Abstract
A simple online headspace solid-phase microextraction (HS-SPME) coupled with the gas chromatography-mass spectrometry (GC-MS) method was developed for simultaneous determination of trace amounts of nine estrogenic odorant alkylphenols and chlorophenols and their derivatives in water samples. The extraction conditions of HS-SPME were optimized including fiber selection, extraction temperature, extraction time, and salt concentration. Results showed that divinylbenzene/Carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber was the most appropriate one among the three selected commercial fibers, and the optimal extraction temperature, time, and salt concentration were 70 °C, 30 min, and 0.25 g/mL, respectively. The developed method was validated and showed good linearity (R 2 > 0.989), low limit of detection (LOD, 0.002–0.5 μg/L), and excellent recoveries (76–126 %) with low relative standard deviation (RSD, 0.7–12.9 %). The developed method was finally applied to two surface water samples and some of these target compounds were detected. All these detected compounds were below their odor thresholds, except for 2,4,6-TCAS and 2,4,6-TBAS wherein their concentrations were near their odor thresholds. However, in the two surface water samples, these detected compounds contributed to a certain amount of estrogenicity, which seemed to suggest that more attention should be paid to the issue of estrogenicity rather than to the odor problem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of their potential adverse effect on humans and wildlife, endocrine-disrupting compounds (EDCs) have drawn much attention in the last decades. The term EDCs covers a broad class of compounds, including natural/synthetic estrogens, natural/synthetic androgens, phytoestrogens, mycoestrogens, progestins, and industrial chemicals (Liu et al. 2011, 2015a, b, c). Estrogens are compounds with the strongest estrogenic potencies, while their concentrations in our environment are normally low. On the contrary, most estrogenic industrial chemicals are very weak EDCs, but their concentrations in the environment are normally high. Therefore, the potential adverse effects of these estrogenic industrial chemicals on our environment cannot be neglected.
With increasing attentions on EDCs and related progress in their potential health effects, some of them have been listed as the new items in the latest drinking water regulations in China and Japan. For example, bisphenol A, diethyl phthalate, and dibutyl phthalate are three EDCs that have been listed in the latest Chinese drinking water quality standard (Standardzation Administration of China (SAC) 2006), while 17β-estradiol (E2) and other four EDCs have been listed as the potential new items in the latest Japanese drinking water quality standard (Ministry of Health, Labour and Welfare of Japan (MHLWJ) 2015). 4-n-butylphenol (4-n-BP), 2-tert-butylphenol (2-t-BP), 4-tert-butylphenol (4-t-BP), and 2, 4-di-tert-butylphenol (2, 4-d-t-BP) have been reported to be estrogenic industrial chemicals (Blair et al. 2000; Liu et al. 2009a; Nishihara et al. 2000), which are four important alkylphenols that have been widely used for production of phenolic, polycarbonate, and epoxy resins (Kuruto-Niwa et al. 2005; Toyama et al. 2010). They have been in use for at least 50 years and reported to be widely distributed in different aquatic environments, including river waters (Cui et al. 2015; Kuruto-Niwa et al. 2005), river/marine sediments (Chen et al. 2005; Duan et al. 2014), and wastewaters (Berge et al. 2014; Padilla-Sanchez et al. 2011). They also have been found in different foods including dairy milk (Kilic and Lindsay 2005). Recently, they received new attention as important odorant compounds in drinking water, and one of their important sources is leaching from drinking water pipes in the distribution systems (Ryssel et al. 2015; Skjevrak et al. 2003). The reported concentrations of 2,4-d-t-BP in drinking water leached from drinking water pipes were up to 368 μg/L (Loschner et al. 2011), and the level was already over its odor threshold of 200 μg/L (Tao and Zhang 2010). When their concentrations in drinking water overpass the odor threshold, they can cause odor problems in drinking water. For example, 2-t-BP was reported to be the odor-causing compound in a famous drinking water odor incident that occurred at the end of 2013 in Hangzhou, China, in which the odor problem lasted for several months and over 1.2 million inhabitants were affected (Liu et al. 2016). As their concentrations in water are generally low, it is quite important to develop a fast and efficient analytical method.
