Abstract
Plant leaves can intercept and directly absorb nanoparticles (NPs) that deposit on their surface, which can lead severe phytotoxicity. However, there is a large blind spot when it comes to the fate and phytotoxicity of NPs after leaf exposure, even though foliar uptake is likely to occur. In this study, lettuce leaves (Lactuca sativa L. var. ramosa Hort.) were exposed to different concentrations of copper-oxide NPs (CuO-NPs, 0, 100, and 1000 mg L−1) for 5, 10, and 15 days. Foliar uptake, subcellular distribution, chemical forms, and impact of CuO-NPs on nutrient status, antioxidant systems, and lettuce growth were examined. Substantially elevated Cu levels were observed in lettuce leaves (up to 6350 mg kg−1), which was one magnitude greater than that in the roots (up to 525 mg kg−1). Cu translocation factors from leaves to roots ranged from 1.80 to 15.6%. The application of CuO-NPs severely inhibited lettuce growth and altered the nutrient status in plants (especially Mn, K, and Ca). Moreover, CuO-NPs increased H2O2 generation, malonaldehyde level (on the 5th and 10th day of exposure), and catalase activity (on the 15th day of exposure) in lettuce leaves. The Cu concentrations in subcellular fractions were ranked: cell wall ≈ organelles > soluble fraction in lettuce leaves, and organelles > cell wall > soluble fraction in lettuce roots. Undissolved Cu forms were predominant in lettuce, which may have helped to reduce the Cu’s mobility and phytotoxicity in the plant. The findings of this study will be of great interest in areas with high levels of metal-NPs in the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Given the wide range of nanoparticle (NP) uses in environmental, commercial, and medical areas, NP production has reached its highest industrial scale (Anjum et al. 2015; Rippner et al. 2018). The worldwide production of Cu-based NPs was estimated to be ~ 200 t every year in 2010 and continues to increase (Keller et al. 2013). Due to the ultrafine size and property of Cu-based NPs, it is widely used in solar cells, gas sensors, catalysts, electronics, lubricant oils, polymers/plastics, and pigments (Anjum et al. 2015). In particular, copper-oxide NPs (CuO-NPs) are increasingly used in antimicrobial products or formulations, because of its antimicrobial properties. For instance, CuO-NPs are used as biocide in paints, plastics, and textiles (Perreault et al. 2014b). They are also used in agriculture for plant protection, for example, to resist phytopathogenic fungus on vines (Gogos et al. 2012; Trujillo-Reyes et al. 2014). Owing to its multifarious uses and high potential to enter different environmental compartments, CuO-NPs have been the major focus in recent bio-toxicity studies (Fedorenko et al. 2020; Ke et al. 2017; Rajput et al. 2020; Tamez et al. 2019).
Plants are represented as the greatest interface between the biosphere and environment. They provide a very large surface area for NP exposure via above-ground biomass and roots (Dietz and Herth 2011; Singh et al. 2017). The scientific community has paid more attention to study the uptake of metal-NPs by plant roots. It is known that natural atmospheric NPs from eolic or volcanic sources can interact with plants through their foliar organs (Hong et al. 2014; Xiong et al. 2019). A portion of engineered NPs released into the environment likely reaches the leaves of plants via wind dispersion (Hong et al. 2014; Uzu et al. 2009). For instance, air-dispersed NPs may penetrate into leaves through stomatal openings, aqueous pores, ectodesmata, cuticular cracks, and lenticels (Pullagurala et al. 2018). However, the research field has a large blind spot when it comes to the fate and phytotoxicity of NPs after leaf exposure, even though this pathway is a very common occurrence.
Although no exact data is available on the level of CuO-NPs in the atmosphere with respect to the increasing levels of CuO-NPs released into the environment, plant leaves are exposed to unusually high concentrations of CuO-NPs. Adhikari et al. (2016) revealed that 8 ppm foliar spray and 0.02 ppm root exposure of CuO-NPs can enter into plant cells, be easily absorbed by plants, and regulate different enzyme activities to enhance plant growth. According to Hong et al. (2015b), the leaves of cucumber seedlings were exposed to engineered CuO-NPs at a level of 50, 100, and 200 mg/L. The results showed that plants undergo phytotoxic symptoms observed as a decrease in transpiration rate and net photosynthesis rate in seedling leaves when exposed to higher levels of CuO-NPs. Also, metal oxide NP exposure was responsible for variation in nutrient/water uptake and developmental processes in plants, resulting in differences in final biomass and yield (Du et al. 2016). The main toxicity mechanisms of metal-NPs are cellular oxidative stress and metabolic dysfunction (Chang et al. 2012; Perreault et al. 2014a).
Moreover, despite recent efforts, few reports are available on Cu distribution patterns and chemical speciation in plants in response to CuO-NP stress after foliar exposure. Metals are distributed in different cell organelles and are transformed into different metal species once taken up by plants. The most important mechanisms responsible for the detoxification in plants refer to the retention of metals in plant cell walls, compartmentalization of metals in organelles, and the blocking of metal transportation in different tissues (Li et al. 2016; Liu et al. 2014). Metal species including inorganic, water-soluble, pectate- and protein-integrated, undissolved phosphate, and oxalate forms govern the biogeochemical behavior of metals inside plants (Lai 2015; Shahid et al. 2017). Therefore, a critical step is investigating the subcellular distribution and chemical speciation of Cu in lettuce, which will contribute to understanding the fate of CuO in lettuce.
In order to safely expand the multidisciplinary use of CuO-NPs, while taking into account the recent information obtained from biochemical and metabolic studies, this study focuses on the air-plant system to explore mechanisms of CuO-NP toxicity and tolerance in plants. In this study, lettuce leaves (Lactuca sativa L. var. ramosa Hort.) were exposed with a series of CuO-NP concentrations (0, 100, and 1000 mg L−1) for 5, 10, and 15 days. Our study will explore: (1) foliar accumulation of CuO-NPs and the translocation factor from leaf to root, (2) effect of CuO-NPs on the plant nutrient content (Fe, Zn, Mn, K, and Ca), growth, and antioxidant system, (3) Cu distribution in the plant subcellular fraction, and (4) chemical speciation of Cu in plants. This study will give us a better understanding of CuO-NP toxicity after foliar uptake and provide valuable information for further research on how to reduce the phytotoxicity of metal-NPs, while increasing the yield and quality of crops.
Methods and materials
CuO-NPs characterization
The CuO-NPs used for plant exposure in this study were from a commercial product (Sigma-Aldrich®, CAS 1317-38-0). The morphology of the CuO-NPs was investigated by transmission electron microscopy (TEM, JEM-1400 Plus, Japan) coupled with energy-dispersive spectrometer (EDS, Aztec X-Max 80, OXFORD). CuO-NPs (2 mg) were suspended in ethanol (10 mL) and ultrasonicated in a water bath at 240 W and 40 kHz (Ultrasonic vibration generator, JP-040, Jiemeng, China) for 5 min before TEM observation. The specific surface area of CuO-NP was characterized in our previous study according to the Brunauer, Emett et Teller (BET) method (Goix et al. 2014; Xiong et al. 2019). The CuO-NP suspensions (100 mg L−1 and 1000 mg L−1) were prepared by suspending CuO-NPs in ultrapure water, followed by sonication in a water bath at 240 W and 40 kHz (Ultrasonic vibration generator, JP-040, Jiemeng, China) for 30 min. The size distribution and zeta potential of the CuO-NP dispersion in ultrapure water at the exposure concentrations were estimated using a Nanosizer (Malvern Zetasizer Nano ZS90, Britain).
