ROS scavenging through AsA-GSH cycle (Foyer et al. 2001). GSH biosynthesis takes place in two steps. First, l-cysteine is conjugated to l-glutamate by γ-GCS forming γ-glutamylcysteine (Volohonsky et al. 2002). In the second step, glycine is added by GSS (Gupta et al. 2005). Moreover, GR is responsible for maintaining a high reduction state of the GSH pool (Anderson and Davis 2004; Murgia et al. 2004). Major ROS-scavenging mechanisms of plants include SOD, APX, CAT and GSTs (Nemat Alla 1995, 2000; Polidoros and Scandalios 1999; Mittler 2002; Hassan and Nemat Alla 2005; Nemat Alla and Hassan 2006). SOD is responsible for the elimination of O −2 generated in plant cells to H2O2. The generated H2O2 is removed by peroxidases and CAT (Polidoros and Scandalios 1999; Geoffroy et al. 2002; Murgia et al. 2004; Hassan and Nemat Alla 2005). GSTs and GSPX not only detoxify ROS but also enhance the conjugation of several herbicides with GSH (Dixon et al. 1995; Anderson and Davis 2004). Some isoforms of GST show dual activity and can also function as GSPX (Edwards et al. 2000; Vontas et al. 2002). Anderson and Davis (2004) stated that GST, GSPX and GR are enzymes that utilize GSH to play an important role in plant defense mechanism. Therefore, this work was aimed to ascertain the changes of antioxidants and GSH-associated enzymes following the induction of oxidative stress in maize by isoproturon.
Materials and methods
Plant materials and growth conditions
Maize grains (Zea mays L., hybrid 351) were germinated in sand/clay soil (1/1, v/v) in pots (25 cm diameter × 20 cm height). Pots, after being left in dark for 3 d, were transferred to a 14 h photoperiod with 450–500 μmol m−2 s−1 photosynthetic photon flux density, 75–80% RH, and 28/14°C d/night regime. When seedlings were 10 d old, the pots were divided into three groups; one to serve as a control and the second for isoproturon treatment at RFD (2.5 kg ha−1). The rate per hectare was calculated according to the surface area per pot and the herbicide was solubilized in a suitable amount of water enough to spray the surface area of each pot in one direction and crosswise. Shoots were collected just before the herbicide application (used for zero time) and also after 2, 4, 8, 12, 16 and 20 d, washed with copious amounts of water and blotted dry. The changes of most parameters were observed mainly between first and fourth d, however, a recovery was detected in some parameters mainly after the 12th d. Therefore, the third group treated with 0.5-, 1.0-, 1.5- and 2.0-fold RFD of isoproturon was performed to study the dose effects on the eighth d after treatment; after the herbicide had affected the plant and before the recovery was set in. For the extraction of the following parameters, leaves were weighed and separately homogenized with mortars and pestles on ice at about 4°C in the appropriate extraction buffer. Extraction and centrifugation were performed under cold condition.
Determination of H2O2
H2O2 was extracted in 200 mM perchloric acid, centrifuged at 5,000×g for 10 min and the supernatant was neutralized with 4 M KOH. After centrifugation at 3,000g for 5 min, the supernatant was loaded onto Dowex 1X8-100 anion exchange resin and eluted with distilled water. H2O2 was assayed in 12.5 mM 3-dimethylaminobenzoic acid in 375 mM phosphate buffer (pH 6.5), 1.3 mM 3-methyl-2-benzothiazolinone hydrazone and 0.25 units horseradish peroxidase (Okuda et al. 1991). The increase in absorbance at 590 nm was monitored for 3 min.
Determination of AsA, GSH and thiol
AsA was extracted in 62.5 mM metaphosphoric acid, centrifuged at 12,000g for 20 min and filtered through a 0.5 μm FH-type Millipore filter. The filtrate was loaded onto an aminex HPX-87H ion exclusion column (300 × 7.8 mm, Bio-rad) connected to analytical HPLC system, and eluted with 4.5 mM H2SO4 at a flow rate of 0.5 ml min−1. The elution of AsA was detected at 245 nm (Ahn et al. 1999).