Different analytical methods including HPLC, gas chromatography (GC), LC-MS, gas chromatography-mass spectrometry (GC-MS), and LC-MS/MS have been reported to be effective for the analysis of alkylphenols in different samples (Crescenzi et al. 1995; Laborie et al. 2016; Lopez-Darias et al. 2010; Tan and Mohd 2003; Wang and Deng 2016). Among them, GC-MS is the most widely applied (Grzeskowiak et al. 2016). To concentrate the analytes, different sample preparation methods including solid phase extraction, liquid-liquid extraction, water-based gas purge microsyringe extraction, liquid-liquid microextraction, and solid-phase microextraction (SPME) have been adopted (Carabias-Martinez et al. 2006; Llompart et al. 2002; Padilla-Sanchez et al. 2011; Yang et al. 2013; Zhu et al. in press). Among these sample preparation methods, SPME is the most widely applied method, which uses non-organic solvents, takes less volume of the sample, and is robust and sensitive (Plotka-Wasylka et al. 2015). To improve its accuracy and sensitivity, the phenolic analytes are often derivatized before the analysis (Liu et al. 2010a, b). However, in Li et al. (2001), it seems that the improvement is not evident for alkylphenols. Moreover, the addition of the derivatization step is inconvenient and takes extra time and thus is unfavorable for the analysis of alkylphenols. To overcome this inconvenient step, a post-derivatization process after the injection of the analytes to GC was reported by Basheer and Lee (2004). However, the residual derivative reagent would influence the sensitivity (Li et al. 2001).
With the improvement of both sample preparation and automation, it is necessary and important to develop an online analytical method. However, to the best of our knowledge, no method is available for the online analysis of alkylphenols yet. Therefore, the main objective of this study was to develop an online analytical method for the above four mentioned estrogenic odorant alkylphenols, in which an online headspace solid-phase microextraction (HS-SPME) coupled with GC-MS was applied. Except for the four alkylphenols, two chlorophenols, 2-chlorophenol (2-CP), and 2-chloro-4-methylphenol (2-C-4-MP), were included, both of which are odorant compounds showing estrogenic potencies and are widely distributed in the natural environment (Blair et al. 2000; Cargouet et al. 2007; Czaplicka 2004; Nishihara et al. 2000). Meanwhile, three phenolic derivatives (4-chloroanisole (4-CAS), 2,4,6-trichloroanisole (2,4,6-TCAS), 2,4,6-tribromoanisole (2,4,6-TBAS) were also included, which are important odorant compounds with very low odor thresholds (Young et al. 1996; Diaz et al. 2005). To obtain the proper analytical conditions, fiber selection, extraction temperature and time, and salinity were optimized. Finally, the developed method was validated and applied to surface water samples.
Materials and methods
Target analytes
The physicochemical properties, odor thresholds, and estrogenic potencies of the nine target analytes used in this study are given in Table 1.
Reagents and materials
The standard compounds of 2-CP, 4-CAS, 2-C-4-MP, 2-t-BP, 4-t-BP, 2,4,6-TCAS, 4-n-BP, 2,4-d-t-BP, and 2,4,6-TBAS were obtained from ANPEL Laboratory Technologies (Shanghai) Inc. They had a purity of at least 99 %. Stock solutions of individual standards at a concentration of 1000 mg/L were obtained by dissolving 0.01 g standard target into10-mL methanol. The mixed stock standard solution of nine odorant phenolic compounds was prepared in methanol at 10 mg/L, which was stocked in the dark at −20 °C. Sodium chloride (analytical grade, China) was pre-purified in a furnace oven at 450 °C for 2 h before its use. Deionized water was obtained from a Milli-Q Reference (Merck Millipore, USA) water purification system. Headspace vials (20 mL) were purchased from Agilent technologies (USA).
Three SPME extraction fibers including Stable-flex DVB/CAR/PDMS (50/30 μm), Stable-flex Carboxen/polydimethylsiloxane (Carboxen/PDMS, 85 μm), and Metal alloy Carbowax-Polyethylene Glycol (PEG, 60 μm) were purchased from Supelco (Sigma-Aldrich, USA).
