Abstract
Bisphenol analogues (BPs) including bisphenol a (BPA) have been broadly utilized as industrial feedstocks and unavoidably discharged into water bodies. However, there is little published data on the occurrence, distribution, and environmental risks of other BPs in surface water. In this study, ten BPs besides BPA were analyzed in surface water from the Pearl River, South China. Among these detected BPs, BPA, bisphenol F (BPF), bisphenol AF (BPAF), and bisphenol S (BPS) were the most frequently detected compounds. The median concentrations of the measured BPs were ranked in the order of BPA (34.9 ng/L) > BPS (24.8 ng/L) > BPAF (10.1 ng/L) > bisphenol F (BPF) (9.0 ng/L) > bisphenol B (BPB) (7.6 ng/L) > bisphenol C (BPC) (1.2 ng/L). Among them, BPA and BPS were predominant BPs, contributing 68% of the total ten BPs in surface water of the Pearl River. These results demonstrated that BPA and BPS were the most extensively utilized and manufactured BPs in this region. The source analysis of BPs suggested that the BPs may be originated from domestic wastewater, wastewater treatment plant (WWTP) effluent, and the leaching of microplastic in surface water of the Pearl River. The calculated BP-derived estrogenic activity exhibited low to medium risks in surface water, but their combined estrogenic effects with other endocrine disrupting compounds should not be ignored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is a high-yield chemical, primarily utilized as an industrial feedstock to produce epoxy resins and polycarbonate plastics (Huang et al. 2012). Numerous studies have documented the various toxicities of BPA, covering reproductive toxicity, genotoxicity, neurotoxicity, embryotoxicity, and endocrine disruption (Chen et al. 2016; Rochester and Bolden 2015; Huang et al. 2018; Vandenberg et al. 2007; Gu et al. 2022). Therefore, considering the harmful health effects of BPA, countries like China, Canada, Japan, and the European Union have banned its usage in some consumer products (Huang et al. 2017; Wang et al. 2019). To further restrict the risk of BPA, Canada has proposed a BPA discharge standard of 1.75 μg/L for treated wastewater since 2009, and new regulations were proposed in 2010 requiring facilities to develop and implement plans to limit environmental release of BPA, which became the first country that tried to reduce BPA risk for human, wildlife, and ecosystem (Flint et al. 2012). To further protect human’s health, BPA has been regulated in National Drinking Water Quality Standard of China, Japan, and European Union (Yuan et al. 2016; 2018; Liu et al. 2021).
To fulfill the market demand, bisphenol analogues with chemical structures similar to BPA have been widely used, and some of them are gradually being used as substitutes for BPA (Chen et al. 2016; Delfosse et al. 2012; Liao et al. 2012; Xue et al. 2015; Banderakhshan et al. 2022). Bisphenol F (BPF), bisphenol S (BPS), and bisphenol AF (BPAF) are the major alternatives of BPA in the manufacture of epoxy resins and polycarbonate plastics. BPF has a variety of usages, such as adhesives, liners, varnishes, and plumbing, as well as in food packaging and dental sealants. BPS is usually applied in thermal papers, can coatings, and dye additives. BPAF is used in electronics, optical fibers, waveguides, and high-performance monomers. Because of the broad use of basic applications extending from food packaging to thermal papers, high quantities of BPs are inevitably emitted into the water bodies through various routes such as industrial effluent and domestic sewage (Erjavec et al. 2016; Yan et al. 2017; Chiriac et al. 2021).
BPA analogues have also been proven to be detrimental to human health and associated with cardiovascular diseases, cancer, obesity, fertility, and allergies. BPS was demonstrated to promote breast cancer progression by altering the level of DNA methylation in the promoter regions of breast cancer–related genes (Huang et al. 2019). Urinary levels of BPAF and BPS were suggested to be significantly associated with type-2 diabetes mellitus (T2DM) (Duan et al. 2018). Moreover, the positive association between urinary BPF and asthma was primarily found in adults, while the correlation between asthma and BPS was only observed in men (Mendy et al. 2020). Overall, these findings have indicated that BPA analogues are not necessarily safe as the replacement compounds of BPA (Helies-Toussaint et al. 2014; Kitamura et al. 2005; Svajger et al. 2016). Thus, it is important to analyze the occurrence of BPs in surface water bodies for their environmental protection and administration (Xie et al. 2022).
The Pearl River Delta is one of the most economically prosperous and populated regions in China. The Pearl Rivers are the critical source of potable water for Guangzhou and its nearby towns. Moreover, this region has been the hub for manufacturing and other industries with a high consumption of BPs in the past decades (Chen et al. 2019). However, to the best of our knowledge, there are only three related publications on the occurrence, distribution, and environmental risks of BPs in the aquatic environment of this region (Zhao et al. 2019; Chen et al. 2020; Huang et al 2020). Among these, the main purpose of Zhao et al. (2019) was to investigate the concentration of BPs in sea and estuary water, while Chen et al. (2020) only collected 10 water samples of the Pearl River to check the river water’s safety to swimmers. In the work of Huang et al. (2020), as many as 38 river water samples were collected, in which urban rainfall runoff was proven to be an important source to receiving water. However, the spatial distribution of BPs in the Pearl River remains unknown, especially in long dry season without influence of rainfall runoff. Therefore, the main purpose of this study was to investigate the spatial distribution of ten BPs in the surface water of the Pearl River without influence of rainwater runoff, along which possible sources of BPs were analyzed.