GSH was extracted in 5% (w/v) TCA containing 10 mM EDTA and centrifuged at 12,000g for 15 min. GSH was assayed in 100 mM phosphate buffer (pH 6.8), 10 mM EDTA, 1 mM CDNB and 1 U equine GST. The absorbance was recorded at 340 nm before commencing the reaction and after the reaction had completed (Anderson and Gronwalds 1991).
The extraction of thiol was performed in 20 mM EDTA. The extracts were centrifuged at 12,000×g for 15 min. Total thiol was measured in 200 mM Tris–HCl (pH 8.2), 10 mM DTNB and absolute methanol and the absorbance was read at 412 nm (Sedlak and Lindsay 1968). The quantity of thiol was calculated from the extinction coefficient ε = 13,100 mM−1 cm−1. Non-protein thiol was measured in 50% (w/v) TCA, mixed and centrifuged at 10,000×g for 15 min. The protein-bound thiol was calculated by subtracting the non-protein from total thiols.
Enzyme assays
SOD was extracted in 50 mM phosphate (pH 7.8), 0.1% (w/v) BSA, 5.5 mM AsA, and 8 mM β-mercaptoethanol. SOD was assayed in 50 mM phosphate (pH 7.8), 9.9 mM l-methionine, 0.057 mM NBT, 0.025% (w/v) Triton ×-100 and 0.1 mM riboflavin (Beyer and Fridovich 1987). The photoreduction of NBT was measured at 560 nm. One unit was defined as the quantity that causes 50% inhibition of NBT photoreduction.
CAT was extracted in 50 mM phosphate buffer (pH 7), and 1 mM DTT. CAT was evaluated spectrophotometrically by determining the consumption of H2O2 at 240 nm in 50 mM phosphate buffer (pH 7.5) and 200 mM H2O2 (Aebi 1984). One unit was defined as the decomposition of 1 μmol H2O2 min−1.
APX was extracted in 0.1 M Tricine-KOH buffer (pH 8), 1 mM DTT, 10 mM MgCl2, 50 mM KCl, 1 mM EDTA, 0.1% (w/v) Triton ×-100 and 0.28 mM PMSF. APX was evaluated at 270 nm in 50 mM phosphate buffer (pH 7.5), 40 mM Na ascorbate and 200 mM H2O2 (Nakano and Asada 1981). One unit was equivalent to 1 μmol of AsA oxidized min−1.
GPX was extracted in 220 mM Tris–HCl (pH 7.4), 250 mM sucrose, 50 mM KCl, 1 mM MgCl2, 160 mM β-mercaptoethanol and 0.57 mM PMSF. GPX was assayed in 20 mM Na acetate (pH 5), 30 mM H2O2, and 2 mM guaiacol. The absorption at 470 nm was recorded and the activity was calculated using the extinction coefficient of 26.6 mM−1 cm−1 (Ranieri et al. 1997). The activity was converted to peroxidase units using horseradish peroxidase.
The extraction of γ-GCS and GSS was performed in 50 mM Tris–HCl (pH 7.5) containing 40 mM PMSF and 2% (w/v) PVPP. The extracts were centrifuged at 15,000×g for 20 min (Aravind and Prasad 2005). The assay of γ-GCS was performed in 20 mM Tris–HCl (pH 8.2), 20 mM sodium glutamate, 20 mM l-amino butyrate, 40 mM Na2-EDTA, 0.4% (w/v) BSA, 20 mM MgCl2 and 50 mM Na2-ATP (Nagalakshmi and Prasad 2001). The reaction mixture was incubated at 37°C for 30 min and the reaction was stopped by TCA to estimate phosphate content at 660 nm. γ-GCS unit was expressed as the release of 1 μmol of phosphate min−1.