Seed germination and plant growth
Lettuce seeds were surface-sterilized with 4% sodium hypochlorite (NaClO) for 10 min, rinsed three times with distilled water, and then cultured for germination (one week) (Haslett et al. 2001). After germination, the plants were grown with half-strength Hoagland’s nutrient solution for 2 weeks under hydroponic conditions. Foliar transfer experiments were carried out with a day/night temperature range of 25 ± 2 °C (16 h)/20 ± 2 °C (8 h). The light intensity in the controlled chamber was 425 ± 50 photons µmol m−2 s−1, and the relative humidity was controlled to 65 ± 5%.
Three-week-old plants were exposed to different concentrations of CuO-NPs. The CuO-NP treatments were applied on plant leaves: a control condition without particles (0 mg L−1), 100 mg L−1, and 1000 mg L−1, corresponding to a quantity of CuO-NPs approximately 0, 8, and 80 µg plant−1 day−1. Application of CuO-NPs was conducted by applying droplets of CuO-NP suspension to the adaxial surface of lettuce leaves using a pipette. A total of 40 µL were deposited on every plant every 12 h. The cultivation devices were pots with lids that had a small hole. Dry sponge mats were added to the small holes to avoid root contact with CuO-NPs.
Leaf and root samples were collected and prepared for analysis 5, 10, and 15 days after treatment. Each treatment was replicated five times. Plant growth was assessed by shoot length, root length, and plant biomass, which were measured at every time point (0, 5, 10, and 15 days). The shoot growth, root growth, and increased weight were expressed as the increased shoot (or root) length and weight on the 5th, 10th, and 15th day in comparison with the 1st day of exposure.
Metal accumulation in plant tissues
Sample preparation for determining elemental content within the plants was carried out, according to Xiong et al. (2014b). After harvest, the leaves and roots were separated and washed carefully to remove surface particles. Around 0.1 g of homogenized dry plant samples were digested with a 6 mL mixture of HNO3:H2O2 (5:1 v/v) at 200 °C for 30 min (Microwave oven, Model MDS-6, Preekem Scientific Instruments Co., Ltd., Shanghai, China). After filtration, Cu, Zn, Fe, Mn, K, and Ca levels in the plant tissues were quantified by flame atomic absorption spectrophotometer (FAAS, Hitachi Z-5300, Japan). Three replicates were analyzed for each different experimental condition.
The Cu translocation factor (TF) from leaves to roots was calculated as follows, according to a previous report (Xiong et al. 2014b):
where [Cu]roots and [Cu]leaves represent metal concentration in roots and leaves, respectively.
Oxidative stress assessment
Reactive oxygen species (ROS) generation: H2O2 content
H2O2 in lettuce leaves was determined by the ROS-sensitive dye 3′3′-diaminobenzidine (DAB) following the method described by Ma et al. (2015a, b, c) and Shaw and Hossain (2013). The plant leaves and roots from the control and NP-treated plants were incubated in 1 mg mL−1 DAB-HCl (pH 3.8) overnight (about 9 h). Chlorophyll was removed by infiltration with 95% ethanol solution followed by boiling in a water bath (95 °C) for 5 min. After washing, tissue images were captured using a Leica DM3000 fluorescence microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany) equipped with a digital camera (Leica DFC7000 T). The deep-brown polymerization product indicated the H2O2 accumulation after reacting with DAB. The H2O2 generation in plant leaves was quantified by the image processing and analysis software Image J 1.46r. A modified method was used for quantitative H2O2 measurements in the plant roots (Kotchoni et al. 2006). After DAB dyeing, the root samples were homogenized in 1 mL 0.2 M HClO4 in a pre-cooled mortar and centrifuged at 4 °C (10,000 g) for 10 min. The absorbance of the supernatants was then measured at a wavelength of 450 nm (DU®730 Nucleic Acid/Protein Analyzer, BECKMAN COULTER®).
Membrane lipid peroxidation
Membrane lipid peroxidation in lettuce leaves was determined by measuring malondialdehyde (MDA) content. MDA content was determined through a color reaction with thiobarbituric acid (TBA), followed by a measurement of optical density of the reaction solution (Nazari et al. 2012). Plant leaves (400 mg) were homogenized in 4 mL of 10% (w/v) of trichloroacetic acid and centrifuged at 4000 rpm for 10 min. According to the method described by Ma et al. (2015a), 2 mL of the extracts reacted with 2 mL of 0.6% TBA at 95 °C in a boiling water bath for 15 min. Then, the mixture was cooled rapidly on an ice bath and centrifuged at 4000 rpm for 15 min before measuring the absorbance of the supernatant at 450 nm, 532 nm, and 600 nm. The concentration of MDA was calculated as follows:
where A450, A532, and A600 are the absorbances at 450 nm, 532 nm, and 600 nm, respectively.
Catalase (CAT) activity
The CAT activity was measured by the method of Dimkpa et al. (2012) and Hong et al. (2015a). About 1 g of plant sample (leaf or root) was individually extracted in 100 mM sodium phosphate buffer (pH 7.5) containing 5 mM DTT and 5% PVP followed by centrifugation at 1200 g for 30 min (SIGMA Laborzentrifugen 3K15, Germany). The supernatant was separated after centrifugation, and 20 mM H2O2 was added. The decrease in absorbance of H2O2 was read at 240 nm for 4 min through a UV spectrophotometer. CAT activity (U/g FW) was obtained from the slope of the H2O2 absorbance curve and normalized to the protein concentration.
Subcellular distribution
Cells were separated into three different fractions: cell wall, organelle, and soluble fraction, as described by Li et al. (2016). Fresh tissue (4 g) was homogenized in pre-cooled medium (50 mM Tris–HCl, 250 mM sucrose, and 1.0 mM DTE (C4H10O2S2), pH 7.5) at 4 °C. The homogenate was centrifuged at 340 g for 10 min (4 °C), and the precipitate was obtained and defined as the ‘cell wall fraction’. The supernatant was centrifuged at 20,000 g for 45 min (4 °C). The deposit was referred to as the ‘organelle fraction’ (excluding vacuoles), and the supernatant solution as the ‘soluble fraction’ (including vacuoles). The three subcellular fractions were collected, oven-dried, acidic digested, and subjected to Cu analysis by FAAS.
Chemical speciation
Chemical speciations of Cu were extracted by sequential extraction procedure in the following order: 80% ethanol, deionized water (dH2O), 1 M NaCl, 2% acetic acid (HAc), 0.6 M HCl, and the last remaining residue, as conducted in Zou et al. (2019). First, fresh tissue (2 g) was homogenized in 80% ethanol (20 mL) and shaken for 18 h at room temperature. The homogenate was centrifuged at 5000 g for 10 min to obtain the first supernatant solution. The deposition was extracted again with the same extraction solution by repeating the procedure two more times. The three supernatants were then pooled, and the residue was extracted with the next extraction solution (Li et al. 2016). Each pooled solution was oven-evaporated to a constant weight, digested, and then subjected to Cu analysis by FAAS.
Statistical analysis
For each exposure condition, statistical analyses were carried out on the means of five (or three) replicates, and the data are presented as the mean ± standard deviation (SD). Analysis of variance (ANOVA) was performed using Duncan’s test at p < 0.05 with SPSS 18.0 software. The differences between control conditions and treatments were subjected to the independent samples t test. The correlation between different parameters was analyzed by partial correlation coefficient (r-value), and the significance levels were set at p < 0.05 (*) and p < 0.01 (**).
