Abstract
Effects of polyethylene glycol (PEG)-induced water stress on the activities of total leaf superoxide dismutase (SOD) and chloroplast SOD (including thylakoid-bound SOD and stroma SOD) are described in white clover (Trifolium repens L.) grown in solution culture from rooted cuttings. Both leaf SOD and chloroplast SOD activities were markedly enhanced with increasing concentration of PEG stress, generating osmotic potentials around the roots 0, −0.5, −1.0, −1.5 MPa. The effects increased with time up to 72 h. Chloroplast Fe-containing SOD represented about 30% of the total leaf SOD activity in the control plants and a significant increase in chloroplast SOD activity was found during the stress period. This accounted for about 35.5–71.1% of the total leaf SOD activity. The proportion of chloroplast SOD in total leaf SOD not only increased with the decreasing of osmotic potential, but also increased with incubation time. Furthermore, the increase in thylakoid-bound SOD activity was much higher than that of stroma SOD in chloroplast of plants under water stress. The enhanced chloroplastic SOD activity, especially thylakoid-bound SOD activity, demonstrated in Trifolium repens suggests that Fe-SOD located in chloroplasts play a more important role than cytosolic Cu/Zn-containing SODs in scavenging O2 −.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On account of its excellent forage quality and high animal-feed value, Trifolium repens L. is the most important forage legume and cover crop. It is extensively grown in the temperate and the subtropical regions of the World. T. repens is agronomically highly valuable because it can add considerable amounts of nitrogen to degraded soils using nitrogen fixed by the bacterium, Rhizobium trifoli in the roots.
Water is essential for plant metabolism and any limitation in its availability affects almost all plant function, including the assimilation and partitioning of carbon (Cabuslay et al. 2002; Wu et al. 2007). Little, however, is known concerning T. repens responses to water deficit. In this study, the responses of the white clove cultivar ‘Syrian Selection’ to polyethylene glycol (PEG)-induced water stress were detected.
In addition to problems created by water shortage itself, plants subjected to water stress undergo increased exposure to activated forms of oxygen and accumulation of free radicals associated that causes damage to membranes by peroxidation (Smirnoff 1993). Both drought and salinity induce water deficit and consequently stomatal closure, a process that reduces CO2 availability for photosynthesis. As a consequence of such condition and under intense sunlight, the rate of production of reducing power is higher than the rate of re-oxidation, mainly by CO2 reduction thereby resulting in excessive reactive oxygen species (ROS) in the chloroplasts, that can result in photoinhibition and photooxidation damage (Asada 1999).
Drought-tolerant plants have well-developed defense systems against ROS, involving both enzymatic and non-enzymatic mechanisms. SOD has a central role in the antioxidant defense network. SOD is a key enzyme lowering concentrations of superoxide (Slooten et al. 1998). SOD catalyzes the disproportion of superoxide radicals (O2 −) to yield molecular oxygen and hydrogen peroxide (H2O2). The control of the steady-state O2 − levels by SOD is an important protective mechanism against cellular oxidative damage, since O2 − acts as a precursor of more cytotoxic or highly reactive oxygen derivatives, such as peroxynitrite or HO (Halliwell and Gutteridge 1999). Therefore, SOD is usually considered the first line of defense against oxidative stress, and increased SOD activity is correlated with increased protection from damage associated with environmental stress (Sigaud-Kutner et al. 2002; Pang et al. 2005). The involvement and the role of antioxidants in protection against oxidative stress have been demonstrated using transgenic plants (Foyer et al. 1994). Transgenic plants over expressing SOD showed increase tolerance to oxidative stress induced by light and methyl viologen (Bowler et al. 1994; Perl et al. 1993; Gupta et al. 1993), chilling (McKersie et al. 1993), ozone (Van Camp et al. 1994a, b), water-deficit (Foyer et al. 1994), salt stress (Tanaka et al. 1999) and anoxia (Biemelt et al. 2000). Over-production of Fe-SOD in tobacco chloroplasts was more effective in protecting against methyl viologen-induced damage than overproduction of Mn-SOD (Van Camp et al. 1994a, b).