Online HS-SPME procedure
The online HS-SPME was performed by an RTC PAL auto-sampler (CTC, Switzerland), which was equipped with a 30-sample tray for 20 mL vials, an SPME fiber conditioning station, and a temperature-controllable mixer tray. The fiber was first conditioned before the SPME with the manufacturer’s recommendations by inserting it to the conditioning station and each time, the fiber was cleaned by heating it at 270 °C for 20 min. A blank test was performed to make sure that there is no residual of target compounds on the fiber after the heat pre-conditioning. The water sample volume used each time in this study was 10 mL. When the mixed standard solution or surface water samples was added to the 20-mL vial with certain NaCl concentration, the vial was immediately and tightly sealed using a screw cap with a PTFE silicone-faced septum. When the temperature of the mixer tray reached the set temperature, the vial was stabilized for 5 min. Then, the fiber was inserted through the septum and kept in the HS of the vial for a certain time interval. During the extraction, the vial was shaken at a fixed speed of 250 rpm. After the extraction, the fiber with the target compounds was desorbed in the injector. The desorption temperature and time were 250 °C and 5 min, respectively.
GC-MS analysis
GC/MS analysis was performed using an Agilent 6890P GC coupled to an Agilent 5973 N mass-selective detector (Agilent Technologies, USA). Separation was accomplished on a fused silicon column (DB-5MS, 30 m*0.25 mm id, 0.25-μm film thickness, Agilent Company). Helium (purity >99.999 %) was used as the carrier gas at a flow rate of 1 mL/min. The oven temperature was initially set at 35 °C, and then programmed at 10 °C/min to 260 °C. The inlet, transfer line, and ion source were set at 270, 280, and 230 °C, respectively. The sample was injected in a splitless mode. The mass detector was operated in the electron ionization mode at 70 eV. The time for solvent delay was set to 6 min. The total GC-MS analysis time was about 23 min. The retention time, qualitative ions, and quantitative ion are listed in Table 2.
Results and discussion
Optimization of HS-SPME condition
The HS-SPME of the nine target compounds was carried out from the headspace of the water solution at a spiked concentration of 10 μg/L. The extraction performances were checked with the main extraction parameters. They were different fibers, extraction temperature, extraction time, and salt concentration, through which the optimal extraction condition was determined.
Determination of optimal fibers
The type of stationary phase, the polarity, and the thickness of the fiber are crucial to the extraction efficiency. Three commercial fibers DVB/CAR/PDMS, Carboxen/PDMS, and PEG from Supelco were selected. They are said to be effective for the extraction of volatile and semi-volatile compounds by the provider, which have been proven to be effective by many researchers (Llompart et al. 2002; Plotka-Wasylka et al. 2015). With the extraction at 60 °C for 20 min, all the three fibers showed excellent extraction performance, and high abundance of peaks for the nine target compounds were obtained as shown in Fig. 1. By comparison, DVB/CAR/PDMS gave the best extraction, followed by Carboxen/PDMS, with PEG giving the least extraction (Fig. 2). Therefore, DVB/CAR/PDMS was considered as the most appropriate fiber for the selected nine targets.
Optimization of extraction temperature
Temperature is an important factor for extraction. Different extraction temperatures ranging from 30 to 75 °C were examined. Figure 3 showed the changing of peak areas of the nine targets at a concentration of 10 μg/L, which was plotted as a function of temperature (30, 40, 50, 60, 70, and 75 °C) at a fixed extraction time of 20 min. The results clearly indicated that the extraction efficiencies increased with the increasing of the extraction temperature. The extraction performance was greatly increased, especially when the extraction temperature was increased from 60 to 70 °C. However, a sudden drop for the extraction performance was observed when the extraction temperature was further increased to 75 °C. The distribution constants of the nine targets between the headspace and aqueous phase increase as the temperature increases; thus, it is not difficult to understand that the extraction performance increased when the extraction temperature increased. The reason for the decreased extraction performance is not yet clear when the extraction temperature was further increased from 70 to 75 °C. One possible reason is that this might result from the decreased partition coefficient of the nine targets between the headspace and the fiber as adsorption is generally an exothermic process (Bagheri et al. 2007; Lord and Pawliszyn 2000). Based on the above experimental results, the optimal extraction temperature was determined at 70 °C.
Optimization of extraction time
Fiber extraction time is another important factor for extraction performance. The extraction time was optimized using the peak areas of the nine target compounds as a function of the fiber exposure time. The SPME fiber was exposed to the headspace of the aqueous solution spiked with 10 μg/L of the nine target compounds for 10–35 min at a fixed temperature of 70 °C. As shown in Fig. 4, the results indicated that 30 min was the optimal time for extraction.
