Abstract
Bisphenol compounds (BPs) are difficult to degrade and highly toxic that can cause a wide range of biotic endocrine disrupting effects. Clarifying Zhushan Lake and Meiliang Lake (typical areas of Taihu Lake) with BPs pollution is important for protecting water safety and human health. Surface water, sediments, and fish samples were collected and analyzed to determine the occurrence, sources, and ecological risks of bisphenol Z (BPZ), bisphenol C (BPC), bisphenol F (BPF), and bisphenol S (BPS). Surface water and sediment samples revealing average BPs concentrations of 1227 ng/L and 11 ng/g (dry weight), respectively. BPS had the highest detection rate among the four BPs, followed by BPF. The pollution level of BPs was the highest in the middle and upper reaches, and there was a downward trend in the lower reaches. In wild fish, contaminants were found to be highest in muscle and gill tissues, indicating that fish are in short-term exposure. Pearson and stable isotope analyses showed that BPF and BPS had similar origins, and sediments were found to be the primary source of the pollutants. Finally, the ecological and human health risk assessments showed current low-risk levels, but the risks are still increasing and require attention. The results can be used for providing valuable information for the water quality management and BPs pollution control in Taihu Lake. Additionally, it functions as a forewarn for BPs producing and applying.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bisphenol A (BPA) is a common endocrine disruptor and its negative effects on the reproductive, nervous, and endocrine systems of aquatic organisms and humans have been well-documented (Andujar et al. 2019; Kiwitt et al. 2021; Meeker et al. 2010). Banning the sale of products containing BPA has led to the increased use of potential substitutes such as bisphenol Z (BPZ), bisphenol C (BPC), bisphenol F (BPF), and bisphenol S (BPS). Previous studies have detected bisphenol compounds (BPs) in various regions across the globe and their negative impact on organisms. A decreasing ratio of BPA to the total BPs was shown, indicating a worsening pollution status of its substitutes, particularly BPF and BPS (Liu et al. 2017).
The Tamagawa River in Japan was found to have BPF concentrations as high as 2850 ng/L, while the Adyar River in India had BPS concentrations of 7.2 µg/L (Yamazaki et al. 2015). Similarly, to the Yellow River Basin, the Yangtze River Basin of China had an average concentration of BPA alternatives in sediments of 1.5–3.2 ng/g (Zhao et al. 2020). BPs not only resist degradation but also accumulate in organisms through contact and ingestion, causing a host of damages including stunted growth, cytotoxicity and oxidative damage (Chen et al. 2016; Lee et al. 2013; Usman et al. 2019). When BPs enter the waters, they will hinder the normal development of aquatic biological nervous system (Wang et al. 2013). BPs have also been shown to have estrogenic effects and may pose a potential threat to ecosystems. Exposure to a certain number of BPs will lead to animal hypothalamus, pituitary and other hyperfunction (Feng et al. 2016). Given this, it is imperative to evaluate the potential impact of these alternatives and develop strategies to minimize environmental exposure to bisphenol compounds to mitigate their negative effects.
Taihu Lake, the third largest freshwater lake in China, is an important part of the Yangtze River Delta located in the south of Jiangsu Province (Su et al. 2021). It is situated between the cities of Wuxi, Suzhou, and Huzhou. The coordinates of Taihu Lake are approximately 31.3086° N latitude and 120.2143° E longitude. It covers an area of about 2338 square kilometers, making it one of the largest freshwater lakes in China. The lake stretches across a vast region and is surrounded by a mix of urban areas, agricultural land, and natural landscapes. The region is densely populated, economically developed, with high levels of urbanization and industrialization (Wang et al. 2022). Several studies have investigated the presence and characteristics of persistent organic pollutants (POPs) in the waters of Taihu Lake, including polycyclic aromatic hydrocarbons, phthalic acid esters, and antibiotics (Kong et al. 2022; Luo et al. 2021; Wang et al. 2018). However, there is a relative lack of studies on BPs pollution in the lake, including their concentration, trends, and behavior in various compartments. Therefore, further research is urgently needed to supplement the existing data and comprehensively understand the degree and impact of BPs pollution in Taihu Lake.
This study analyzes the contents and spatial distributions of four BPs in typical areas of Taihu Lake, investigates the correlation between the BPs in fish and environmental levels, and provide a preliminary assessment of the ecological and human health risks associated with BPs exposure. Additionally, the stable isotope analysis on various samples was implemented to trace the origins of the fish and gain valuable insights into the sources contributing to the fish population in the lake, whose aim is to offer scientific evidence and data to support BPs risk management and environmental quality control measures in the Taihu Lake region. For this purpose, several hypothesizes are formulated: (1) a certain number of BPs will be detected in different samples; (2) BPS and BPF may dominant the four BPs; (3) BPs distribution in various reaches of lake and organs of fish will be different; (4) sediments may be more possible source of BPs in fish; (5) the potential health risks posed by BPs should be paid more attention.