Materials and methods
Chemicals and reagents
A total of ten BPs were chosen as the target chemicals, including BPA, BPB, BPC, BPE, BPF, BPP, BPS, BPZ, BPAF, and BPAP. Some fundamental information on these ten BPs is shown in Table S1. Deuterated bisphenol A (BPA-d16) was applied as the internal standard (IS). Information for the purity of each BP and supplies is available in our previous study (Wang et al. 2021).
Study area and sample collection
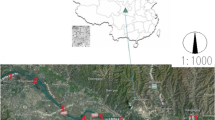
The Pearl River system is highly complicated, and the river networks are intertwined (Fig. 1). Generally, the river flows across Guangzhou city with a population of about 15 million (China Statistics Bureau 2020). The Pearl River branches into two tributaries at the turning point of S4, flowing through Guangzhou city and then converging into one river at Huangpu Port. Liuxi Reservoir is located in the northeastern of Guangzhou city that belongs to Liuxi National Forest Park. The Pearl River is a crucial potable water resource for Guangzhou city and its nearby townships.
In total, 25 sites were selected in the Pearl River, including 17 mainstream sites, 7 tributary sites, and 1 control site (Liuxi reservoir, S0) (Table S2). The sample sites were the same as our previous work (Tang et al. 2021a) and only one water sample was collected in each site. At each site, grab samples (2 L) were gathered from 0.5 m below the water surface in amber glass bottles. Water samples were immediately delivered on ice to the laboratory and kept at − 20 °C before pretreatment. The collected water samples were usually treated using solid phase extraction (SPE) within 48 h. All water samples were obtained in December of 2020, which was the dry season in South China and there had been no evident precipitations for at least one month before water sample collection.
Chemical analysis
Extraction, derivatization, and GC–MS analysis
The methods for extraction, derivatization, and instrumental analysis were performed following our previous study (Wang et al. 2020). In brief, water samples were filtered through 0.45-μm glass fiber filters and spiked with the internal standard (50 μL of 1 mg/L each). The filtered water samples were then loaded onto an Oasis HLB SPE cartridge (225 mg, Waters, USA) with an AQUA Loader 3 (GL Science, Japan) at a flow rate of 10 mL/min. After the sample loading, the cartridges were dried under a vacuum and then eluted with 5 mL methanol. The eluates were dried under a mild stream of N2 in preparation for derivatization. BPs were analyzed by GC–MS, using an Agilent 7890B series gas chromatograph with a 5977B single quadrupole mass spectrometer (Agilent, USA). Detailed descriptions about the derivatization steps and instrumental analysis of BPs are provided in the Supporting Information.
Quality assurance and quality control
A procedure blank, solvent blank, and 100 ng/mL mixed standard sample were performed sequentially every 12 injections during the testing of each batch of samples to check out background contaminants, residues, and instrument properties. The calibration curve was prepared using the 7-point quadratic regression ranging from 5 to 200 μg/L. The coefficients of determination (R2) > 0.993 showed the excellent linearity for the standard calibration curve. Quantification of BPs was determined by an internal standard method. For each target compound, limits of detection (LODs) and limits of quantification (LOQs) were obtained by the standard deviation (SD) of triple and tenfold the seven replicate injections of the lowest concentration of the standard calibration curve. The method recoveries were obtained by spiking standards at 20, 100, and 200 ng/L into the river water sample. Detail recoveries, LODs, and LOQs of target bisphenol analogues are described in Table 1
Risk assessment
Estrogenic activity assessment
Since the Pearl River is the primary supply of potable water for the population and BPs induce similar estrogenic effects, estrogen equivalent (EEQ) is also employed to assess the risk of BPs in water bodies (Eldridge et al. 2007; Konkel 2013). The chemically calculated EEQ of the target ten BPs is calculated with Eq. (1). Pollutants are considered to be harmful to the endocrine systems of organisms in the aquatic environment when EEQ is greater than 1.0 ng E2/L (Yan et al. 2017; Tang et al. 2021a, b).
where Ci indicates the levels of the target BPs in water samples and EEFi represents the proportion of the median effective concentration (EC50) of each chemical to the EC50 of 17β-estradiol. As shown in Table S1, each BP has different estradiol equivalency factors and varied considerably. Hence, the choice of estradiol equivalency factor may be significantly different. In the present study, the maximum reported estradiol equivalency factor was employed for each target chemical.
Eco-toxicity assessment
The risk quotient (RQ) method was used to assess the eco-toxicity of the selected BPs in the water bodies (Xiong et al. 2016; Yan et al. 2017; Zhao et al. 2015). RQi value is the proportion of the maximum measured environmental concentration (MECi) to the predicted no-effect concentration (PNECi), depicted in the following equation.
The ΣRQ was the sum of RQ of each separately measured contaminant (RQi) at each sampling site (Eq. (2)). Generally, environmental risks are classified as high risk (RQ > 1.0), medium risk (0.1 < RQ < 1.0), and low risk (0.01 < RQ < 0.1) (Blair et al. 2013; Palma et al. 2014). The PNEC values are usually obtained from dividing the minimum toxicity value (i.e., lowest short-term L(E)C50 or long-term NOEC value) in the literature by an assessment factor (AF) of 1000, 100, 50, or 10. The toxicity values of the present study were derived from published studies, as shown in Table S4.
