Abstract
“Swimming across the Pearl River” is an annual large-scale sporting event with great popularity in Guangzhou. To reduce the risk of swimmers’ exposure to various contaminants in the Pearl River during swimming activities, the local government limits direct sewage and effluent discharge from urban channels during the event. However, the impact of discharge reduction on some contaminants of emerging concern (CECs), such as organophosphorus flame retardants (OPFRs), bisphenol analogues (BPs), and triclosan remains unknown. In the present study, the concentrations of CECs, as well as ammonia-nitrogen (NH3-N), dissolved organic carbon, and chemical oxygen demand, were measured in aqueous and suspended particulate matter (SPM) from the Guangzhou reaches of the Pearl River. The concentration ranges of sixteen OPFRs, eight BPs, and triclosan were 21.2–91.0, 8.46–37.3, and 1.47–5.62 ng/L, respectively, in aqueous samples, and 25.2–492, 14.0–86.3, and 0.69–17.5 ng/g, respectively, in SPM samples. Hydrophobic and π-π interactions could be contributing to the distribution of CECs. Principal component analysis indicated that consumer materials, manufacturing, and domestic sewage might be the main sources of the CECs. In addition, our study showed that the concentrations of CECs did not change considerably before or after discharge reduction activities, although NH3-N showed a substantial decrease following pollution control measure. The results demonstrated that temporary reductions of contaminant discharges to the Pearl River had only limited effect on the levels of CECs. Further research is needed to investigate the distributions and potential health risks of CECs in the Pearl River.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Situated in the subtropical zone of southern China, the Pearl River Delta (PRD), one of the most densely populated and economically developed regions in China, covers an area of 46.1 × 104 km2 and has a population of over 56 million (Zhang et al. 2020). Meanwhile, the river also has a highly complex water network that receives substantial rainfall during the summer (Wang et al. 2015a). Consequently, all types of pollutants are introduced to the Pearl River through various sources and pathways (Ouyang et al. 2005). Such sources include contaminants of emerging concern (CECs) from urban effluents of wastewater treatment plants (Zeng et al. 2014), hospital and aquaculture zone effluents (Xu et al. 2007), domestic sewage, and run-off from urban and agricultural areas (Peng et al. 2017a).
The Pearl River was one of the most polluted rivers in the 1990s (Tan et al. 1996). Beginning in the early twenty-first-century, the government established effective interventions to reduce the emissions of pollutants to improve water quality. An annual sports event, “Swimming across the Pearl River” has also been held in the summer since 2006, to show to the public that the once-contaminated river has been cleaned (Yang et al. 2007). However, the Pearl River watershed has still been suffering from pollution in recent years (Lai et al. 2019; Peng et al. 2017a, b; Pintado-Herrera et al. 2017; Zhao et al. 2019). In order to reduce the risk of pollutant exposure to swimmers during the event, the local government of Guangzhou restricted direct sewage discharge upstream, as well as effluent discharged from urban channels to the Pearl River at least 2 months prior to the event. However, the impact of such discharge reductions on the occurrences of CECs in the Pearl River remains unknown.
CECs include a broad list of synthetic substances in global use, such as flame retardants and plasticizers, endocrine-disrupting compounds (estrogens and steroid hormones), and pharmaceuticals and antibacterial agents, which are transported to the environment by human activities (Lai et al. 2019; Stuart et al. 2012). Such chemicals have not previously been considered significant in terms of their occurrences and distributions in the environment. For example, the US EPA has derived statutory guideline values for about 125 contaminants in drinking water; no CECs are on the list (US EPA, 2010). However, they are now frequently detected and may have potentially negative consequences on wild animals and humans, even at low levels (Li et al. 2019; Stuart et al. 2012). Three type of CECs, including organophosphorus flame retardants (OPFRs), bisphenol analogues (BPs), and triclosan (TCS), are widely used in the PRD regions and have attracted increasing concern among researchers and local government (Coogan et al. 2007; Jin and Zhu 2016; Liu et al. 2016; Van der Veen and de Boer 2012). Exposure to OPFRs (triphenyl phosphate, TPhP) retarded ovarian development and significantly reduced egg production in Japanese Medaka (Oryzias latipes), even at environmentally relevant concentrations, and may also have population-level impacts on wild fish (Li et al. 2019). Some widely used alternatives of bisphenol A (BPA), such as bisphenol S (BPS), bisphenol AF (BPAF), bisphenol F (BPF), and bisphenol P (BPP), have also been shown to display estrogenic and antiestrogenic activities similar to or greater than that of BPA (Chen et al. 2016). TCS is a synthetic broad-spectrum antibacterial agent commonly used in personal care products and has also raised concerns recently regarding its potential for endocrine disruption (Fan et al. 2019; Zhao et al. 2010). Although CECs were detected at relatively low concentrations, ranging from pg/L to ng/L in aquatic ecosystems, such chemicals may still be toxic and produce potential adverse effects on ecosystems and human health (Jurado et al. 2012).
As compared with traditional organic pollutions, such as persistent organic pollutants and pesticides, investigations of the sources, occurrences, and fates of CECs are limited and research is scarce (Kim et al. 2017; Zhao et al. 2019). For example, 14 types of OPFRs and plasticizers were studied in surface water samples obtained from the Pearl River in 2013, with concentrations ranging from 15 to 1790 ng/L. The high frequency and concentration of tris (2-chloroethyl) phosphate (TCEP) detected along the coast of China may potentially cause adverse effects to aquatic organisms (Lai et al. 2019). Eight bisphenol analogues were detected in aqueous samples, suspended particulate matter (SPM) samples, and organisms in the Pearl River Estuary in 2017; the results indicated that the water pollution of BPs in the Pearl River Estuary’s coast may be worse than that in Europe and the Antarctic (Zhao et al. 2019). Moreover, two antimicrobial agents were investigated in the Pearl River system in 2007–2008, one of which, TCS, posed median risks in the Pearl River and Liuxi Rivers, and high risks in the Shijing River (Zhao et al. 2010). The rapid urbanization and intensified human activities of the PRD region are the main causes of these anthropogenic contaminants being released into aquatic environment (Peng et al. 2017a, b; Gong et al. 2019), and studies indicated that sewage treatment systems cannot completely remove these emerging pollutants (Garcia-Rodríguez et al. 2014; Kim et al. 2017; Schreder and La Guardia 2014). Although a series of measures have been implemented to control discharge, for example, constructing of sewage treatment stations and sewage pipe networks to improve water quality, there is still a lack of quantitative data to accurately assess the effects of such control measures on the occurrences and fates of CECs (Li et al. 2017).