Materials and Methods
Chemicals and Materials
We obtained BPS (purity = 99%), BPC (purity > 98.0%), and BPZ (purity ≥ 98.0%) from Shanghai Aldin Reagent Co., Ltd., Shanghai, China. BPF (purity = 98%) was obtained from Shanghai Maclin Biochemical Technology Co., Ltd., Shanghai, China. For a stable isotope internal standard, we purchased BPS-13C12 (13C12H10O4S) (99.0%) and BPA-13C12 (13C12H16O2) (99.0%) from A ChemTek, Inc., MA, USA. We used dimethyl sulfoxide (DMSO) from Shanghai Lingfeng Chemical Reagent Co., Ltd., Shanghai, China. The bottom sampler (330 Van Veen) was purchased from Qingdao Watertools Technologies Co., Ltd., Shandong, China. The surface water sampler (WB-SS) was purchased from Beijing Purity Instrument Co., Ltd., Beijing, China. The SPE column (Generik H2P) was purchased from Sepax Technologies, Inc., DE, USA. Furthermore, we employed HPLC-grade methanol, acetonitrile, dichloromethane, and normal hexane throughout the experiment, along with other chemicals of analytical grade, such as normal saline and ethanol.
Sampling Methods
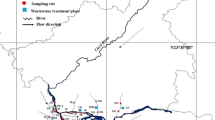
Previous literature suggests that the North Taihu Lake area is more polluted than other parts of the lake (Yang et al. 2011). The region belongs to the subtropical monsoon climate, which is dominated by plains and water networks. It has sufficient light and rainfall, and the four seasons are quite different and independent. The average and maximum water depth of North Taihu Lake is 1.89 m and 2.6 m, and the water surface evaporation is 842 mm. In general, the annual precipitation range is 1048–1920 mm, and the rainy season is from May to September (Kang et al. 2015). For this study, we selected 20 sampling sites (119° 59ʹ–120° 11ʹ N, 31° 20ʹ–31° 31ʹ E) in Zhushan Lake and Meiliang Lake in December 2021. Sampling sites S1-S14 were in Zhushan Lake, and S15–S20 were in Meiliang Lake. Table S1 provides detailed information on each site’s water quality parameters. To collect water samples, we used a stainless steel bucket washed thrice with lake water, sampling 0.5 m below the surface, and storing in pre-cleaned 2 L brown glass bottles. Using stainless steel tools, we collected surface sediment samples and transported them along with water samples in a cooler to the laboratory. Since Taihu Lake enforces a ten-year ‘fishing ban,’ we selected four common fish species in the lake—Channa argus, Carassius auratus, Parabramis pekinensis, and Hemiculter leucisculus—based on their feeding habits and bought them at Changzhou Fresh Market in December 2022. After verifying weight and length (Table S2), we immediately dissected the biological samples at the laboratory. While water samples remained at 4 °C, sediment and biological samples were stored at − 20 °C.
Sample Extraction and Instrument Analysis
We employed SPE with minor modifications (Kong et al. 2022) to pretreat the water samples. Firstly, we activated the H2P cartridges (500 mg, Sepax, USA) successively with 10 mL methanol and 10 mL Milli-Q water. Next, we filtered 500 mL water samples through a 0.45 μm mixed cellulose esters membrane filter, then adjusted the pH to 3 with 0.1 mol/L hydrochloric acid and added 50 ng of the internal standard mixture. We then passed the filtrates through the activated cartridges at a rate of approximately 5 mL/min, eluting them with 10 mL methanol. Subsequently, we concentrated the eluent to near dryness with a water bath nitrogen blower, and finally, redissolved it to 1 mL with methanol for instrumental analysis.
The sediment samples were thoroughly cleaned by removing stones, leaves, and other debris, then further processed using the quartering method, freeze-drying, grinding, and crushing, and filtering through an 80-mesh sieve. Each sediment sample (5 g) was transferred to a 50 mL polytetrafluoroethylene (PTFE) centrifuge tube, and a 25 mL mixture of methylene chloride and hexane (4:1, v/v) was added. The mixture was vortexed for 30 s, extracted via ultrasound for 30 min, and centrifuged at 4000 rpm/min for 10 min. The supernatant was collected, and these extraction steps were repeated thrice. The supernatants were combined, concentrated using a rotary evaporator, and diluted with Milli-Q water to 500 mL to extract BPs using the aforementioned SPE method. For the fish samples, after the dissection and storage, 5 g of each freeze-dried sample was similarly processed.
The BPs were analyzed using an ultra-high-performance liquid chromatograph coupled with a triple quadrupole liquid mass spectrometer. An electrospray ionization source was utilized in negative ion mode for multiple reaction monitoring (MRM). The mobile phase consisted of Milli-Q water (A) and methanol (B), with a flow rate of 0.2 mL/min. Chromatographic separation was achieved via a Waters Atlantis T3 Column, while the column temperature was maintained at 25 °C and the injection volume was set to 10 μL.
Quality Control and Quality Assurance
To ensure the accuracy and validity of the results, rigorous scientific protocols were employed. The internal standard method described by Han et al. (2022) was implemented to quantify BPs. The limits of detection (LODs) and limits of quantification (LOQs) were assessed to determine method sensitivity, with correlation coefficients (R2) above 0.998. The LODs and LOQs in water samples ranged from 0.22–3.1 ng/L and 0.72–10 ng/L, respectively. Quality control measures were implemented throughout the pretreatment and analysis procedures, such as method blank, spiked blank, and sample parallel sampling. The recovery experiment was repeated six times, yielding relative standard deviations (RSDs) of less than 20%. No target compounds were detected in any of the blank samples. The recoveries of BPs were between 91.4 and 103% in water samples, 87.8 and 110.9% in sediments, and 85.9 and 97.5% in biological samples.