Data analysis
In the statistical analysis, the concentrations of BPs below their LODs were set one-half of their corresponding LODs (Luo et al. 2018, 2020). Statistical analyses were performed with SPSS version 17.0 software package. All of the plots were created with Origin version 8.1. The statistical significance was set at p < 0.05 or below.
Result and discussion
Concentrations of bisphenol analogues in Pearl River
Table 2 shows the range, mean, median, and detection frequency of each BP in the Pearl River water. At least one BP was detected in each water sample. The levels of the total ten BPs (ΣBPs) in the Pearl River water varied from 36.6 to 261.8 ng/L with mean (median) concentration of 114.0 (89.8) ng/L. Among the determined ten BPs, BPA, BPAF, BPF, and BPS were widely detected with detection frequencies of 100%, 92%, 84%, and 72%, respectively, while the respective detection frequencies of BPB, BPC, BPZ, and BPAP were 52%, 44%, 4%, and 4%. BPE and BPP were not detected in any water sample. Among the detected BPs, the median concentration was ranked in the order of BPA (34.9 ng/L) > BPS (24.8 ng/L) > BPAF (10.1 ng/L) > BPF (9.0 ng/L) > BPB (7.6 ng/L) > BPC (2.7 ng/L). However, BPZ and BPAP were only detected in one water sample with the concentration approaching their LOQs. Though their usage data are hardly available, the detected results of this work suggested that BPs were widely used in Guangzhou, leading to their wide distribution in the Pearl River.
Figure 2 shows the concentration contribution ratio of each BP to the ΣBPs. Based on their median concentration, BPA contributed about 39%, followed by BPS with a contribution ratio of 27%, BPAF (11%), and BPF (10%), while the total contribution ratio of BPA, BPS, and BPAF was over 70%. The contribution ratio of BPS was close to that of BPA, which suggested that usage of BPS has been rapidly increased in Guangzhou city in recent years, since BPs i are structurally similar to BPA, and numerous studies have confirmed the BPs as an kind of EDCs (Van Leeuwen et al. 2019). Hence, the environmental risks of BPs in surface water should be paid more attention.
Concentration comparison with previous studies
Recently, the levels of BPs in river water have been investigated in several regions, especially for BPA, and these studies were in comparison with the current work (Table 3). The concentrations of BPA in this research were less than those in previous studies in the Pearl River system (Huang et al. 2020), but comparable to those in water samples from the Hunhe River (40 ng/L), Haihe River (42 ng/L), Liaohe River (47 ng/L), West River (43 ng/L), and Yellow River (33/44 ng/L) in China (Jin and Zhu 2016; Patrolecco et al. 2006; Yamazaki et al. 2015; Zhao et al. 2020). Mean BP concentrations (39 ng/L) in this work were greater than those reported from China and Japan (Huang et al. 2020; Yamazaki et al. 2015), but lower than those in India (Yamazaki et al. 2015). The mean levels of BPAF (18 ng/L) in the present study were less than those found from the Yellow River (40/24 ng/L; dry/wet season) (Zhao et al. 2020), but higher than those of other previous studies (Huang et al. 2020; Jin and Zhu 2016). Other BPs were detected with low levels in this work, comparable to those of other studies from China (Huang et al. 2020; Jin and Zhu 2016). In comparison with the concentrations of BPA, BPS, and BPF in the Pearl River in 2015, recorded by Huang et al. (2020), the levels of BPA have been decreased dramatically. Still, the concentrations of BPS and BPF were basically unchanged. This may be attributed to the ban on related applications of BPA in China (Qiu et al. 2021).
Spatial distribution and potential sources of BPs
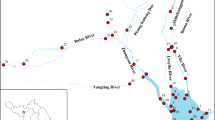
The spatial distribution of BPs in this region (Table 2) showed that the ΣBPs in the Pearl River water varied considerably in the range of 37–262 ng/L with mean levels of 90 ng/L. For the Pearl River, the higher levels for BPA, BPS, and BPAF occurred at sites S11-S13, S20-S21, and S23, while the greatest levels of BPA (126.0 ng/L) was found at site S23, the maximum concentrations of BPS (124.4 ng/L) was observed at site S13, with the largest concentrations of BPAF (123.1 ng/L) was measured at site S20. These sites are located in the branches of the Pearl River, adjacent to industrial zones, resulting in large quantities of industrial wastewater being released into the river with incomplete removal. Compared to other sites, the levels of BPs are well-distributed in surface water of the Pearl River, and the main contribution of BPs may be from domestic pollution. Unexpectedly, trace amounts of BPs were detected at site S0, which is an essential potable water source, raising concerns about the risk of human exposure. Exposure to these compounds may result in estrogenic effects and reproductive effects, or jeopardize the cognitive abilities of children (McDonough et al. 2021). We speculate that some of the BPs in site S0 may come from various sources, involving photo-/microbial degradation from parent chemicals, existing as industrial additives in consumer products.