To understand the impact of anthropogenic activities on the occurrences and fates of CECs in the Pearl River, and to reveal the effects of discharge reduction measures on water quality, especially on CECs levels, the concentrations of CECs and water quality indices (WQIs) were measured in water obtained from the Guangzhou reaches of the Pearl River. Samples were collected before and after the implementation of discharge reduction measures in preparation of the “Swimming across the Pearl River” event. The partitioning of CECs between aqueous and SPM samples was also investigated to reveal the adsorptive partitioning characteristics of pollutants. The discharge control measures implemented during the “Swimming across the Pearl River” event offered an invaluable opportunity to investigate how the water quality of the Pearl River was influenced by sewage effluents from urban channels. These results will provide useful lessons for urban water management departments. To our knowledge, this is the first report on the influence of discharge reductions on the occurrences and fates of CECs in urban rivers that flow across megacities.
Materials and methods
Chemicals and reagents
In the present study, sixteen OPFR standards, including tris(1,3-dichloro-2 propyl)phosphate (TDCP), tris(2-chloroethyl) phosphate (TCEP), 2-ethylhexyl diphenyl phosphate (EDP), tris(2-butoxyethyl) phosphate (TBEP), tributyl phosphate (TBP), tripropyl phosphate (TPrP), tri-p-cresyl phosphate (p-TCP), tri-m-cresyl phosphate (m-TCP), tri-o-cresyl phosphate (o-TCP), tris(2-isopropylphenyl) phosphate (TiPPP), triphenyl phosphate (TPhP), TCPP, bisphenol A bis(diphenyl phosphate) (BPADP), triethylphosphate (TEPP), tris(1-Chloro-2-propyl) phosphate (TiCPP), and tris(2,3-dibromopropyl)phosphate (TDBPP), were purchased from AccuStandard (New Haven, USA). The standards of eight bisphenol analogues, including BPA, BPAF, BPS, BPF, BPP, bisphenol AP (BPAP), bisphenol Z (BPZ), and tetrabromobisphenol A (TBBPA), were also obtained from AccuStandard (New Haven, USA). TCS was obtained from Dr. Ehrenstorfer (Augsburg, Germany). Isotopically labeled standards d12-TCEP, d15-TEPP, d15-TPhP, d27-PATE (phosphoric acid tributyl), and 13C12-BPA were manufactured by AccuStandard (New Haven, USA). d3-TCS was supplied by CDN Isotopes Inc. (Quebec, Canada). All other organic solvents were HPLC-grade or higher, and were purchased from ANPEL (Shanghai, China).
Discharge reduction details
As reported by Peng et al. (2017b), the production of domestic wastewater in PRD region was 7 billion m3 per year, with about 10% of the domestic wastewater not treated before discharge. The direct discharge of the untreated domestic sewage via pipelines into a water body might cause pollution of the Pearl River, which would further pose potential health risks because of exposure to pollutants during swimming activities. The “Swimming across the Pearl River” event is held every July, which is the rainiest period of Guangzhou. Therefore, the contribution of the urban surface runoffs to the pollutants in the Pearl River water cannot be ignored. To reduce the concentrations of pollutants in the water body and to lower the risk of exposure for swimmers, the local government has taken several discharge reduction measures. For example, all of the channels within 10-km upstream of the swimming area of Pearl River were blocked to prevent domestic sewage discharge 2 months before the event. These domestic sewages would be piped into two nearby large wastewater treatment plants (WWTP), Liede WWTP and Datansha WWTP, for their final treatment. The effluent from the WWTP should reach the class I standard for pollutant discharge of China (GB18918-2002, Standards for Urban Sewage Treatment Plants). However, the wastewater can still directly go into channels by run-off and secretly discharge wastewater.
Sample collection
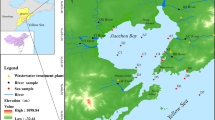
Surface water samples were collected from the Guangzhou reaches of the Pearl River at 9:00 to 10:00 am on May 27, June 20, July 2, and July 9, 2019. The swimming event was held on July 18 of the same year. The first sampling time was before discharge reduction activities occurred. In addition, prior to the last sampling, better-quality Beijiang water was introduced upstream to dilute the concentrations of pollutants in the Pearl River. The sampling sites (Z1–Z10) in our study are shown in Fig. 1. Surface water samples were collected using a stainless-steel bucket and then stored in 10-L wide-mouth amber glass bottles. After sampling, the samples were immediately transported to our laboratory. All samples were filtered through pre-cleaned glass fiber filters (GF/F, 0.7-μm pore size, baked at 450 °C for 4 h before use; Whatman, UK) to separate the SPM. The filters holding the SPM samples were frozen at − 20 °C until analysis. The filtrates were stored at 4 °C prior for further treatment, which was accomplished within 48 h of sampling.
Sample treatment protocols
Two liters of each water sample was divided into two sub-samples. One part was used to determine phenolic chemicals, such as BPs and TCS, and the remainder was used for the determination of OPFRs. For the phenolic chemicals, filtered water samples (1 L) and blank samples were spiked with the surrogate standards (13C12-BPA, d3-TCS), and loaded onto Oasis HLB cartridges (500 mg, 6-mL volume, Waters, USA) that were pre-conditioned sequentially with 5 mL of ethyl acetate (ETAC), 5 mL of methanol (MeOH), and 10 mL of Milli-Q water, at a flow rate of approximately 10 mL/min. Subsequently, cartridges were washed with 10 mL of MeOH/H2O (10:90, v/v) to remove interferences and then dried by a vacuum pump. The analytes were eluted with 10 mL of methanol/dichloromethane (MeOH:DCM, 50:50, v/v). The extracts were evaporated to dryness under a gentle stream of nitrogen, and then redissolved in 200 μL of methanol for instrumental analysis. For OPFRs, filtered water samples (1 L) and blanks were spiked with the surrogate standards (d27-PATE, d15-TEPP, d15-TCEP, and d12-TPhP), and a similar pretreatment to that used for phenolic chemicals was used; the only differences were that the target compounds were eluted with 10-mL ETAC/DCM (50:50, v/v) and without the wash step using 10 mL of MeOH/H2O (10:90, v/v).