Parameter Measurement and Statistical Analysis
In accordance with the EU technical guidance on environmental risk assessment, the ecotoxicity of four BPs in the study area was evaluated using the risk quotient (RQ) method, calculated as shown in Formulas (1) and (2). The RQ value is the ratio of the measured environmental concentration (MEC) to the predicted no-effect concentration (PNEC), which is typically determined by dividing toxicity data (LC50, EC50, NOEC, etc.) by assessment factors (AF) ranging from 10 to 1000 (Liu et al. 2020a; Yan et al. 2013). The LC50 values obtained from prior experiments were used as calculating data in this study, as reported by Han et al. (2021). An RQ < 0.01, 0.01 < RQ < 1, and RQ > 1 indicated low, medium, and high ecological risk, respectively (Liu et al. 2020b).
To assess the impact of BPs on human health, the estrogen toxicity equivalent (EEQ) was employed. This was calculated by multiplying the concentrations of measured BPs with their respective estradiol equivalence factor (EEF), as described by Yan et al. (2017). When EEQ values exceed 1.0 ng/L, it suggests a potential risk to human health (Liao et al. 2012b). The calculation formula is as follows:
where it is the total estradiol equivalent quantity, the concentration of the target compounds in the samples, and EEFi is the estradiol equivalency factor.
Statistical analyses and data visualization were performed using SPSS Statistics 20 and ArcGIS 10.3, respectively.
Results and Discussions
Concentrations of BPs in Surface Water and Sediments
The concentration of BPs in surface water and sediments was explored to provide an intuitive assessment of their distribution. Table S3 shows the distribution of BPs in surface water and sediments. BPS had a detection frequency of 100% in surface water, followed by BPF and BPC with 90% each. BPZ had the lowest detection frequency at 80%. The ΣBPs content in surface water ranged from 53 to 5518 ng/L, with an average of 1227 ng/L. Among all BPs, BPS had the highest average concentration at 914 ng/L, accounting for 75% of the total. In contrast, the concentration of BPZ and BPC was one to two orders of magnitude lower than the other two BPs, possibly due to their octanol–water partition coefficient (log Kow) (Jonker 2016). Research has shown that BPA is a highly produced chemical in China, with an annual output of around 167,000 tons. However, BPF and BPS have gradually replaced BPA since 2008 (Liao et al. 2012d). The overall demand for BPF and BPS has increased over the past decade, with pollution concentrations that are 240 and 100 times higher, respectively, than those in 2013. BPZ and BPC have also been detected since then (Jin and Zhu 2016), indicating that the use of BPs in the Taihu Lake area is increasing. In 2013, BPF, BPS, and BPZ in Taihu Lake was ND–5.6, 0.3–67, and ND ng/L and increased to ND–1600, 4.5–1600, and ND–17 ng/L in 2017 (Jin and Zhu 2016; Yan et al. 2017). A similar pattern was also observed in previous studies of Taihu Lake surface water, where BPF and BPS were the dominant BPs (Si et al. 2019; Zhou et al. 2020b).
Compared to water samples, sediment samples provide more stable and representative measurements of pollutant concentrations. In sediments, the detection frequencies of BPS (95%), BPF (90%), BPC (65%), and BPZ (65%) gradually decreased. The ΣBPs in the sediment group ranged from not detected to 42 ng/g, which was significantly lower than that in the surface water group. BPF and BPS were the principal pollutants with contribution rates of 36% and 51%, respectively. The average concentrations of BPF and BPS in sediments were 3.9 ng/g and 5.6 ng/g, respectively, which were consistent with the study results from surface water. It is worth noting that BPZ and BPC were first detected in sediments from Taihu Lake in 2015, and the concentration of these pollutants has increased by more than five times since then (Chen et al. 2017). The results of the study suggest that even though pollutants such as BPZ and BPC have not been growing as fast in the environment as some of the less toxic alternatives to BPA, they are still significant causes for concern due to their moderate toxicity. Although the half-lives of BPs are not long compared to other endocrine disruptors, they are known as “pseudo-persistent chemicals” because they have a wide distribution in the environment and are continuously released from daily production and life, leading to their accumulation. Furthermore, environmental factors such as pH have a significant impact on the degradation and accumulation of BPs. Studies have shown that BPs are more difficult to degrade under acidic conditions, while the opposite is true under alkaline conditions, and are more easily accumulated in sediments (Shehab et al. 2020).
There have been several reports on the levels of BP pollutant concentrations in rivers and lakes both domestically and abroad. By analyzing literature data, BPs concentrations detected in the Louma River, Liao River, and Pearl River basins in China ranged from 4.4 to 639 ng/L (Jin and Zhu 2016; Peng et al. 2007), which was similar to the concentration levels in the present study. BPs were also detected in the United States, India, South Korea, and other places, but the concentration levels were not similar, indicating regional differences in the use of BPs (Liao et al. 2012a; Yamazaki et al. 2015). Among these regions, South Korea was found to have the most serious sediment pollution levels with concentrations ranging from ND–1970 ng/g attributed to surrounding chemical plants (Hyo-Bang et al. 2012). Interestingly, water collected from Tokyo Bay, Edogawa River, and other locations in Japan mostly contained BPF as the main pollutant with concentrations hundreds to thousands of times higher than other BPs. Notably, the BPF concentration in the surface water of the Tamagawa River was measured as high as 2850 ng/L, which might be associated with different industrial structures in the area (Liao et al. 2012c). These findings suggest that BPs pollution in the north of Taihu Lake Basin remains below foreign studies’ levels. Nonetheless, concentrations of BPs are increasing worldwide. Compared to surface water, sediments pose a greater ecological risk as the primary carrier of BPs and require more attention.