The assay of GSS was carried out in 100 mM Tris–HCl (pH 8.0) containing 50 mM KCl, 20 mM MgCl2, 2 mM EDTA, 10 mM ATP, 2.5 mM DTT, 5 mM glycine and 5 mM γ-glutamylcysteine (Volohonsky et al. 2002). The reaction mixtures were incubated at 37°C and GSH was determined every 5 min up to 20 min. GSS unit was expressed as 1 μmol of GSH formed min−1.
GST was extracted in 100 mM Tris–HCl (pH 7.5), 2 mM EDTA, 14 mM β-mercaptoethanol and 7.5% PVPP. After centrifugation at 15,000g for 15 min, ammonium sulfate was added to 80% saturation and the protein pellets were collected (Dixon et al. 1995). Routine assay of GST was performed in 100 mM phosphate (pH 6.5), 5 mM GSH and 1 mM CDNB. The absorbance at 340 nm was measured and the activity was calculated by the extinction coefficient ε = 9.6 mM−1 cm−1 (Ando et al. 1988). Moreover, a series of HPLC assays were conducted to measure GST activities towards the herbicides alachlor, metolachlor and atrazine as substrates. The activities were evaluated from the determination of the residual non-conjugated herbicide. A sample of about 2 mg of protein was incubated with 10 μmol of GSH and 160 nmol of the herbicide for 2 h at 35°C. The reaction was stopped in a dry ice-acetone bath and the mixture was then lyophilized, extracted in methanol, centrifuged at 48,000×g for 10 min and injected into the HPLC. The following isocratic conditions were employed: mobile phase, water/acetonitrile (1/9, v/v); flow rate, 1 ml min−1; detection, 220 nm. Two control tests were carried out, either GSH or the enzyme was left out to check possible herbicide losses by either non-conjugating reactions or the non-enzymatic GSH conjugation.
GSPX was extracted in 100 mM Tris–HCl (pH 7.5), 1 mM EDTA and 2 mM DTT. The extracts were centrifuged at 15,000×g for 20 min. The reaction mixture constituted of 100 mM phosphate (pH 7.0), 2% (w/v) Triton ×-100, 0.24 U GR, 1 mM GSH, 0.15 mM NADPH, and 1 mM cumene hydroperoxide. After incubation at 30°C for 10 min, the rate of NADPH oxidation was measured by monitoring the absorbance at 340 nm for 3 min and calculated from the extinction coefficient ε = 6.2 mM−1 cm−1 (Nagalakshmi and Prasad 2001).
Extraction of GR was performed in 100 mM phosphate (pH 7.5) and 0.5 mM EDTA. The extracts were centrifuged at 15,000×g for 20 min. The reaction mixture contained 100 mM phosphate (pH 7.5), 0.5 mM EDTA, 0.75 mM DTNB, 0.1 mM NADPH and 1 mM oxidized glutathione (Smith et al. 1988). The reaction mixture was incubated at 35°C meanwhile absorbance at 412 nm was being measured up to 5 min. GR unit was expressed as 1 μmol of thiobis-2-nitrobenzoic acid formed min−1.
Protein was determined according to Bradford (1976). All values are means (±SD) of at least six replications from two independent experiments. The full data were statistically analyzed using the least significant difference (LSD) test at 5% level (Snedecor and Cochran 1980).
Results and discussion
Figure 1 shows that RFD of isoproturon significantly accumulated H2O2 in maize leaves during the entire experimental period as compared with control. The magnitude of accumulation increased with the elapse of time. Moreover, all doses of isoproturon (0.5–2.0-fold RFD) caused substantial and consistent increases ranged from 139 to 383%. Curve fitting equations indicate that 50% accumulation of H2O2 was induced by about 0.54 RFD of isoproturon.