For the SPM samples, after samples were lyophilized for 24 h and spiked with surrogate standards, they underwent ultrasonic extraction with 30 mL of MeOH:DCM (50:50, v/v) three times. The extracts were then combined and rotary evaporated to 1 mL, subsequently purified using Oasis HLB cartridges (500 mg, 6 mL, Waters, USA) as described above without washing, and samples were not undergoing dilution before passing through the cartridges. The targets, including phenolic chemicals and OPFRs, were eluted with 10 mL of MeOH/DCM (50:50, v/v), evaporated to dryness, and reconstituted with 200 μL, as described above. It is noted that SPM samples were analyzed twice with different analysis methods for phenolic chemicals and OPFRs.
Determination of dissolve organic carbon and WQIs
The contents of dissolved organic carbon (DOC) in surface water were determined using a TOC-VCPH analyzer (Shimadzu, Japan). After acidification with diluted hydrochloric acid solution to pH = 3, 50 mL of filtrate was analyzed.
Two parameters of WQIs, ammonia-nitrogen (NH3-N) and chemical oxygen demand (COD), were also measured according to the National Standards of Surface Water Testing Standards GB7481-87 and GB11914-89, respectively.
Instrumental analysis
The qualitative and quantitative analyses of CECs were performed using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) with a 1260 Infinity LC (Agilent Technologies, USA) coupled to a 6470 Triple Quad LC-MS/MS system (Agilent Technologies, USA). A C18 column (Poroshell 120 EC-C18, 100 mm × 4.6 mm, 2.7 μm; Agilent Technologies, USA) was used for the separation of analytes. The details of the HPLC conditions and MS/MS parameters are provided in the Supplemental Information.
Quality assurance and quality control
One procedural blank and one spiked sample were included with each batch of 12 field samples. The recoveries of CECs in the spiked samples ranged from 61.9 to 120%. The limits of detection (LOD) and quantification (LOQ) were defined as threefold and tenfold of the signal-to-noise ratio, respectively. Detailed recoveries, LOD, and LOQ are provided in Table S2.
Calculations and statistical analysis
To describe the distribution of CECs between aqueous and SPM samples, distribution coefficients (Kd) were calculated using following equation (Wang et al. 2018):
where Cs and Ca are the concentrations of target compounds in SPM samples (ng/g dw) and aqueous samples (ng/L), respectively.
Principal component analysis (PCA) was used for source apportionment to identify the possible sources. Correlation analyses were calculated to evaluate correlations between the Kd of individual CECs and KOW. A p value less than 0.05 was considered statistically significant.
Results and discussion
Occurrences and distributions of the CECs in aqueous samples
Among 16 OPFRs analyzed, BPADP, TiPPP, and TDBPP were below the detection limit, while the other congeners were all detected in aqueous samples (Table 1). The sum of the concentrations of OPFRs in the aqueous phase ranged from 21.2 to 91.0 ng/L, with a mean value of 41.0 ng/L. TBP (mean, 9.21 ng/L) was the dominant compound, followed by TCEP (8.72 ng/L), TBEP (6.22 ng/L), and TCPP (3.90 ng/L). OPFRs, as alternatives of brominated flame retardants, have been widely applied in textile, plastics, and many other products (Wang et al. 2018). Their large consumption has caused the elevated levels of OPFRs in the environment media, and it has been frequently detected in aquatic/sediment systems (Van der Veen and de Boer 2012). Specifically, TBP has an annual production of approximately 3000–5000 tons (Nancharaiah et al. 2015). TCEP annual production on a global scale is about 4000 tons (Kristin and Wilhelm 2009) and TBEP has a global production of about 5000–6000 tons per year (Ma et al. 2016). The high concentrations of the chemicals found in the Pearl River may be due to the large consumption of commercial products in the PRD regions. As a comparison, the present results were much lower than those from the Bohai Sea (9.6–1549 ng/L) (Wang et al. 2015b), Taihu Lake (166–1530 ng/L) (Wang et al. 2018), and Pearl River Estuary (14.9–1790 ng/L) (Lai et al. 2019) (Table S4). Moreover, the levels found in the present study were lower than those found in coastal and marine surface waters from Germany (Bollmann et al. 2012) and river water from Spain (Cristale et al. 2013). However, pollutant levels in this study were higher than those in river water from Sweden (Gustavsson et al. 2019). Nevertheless, the contamination of OPFRs in aqueous samples from the Guangzhou reaches of the Pearl River is at relatively low levels.
The total concentrations of BPs ranged from 8.46 to 37.3 ng/L, with a mean value of 19.5 ng/L. Among eight BPs measured, only BPA, BPF, BPS, and TBBPA were detected in all of the aqueous phase samples, with concentration ranges of 2.57–30.1, 1.66–7.48, 0.50–2.47, and 0.26–0.77 ng/L, respectively, with mean values of 13.2, 4.65, 1.28, and 0.34 ng/L, respectively. BPA accounted for 69.0% of the total concentrations of BPs. The dominance of BPA in BPs indicated that BPA is still widely used in the PRD region. Many studies reported the occurrence of BPs in the surface water around the world (Table S5); however, the range of BPA in the present investigation was lower than those previously reported from the Pearl River Estuary (9.5–173 ng/L), Pearl River (n.d.–98.0 ng/L, n.d.—not detected), and West River (n.d.–43.0 ng/L). The observed difference might be attributed to the different sampling locations, different river water flow rates, and seasonal variations in this study compared with others (Liu et al. 2017). The range of BPA concentrations in the Pearl River of the present study was relatively higher than that from Taihu Lake (4.2–14.0 ng/L) reported by Jin and Zhu (2016), but lower than the concentrations of other studies from Taihu Lake (19.0–560 ng/L) (Liu et al. 2017; Yan et al. 2017). Nevertheless, the concentration of BPF (2.1–7.5 ng/L) was higher than that from Taihu Lake (n.d.–5.6 ng/L), while the concentration of BPS (0.5–6.9 ng/L) was lower than that of Taihu Lake (0.28–67.0 ng/L) in the study of Jin and Zhu (2016). The different compositions of BPs between the Pearl River and Taihu Lake suggest that different BPA alternatives might be used in these two regions, and the releasing from various sources and with different fluxes would also affect the composition characteristics of target pollutants. Compared with other countries such as Japan, Korea, and India (Table S5), the concentrations of BPs from the Guangzhou reaches of the Pearl River were relatively lower. In addition, the composition profiles varied substantially, which may be due to the different types of commercial BP products used among the different areas, and that need further studies to confirm.