WWTP effluents are considered to be the potential source of BPs in the aquatic environment. To confirm this, the selected BPs in the upstream and downstream river waters of the seven municipal WWTPs were further compared. As shown in Fig. 3, the levels of BPA, BPS, and ΣBPs in the downstream river waters of XT WWTP were significantly higher than that of the upstream river water. However, the concentration of ΣBPs between the upstream and downstream river waters of other WWTPs has no obvious statistical relationship. In South China, industrial wastewater is initially processed in factory sewage treatment facilities and then further treated in WWTPs. The XT WWTP in the present study received both industrial and domestic sewage, while other WWTPs only received domestic sewage. Therefore, the XT WWTP effluent may have a higher concentration of BPs, leading to elevated concentrations of BPs in downtown water. The study of Huang et al. (2020) reported higher concentrations of BPs in the XT WWTP effluent than those of the other WWTPs. The results seemed to suggest that the WWTP effluent is an essential potential source of BPs. However, the concentrations of the total BPs between the upstream and downstream river waters of the other WWTPs have no obvious statistical relationship (p>0.05). There are several possible reasons for this, including the low concentration of BPs in WWTP effluent and the different half-life of BPs.
BPs are extensively applied in household products and urban infrastructure. As a result, BPs will be unavoidably discharged into the receiving streams through the urban rainfall runoff. A previous study suggested that the daily mass load of BPA in runoff was 2.7 times greater than that in WWTP effluents in the UK (Wilkinson et al. 2016), indicating that rainfall runoff was an important source of BPA. Consistently, other monitoring results also suggested that rainfall runoff was the main source of pharmaceuticals and personal care products (PPCPs) in the rivers. In spite of the diluting effect, PPCP levels were increased in the receiving rivers (Liu et al. 2018; Mei et al. 2018). In this study, sampling was collected during the dry season, and it has not rained for more than a month. Thus, the BPs entering rivers through rainfall runoff were unlikely to be the critical source in this study.
In addition to WWTP effluent and rainfall runoff, microplastics may be potential sources for BPs in surface water due to the release of monomers during the aging process. Numerous studies have shown that BPA will be available for leaching from microplastics in seawater and surface water (Notardonato et al. 2019; Sajiki and Yonekubo 2004; Xu et al., 2020) Meanwhile, some available studies have investigated the presence of microplastics in surface water in China, including Yongjiang River, Pearl River, Hanjiang River, and Yangtze River (Zhang et al. 2020; Lin et al. 2018; Wang et al. 2017), with the abundance ranges of 379–8920 items/m3. Thus, microplastics may contribute to BPs in surface water, although more knowledge is needed to verify the hypothesis.
As shown in Table 4, the correlations between the levels of these BPs were also performed. In the Pearl River, the correlation analysis suggested that there was a significant positive relationship between BPA and BPF (R = 0.440, p < 0.05), as well as BPA and BPS (R = 0.662, p < 0.01). All of these results indicated that they had shared similar contamination sources. The positive correlations between BPBand BPF, as well as BPB and BPS (R > 0.4, p < 0.05) were observed. BPF has significant positive relationships with BPS (R = 0.652, p < 0.01), and BPZ has significant positive relationships with BPAP (R = 0.898, p < 0.01).
Based on the distribution of residential regions and industrial zones around the Pearl River, principal component analysis (PCA) was used to analyze the BPs in the Pearl River to determine their sources. In the Pearl River, the clustering could be recognized at least in the score plot of PCA: (1) S2 and S3, which had the minimum levels of BPA; S1, S4, and S9, which had the lowest levels of BPAF; S18, which contained the highest concentration of BPZ and BPAP; and (4) all of the other sampling sites.
Estrogenic activities and ecological risk assessment
Estrogenic activity assessment
The BP-derived EEQ and the sum of EEQ for each sampling point were calculated (Fig. 4). For the Pearl River, ΣEEQ varied from 0.050 to 0.284 ng E2/L. Recent research reported that the ranges of ΣEEQ were 0.004–0.18 and 0.005–0.11 ng E2/L for surface water in the Pearl River system in wet and dry seasons, which were similar to the current results (Huang et al. 2020). These ΣEEQ were well below 1.0 ng E2/L, suggesting that there was no detectable estrogenic risk for BPs in surface water of the Pearl River. In addition to BPs, numerous other chemicals in the surface water, such as alkylphenols, phthalates, and polychlorinated biphenyls, as well as natural and synthetic estrogens (E1, E2, and EE2), have estrogenic effects (Tang et al. 2020; Zhao et al. 2021). Consequently, BPs may contribute only a portion of the total estrogenic activity in surface water (Fig. 5).
Eco-toxicity assessment
The risk quotient (RQ) method was applied to evaluate the eco-toxicity of BPs in the Pearl River. The total RQ of each BP was computed to investigate the potential integrated impact of these contaminants. For the Pearl River, the range of ΣRQ was 0.011–0.148. The results showed that none of the sampling sites in the Pearl River had high risks to aquatic organisms (ΣRQ < 1.0), and only 12% of the monitored water samples presented moderate risks to the river ecosystem. Among the ten BPs, BPC had a comparatively greater RQ value for all the samples sites with the highest contribution of 39%. Previous studies have demonstrated that some BPs, i.e., BPF, BPS, and BPAF, etc., had similar estrogenic effects and anti-androgenic effects to that of BPA (Van Leeuwen et al. 2019). Meantime, risks may be significantly enhanced due to their elevated combined effects of complex estrogenic contaminants in the aquatic environment (Li et al. 2018). Therefore, it is vital to take more effective actions to decrease the discharge of BPs (Fig. 6).