Influence of salt addition
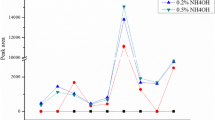
It has been proved that increasing ionic strength would increase phase separation through sorting out phenomena (Paleologos et al. 2005). Therefore, the influence of salt addition on extraction efficiency was also examined. NaCl was used as the salt in this experiment, and five different salt concentration gradients ranging from 0 to 0.3 g/mL were investigated at a fixed temperature and extraction time (70 °C and 30 min, respectively). As shown in Fig. 5, the peak areas of the nine target compounds had no significant change when addition of the salt concentration was increased from 0 to 0.1 g/mL, but after then, the corresponding peak areas greatly increased. With the salt concentration at 0.25 g/mL, the peak areas of the nine target compounds were the highest. When addition of the salt concentration was further increased, a sharp or slow decrease for the peak areas of the nine target compounds was observed. Therefore, the salt concentration at 0.25 g/mL was selected as the most suitable condition.
Method validation and application to real samples
The developed method using the above optimized conditions was validated with respect to linear range, coefficient of determination, limit of detection (LOD), and recoveries. Standard calibration curves were established by spiking the nine target compounds to purified water or surface water at six different concentration levels, which showed satisfactory linearity based on the external standard method (Table 3). LOD and LOQ were calculated based on three times (S/N = 3) and ten times (S/N = 10) of the signal-to-noise ratio, in which the calibrations were calculated at the lowest calibration concentration of 1 μg/L. As shown in Table 3, LOD ranged from 0.002 to 0.5 μg/L, while LOQ ranged from 0.006 to 1.7 μg/L. Their LOD values were all below their odor thresholds, so were their LOQ values (except for 2,4,6-TCAS and 2,4,6-TBAS in which their LOQ values for surface water were slightly above their odor thresholds). The low detection limits suggested that the developed method is satisfactory for odor problem screen in drinking water. To further validate the developed method, recovery experiments were carried out by spiking three known amounts of the standard mixture to purified and surface water. To prevent potential pollution, the same purified water or surface water were also monitored without spiking. When the calculation was performed for recovery efficiency, the concentrations of the controls without spiking were excluded. As shown in Table 4, excellent recovery efficiencies were obtained with low RSD.
The developed method was applied to determination of the nine target compounds in two surface waters, which are used as the two important sources for drinking water production. All samples were collected at the beginning of 2016. As shown in Tables 1 and 5, some of the nine target compounds were detected, but none of them were above their odor thresholds except for 2,4,6-TCAS and 2,4,6-TBAS, wherein their concentrations were near their odor thresholds. With the estrogenic potencies of the target compounds and their detected concentrations, the estrogen equivalent (EEQ) in the monitored surface water samples can be calculated as the equation below (Liu et al. 2009b, c).
where EP and c denote the estrogenic potency of one specific target compound and the corresponding detected concentration, respectively. The unit of EEQ is ngE2/L. With their EP values in Table 1 and their detected concentrations in Table 5, EEQ in the two surface water samples could be calculated. The calculated EEQ levels of the surface water A and B were about 0.15 and 0.17 ngE2/L, which were all relatively low. Soares et al. (2009) reported that egg mortality and other adverse effects in late gastrulation and/or early organogenesis in zebrafish were observed at very low ethynyl estradiol (EE2) exposure concentration of 0.19 ng/L, in which the calculated EEQ level was 0.27 ngE2/L with its EP of 1.4 (Liu et al. 2009a). The calculated EEQ levels of the two surface water samples were about half of the exposure level in Soares et al. (2009). As the surface waters are two important sources for drinking water production, for safety consideration, the latent potential adverse effects of these detected estrogenic compounds on the consuming residents should be evaluated if these compounds cannot be well removed during the drinking water treatment process. To the best of our knowledge, there is no information on the removal of perforation of drinking water treatment process (DWTP) on alkylphenols. In a batch study, the removal efficiencies of nanofiltration membranes on three alkylphenols of 2-t-BP, 4-t-BP, and 4-n-BP were only 15–82 % (Jung et al. 2007), which seemed to suggest that alkylphenols might not be readily removed by DWTP. The fact suggested that the surface water deserves more attention on estrogenic compounds rather than the screening for odor problem.