Results and discussion
CuO-NPs characterization
The characterization of the CuO-NPs is shown in Fig. S1 and Table S1. The primary particle size of CuO-NPs ranged from 40 to 200 nm as determined from four different TEM imaging (Fig. S1a), EDS spectra showed that the nanoparticles consist of Cu and O elements (Fig. S1b). The specific surface area of CuO-NP was 12.6 m2 g−1, as documented in our previous study (Goix et al. 2014; Xiong et al. 2019). The particle size distribution in ultrapure water for 100 and 1000 mg L−1 CuO-NPs was 642 and 1559 nm, respectively (Fig. S1c, d, and Table S1). The zeta potentials in ultrapure water were 16.1 and 9.9 mV for 100 and 1000 mg L−1 CuO-NPs, respectively (Fig. S1e, f, and Table S1).
Cu accumulation in plant tissues and influences on nutrient elements
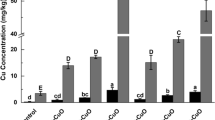
Lettuce accumulated high concentrations of Cu after foliar exposure to CuO-NPs (Fig. 1a). Cu concentration in the control groups was only 4.0–14.0 mg kg−1 in leaves. The concentration increased to 414–659 mg kg−1 when treated with 100 mg L−1 of CuO-NPs and finally increased up to 3100–6350 mg kg−1 when treated with 1000 mg L−1 of CuO-NPs. The TF of Cu ranged between 1.80 and 15.6% after CuO-NP treatment. Cu concentration in roots was 5.46–26.0 mg kg−1 in the control groups. The concentration increased to 7.46–83.6 mg kg−1 and 102–525 mg kg−1 after being treated with 100 and 1000 mg L−1 of CuO-NPs, respectively.
Metal concentrations in lettuce leaf and root (DW) after 5, 10, and 15 days of foliar exposure to CuO-NPs (0, 100, and 1000 mg L−1): Cu (a), Fe (b), Zn (c), Mn (d), K (e), and Ca (f) concentration. Values are expressed as the mean of three replicates (± SD) for each treatment; the different lowercase letters indicate significant difference in leaf or root at p < 0.05
Cu concentration in plant tissue was significantly positively correlated with exposure dose (r = 0.93 and 0.66, respectively, in leaf and root, p < 0.01). Cu concentration in leaves was weakly correlated with exposure duration (r = 0.22, p > 0.05), but was moderately associated with exposure duration in plant roots (r = 0.44, p < 0.05). Cu concentration in lettuce leaves and roots was positively correlated (r = 0.72, p < 0.01). In the present study, lettuce leaves were treated with CuO-NPs and washed carefully before determining metal concentration, and roots were protected with a plastic cover on top of the cultivation device to avoid directly contact with NPs. The result indicates that after foliar exposure, Cu could be absorbed by plant leaves and transferred to plant roots by dosage and duration-dependent methods.
Metal-NP uptake by plant leaves were also observed in several other studies (Du et al. 2016; Natasha et al. 2019; Shahid et al. 2017). According to Hong et al. (2015b), after foliar exposure to CuO and CeO2, Cu and Ce were assimilated by cucumber leaves and were transferred to stems and other tissues. Adhikari et al. (2016) reported that Cu NPs could enter into the cells of maize both through their leaves and roots, and the entry of Cu NPs across the cell membrane was confirmed by transmission electron microscopy images. It is believed that the uptake of air-dispersed NPs functions directly from the penetration and transport via the stomatal openings (Pullagurala et al. 2018; Raliya et al. 2016). According to the previous references, both NPs and the dissolved Cu could transfer within plants. However, NP uptake quantification in different plant parts remains a challenge (Singh and Kumar 2020). Due to their small size and large surface energy values, NPs are prone to aggregation in the aqueous phase and may affect bioavailability and toxicity of NP to plants. In addition, NPs have a tendency to remain around the epidermis of the plant tissue. The chemical speciation changes during metal transfer. It is easier for the soluble Cu to transport over long distances. Overall, NP internalization depends on many factors like type, size, surface charge, the presence of other NPs, and plant anatomy. The results of Cu accumulation showed that plants can absorb large quantities of NPs by their leaves and are able to reduce airborne particulate matter by capturing particles with their leaves (Liu et al. 2018; Wang et al. 2015a, b; Xie et al. 2018; Xiong et al. 2014a). However, excess metal particles in plants could also lead to severe phytotoxicity and potential risk of food safety.
Nutrient elements (Fe, Zn, Mn, K, and Ca) in lettuce leaves and roots after 5, 10, and 15 days of exposure to CuO-NPs (0, 100, and 1000 mg L−1) are presented in Fig. 1b–f. Fe concentration first increased and then decreased in plant leaves after CuO-NP exposure, whereas Fe concentration in plant roots increased significantly with exposure dosage (r = 0.43, p < 0.05) and exposure duration (r = 0.40, p < 0.05) during the entire experimental procedure (Fig. 1b). Zn concentration remained constant in plant leaves after CuO-NP exposure when compared to the control groups, but increased with exposure duration (r = 0.84, p < 0.01). Zn concentration in plant roots changed drastically, decreasing after 5 and 10 days of exposure and increasing on the 15th day (Fig. 1c). Mn concentration significantly decreased in plant leaves after 15 days of exposure and significantly decreased during the whole exposure duration in plant roots (Fig. 1d). K concentration in plant leaves was negatively correlated with exposure dose (r = −0.43, p < 0.05) and significantly decreased after 15 days of exposure to CuO-NPs. However, the K concentration varied greatly in plant roots. There was no correlation between K concentration in plant roots, and both exposure dose and exposure duration (Fig. 1e). Ca concentration significantly decreased in plant leaves after 15 days of exposure to CuO-NPs, but remained constant in plant roots (Fig. 1f).
In conclusion, Cu caused the impaired uptake of essential elements to varying levels, including inhibiting the content of Mn, K, and Ca in plant leaves as well as the content of Mn in plant roots after 15 days of exposure to CuO-NPs. Fe content significantly increased in plant roots after CuO-NP treatment. The effects of metal-NPs on nutrient status have also been reported in many previous studies. According to Le Van et al. (2016), the nutritional status of cotton was changed by nano-CuO (30 ± 10 nm; 10, 200, and 1000 mg L−1), where the uptake of Mn, Zn, Fe, Mg, Mo, and B was significantly reduced. Nano-Cu/CuO (10 and 20 mg L−1, treated for 15 days) increased the uptake of Cu, Al, and S, but decreased the uptake of Mn, Ca, P, and Mg in the treated lettuce (Trujillo-Reyes et al. 2014). Taken together, NPs can influence the concentration of macro/micronutrient elements in plants, and the outcome is usually harmful.
The study of Rodrigo-Moreno et al. (2013a) suggested that Cu transport into the cytosol can produce highly toxic hydroxyl radicals (OH·) and regulate plasma membrane OH· -sensitive Ca2+ and K+ channels in Arabidopsis roots. In this context, the activation of Ca2+ and K+ efflux channels may result in Ca2+ and K+ leakage from cells, even causing programmed cell death (Demidchik et al. 2010). As essential micronutrients, the transition metals Fe, Zn, or Mn act as cofactors in many fundamental plant cell proteins (Rodrigo-Moreno et al. 2013b). As a component of the Mn-superoxide dismutase (SOD) enzyme, Mn plays a vital role in redox reactions that protect plants from the damaging effects of ROS. Moreover, Mn is essential for the oxygen evolution on the lumenal side of Photosynthesis II. Mn deficits may change the oxygen evolution rate, disturb photosynthesis, and inhibit plant growth and development (Dalcorso et al. 2014; Yruela 2013). Other symptoms of Mn deficiency are chlorosis in young leaves, lower grain yields, and shorter roots due to the reduction in pollen fertility and carbohydrate production (Dalcorso et al. 2014). Thus, Mn deficiency may cause damage in plants at different levels. As a component of Cu/ZnSOD, ascorbate peroxidase (APX), and CAT, the Zn element is indispensable for scavenging superoxide radicals (O2·−) and H2O2 (Moretto et al. 2018). The variation of Zn may influence the antioxidase system in lettuce. Concentrations of other macro- (e.g., P, K, and S) and micro- (e.g., Fe and Cu) nutrients can be affected by Zn homeostasis (Jain et al. 2013; Nair and Chung 2017). The variation in nutrient content, along with hormone levels, also affects root morphology, such as lateral root formation or root branching (Jain et al. 2013). As a result of root damage, the essential element absorption from Hoagland’s nutrient solution by a plant’s roots may be inhibited.