Conclusion
Since BPA has been restricted in many countries for food-contact-related products, several BPs, including BPF, BPS, and BPAF, have been widely used. A large number of BPs were found in surface water of the Pearl River. The predominant BPs were BPA, BPF, BPS, and BPAF. The results of the study on the spatial distribution and possible sources of BPs suggested that the distribution of BPs was comparatively uniform in surface water. This study also demonstrated no high risk of BPs in surface water samples, but their combined estrogenic effects with other EDCs should not be ignored. Further research is recommended to clearly understand the combined estrogenic effects in surface water, which would provide valuable information to understand the fate of BPs in the aquatic environment.
Data availability
Not applicable.
References
Banaderakhshan R, Kamp P, Breul L, Steinbichl P, Hartmann C, Furhacker M (2022) Bisphenol A and its alternatives in Austrian thermal paper receipts, and the migration from reusable plastic drinking bottles into water and artificial saliva using UHPLC-MS/MS. Chemosphere 286:131842. https://doi.org/10.1016/j.chemosphere.2021.131842
Blair BD, Crago JP, Hedman CJ, Klaper RD (2013) Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 93:2116–2123. https://doi.org/10.1016/j.chemosphere.2013.07.057
Boyd GR, Palmeri JM, Zhang SY, Grimm DA (2004) Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA. Sci Total Environ 333:137–148. https://doi.org/10.1016/j.scitotenv.2004.03.018
Cesen M, Ahel M, Terzic S, Heath DJ, Heath E (2019) The occurrence of contaminants of emerging concern in Slovenian and Croatian wastewaters and receiving Sava river. Sci Total Environ 650:2446–2453. https://doi.org/10.1016/j.scitotenv.2018.09.238
Chen MY, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17:80–86. https://doi.org/10.1002/tox.10035
Chen D, Kannan K, Tan HL, Zheng ZG, Feng YL, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-a review. Environ Sci Technol 50:5438–5453. https://doi.org/10.1021/acs.est.5b05387
Chen WW, Chen YJ, Huang HF, Lu YJ, Khorram MS, Zhao WY, Wang DQ, Qi SH, Jin B, Zhang G (2019) Occurrence of N-nitrosamines in the Pearl River delta of China: characterization and evaluation of different sources. Water Res 164:114896. https://doi.org/10.1016/j.watres.2019.114896
Chen P, Zhong Y, Chen K, Guo C, Gong J, Wang D, Yang Y, Ma S, Yu Y (2020) The impact of discharge reduction activities on the occurrence of contaminants of emerging concern in surface water from the Pearl River. Environ Sci Pollut Res 27:30378–30389. https://doi.org/10.1007/s11356-020-09295-x
China Statistics Bureau, 2020. http://www.stats.gov.cn. Accessed 13 Nov 2021
Chiriac FL, Paun I, Pirvu F, Pascu LF, Galaon T (2021) Occurrence and fate of bisphenol A and its congeners in two wastewater treatment plants and receiving surface waters in Romania. Environ Chem Toxicol 40:435–446. https://doi.org/10.1002/etc.4929
Delfosse V, Grimaldi M, Pons JL, Boulahtouf A, le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P (2012) Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. P Natl Acad Sci USA 109:14930–14935. https://doi.org/10.1073/pnas.1203574109
Duan YS, Yao YM, Wang B, Han LP, Wang L, Sun HW, Chen LM (2018) Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ Pollut 243:1719–1726. https://doi.org/10.1016/j.envpol.2018.09.093
Eldridge ML, Sanseverino J, Layton AC, Easter JP, Schultz TW, Sayler GS (2007) Saccharomyces cerevisiae BLYAS, a new Bioluminescent bioreporter for detection of androgenic compounds. Appl Environ Microb 73:6012–6018. https://doi.org/10.1128/Aem.00589-07
Erjavec B, Hudoklin P, Perc K, Tisler T, Dolenc MS, Pintar A (2016) Glass fiber-supported TiO2 photocatalyst: efficient mineralization and removal of toxicity/estrogenicity of bisphenol A and its analogs. Appl Catal B-Environ 183:149–158. https://doi.org/10.1016/j.apcatb.2015.10.033
Flint S, Markle T, Thompson S, Wallace E (2012) Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manag 104:19–34. https://doi.org/10.1016/j.jenvman.2012.03.021
Gong J, Ran Y, Chen DY, Yang Y, Ma XX (2009) Occurrence and environmental risk of endocrine-disrupting chemicals in surface waters of the Pearl River, South China. Environ Monit Assess 156:199–210. https://doi.org/10.1007/s10661-008-0474-4
Gu J, Guo M, Yin X, Huang C, Qian L, Zhou L, Wang Z, Wang L, Shi L, Ji G (2022) A systematic comparison of neurotoxicity of bisphenol A and its derivatives in zebrafish. Sci Total Environ 805:150210. https://doi.org/10.1016/j.scitotenv.2021.150210
Helies-Toussaint C, Peyre L, Costanzo C, Chagnon MC, Rahmani R (2014) Is bisphenol S a safe substitute for bisphenol A in terms of metabolic function? An in vitro study. Toxicol Appl Pharm 280:224–235. https://doi.org/10.1016/j.taap.2014.07.025