Conclusions
A simple, rapid, and sensitive online HS-SPME-GC/MS method was developed for the simultaneous determination of the nine estrogenic odorant alkylphenols and chlorophenols and their derivatives. Extraction conditions of the HS-SPME were optimized. The most appropriate fiber was DVB/CAR/PDMS among the three commercial available fibers. The optimized extraction temperature, extraction time, and addition of salt concentration were 70 °C, 30 min, and 0.25 g/mL, respectively. The developed method was validated and applied to monitor two surface waters. Some of the target compounds were detected, but none of the detected target compounds exceed the odor thresholds except for 2,4,6-TCAS and 2,4,6-TBAS wherein their concentrations were near their odor thresholds. However, the detected nine target compounds in the two surface water samples contributed to certain amounts of estrogenicity. This fact indicated that more attention should be paid to the estrogenic effects rather than to the odor issue.
References
Akahori Y, Nakai M, Yamasaki K, Takatsuki M, Shimohigashi Y, Ohtaki M (2008) Relationship between the results of in vitro receptor binding assay to human estrogen receptor α and in vivo uterotrophic assay: comparative study with 65 selected chemicals. Toxicol in Vitro 22:225–231
Basheer C, Lee H (2004) Analysis of endocrine disrupting alkylphenols, chlorophenols and bisphenol A using hollow-protected liquid-phase microextraction coupled with injection port-derivatization gas chromatography-mass spectrometry. J Chromatogr A 1057:163–169
Bagheri H, Aghakhani A, Es-haghi A (2007) Sol-gel-based SPME and GC-MS for trace determination of geosmin in water and apple juice samples. Chromatographia 66:779–783
Berge A, Gasperi J, Rocher V, Gras L, Coursimault A, Moilleron R (2014) Phthalates and alkylphenols in industrial and domestic effluents: case of Paris conurbation (France). Sci Total Environ 488–489:26–35
Blair RM, Fang H, Branham WS, Hass BS, Dial SL, Moland CL, Tong WD, Shi LM, Perkins R, Sheehan DM (2000) The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci 54:138–153
Carabias-Martinez R, Rodriguez-Gonzalo E, Revilla-Ruiz P (2006) Determination of endocrine-disrupting compounds in cereals by pressurized liquid extraction and liquid chromatography–mass spectrometry: study of background contamination. J Chromatogr A 1137:207–215
Cargouet M, Perdiz D, Levi Y (2007) Evaluation of the estrogenic potential of river and treated waters in the Paris area (France) using in vivo and in vitro assays. Ecotoxicol Environ Saf 67:149–156
Chen B, Mai BX, Duan JC, Luo XJ, Yang QS, Sheng GY, Fu JM (2005) Concentrations of alkylphenols in sediments from the Pearl River estuary and South China Sea, South China. Mar Pollut Bull 50:993–997
Crescenzi C, Di Corcia A, Samperi R, Marcomini A (1995) Determination of nonionic polyethoxylate surfactants in environmental waters by liquid chromatography/electrospray mass spectrometry. Anal Chem 67:1797–1804
Cui M, Qiu J, Li Z, He M, Jin M, Kim J, Quinto M, Li D (2015) An etched stainless steel wire/ionic liquid-solid phase microextraction technique for the determination of alkylphenols in river water. Talanta 132:564–571
Czaplicka M (2004) Sources and transformations of chlorophenols in the natural environment. Sci Total Environ 322:21–39
Diaz A, Fabrellas C, Ventura F (2005) Determination of the odor threshold concentrations of chlorobrominated anisoles in water. J Agric Food Chem 53:383–387
Duan XY, Li YX, Li XG, Zhang DH, Gao Y (2014) Alkylphenols in surface sediments of the Yellow Sea and East China Sea inner shelf: occurrence, distribution and fate. Chemosphere 107:265–273
Grzeskowiak T, Czarczynska-Goslinska B, Zgoła-Grzeskowiak A (2016) Current approaches in sample preparation for trace analysis of selected endocrine-disrupting compounds: focus on polychlorinated biphenyls, alkylphenols, and parabens. Trends Anal Chem 75:209–226