For TBBPA, the water samples in the present study had higher concentrations than the Beijiang River (0.020–0.270 ng/L) (Xiong et al. 2016), slightly lower than those found in Taihu Lake (n.d.–1.12 ng/L), and much lower than those of the Xiaoqing River (6.0–113 ng/L), Qinghe River (23.9–224 ng/L), and Chaohu Lake (850–4870 ng/L) (Lan et al. 2019; Yang et al. 2012; Yin et al. 2011). The results are consistent with previous studies that found higher TBBPA levels in northern China than southern China. Compared with other countries (Table S6), relatively lower levels of TBBPA were reported in the Prédecelle River in France (< 0.035–0.068 ng/L) (Labadie et al. 2010) and lakes in England (< 0.14–3.2 ng/L) (Harrad et al. 2009). However, much higher levels were found in several rivers and lakes in Poland (260–490 ng/L) (Kowalski and Mazur 2014).
TCS was also detected in all water samples in this study. The concentrations of TCS ranged from 1.47 to 5.62 ng/L with a mean of 2.85 ng/L. The concentrations of TCS in the present study was much lower than those in the Yangzte River, Hai River, Yellow River, and Dongjiang River, China, where the range of concentrations was n.d. − 65.6, n.d. − 34.4, n.d. − 64.7, and <LOQ − 170 ng/L, respectively (Ma et al. 2018; Zhao et al. 2013). Also, the level of TCS in the present study was much lower than those found in the Pearl River (1.51–478 ng/L, 2007–2009) (Zhao et al. 2013) and urban rivers in Guangzhou City (35–1023 ng/L, 2005–2006) (Peng et al. 2008), which might indicate that the level of TCS in the Pearl River is gradually decreasing with the globally phasing out of triclosan since 2014 (Dhillon et al. 2015). The decreasing trends in concentrations of TCS were also observed in sediment from the Pearl River Estuary, with concentration range of TCS found from <LOQ to 1329 ng/g in 2010, then was dropped dramatically in 2017, which maximum concentration was 41.7 ng/g (Peng et al. 2017b; Zhao et al. 2010). The occurrences of TCS in other countries are shown in Table S7. Specifically, TCS levels found in the present study were higher than those found in the Ebro River in Spain (n.d.–2.0 ng/L) (Gorga et al. 2013), similar to those in several rivers in the USA (3.5–5.3 ng/L) (Kumar et al. 2010), and lower than those from rivers in Australia (< 3–75 ng/L) (Ying and Kookana 2007) and Japan (n.d.–134 ng/L) (Nishi et al. 2008). Overall, the contamination of TCS in the Pearl River was at a moderate level, compared with other regions.
Occurrence and distribution of CECs in SPM
SPM is ubiquitously found in aquatic environments, and it is an important matrix for the absorption of CECs due to the affinity of CECs in SPM (Zhao et al. 2019). The concentrations of target compounds in SPM samples from the Pearl River are shown in Table 1. Among 16 OPFRs analyzed, TDCP, TPrP, TiPPP, and TDBPP were below the detection limit. The detection frequencies of TEPP, TCPP, and TiCPP were 62.5%, 87.5%, and 85.0%, respectively. The remaining compounds were detected in all SPM samples. BPADP was found in all SPM samples; however, it was below the limit of detection in aqueous samples. This finding may be attributed to the large molecular weight and the many hydrophobic benzene rings resulting in BPADP being hydrophobic, thus leading to its low solubility in water. The logKOW of BPADP (8.29) was also much higher than that of other OPFR isomers (Bergman et al. 2012) (Table S8). The cumulative concentrations of the 12 OPFRs detected in SPM samples ranged from 25.2 to 492 ng/g, with an average of 133.4 ng/g. Similar to aqueous phase samples, TBP (mean: 86.7 ng/g) was predominant in the SPM samples, followed by BPADP, TCEP, and TCPP, with means of 17.7, 13.1, and 4.91 ng/g, respectively.
The available data on the occurrences of OPFRs in SPM was limited prior to this study. As reported by Gustavsson et al. (2019), the concentrations of particulates in surface water from the Krycklan Catchment Study area ranged from the LOD of the analytical method to 3.8 ng/L. Additionally, Zha et al. (2018) reported the concentrations of suspended sediments in the Yangtze River ranged from 26.4 to 29.0 ng/g. Those two reports found lower levels of OPFRs in water than the present study. However, much higher levels of OPFRs (394–2343 ng/g dw) were reported from WWTP (Kim et al. 2017; Liang and Liu 2016). Considering the limited data on OPFRs in SPM, additional studies are needed.
The cumulative concentrations of BPs in SPM ranged from 14.0 to 86.3 ng/g, with a mean of 32.1 ng/g. Similar to aqueous phase samples, only BPA, BPF, BPS, and TBBPA were detected in all SPM samples (Table 1). Their concentrations ranged from 5.72 to 26.1, 6.44 to 49.5, 0.19 to 1.48, and 1.52 to 9.24 ng/g, respectively, with a mean of 14.6, 16.9, 0.55, and 4.47 ng/g, respectively. Compared with data from the Pearl River Estuary (69.4–157 ng/g) (Zhao et al. 2019), the BPA concentrations in SPM samples in the current study were lower (Table S5). BPA concentrations in SPM samples in this study were also lower than those found in the Yangtze River (n.d.–364 ng/g) (Liu et al. 2016) and Taihu Lake (n.d. to 2682 ng/g) (Zhang et al. 2014). BPA concentrations in SPM samples in the current study were also lower than those found in the Thermaikos Gulf where the concentrations of BPA in SPM ranged from 26 to 160 ng/g (Arditsoglou and Voutsa 2012). Unfortunately, although the concentrations of BPF were higher than those of BPA in the present study, the data for other bisphenol analogues in SPM are limited in the literature. In a study of the Pearl River Estuary by Zhao et al. (2019), extremely high levels of BPs were observed (868–10,800 ng/g). Comparatively, much lower concentrations of BPs ranging from 47.5 to 353 ng/g in SPM samples were observed by Zheng et al. (2019) in the Yangtze River and an urban river in Nanjing. To our knowledge, with the exception of TBBPA, there have been only two reports on the concentrations of other BPs in SPM (Zhao et al. 2019; Zheng et al. 2019).