Huang YQ, Wong CKC, Zheng JS, Bouwman H, Barra R, Wahlstrom B, Neretin L, Wong MH (2012) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99. https://doi.org/10.1016/j.envint.2011.04.010
Huang RP, Liu ZH, Yuan SF, Yin H, Dang Z, Wu PX (2017) Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ Pollut 230:143–152. https://doi.org/10.1016/j.envpol.2017.06.026
Huang RP, Liu ZH, Yin H, Dang Z, Wu PX, Zhu NW, Lin Z (2018) Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: a thorough literature review. Sci Total Environ 626:971–981. https://doi.org/10.1016/j.scitotenv.2018.01.144
Huang W, Zhao C, Zhong H, Zhang SD, Xia YJ, Cai ZW (2019) Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ Pollut 246:697–703. https://doi.org/10.1016/j.envpol.2018.12.084
Huang Z, Zhao JL, Yang YY, Jia YW, Zhang QQ, Chen CE, Liu YS, Yang B, Xie LT, Ying GG (2020) Occurrence, mass loads and risks of bisphenol analogues in the Pearl River Delta region, South China: urban rainfall runoff as a potential source for receiving rivers. Environ Pollut 263:114361. https://doi.org/10.1016/j.envpol.2020.114361
Jin HB, Zhu LY (2016) Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res 103:343–351. https://doi.org/10.1016/j.watres.2016.07.059
Jin XL, Jiang GB, Huang GL, Liu JF, Zhou QF (2004) Determination of 4-tert-octylphenol, 4-nonylphenol and bisphenol A in surface waters from the Haihe River in Tianjin by gas chromatography-mass spectrometry with selected ion monitoring. Chemosphere 56:1113–1119. https://doi.org/10.1016/j.chemosphere.2004.04.052
Kang JH, Kondo F (2006) Bisphenol A in the surface water and freshwater snail collected from rivers around a secure landfill. B Environ Contam Tox 76:113–118. https://doi.org/10.1007/s00128-005-0896-4
Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84:249–259. https://doi.org/10.1093/toxsci/kfi074
Konkel L (2013) BPA as a mammary carcinogen early findings reported in rats. Environ Health Persp 121:A284–A284. https://doi.org/10.1289/ehp.121-A284
Kuch HM, Ballschmiter K (2001) Determination of endocrine-disrupting phenolic compounds and estrogens in surface and drinking water by HRGC-(NCI)-MS in the picogram per liter range. Environ Sci Technol 35:3201–3206. https://doi.org/10.1021/es010034m
Li D, Chen HX, Bi R, Xie HB, Zhou Y, Luo YJ, Xie LT (2018) Individual and binary mixture effects of bisphenol A and lignin-derived bisphenol in Daphnia magna under chronic exposure. Chemosphere 191:779–786. https://doi.org/10.1016/j.chemosphere.2017.10.022
Liao CY, Liu F, Moon HB, Yamashita N, Yun SH, Kannan K (2012) Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol 46:11558–11565. https://doi.org/10.1021/es303191g
Lin L, Zuo LZ, Peng JP, Cai LQ, Fok L, Yan Y, Li HX, Xu XR (2018) Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci Total Environ 644:375–381. https://doi.org/10.1016/j.scitotenv.2018.06.327
Liu WR, Yang YY, Liu YS, Zhao JL, Zhang QQ, Yao L, Zhang M, Jiang YX, Wei XD, Ying GG (2018) Biocides in the river system of a highly urbanized region: a systematic investigation involving runoff input. Sci Total Environ 624:1023–1030. https://doi.org/10.1016/j.scitotenv.2017.12.225
Liu YX, Su WC, Zhu YL, Xiao L, Hu TS (2020) Endocrine disrupting compounds in the middle and lower reaches of the Lhasa River Basin: occurrence, distribution, and risk assessment. Sci Total Environ 727:138694. https://doi.org/10.1016/j.scitotenv.2020.138694
Liu ZH, Dang Z, Liu Y (2021) Legislation against endocrine-disrupting compounds in drinking water: essential but not enough to ensure water safety. Environ Sci Pollut Res 28:19505–19510. https://doi.org/10.1007/s11356-021-12901-1
Luo Q, Liu ZH, Yin H, Dang Z, Wu PX, Zhu NW, Lin Z, Liu Y (2018) Migration and potential risk of trace phthalates in bottled water: a global situation. Water Res 147:362–372. https://doi.org/10.1016//j.watres.2018.10.002
Luo Q, Liu ZH, Yin H, Dang Z, Wu PX, Zhu NW, Lin Z, Liu Y (2020) Global review of phthalates in edible oil: an emerging and nonnegligible exposure source to human. Sci Total Environ 704:135369. https://doi.org/10.1016/j.scitotenv.2019.135369
McDonough CM, Xu HS, Guo TL (2021) Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: insights from a multi-species comparison. Crit Rev Toxicol 51:283–300. https://doi.org/10.1080/10408444.2021.1908224
Mei XB, Sui Q, Lyu SG, Wang D, Zhao WT (2018) Pharmaceuticals and personal care products in the urban river across the megacity Shanghai: occurrence, source apportionment and a snapshot of influence of rainfall. J Hazard Mater 359:429–436. https://doi.org/10.1016/j.jhazmat.2018.07.081
Mendy A, Salo PM, Wilkerson J, Feinstein L, Ferguson KK, Fessler MB, Thorne PS, Zeldin DC (2020) Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environ Res 183:108944. https://doi.org/10.1016/j.envres.2019.108944