Cu toxicity on plant growth
CuO-NPs significantly inhibit lettuce growth after foliar exposure. Lettuce exhibited characteristics of slow growth, weak leaves, and undeveloped roots when compared with the control plants (Fig. 2a). The shoot growth and increased plant dry weight (DW) was inhibited by 46–76% and 25–79%, respectively, and significantly decreased after 10 days of exposure. Root growth was inhibited by 1–53% and significantly decreased after 15 days of exposure (Fig. 2b–d). The toxicity was more severe with an increase in exposure duration and exposure dose. According to the data of Cu accumulation in plant tissue, we found that shoot growth and plant DW was prevented only when Cu concentration in the plant leaves was above 414 mg L−1. Similarly, root growth was observably inhibited only when Cu concentration in plant roots reached 525 mg L−1.
Growth status of lettuce after 15 days of foliar exposure to CuO-NPs (0,100, and 1000 mg L−1) (a); Shoot growth (b), root growth (c), and increased weight (d) of lettuce after 5, 10, and 15 days of foliar exposure to CuO-NPs (0, 100, and 1000 mg L−1). Values are expressed as the mean of five replicates (± SD) for each treatment; the different lowercase letters indicate significant difference at p < 0.05
The effect of NPs in higher plants have been reported in a growing number of recent studies, including both positive (Hussain et al. 2018; Rossi et al. 2019) and negative effects (Rizwan et al. 2017; Xiong et al. 2017). However, numerous studies have demonstrated that CuO-NPs at different concentrations adversely affect plant growth in lettuce, radish, cucumber, rice, alfalfa, maize, and barley, in a dosage-dependent manner (Adhikari et al. 2016; Hong et al. 2015a; Rizwan et al. 2017; Shaw et al. 2014; Shaw and Hossain 2013; Wang et al. 2015a; Wu et al. 2014). For example, Dimkpa et al. (2012) reported that CuO-NPs (500 mg kg−1, treated for 14 days) reduced the shoot and root length of wheat by 13% and 59%, respectively, and their roots were thinner and more brittle than the control and exhibited necrotic spots. Similarly, lettuce and alfalfa exposed to CuO-NPs that were suspended in Hoagland’s nutrient solution (20 ppm) for 15 days reduced their root length by 50% and 48%, respectively, and brown coloration observed in the roots as compared to the control plants (Hong et al. 2015a). Xiong et al. (2017) reported that high foliar Cu uptake by lettuce decreased water content, net photosynthesis level, and plant weight after exposure to CuO-NPs (0, 10, or 250 mg per plant) for 15 days. However, most of these studies focused on seed germination or root exposure to NPs. The present study shows how foliar exposure to CuO-NPs can also lead to serious phytotoxicity, which is an important milestone toward understanding the mechanisms involved in toxicity of leaf exposure to metal-NPs.
According to our previous study, we found that most CuO-NPs were deposited as micrometric aggregates either on the leaf surface or in the stomata (Xiong et al. 2017). The present study also discovered that there was 2.3–74% (mean 37%) of the applied NPs on the surface of lettuce leaves (Table S2). In the previous study of Zhou et al. (2011), they found that CuO-NPs were deposited on plant root surfaces both by adsorption and mechanical adhesion; the adsorption of CuO-NPs increased with increasing exposure concentrations in the range of 5.0–200 mg L−1. The formation of CuO aggregates on foliar surfaces could lead to structural damages such as deformation of stomata in Lactuca sativa (Xiong et al. 2017) and may further reduce stomatal conductance, decrease light-harvesting, influence the structure and function of the chloroplasts, and, finally, result in an inhibition in photosynthesis, and, hence, plant growth. Vice versa, the reduction in shoot and root growth can decrease the surface area for photosynthetic rate and water uptake and consequently affect overall plant performance (Rajput et al. 2020).
Leafy lettuce is a widely cultivated vegetable in many countries. It is typically eaten cold and raw in salads, hamburgers, and many other dishes (Sani et al. 2011). In the present study, substantially elevated Cu levels were observed in the plants and CuO-NPs seriously inhibited lettuce growth, probably by forming Cu aggregates on the leaf surface, disturbing the nutrient content (especially Mn, K, and Ca) and inducing oxidative damage in lettuce. Thus, the quality of lettuce was deeply affected. Cu accumulation in vegetables followed by ingestion may result in a significant contribution to the human daily intake of inorganic Cu. According to the study of Dimkpa et al. (2012), Hong et al. (2015b), and Wang et al. (2016), CuO-NPs have been reported to reduce the growth of wheat (Triticum aestivum), cucumber (Cucumis sativus), and Arabidopsis thaliana. It also significantly increased Cu accumulation in plant tissue, which may cause potential health effects on the food chain. The study of Keller et al. (2018) found that after leaf exposure, lettuce retained a high amount of CuO-NPs on the leaf surface even after washing. The data indicate that certain fractions of CuO-NPs are taken up by plants which may result in undesirable accumulation in edible plant tissues, ultimately exposing humans via the food chain.
Oxidative stress assessment
ROS generation
It is well reported that NPs can induce ROS generation and oxidative damage in plants (Ma et al. 2015a, b, c; Xia et al. 2008). H2O2 is one of the important representatives of ROS. It is relatively stable compared to other types of ROS. Signals such as hormones, biotic, and abiotic stress can stimulate the production and accumulation of H2O2 in plant cells.
After DAB staining, NP-stressed lettuce leaves exhibited brown or dark brown spots that pinpointed H2O2 deposits. The location of this brown polymerization was observed easily by the naked eye (Fig. 3a). On the 5th day, the brown spots were mainly present in the central veins of leaves. On the 15th day, entire leaf surfaces were covered by the brown polymerization product. No such spots were detected in the control leaves throughout the experiment. The presence of dark brown spots indicated serious oxidative burst under CuO-NP stress. Corresponding quantitative H2O2 measurements are shown in Fig. 3b. The relative intensity increased both with exposure dosage and exposure duration, which is consistent with the observed DAB dyeing results. Shaw and Hossain (2013) also investigated the foliar H2O2 accumulation under nano-CuO stress. After histochemical staining with DAB, the dark brown spots were visualized on rice leaves, indicating severe oxidative burst under nano-CuO stress.
H2O2 generation (indicated by dark brown spots) in lettuce leaves as a function of exposure duration (5, 10, and 15 days) and CuO-NPs dose (0, 100, and 1000 mg L−1) deposited on leaves (a), and the corresponding quantitative results (b). Values are expressed as the mean of three replicates (± SD) for each treatment; the different lowercase letters indicate significant difference at p < 0.05
Lettuce roots exhibited brown polymerization product both in the control and CuO-NP exposed groups (Fig. S2a). The quantitative H2O2 measurements in plant roots are shown in Fig. S2b. On the 5th and 10th day, the absorbance at 450 nm increased after exposure to CuO-NPs. On the 15th day, no significant difference was observed between treatment groups and the control. The amount of H2O2 generation in roots was more pronounced in the first 10 days and was alleviated on the 15th day.