Spatial Distributions of BPs in Surface Water and Sediments
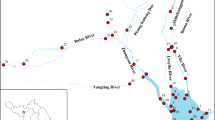
Figure 1 shows the spatial distribution of BPs in surface water and sediments of Zhushan Lake and Meiliang Lake. In Zhushan Lake, the highest pollution levels of BPs were found in the inlet tributaries to the center of the lake on the east and west sides, while pollution levels were lower upstream and at the outlet of the lake. Sampling sites with high pollution levels were mainly concentrated in the middle part of the lake. Site S13 had the highest concentration (6.7–4239 ng/L) while site S11 had the lowest concentration (nd–153 ng/L), with a difference of about 30 times. Other noteworthy sampling sites include S9 (1.5–2730 ng/L), S10 (0.80–1755 ng/L), and S14 (12–2788 ng/L), with pollution concentrations 23, 18, and 19 times that of S11, respectively. S9 is located near Zhuxi Industrial Park in Yixing City, while S13 and S14 are adjacent to several textile printing and dyeing enterprises and chemical enterprises in Wuxi City, and S10 is between the three points at the mouth of the lake. The survey data indicate that the main sources of BPs in the lake are wastewater discharge from metallurgical, printing and dyeing, chemical, and other enterprises in the vicinity and the degradation of organic matter produced by human activities. This is closely related to the high pollution levels of BPs in the middle part of Zhushan Lake and the positive correlation between BPS and BPF (Zhong et al. 2010). Additionally, slow water flow due to insufficient upstream water also contributed to the accumulation of pollutants near the aforementioned sites. Lower surface water pollution levels downstream to the center of the lake may be due to the dilution and purification of industrial effluents (Lu et al. 2020).
The spatial distribution of BPs in sediments followed a trend similar to that of surface water, with pollution levels increasing from upstream to the middle part of the lake and then decreasing downstream. However, the concentrations of each pollutant in sediments were generally low, likely due to the “silt clearing project” implemented in Taihu Lake in recent years. This project may have contributed to some extent to improvement in water quality (Xia et al. 2014). Meiliang Lake (S15–S20) had a much lower overall pollution level, with surface water and sediment pollution ranges of nd–588 ng/L and nd–3.5 ng/g, respectively. This could be attributed to the fact that there are few industrial concentration areas surrounding the lake, resulting in less impact from industrial wastewater discharge. It is also possible that other rivers around Wuxi may have carried pollutants away from Meiliang Lake, reducing the effects of human activities and industrial production on the lake’s water quality.
Bioaccumulation of BPs in Common Fish
To provide a more comprehensively representation of the distribution of fish in the Taihu Lake Basin, this study also purchased 16 fish from the fresh market near Taihu Lake. After determining the source and fishing time, the concentrations of BPs in five tissues and organs (muscle, brain, liver, kidney, gill) in four fish species (Channa argus, Carassius auratus, Parabramis pekinensis, Hemiculter leucisculus) were compared. The results showed that the concentrations of BPs in different fish organs followed the same trend, with the highest concentrations found in muscle, followed by gill, liver, brain, and kidney. The frequencies of detection for the four BPs were 76% for BPF, 65% for BPS, 41% for BPZ, and 39% for BPC, respectively.
Figure 2 shows that the fish had the highest levels of BPs in their muscle and gills, measuring 13 and 10 ng/g, respectively. The gills, being the first filtering organ of a fish’s body, have a significant exchange of substances with the outside environment. This suggests that the testing fish accumulated BPs mainly through respiration and food acquisition and were exposed for a short period, rather than deep accumulation. This is likely due to the high mobility of the lake water, which partially self-purifies BPs in the body. The liver is the main organ for metabolizing and transforming drugs, poisons, and environmental pollutants in a fish’s body. The kidney is the main organ responsible for filtration metabolism and reabsorption, and hence is a site of BP accumulation, as seen in the detection of medium and low concentrations of BPs in the liver (7.5 ng/g) and kidney (3.1 ng/g), respectively. Interestingly, BPZ and BPC were not detected in all brain tissues, indicating their inability to invade the fish brain through the blood–brain barrier. However, the levels of the other two substances in the brain were higher than those in the kidney (Liu et al. 2022).
The concentration of BPs in four wild fish species were ranked in the order Channa argus > Parabramis pekinensis > Hemiculter leucisculus > Carassius auratus, with a range from 0.16 to 7.2 ng/g. The concentrations of BPS, BPF, BPZ, and BPC in Channa argus were 4.1, 3.2, 3.1, and 5.9 times higher than in Parabramis pekinensis, Hemiculter leucisculus, and Carassius auratus, respectively. The trend of enrichment concentrations generally followed feeding habits, with carnivorous fish exhibiting the highest levels of BPs, followed by herbivorous fish, and omnivorous fish at the lowest level. BPs tend to be enriched in sediments such as bottom mud, water plants, and semi-decayed plant residues that strongly adsorb them (Zhou et al. 2020a). Thus, the enrichment of BPs in fish is highly correlated with their feeding habits and their position in the trophic levels of the food chain.
BPs Source Analysis
Correlation and Principal Component Analysis
Correlation analysis is a commonly used analytical method for pollutant source analysis. The degree of correlation between pollutants reflects the proximity of their source pathways (Guo et al. 2012). Table 1 presents the Pearson correlation of BPZ, BPC, BPF, and BPS in various carriers. At the p < 0.01 level, the pairs of BPS-BPC, and BPS-BPF were extremely correlated and demonstrated similarity in surface water. In sediments, the pair of BPF-BPS was significantly correlated. At the p < 0.05 level, the following pairs of BPF-BPZ, BPF-BPC, and BPS-BPC were significantly correlated and demonstrated similarities.