H2O2 leads to a variety of cellular responses that are dose dependent. A high dosage results in hypersensitive cell death whereas a low dosage blocks cell cycle progression and functions as a developmental signal for the onset of secondary wall differentiation (Kurama et al. 2002). Really, H2O2 content increased under similar stress conditions (Luna et al. 2005). Nevertheless, H2O2 accumulation in maize by isoproturon might be due to an increased production and/or decreased degradation. The present results declare the dose-dependent influence of isoproturon on H2O2 accumulation. The higher the herbicide dose, the greater the accumulation of H2O2. Consequently, the increase in H2O2 production and/or the decrease in its degradation could be subsequently elevated with the high doses. The production of H2O2 is controlled by SOD while its degradation depends mainly on CAT, APX and GPX. Therefore, the greater accumulation of H2O2 would point to an increase in the SOD activity and/or a reduced activity of CAT, APX and GPX.
As shown in Fig. 2, SOD activity was significantly enhanced by isoproturon up to the 12th d of treatment. Similar inductions of CAT activity were detected only during the first 4 d, however, the activity was significantly reduced from the 12th d. On the other hand, APX activity was significantly reduced up to the end of the experiment, nevertheless, no changes were mostly observed during the first 4 d. On the contrary, GPX activity was significantly higher in treated samples than control during the first 4 d, thereafter, the enzyme activity was significantly reduced from the 12th d. The dose-dependent curves show enhancements in SOD and GPX activities by both 0.5- and 1.0-fold RFD of isoproturon, however, 1.5- and 2.0-fold RFD reduced the enzyme activities. On the other hand, CAT and APX activities were diminished by all doses; the magnitude of diminution was augmented as the herbicide dose increased.
SOD is a key enzyme in protecting cells against oxidative stress. Overproduction of SODs in plant chloroplasts led to protection against herbicides (Iannelli et al. 1999). However, higher doses of isoproturon reduced the SOD activity. Thus H2O2 high levels induced by high doses might result from the drop in its degradation rather than an increase in its formation. As expected, the activities of H2O2-scavenging enzymes were reduced by high doses. Under these conditions, where CAT, APX and GPX were diminished, the cell is not fully competent to remove H2O2, which would accumulate to toxic levels. Therefore, the deleterious effects of high doses seemed obvious. On the other hand, there was a correlation between the resistance of maize to paraquat and the increased activities of SOD and peroxidases (Iannelli et al. 1999). In addition, Pyon et al. (2004) reported that the activities of SOD, CAT and peroxidases were higher in paraquat-resistant Erigeron canadensis than in susceptible biotype. The changes in CAT and peroxidases could be related to their cytochemical localization. De Felipe et al. (1988) studied the cytochemical localization of CAT and peroxidases in the mesophyll of Lolium rigidum treated with isoproturon. They observed that the number of peroxisomes increased when growing in the presence of 3.4 × 10−5 M isoproturon. Also, Geoffrey et al. (2002) found that CAT and APX of Scenedesmus obliquus were significantly stimulated by oxyfluorfen. Therefore, the decreases in CAT and peroxidases activities indicate, if any, deficiency in H2O2 detoxification. The detoxification of H2O2 and the other ROS depends moreover on AsA-GSH cycle.
Results presented in Fig. 3 show that isoproturon resulted in significant increases in AsA and GSH contents only during the first 4 d of treatment. Thereafter, the levels retracted and even became lower than control from the 12th d onward. There were increases in AsA and GSH by low doses, high doses in contrast led to great diminutions. On the other hand, different forms of thiol have roles as detoxicants and antioxidants (Potters et al. 2002). It is obvious from Fig. 4 that isoproturon treatment led to high increases in protein-bound, non-protein and total thiol contents in maize. The magnitude of increase seemed higher at the start. Protein-bound and total thiol fractions were elevated by isoproturon during the first 8 d of treatment. Non-protein thiol was significantly increased only during the first 4 d. The dose-dependent curves show that 0.5 and 1.0 RFD resulted in significant enhancements in both protein-bound and total thiol whereas non-protein thiol was increased only by 0.5 RFD.
