Abstract
Nanoparticles (NPs) can be used in several ways in agriculture, including increasing production rates and improving nutritional values in plants. The present study aims to clarify how biogenic copper oxide nanoparticles (CuO NPs) applied by two routes of exposure (foliar spray and soil irrigation) affect the elemental uptake by lettuce. In vivo experiments using lettuce (n = 4) were performed with CuO NPs in comparison with copper salt (CuSO4), considering a final mass added of 20 mg of CuO per plant. The elemental composition of roots was mostly affected by the soil irrigation exposure for both Cu forms (NPs and salt). Neither Cu form added by soil irrigation was translocated to leaves. Copper concentration in leaves was mainly affected by foliar spray exposure for both Cu forms (NPs and salt). All Cu forms through foliar spray were sequestered in the leaves and no translocation to roots was observed. Foliar spray of CuO NPs caused no visual damage in leaves, resulted in less disturbance of elemental composition, and improved dry weight, number of leaves, CO2 assimilation, and the levels of K, Na, S, Ag, Cd, Cr, Cu, and Zn in leaves without causing significant changes in daily intake of most elements, except for Cu. Although Cu concentration increased in leaves by foliar spray of CuO NPs, it remained safe for consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, nanotechnology has received considerable attention as a feasible tool in many areas of knowledge, including agriculture (Gajjar et al. 2009; Wang et al. 2016). Previous studies indicated the potential use of metallic and mining reject nanoparticles (NPs) to increase agricultural production rates (Dalmora et al. 2016a, b; Ferrari et al. 2019; Gredilla et al. 2019; Plata et al. 2021), the efficiency of natural resources use, reduction in waste generation, development of fertilizers, nanopesticides, nanostructured materials for the treatment of agricultural waste and nanosensors (Adisa et al. 2019; Arruda et al. 2015; Kah et al. 2019; Khot et al. 2012; Rodrigues et al. 2017; White and Gardea-Torresdey 2018).
Copper-based NPs in particular have drawn considerable attention for agriculture purposes due to their antimicrobial properties (Hong et al. 2015; Servin et al. 2017). Moreover, Cu is one of the essential trace elements that play a significant role in the regulation of many physiological and biochemical processes in plants (Kaewchangwat et al. 2017; Singh et al. 2013). However, Cu deficiency can harm plant metabolism, resulting in low crop yield and physiological disturbance (Kaewchangwat et al. 2017; Singh et al. 2013). In addition, most Cu compounds are highly toxic (Zamberlan et al. 2020).
Inorganic NPs have been investigated as alternatives to alleviate the undesirable effects of the accumulation of some potentially toxic elements (PTEs); for example, Si alleviated the effects of Cr in peas (Tripathi et al. 2015), As in maize (Tripathi et al. 2016), Cd in rice (Rizwan et al. 2019), and Pb and Cd in sunflower (Mousavi et al. 2018). In addition, TiO2 NPs alleviated the effects of Cd in soybean (Singh and Lee 2016), Pb in rice (Cai et al. 2017), and Cd in rice (Rizwan et al. 2019). The application of NPs in the presence of these PTEs may also result in the reduction of some elements essential to plant nutrition such as Ca, N, P, Zn, Mn, Fe, S, K, and Na (Hayes et al. 2020; Hu et al. 2020). Therefore, studies have presented a great diversity of negative and positive effects after the application of inorganic synthetic and natural NPs (Abollino et al. 2007; Cortés et al. 2020; Oliveira et al. 2019; Ramos et al. 2020).
It is important to understand the application effects of NPs on the absorption of essential elements and PTEs in lettuce due to the high consumption of this vegetable worldwide (Hong et al. 2015). Lettuce is well known to accumulate PTEs, such as Cd and Pb (Zhang et al. 2013). The effects of commercial copper oxide (CuO) NPs on the elemental composition of lettuce have been investigated (Hong et al. 2015; Zhao et al. 2016a). The exposure of hydroponic lettuce to CuO NPs (20 mg L-1) resulted in higher absorption of Cu, reduced P and Fe accumulation in shoots, and increased S in the roots (Hong et al. 2015). Zhao et al. (2016a) exposed the lettuce leaves grown in soil to commercial Cu(OH)2 nanopesticides for 1 month and reported a significant accumulation of K and Zn in vascular tissues and a decrease in Mg.
In this study, we evaluated the effects of CuO NPs synthesized by the green chemical route on lettuce. This biologically friendly route involved the use of green tea extract as a reducing and coating agent. Green tea extract has antioxidant phytochemicals such as catechin, which allow CuO NP formation (Rolim et al. 2019a). In addition, antioxidant molecules derived from green tea also act as coating agents to avoid NP aggregation, thereby increasing their biocompatibility. Studies into the effects of the exposure of lettuce to biogenic CuO NPs are still scarce. Considering this scenario, the present study aimed to clarify how biogenic CuO NPs applied by different routes of exposure (foliar spray and soil irrigation) interact with different elements. The objectives were (1) to synthesize and characterize biogenic CuO NPs, (2) to evaluate the effect of CuO NPs and CuSO4 on productivity and elemental concentrations on lettuce grown in pots under greenhouse conditions, and (3) to estimate the daily nutrient intake in lettuce tissues. To the best of our knowledge, this is the first report to compare the foliar spray and soil irrigation administration of green tea synthesized CuO NPs compared to CuSO4 on lettuce growth and metal uptake.
Materials and methods
Reagents
Copper chloride II (CuCl2) used for the synthesis of NPs and copper sulfate (CuSO4) used for plant application were purchased from Sigma–Aldrich (St. Louis, MO, USA). Green tea powder (Camellia sinensis) used for green synthesis was obtained from Sumioka Shokuhin Kabushikikaisha (Hiraguti, Japan). Sodium hydroxide (NaOH) was obtained from Synth (Diadema, SP, Brazil). Nitric acid (HNO3) (Synth, São Paulo, Brazil) was previously purified with the SavillexTM DST-1000 sub-boiling distillation system (Minnetonka, USA). High purity deionized water (resistivity 18.2 MΩ cm-1) used throughout the experiments was obtained by the Elga water purification system (ELGA, Ubstadt-Weiher, Germany).
Green synthesis of CuO nanoparticles
An aqueous solution of CuCl2 (93.0 mmol L−1) was added dropwise into a suspension of green tea extract (2.5 mg mL−1). The volumetric proportion of CuCl2 and green tea extract was 1 to 2, respectively. The pH of the final suspension was adjusted to 5.5 using NaOH (1 mol L−1), then the mixture was stirred for 15 min by magnetic stirring. The final mixture was centrifuged. The black proportion of precipitate of CuO nanoparticles (CuO NPs) was freeze-dried (Pelegrino et al. 2020).
Characterization of CuO NPs
The hydrodynamic size, polydispersity index (PDI), and zeta potential values of CuO NPs were analyzed by dynamic light scattering (DLS, Zetasizer Nano ZS, Malvern Instruments Co, UK) (Pelegrino et al. 2020; Rolim et al. 2019a, b). The morphology of CuO NPs was obtained by transmission electron microscopy (TEM) at 20 kV (JEM-2100 TEM, Jeol Ltd.) and analyzed using the ImageJ software (Pelegrino et al. 2020; Rolim et al. 2019a). Copper concentration of the biosynthesized CuO NPs was determined by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7900, Hachioji, Japan). The extraction method and Cu analysis were performed according to Souza et al. (2019). Briefly, triplicate samples of approximately 15 mg of CuO NP powder each were placed in 50-mL Teflon tubes. Then, 4 mL of HNO3 (65% v v-1) were added and the tubes were closed. The extractions were carried out for 2 h at room temperature (25 °C). The volume was made up to 50 mL with type 1 water. Finally, the solution was diluted 1000 times and Cu concentration was determined by ICP-MS. This procedure was performed in triplicate. Similar analytical procedures have been reported by several previous works (Duarte et al. 2019; Oliveira et al. 2018; Gasparotto et al. 2018; Nordin et al. 2018; Wilcox et al. 2015).
Soil characterization
The soil samples (n = 3) were collected from an urban agriculture field in the vicinity of a petrochemical plant in Santo André, SP, Brazil (23° 64′ S; 46° 49′ W). This site has been used as an urban garden for the last three decades, and the soil is under the influence of several metals released into the environment by the petrochemical industry and vehicular traffic. To simulate a field condition, the soil was prepared just exactly as the farmers on the site recommended as follows: a bed was separated to provide enough soil amounts for characterization and pot experiments. It was prepared by mixing the local soil and organic amended compounds (compost and spent mushroom substrate) (1:1) and sieving (2 mm).
For soil characterization, approximately 1000 kg was collected. Then, the soil was oven-dried at 40 °C until constant mass, quartered, homogenized, and sieved with a stainless mesh (2 mm). Soil texture, pH, organic matter (OM), cation exchange capacity at pH 7 (CEC), sum of bases (SB), base saturation (V %), and potential acidity (H + Al) were determined according to Embrapa (Donagema et al. 2011; van Raij et al. 2001).