Membrane lipid peroxidation
MDA is a marker of lipid peroxidation in the membrane (Canivet et al. 2015). After 5 days of CuO-NP exposure, MDA level increased in both 100 and 1000 mg L−1 CuO-NP exposure groups. On the 10th day, the MDA level rised only in the 1000 mg L−1 CuO-NP exposure group. After 15 days, there was no significant difference between the control and CuO-NP treatment groups (Fig. S3).
The effects of NPs on lipid peroxidation were different depending on the type of NPs and biological models studied (Canivet et al. 2015). For instance, Shaw and Hossain (2013) reported that CuO-NP increased MDA level in rice leaves after 7 and 14 days. Similarly, CuO-NP significantly increased MDA content in the leaves of Arabidopsis thaliana after 4 days of exposure (Ke et al. 2017). However, Ghosh et al. (2010) highlighted that TiO2 NP at 4 mM increased the MDA concentration in Allium cepa roots, but decreased MDA level when the exposure dose was increased to 10 mM. Plants have a complex network of metal trafficking pathways to regulate Cu homeostasis appropriately in response to environmental CuO-NP level variation. In this study, MDA levels increased in the first 10 days and then decreased. We speculate that with the increase exposure duration, the plant activated its defense/detoxification mechanisms to scavenge oxidative stress caused by CuO-NPs, alleviating the damage to the membrane system.
CAT activity
CAT is a tetrameric heme-containing enzyme that is able to dismutate H2O2 into H2O and O2 directly. It is required for ROS detoxification under stressful conditions (Garg and Manchanda 2009).
The CAT activity in lettuce leaves was not significantly different between the control and CuO-NP treatment groups for the first 5 days. After 10 and 15 days of exposure, CAT activity of the treated groups increased, but only significantly increased for the 1000 mg L−1 CuO-NP treatment group after 15 days of exposure (Fig. S4). The CAT activity of lettuce leaves was significantly positively correlated with exposure dose (r = 0.45, p < 0.05). In lettuce roots, the CAT activity remains constant on the 5th day, and slightly increased on the 15th day.
Previous studies have documented that NPs applied to plants can modulate the activity of stress enzymes (Hong et al. 2014). According to Kim et al. (2012), Nekrasova et al. (2011), and Trujillo-Reyes et al. (2014) CuO-NP treatments increased CAT activity in lettuce roots, cucumber plants, and Elodea densa Planch, respectively. Oxidative stress in CuO-NP-treated wheat was evident by the increase of lipid peroxidation and increase of peroxidase and CAT activities in roots (Dimkpa et al. 2012). The present study suggests that CuO-NPs in contact with plant leaves can modify the antioxidant enzyme activity in lettuce plants, and that the increase in CAT activity is predominantly responsible for the maintenance of the cellular redox state.
In conclusion, in vivo detection of H2O2 in the form of dark brown spots in leaves indicates severe oxidative burst under CuO-NP stress. Enhanced CAT activity under CuO-NP stress is an indication of the activation of plant defense mechanisms to combat oxidative stress damage. The increased CAT activity in lettuce leaves may explain the decrease in MDA level on the 15th day. However, the oxidative stress is still severe in lettuce leaves, as evident from the increased H2O2 production during the entire exposure period, which could be one of the main factors underlying the CuO-NP caused anomalies.
Subcellular distribution
In lettuce leaves, there was a significant increase of Cu concentration in cell walls (90–691 mg kg−1 DW) and organelles (79–467 mg kg−1 DW) after CuO-NP treatment, which was 16.7–213 times and 6.46–40.2 times that of the control groups, respectively (Fig. 4a). Cu concentration in soluble fraction also increased after CuO-NP exposure, but not significantly, and it was positively correlated with exposure duration (r = 0.68, p < 0.01). Cu concentration in cell walls and organelles were 18–264 and 31–214 times higher than that in soluble fraction, respectively. The proportion of Cu accounted for 34–46% in cell walls, 9–26% in organelles, and 39–57% in soluble fraction, in the control groups (Fig. S5a). However, after CuO-NP treatment, the majority of Cu was present in cell walls (82%–97%), compared to organelle (2%–15%) and soluble fraction (0.8%–9%) (Fig. S5a).
Subcellular distribution of Cu in lettuce leaves (a) and roots (b) as a function of exposure duration (5, 10, and 15 days) and CuO-NPs dose (0, 100, and 1000 mg L−1) deposited on leaves. Values are expressed as the mean of three replicates (± SD) for each treatment; the different lowercase letters indicate significant difference at p < 0.05
In lettuce roots, Cu concentration in cell walls increased both with exposure duration and exposure dose (Fig. 4b). Cu concentration in the organelles significantly increased after 5 and 10 days of exposure, but decreased after 15 days of CuO-NP exposure. Cu concentration in soluble fraction was not correlated with exposure dose, but significantly correlated with exposure duration. Moreover, Cu concentration in the organelles of lettuce roots was substantially higher than in the cell walls and soluble fraction. The proportion of Cu in the control groups accounted for 34–46% in cell walls, 9–26% in organelles, and 39–57% in soluble fraction, respectively (Fig. S5b). Similar to the control groups, the proportions of Cu in cell walls, organelles, and soluble fraction were 26%–45%, 14%–26%, and 30%–55%, respectively, after CuO-NP treatment (Fig. S5b). In this study, lettuce roots were not directly contacted with CuO-NPs (foliar exposure). Cu in roots was mainly transferred from leaves perhaps by the apoplast and symplastic pathways (Rajput et al. 2019). Thus, the subcellular distribution in the roots was different from the leaves.
To some extent, the subcellular distribution may reflect the toxicity of CuO-NPs and detoxification mechanisms of the plant. The cell wall is considered the first barrier of protection for the protoplast from the toxic effects of allogenic material (Weng et al. 2012). Nanoparticles more than 20 nm in diameter can barely penetrate through cell walls, especially if agglomeration is present (Ma et al. 2015b; Rondeau-Mouro et al. 2008). Moreover, the hydroxyl, carboxyl, amino groups, sulfhydryl groups, and aldehyde groups of protein and polyose in plant cell walls can bind metal cations by complexation, precipitation, and ion exchange in order to limit their transport across the cytomembrane (Li et al. 2016; Wang et al. 2009). Cu compartmentalization in the cell wall of lettuce leaves may be one of the mechanisms inhibiting plant Cu diffusion.
However, NP exposure caused ROS production or other effects that may alter cell wall structure, i.e., reduce cell wall thickness, trigger cell wall loosening, or change cell wall pore size (Kim et al. 2014; Ma et al. 2015b). Thus, NPs may pass through the cell wall and localize inside cells. As NPs have excellent adsorption property, we speculate that NPs may first be adsorbed in the plant cell wall and plasma membrane. With increasing exposure dose, NPs were transferred from the plasma membrane to the organelle membrane through endocytosis. Only little was dissociated in soluble fraction. Therefore, the Cu concentration in plant organelles was significantly higher compared to the control. However, further unambiguous assignments could not be made from our recent data. There is already some research demonstrating the endocytosis of NPs by plant cells. The study of Wang et al. (2011, 2012) observed an endosome (~ 500 nm) in maize cells and uptake of CuO-NPs into the alga Microcystis aeruginosa by endocytosis. Liu et al. (2009) reported that carbon nanotubes (SWNT/FITC) effectively translocated into Nicotiana tabacum L.cv. Bright Yellow (BY-2) cells. When plant cells were pretreated with the inhibitor of endocytosis, the cellular fluorescence of SWNT/FITC decreased significantly (~ 64%); Thus, they proposed that SWNT/FITC was taken up by fluidic-phase endocytosis. Based on the previous studies, endocytosis is one of the likely pathways for particle uptake. Once entered into the plant cells, the particles may redistribute or transfer to other tissues.