AsA and GSH act to detoxify ROS through active enzymatic pathways. AsA plays an important role in scavenging free radicals in plants. GSH is regarded as a key component of antioxidant defenses in most aerobic organisms and moreover to be limiting for tolerance to herbicides (Foyer et al. 2001). The inductions of AsA and GSH by low doses might increase plant defense to oxidative stress. The reverse is really true; the diminutions caused by 1.5- and 2.0-fold RFD would negatively affect the plant defense mechanisms. Therefore, the incipient increased GSH and AsA levels followed thereafter by consistent decreases might point to a malfunction and probably disable the antioxidative defense mechanism by the elapse of time as well as high doses. Thiol pool functions as the stress indicator and plays several roles in oxidative stress control and protection against xenobiotics and heavy metals (Nemat Alla and Hassan 1998; Mendoza-Cózatl and Moreno-Sánchez 2006). Farago et al. (1993) found that the decreased GSH levels in maize shoot enhance the susceptibility to metolachlor. The present results showed that thiol and GSH were induced by isoproturon only during the first few d and by low doses. The induction in thiol and GSH would enhance the detoxification of both herbicides and ROS as well as the formation of any metal-binding protein (Rauser 2000; Aravind and Prasad 2005).
GSH is synthesized from the combined action of γ-GCS and GSS (Volohonsky et al. 2002; Gupta et al. 2005). Figure 5 shows that the activities of γ-GCS and GSS were significantly increased by isoproturon during the first 8 and 4 d, respectively. The magnitude of increase was nullified by increasing the herbicide dose so that 1.5 and 2.0 RFD resulted in significant reductions. Such trend was most likely related to GSH changes confirming their essential role for GSH synthesis which, in turn, would favor the responsibility as antioxidant through GSH-AsA cycle or as detoxicant to accelerate the detoxification mechanisms of xenobiotics. Nevertheless, high doses decreased GSH and the connected enzymes causing a failure in detoxification mechanisms. Of the detoxification enzymes are the GSTs which are enhanced under certain conditions to increase plant defense against several biotic and abiotic agents. GSTs play a role in antioxidant metabolism (Polidoros and Scandalios 1999; Hassan and Nemat Alla 2005). Moreover, GSTs catalyze the conjugation of some xenobiotics with GSH.
Figure 6 shows the activities of GST towards CDNB, alachlor, metolachlor and atrazine as substrates [GST(CDNB), GST(ALA), GST(MET) and GST(ATR), respectively]. In general, isoproturon provoked significant reductions in activities of GST(CDNB), GST(ALA) and GST(MET) during the experimental period, however, no variations from control were detected during the first 4 d of treatment. GST(ATR) activity seemed unaffected by isoproturon all over the experimental period. Curve fitting indicates that activities of GST isoforms were generally decreased with increasing isoproturon doses. GST(CDNB) and GST(MET) isoforms seemed more resistant to isoproturon followed by GST(ATR) whereas GST(ALA) appeared the most sensitive. However, the reductions in GSTs activities by isoproturon were accompanied by general retractions of GSH. These findings could point to indicate the negative effects of isoproturon on antioxidants and detoxicants. In spite of the incipient increases in GSH and other thiol forms as well as activities of GST, γ-GCS and GSS, the deleterious effects of high doses and/or with the elapse of time appeared obvious.