Compared with aqueous phase samples, SPM samples contained higher concentrations of TBBPA, which was attributed to the high logKOW (7.20) and strong capacity to bind particulate matter (Liu et al. 2016). Compared with data from other regions (Table S6), the concentrations of TBBPA in SPM in the current study were consistent with those found in a previous study around the Dongjiang River (n.d.–1.6 ng/g) (He et al. 2013). However, much higher TBBPA levels in the range of n.d.–892 ng/g were observed by Liu et al. (2016) in SPM samples from Taihu Lake. Thus, TBBPA levels in the Pearl River were moderate compared with several rivers in Europe (<LOD–9.44) (Kotthoff et al. 2017).
TCS was found in all SPM samples, and ranged from 0.69 to 17.5 ng/g, with an average of 6.35 ng/g. Such concentrations were much lower than those found in a previous study in the Pearl River, in which TCS levels ranged from n.d. to 1162 ng/g (Fan et al. 2019). TCS levels in this study were also lower than those found in the Yellow River, Dongjiang River, Hai River, and Liao River, which ranged from <LOQ to 10.3, from n.d. to 297, from <LOQ to 3.9, and from <LOQ to 46.7 ng/L, respectively (Zhao et al. 2013). Overall, the TCS contamination in SPM samples from the Pearl River identified in this study was relatively lower than other water sources globally.
Partitioning of CECs between water and SPM
To investigate the distribution of the target compounds between aqueous and SPM phases, and the possible mechanisms for such distributions, the logKd values of the chemicals were calculated and are shown in Table S8. The average logKd values of the detected OPFRs ranged from 2.44 to 3.72, while they were 1.65 to 4.11 for BPs, and 3.33 for TCS. As shown in Fig. 2, the calculated logKd displayed a significant correlation with logKOW (R2 = 0.648, p < 0.05). The results demonstrated that the partitioning of CECs between aqueous and SPM phases was strongly influenced by hydrophobic interactions, and the absorption into the SPM phase by this interaction is one of the most important fates of CECs in water (Wang et al. 2018). Among the six alkyl-OPFRs and five aryl-OPFRs detected, the average logKd value of aryl-OPFRs (3.48) was higher than that of alkyl-OPFRs (2.56). Subtle non-covalent forces such as π-π interactions between aromatic rings of aryl-OPFRs and organic carbon in SPM might have played an import role in the field investigation (Gong et al. 2016). The data we obtained also follow the suggested theory that π-π interactions contribute to the sorption of CECs in SPM.
The results indicated that substituent groups might have important effects on Kd value. For example, for alkyl-OPFRs, we found that the logKd of the straight-chain compound, TCPP (2.70), was higher than that of the branched isomer, TiCPP (2.58) (Table S8), which indicated that the structures of the chemicals also influence their distribution. Similar results were also observed by Wang et al. (2018) who found that the logKOC (KOC was Kd corrected by organic carbon) of TNBP was higher than that of the corresponding branched isomer, TIBP. Furthermore, the logKd of brominated compounds was higher than that of the corresponding non-brominated compounds. For example, the logKd of 4.11 for TBBPA was higher than that of 3.07 for BPA (Table S8). In addition, the partition is a dynamic process in the field. Other factors, such as colloids, particulate minerals, and ion exchange, among others, may also affect the distribution of CECs (Gong et al. 2019).
Principal component analysis and source implications
PCA was performed to investigate the possible sources of CECs in the Pearl River. The Kaiser-Meyer-Olkin (KMO) test conducted shows that the score for these CECs in aqueous-phase was lower than 0.6, indicating that data is not suited for factor analysis (Karpuzcu et al. 2014). Thus, the PCA was performed only using the concentrations data of CECs in the SPM phase; a score plot is also shown in Fig. 3. The first principal component (PC1) explained 30.1% of the total variance with high loadings of TCPP (0.961), TiCPP (0.961), TCEP (0.779), EDP (0.745), TPhP (0.735), and TBEP (0.663). TiCPP is mainly used in flexible polyurethane and in PVC wallpaper (Yadav et al. 2018). TBEP is applied as an additive in synthetic rubber and a leveling agent in floor polish (Anneli et al. 2003). TCEP is a typical plasticizer and flame retardant in polyurethane foams, and EDP is the main componnent of food packaging and paints (Brommer 2014; Van der Veen and de Boer 2012). Most of these chemical correlations were probably due to the application of flame retardants and plasticizers. Thus, PC1 indicated that these OPFRs were widespread in the Pearl River, resulting from release, abrasion, and dissolution from consumer materials, which contain flame retardants and plasticizers. The second principal component (PC2) explained 21.4% of total variance with high loadings of p-TCP (0.984), m-TCP (0.979), and o-TCP (0.976). High loading on TCP indicated they were widespread in the Pearl River. These chemical has been widely used in hydraulic fluids and lubricants (Van der Veen and de Boer 2012). Therefore, PC2 may suggest a contribution of manufacturing. The third principal component (PC3) explained 20.1% of total variance with high loadings of BPS (0.841), TCS (0.839), BPF (0.808), and BPA (0.539). BPA and its alternatives, BPS, BPF, are widely used as plasticizer in our daily consumer products, including polyvinyl chloride, food packaging, and thermal receipts, among other items (Chen et al. 2016). TCS is one of the antimicrobial agents primarily used in personal care products, such as disinfecting handsoaps, toothpastes, and body washes (Zhao et al. 2010). These chemicals were substituent of product for household use. Consequently, PC3 may reflect the sources of domestic sewage discharged into the Pearl River. The three major components accounted for 71.6% of the total variance of CEC concentrations in the SPM samples and suggested that CECs enter the Pearl River through multiple pathways.