Chemical speciation
Cu concentration of five different chemical speciations in lettuce leaves and roots increased with increasing the CuO-NP dose (Fig. 5). In lettuce leaves, Cu concentration followed this order: HCl > HAc > NaCl > dH2O > ethanol extraction. According to the report of Li et al. (2016), the HCl, HAc, and NaCl-extractable Cu are referred to as the “insoluble form,” which are mainly Cu oxalate, pectate- and protein-integrated Cu, and undissolved Cu phosphate. The dH2O and ethanol-extractable Cu are referred to as the “soluble form,” which contains nitrate/nitrite, chloride, cupric chlorate, soluble Cu-organic acid complexes, and Cu(H2PO4)2 (Li et al. 2016). The “insoluble form” was the predominant form in lettuce leaves. In the roots, ethanol-extractable Cu was the predominant form during the first 5 days of exposure. However, the concentration of ethanol-extractable Cu in roots decreased, whereas the “insoluble form” of Cu (NaCl, HAc, and HCl-extractable Cu) increased with increasing exposure duration. Foliar Cu concentration in the residue was positively correlated with exposure dose. Radicular Cu concentration in the residue was negatively correlated with exposure dose.
Chemical speciation of Cu in lettuce leaves (L) and roots (R) as a function of exposure duration (5, 10, and 15 days) and CuO-NPs dose (0, 100, and 1000 mg L−1) deposited on leaves. Cu concentration in ethanol (a), dH2O (b), NaCl (c), HAc (d), and HCl (e) extraction, and in the residue (f). Values are expressed as the mean of three replicates (± SD) for each treatment; the different lowercase letters indicate significant difference in leaf or root at p < 0.05
The chemical speciation of metals is closely related to their bioavailability and toxicity, compartmentation, and homeostasis inside plants (Schreck et al. 2014; Shahid et al. 2017). Different chemical forms of metal showed distinguishing toxicity severity and migration capability (Fu et al. 2011). Normally, metal in inorganic form and water-soluble form have a higher transferability and stronger phytotoxicity than pectate- and protein-integrated metals, undissolved metal phosphates, and residues (Zou et al. 2019). In this study, the largest proportion of Cu was accounted for by insoluble forms in lettuce leaves. The results indicate that plant leaves may combine Cu by complexation to lower its mobility and toxicity. The soluble form of Cu transfers from leaves to roots in the beginning. However, with increasing exposure duration, plant roots could also combine and transfer Cu into “insoluble form” in order to reduce its mobility and phytotoxicity. According to the study of Fu et al. (2011), we hypothesized that plant Cu may chelate with some specific polar material in plant cells, such as carboxyl or hydroxyl, leading to a reduction in its toxicity by forming a non-toxic complex.
Conclusion
From the results and the above discussion, it is concluded that lettuce leaves accumulated significant Cu concentration and transferred Cu to the root tissues. Cu accumulation influenced the composition and content of nutrient elements in both plant leaves (edible part) and roots. Prolonged CuO-NP treatment triggered oxidative burst (H2O2 generation, high MDA content), with subsequent alteration to antioxidant responses (CAT activity). After Cu uptake by plant leaves, Cu primarily accumulated in cell walls and organelles and was mostly present in undissolved Cu forms. Plants may decrease the toxicity of CuO-NPs by implementing cell wall barriers and by lowering the mobility of Cu. The application of CuO-NPs led to a marked suppression of plant growth in lettuce.
These results are of great significance for the comprehensive understanding of the ecological risks of CuO-NPs in the atmosphere. They provide a theoretical basis for the safe production, use, consumption, and disposal of metal-NPs and will be helpful in generating new techniques and methods for further research on how to reduce the phytotoxicity of metal-NPs in order to increase the yield and quality of crops.
References
Adhikari, T., Sarkar, D., Mashayekhi, H., & Xing, B. (2016). Growth and enzymatic activity of maize (Zea mays L.) plant: Solution culture test for copper dioxide nano particles. Journal of Plant Nutrition, 39(1), 99–115.
Anjum, N. A., Adam, V., Kizek, R., Duarte, A. C., Pereira, E., Iqbal, M., et al. (2015). Nanoscale copper in the soil—Plant system—Toxicity and underlying potential mechanisms. Environmental Research, 138, 306–325.
Canivet, L., Dubot, P., Garçon, G., & Denayer, F.-O. (2015). Effects of engineered iron nanoparticles on the bryophyte, Physcomitrella patens (Hedw.) Bruch & Schimp, after foliar exposure. Ecotoxicology and Environmental Safety, 113, 499–505.
Chang, Y., Zhang, M., Xia, L., Zhang, J., & Xing, G. (2012). The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials, 5, 2850–2871.
Dalcorso, G., Manara, A., Piasentin, S., & Furini, A. (2014). Nutrient metal elements in plants. Metallomics, 6(10), 1770–1788.
Demidchik, V., Cuin, T. A., Svistunenko, D., Smith, S. J., Miller, A. J., Shabala, S., et al. (2010). Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science, 123(9), 1468–1479.
Dietz, K. J., & Herth, S. (2011). Plant nanotoxicology. Trends in Plant Science, 16(11), 582–589.
Dimkpa, C. O., McLean, J. E., Latta, D. E., Manangon, E., Britt, D. W., Johnson, W. P., et al. (2012). CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. Journal of Nanoparticle Research, 14(9), 1125.
Du, W., Tan, W., Peralta-Videa, J. R., Gardea-Torresdey, J. L., Ji, R., Yin, Y., et al. (2016). Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiology and Biochemistry, 110, 210–225.
Fedorenko, A. G., Minkina, T. M., Chernikova, N. P., Fedorenko, G. M., Mandzhieva, S. S., Rajput, V. D., et al. (2020). The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum). Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00530-5.
Fu, X., Dou, C., Chen, Y., Chen, X., Shi, J., Yu, M., et al. (2011). Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. Journal of Hazardous Materials, 186(1), 103–107.
Garg, N., & Manchanda, G. (2009). ROS generation in plants: Boon or bane? Plant Biosystems—An International Journal Dealing with all Aspects of Plant Biology: Official Journal of the Societa Botanica Italiana, 143(1), 81–96.
Ghosh, M., Bandyopadhyay, M., & Mukherjee, A. (2010). Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere, 81(10), 1253–1262.
Gogos, A., Knauer, K., & Bucheli, T. D. (2012). Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. Journal of Agricultural and Food Chemistry, 60, 9781–9792.
Goix, S., Lévêque, T., Xiong, T. T., Schreck, E., Baeza-Squiban, A., Geret, F., et al. (2014). Environmental and health impacts of fine and ultrafine metallic particles: Assessment of threat scores. Environmental Research, 133, 185–194.
Haslett, B. S., Reid, R. J., & Rengel, Z. (2001). Zinc mobility in wheat: Uptake and distribution of zinc applied to leaves or roots. Annals of Botany, 87(3), 379–386.
Hong, J., Peralta-Videa, J. R., Rico, C., Sahi, S., Viveros, M. N., Bartonjo, J., et al. (2014). Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environmental Science and Technology, 48, 4376–4385.
Hong, J., Rico, C. M., Zhao, L., Adeleye, A. S., Keller, A. A., Peralta-Videa, J. R., et al. (2015a). Toxic effects of copper-based nanoparticles or compounds to lettuce (Lactuca sativa) and alfalfa (Medicago sativa). E Environmental Science. Processes and Impacts, 17(1), 177–185.