Changes of GST activities towards CDNB [GST(CDNB)], alachlor [GST(ALA)], metolachlor [GST(MET)] and atrazine [GST(ATR)] as substrates in leaves of 10-d-old maize seedlings during the following 20 d from treatment with RFD of isoproturon a–d. e–h Dose-dependent curves on the eighth d after treatment. Data are mean ± SD. Vertical bars represent LSD at 5% level
Some isoforms of GST showed dual activity and can also function as GSPX (Edwards et al. 2000; Volohonsky et al. 2002; Vontas et al. 2002). Moreover, Ye et al. (2000) reported that two distinctly different types of GSPX activity exist in plants; those types having only GSPX activity, and those having dual GST/GSPX activities. GSPX is a part of the arsenal of the protective enzymes, which respond to stress (Aravind and Prasad 2005). In addition, GR is responsible for sustaining the reduced status of GSH (Yoon et al. 2005). Thus, GSH production would depend not only on the activities of γ-GCS and GSS but also on GR activity.
The activities of GSPX and GR were significantly reduced by isoproturon during the first 16 and 12 d, respectively (Fig. 7). Moreover, all doses reduced the activity of both enzymes. The greater the dose, the more the activity reduction was. Therefore, the reduction of GSPX would retard the detoxification of xenobiotics and ROS. Although the concentration of GSH and the enzymes connected with its synthesis rose in the early phase of the experiment, there was no increase in GST and GSPX activities pointing that the detoxification of isoproturon follows other metabolic routes than conjugation with GSH. Indeed, isoproturon metabolism is catalyzed by cytochrome P450 monooxygenase (Singh et al. 1998; Devine and Preston 2000). The predominant metabolites of isoproturon were identified as monodesmethyl–isoproturon, 2-hydroxy-isoproturon and 2-hydroxy-monodesmethyl-isoproturon (Glaβgen et al. 1999). On the other hand, the decrease in GR activity could not preserve enough GSH level for performing its functionality well. Gehin et al. (2006) reported that the glyphosate-caused depletion of GSH was accompanied with GSPX disorders. Moreover, Zabalza et al. (2006) concluded that the enhancement of the GSH content detected in imazethapyr-treated pea roots can be related to the detected increase of GR activity.
In conclusion, treatment of maize with isoproturon accumulated H2O2 throughout the experimental period and meanwhile temporarily increased AsA, GSH and thiol fractions. Such incipient increases were also detected, to some extent, for activities of SOD, CAT, APX, GPX, γ-GCS and GSS. In contradiction, activities of GSPX, GR and GSTs [GST(CDNB), GST(ALA) and GST(MET)] were generally reduced by isoproturon with no changes in GST(ATR). High doses greatly dropped the enzymatic and non-enzymatic antioxidants. These observations confirm the existence of oxidative stress induced in maize by isoproturon, the status that appeared less evident in the early phase of the experiment and by using low doses. High doses indicated that H2O2 accumulation was the result of delay in its scavenging. On the other hand, there was no increase in GST and GSPX activities in the early phase of the experiment although GSH and the activities of enzymes connected with its synthesis rose. These findings confirm that the detoxification of isoproturon follows other metabolic routes than conjugation with GSH. The elevated GSH appeared resulted from γ-GCS and GSS activities rather than GR activity. With the elapse of time and/or using high doses, γ-GCS, GSS and GR activities were greatly reduced decreasing therefore GSH with a subsequent lowering in ROS detoxification. Consequently, the protective mechanism by antioxidant defense system seemed to be delayed as time of treatment was prolonged and/or the herbicide dose was increased.