Jung YJ, Kiso Y, Park HJ, Nishioka K, Min KS (2007) Rejection properties of NF membranes for alkylphenols. Desalination 202:278–285
Kilic M, Lindsay RC (2005) Distribution of conjugates of alkylphenols in milk from different ruminant species. J Dairy Sci 88:7–12
Kuruto-Niwa R, Nozawa R, Miyakoshi T, Shiozawa T, Terao Y (2005) Estrogenic activity of alkylphenols, bisphenol S, and their chlorinated derivatives using a GFP expression system. Environ Toxicol Pharmacol 19:121–130
Laborie S, Moreau-Guigon E, Alliot F, Desportes A, Oziol L, Chevreuil M (2016) A new analytical protocol for the determination of 62 endocrine-disrupting compounds in indoor air. Talanta 147:132–141
Li D, Park J, Oh JR (2001) Silyl derivatization of alkylphenols, chlorophenols, and bisphenol A for simultaneous GC/MS determination. Anal Chem 73:3089–3095
Liu ZH, Kanjo Y, Mizutani S (2009a) Removal mechanisms of endocrine disrupting compounds (EDCs) in wastewater treatment-physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–738
Liu ZH, Kanjo Y, Mizutani S (2009b) Urinary excretion rates of natural estrogens and androgens from humans, and their occurrence and fate in the environment: a review. Sci Total Environ 407:4975–4985
Liu ZH, Ito M, Kanjo Y, Yamamoto A (2009c) Profiles and removal of endocrine disrupting chemicals by using an ER/AR competitive ligand binding assay and chemical analyses. J Environ Sci 21:900–906
Liu ZH, Hashimoto T, Okumura Y, Kanjo Y, Mizutani S (2010a) Simultaneous analysis of natural free estrogens and their conjugates in wastewater by GC-MS. Clean: Soil, Air, Water 38:181–188
Liu ZH, Kanjo Y, Mizutani S (2010b) Deconjugation characteristics of natural estrogen conjugates by acid-catalyzed solvolysis and its application for wastewater samples. J Environ Monit 12:1594–1600
Liu ZH, Ogejo JA, Pruden A, Knowlton KF (2011) Occurrence, fate and removal of synthetic oral contraceptives (SOCs) in the natural environment: a review. Sci Total Environ 409:5149–5161
Liu ZH, Lu GN, Yin H, Dang Z, Littier H, Liu Y (2015a) Sample-preparation methods for direct and indirect analysis of natural estrogens. Trends Anal Chem 64:149–164
Liu ZH, Lu GN, Yin H, Dang Z, Rittmann B (2015b) Removal of natural estrogens and their conjugates in municipal wastewater treatment plants: a critical review. Environ Sci Technol 49:5288–5300
Liu ZH, Lu GN, Yin H, Dang Z (2015c) Do we underestimate the concentration of estriol in raw municipal wastewater? Environ Sci Pollut Res 22:4753–4758
Liu ZH, She PY, Wei XN, Duan L, Yin H, Dang Z (2016) New drinking water quality standards in Japan and discussions. China Water Wastewater 32:8–10 (in Chinese)
Llompart MA, Lourido M, Landin P, Garcia-Jares C, Cela R (2002) Optimization of a derivatization–solid-phase microextraction method for the analysis of thirty phenolic pollutants in water samples. J Chromatogr A 963:137–148
Lopez-Darias J, Pino V, Meng Y, Anderson JL, Afonso AM (2010) Utilization of a benzyl functionalized polymeric ionic liquid for the sensitive determination of polycyclic aromatic hydrocarbons; parabens and alkylphenols in waters using solid-phase microextraction coupled to gas chromatography–flame ionization detection. J Chromatogr A 1217:7189–7197
Lord H, Pawliszyn J (2000) Evaluation of solid-phase microextraction technology. J Chromatogr A 885:153–193
Loschner D, Rapp T, Schlosser FU, Schuster R, Stottmeister E, Zander S (2011) Experience with the application of the draft European Standard prEN 15768 to the identification of leachable organic substances from materials in contact with drinking water by GC-MS. Anal Methods 3:2547–2556
MHLWJ (2015) Japanese drinking water quality standard (in Japanese). http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/topics/bukyoku/kenkou/suido/kijun/kijunchi.html. Accessed on 21 May 2016.