Impact of discharge reduction activities on the Pearl River
During the discharge reduction period, sample collection was performed a total of four times. The first collection occurred prior to discharge reduction activities on May 27, 2019. The following three samplings occurred between June 20 and July 9, 2019, after discharge reduction occurred. The variations in the concentrations of CECs and WQIs before and after the occurrence of discharge reduction activities are shown in Fig. 4 and Fig. S1, respectively. During this campaign, we did not observe significant changes of CECs in the aqueous phase, SPM phase, or in the total bulk concentration, which included the aqueous and SPM phases combined. The reason still remains unclear why there was no observable change in CECs, but it may be due to the similar levels of dissolved organic carbon during sampling time interval (Fig. S1). A possible major component could be humic substances (for example, fulvic acid), which are hydrophilic; thus, humic substances might have served as carriers for CECs in the river. The interaction of CECs with various functional groups from DOC might influence its final adsorption and desorption behaviors (Katagi 2006). In addition, the release of CECs from the bottom sediment might have also contributed to the water concentrations of CECs, as relatively higher concentrations of CECs were reported in sediments from the Pearl River (Gong et al. 2011; Pintado-Herrera et al. 2017). However, it was not within the scope of the study to collect the sediment samples in tandem. Therefore, it warrants future studies on the effects of the sediment on the CEC concentration in water and SPM in the Pearl River.
However, NH3-N levels in the water were significantly decreased. The concentrations of NH3-N decreased from 5.57 mg/L in the first sampling time to 0.59, 2.76, and 1.03 mg/L of the followed three times after discharge reduction restriction was put in place, and they reduced by 89.5%, 50.5%, and 81.5%, respectively. Thus, those data indicated that urban channels might be the predominant sources of NH3-N pollution in the Pearl River. Given that less industrial and agricultural activities occur around urban channels, the pollution of NH3-N may be due to the domestic sewage of nearby residents. Similar to CECs, COD and DOC did not show large changes during the four sampling times, which may indicate that their presence was from other sources, and urban channels are not the main sources of COD and DOC pollution in the Pearl River. Therefore, the primary pollution source of COD and DOC could be a non-point source, such as urban run-off (Yan et al. 2019). It is notable that obvious decreases of CECs in SPM samples were observed from the fourth sampling. The transfer of water from the Beijiang River to the Pearl River on July 8 may have influenced the concentration of CECs. However, additional evidence is required to confirm this hypothesis. Nevertheless, the present study demonstrated that restricting the discharge of urban channels into the Pearl River from Guangzhou reaches exhibited a limited effect on water quality. Moreover, the findings of this study also suggest that urban channels are not the main sources of CEC pollution in the Pearl River. To further improve water quality, and in particular, to reduce the levels of CECs in the Pearl River, additional control measures should be implemented.
Conclusions
To understand the distribution of CECs between aqueous and SPM samples, and to investigate the influence of discharge reduction on CECs levels in the Pearl River, the present study analyzed the concentrations of CECs in aqueous and SPM samples from the Pearl River before and after the discharge reduction actions. OPFRs, BPs, and TCS were all detected in both aqueous and SPM samples. Hydrophobic and π-π interactions might be correlated to the partitioning of CECs between aqueous and SPM phases. Restricting the discharge of urban channels has a relatively small impact on the concentration of organic pollutants in the Pearl River, especially for CECs, although it has a substantial impact on NH3-N. Water transfer may be an alternative approach to decreasing the levels of pollution, although additional studies are required to confirm the utility of such an approach. This investigation of discharge reduction during the “Swimming across the Pearl River” event can provide policy makers more science-based evidence about the effectiveness of control measures and assist such individuals in making science-based decisions for the next event. Due to the limitation of the sample size and the sampling period, further research is needed to investigate the impact of discharge reduction in a longer time scale using paired water and sediment samples. Nevertheless, according to the present results, new controls should be implemented to further improve the water quality, with additional discharge control measures for other areas and other entryways, such as industrial and domestic wastewater, should be considered.
References
Anneli M, Barbro A, Haglund P (2003) Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53(9):1137–1146
Arditsoglou A, Voutsa D (2012) Occurrence and partitioning of endocrine-disrupting compounds in the marine environment of Thermaikos Gulf, Northern Aegean Sea. Greece Mar Pollut Bull 64(11):2443–2452
Bergman Å, Rydén A, Law RJ, de Boer J, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, den Eede NV, Ven der Veen I (2012) A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ Int 49:57–82
Bollmann UE, Möller A, Xie ZY, Ebinghaus R, Einax JW (2012) Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters. Water Res 46(2):531–538
Brommer S (2014) Characterizing human exposure to organophosphate ester flame retardants. A PhD Thesis Submitted to Birmingham Division of Environmental Health and Risk Management College of Life and Environmental Sciences. School of Geography, Earth and Environmental Sciences, The University of Birmingham, Edgbaston, B15 2TT,United Kingdom
Chen D, Kannan K, Tan H, Zheng Z, Feng YL, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity-a review. Environ Sci Technol 50(11):5438–5453
Coogan MA, Edziyie RE, La Point TW, Venables BJ (2007) Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67(10):1911–1918
Cristale J, García Vázquez A, Barata C, Lacorte S (2013) Priority and emerging flame retardants in rivers: occurrence in water and sediment, daphnia magna toxicity and risk assessment. Environ Int 59:232–243