The high correlation between BPF and BPS in both water and sediment suggests that they likely share similar sources. This is plausible given their higher stability and persistence in various aqueous media (Chen et al. 2016). BPC showed a significant correlation with BPS in water, but not particularly in sediment. Moreover, BPZ was only found to correlate with BPF in sediments, suggesting a more diverse and uncertain origin of BPZ and BPC.
To further ascertain the pollution sources of BPs in the study area, a principal component analysis (PCA) was conducted (Table S4). Two principal components with eigenvalues > 1 were extracted, and their cumulative variances reached 82.2% and 76.5%, respectively. As shown in Table S5, the first principal component of surface water had a robust positive correlation with BPS, BPF, and BPC, while the second component showed a high positive load factor only for BPZ content, indicating that BPZ in surface water may have an independent source, distinct from the other three BPs. Conversely, the first principal component in sediments was highly correlated with BPF and BPS, suggesting that the BPC in sediments may have a different source from that in surface water, which possibly links to its adsorption capacity and demands further exploration.
Spearman Correlation Matrix Between Conventional Water Quality Parameters and BPs Concentration in Surface Water and Sediments
In order to examine the association between the concentration of BPs in surface water and sediments and conventional water quality parameters, Spearman analysis was employed in the study. Figure 3 illustrates the results obtained from this non-parametric measurement method (Han et al. 2020).
The water quality parameters that were analyzed included TN (0.62–3.08 mg/L), TP (0.03–0.09 mg/L), NH3-N (0.05–0.72 mg/L), DO (9.4–15.4 mg/L), and Chla (2.0–33.7 mg/L). TP, TN and NH3-N are important factors that contribute to the eutrophication of water bodies. Additionally, algae consume DO in the water, and low levels of DO can lead to the death of aquatic organisms. The faster phytoplankton grows, the higher the Chla in the water. These parameters can affect the distribution and concentration of pollutants to some extent. According to the Spearman correlation coefficient, there are strong significant correlations between four pairs among the conventional water quality parameters: NH3-N and TN (R = 0.86, p < 0.01), NH3-N and TP (R = 0.69, p < 0.05), DO and TP (R = − 0.96, p < 0.001), and DO and NH3-N (R = − 0.80, p < 0.01). These results suggest that the water quality parameters are homogeneous across water quality parameters due to the fluidity and complexity of the lake. However, certain connections can still be observed. For instance, the amount of DO is an indicator of the self-purification ability of the lake. DO was observed to have a negative correlation with TN, TP and NH3-N in this study, indicating that high dissolved oxygen levels are beneficial to degrading various pollutants in the lake (Coffin et al. 2021).
Chla is an important parameter that responds to eutrophication in water bodies. It was observed that Chla was negatively correlated with TN, TP, and other indicators. This is likely because poor water quality leads to increased demand for nutrients, such as nitrogen and phosphorus, from algae during water bloom outbreaks. The death of algal plants can result in a significant increase in nitrogen and phosphorus content, and a decrease in Chla. Other indicators, such as transparency and pH, also affect the eutrophication status (Pang et al. 2021). Apart from BPZ in surface water, all BPs concentrations were negatively correlated with indicators such as nitrogen and phosphorus, and positively correlated with dissolved oxygen. Additionally, it’s worth mentioning that BPZ, BPC, BPS in sediments, and BPS in surface water all showed significant positive correlations with NH3-N, indicating that surface water and sediment BP distribution may be affected by water quality and environmental conditions.
Stable Isotope Method Analysis
Analyzing the stable carbon and nitrogen isotopes in wild fish tissues can help us understand their feeding sources and the changes in the water environment. Based on the flow characteristics of the lake, the sampling sites were divided into five areas: Z1 (S1, S2, S3, S4, S14), Z2 (S5, S6, S7, S8, S13), Z3 (S9, S10, S11, S12), Z4 (S15, S19, S20), and Z5 (S16, S17, S18). The fish samples were grouped into four ccategories according to species (Z6, Carassius auratus, Z7, Hemiculter leucisculus, Z8, Channa argus, Z9, Parabramis pekinensis). The δ13C and δ15N in solid and water samples were measured using EA-IRMS (Gdam et al. 2022).
As shown in Table 2, the δ13C values in solid and liquid samples were − 30.38‰ to − 21.48‰ (8.9‰), and − 16.62‰ to − 11.8‰ (4.82‰), respectively. The δ15N values were 6.74‰ to 10.57‰ (3.83‰) and 34.9‰ to 40.6‰ (5.70‰), respectively. The δ13C value in water samples was significantly higher than that of solid samples, while the δ15N value was 3–4 times higher than that of solid samples. These results suggest that the stable isotope values in fish tissues are more similar to those in sediments, indicating that fish are more likely to consume food from the sediments rather than surface water. Additionally, the δ13C value of Channa argus was the lowest, while the δ15N value of Hemiculter leucisculus was the highest, suggesting that these two species have different food sources compared to other fish species. Geographically, Hemiculter leucisculus and Channa argus are more likely to be found downstream near the center of Taihu Lake, while Carassius auratus and Parabramis pekinensis are more likely to be found upstream near the shore.