To understand better the mechanisms underlying drought resistance in T. repens, the effects of PEG-induced water stress on activities of SOD in T. repens cultivar ‘Syrian Selection’ were studied. Both leaf SOD and chloroplast SOD activities were markedly enhanced with the increasing of PEG or with time, and the proportion of chloroplast SOD in total leaf SOD not only increased with the decreasing of osmotic potential, but also increased with incubation time. The enhanced leaf and chloroplast SOD activities demonstrated in T. repens suggest that T. repens seedlings may have higher potential to scavenge O2 − produced in situ in the chloroplasts, which may lead to higher tolerance to water stress.
Materials and methods
Plant material and water stress treatments
Seedlings of white clover (Trifolium repens L.) cultivar ‘Syrian Selection’ were established as apical cuttings consisting of 2–3 nodes rooted in a sand–peat potting mix. When a significant root system had developed, plants were transplanted to plastic jugs of half-strength Hoagland nutrient solution. According to Verslues et al. (1998), PEG used to impose low water potentials in solution culture decreased O2 movement by increasing solution viscosity, so supplemental oxygenation was required to avoid hypoxia in PEG solutions. In this experiment, the solution was aerated by bubbling with pressurized air through a perforated plastic tube extending along the bottom of the jug, the flow rate was 1,000 mL min−1. The ambient temperature was 25 ± 2°C, relative humidity was 60%, photoperiod was 14 h light/10 h dark and photon flux density was 200 μmol m−2 s−1. When plants reached a sufficient size, comprising at least 8–10 mature stolons, water stress was imposed.
For water stress treatments, T. repens plants were culture in half-strength Hoagland nutrient solution containing polyethylene glycol 6000 solutions of osmotic potential: −0.5, −1.0 and −1.5 MPa. After 24, 48 and 72 h of incubation, T. repens leaves and chloroplasts were assayed for SOD activities and isozymes.
Preparation of leaf SOD extracts and SOD activity assay
One gram of plant material was homogenized at 4°C in 2 mL of medium: 100 mmol L−1 K-phosphate buffer (pH 7.8) containing 3 mmol L−1 MgSO4, 3 mmol L−1 EDTA and 2% (W/V) polyvinyl polypyrrolidone (PVP). The homogenate was centrifuged at 20,000g (Eppendorf Centrifuge 5417R) for 5 min at 4°C. The supernatant was used for determination of SOD and protein content.
In the presence of SOD the photochemical reduction of nitro blue tetrazolium (NBT) is inhibited and the level of inhibition was used to quantify the enzyme. SOD was assayed according to Giannopolitis and Ries (1977) with some modifications. The reaction medium was composed of 50 mmol L−1 K-phosphate buffer (pH 7.8), 0.1 mmol L−1 EDTA, 15 mmol L−1 methionine, 60 μmol L−1 riboflavin, 2.25 mmol L−1 NBT and an appropriate aliquot of extract in a final volume of 4 mL. The reaction mixture was illuminated with light intensity of 72 μmol m−2 s−1 for 15 min and turning the lights off stopped the reaction. A control reaction was always performed wherein all the steps and components were exactly the same as described above, except that crude enzyme was replaced with an equal volume of phosphate buffer (pH 7.8). Assays were always carried out at 25°C. The reaction was measured at 560 nm. One unit of enzyme activity was defined as the quantity of enzyme that reduced the absorbance reading of samples to 50% in comparison to tubes lacking enzymes.
The protein content was determined by using bovine serum albumin as standard according to Bradford (1976).