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- CAT:
-
Catalase
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- d:
-
Day(s)
- DTNB:
-
5,5-dithiobis-(2-nitrobenzoic acid)
- DTT:
-
Dithiothritol
- γ-GCS:
-
γ-Glutamylcysteine synthetase
- GPX:
-
Guaiacol peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSPX:
-
Glutathione peroxidase
- GSS:
-
Glutathione synthetase
- GST:
-
Glutathione S-transferase
- NBT:
-
Nitroblue tetrazolium
- PMSF:
-
Phenyl methyl sulphonyl fluoride
- PVPP:
-
Polyvinylpolypyrrolidone
- RFD:
-
Recommended field dose
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahn YO, Kwon SY, Lee HS, Park IH, Wak SS (1999) Biosynthesis and metabolism of vitamin C in suspension cultures Scutellaria baicalensis. J Biochem Mol Biol 32:451–455
Anderson JV, Davis DG (2004) Abiotic stress alters transcript profiles and activity of glutathione S-transferase, glutathione peroxidase, and glutathione reductase in Euphorbia esula. Physiol Plant 120:421–433
Anderson MP, Gronwalds JW (1991) Atrazine resistance in velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol 96:107–109
Ando K, Honma M, Chiba S, Tahara S, Mizutani JK (1988) Glutathione transferase from Mucor javanicus. Agric Biol Chem 52:135–139
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate–glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Beyer WF, Fridovich Y (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–154
De Felipe MR, Lucas MM, Pozuelo JM (1988) Cytochemical study of catalase and peroxidase in the mesophyll of Lolium rigidum plants treated with isoproturon. J Plant Physiol 132:67–73
Devine MD, Preston C (2000) The molecular basis of herbicide resistance. In: Cobb AH, Kirkwood RC (eds) Herbicides and their mechanisms of action. Academic, CRC, Sheffield pp 72–104
Dixon DP, Edwards R, Robinson NJ, Fordham-Skelton AP, Cole DJ (1995) Spectrum of herbicide reactive glutathione transferases in maize. Bright Crop Prot Conf Weeds UK 255–260
Edwards R, Dixon DP, Walbot V (2000) Plant glutathione s-transferases: enzymes with multiple functions in sickness and in health. Trend Plant Sci 5:193–198
Farago S, Kreuz K, Brunold C (1993) Decreased glutathione levels enhance the susceptibility of maize seedlings to metolachlor. Pestic Biochem Physiol 47:199–205
Foyer CH, Theodoulou FL, Delrot S (2001) The function of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci 4:486–492
Gehin A, Guyon C, Nicod L (2006) Glyphosate-induced antioxidant imbalance in HaCaT: the protective effect of Vitamins C and E. Environ Toxic Pharmac 22:27–34
Geoffroy L, Teisseire H, Couderchet M, Vernet G (2002) Effect of oxyfuorfen and diuron alone and in mixture on antioxidative enzymes of Scenedesmus obliquus. Pestic Biochem Physiol 72:178–185
Glaβgen WE, Komoβa D, Bohnenkamper O, Haas M, Hertkorn N, May RG, Szymczak W, Sandermann H Jr (1999) Metabolism of the herbicide isoproturon in wheat and soybean cell suspension cultures. Pestic Biochem Physiol 63:97–113
Gupta S, Srivastava AK, Banu N (2005) Setaria cervi: Kinetic studies of filarial glutathione synthetase by high performance liquid chromatography. Exp Paras 111:137–141
Hassan NM, Nemat Alla MM (2005) Oxidative stress in herbicide-treated broad bean and maize plants. Acta Physiol Plant 27:429–438
Iannelli AM, Van Breusegem F, Van Montagu M, Inze D, Massacci A (1999) Tolerance to low temperature and paraquat-mediated oxidative stress in two maize genotypes. J Exp Bot 50:523–532
Kurama EE, Fenille RC, Rosa VE Jr, Rosa DD, Ulian EC (2002) Mining the enzymes involved in the detoxification of reactive oxygen species (ROS) in sugarcane. Mol Plant Path 3:251–259
Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH (2005) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56:417–423
Mendoza-Cózatl DG, Moreno-Sánchez R (2006) Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J Theor Biol 238:919–936
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Murgia I, Tarantino D, Vannini C, Bracale M, Carravieri S, Soave C (2004) Arabidopsis thaliana plants overexpressing thylakoidal ascorbate peroxidase show increased resistance to paraquat-induced photooxidative stress and to nitric oxide-induced cell death. Plant J 38:940–953