In addition, several chemical elements were quantified in soil (Ag, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mo, Na, Ni, P, Pb, S, Sb, Se, U, V, and Zn) sieved with a stainless mesh (150 mm). For this purpose, the United States Environmental Protection Agency (USEPA) 3051A procedure (US-EPA Method 3051A 2007) was performed, with some modifications according to Segura et al. (2016) and Suda and Makino (2016). About 500 mg of soil was pre-digested for 48 h with 10 mL of concentrated HNO3. Then, the samples were heated in a digesting block at 175 oC for 15 min. The digested samples were diluted to 50 mL with type 1 water.
The elements were determined using an inductively coupled mass spectrometer (Agilent 7900, Hachioji, Japan). A stock solution containing all elements (10 mg L-1) (Perkin Elmer, USA) was used to prepare the calibration curve according to Paniz et al. (2018). To ensure the reliability of the results (accuracy and precision), the certified reference materials TILL-4 (soil) (Canadian Certified Reference Materials, Vancouver, Canada) were analyzed. The results of the analysis of the CRMs were statistically consistent with the certified values.
The mineralogical composition identification of the soil was determined by the powder method using an X-ray diffractometer (PANalytical, model: Xpert Pro, The Netherlands) with an X’Celerator detector using Cu-Kα radiation (λ = 1,542 Å, 2,2 kW). Crystalline phase identification was done by comparison with the ICDD (International Center for Diffraction Data) and PANalytical inorganic crystal structure databases.
In vivo bioassays
Lettuce seedlings (Lactuca sativa L. (cv. Vanda)) were grown under greenhouse conditions controlling for temperature and humidity (25 oC and 50–60%, respectively). Seedlings were grown for 30 days and transplanted to individual pots of 3.4 dm3. Plants were watered daily at ~ 60% of their field capacity. We focus on the comparison of CuO NPs and CuSO4 salt applied in two different exposure routes: (i) spray on leaves (foliar application) and (ii) soil irrigation water directly added to soil by using an automatic pipette (soil irrigation). The treatments were CuO NPs by (i) foliar spray application and (ii) soil irrigation; CuSO4 by (iii) foliar spray application and (iv) soil irrigation; and (v) control without Cu exposure. The 5 groups were divided to contain 4 replicates randomly distributed in blocks composed of 3 rows of 10 m spaced 0.30 m apart.
Particles were added to diluent (water), and suspensions were sonicated for 10 min at 2800 rpm. The treatments were applied after 2 weeks of acclimatization; thus, the exposure started at day 45. The approximate volume amount for each pot was 15 mL per event. The exposure frequency was twice per week. A total of eight sprays were done by harvest time and the final concentration added by the treatment was 20 mg of CuO per plant and the equivalent mass of Cu was calculated to define the mass of CuSO4 added per plant. This concentration was defined based on previous studies by our research group (Pelegrino et al. 2020). At day 75, the plants were harvested.
Growth parameters
Fresh and dry weight, longitudinal and radial length, and number of leaves
After the in vivo bioassays, the lettuce samples were measured using a metric scale as to the longitudinal and radial length (cm), fresh and dry weight (FW, DW) (g) (excluding senescent leaves, if any) and the number of leaves was counted manually during the harvest stage.
Leaf gas exchange
Leaf gas exchange was measured at the end of the experiment, 2 days after the 8th application. For this, we used an infrared gas analyzer (Li-6400, Licor, Lincoln, NE, USA) attached to a modulated fluorometer (6400-40 LCF, Licor, Lincoln, NE, USA). Leaf CO2 assimilation (A), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration (E) were measured under PPFD of 500 μmol m-2 s-1 and an air CO2 concentration of 400 μmol mol-1. The plants were well hydrated before the measurements. The instantaneous carboxylation efficiency (k) was given by the rate between leaf CO2 assimilation and intracellular CO2 partial pressure (A/ Ci). The measurements were performed between 8:30 and 11:00 a.m. The vapor pressure difference between leaf and air (VPDL) was 2.2 ± 0.3 kPa and leaf temperature was 29.0 ± 1.0 °C during the evaluations.
Elemental determination in lettuce and soil
At the end of the experiment, the heads of lettuce were separated from the roots. Then, the root samples were separated from the soil. The plant samples (leaves and roots) were washed under running water and the excess water was manually removed. In the laboratory, plant samples and soil were oven-dried at 40 °C until constant mass. Finally, soil samples were quartered, homogenized, and sieved with a stainless mesh (150 mm).
After drying, plant tissues were prepared and analyzed according to Paniz et al. (2018). The roots and leaves were cut into fine pieces. About 200 mg of sample was pre-digested for 48 h in 2 mL of concentrated HNO3 previously purified in a sub-boiling distillation system. The samples were heated in a graphite digester block for 4 h at 95 °C. After cooling at room temperature, the samples were diluted in 40 mL with type 1 water. Soil samples were prepared and analyzed as described in detail in Section 2.4.
The elements were determined using an inductively coupled mass spectrometer (Agilent 7900, Hachioji, Japan). A stock solution containing all elements (10 mg L-1) (Perkin Elmer, USA) was used to prepare the calibration curve according to Paniz et al. (2018). To ensure the reliability of the results (accuracy and precision), the certified reference materials (CRMs) NIST 1573 (tomato leaves) (National Institute of Standards and Technology, Gaithersburg, MD, USA) were analyzed. These materials were subjected to the same methods used for the analysis of plant tissues. Moreover, blank samples were assayed throughout all experiments to check for possible contamination problems. The results of the analysis of the CRMs were statistically consistent with the certified values.
Estimated daily intake of chemical elements from lettuce
The estimated daily intake of some elements (those that presented significant differences among pot groups) through lettuce consumption was calculated by Eq. 1 (Xu et al. 2013).
where EDI is the estimated daily intake for each element, expressed as mg day-1. DI represents the average daily intake of lettuce, which was assumed to be 40 g day-1 FW (approximately four leaves). This value is in good agreement with the daily lettuce consumption in the USA of 41 g day-1 FW (Zhao et al. 2016a). Finally, Celement is the average element concentration in lettuce leaves (n = 4) in mg g-1 FW.
Statistical analysis
The dataset consisted of 72 sample cases (4 replicates × 3 treatments × 2 exposure routes × 3 matrices) by 24 variables (elemental content) distributed by treatment (control, CuO NPs, and CuSO4), exposure (foliar and soil irrigation), and matrix (root and leaf). A two-way analysis of variance (ANOVA) was applied by sample matrices (M) where metals were measured to test if statistical differences were observed by the considered factor: treatment (T), exposure route (E), and their interaction (E × T). In all the tests, a significance level of 0.05 was considered.
Results and discussion
Characterization of CuO NPs
The hydrodynamic size of CuO NPs was 74.6 ± 1.5 nm with a PDI of 0.28 ± 0.06 and a zeta potential of -20.8 ± 0.5 mV. These values are similar to those reported for commercial CuO NPs (Hong et al. 2015; Singh and Kumar 2016). The total copper content in CuO NPs (mass ratio) obtained by ICP-MS was 52 ± 5%. Figure 1a and b shows representative images of CuO NPs using TEM. The NPs had a spherical shape and were well dispersed (Fig. 1a, b) with an average size of 13.0 ± 0.1 nm (Fig. 1c). Other studies using green synthesis also showed a similar NP shape, for example, Dey et al. (2019) showed spherical shape CuO NPs synthesized using Azadirachta indica leaves, and Naika et al. (2015) showed similar results for CuO NPs synthesized using Gloriosa superba L. extract. These results are similar to our previously published paper (Pelegrino et al. 2020).
Thus, the green synthesis of CuO NPs was successfully performed and yielded NPs with good stability. Moreover, it was possible to obtain NPs with a size and shape similar to commercial NPs with the advantage of using a green synthesis and increasing biocompatibility due to the green tea coating.
Soil characterization
The geochemical composition of the soil used in the pot experiment is reported on the supplementary material (Table S1). In general, most samples consist of moderately to slightly acidic soils, with a pH of 5.9 on average. The results showed about 338 g kg-1 of clay, high sand content 477 g kg-1 on average and, in general, a median silt content with an average of 186 g kg-1. The soil texture was classified as sandy-clay-loam soil, which is considered a soil of moderately fine texture (Dos Santos et al. 2018), and PTE retainability is generally higher in fine-textured soils than coarse-textured soils due to the presence of more pore spaces (McBride et al. 2014).
The OM content was 58 g kg-1, which is considered high for tropical soils (Fadigas et al. 2002) facilitating PTE and NP attachment. The CEC, which expresses the soil’s capacity to retain cations, showed an average of 185 mmolc dm-3; this value is also considered high for tropical soils according to Fadigas et al. (2002). These authors studied 162 Brazilian clayey soils and observed that 79% of the samples had a CEC lower than 100 mmolc dm-3. The high CEC value of the soil used in our study may be attributed to the high OM content and to the presence of clay minerals.
The X-ray diffraction results (Fig. S1) of soil showed the presence of higher intensity peaks of quartz (SiO2) and gibbsite (Al(OH)3). Clay minerals such as kaolinite (Al2(Si2O5)(OH)4), vermiculite (Mg3Si4O10(OH)2), and muscovite (KAl2((Si3Al)O10(OH)2) were also identified. A lower abundance of anatase (TiO2) was observed.
Primary minerals such as kaolinite clays tend to have moderate CEC (10–150 mmolc dm-3), while vermiculite has a 2:1 crystalline structure with high CEC (1000–2000 mmolc dm-3) (Brady et al. 2008), which is a consequence of surface and interlayer ion exchange processes as well as isomorphic substitutions. Such properties, associated with high surface areas, have made these materials capable of attaching PTEs depending on soil acidity and may be also a target of attachment of metallic NPs (Geitner et al. 2020).