Isolation of chloroplast SOD and SOD activity assay
Chloroplasts were isolated according to the method of Sgherri et al. (2000) with some modifications. Twenty grams of T. repens leaves were placed at −15°C for 1 h, and then washed with cold deionized water and homogenized in ice-cold grinding medium containing 330 mmol L−1 sorbitol, 50 mmol L−1 MES (pH 6.1), 10 mmol L−1 NaCl, 2 mmol L−1 MgCl2, 2 mmol L−1 EDTANa2, 0.5 mmol L−1 KH2PO3 and 2 mmol L−1 sodium ascorbate. The homogenate was filtered through eight layers of cheesecloth and centrifuged at 300g for 30 s. The supernatant was centrifuged at 1,500g for 2 min, then the supernatant was discarded and the pellet obtained was suspended in 3-mL suspension medium (330 mmol L−1 sorbitol, 50 mmol L−1 HEPES (pH 7.6), 10 mmol L−1 NaCl, 2 mmol L−1 MgCl2, 2 mmol L−1 EDTA-Na2, 0.5 mmol L−1 KH2PO3, 2 mmol L−1 sodium ascorbate). Three milliliters of chloroplast suspension was loaded onto 4 mL of 40% Percoll and centrifuged at 2,500g for 60 s. The lowest layer, containing intact chloroplasts, was removed and washed three times with the suspension medium. One fraction was resuspended with 1-mL suspension medium containing 0.1% Triton X-100, which is used in total SOD activity assay. The other fraction was re-suspended in a hypotonic medium (the same as the suspension medium except that sorbitol concentration was 4 mmol L−1) in order to break the chloroplasts and release the stromal enzyme. After centrifugation at 20,000g for 5 min, the supernatant is used as stromal SOD extracts and thylakoids were included in the pellet for solubilizing membrane-bound SOD. Thylakoid-bound SOD was solubilized as reported in Navari-lzzo et al. (1998). Thylakoid-bound SOD was extracted by incubating membranes with 0.1% Triton X-100 and 1.5 mmol L−1 DTT for 30 min (Hayakawa et al. 1985). All the steps were carried out at 4°C.
Identification of SOD isozymes
SOD isozymes from T. repens leaves and chloroplasts were separated by non-denaturating polyacrilamide gel electrophoresis (Laemmli 1970). Samples (40 mg protein per slot) were loaded on 10% gels. The gels were run at 4°C. SOD isozymes were localized on the gels by the method of NBT reduction by superoxide radicals generated photochemically (Beauchamp and Fridovich 1971). After electrophoresis, the gels were covered with a solution containing nitro blue tetrazolium (0.25 mg mL−1) and riboflavin (0.1 mg mL−1), and exposed to light. SOD activity in gels was visualized as achromatic bands by staining with NBT. The three types of SOD, Mn-SOD, Fe-SOD and Cu/Zn-SOD were identified using specific inhibitors. Before staining, zymograms were incubated at 25°C for 30 min, separately, in solutions of 5 mmol L−1 H2O2, 3 mmol L−1 KCN or both inhibitors. The sensitivity of Cu/Zn-SOD to cyanide (KCN) has been used as a diagnostic tool to distinguish Cu/Zn-SOD from Fe-SOD and Mn-SOD that are unaffected by cyanide. Likewise, Fe-SOD is irreversibly inactivated by H2O2, whereas Mn-SOD is resistant to both inhibitors (Baum and Scandalios 1979).
Activity staining patterns of the negatively SOD gels were analyzed using a FB 910 Densitometer (FisherBiotech, USA) and a computer-aided image analysis system. The isozyme activity in percentage was quantified by recording the transmittance of the gels and integrating the activity areas under the transmittance peaks. The isozyme activity was obtained by multiplying each percentage by the total SOD activity of extracts (Hernandez et al. 1994).
Results
SOD isozyme patterns
Extracts of soluble proteins from T. repens seedlings were analyzed on the non-denaturing polyacrylamide gel in order to differentiate Cu/Zn-, Mn-, and Fe-SOD. According to inhibitor sensitivities, Mn-SOD (neither inhibited by 3 mmol L−1 KCN nor 5 mmol L−1 H2O2), Fe-SOD (inhibited by 5 mmol L−1 H2O2) and Cu/Zn-SOD (inhibited by 3 mmol L−1 KCN and 5 mmol L−1 H2O2) were examined. Three kinds of electrophoretically different isozymes were present in the leaves of T. repens. As judged by their sensitivity towards KCN or H2O2, they were identified as one Mn-SOD, one Fe-SOD and three Cu/Zn-SODs. Band B was fully inhibited in the presence of 5 mmol L−1 H2O2, but not affected by 3 mmol L−1 KCN, the broad band C was very sensitive to both KCN and H2O2, while the upper band (A) was insensitive to both inhibitors, indicating that the band A was Mn-SOD, the band B was Fe-SOD and the broad band C with high electrophoretic mobility contained Cu/Zn-SODs (Fig. 1). On the right-hand side, the activity bands were named according to the inhibitor sensitivities of the respective SOD proteins (Fig. 1, rows 2, 3). According to the isoform patterns observed in T. repens leaves, 20, 30 and 50% of the total SOD activity can be ascribed to the activities of Mn-SOD, Fe-SOD and Cu/Zn-SODs, respectively.