Nagalakshmi N, Prasad MNV (2001) Reponses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867
Nemat Alla MM (1995) Glutathione regulation of glutathione S-transferase and peroxidase activity in herbicide-treated Zea mays. Plant Physiol Biochem 33:185–192
Nemat Alla MM (2000) The influence of naphthalic anhydride and 1-aminobenzotriazole on maize resistance to herbicides: A possible role for glutathione S-transferase in herbicide persistence and detoxification. Agric Med 130:18–26
Nemat Alla MM, Hassan NM (1998) Efficacy of exogenous GA3 and herbicide safeners in protection of Zea mays from metolachlor toxicity. Plant Physiol Biochem 36:809–815
Nemat Alla MM, Hassan NM (2006) Changes of antioxidants levels in two maize lines following atrazine treatments. Plant Physiol Biochem 44:202–210
Okuda T, Masuda Y, Yamanaka A, Sagisaka S (1991) Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 97:1265–1267
Polidoros AN, Scandalios JG (1999) Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L.). Physiol Plant 106:112–120
Potters G, De Gara L, Asard H, Horemans N (2002) Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol. Biochem 40:537–548
Pyon JY, Piao RZ, Roh SW, Shin SY, Kwak SS (2004) Differential levels of antioxidants in paraquat-resistant and -susceptible Erigeron Canadensis biotypes in Korea. Weed Biol Manag 4:75–80
Ranieri A, Castagna A, Lorenzini G, Soldatini GF (1997) Changes in thylakoid protein patterns and antioxidant levels in two wheat cultivars with different sensitivity to sulphur dioxide. Environ Exp Bot 37:125–135
Rauser WE (2000) The role of thiols in plants under metal stress. In: Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidian JC (eds) Sulfur nutrition and sulfur assimilation in higher plants. Paul Haupt, Bern, pp 169–183
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue by Ellman’s reagent. Anal Biochem 25:192–208
Singh S, Kirkwood RC, Marshall G (1998) Effect of the monooxygenase inhibitor piperonyl butoxide on the herbicidal activity and metabolism of isoproturon in herbicide resistant and susceptible biotypes of Phalaris minor and wheat. Pestic Biochem Physiol 59:143–153
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5-dithiobis-(2-nitrobenzoic acid). Anal Biochem 175:408–413
Snedecor W, Cochran G (1980) Statistical methods, 7th edn. The Iowa State University Press, Ames
Volohonsky G, Tuby CNYH, Porat N, Wellman-Rousseau M, Visvikis A, Leroy P, Rashi S, Steinberg P, Stark A (2002) A spectrophotometric assay of γ-glutamylcysteine synthetase and glutathione synthetase in crude extracts from tissues and cultured mammalian cells. Chem Biol Interact 140:49–65
Vontas JG, Small GJ, Nikou DC, Ranson H, Hemingway J (2002) Purification, molecular cloning and heterologous expression of a glutathione S-transferase involved in insecticide resistance from the brown plant hopper Nilaparvata lugens. Biochem J 362:329–337
Ye B, Faltin Z, Ben-Hyyim G, Eshdat Y, Gressel J (2000) Correlation of glutathione peroxidase to paraquat/oxidative stress resistance in Conyza determined by direct fluorometric assay. Pestic Biochem Physiol 66:182–194
Yoon HS, Lee IA, Lee H, Lee BH, Jo J (2005) Overexpression of a eukaryotic glutathione reductase gene from Brassica campestris improved resistance to oxidative stress in Escherichia coli. Biochem Biophys Res Commun 326:618–623
Zabalza A, Gaston S, Sandalio LM, del Río LA, Royuela M (2006) Oxidative stress is not related to the mode of action of herbicides that inhibit acetolactate synthase. Environ Exp Bot 59:150–159
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Rights and permissions
About this article
Cite this article
Nemat Alla, M.M., Hassan, N.M. Changes of antioxidants and GSH-associated enzymes in isoproturon-treated maize. Acta Physiol Plant 29, 247–258 (2007). https://doi.org/10.1007/s11738-007-0031-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0031-8