Nazifa TH, Kristanti RA, Ike M, Kuroda M, Hadibarata T (2020) Occurrence and distribution of estrogenic chemicals in river waters of Malaysia. Toxicol Env Health 12:65–74. https://doi.org/10.1007/s13530-020-00036-8
Notardonato I, Protano C, Vitali M, Bhattacharya B, Avino P (2019) A method validation for simultaneous determination of phthalates and bisphenol A released from plastic water containers. Appl Sci 9:2945. https://doi.org/10.3390/app9142945
Palma P, Kock-Schulmeyer M, Alvarenga P, Ledo L, Barbosa IR, de Alda ML, Barcelo D (2014) Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci Total Environ 488:208–219. https://doi.org/10.1016/j.scitotenv2014.04.088
Patrolecco L, Capri S, De Angelis S, Pagnotta R, Polesello S, Valsecchi S (2006) Partition of nonylphenol and related compounds among different aquatic compartments in Tiber River (Central Italy). Water Air Soil Poll 172:151–166. https://doi.org/10.1007/s11270-005-9067-9
Qiu WH, Liu SA, Chen HH, Luo SS, Xiong Y, Wang XJ, Xu BT, Zheng CM, Wang KJ (2021) The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J Hazard Mater 406:124303. https://doi.org/10.1016/j.jhazmat.2020.124303
Rochester JR, Bolden AL (2015) Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Persp 123:643–650. https://doi.org/10.1289/ehp.1408989
Ruan T, Liang D, Song SJ, Song MY, Wang HL, Jiang GB (2015) Evaluation of the in vitro estrogenicity of emerging bisphenol analogs and their respective estrogenic contributions in municipal sewage sludge in China. Chemosphere 124:150–155. https://doi.org/10.1016/j.chemosphere.2014.12.017
Sajiki J, Yonekubo J (2004) Leaching of bisphenol A (BPA) from polycarbonate plastic to water containing amino acids and its degradation by radical oxygen species. Chemosphere 55:861–867. https://doi.org/10.1016/j.chemosphere.2003.11.065
Santhi VA, Sakai N, Ahmad ED, Mustafa AM (2012) Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci Total Environ 427:332–338. https://doi.org/10.1016/j.scitotenv.2012.04.041
Svajger U, Dolenc MS, Jeras M (2016) In vitro impact of bisphenols BPA, BPF, BPAF and 17 beta-estradiol (E2) on human monocyte-derived dendritic cell generation, maturation and function. Int Immunopharmacol 34:146–154. https://doi.org/10.1016/j.intimp.2016.02.030
Tang Z, Liu ZH, Wang H, Dang Z, Yin H, Zhou Y, Liu Y (2020) Trace determination of eleven natural estrogens and insights from their occurrence in a municipal wastewater treatment plant and river water. Water Res 182:115976. https://doi.org/10.1016/j.watres.2020.115976
Tang Z, Liu ZH, Wan YP, Wang H, Dang Z, Liu Y (2021a) Far-less studied natural estrogens as ignored emerging contaminants in surface water: insights from their occurrence in the Pearl River, South China. Acs Est Water 1:1776–1784. https://doi.org/10.1021/acsestwater.1c00109
Tang Z, Liu ZH, Wang H, Dang Z, Liu Y (2021b) A review of 17 alpha-ethynylestradiol (EE2) in surface water across 32 countries: sources, concentrations, and potential estrogenic effects. J Environ Manage 292:112804. https://doi.org/10.1016/j.jenvman.2021.112804
van Leeuwen SPJ, Bovee TFH, Awchi M, Klijnstra MD, Hamers ARM, Hoogenboom RLAP, Portier L, Gerssen A (2019) BPA, BADGE and analogues: a new multi-analyte LC-ESI-MS/MS method for their determination and their in vitro (anti)estrogenic and (anti)androgenic properties. Chemosphere 221:246–253. https://doi.org/10.1016/j.chemosphere.2018.12.189
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177. https://doi.org/10.1016/j.reprotox.2007.07.010
Wang WF, Ndungu AW, Li Z, Wang J (2017) Microplastics pollution in inland freshwaters of China: a case study in urban surface waters of Wuhan, China. Sci Total Environ 575:1369–1374. https://doi.org/10.1016/j.scitotenv.2016.09.213
Wang H, Liu ZH, Zhang J, Huang RP, Yin H, Dang Z, Wu PX, Liu Y (2019) Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci Total Environ 692:107–116. https://doi.org/10.1016/j.scitotenv.2019.07.134
Wang H, Liu ZH, Tang Z, Zhang J, Yin H, Dang Z, Wu PX, Liu Y (2020) Bisphenol analogues in Chinese bottled water: quantification and potential risk analysis. Sci Total Environ 713:136583. https://doi.org/10.1016/j.scitotenv.2020.136583
Wang H, Liu ZH, Tang Z, Zhang J, Dang Z, Liu Y (2021) Possible overestimation of bisphenol analogues in municipal wastewater analyzed with GC-MS. Environ Pollut 273:116505. https://doi.org/10.1016/j.envpol.2021.116505
Wilkinson JL, Swinden J, Hooda PS, Barker J, Barton S (2016) Markers of anthropogenic contamination: a validated method for quantification of pharmaceuticals, illicit drug metabolites, perfluorinated compounds, and plasticisers in sewage treatment effluent and rain runoff. Chemosphere 159:638–646. https://doi.org/10.1016/j.chemosphere.2016.06.039