The base saturation was high, 82%, and the soil can be classified according to the current Brazilian soil classification system as eutrophic soil (V ≥ 50%) (Dos Santos et al. 2018). The concentration of exchangeable phosphorus in the urban garden soil samples was 274 mg dm-3, which is considered a sufficient value for productive cultivation (Van Raij 2011). Overall, this soil presented characteristics to supply nutrients to cultivation, since the farmers from this urban agriculture field, where the soil was collected, periodically use acidity correctives, mineral, and organic fertilizers. However, this can facilitate the incursion of contaminants such as PTEs.
Total contents of PTEs in the soil rank in the following order: Ba>Zn > Cu > V > Pb > Cr > Ni > As>Co > Mo > Se > Sb > Cd > Ag. There is no international consensus regarding soil PTE guidelines. The barium level (662 mg kg-1) is above the intervention value for agricultural purposes (500 mg kg-1) described by the local environmental protection agency (Environmental Protection Agency of the State of São Paulo, Brazil) (CETESB 2016). This represents the amount of an element in the soil or groundwater that may pose direct or indirect potential risks to human health. Copper, Se, Pb, and Zn levels are above the São Paulo State Prevention Values (SP-PV), but are still considered safe for agricultural activities (CETESB 2016). High levels of Ba were previously reported in the Brazilian urban soil studies (Figueiredo et al. 2011; Lange et al. 2017; Lange et al. 2018), since this element is applied in manufactured materials such as tiles, glass, bricks, pigments, paints, brake lines, and auto parts.
Productivity of lettuce
Fresh and dry weight, longitudinal and radial length, and number of leaves
The productivity parameters of lettuce plants were evaluated by FW and DW, longitudinal and radial length, and the number of leaves (Fig. 2a–e). A two-way ANOVA (data not presented) showed that the effect of treatment was prevalent for DW (CuO NPs significantly improved DW among pot groups). Conversely, the number of leaves was affected mostly by the route of exposure, where foliar spray improved the number of leaves among pot groups. A significant increase in DW (46%) and in the number of leaves (18%) was observed in the group exposed to CuO NPs by foliar spray compared to the control (Fig. 2a, d).
Neither CuO NP nor CuSO4 application by soil irrigation caused any significant changes in productivity parameters (Fig. 2). Foliar spray led to a significant increase (8 ± 1%) in fresh weight, regardless of the form of Cu (Nano or salt). For longitudinal length, there was a statistically significant difference among the treatments (p < 0.05): CuSO4-treated pots significantly improved longitudinal length among pot groups. Neither treatment, exposure, nor their combined effect affected the radial length. Figure S2 shows representative images of different treatments at the final application (8th application).
Previous studies reported positive and negative impacts of NPs on productivity parameters. The application of CuO NPs enhanced root length and biomass of lettuce (Hong et al. 2015; Wang et al. 2019). However, several studies have described commercial CuO NPs application as reducing root and longitudinal length, biomass, and water content in lettuce (Dimkpa et al. 2013; Hong et al. 2015; Trujillo-Reyes et al. 2014). Hafeez et al. (2015) evaluated the use of biogenic Cu NPs (20 nm) in wheat growth. The concentration of 30 mg kg-1 doubled the leaf area and the chlorophyll content. On the other hand, concentrations of Cu NPs above 30 mg kg-1 can result in phytotoxic effects on the plants (Hafeez et al. 2015). In comparison with the results obtained by other inorganic NPs, the use of TiO2 NPs in spinach doubled the FW and DW compared to the control (Yang et al. 2007). Furthermore, the application of four rare earth oxide NPs (CeO2, La2O3, Gd2O3, and Yb2O3) implied in a reduction of 75% to 90% of length in lettuce plants (Ma et al. 2010). Our findings suggested that foliar spray of the biogenic CuO NPs has beneficial impacts on lettuce productivity.
Leaf gas exchange
The leaf gas exchange markers studied were CO2 assimilation, transpiration, and time-course of stomatal conductance (Fig. 3). Figure 3 a shows that CO2 assimilation slightly increased after NP exposure via foliar application, and as previously discussed, this pot group also increased the DW and number of leaves (Fig. 2a, d). Therefore, this treatment/exposure did not present toxicity to lettuce; actually, it showed a positive effect on productivity and CO2 assimilation. However, CO2 assimilation was not affected for CuSO4-treated pots exposed by foliar spray. Via soil irrigation, the NPs did not change the CO2 assimilation; however, the application of CuSO4 significantly decreased it in comparison with control. Rawat et al. (2018) showed similar results after treating bell pepper. They also reported a decrease in CO2 assimilation after salt application and no disturbance of it with Cu NPs. The CO2 assimilation was 34% higher using foliar application than soil irrigation (Fig. 3a).
Small changes without statistical significance were observed for transpiration (Fig. 3b). For this parameter, there was a high variability between measurements, which may be related to changes in environmental conditions when each plant was measured. The stomatal conductance slightly increased after NP and salt treatments via foliar and soil irrigation exposure. In addition, it was 28% higher using the foliar than soil irrigation application (Fig. 3c). NPs applied via foliar spray could cause entrapment of NPs in the cuticle or encompass penetration through stomata (Priyanka et al. 2019). This did not occur in our study, since the results showed a non-significant difference between stomatal conductance treated with NPs and salt via foliar or soil irrigation in comparison with control (Fig. 3c). Overall, all treatments showed a nontoxic effect for lettuce regarding its productivity and gas exchange parameters.
Determinations of macro and trace elements in samples
Regarding the elemental average concentrations in leaves and roots, the detailed information is reported in Table 1. As seen in this table, elemental content is influenced by treatments and exposure in different ways compared to the control. Soil elemental composition was also determined after harvest (Table S2). The results showed that Cu concentration in soil was slightly lower in all treatment samples (ranging from 89 to 101 mg kg-1) than the control (102 to 104 mg kg-1); thus, Cu-treated pots did not cause a significant increase of Cu in soil.
Exposure and treatment effects on elemental variability
The elemental concentration variability by the exposure route (E), by the treatment (T), and their combined effect (E x T) was assessed by a two-way ANOVA (Tables S3 and S4). Figure 4 summarizes the significant changes in the elemental concentration of root and leaf tissues compared to the corresponding controls, as proposed previously in the study by Cota-Ruiz et al. (2020). The results showed that (T), (E), and the interactions between them all had significant effects on the leaf and root mineral profile.
Macro and trace elements results in roots and leaves of lettuce treated with different Cu compounds (NPs or salt) via two routes of exposure (foliar spray and soil irrigation). Mean significant increments compared to the control are signalized by (+) and reductions (-), and unaffected (equal symbol) (n = 4)
Neither treatment, exposure, nor their combined effect influenced As, Ba, Cd, Co, Cr, Fe, Mn, Na, Ni, Li, Pb, Sn, or U concentrations in roots (Table 1). Copper, P, Sb, and V concentrations in roots were significantly affected only by (E), mainly by soil irrigation, except for Sb, the foliar application of which displayed a more significant effect than soil irrigation. The effect of (T) (CuO NPs) was only significant on K concentrations in roots. Treatments (NPs and salt) exhibited a more significant effect than (E) for Mo, S, and Zn. Calcium content in roots was significantly affected by (E), (T) and their interaction (E × T); however, (T) (both NPs and salt) had a more significant effect than the other two factors. Magnesium and Ag contents in roots were significantly affected by (E) and (E × T); however, for Ag, the (E) (soil irrigation) showed a more significant effect than (E x T). Overall, comparing the F values of these various factors, exposure (E), mainly soil irrigation, displayed a more significant effect than (T) and (E × T) on the root mineral profile.
Neither treatment, exposure, nor their combined effect altered Co, Li, Mn, Pb, Sn, U, or V concentrations in leaves (Table 1) and were not represented in Fig. 4. To sum up, the influence of treatment was the main factor of most elements’ variability in leaves, except for Cu. Overall, CuSO4-treated pots presented more alterations on the leaf mineral profile than CuO NP-treated pots (Fig. 4). In leaves, Na, Mg, P, K, Ca, Cd, Cr, Zn, As, Se, Mo, Cd, and Ba concentrations were significantly affected only by (T) as follows: (i) Cr concentrations were significantly affected by CuO NP application; (ii) As, Mo, P, and Se concentrations were significantly affected by CuSO4 application and (iii) Na, K, Mg, Ca, Cd, Zn, and Ba were affected by both Cu forms (CuO NPs and CuSO4); (iv) FeS and Ni in leaves were significantly affected by both (E) and (T) effects, mainly by both treatments (CuO NPs and CuSO4) and a less significant effect by soil irrigation route of exposure was observed; (v) Cu, Ag, and Sb were significantly affected by (E), (T) and their interaction (E × T). Nevertheless, for Cu, the effect of foliar spray was the most pronounced factor in leaves, for Ag, it was the treatment effect (CuO NPs and CuSO4), and for Sb, it was the CuSO4 application.