Ecological Risk Assessment
The ecological balance of the southern Jiangsu Basin is maintained by Taihu Lake’s unique protective effect, and assessing the ecological risk of Taihu Lake (Southern Jiangsu section) is critical due to the reproductive toxicity of BPs to aquatic organisms. Zebrafish was considered the most susceptible species based on the worst-case scenario, and its acute toxicity (AF = 1000) was used to calculate the Predicted No Effect Concentration (PNEC). Figure 4 shows the risk assessment results for aquatic organisms calculated from toxicity data and BP concentrations in surface water.
All four BPs showed a RQ of < 1, indicating a low risk to aquatic organisms. The ΣRQs for fish ranged from 0.0013 to 0.1759, with a mean of 0.047. Except for S9, S10, and S13, the ΣRQs showed a low risk to fish. The contribution rate to the total risk was highest for BPF (70.6%), followed by BPC (13.6%), BPS (13.0%) and BPZ (2.8%). The RQs of BPF at most sampling points were below 0.01, but at S9, S10 and S13, they were 0.1406, 0.1527 and 0.1024, respectively, indicating moderate environmental risk. Lyu et al. (2023) explored bisphenol exposures and associated health risk among Japanese women living in the Kyoto area from 1993 to 2016, founding BPF also was the most important risk driver. BPS only showed low risk at four sampling sites (S9, S10, S13, S14). The ecological risk of BPZ at all sampling sites was not significant, and BPC showed a low risk only at S8, S10, and S13. Overall, BPs in the southern Jiangsu section of Taihu Lake posed low risks to aquatic organisms. The ecological risk of BPs from Zhushan Lake increased with the flow direction and decreased in the central direction of Taihu Lake, which was consistent with the spatial distribution of BPs concentration. While the water quality of Meiliang Lake was better, more than half of the sites were identified as low risk.
The study also assessed the potential human health risks of the four BPs by calculating the EEQt of each sampling site. The EEQt was calculated based on the estrogen equivalent factor of each BP, as reported by Yan et al. (2017). According to the results shown in Figure S2, the maximum EEQt in the southern Jiangsu section of Taihu Lake was 0–0.0669 ng E2/L, which is lower than the maximum allowable value of 1 ng E2/L. Therefore, the risk to human health is not significant. The highest EEQt values were found at S11, followed by S10, and BPF and BPS remained the main contributors. Interestingly, the average EEQt of BPF was 30 times higher than that of BPS. It is important to note that this study only considered the concentration of BPs in water, while ecological and human health risks may also arise due to the accumulation of BPs in other media carriers and food chains. Karolina et al. (2023) also indicated the presence of BPs in the aquatic environment can lead to unexpected effects on aquatic organisms.
Practical Applications
There has been increasing concern about the potential health risks associated with BPs in recent years. Numerous studies have been conducted to understand the effects of BPs on human health and the environment. Here are some of the practical applications of the study: (1) examining the presence, persistence, and potential ecological impacts of BPs in aquic ecosystem; (2) providing valuable insights into the mechanisms of action, exposure routes, and potential health effects associated with BPs; (3) playing a vital role in shaping regulatory actions and policies worldwide; (4) spurring the development and useful of alternative materials that are deemed safer.
Environmental Implications
The emission of BPs contributes to the increase of new pollutants in the environment, causing estrogen effects. Zhushan Lake and Meiliang Lake are two important positions affecting the water quality in Taihu Lake. Herein, we explored the four BPs in Zhushan Lake and Meiliang Lake. Here are some of the implications of the study: (1) theoretical implications: the detection of BPs helping to assess risks and developing strategies for mitigation; the potential health effects associated with BPs helping to inform public health policies and regulations; (2) practical implications: the detection of BPs in the environment highlighting the need for safe alternatives and leading to the development of BPs products; many countries implementing restrictions and bans on the use of BPs in specific applications.
Conclusions
This study investigated the presence and concentrations of four types of BPs (BPZ, BPC, BPF, and BPS) in Taihu Lake’s surface water, sediment, and wild fish. The results revealed wide-spread presence of these BPs, with varying concentrations across the different compartments. BPS and BPF were identified as the major contributors among the BPs analyzed. Ecological risk analysis indicated that BPF posed a higher risk contribution than the other BPs, accounting for 70.6% of the total risk. Moreover, the significant correlations observed between BPS and BPF in surface water and sediment suggested a common source of contamination. Wild fish muscle tissue exhibited the highest mean concentration of BPs, indicating the potential bioaccumulation of these substances. However, it was unlikely for BPZ and BPC to cross the blood–brain barrier into the fish brain. Stable isotope results further suggested that wild fish primarily fed on sediment. These findings underscore the importance of conducting further investigations into the potential risks associated with BPs.
Despite the valuable insights gained from this study, it also presented certain limitations which should be taken into consideration. Some main limitations of the study include: (1) The study focused on two specific areas of Taihu Lake, which may not fully represent the entire lake system. (2) It did not extensively explore the potential effects of seasonal variations on the occurrence and distribution of BPs. (3) It did not explicitly determine the exact sources of BPs in Taihu Lake. (4) It was lack of ecotoxicological assessment. These limitations provide opportunities for future developments and research in this area. Several potential future research could be explored: (1) Conducting long-term monitoring of BPs in Taihu Lake would provide valuable insights into temporal trends and variations in pollution levels. (2) Further investigations into the sources of BPs in the lake could involve the use of advanced techniques such as fingerprinting, source apportionment modeling, and identification of specific industries or activities contributing to the BP pollution. (3) Investigating the transfer of BPs through different trophic levels would provide insights into their persistence and potential health impacts. (4) Explore the effects of BPs on non-target organisms, including vulnerable species and sensitive life stages. Addressing the limitations identified in the study and pursuing future research directions will provide a more comprehensive understanding of the pollution levels of BPs in Taihu Lake and contribute to the development of effective pollution control strategies.