Xie J, Zhao N, Zhang Y, Hu H, Zhao M, Jin H (2022) Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 287:132218. https://doi.org/10.1016/j.chemosphere.2021.132218
Xiong JK, Li GY, An TC, Zhang CS, Wei CH (2016) Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang River, South China. Environ Pollut 219:596–603. https://doi.org/10.1016/j.envpol.2016.06.021
Xu ZN, Xiong X, Zhao YH, Xiang W, Wu CX (2020) Pollutants delivered every day: Phthalates in plastic express packaging bags and their leaching potential. J Hazard Mater 384:121282. https://doi.org/10.1016/j.jhazmat.2019.121282
Xue JC, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K (2015) Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res 137:120–128. https://doi.org/10.1016/j.envres.2014.12.007
Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PKS, Moon HB, Jeong Y, Kannan P, Achyuthan H, Munuswamy N, Kannan K (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotox Environ Safe 122:565–572. https://doi.org/10.1016/j.ecoenv.2015.09.029
Yan ZY, Liu YH, Yan K, Wu SM, Han ZH, Guo RX, Chen MH, Yang QL, Zhang SH, Chen JQ (2017) Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 184:318–328. https://doi.org/10.1016/j.chemosphere.2017.06.010
Yuan SF, Liu ZH, Lian HX, Yang CT, Lin Q, Yin H, Dang Z (2016) Simultaneous determination of estrogenic odorant alkylphenols, chlorophenols, and their derivatives in water using online headspace solid phase microextraction coupled with gas chromatography-mass spectrometry. Environ Sci Pollut Res 23:19116–19125. https://doi.org/10.1007/s11356-016-7107-1
Yuan SF, Liu ZH, Lian HX, Yang CT, Lin Q, Yin H, Lin Z, Dang Z (2018) Fast trace determination of nine odorant and estrogenic chloro- and bromo-phenolic compounds in real water samples through automated solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. Environ Sci Pollut Res 25:3813–3822. https://doi.org/10.1007/s11356-017-0816-2
Zhang X, Leng YF, Liu XN, Huang K, Wang J (2020) Microplastics’ pollution and risk assessment in an urban river: a case study in the Yongjiang River, Nanning City, South China. Expos Health 12:141–151. https://doi.org/10.1007/s12403-018-00296-3
Zhao JL, Ying GG, Wang L, Yang JF, Yang XB, Yang LH, Li X (2009) Determination of phenolic endocrine disrupting chemicals and acidic pharmaceuticals in surface water of the Pearl Rivers in South China by gas chromatography-negative chemical ionization-mass spectrometry. Sci Total Environ 407:962–974. https://doi.org/10.1016/j.scitotenv.2008.09.048
Zhao JL, Chen XW, Yan B, Wei C, Jiang YX, Ying GG (2015) Estrogenic activity and identification of potential xenoestrogens in a coking wastewater treatment plant. Ecotox Environ Safe 112:238–246. https://doi.org/10.1016/j.ecoenv.2014.11.012
Zhao X, Zhang H, Chen ZL, Wang XC, Shen JM (2020) Spatial and temporal distributions of bisphenol analogues in water and sediment from the Lanzhou section of the Yellow River. China Arab J Geosci 13:1115. https://doi.org/10.1007/s12517-020-06122-y
Zhao JL, Huang Z, Zhang QQ, Ying-He L, Wang TT, Yang YY, Ying GG (2021) Distribution and mass loads of xenoestrogens bisphenol a, 4-nonylphenol, and 4-tert-octylphenol in rainfall runoff from highly urbanized regions: a comparison with point sources of wastewater. J Hazard Mater 401:123747. https://doi.org/10.1016/j.jhazmat.2020.123747
Zhao X, Qiu WQ, Zheng Y, Xiong J, Gao C, Hu S (2019) Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China. Ecotoxicology and Environmental Safety 180:43–52
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21577040; No. 21107025); Science and Technology Program of Guangzhou, China (No.201904010100; No. 201510010162); special funds for public welfare research and capacity building in Guangdong Province (No. 2015A020215003), Zhongshan Public Water Co. LTD for water micropollutant project (No. ZPW-2020-A-010); and the Guangdong Science and Technology Program (2020B121201003).
Author information
Authors and Affiliations
Contributions
Hao Wang, data analysis and original draft preparation; Zhao Tang, data analysis; Ze-hua Liu, supervisor, funding support, writing review; Feng Zeng, co-supervisor, equipment support; Jun Zhang, sample collection; Zhi Dang, writing review.
Corresponding authors
Ethics declarations
Ethical approval.
Not applicable.
Consent to participate
All authors have given consent to their contribution.
Consent for publication
All authors have agreed with the content and all have given explicit consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Tang, Z., Liu, Zh. et al. Occurrence, spatial distribution, and main source identification of ten bisphenol analogues in the dry season of the Pearl River, South China. Environ Sci Pollut Res 29, 27352–27365 (2022). https://doi.org/10.1007/s11356-021-17647-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-17647-4