Hong, J., Wang, L., Sun, Y., Zhao, L., Niu, G., Tan, W., et al. (2015b). Foliar applied nanoscale and microscale CeO2 and CuO alter cucumber (Cucumis sativus) fruit quality. Science of the Total Environment, 563–564, 904–911.
Hussain, A., Ali, S., Rizwan, M., Zia ur Rehman, M., Javed, M. R., Imran, M., et al. (2018). Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environmental Pollution, 242, 1518–1526.
Jain, A., Sinilal, B., Dhandapani, G., Meagher, R. B., & Sahi, S. V. (2013). Effects of deficiency and excess of zinc on morphophysiological traits and spatiotemporal regulation of zinc-responsive genes reveal incidence of cross talk between micro- and macronutrients. Environmental Science and Technology, 47(10), 5327–5335.
Ke, M., Zhu, Y., Zhang, M., Gumai, H., Zhang, Z., Xu, J., et al. (2017). Physiological and molecular response of Arabidopsis thaliana to CuO nanoparticle (nCuO) exposure. Bulletin of Environmental Contamination and Toxicology, 99(6), 713–718.
Keller, A. A., Huang, Y., & Nelson, J. (2018). Detection of nanoparticles in edible plant tissues exposed to nano-copper using single-particle ICP-MS. Journal of Nanoparticle Research, 20(4), 101.
Keller, A. A., McFerran, S., Lazareva, A., & Suh, S. (2013). Global life cycle releases of engineered nanomaterials. Journal of Nanoparticle Research, 15(6), 1692.
Kim, J.-H., Lee, Y., Kim, E.-J., Gu, S., Sohn, E. J., Seo, Y. S., et al. (2014). Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environmental Science and Technology, 48(6), 3477–3485.
Kim, S., Lee, S., & Lee, I. (2012). Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water, Air, and Soil pollution, 223, 2799–2806.
Kotchoni, S. O., Kuhns, C., Ditzer, A., Kirch, H., & Bartels, D. (2006). Over-expression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant, Cell and Environment, 29, 1033–1048.
Lai, H. Y. (2015). Subcellular distribution and chemical forms of cadmium in Impatiens walleriana in relation to its phytoextraction potential. Chemosphere, 138, 370–376.
Le Van, N., Ma, C., Shang, J., Rui, Y., Liu, S., & Xing, B. (2016). Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere, 144, 661–670.
Li, H., Luo, N., Zhang, L. J., Zhao, H. M., Li, Y. W., Cai, Q. Y., et al. (2016). Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Science of the Total Environment, 571, 1183–1190.
Liu, J., Cao, Z., Zou, S., Liu, H., Hai, X., Wang, S., et al. (2018). An investigation of the leaf retention capacity, efficiency and mechanism for atmospheric particulate matter of five greening tree species in Beijing, China. Science of the Total Environment, 616–617, 417–426.
Liu, Q., Chen, B., Wang, Q., Shi, X., Xiao, Z., Lin, J., et al. (2009). Carbon nanotubes as molecular transporters for walled plant cells. Nano Letters, 9(3), 1007–1010.
Liu, J., Qu, P., Zhang, W., Dong, Y., Li, L., & Wang, M. (2014). Variations among rice cultivars in subcellular distribution of Cd: The relationship between translocation and grain accumulation. Environmental and Experimental Botany, 107, 25–31.
Ma, C., Chhikara, S., Minocha, R., Long, S., Musante, C., White, J. C., et al. (2015a). Reduced silver nanoparticle phytotoxicity in crambe abyssinica with enhanced glutathione production by overexpressing bacterial γ-glutamylcysteine synthase. Environmental Science and Technology, 49(16), 10117–10126.
Ma, C., White, J. C., Dhankher, O. P., & Xing, B. (2015b). Metal-based nanotoxicity and detoxification pathways in higher plants. Environmental Science and Technology, 49(12), 7109–7122.
Ma, Y., Zhang, P., Zhang, Z., He, X., Li, Y., Zhang, J., et al. (2015c). Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicology, 9, 262–270.
Moretto, R., Aurélio, M., & Arruda, Z. (2018). Evaluation of changes in the macro and micronutrients homeostasis of transgenic and non-transgenic soybean plants after cultivation with silver nanoparticles through ionomic approaches. Journal of Trace Elements in Medicine and Biology, 48(April), 181–187.
Nair, P. M. G., & Chung, I. M. (2017). Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Science of the Total Environment, 575, 187–198.
Natasha, N., Shahid, M., Dumat, C., Khalid, S., Rabbani, F., Farooq, A. B. U., et al. (2019). Foliar uptake of arsenic nanoparticles by spinach: an assessment of physiological and human health risk implications. Environmental Science and Pollution Research, 26(20), 20121–20131.
Nazari, M., Maali Amiri, R., Mehraban, F. H., & Khaneghah, H. Z. (2012). Change in antioxidant responses against oxidative damage in black chickpea following cold acclimation. Russian Journal of Plant Physiology, 59(2), 183–189.
Nekrasova, G. F., Ushakova, O. S., Ermakov, A. E., Uimin, M. A., & Byzov, I. V. (2011). Effects of copper(II) ions and copper oxide nanoparticles on Elodea densa Planch. Russian Journal of Ecology, 42(6), 458–463.
Perreault, F., Popovic, R., & Dewez, D. (2014a). Different toxicity mechanisms between bare and polymer-coated copper oxide nanoparticles in Lemna gibba. Environmental Pollution, 185, 219–227.
Perreault, F., Samadani, M., & Dewez, D. (2014b). Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology, 8(4), 374–382.
Pullagurala, V. L. R., Rawat, S., Adisa, I. O., Hernandez-Viezcas, J. A., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2018). Plant uptake and translocation of contaminants of emerging concern in soil. Science of the Total Environment, 636, 1585–1596.
Rajput, V., Minkina, T., Ahmed, B., SushKova, S., Singh, R., Soldatov, M., et al. (2020). Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: Critical review of the state of the science and future perspectives. Reviews of Environmental Contamination and Toxicology, 252, 51–96.
Rajput, V. D., Minkina, T., Sushkova, S., Mandzhieva, S., Fedorenko, A., Lysenko, V., et al. (2019). Structural and ultrastructural changes in nanoparticle exposed plants. In R. Pudake, N. Chauhan, & C. Kole (Eds.), Nanoscience for sustainable agriculture (pp. 281–295). Cham: Springer. https://doi.org/10.1007/978-3-319-97852-9_13.
Raliya, R., Franke, C., Chavalmane, S., Nair, R., Reed, N., & Biswas, P. (2016). Quantitative understanding of nanoparticle uptake in watermelon plants. Frontiers in Plant Science, 7, 1–10.
Rippner, D. A., Green, P. G., Young, T. M., & Parikh, S. J. (2018). Dissolved organic matter reduces CuO nanoparticle toxicity to duckweed in simulated natural systems. Environmental Pollution, 234, 692–698.
Rizwan, M., Ali, S., Qayyum, M. F., Ok, Y. S., Adrees, M., Ibrahim, M., et al. (2017). Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. Journal of Hazardous Materials, 322, 2–16.
Rodrigo-Moreno, A., Andrés-colás, N., Poschenrieder, C., Gunsé, B., Peñarrubia, L., & Shabala, S. (2013a). Calcium- and potassium-permeable plasma membrane transporters are activated by copper in Arabidopsis root tips: linking copper transport with cytosolic hydroxyl radical production. P Plant, Cell and Environment, 36, 844–855.