Data Availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- BP:
-
Bisphenol
- BPZ:
-
Bisphenol Z
- BPC:
-
Bisphenol C
- BPF:
-
Bisphenol F
- BPS:
-
Bisphenol S
- BPA:
-
Bisphenol A
- POPs:
-
Persistent organic pollutants
- DMSO:
-
Dimethyl sulfoxide
- PTFE:
-
Polytetrafluoroethylene
- MRM:
-
Multiple reaction monitoring
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- RSD:
-
Relative standard deviation
- RQ:
-
Risk quotient
- MEC:
-
Measured environmental concentration
- PNEC:
-
Predicted no-effect concentration
- AF:
-
Assessment factor
- EEQ:
-
Estrogen toxicity equivalent
- EEF:
-
Estradiol equivalence factor
References
Andujar N, Galvez-Ontiveros Y, Zafra-Gomez A, Rodrigo L, Alvarez-Cubero MJ, Aguilera M et al (2019) Bisphenol A analogues in food and their hormonal and obesogenic effects: a review. Nutrients 11:350–353
Chen D, Kannan K, Tan HL, Zheng ZG, Feng YL, Wu Y et al (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-a review. Environ Sci Technol 50:5438–5453
Chen M-H, Guo M, Xu H-Z, Liu D, Cheng J, Li J et al (2017) Distribution characteristics and potential risk of bisphenol analogues in surface water and sediments of Lake Taihu. Environ Sci 38:2793–2800
Coffin M, Knysh KM, Roloson SD, Pater CC, Theriaul E, Cormier JM et al (2021) Influence of nutrient enrichment on temporal and spatial dynamics of dissolved oxygen within northern temperate estuaries. Environ Monit Assess 193:804–804
Feng YX, Jiao ZH, Shi JC, Li M, Guo QZ, Shao B (2016) Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere 147:9–19
Gdam A, Maldo B, Mpr C, Vdcda A, Mjvb A (2022) Origin geographical classification of green coffee beans (Coffea arabica L.) produced in different regions of the Minas Gerais state by FT-MIR and chemometric—ScienceDirect. Curr Res Food Sci 112:68–74
Guo G, Wu F, Xie F, Zhang R (2012) Spatial distribution and pollution assessment of heavy metals in urban soils from southwest China. J Environ Sci 24:9 (in Chinese)
Han QF, Zhao S, Zhang XR, Wang XL, Song C, Wang SG (2020) Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China. Environ Int 138:105551–105552
Han Y, Fei YM, Wang M, Xue Y, Chen H, Liu YX (2021) Study on the joint toxicity of BPZ, BPS, BPC and BPF to zebrafish. Molecules 26:52–54
Han Y, Fei YM, Du ED, Wang MX, Xue YG, Liu YX (2022) Determination of 4 bisphenol environmental hormone in zebrafish exposure system by solid phase extraction-ultra performance liquid chromatography-tandem mass spectrometry. Res Environ Sci 35(2):499–507 (in Chinese)
Hyo-Bang M, Minkyu C (2012) Contamination and potential sources of polybrominated diphenyl ethers (PBDEs) in water and sediment from the artificial Lake Shihwa, Korea. Chemosphere 65:765–769
Jin HB, Zhu LY (2016) Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res 103:343–351
Jonker MTO (2016) Determining octanol-water partition coefficients for extremely hydrophobic chemicals by combining “slow stirring” and solid-phase microextraction. Environ Toxicol Chem 35:1371–1377
Kang C, Kuba T, Hao A, Iseri Y (2015) Antioxidant responses of Vallisneria asiatica to eutrophic sediments in Lake Taihu, China. Bull Environ Contam Toxicol 95:194–199
Kiwitt-cárdenas J, Adoamnei E, Arense-gonzalo J et al (2021) Associations between urinary concentrations of bisphenol A and sperm DNA fragmentation in young men. Environ Res 199:111289
Kong M, Xing L, Yan R, Li J, Zhang Y, Li A et al (2022) Spatiotemporal variations and ecological risks of typical antibiotics in rivers in flowing into Taihu Lake, China. J Environ Manag 309:114699
Lee S, Liu X, Takeda S, Choi K (2013) Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere 93:434–440
Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB et al (2012a) Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 46:6860–6866
Liao C, Liu F, Kannan K (2012b) Bisphenols, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 46:6515–6522
Liao C, Liu F, Moon HB, Yamashita N, Yun S, Kannan K (2012c) Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol 46:11558–11565
Liao CY, Liu F, Guo Y, Moon HB, Nakata H, Wu Q et al (2012d) Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46:9138–9145
Liu YH, Zhang SH, Song NH, Guo RX, Chen MH, Mai DN et al (2017) Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): implications for ecological and human health risk. Sci Total Environ 599:1090–1098
Liu N, Jin X, Feng C, Wang Z, Wu F, Johnson AC et al (2020a) Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: a proposed multiple-level system. Environ Int 136:105454–105454
Liu Y, Feng M, Wang B, Zhao X, Guo R, Bu Y et al (2020b) Distribution and potential risk assessment of antibiotic pollution in the main drinking water sources of Nanjing, China. Environ Sci Pollut Res Int 27:21429–21441