Silver, Cu, Fe, and S had the greatest changes by both effects (T) and (E) among all the elements (Fig. 5a–d). This is an intriguing finding and merits further study to evaluate the processes involved in these elemental dynamics in “soil-plant system” under CuO NP exposure, because they are commonly associated in several geochemical reactions and minerals (Davis et al. 2001; Mantha et al. 2019; Stanley 1987) as well as in the biochemical reactions in roots and shoots (Dimkpa et al. 2012).
As the concentrations of Co, Li, Pb, Sn, and U in roots and leaves were statistically similar among the exposed groups and control, they were not discussed further in this study.
Macroelements (Ca, K, Mg, Na, P, and S)
As previously described in Section 3.4.1, the elemental concentration in roots was mainly affected by the soil irrigation route of application. Sodium concentration in roots was not affected. Only the foliar exposure of CuO NPs caused a slight increase in S concentration in roots (19%). Calcium increased significantly in the roots (~ 20%) due to exposure by soil irrigation (NPs or salt). Nanoparticles caused no alteration in Mg content in roots and Cu salt soil irrigation increased it (30%). CuO NP exposure via soil irrigation caused a significant increase in P and K concentration in roots (36% and 27%, respectively) (Fig. 4).
Overall, the concentration of macroelements in leaves from the groups exposed to Cu (NPs or Salt) was higher than in the control samples (Fig. 4). No changes were observed for Ca and Mg concentrations in CuO NP-treated pots exposed by foliar spray and for P concentrations in CuO exposure compared to the control. In general, the increase in macroelement concentration in leaves was higher in CuSO4-treated pots than in CuO NP-treated pots, regardless of the route of application. Comparing the two routes of application, the soil irrigation route caused a higher increase in the concentration of these macroelements.
Contrary to our findings, Hong et al. (2015) have found that the application of CuO NPs (< 100 nm) at 20 mg L-1 in lettuce decreased P in roots and leaves. Cota-Ruiz et al. (2020) reported that P and S contents were reduced in bulk and ionic Cu-exposed (80 and 280 mg kg-1) alfalfa plants compared to the controls. Copper NPs (100–500 mg kg−1 of sand for 7 days) decreased the Ca and Mg contents in leaves of bean plants (Dimkpa et al. 2015) and Cu NPs (20 mg L−1 for 15 days) decreased concentrations of P, Ca, and Mg in lettuce (Trujillo-Reyes et al. 2014). The application of TiO2 NPs (50–400 mg L-1) in lettuce (Lactuca sativa L.) reduced the content of Ca (35.7%), Mg (27.2%), and Na (15.4%) compared to the control (Hu et al. 2020).
On the other hand, Hayes et al. (2020) evaluated the application of Al2O3 NPs in lettuce and reported increased concentrations of Mg, P, and Ca compared to the control. CuO NPs (150 mg kg-1) on green onion increased root Ca (86%), bulb Ca (74%), and bulb Mg content in roots and leaves as well (108%) (Wang et al. 2020). However, at the same concentration, the salt application reduced Ca (~ 65%) and Mg (~ 30%) concentrations in roots (Wang et al. 2020).
Beyond the comparison among treatments and control, it was essential to evaluate if the nutrient content on leaves was suitable for lettuce plant nutrition. In comparison with the determined values: Mg (2.99–4.34 g kg-1), S (3.92–5.96 g kg-1), and Ca (14.32–22.75 g kg-1) in the leaves, these elements are within the range recommended by Malavolta (1980), Pais and Jones Jr (1997), and Furlani (2004) as follows: Mg (3–5 g kg-1), S (1–5 g kg-1), and Ca (10–50 g kg-1). However, P (10.31–13.81 g kg-1) and K (82.86–137.43 g kg-1) were higher than the recommended values by these authors, P (1–3 g kg-1), K (8–50 g kg-1), but also in the control samples, most probably due to the high levels of these elements in the soil composition of the urban garden (Table S1).
Trace elements (Ag, As, Ba, Cd, Cr, Cu, Fe, Mo, Ni, Sb, Se, V, and Zn)
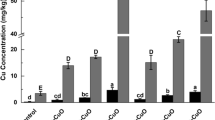
As expected, Cu concentration increased in leaves after the application of CuO NPs and CuSO4 (Fig. 5b) compared to the control. As mentioned before, this increase was more intense with foliar spray than soil irrigation for both materials (CuO NPs and CuSO4). Foliar Cu accumulation in leaves was similar regardless of the Cu form applied (nano 45 ± 7 mg kg-1 or salt 55 ± 4 mg kg-1). The accumulated Cu was mostly inside the tissues, since the lettuces were washed thoroughly; the same was observed by Zhao et al. (2016a) after they exposed lettuce to Cu(OH)2 nanopesticide by foliar spray.
According to Anjum et al. (2015), levels of 20–30 mg Cu kg-1 in leaves (DW) were considered toxic for most crop species. However, no chlorosis spots or loss of productivity were observed for lettuce heads from CuO NPs by foliar spray exposure. This result may be positive for plant development, since Cu homeostasis is essential for proper plant growth because it is important for the cuproproteins cofactor. Zhao et al. (2016c) observed that after the exposure of lettuce leaves to foliar applications of Cu(OH)2 nanopesticides, even at high concentrations of Cu (823–2501 mg kg-1), the lettuce leaves did not present any visible toxic symptoms throughout the entire exposure period. In our study, chlorosis spots were observed on leaves after CuSO4 foliar spray application. This may occur due to the affinity of ionic copper to complex with sulfhydryl groups present in amino acids or antioxidants such as reduced glutathione (GSH), and these complexes are phytotoxic, being involved in GSH oxidation via interaction with thiol groups (Dimkpa et al. 2012).
None of the soil irrigation groups (nano 6.0 ± 2.0 mg kg-1 or salt 6.5 ± 0.2 mg kg-1) promoted any considerable increase on the Cu accumulation in leaf tissues compared with the controls (5.2 ± 0.4 mg kg-1) and the foliar exposure produced 8-fold more Cu in leaves than soil irrigation. The results observed for exposure via soil irrigation may be attributed to the fact that the soil used for the pot experiment was slightly acidic. In this kind of environment, CuO NPs are dissolved and act similarly to dissolved Cu; in the long term, they can transform into Cu bound to FeO(OH) or OM transforms into Cu2S (Peng et al. 2017; Sekine et al. 2017).
Similar results were observed by Servin et al. (2017). They evaluated the bioaccumulation of CuO in NPs, bulk, and ionic form (0–400 mg kg-1) added into soil, by lettuce exposed for 70 days. The soil used by these authors (sandy loam, 69% sand, 22% silt, 8.6% clay, 4.3% OM, pH 5.9, CEC 186 mmolc dm-3) was very similar to the soil used in this present study and they observed concentrations of Cu from 7.0 ± 3.0 (control-unexposed) to 9.0 ± 1.7 mg kg-1 (NPs) and 9.0 ± 1.6 mg kg-1 (ion). The Cu concentrations added in soil by Servin et al. (2017) were much higher than ours and this may indicate that lettuce exposed to Cu in soil, regardless of the form (nano or salt) has a maximum capacity to accumulate Cu in leaves. Early studies have reported that Cu translocation to edible parts of lettuce seemed to be well regulated, as their concentrations were fairly constant (Ginocchio et al. 2002).
In this study, root concentrations of Cu varied widely, especially in samples of soil irrigation treatments from 54 to 64 mg kg-1 (control), 64 to 118 mg kg-1 (CuO NPs), and 67 to 111 mg kg-1 (CuSO4) (Table 1). The Cu translocation from roots to leaves for soil irrigation treatments (NPs or salt) was similar to the control samples (7 to 8%), which means that the accumulation of Cu in roots was around 92%. Previously, other studies reported Cu accumulation rates ranging from 87 to 99% in the roots, with very low translocation to leaves for lettuce, alfalfa, and cilantro (Hong et al. 2015; Servin et al. 2017; Zuverza-Mena et al. 2015).
According to Fig. 4, foliar exposure of Cu (NPs or salt) did not cause a disturbance in trace element concentrations in roots, except for Sb, in which the concentration was reduced in CuSO4-treated pots. Soil irrigation exposure, regardless of the treatment (NPs or salt), increased the concentrations of Ag, Mo, V, and Zn (from 20 to 36%) in roots compared to the control.
In leaves, after soil irrigation, Ag content was significantly smaller (54%) than the control when NPs and Cu salt were applied (Fig. 5a). On the other hand, Ag increased with the foliar NP application (31%) and decreased (69%) by the foliar salt application when compared to the levels of the control samples. This is an interesting finding, since the accumulation of Ag in plants exposed to CuO NPs was very distinct depending on the route of application. The Ag translocation mechanism is most probably associated with the same processes of free Cu translocation, and the lettuce mechanism to accumulate free Cu in roots also probably triggers Ag accumulation in roots. Copper and Ag have similar geochemical behavior. Silver rarely occurs in nature in a soluble form; thus, available Ag for plant uptake is restricted (Nguyen et al. 2017). According to Settimio et al. (2014), the labile forms of Ag in soil fractions are Ag+, reversibly sorbed Ag+ to Fe-oxohydroxides and organic S of OM; Ag+ weakly complexed with other soil solution ligands (L); the non-labile Ag is solid: metallic Ag, AgCl, and Ag2S; and Ag irreversibly bound to organic S and Fe-oxohydroxides (surface precipitated or fixed within crystal lattices). Excess of free Cu is known to stimulate the release of amino acids, carboxyl and amino groups, organic acids, glutathione, cysteine, to complex, or chelate with Cu with other root exudates that can bind and reduce the availability of Cu and Ag to leaves (Zhao et al. 2016b).