Differentiation of Cu/Zn-, Mn-, and Fe-SOD in leaf extract of control unstressed T. repens plants. Forty-mg-protein were loaded and separated on the non-denaturing polyacrylamide gel and stained with NBT. Row 1 crude enzyme extract; row 2 crude enzyme extract treated with 3 mmol L−1 KCN; row 3 crude enzyme extract treated with 5 mmol L−1 H2O2; row 4 enzyme extract from isolated chloroplasts. Band A Mn-SOD; band B chloroplastic Fe-SOD; band C cytosolic Cu/Zn-SODs
Electrophoretic pattern of SOD isolated from chloroplasts was shown in Fig. 1, row 4. The band B was the main SOD in chloroplasts. Thus, the band C was very likely to be cytosolic Cu, Zn-containing SOD and the band B Fe-containing SOD located in chloroplasts.
Effects of water stress on leaf SOD activity
When seedlings of T. repens were subjected to water stress by incubation with PEG solutions of different concentrations, the staining intensities of the SOD isozymes in gels were proportionally enhanced with the decreasing of osmotic potential (Fig. 2). No novel SOD isoenzyme band was apparent in extracts from PEG-treated plants compared to control plants. Mn-SOD activity decreased while Fe-SOD activity significantly increased as responses to PEG-treatment. Cu/Zn-SODs exhibited the highest inherent activity; however, only one Cu/Zn-SOD isoenzyme activity increased in response to water stress.
Effect of polyethylene glycol-induced water stress on the activities of superoxide dismutase isozymes in leaves of T. repens after 48 h. The non-denaturing polyacrylamide gel was loaded with 40 mg protein per slot and negatively stained with NBT. Lanes 1, 2, 3 and 4 represent Control, −0.5, −1.0, and −1.5 MPa, respectively
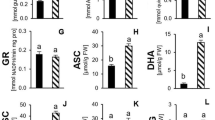
Electrophoretic observations were confirmed by total leaf SOD and chloroplast SOD activities. As shown in Fig. 3, leaf SOD dramatically increased with increasing water stress. Upon imposition of water stress at 48 h, the total SOD activity increased by 25.3, 35.6 and 53.5% at −0.5, −1.0 and −1.5 MPa, respectively. Furthermore, leaf SOD activity also increased with incubation time. Incubation of T. repens plants for 24, 48 and 72 h led to a gradual increase in the activity of leaf total SOD. For example, at −1.5 MPa, the highest effect was found 72 h after water deficit—the activity was about 72.3% higher than in the control plants (Fig. 3a).
Leaf SOD activity a, total chloroplast SOD activity b, thylakoid-bound SOD activity c and stroma SOD activity of chloroplast d in leaves of T. repens exposed to different levels of polyethylene glycol-induced water stress (0, −0.5, −1.0, −1.5 MPa) after 24, 48 and 72 h. Data points are mean ± SE (n = 5)
Effects of water stress on chloroplast SOD activity
To examine the response of chloroplast SOD activity to water stress, total chloroplast SOD activity was determined. As shown in Fig. 3b, under water stress, the total chloroplast SOD activity was markedly enhanced with decreasing osmotic potential or with time in T. repens seedlings. In the control plants, the chloroplast Fe-containing SOD represented about 30% of the total leaf SOD activity. This is consistent with the results of the former electrophoresis (Fig. 1). The proportion of chloroplast SOD in total leaf SOD not only increased with the decreasing osmotic potential, but also increased with incubation time. For example, at −1.0 MPa, the percentages of chloroplast SOD in total leaf SOD activity were 36.5, 46.5 and 62.7% at 24, 48 and 72 h, respectively. While at 48 h, the percentages of chloroplast SOD in total leaf SOD activity were 40.2, 46.5 and 59.7% at −0.5, −1.0 and 1.5 MPa, respectively, whereas the chloroplast SOD in the control plants remained unchanged. These results suggest that water stress influences differentialy chloroplastic and cytosolic SODs. Enhanced Fe-SOD located in chloroplasts may play an even more important role than cytosolic SODs in scavenging O2 − with in situ amounts produced in the chloroplasts increasing with the magnitude of water stress.