Rodrigo-Moreno, A., Poschenrieder, C., Shabala, S., Science, E., Fi, S. F., Vegetal, F., et al. (2013b). Transition metals: A double edge sward in ROS generation and signaling. Plant Signaling and Behavior, 8(3), 1–5.
Rondeau-Mouro, C., Defer, D., Leboeuf, E., & Lahaye, M. (2008). Assessment of cell wall porosity in Arabidopsis thaliana by NMR spectroscopy. Journal of Biological Macromolecules, 42(2), 83–92.
Rossi, L., Fedenia, L. N., Sharifan, H., Ma, X., & Lombardini, L. (2019). Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiology and Biochemistry, 135, 160–166.
Sani, H. A., Tsafe, A. I., Bagudo, B. U., Itodo, A. U., & Chemistry, A. (2011). Toxic metals uptake by Spinach (Spinacea oleracea) and Lettuce (Lactuca sativa) cultivated in Sokoto: A comparative study. Pakistan Journal of Nutrition, 10(6), 572–576.
Schreck, E., Dappe, V., Sarret, G., Sobanska, S., Nowak, D., Nowak, J., et al. (2014). Foliar or root exposures to smelter particles: Consequences for lead compartmentalization and speciation in plant leaves. Science of the Total Environment, 476–477, 667–676.
Shahid, M., Dumat, C., Khalid, S., Schreck, E., Xiong, T., & Niazi, N. K. (2017). Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. Journal of Hazardous Materials, 325, 36–58.
Shaw, A. K., Ghosh, S., Kalaji, H. M., Bosa, K., Brestic, M., Zivcak, M., et al. (2014). Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environmental and Experimental Botany, 102, 37–47.
Shaw, A. K., & Hossain, Z. (2013). Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere, 93(6), 906–915.
Singh, D., & Kumar, A. (2020). Quantification of metal uptake in Spinacia oleracea irrigated with water containing a mixture of CuO and ZnO nanoparticles. Chemosphere, 243, 125239.
Singh, S., Vishwakarma, K., Singh, S., Sharma, S., Dubey, N. K., Singh, V. K., et al. (2017). Understanding the plant and nanoparticle interface at transcriptomic and proteomic level: A concentric overview. Plant Gene, 11, 265–272.
Tamez, C., Hernandez-Molina, M., Hernandez-Viezcas, J. A., & Gardea-Torresdey, J. L. (2019). Uptake, transport, and effects of nano-copper exposure in zucchini (Cucurbita pepo). Science of the Total Environment, 665, 100–106.
Trujillo-Reyes, J., Majumdar, S., Botez, C. E., Peralta-Videa, J. R., & Gardea-Torresdey, J. L. (2014). Exposure studies of core-shell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: Are they a potential physiological and nutritional hazard? Journal of Hazardous Materials, 267, 255–263.
Uzu, G., Sobanska, S., Aliouane, Y., Pradere, P., & Dumat, C. (2009). Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environmental Pollution, 157(4), 1178–1185.
Wang, L., Gong, H., Liao, W., & Wang, Z. (2015a). Accumulation of particles on the surface of leaves during leaf expansion. Science of the Total Environment, 532, 420–434.
Wang, Z., Li, J., Zhao, J., & Xing, B. (2011). Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environmental Science and Technology, 45(14), 6032–6040.
Wang, S., Liu, H., Zhang, Y., & Xin, H. (2015b). The effect of CuO NPs on reactive oxygen species and cell cycle gene expression in roots of rice. Environmental Toxicology and Chemistry, 34(3), 554–561.
Wang, Z., Xie, X., Zhao, J., Liu, X., Feng, W., White, J. C., et al. (2012). Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.). Environmental Science and Technology, 46(8), 4434–4441.
Wang, Z., Xu, L., Zhao, J., Wang, X., White, J. C., & Xing, B. (2016). CuO nanoparticle Interaction with Arabidopsis thaliana: Toxicity, parent-progeny transfer, and gene expression. Environmental Science Technology, 50(11), 6008–6016.
Wang, J., Yuan, J., Yang, Z., Huang, B., Zhou, Y., Xin, J., et al. (2009). Variation in cadmium accumulation among 30 cultivars and cadmium subcellular distribution in 2 selected cultivars of water Spinach (Ipomoea aquatica Forsk.). Journal of Agricultural and Food Chemistry, 57(19), 8942–8949.
Weng, B., Xie, X., Weiss, D. J., Liu, J., Lu, H., & Yan, C. (2012). Kandelia obovata (S., L.) Yong tolerance mechanisms to cadmium: Subcellular distribution, chemical forms and thiol pools. Marine Pollution Bulletin, 64(11), 2453–2460.
Wu, S. G., Huang, L., Head, J., Ball, M., Tang, Y. J., & Chen, D. (2014). Electrospray facilitates the germination of plant seeds electrospray facilitates the germination of plant seeds. Aerosol and Air Quality Research, 14, 632–641.
Xia, T., Kovochich, M., Liong, M., Ma, L., Shi, H., Yeh, J. I., et al. (2008). Comparison of the mechanism of toxicity nanoparticles based on dissolution and oxidative stress properties. ACS Nano, 2(10), 2121–2134.
Xie, C., Kan, L., Guo, J., Jin, S., Li, Z., Chen, D., et al. (2018). A dynamic processes study of PM retention by trees under different wind conditions. Environmental Pollution, 233, 315–322.
Xiong, T., Dumat, C., Dappe, V., Vezin, H., Schreck, E., Shahid, M., et al. (2017). Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environmental Science and Technology, 51(9), 5242–5251.
Xiong, T.-T., Leveque, T., Austruy, A., Goix, S., Schreck, E., Dappe, V., et al. (2014a). Foliar uptake and metal(loid) bioaccessibility in vegetables exposed to particulate matter. Environmental Geochemistry and Health, 36(5), 897–909.
Xiong, T., Leveque, T., Shahid, M., Foucault, Y., & Dumat, C. (2014b). Lead and cadmium phytoavailability and human bioaccessibility for vegetables exposed to soil or atmospheric pollution by process ultrafine particles. Journal of Environment Quality, 43(5), 1593–1600.
Xiong, T., Zhang, T., Dumat, C., Sobanska, S., Dappe, V., Shahid, M., et al. (2019). Airborne foliar transfer of particular metals in Lactuca sativa L.: translocation, phytotoxicity, and bioaccessibility. Environmental Science and Pollution Research, 26(20), 20064–20078.
Yruela, I. (2013). Transition metals in plant photosynthesis. Metallomics, 5(9), 1090–1109.
Zhou, D., Jin, S., Li, L., Wang, Y., & Weng, N. (2011). Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions. Journal of Environmental Science, 23(11), 1852–1857.
Zou, C., Sha, Y., Ding, D., Li, G., Cui, Y., Hu, N., et al. (2019). Aspergillus niger changes the chemical form of uranium to decrease its biotoxicity, restricts its movement in plant and increase the growth of Syngonium podophyllum. Chemosphere, 224, 316–323.
Acknowledgements
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant No. 41701572), as well as China Postdoctoral Science Foundation (Grant No. 2017M612684) to Tiantian Xiong, and Guangdong Pearl River Scholar Funded Scheme (2012) to Shaoshan Li. We are grateful to MogoEdit for the English editing of the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 41701572), China Postdoctoral Science Foundation (Grant No. 2017M612684), and Guangdong Pearl River Scholar Funded Scheme (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiong, T., Zhang, T., Xian, Y. et al. Foliar uptake, biotransformation, and impact of CuO nanoparticles in Lactuca sativa L. var. ramosa Hort.. Environ Geochem Health 43, 423–439 (2021). https://doi.org/10.1007/s10653-020-00734-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00734-9