In leaves (Fig. 4), there was an increase in Cd and Zn concentrations in all pot groups compared to the control (from 13 to 25%). CuO NP-treated pots exposed by foliar spray caused an increase in Cd, Cr, and Zn levels in leaves. Arsenic, Mo, and Se concentrations in leaves were increased only in the CuSO4-treated pots by both routes of exposure. This is noteworthy because these three elements can commonly exist as oxyanions in soil-oxidizing environments. Soil irrigation exposure caused an increase in Ni (71% NPs and 40% salt) and an increase in Sb in leaves (100%), only for CuSO4-treated pots. Iron content in leaves increased in almost all pot groups, except in CuO NP-treated pots exposed by foliar spray.
The soil collected from an urban garden used for our pot experiment presented a high level of Ba (Table S1), a fact also observed in soils from other urban gardens (McBride et al. 2014; Nabulo et al. 2010). Thus, an undesirable effect of CuO NP exposure in our study would be a higher accumulation of Ba in leaves. Despite that, Ba content in leaves increased in almost all pot groups, except in CuO NP-treated pots exposed by foliar spray. Nevertheless, the range of concentration found in our study (9 to 13 mg kg-1) for this element is in good agreement with the mean value (12 mg kg-1) obtained for leafy green vegetables purchased in New York supermarkets (McBride et al. 2014).
Opposite findings were reported regarding the role of NPs on Cr uptake in plants. Magnetite NPs (López-Luna et al. 2016) and biogenic CuNPs (Noman et al. 2020b) were found to reduce the translocation rate of this element from root to shoots. Also, similarly to our data, Noman et al. (2020a) reported a decreased Cd translocation by 49.62 % in wheat plants exposed to CuNPs added into soil. John (1976) claims that if the K level in plants is high, this could inhibit the Cd in leaves. In our study, the highest level of Cd in leaves was observed for foliar NP treatment, which presented the lowest level of K in leaves (Table 1).
Very scarce information about the effect of NPs on Mo accumulation in plants is available. Martínez-Fernández et al. (2016) exposed Helianthus annuus L. to Fe oxide NPs by hydroponic culture solution, and the concentration of Mo in the roots increased with the dose of NPs. In another study, with bell pepper (Capsicum annum L.) plants exposed to CuO NPs and CuCl2 exposure, Mo uptake and transport of ionic copper treatment was probably supported by the P transporters (Rawat et al. 2018). The uptake, metabolism, and enzymatic mechanisms for Mo and Fe affect each other (Bittner 2014), and Mo is said to share some transporters with S and P (Nie et al. 2014). In our study, these four elements (Mo, Fe, P, and S) presented a similar pattern of accumulation, especially for the soil irrigation group (Table 1).
Estimated daily intake of chemical elements from lettuce
Since lettuce is one of the most acquired and consumed vegetables in Brazil and worldwide (Canella et al. 2018; Kim et al. 2016), it is important to assess the contribution of the applied treatments/exposure to reach the dietary reference intakes (DRIs) of nutrients, as well as its influence on the intake of PTEs. Table 2 shows this information for some elements that presented significantly differences among pot groups and available information about dietary reference dose according to international regulations.
For all elements except Cu, small differences were observed in the EDI between pot groups (less than twice from one group to another). However, the foliar application of salt increased Cu EDI about 10 times in the control, changing from 2.30 to 24.2% of the supplied percentage of the DRI of this element. The EDI for Cu ranged from 0.021 (control) to 0.181 mg day-1 (NPs via foliar application) and 0.218 mg day-1 (salt via foliar application), which is within recommended intake guidelines of 0.9 mg Cu/ person-day, thus safe for human consumption. Higher values were reported by Zhao et al. (2016a). These authors investigated the impact of Cu(OH)2 nanopesticide on the nutritional value of lettuce and observed a considerable increase in the EDI of Cu with exposure; the leaves accumulated around 1350–2010 mg Cu kg-1 DW after 30 days of foliar exposure. The EDI reported by them was 0.021 mg day-1 for the control, 2.2 mg day-1 for the low level of exposure, and 3.3 mg day-1 for the high level (Zhao et al. 2016a).
For Fe, Na, and Zn, the EDI did not represent a considerable percentage of the DRI in any pot group (Table 2). On the other hand, for Mg, P, and Ca, the consumption of lettuce can contribute with 2.84 to 9.58% of the DRI. Moreover, the ingestion can contribute to 9.75 to 21.1% of the DRI for K, so lettuce can be a good source of K. The salt application by soil irrigation provided the highest EDI values for Na, Mg, P, K, and Ca, while for Zn, both treatments provided the same EDI (Table 2). Thus, CuO NP exposure for both routes of application in lettuce did not cause a considerable change (less than 1.5-fold compared to the control) on Na, Ca, Zn, Mg, K, and P supply. Moreover, Kemi et al. (2010) suggested that the Ca/P ratio in food must be 1.0 and that a low dietary Ca/P ratio may interfere with the homeostasis of Ca metabolism in humans. Our results showed that the Ca/P ratio was higher than 1 for all treatments ranging from 1.4 (control) to 1.8 (salt foliar exposure).
For Cd, the treatments did not affect EDI significantly. The percentages of the provisional tolerable daily intake (PTDI) provided varied from 0.96 to 1.20%, which is very low. Fortunately, the consumption of lettuce obtained in these pot trials did not pose a risk to consumers due to Cr ingestion, since the highest EDI (0.00192 NPs exposure by soil irrigation) represents 5.47 to 9.58% of the DRI for Cr (Table 2). On average, the EDI values observed in our study are similar to the value reported (0.00144) by Pillay and Jonnalagadda (2007) for lettuce from various supermarkets in Durban (South Africa).
Conclusion
Green tea-synthesized CuO NPs were found to have spherical shape and small size. Lettuce plants were exposed in a pot experiment to fixed concentrations of copper, as CuO NPs and CuSO4 by foliar spray, and soil irrigation as application routes. For lettuce, exposure via foliar application improved the number of leaves and DW and caused a slight improvement in gas exchange. Moreover, all the lettuce exposed to copper nano or salt form showed changes in elemental accumulation in plant tissues (leaves and roots), mainly increasing their concentration in roots and leaves. However, foliar application of NPs was the one that resulted in less disturbance of lettuce mineral profile. Copper salt exposure resulted in a more aggressive alteration of the mineral profile compared to the control, regardless of the route of application, and caused necroses in leaves by foliar spray exposure. CuO NPs application via foliar spray caused accumulation of K, Na, S, Ag, Cd, Cr, Cu, and Zn in leaves. Nevertheless, this increase was not of concern to consumers. Further studies are required to evaluate the impact of these CuO NPs under field conditions and their fate in the environment. Also, it is strongly recommended that the effects on enzymatic, molecular, and genetic levels of lettuce plants be assessed, but these topics are beyond the scope of this study.
Data availability
All data supporting the conclusions of this article are provided as figures, tables, and supplementary tables and figures.