Similar to total chloroplast SOD activity, both the activities of thylakoid-bound SOD and stroma SOD increased with decreasing osmotic potential or with time (Fig. 3c ,d). The thylakoid-bound SOD activity was much higher than the stroma SOD activity in T. repens seedlings. For example, the activity of thylakoid-bound SOD was 28.8 U mg−1 pro h−1, while the activity of stroma SOD was 12.10 U mg−1 pro h−1 when seedlings grown in solution with osmotic potential of −1.0 MPa at 48 h. That is to say, the activity of thylakoid-bound SOD constituted the major part of total chloroplast SOD activity. Furthermore, the increases in the thylakoid-bound SOD activity were much higher than in the stroma SOD activity under water stress. For example, the increase in thylakoid-bound SOD activity was 14.8 U mg−1 pro h−1, while that of stroma SOD activity was 6.36 U mg−1 pro h−1 at −1.0 MPa at 48 h. The activity of thylakoid-bound SOD was distinctly enhanced at −1.0 MPa and represented 70% of total chloroplast SOD. These results indicate T. repens seedlings have a high ability to scavenge ROS in situ produced in the chloroplasts when subjected to the PEG-induced water stress.
Discussion
Abiotic stress accounts for more plant productivity loss than any other factor (Boyer 1982). At the present, our ability to improve plant stress tolerance is limited by a poor understanding of the inherent complexity of stress physiology and adaptation processes. In the present work, the responses of the SOD enzymes to PEG-induced water stress suggest that oxidative stress is an influential component of water stress on T. repens plants. The data also suggest that SOD plays a significant role in resistance to water stress in T. repens.
In different species, tolerance to water stress has been linked to increased enzymatic defenses against oxygen radicals, together with synthesis of free radical scavengers (Smirnoff 1993; Zhang and Kirkham 1994). Variable responses of SOD to water deficit have been reported. Enhanced SOD activity in pea (Moran et al. 1994), wheat (Zhang and Kirkham 1994) and tobacco (Van Rensburg and Krüger 1994) was found when drought stress was applied. However, most of these studies did not distinguish between the activities of various SOD isoforms. It is known that enzymes having a multiple intracellular distribution are present as different isoenzyme forms and targeted to different cell compartments (Damodara and Venkaiah 1984). Clearly therefore, analysis of the activity of individual SOD isozymes is important, because it can help to understand how each stress may affect different subcellular compartments (Scandalios 1993). Three SOD isozymes in potato species have been described (Bowler et al. 1994). Cu/Zn-SOD isozymes are present in both cytosol and chloroplasts, Mn-SOD in mitochondria and glyoxisomes, and Fe-SOD in chloroplasts and peroxisomes (Scandalios 1993). All the SODs are nuclear coded and transported to their organellar locations by means of NH2-terminal targeting sequences (Bowler et al. 1994). In the chloroplast, superoxide dismutase and ascorbate peroxidase enzymes exist in both soluble and thylakoid-bound forms. Thylakoid-bound forms of SOD and APX may efficiently detoxify O2 − and H2O2 at their site of production (Bowler et al. 1994) and prevent inactivation of Calvin cycle enzymes (Kaiser 1979). Soluble forms of SOD and APX react with O2 − and H2O2 that diffuse into the stroma from the thylakoid membrane (Niyogi 1999).