Dhillon GS, Kaur S, Pulicharla R, Brar SK, Cledón M, Verma M, Surampalli RY (2015) Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health 12:5657–5684
Fan JJ, Wang S, Tang JP, Zhao JL, Wang L, Wang JX, Liu SL, Li F, Long SX, Yang Y (2019) Bioaccumulation of endocrine disrupting compounds in fish with different feeding habits along the largest subtropical river, China. Environ Pollut 247:999–1008
Garcia-Rodríguez A, Matamoros V, Fontàs C, Salvadó V (2014) The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—a review. Environ Sci Pollut Res 21:11708–11728
Gong J, Duan DD, Yang Y, Ran Y, Chen DY (2016) Seasonal variation and partitioning of endocrine disrupting chemicals in waters and sediments of the Pearl River system, South China. Environ Pollut 219:735–741
Gong J, Ran Y, Chen DY, Yang Y (2011) Occurrence of endocrine-disrupting chemicals in riverine sediments from the Pearl River Delta, China. Mar Pollut Bull 63(5−12):556–563
Gong J, Ran Y, Zhang DN, Chen DY, Li HY, Huang YD (2019) Vertical profiles and distributions of aqueous endocrine-disrupting chemicals in different matrices from the Pearl River Delta and the influence of environmental factors. Environ Pollut 246:328–335
Gorga M, Petrovic M, Barceló D (2013) Multi-residue analytical method for the determination of endocrine disruptors and related compounds in river and waste water using dual column liquid chromatography switching system coupled to mass spectrometry. J Chromatogr A 1295:57–66
Gustavsson J, Wiberg K, Nguyen MA, Josefsson S, Laudon H, Ahrens L (2019) Seasonal trends of legacy and alternative flame retardants in river water in a boreal catchment. Sci Total Environ 692:1097–1105
Harrad S, Abdallah MAE, Rose NL, Turner SD, Davidson TA (2009) Current-use brominated flame betardants in water, sediment, and fish from English lakes. Environ Sci Technol 43(24):9077–9083
He MJ, Luo XJ, Yu LH, Wu JP, Chen SJ, Mai BX (2013) Diasteroisomer and enantiomer-specific profiles of hexabromocyclododecane and tetrabromobisphenol a in an aquatic environment in a highly industrialized area, South China: vertical profile, phase partition, and bioaccumulation. Environ Pollut 179:105–110
Jin HB, Zhu LY (2016) Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res 103:343–351
Jurado A, Vàzquez-Suñé E, Carrera J, de Alda M.L, Pujades E, Barceló D (2012) Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Sci Total Environ, 440:82–94
Karpuzcu ME, Fairbairn D, Arnold WA, Barber BL, Kaufenberg E, Koskinen WC, Novak PJ, Rice PJ, Swackhamer DL (2014) Identifying sources of emerging organic contaminants in a mixed use watershed using principal components analysis. Environ Sci: Processes Impacts 16:2390
Katagi T (2006) Behavior of pesticides in water-sediment systems. Rev Environ Contam Toxicol 187:133–125
Kim UJ, Oh JK, Kannan K (2017) Occurrence, removal, and environmental emission of organophosphate flame retardants/plasticizers in a wastewater treatment plant in New York State. Environ Sci Technol 51(14):7872–7880
Kotthoff M, Rüdel H, Jürling H (2017) Detection of tetrabromobisphenol A and its mono- and dimethyl derivatives in fish, sediment and suspended particulate matter from European freshwaters and estuaries. Anal Bioanal Chem 409(14):3685–3694
Kowalski B, Mazur M (2014) The simultaneous determination of six flame retardants in water samples using SPE pre-concentration and UHPLC-UV method. Water Air Soil Pollut 225(3):1866
Kristin Q, Wilhelm P (2009) Temporal concentration changes of DEET, TCEP, terbutryn, and nonylphenols in freshwater streams of Hesse, Germany: possible influence of mandatory regulations and voluntary environmental agreements. Environ Sci Pollut Res 16:630–640
Kumar KS, Priya SM, Peck AM, Sajwan KS (2010) Mass loadings of triclosan and triclocarbon from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Arch Environ Contam Toxicol 58(2):275–285
Labadie P, Tlili K, Alliot F, Bourges C, Desportes A, Chevreuil M (2010) Development of analytical procedures for trace-level determination of polybrominated diphenyl ethers and tetrabromobisphenol a in river water and sediment. Anal Bioanal Chem 396(2):865–875
Lai NLS, Kwok KY, Wang XH, Yamashita N, Liu GJ, Leung KMY, Lam PKS, Lam JCW (2019) Assessment of organophosphorus flame retardants and plasticizers in aquatic environments of China (Pearl River Delta, South China Sea, Yellow River Estuary) and Japan (Tokyo Bay). J Hazard Mater 371:288–294
Lan J, Shen Z, Gao W, Liu A (2019) Occurrence of bisphenol-A and its brominated derivatives in tributary and estuary of Xiaoqing River adjacent to Bohai Sea, China. Mar Pollut Bull 149:110551
Li JF, Yang LS, Zhou WZ, Wang CJ (2017) Case discussion of building sewage pipe into the utility tunnel in New City Zone. China Water & Wasterwater 33(24):44–47
Li Y, Chen R, He J, Ma H, Zhao F, Tao S, Liu J (2019) Triphenyl phosphate at environmental levels retarded ovary development and reduced egg production in Japanese Medaka (Oryzias latipes). Environ Sci Technol 53(24):14709–14715
Liang K, Liu JF (2016) Understanding the distribution, degradation and fate of organophosphate esters in an advanced municipal sewage treatment plant based on mass flow and mass balance analysis. Sci Total Environ 544:262–270
Liu D, Liu JN, Guo M, Xu HZ, Zhang SH, Shi LL, Yao C (2016) Occurrence, distribution, and risk assessment of alkylphenols, bisphenol A, and tetrabromobisphenol A in surface water, suspended particulate matter, and sediment in taihu lake and its tributaries. Mar Pollut Bull 112(1−2):142−150
Liu YH, Zhang SH, Ji GX, Wu SM, Guo RX, Cheng J, Yan ZY, Chen JQ (2017) Occurrence, distribution and risk assessment of suspected endocrine-disrupting chemicals in surface water and suspended particulate matter of Yangtze River (Nanjing section). Ecotoxicol Environ Saf 135:90–97
Ma XQ, Wan YJ, Wu MY, Xu Y, Xu Q, He ZY, Wei X (2018) Occurrence of benzophenones, parabens and triclosan in the Yangtze River of China, and the implications for human exposure. Chemosphere 213:517–525
Ma ZY, Tang S, Su GY, Miao YQ, Liu HL, Xie YW, Giesy JP, Saunders DMV, Hecker M, Yu HX (2016) Effects of tris (2-butoxyethyl) phosphate (TBOEP) on endocrine axes during development of early life stages of zebrafish (Danio rerio). Chemosphere 144:1920–1927
Nancharaiah YV, Kiran Kumar Reddy G, Krishna Mohan TV, Venugopalan VP (2015) Biodegradation of tributyl phosphate, an organosphate triester, by aerobic granular biofilms. J Hazard Mater 283:705–711