References
Abollino O, Giacomino A, Malandrino M, Mentasti E (2007) The efficiency of vermiculite as natural sorbent for heavy metals. Application to a contaminated soil. Water Air Soil Pollut 181(1-4):149–160. https://doi.org/10.1007/s11270-006-9286-8
Adisa IO, Pullagurala VLR, Peralta-Videa JR, Dimkpa CO, Elmer WH, Gardea-Torresdey JL, White JC (2019) Recent advances in nano-enabled fertilizers and pesticides: a critical review of mechanisms of action. Environ Sci Nano 6:2002–2030. https://doi.org/10.1039/c9en00265k
Anjum NA, Adam V, Kizek R, Duarte AC, Pereira E, Iqbal M, Lukatkin AS, Ahmad I (2015) Nanoscale copper in the soil-plant system—toxicity and underlying potential mechanisms. Environ Res 138:306–325. https://doi.org/10.1016/j.envres.2015.02.019
Arruda SCC, Diniz Silva AL, Moretto Galazzi R, Antunes Azevedo R, Zezzi Arruda MA (2015) Nanoparticles applied to plant science: a review. Talanta 131:693–705. https://doi.org/10.1016/j.talanta.2014.08.050
Bittner F (2014) Molybdenum metabolism in plants and crosstalk to iron. Front Plant Sci 5:1–7. https://doi.org/10.3389/fpls.2014.00028
Brady NC, Weil RR, Weil RR (2008) The nature and properties of soils. Prentice Hall, Upper Saddle River, pp 662–710
Cai F, Wu X, Zhang H, Shen X, Zhang M, Chen W, Gao Q, White JC, Tao S, Wang X (2017) Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). NanoImpact 5:101–108. https://doi.org/10.1016/j.impact.2017.01.006
Canella DS, Louzada MLDC, Claro RM, Costa JC, Bandoni DH, Levy RB, Martins APB (2018) Consumption of vegetables and their relation with ultra-processed foods in Brazil. Rev Saude Publica 52:50. https://doi.org/10.11606/S1518-8787.2018052000111
CETESB (2016) Environmental Company of the State of São Paulo. Guiding Values for soils and water underground in the State of São Paulo. https://cetesb.sp.gov.br/aguas-subterraneas/wpcontent/uploads/sites13/2013/11 /tabela_vos_2016_site.pdf. Accessed 20 June 2020
Cortés A, Silva LFO, Ferrari V, Taffarel SR, Feijoo G, Moreira MT (2020) Environmental assessment of viticulture waste valorisation through composting as a biofertilisation strategy for cereal and fruit crops. Environ Pollut 264:114794. https://doi.org/10.1016/j.envpol.2020.114794
Cota-Ruiz K, Ye Y, Valdes C, Deng C, Wang Y, Hernández-Viezcas JA, Duarte-Gardea M, Gardea-Torresdey JL (2020) Copper nanowires as nanofertilizers for alfalfa plants: understanding nano-bio systems interactions from microbial genomics, plant molecular responses and spectroscopic studies. Sci Total Environ 742:140572. https://doi.org/10.1016/j.scitotenv.2020.140572
Dalmora AC, Ramos CG, Querol X, Kautzmann RM, Oliveira MLS, Taffarel SR, Moreno T, Silva LF (2016a) Nanoparticulate mineral matter from basalt dust wastes. Chemosphere 144:2013–2017. https://doi.org/10.1016/j.chemosphere.2015.10.047
Dalmora AC, Ramos C, Oliveira M, Teixeira E, Kautzmann R, Taffarel S, De Brum I, Silva LF (2016b) Chemical characterization, nano-particle mineralogy and particle size distribution of basalt dust wastes. Sci Total Environ 539:560–565. https://doi.org/10.1016/j.scitotenv.2015.08.141
Davis A, Helgen SO, McNulty T (2001) Discriminating between copper and silver mill tailings in Silver Bow Creek overbank deposits, Butte, Montana, U.S.A. Environ Forensic 2:249–259. https://doi.org/10.1006/enfo.2000.0041
Del Valle HB, Yaktine AL, Taylor CL, Ross AC (Eds.) (2011) Dietary reference intakes for calcium and vitamin D. National Academies Press
Dey A, Manna S, Chattopadhyay S, Mondal D, Chattopadhyay D, Raj A, Das S, Bag BG, Roy S (2019) Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-α and caspases signaling pathway against cancer cells. J Saudi Chem Soc 23:222–238. https://doi.org/10.1016/j.jscs.2018.06.011
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2012) Bioactivity and biomodification of Ag, ZnO, and CuO nanoparticles with relevance to plant performance in agriculture. Ind Biotechnol 8:344–357. https://doi.org/10.1089/ind.2012.0028
Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI, Anderson AJ (2013) Fate of CuO and ZnO nano- and microparticles in the plant environment. Environ Sci Technol 47:4734–4742. https://doi.org/10.1021/es304736y
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2015) Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 24:119–129. https://doi.org/10.1007/s10646-014-1364-x
Donagema GK, de Campos DB, Calderano SB, Teixeira WG, Viana JM (2011) Manual de métodos de análise de solo. Embrapa Solos-Documentos (INFOTECA-E)
Dos Santos HG, Jacomine PT, Dos Anjos LHC, de Oliveira VA, Lumbreras, JF, Coelho MR, Almeida JA, Filho JCA, Oliveira JB, Cunha TJF (2018) Brazilian soil classification system. Embrapa Solos-Livro técnico (INFOTECA-E)
Duarte AL, DaBoit K, Oliveira ML, Teixeira EC, Schneider IL, Silva LFO (2019) Hazardous elements and amorphous nanoparticles in historical estuary coal mining area. Geosci Front 10(3):927–939. https://doi.org/10.1016/j.gsf.2018.05.005
EFSA (2011) Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion: statement on tolerable weekly intake for cadmium. EFSA J 9(2):1975 [19 pp.]. https://doi.org/10.2903/j.efsa.2011.1975
Fadigas FDS, Amaral-Sobrinho NMBD, Mazur N, Anjos LHCD, Freixo AA (2002) Natural contents of heavy metals in some Brazilian soil classes. Bragantia 61(2):151–159. https://doi.org/10.1590/S0006-87052002000200008
Ferrari V, Taffarel SR, Espinosa-Fuentes E, Oliveira MLS, Saikia BK, Oliveira LFS (2019) Chemical evaluation of by-products of the grape industry as potential agricultural fertilizers. J Clean Prod 208:297–306. https://doi.org/10.1016/j.scitotenv.2015.08.141
Figueiredo AMG, Tocchini M, dos Santos TF (2011) Metals in playground soils of Sao Paulo city, Brazil. Procedia Environ Sci 4:303–309. https://doi.org/10.1016/j.proenv.2011.03.035
Furlani AMC (2004) Nutrição mineral [Mineral nutrition]. In: Kerbauy GB (ed) Fisiologia vegetal [Plant physiology],1stedn. Guanabara Koogan, Rio de Janeiro, pp 40-75
Gajjar P, Pettee B, Britt DW, Huang W, Johnson WP, Anderson AJ (2009) Antimicrobial activities of commercial nanoparticles against an environmental soil microbe, Pseudomonas putida KT2440. J Biol Eng 3:1–13. https://doi.org/10.1186/1754-1611-3-9
Gasparotto J, Chaves PR, da Boit MK, da Rosa-Siva HT, Bortolin RC, Silva LFO, Rabelo TK, Silva J, da Silva FR, Nordin AP, Soares K, Borges MS, Gelain DP, Moreira JCF (2018) Obese rats are more vulnerable to inflammation, genotoxicity and oxidative stress induced by coal dust inhalation than non-obese rats. Ecotoxicol Environ Saf 165:44–51. https://doi.org/10.1016/j.ecoenv.2018.08.097
Geitner NK, Hendren CO, Cornelis G, Kaegi R, Lead JR, Lowry GV et al (2020) Harmonizing across environmental nanomaterial testing media for increased comparability of nanomaterial datasets. Environ Sci Nano 7(1):13–36. https://doi.org/10.1039/c9en00448c
Ginocchio R, Rodríguez PH, Badilla-Ohlbaum R, Allen HE, Lagos GE (2002) Effect of soil copper content and pH on copper uptake of selected vegetables grown under controlled conditions. Environ Toxicol Chem 21:1736–1744. https://doi.org/10.1002/etc.5620210828
Gredilla A, Fdez-Ortiz de Vallejuelo S, Rodriguez-Iruretagoiena A, Gomez L, Oliveira MLS, Arana G, De Diego A, Madariaga JM, Silva LFO (2019) Evidence of mercury sequestration by carbon nanotubes and nanominerals present in agricultural soils from a coal fired power plant exhaust. J Hazard Mater 378:120747. https://doi.org/10.1016/j.jhazmat.2019.120747
Hafeez A, Razzaq A, Mahmood T, Jhanzab HM (2015) Potential of copper nanoparticles to increase growth and yield of wheat. J Nanosci Adv Technol 1:6–11. https://doi.org/10.24218/jnat.2015.02
Hayes KL, Mui J, Song B, Sani ES, Eisenman SW, Sheffield JB, Kim B (2020) Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: a comparison study to phytotoxic aluminum ions. Sci Total Environ 719:137393. https://doi.org/10.1016/j.scitotenv.2020.137393
Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL (2015) Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). Environ Sci Process Impacts 17:177–185. https://doi.org/10.1039/c4em00551a
Hu J, Wu X, Wu F, Chen W, Zhang X, White JC, Li J, Wan Y, Liu J, Wang X (2020) TiO2 nanoparticle exposure on lettuce (Lactuca sativa L.): dose-dependent deterioration of nutritional quality. Environ Sci Nano 7(2):501–513. https://doi.org/10.1039/c9en01215j
Institute of Medicine (1997) Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. The National Academies Press, Washington, DC, p 10.17226/5776
Institute of Medicine (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. The National Academies Press, Washington, DC. https://doi.org/10.17226/10026
John MK (1976) Interrelationships between plant cadmium and uptake of some other elements from culture Solutions by oats and lettuce. Environ Pollut 11:85–95