Yu and Rengel (1999) found in water-stressed lupins that total SOD activity markedly increased due to Cu/Zn-SOD and Fe-SOD activity that rose with increasing stress. When water stress was relieved, the activity of Cu/Zn-SOD returned to the control level, while the Fe-SOD activity remained above the control level. In contrast, Mn-SOD activity was not affected during the course of drought stress. Yu and Rengel suggested that Cu/Zn-SOD and Fe-SOD, but not Mn-SOD are involved in the drought-induced oxidative stress events in the lupin. In this study, no novel SOD isoenzyme was apparent in PEG-treated plants compared to control plants (Figs. 1, 2) although total leaf SOD and chloroplast SOD activities both markedly increased when T. repens was exposed to PEG-induced water stress (Fig. 3a, b). The proportion of chloroplast SOD in total leaf SOD not only increased with the decreasing of osmotic potential but also increased with incubation time (Fig. 3b). So Fe-SOD located in chloroplasts play a more important role than cytosolic Cu/Zn-containing SODs in scavenging O2 − produced in the chloroplasts during water deficiency.
Two important sources of oxidative stress in photosynthetic organisms are the chloroplast and the mitochondria. In chloroplasts, the presence of electron flux, high oxygen concentration and metal ions can enhance the generation of ROS, particularly O2 − and O 12 (Foyer 1996). Water deficit is known to enhance O2 − production in the chloroplast (Asada 1999) and to be associated with greater activity of chloroplast SOD over time. Thylakoids are considered to be major sites of superoxide production because of the simultaneous presence in chloroplasts of high oxygen concentrations and an electron transport system (Sgherri et al. 2000). Here, it was found that the increase in chloroplast Fe-SOD activity and the increase in magnitude of water stress were closely related (Fig. 3b). The activity of thylakoid-bound SOD constituted the major part of total chloroplast SOD activity and was effectively enhanced by water stress (Fig. 3c, d). Consequently, the enhancement of SOD activity in the chloroplasts of T. repens, especially thylakoid-bound SOD activity appears to play an important role in resisting oxidative stress induced by water deficit.
References
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. doi:10.1146/annurev.arplant.50.1.601
Baum JA, Scandalios JG (1979) Developmental expression and intracellular localization of superoxide dismutase in maize. Differentiation 13:133–140. doi:10.1111/j.1432-0436.1979.tb01576.x
Beauchamp CO, Fridovich I (1971) Superoxide dismutase. Improved assays and an assay applicable to acrylamide gel. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Biemelt S, Keetman U, Mock HP, Grimm B (2000) Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell Environ 23:135–144. doi:10.1046/j.1365-3040.2000.00542.x
Bowler C, Van Camp W, Van Montagu M, Inze D (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13:199–218. doi:10.1080/713608062
Boyer J (1982) Plant productivity and environment. Science 218:443–444. doi:10.1126/science.218.4571.443
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cabuslay GS, Ito O, Alejar AA (2002) Physiological evaluation of responses of rice. Plant Sci 163:815–827. doi:10.1016/S0168-9452(02)00217-0
Damodara C, Venkaiah B (1984) Studies on isoenzymes of superoxide dismutase from mung bean (Vigna radiata) seedlings. J Plant Physiol 116:279–284
Foyer CH (1996) Free radical processes in plants. Biochem Soc Trans 24:427–433
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defense mechanism studied in transgenic plants. Plant Cell Environ 17:507–523. doi:10.1111/j.1365-3040.1994.tb00146.x
Giannopolitis CN, Ries SK (1977) Superoxide dismutases, I. Occurrence in higher plants. Plant Physiol 59:309–314
Gupta AS, Heinen JL, Holaday AS, Burke JJ, Allen RD (1993) Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA 90:1629–1633. doi:10.1073/pnas.90.4.1629
Hayakawa T, Kanematsu S, Asada K (1985) Purification and characterization of thylakoid-bound Mn-superoxide dismutase in spinach chloroplasts. Planta 166:111–116. doi:10.1007/BF00397393