Liu J, Guo J, Cai Y, Ren J, Lu G, Li Y et al (2022) Multimedia distribution and ecological risk of bisphenol analogues in the urban rivers and their bioaccumulation in wild fish with different dietary habits. Process Saf Environ Prot 164:309–318
Lu J, Zhang C, Wu J, Zhang Y, Lin Y (2020) Seasonal distribution, risks, and sources of endocrine disrupting chemicals in coastal waters: will these emerging contaminants pose potential risks in marine environment at continental-scale? Chemosphere 247:125907–125907
Luo X, Shu S, Feng H, Zou H, Zhang Y (2021) Seasonal distribution and ecological risks of phthalic acid esters in surface water of Taihu Lake, China. Sci Total Environ 768:144517
Lyu ZQ, Harada KH, Kim SM, Fujitani T, Hitomi T, Pan R et al (2023) Temporal trends in bisphenol exposures and associated health risk among Japanese women living in the Kyoto area from 1993 to 2016. Chemosphere 316:137867
Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT et al (2010) Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol 30:532–539
Pang M, Song W, Liu Y, Pang Y (2021) Simulation of the parameters effecting the water quality evolution of Xuanwu Lake, China. Int J Environ Res Public Health 18:369–376
Peng X, Wang Z, Mai B, Chen F, Chen S, Tan J et al (2007) Temporal trends of nonylphenol and bisphenol A contamination in the Pearl River Estuary and the adjacent South China Sea recorded by dated sedimentary cores. Sci Total Environ 384:393–400
Shehab ZN, Jamil NR, Aris AZ (2020) Occurrence, environmental implications and risk assessment of Bisphenol A in association with colloidal particles in an urban tropical river in Malaysia. Sci Rep 10:20360
Si W, Cai Y, Liu J, Shen J, Chen Q, Chen C et al (2019) Investigating the role of colloids on the distribution of bisphenol analogues in surface water from an ecological demonstration area, China. Sci Total Environ 673:699–707
Su H, Shi D, Yang J, Tao Y, Sun F, Wei Y (2021) Distribution characteristics and risk assessment of mercury in sediments from Taihu Lake. Front Environ Sci 9:17–19
Usman A, Ikhlas S, Ahmad M (2019) Occurrence, toxicity and endocrine disrupting potential of Bisphenol-B and Bisphenol-F: a mini-review (vol 312, pg 222, 2019). Toxicol Lett 314:193–193
Wang X, Dong Q, Chen Y et al (2013) Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquat Toxicol 142:104–113
Wang D, Wang Y, Singh VP, Zhu J, Jiang L, Zeng D et al (2018) Ecological and health risk assessment of PAHs, OCPs, and PCBs in Taihu Lake basin. Ecol Ind 92:171–180
Wang JQ, Song YZ, Chen X (2022) Estimation of atmospheric nitrogen deposition to Taihu Lake from taihu watershed, China. Appl Ecol Environ Res 20:1085–1101
Xia J, Su G, Zhang X, Shi W, Giesy JP, Yu H (2014) Dioxin-like activity in sediments from Tai Lake, China determined by use of the H4IIE-luc bioassay and quantification of individual AhR agonists. Environ Sci Pollut Res Int 21:1480–1488
Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PKS, Moon HB et al (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol Environ Saf 122:565–572
Yan C, Yang Y, Zhou J, Liu M, Nie M, Shi H et al (2013) Antibiotics in the surface water of the Yangtze Estuary: occurrence, distribution and risk assessment. Environ Pollut 175:22–29
Yan Z, Liu Y, Yan K, Wu S, Han Z, Guo R et al (2017) Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 184:318–328
Yang L, Zhu L, Liu Z (2011) Occurrence and partition of perfluorinated compounds in water and sediment from Liao River and Taihu Lake, China. Chemosphere 83:806–814
Zhao X, Zhang H, Chen ZL, Wang XC, Shen JM (2020) Spatial and temporal distributions of bisphenol analogues in water and sediment from the Lanzhou section of the Yellow River, China. Arab J Geosci 13:55–57
Zhong W, Wang D, Xu X, Luo Q, Wang B, Shan X et al (2010) Screening level ecological risk assessment for phenols in surface water of the Taihu Lake. Chemosphere 80:998–1005
Zhou LJ, Wang WX, Lv YJ, Mao ZG, Chen C, Wu QL (2020a) Tissue concentrations, trophic transfer and human risks of antibiotics in freshwater food web in Lake Taihu, China. Ecotoxicol Environ Saf 197:110626–110626
Zhou N, Liu Y, Cao S, Guo R, Ma Y, Chen J (2020b) Biodegradation of bisphenol compounds in the surface water of Taihu Lake and the effect of humic acids. Sci Total Environ 723:138164–138164
Acknowledgements
This work was funded by the National Natural Science Foundation of China [21906009], Jiangsu High-level Innovative and Entrepreneurial Talent Introduction Program Special Fund [SCZ2010300004], and The Science and Technology Research Award Package of Changzhou University [KYP2102098C and KYP2202117C].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, Y., Liu, Y., Rong, X. et al. Exposure, Distribution, and Ecological Risk of Four New Bisphenol Analogs in the Typical Lake Region of Taihu Lake. Expo Health 16, 1027–1037 (2024). https://doi.org/10.1007/s12403-023-00608-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-023-00608-2