Kaewchangwat N, Dueansawang S, Tumcharern G, Suttisintong K (2017) Synthesis of copper-chelates derived from amino acids and evaluation of their efficacy as copper source and growth stimulator for Lactuca sativa in nutrient solution culture. J Agric Food Chem 65:9828–9837. https://doi.org/10.1021/acs.jafc.7b03809
Kah M, Tufenkji N, White JC (2019) Nano-enabled strategies to enhance crop nutrition and protection. Nat Nanotechnol 14:532–540. https://doi.org/10.1038/s41565-019-0439-5
Kemi VE, Kärkkäinen MUM, Rita HJ, Laaksonen MML, Outila TA, Lamberg-Allardt CJE (2010) Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. Br J Nutr 103:561–568. https://doi.org/10.1017/S0007114509992121
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70. https://doi.org/10.1016/j.cropro.2012.01.007
Kim MJ, Moon Y, Tou JC, Mou B, Waterland NL (2016) Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J Food Compos Anal 49:19–34. https://doi.org/10.1016/j.jfca.2016.03.004
Lange CN, Figueiredo AMG, Enzweiler J, Castro L (2017) Trace elements status in the terrain of an impounded vehicle scrapyard. J Radioanal Nucl Chem 311:1323–1332. https://doi.org/10.1007/s10967-016-5078-9
Lange CN, Figueiredo AMG, Enzweiler J, Monteiro LR (2018) Potentially toxic elements downward mobility in an impounded vehicle scrapyard. J Radioanal Nucl Chem 316:819–830. https://doi.org/10.1007/s10967-018-5729-0
López-Luna J, Silva-Silva MJ, Martinez-Vargas S, Mijangos-Ricardez OF, González-Chávez MC, Solís-Domínguez FA, Cuevas-Díaz MC (2016) Magnetite nanoparticle (NP) uptake by wheat plants and its effect on cadmium and chromium toxicological behavior. Sci Total Environ 565:941–950. https://doi.org/10.1016/j.scitotenv.2016.01.029
Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z (2010) Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78:273–279. https://doi.org/10.1016/j.chemosphere.2009.10.050
Malavolta E (1980) Elementos de nutrição mineral de plantas [Elements of plant mineral nutrition]. Agronômica Ceres, São Paulo
Mantha H, Schindler M, Hochella MF (2019) Occurrence and formation of incidental metallic Cu and CuS nanoparticles in organic-rich contaminated surface soils in Timmins, Ontario. Environ Sci Nano 6:163–179. https://doi.org/10.1039/C8EN00994E
Martínez-Fernández D, Barroso D, Komárek M (2016) Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ Sci Pollut Res 23:1732–1741. https://doi.org/10.1007/s11356-015-5423-5
McBride MB, Shayler HA, Spliethoff HM, Mitchell RG, Marquez-Bravo LG, Ferenz GS, Russell-Anelli JM, Casey L, Bachman S (2014) Concentrations of lead, cadmium and barium in urban garden-grown vegetables: the impact of soil variables. Environ Pollut 194:254–261. https://doi.org/10.1016/j.envpol.2014.07.036
Mousavi A, Roghani-Mamaqani H, Salami-Kalajahi M, Shahi S, Abdollahi A (2018) Modification of graphene with silica nanoparticles for use in hybrid network formation from epoxy, novolac, and epoxidized novolac resins by sol-gel method: Investigation of thermal properties. Express Polym Lett 12:187–202. https://doi.org/10.3144/expresspolymlett.2018.18
Nabulo G, Young SD, Black CR (2010) Assessing risk to human health from tropical leafy vegetables grown on contaminated urban soils. Sci Total Environ 408:5338–5351. https://doi.org/10.1016/j.scitotenv.2010.06.034
Naika HR, Lingaraju K, Manjunath K, Kumar D, Nagaraju G, Suresh D, Nagabhushana H (2015) Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J Taibah Univ Sci 9:7–12. https://doi.org/10.1016/j.jtusci.2014.04.006
National Academies of Sciences, Engineering, and Medicine (2019) Dietary reference intakes for sodium and potassium. National Academies Press. https://doi.org/10.17226/25353
Nguyen TXT, Amyot M, Labrecque M (2017) Differential effects of plant root systems on nickel, copper and silver bioavailability in contaminated soil. Chemosphere 168:131–138. https://doi.org/10.1016/j.chemosphere.2016.10.047
Nie Z, Hu C, Liu H, Tan Q, Sun X (2014) Differential expression of molybdenum transport and assimilation genes between two winter wheat cultivars (Triticum aestivum). Plant Physiol Biochem 82:27–33. https://doi.org/10.1016/j.plaphy.2014.05.002
Noman M, Ahmed T, Hussain S, Niazi MBK, Shahid M, Song F (2020a) Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J Hazard Mater 398:123175. https://doi.org/10.1016/j.jhazmat.2020.123175
Noman M, Shahid M, Ahmed T, Tahir M, Naqqash T, Muhammad S, Song F, Abid HMA, Aslam Z (2020b) Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol Environ Saf 192:110303. https://doi.org/10.1016/j.ecoenv.2020.110303
Nordin AP, Da Silva J, de Souza CT, Niekraszewicz LA, Dias JF, da Boit K, Oliveira MLS, Grivicich I, Garcia LH, Oliveira LFS, da Silva FR (2018) In vitro genotoxic effect of secondary minerals crystallized in rocks from coal mine drainage. J Hazard Mater 346:263–272. https://doi.org/10.1016/j.jhazmat.2017.12.026
Oliveira ML, da Boit K, Pacheco F, Teixeira EC, Schneider IL, Crissien TJ, Pinto DC, Oyaga RM, Silva LFO (2018) Multifaceted processes controlling the distribution of hazardous compounds in the spontaneous combustion of coal and the effect of these compounds on human health. Environ Res 160:562–567. https://doi.org/10.1016/j.envres.2017.08.009
Oliveira MLS, Ramirez O, Schneider IL, Teixeira EC, Silva LFO (2019) A realistic study of 3D composition of carbon nanotubes and carbonaceous nanocompounds from different soils around coal power plant. Chemosphere 236:124534. https://doi.org/10.1016/j.chemosphere.2019.124534
Pais I, Jones Jr JB (1997) The handbook of trace elements. CRC Press
Paniz FP, Pedron T, Freire BM, Torres DP, Silva FF, Batista BL (2018) Effective procedures for the determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and rare earth elements in plants and foodstuffs. Anal Methods 10:4094–4103. https://doi.org/10.1039/c8ay01295d
Pelegrino MT, Kohatsu MY, Seabra AB, Monteiro LR, Gomes DG, Oliveira HC, Rolim WR, Jesus TA, Batista BL, Lange CN (2020) Effects of copper oxide nanoparticles on growth of lettuce (Lactuca sativa L.) seedlings and possible implications of nitric oxide in their antioxidative defense. Environ Monit Assess 192(4):1–14. https://doi.org/10.1007/s10661-020-8188-3
Peng C, Xu C, Liu Q, Sun L, Luo Y, Shi J (2017) Fate and transformation of CuO nanoparticles in the soil-rice system during the life cycle of rice plants. Environ Sci Technol 51:4907–4917. https://doi.org/10.1021/acs.est.6b05882
Pillay V, Jonnalagadda SB (2007) Elemental uptake by edible herbs and lettuce (Latuca sativa). J Environ Sci Health B 42:423–428. https://doi.org/10.1080/03601230701316416
Plata LF, Ramos CG, Silva MLO, Silva LFO (2021) Release kinetics of multi-nutrients from volcanic rock mining by-products: evidences for their use as a soil remineralizer. J Clean Prod 279:123668. https://doi.org/10.1016/j.jclepro.2020.123668
Priyanka N, Geetha N, Ghorbanpour M, Venkatachalam P (2019) Role of engineered zinc and copper oxide nanoparticles in promoting plant growth and yield: present status and future prospects. In: Ghorbanpour M, Wani S (eds) Advances in Phytonanotechnology, 1st edn., Academic press, pp. 183-201. https://doi.org/10.1016/B978-0-12-815322-2.00007-9
Ramos CG, de Medeiros DDS, Gomez L, Oliveira LFS, Schneider IAH, Kautzmann RM (2020) Evaluation of soil Re-mineralizer from by-product of volcanic rock mining: experimental proof using black oats and maize crops. Nat Resour Res 29(3):1583–1600. https://doi.org/10.1007/s11053-019-09529-x
Rawat S, Pullagurala VLR, Hernandez-Molina M, Sun Y, Niu G, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL (2018) Impacts of copper oxide nanoparticles on bell pepper (Capsicum annum L.) plants: a full life cycle study. Environ Sci Nano 5:83–95. https://doi.org/10.1039/c7en00697g
Rizwan M, Ali S, Rehman MZ, Malik S, Adrees M, Qayyum MF, Alamri SA, Alyemeni MN, Ahmad P (2019) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41:35. https://doi.org/10.1007/s11738-019-2828-7
Rodrigues SM, Demokritou P, Dokoozlian N, Hendren CO, Karn B, Mauter MS, Sadik OA, Safarpour M, Unrine JM, Viers J, Welle P, White JC, Wiesner MR, Lowry GV (2017) Nanotechnology for sustainable food production: promising opportunities and scientific challenges. Environ Sci Nano 4:767–781. https://doi.org/10.1039/c6en00573j
Rolim WR, Pelegrino MT, Lima BA, Ferraz LS, Costa FN, Bernardes JS, Rodigues T, Brocchi M, Seabra AB (2019a) Green tea extract mediated biogenic synthesis of silver nanoparticles: characterization, cytotoxicity evaluation and antibacterial activity. Appl Surf Sci 463:66–74. https://doi.org/10.1016/j.apsusc.2018.08.203
Rolim WR, Pieretti JC, Renó DLS, Lima BA, Nascimento MHM, Ambrosio FN, Lombello CB, Brocchi M, Souza ACS, Seabra AB (2019b) Antimicrobial activity and cytotoxicity to tumor cells of nitric oxide donor and silver nanoparticles containing PVA/PEG films for topical applications. ACS Appl Mater Interfaces 11(6):6589–6604. https://doi.org/10.1021/acsami.8b19021