Nishihara T, Nishikawa J, Kanayama T, Dakeyama F, Saito K, Imagawa M, Takatori S, Kitagawa Y, Hori S, Utsumi H (2000) Estrogenic activities of 517 chemicals by yeast two-hybrid assay. J Health Sci 46:282–298
Padilla-Sanchez JA, Plaza-Bolanos P, Romero-Gonzalez R, Barco-Bonilla N, Martinez-Vidal JL, Garrido-Frenich A (2011) Simultaneous analysis of chlorophenols, alkylphenols, nitrophenols and cresols in wastewater effluents, using solid phase extraction and further determination by gas chromatography–tandem mass spectrometry. Talanta 85:2397–2404
Paleologos EK, Giokas DL, Karayannis MI (2005) Micelle-mediated separation and cloud-point extraction. Trends Anal Chem 2005(24):426–436
Plotka-Wasylka J, Szczepanska N, de la Guardia M, Namiesnik J (2015) Miniaturized solid-phase extraction techniques. Trends Anal Chem 73:19–38
Ryssel ST, Arvin E, Lutzhoft HCH, Olsson ME, Prochazkova Z, Albrechtsen HJ (2015) Degradation of specific aromatic compounds migrating from PEX pipes into drinking water. Water Res 81:269–278
Standardzation Administration of China (SAC) (2006) Chinese Drinking Water Quality Standard (GB5749-2006).
Skjevrak I, Due A, Gjerstad KO, Herikstad H (2003) Volatile organic components migrating from plastic pipes (HDPE, PEX and PVC) into drinking water. Water Res 37:1912–1920
Soares J, Coimbra AM, Reic-Henriques MA, Monteiro NM, Vieira MN, Oliveira JMA, Guedes-Dias P, Fontainhas-Fernandes A, Parra SS, Carvalho AP, Castro FC, Santos MM (2009) Disruption of zebrafish (Danio rerio) embryonic development after full life-cycle parental exposure to low levels of ethinylestradiol. Aquat Toxicol 95:330–338
Tan BL, Mohd MA (2003) Analysis of selected pesticides and alkylphenols in human cord blood by gas chromatograph-mass spectrometer. Talanta 61:385–391
Tao Y, Zhang L (2010) Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT Food Sci Technol 43:1550–1556
Toyama T, Momotani N, Ogata Y, Miyamori Y, Inoue D, Sei K, Mori K, Kikuchi S, Ike M (2010) Isolation and characterization of 4-tert-butylphenol-utilizing Sphingobium fuliginis strains from Phragmites australis rhizosphere sediment. Appl Environ Microbiol 76:6733–6740
Wang X, Deng C (2016) Preparation of C18-functionalized magnetic polydopamine microspheres for the enrichment and analysis of alkylphenols in water samples. Talanta 148:387–392
Yang C, Zhao J, Wang J, Yu H, Piao X, Li D (2013) Water-based gas purge microsyringe extraction coupled with liquid chromatography for determination of alkylphenols from sea food Laminaria japonica Aresh. J Chromatogr A 1300:38–42
Young WF, Horth H, Crane R, Ogden T, Arnott M (1996) Taste and odour threshold concentrations of potential potable water contaminants. Water Res 30:331–340
Zhu X, Zhu Y, Zhang Y, F. Li, S. Xin (in press) Potentiometric and semi-empirical quantum chemical studies on liquid–liquid micro-extraction of 4-tert-butylphenol with trioctyl phosphate clusters, Arabian Journal of Chemistry (doi: 10.1016/j.arabjc.2014.05.012)
Acknowledgments
This work was financially supported by The Key Program of National Natural Science of China (No. 41330639); the Program for National Natural Science Foundation of China (No. 21107025; No. 21577040; U1501234); Special funds for public welfare research and capacity building in Guangdong Province(B2153210); Science and Technology Program of Guangzhou, China (No. 201510010162); and the Fundamental Research Funds for the Central Universities (2014ZM0073) as well as the funding for water odor research (D8144320).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statements
The manuscript has not been submitted to more than one journal for simultaneous consideration. The manuscript has not been published previously (partly or in full). The manuscript is a whole study, and is not split up into several parts to increase the quantity of submissions. No data have been fabricated or manipulated (including images) to support the conclusions. The manuscript is our own work, and no data, text, or theories by others are presented unless cited. All authors know the submission, and consents were obtained before the submission.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Yuan, Sf., Liu, Zh., Lian, HX. et al. Simultaneous determination of estrogenic odorant alkylphenols, chlorophenols, and their derivatives in water using online headspace solid phase microextraction coupled with gas chromatography-mass spectrometry. Environ Sci Pollut Res 23, 19116–19125 (2016). https://doi.org/10.1007/s11356-016-7107-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7107-1