Nishi I, Kawakami T, Onodera S (2008) Monitoring of triclosan in the surface water of the Tone Canal, Japan. Bull Environ Contam Toxicol 80(2):163–166
Ouyang TP, Zhu ZY, Kuang YQ (2005) River water quality and pollution sources in the Pearl River Delta, China. J Environ Monit 7(7):664–669
Peng FJ, Pan CG, Zhang M, Zhang NS, Windfeld R, Salvito D, Selck H, Van den Brink PJ, Ying GG (2017a) Occurrence and ecological risk assessment of emerging organic chemicals in urban rivers: Guangzhou as a case study in China. Sci Total Environ 589:46–55
Peng XZ, Xiong SS, Ou WH, Wang ZF, Tan JH, Jin JB, Tang CM, Liu J, Fan YJ (2017b) Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J Hazard Mater 323:139–146
Peng XZ, Yu YY, Tang CM, Tan JH, Huang QX, Wang ZD (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397:158–166
Pintado-Herrera MG, Wang CC, Lu JT, Chang YP, Chen WF, Li XL, Lara-Martín PA (2017) Distribution, mass inventories, and ecological risk assessment of legacy and emerging contaminants in sediments from the Pearl River Estuary in China. J Hazard Mater 323:128–138
Schreder ED, La Guardia MJ (2014) Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environ. Sci Technol 48(19):11575–11583
Stuart M, Lapworth D, Crane E, Hart A (2012) Review of risk from potential emerging contaminants in UK groundwater. Sci Total Environ 416:1–21
Tan JP, Lin NF, Li SZ, Xianyu KY (1996) The influences of land-base pollution sources on the water quality of Pearl River Estuary. Pearl River 3:35–41
US EPA (2010) National primary drinking water regulations. United States Environmental Protection Agency
Van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88(10):1119–1153
Wang DG, Jiang P, Wang GL, Wang DS (2015a) Urban extent enhances extreme precipitation over the Pearl River Delta, China. Atmos Sci Lett 16(3):310–317
Wang RM, Tang JH, Xie ZY, Mi WY, Chen YJ, Wolschke H, Tian CG, Pan XH, Luo YM, Ebinghaus R (2015b) Occurrence and spatial distribution of organophosphate ester flame retardants and plasticizers in 40 rivers draining into the Bohai Sea, north China. Environ Pollut 198:172–178
Wang XL, Zhu LY, Zhong WJ, Yang LP (2018) Partition and source identification of organophosphate esters in the water and sediment of Taihu Lake, China. J Hazard Mater 360:43–50
Xiong JK, Li GY, An TC, Zhang CS, Wei CH (2016) Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang River, South China. Environ Pollut 219:596–603
Xu WH, Zhang G, Li XD, Zou SC, Li P, Hu ZH, Li J (2007) Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res 41(19):4526–4534
Yadav IC, Devi NL, Li J, Zhang G (2018) Organophosphate ester flame retardants in Nepalese soil: spatial distribution, source apportionment and air-soil exchange assessment. Chemosphere 190:114–123
Yan ZY, Liu YH, Yan K, Wu SM, Han ZH, Guo RX, Chen MH, Yang QL, Zhang SH, Chen JQ (2017) Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: Occurrence, distribution, source apportionment, and ecological and human health risk. Chemosphere 184:318−328
Yan RH, Li LL, Gao JF (2019) Framework for quantifying rural NPS pollution of a humid lowland catchment in Taihu Basin, Eastern China. Sci Total Environ 688:983–993
Yang SW, Wang SR, Wu FC, Yan ZG, Liu HL (2012) Tetrabromobisphenol A: tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ Sci Pollut Res Int 19(9):4090–4096
Yang YJ, Zhong Y, Fu XS, Liu CT, Li XH, Shi J (2007) Whether Guangzhou reach of Zhujiang River will be taken as a swimming area: an investigation of water quality of urban river surge and a certain section. J Environ Health 26(4):332–333
Yin JF, Meng ZH, Zhu YS, Song MY, Wang HL (2011) Dummy molecularly imprinted polymer for selective screening of trace bisphenols in river water. Anal Methods 3(1):173–180
Ying GG, Kookana RS (2007) Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ Int 33(2):199–205
Zeng XY, He LX, Cao SX, Ma ST, Yu ZQ, Gui HY, Sheng GY, Fu JM (2014) Occurrence and distribution of organophosphate flame retardants/plasticizers in wastewater treatment plant sludges from the Pearl River Delta, China. Environ Toxicol Chem 33(8):1720–1725
Zha DP, Li Y, Yang CM, Yao C (2018) Assessment of organophosphate flame retardants in surface water and sediment from a freshwater environment (Yangtze River, China). Environ Monit Assess 190(4):222
Zhang YZ, Meng W, Zhang Y (2014) Occurrence and partitioning of phenolic endocrine-disrupting chemicals (EDCs) between surface water and suspended particulate matter in the north Tai Lake Basin, eastern China. Bull Environ Contam Toxicol 92(2):148–153
Zhang WZ, Zheng XW, Gu P, Wang N, Lai ZN, He J, Zheng Z (2020) Distribution and risk assessment of phthalates in water and sediment of the Pearl River Delta. Environ Sci Pollut Res 27:12550–12565. https://doi.org/10.1007/s11356-019-06819-y
Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L (2010) Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: from source to the receiving environment. J Hazard Mater 179(1−3):215–217
Zhao JL, Zhang QQ, Chen F, Wang L, Ying GG, Liu YS, Yang B, Zhou LJ, Liu S, Su HC, Zhang RQ (2013) Evaluation of triclosan and triclocarban at river basin scale using monitoring and modeling tools: implications for controlling of urban domestic sewage discharge. Water Res 47(1):395–405
Zhao X, Qiu WH, Zheng Y, Xiong JZ, Gao CZ, Hu SY (2019) Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China. Ecotoxicol Environ Saf 180:43–52
Zheng CY, Liu JC, Ren JH, Shen J, Fan J, Xi RY, Chen W, Chen Q (2019) Occurrence, distribution and ecological risk of bisphenol analogues in the surface water from a water diversion project in Nanjing, China. Int J Environ Res Public Health 16(18):3296
Funding
This study was supported by the National Natural Science Foundation of China (41991310), the Local Innovative and Research Teams Project of the Guangdong Pearl River Talent Program (2017BT01Z032), and the Innovation Team Project of the Guangdong Provincial Department of Education (2017KCXTD012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing intersts.
Additional information
Editorial Responsibility: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 66 kb)
Rights and permissions
About this article
Cite this article
Chen, P., Zhong, Y., Chen, K. et al. The impact of discharge reduction activities on the occurrence of contaminants of emerging concern in surface water from the Pearl River. Environ Sci Pollut Res 27, 30378–30389 (2020). https://doi.org/10.1007/s11356-020-09295-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09295-x