Hernandez JA, Del Rio L, Sevilla F (1994) Salt stress-induced changes in superoxide dismutase isozymes in leaves and mesophyll protoplasts from Vigna unguiculata L. New Phytol 126:37–44. doi:10.1111/j.1469-8137.1994.tb07527.x
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, New York
Kaiser WM (1979) Reversible inhibition of the Calvin Cycle and inactivation of oxidative pentose phosphate cycle in isolated intact chloroplasts by hydrogen peroxide. Planta 145:377–382. doi:10.1007/BF00388364
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Li FL, Wu N (2008) Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ Exp Bot 63:248–255. doi:10.1016/j.envexpbot.2007.11.002
McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inze D, D’halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sati6a L.). Plant Physiol 103:1155–1163. doi:10.1104/pp. 103.4.1155
Moran JK, Becana M, Iturbe-Ormaexte I, Frechilla S, Klucas RV, Aparicio-Tejo P (1994) Drought induces oxidative stress in pea plants. Planta 194:346–352. doi:10.1007/BF00197534
Navari-lzzo F, Quartacci MF, Pinzino C, Dalla Vecchia F, Sgherri CLM (1998) Thylakoid-bound and stromal antioxidative enzymes in wheat treated with excess copper. Physiol Plant 104:630–638. doi:10.1034/j.1399-3054.1998.1040416.x
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359. doi:10.1146/annurev.arplant.50.1.333
Pang CH, Zhang SJ, Gong ZZ, Wang BS (2005) NaCl treatment markedly enhances H2O2-scavenging system in leaves of halophyte Suaeda salsa. Physiol Plant 125:490–499
Perl A, Perl-treves R, Galili S, Aviv D, Shalgi E, Malkin S et al (1993) Enhanced oxidative-stress defense in transgenic potato expressing tomato Cu/Zn superoxide dismutases. Theor Appl Genet 85:568–576. doi:10.1007/BF00220915
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol. 101(1): 7–12
Sgherri CLM, Maffei M, Navari-lzzo F (2000) Antioxidative enzymes in wheat subjected to increasing water deficit and rewatering. J Plant Physiol 157:273–279
Sigaud-Kutner TCS, Pinto E, Okamoto OK, Latorre LR, Colepicolo P (2002) Changes in superoxide dismutase activity and photosynthetic pigment content during growth of marine phytoplankters in batch-cultures. Physiol Plant 114:566–571. doi:10.1034/j.1399-3054.2002.1140409.x
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58. doi:10.1111/j.1469-8137.1993.tb03863.x
Tanaka Y, Hibino T, Hayashi Y, Tanaka A, Kishitani S, Takabe T et al (1999) Salt tolerance of transgenic rice overexpressing yeast mitochondrial MnSOD in chloroplast. Plant Sci 148:131–138. doi:10.1016/S0168-9452(99)00133-8
Van Rensburg L, Krüger GHJ (1994) Evaluation of components of oxidative stress metabolism for use in selection of drought tolerant cultivars of Nicotiana tabacum L. Plant Physiol 143:730–737
Van Camp W, Willekens H, Bowler C, Van Montagu M, Inze D, Reupold-Poop P (1994a) Elevated levels of superoxide dismutase protect transgenic plants against ozone damage. Biotechnolgy 12:165–168. doi:10.1038/nbt0294-165
Van Camp W, Herouart D, Willekens H, Takahashi H, Saito K, Van Montagu M et al (1994b) Tissue-specific activity of two manganese superoxide dismutase promotors in transgenic tobacco. Plant Physiol 112:525–535. doi:10.1104/pp. 112.2.525
Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials. Impact Of oxygen availability in polyethylene glycol solutions. Plant Physiol 116:1403–1412. doi:10.1104/pp. 116.4.1403
Wu FZ, Bao WK, Li FL, Wu N (2007) Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ Exp Bot 63:248–255. doi:10.1016/j.envexpbot.2007.11.002
Yu Q, Rengel Z (1999) Drought and salinity differentially influence activities of superoxide dismutases in narrowleafed lupinus. Plant Sci 142:1–11. doi:10.1016/S0168-9452(98)00246-5
Zhang J, Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol 35:785–791
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. B. Jackson.
Rights and permissions
About this article
Cite this article
Chang-Quan, W., Rui-Chang, L. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol Plant 30, 841–847 (2008). https://doi.org/10.1007/s11738-008-0189-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0189-8