Segura FR, Nunes EA, Paniz FP, Paulelli ACC, Rodrigues GB, Braga GÚL, Filho WRP, Barbosa F, Cerchiaro G, Silva FF, Batista BL (2016) Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ Pollut 218:813–825. https://doi.org/10.1016/j.envpol.2016.08.005
Sekine R, Marzouk ER, Khaksar M, Scheckel KG, Stegemeier JP, Lowry GV, Donner E, Lombi E (2017) Aging of dissolved copper and copper-based nanoparticles in five different soils: short-term kinetics vs. long-term fate. J Environ Qual 46:1198–1205. https://doi.org/10.2134/jeq2016.12.0485
Servin AD, Pagano L, Castillo-Michel H, De la Torre-Roche R, Hawthorne J, Hernandez-Viezcas JA, Loredo-Portales R, Majumdar S, Gardea-Torresday J, Dhankher OP, White JC (2017) Weathering in soil increases nanoparticle CuO bioaccumulation within a terrestrial food chain. Nanotoxicology 11:98–111. https://doi.org/10.1080/17435390.2016.1277274
Settimio L, McLaughlin MJ, Kirby JK, Langdon KA, Lombi E, Donner E, Scheckel KG (2014) Fate and lability of silver in soils: effect of ageing. Environ Pollut 191:151–157. https://doi.org/10.1016/j.envpol.2014.04.030
Singh D, Kumar A (2016) Impact of soil irrigation using water containing CuO and ZnO nanoparticles on spinach oleracea grown in soil media. Bull Environ Contam Toxicol 97:548–553. https://doi.org/10.1007/s00128-016-1872-x
Singh J, Lee BK (2016) Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J Environ Manag 170:88–96. https://doi.org/10.1016/j.jenvman.2016.01.015
Singh UM, Sareen P, Sengar RS, Kumar A (2013) Plant ionomics: a newer approach to study mineral transport and its regulation. Acta Physiol Plant 35:2641–2653. https://doi.org/10.1007/s11738-013-1316-8
Souza FM, Paniz FP, Pedron T, Santos MC, Batista BL (2019) A high-throughput analytical tool for quantification of 15 metallic nanoparticles supported on carbon black. Heliyon 5:e01308. https://doi.org/10.1016/j.heliyon.2019.e01308
Stanley CR (1987) Hinsdalite and other products of oxidation at the Daisy Creek stratabound copper-silver prospect, northwestern Montana. Can Mineral 25:213–220
Suda A, Makino T (2016) Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on arsenic and cadmium: a review. Geoderma 270:68–75. https://doi.org/10.1016/j.geoderma.2015.12.017
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198. https://doi.org/10.1016/j.plaphy.2015.07.026
Tripathi DK, Singh S, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2016) Silicon nanoparticles more efficiently alleviate arsenate toxicity than silicon in maize cultiver and hybrid differing in arsenate tolerance. Front Environ Sci 4:46. https://doi.org/10.3389/fenvs.2016.00046
Trujillo-Reyes J, Majumdar S, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2014) Exposure studies of core-shell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard? J Hazard Mater 267:255–263. https://doi.org/10.1016/j.jhazmat.2013.11.067
US-EPA Method 3051A (2007) Microwave assisted acid digestion of sediments, sludges, soils and oils, 3rd edn. US Environmental Protection Agency, Washington, DC, DC
van Raij B (2011) Fertilidade do Solo e Manejo de Nutrientes [Soil fertility and management of nutrients]. INPI, Piracicaba (in Portuguese)
van Raij B, Cantarella H, Andrade JC, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico, Campinas, Brazil. Análise química para avaliação da fertilidade de solos tropicais. Instituto Agronômico, Campinas, SP, Brazil
Wang Y, Sun C, Zhao X, Cui B, Zeng Z, Wang A, Liu G, Cui H (2016) The application of nano-TiO2 photo semiconductors in agriculture. Nanoscale Res Lett 11:1–7. https://doi.org/10.1186/s11671-016-1721-1
Wang Y, Lin Y, Xu Y, Yin Y, Guo H, Du W (2019) Divergence in response of lettuce (var. ramosa Hort.) to copper oxide nanoparticles/microparticles as potential agricultural fertilizer. Environ Pollut Bioavailab 31:80–84. https://doi.org/10.1080/26395940.2019.1578187
Wang Y, Deng C, Cota-Ruiz K, Peralta-Videa JR, Sun Y, Rawat S, Tan W, Reyes A, Hernandez-Viezcas JA, Niu G, Li C, Gardea-Torresdey JL (2020) Improvement of nutrient elements and allicin content in green onion (Allium fistulosum) plants exposed to CuO nanoparticles. Sci Total Environ 725:138387. https://doi.org/10.1016/j.scitotenv.2020.138387
White JC, Gardea-Torresdey J (2018) Achieving food security through the very small. Nat Nanotechnol 13:627–629. https://doi.org/10.1038/s41565-018-0223-y
Wilcox J, Wang B, Rupp E, Taggart R, Hsu-Kim H, Oliveira ML, Cutruneo CMNL, Taffarel S, Silva LFO, Hopps SD, Thomas GA, Hower JC (2015) Observations and assessment of fly ashes from high-sulfur bituminous coals and blends of high-sulfur bituminous and subbituminous coals: environmental processes recorded at the macro-and nanometer scale. Energy Fuel 29(11):7168–7177. https://doi.org/10.1021/acs.energyfuels.5b02033
Xu D, Zhou P, Zhan J, Gao Y, Dou C, Sun Q (2013) Assessment of trace metal bioavailability in garden soils and health risks via consumption of vegetables in the vicinity of Tongling mining area, China. Ecotoxicol Environ Saf 90:103–111. https://doi.org/10.1016/j.ecoenv.2012.12.018
Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119:77–88. https://doi.org/10.1007/s12011-007-0046-4
Zamberlan DC, Halmenschelager PT, Silva LFO, Da Rocha JBT (2020) Copper decreases associative learning and memory in Drosophila melanogaster. Sci Total Environ 710:135306. https://doi.org/10.1016/j.scitotenv.2019.135306
Zhang K, Yuan J, Kong W, Yang Z (2013) Genotype variations in cadmium and lead accumulations of leafy lettuce (Lactuca sativa L.) and screening for pollution-safe cultivars for food safety. Environ Sci Process Impacts 15(6):1245–1255. https://doi.org/10.1039/C3EM00158J
Zhao L, Huang Y, Hannah-Bick C, Fulton AN, Keller AA (2016a) Application of metabolomics to assess the impact of Cu(OH)2 nanopesticide on the nutritional value of lettuce (Lactuca sativa): enhanced Cu intake and reduced antioxidants. NanoImpact 3–4:58–66. https://doi.org/10.1016/j.impact.2016.08.005
Zhao L, Huang Y, Hu J, Zhou H, Adeleye AS, Keller AA (2016b) 1H NMR and GC-MS based metabolomics reveal defense and detoxification mechanism of cucumber plant under nano-Cu stress. Environ Sci Technol 50:2000–2010. https://doi.org/10.1021/acs.est.5b05011
Zhao L, Ortiz C, Adeleye AS, Hu Q, Zhou H, Huang Y, Keller AA (2016c) Metabolomics to detect response of lettuce (Lactuca sativa) to Cu(OH)2 nanopesticides: oxidative stress response and detoxification mechanisms. Environ Sci Technol 50:9697–9707. https://doi.org/10.1021/acs.est.6b02763
Zuverza-Mena N, Medina-Velo IA, Barrios AC, Tan W, Peralta-Videa JR, Gardea-Torresdey JL (2015) Copper nanoparticles/compounds impact agronomic and physiological parameters in cilantro (Coriandrum sativum). Environ Sci Process Impacts 17:1783–1793. https://doi.org/10.1039/c5em00329f
Acknowledgments
The authors thank the Brazilian Nanotechnology National Laboratory/Center for Research in Energy and Materials (LNNano/CNPEM) for TEM analyses. The authors are grateful to the Multiuser Central Facilities (UFABC) for the experimental support. We thank Msc Alessandro Lamarca Urzedo and Dr. Danilo da Cruz Centeno for the technical support.
Funding
This study received funding from Fundação de Amparo à Pesquisa do Estado de São Paulo (grants 2017/05029-8, 2017/20914-8, 2018/08194-2, 2019/26337-8 and 2016/10060-9) to Conselho Nacional de Desenvolvimento Científico e Tecnológico (grants 429555/2018-0, 153204/2018-4, 313117/2019-5 and 404815/2018-9). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES) (grant 88887.363169/2019-00)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
Camila Neves Lange—Conceptualization, Methodology, Analysis, Project administration, Writing - review and editing; Marcio Yukihiro KohatsuMethodology, Analysis, Writing - review and editing; Milena Trevisan Pelegrino—Methodology, Analysis, Writing; Lucilena Rebelo Monteiro—Statistical analysis and manuscript writing; Bruna Moreira Freire—Sample analysis and manuscript writing; Rodrigo Mendes Pereira—Sample analysis and manuscript writing; Paola Fincheira—Sample preparation and writing; Olga Rubilar—Writing - review and editing; Gonzalo Tortella—Writing - review and editing; Bruno Lemos Batista—Conceptualization, Funding acquisition, Writing - review and editing; Tatiane Araújo de Jesus—Conceptualization, Funding acquisition, Writing - review and editing; Amedea Barozzi Seabra—Conceptualization, Methodology, Writing - review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 538 kb)
Rights and permissions
About this article
Cite this article
Kohatsu, M.Y., Pelegrino, M.T., Monteiro, L.R. et al. Comparison of foliar spray and soil irrigation of biogenic CuO nanoparticles (NPs) on elemental uptake and accumulation in lettuce. Environ Sci Pollut Res 28, 16350–16367 (2021). https://doi.org/10.1007/s11356-020-12169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-12169-x