Abstract
This study was focused on physical, petrographical, mineralogical, and chemical characterization of a volcanic-rock mining by-product (dacite rock), as well as on greenhouse experiment with black oats and maize crops to evaluate the potential use of the by-product as soil re-mineralizer. The by-product sample was obtained from a quarry in the Nova Prata mining district in southern Brazil. The particle size distribution of the by-product and soil was determined by sieving. Dacite rock petrographic description was performed on a polished thin section by optical microscopy. The soil and dacite rock mineralogical phases were identified by X-ray diffraction. The by-product and soil chemical composition was determined by X-ray fluorescence. Inductively coupled plasma mass spectrometry was performed to determine potentially toxic elements, As, Cd, Hg and Pb in by-product. Additional chemical compositions of the by-product and soil were analyzed using a scanning electron microscope equipped with an energy dispersive X-ray detector. Black oats and, sequentially maize, crops were cultivated in a typical Hapludox soil treated with the by-product in a greenhouse. Five by-product doses (0, 906, 1813, 3625, and 7251 kg ha−1) were added into pots containing soil, each with seven replications. Responses to treatments were evaluated from dry matter production, nutritional status of the crops, and in the changes in soil properties after 70 days of each cultivation. The results showed that the by-product is composed of plagioclase, K-feldspar, quartz, clinopyroxene, smectites, and opaque minerals with apatite as accessory mineral. The addition of 3625 and 7251 kg ha−1 doses of the by-product substantially increased the dry matter yield in maize leaves. The Ca uptake by maize leaves cultivated in soil with 7251 kg ha−1 dose of the by-product was significantly higher in soil with other doses, and all by-product doses promoted high concentrations of Mg and Ca. The accumulated amounts of Ca, K, Mg and P indicated that they were enough to supply maize nutritional needs. Improvements in soil properties, such as high levels of Ca, K and P and low levels of exchangeable Al and Al saturation were observed. The results of the study suggest that the by-product can be used as soil re-mineralizer. The dacite rock by-product studied here has potential to be an environmental solution to soil fertilization problem because it does not require chemical processing and can be used as it is mined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most tropical soils are acidic and have low fertility (Melfi et al. 1999; Rabel et al. 2018). However, to reduce ecological and economic damage from modern and intensive agriculture in external inputs, especially in highly soluble fertilizers, many alternative measures have been carried out (Ferrari et al. 2019). One of the measures that has been taken was to restore leached or degraded soils by adding rock mining by-products, which would yield the elements lost through leaching (Ramos et al. 2017). With regard to the use of ground rock as fertilizer, several experiments and data have shown that a wide variety of materials, with low environmental impact, can be used as alternative fertilizer for soils (Kronberg et al. 1976; Leonardos et al. 1976; Escosteguy and Klamt 1998; Gillman et al. 2001; Nunes et al. 2014).
Mining is mineral resource extraction, which induces environmental impacts on both the mined and nearby areas (Sánchez-Peña et al. 2018). In general, the by-products of rock mining are discarded in landfills or disposed of in the environment without any treatment processes (Machado et al. 2014). Thus, the application of by-products of volcanic rock mining as soil re-mineralizer can minimize the environmental impact caused by agricultural and mining activity and, hence, provide a wide range of nutrients that result in the improved quality of food and an environmentally safe technique (Ramos et al. 2015). Near agricultural production centers in the State of Rio Grande do Sul (RS) and Santa Catarina (SC), there is a wide distribution of volcanic rock deposits. Therefore, it is of great relevance to conduct research on the agronomic performance of these rocks, especially for plants demanding Si and K (Bergmann et al. 2017).
In the rock aggregate mining sector, fine crushed powders are by-products intended for uses such as asphalt filler. However, such by-products can create environmental problems when the demand is lower than production. In this case, their application in agriculture should be considered as an alternate to re-mineralize nutrient-depleted soils. Mineralogical and chemical characterization and an evaluation of their agronomic performance are required for evaluating the environmental risk of this material (Korchagin et al. 2019). Basalt by-products from quarries in South Brazil are well known as a soil re-mineralizer (Nunes et al. 2014), but dacite has not yet been utilized. The use of crushed rocks (mining by-products) as soil re-mineralizer is a long-standing practice to improve soil properties and increase crops productivity. It is also associated with the reduction in production costs, since re-mineralizers are cheap compared to soluble fertilizers (Silva 2016; Manning and Theodoro 2018).
In 2013, Brazilian law no 12890/2013 included soil re-mineralizers as an input category for agriculture. Soil re-mineralizers are all mineral materials that have undergone only size reduction and size classification by mechanical processes and that change the soil fertility indices by addition of macro- and micro-nutrients for crops and improve the physical or physicochemical properties or the biological activity of soils. In Brazil, soil re-mineralizers have been developed, and Brazilian federal law (Brazil 2013) allows these to be used for crop nutrition, with specifications clearly defined by Normative Instruction (IN) no 5 of the Ministry of Agriculture, Livestock and Supply (Brazil 2016). This approach provides a model that all countries to explore local geological sources and reduce the use on of high solubility fertilizers (Manning and Theodoro 2018).
Several rock powders such as basalt, phonolite, phlogopite, and granite, among others, have been studied to evaluate its potential use as fertilizer (van Straaten 2007). Dacite rock has different chemical and mineral compositions compared to the rocks cited above because of hydrothermal alteration (Meunier et al. 1988; Rosenstengel and Hartmann 2012). No geochemical/mineralogical study has been conducted to date to assess dacite rock by-product for application as re-mineralizer in tropical soil. Information from such study is fundamental for ensuring safe and efficient use of this by-product as soil re-mineralizer. Dacite rock not only contains primary minerals, but also clay minerals and accessory minerals that contain nutrients for plants (e.g., phosphorus, potassium) and may increase soil cation exchange capacity. To be considered and marketed as soil re-mineralizers, dacite rock by-product must meet the specific criteria established by the Ministry of Agriculture, Livestock and Supply (MAPA) (Brazil 2016).
The objective of this study was to evaluate the potential use of the by-product produced from volcanic rock mining in the fertilization of the tropical soil, in the nutrition of the black oats and the maize crops, in a greenhouse located in Nova Santa Rita city, Rio Grande do Sul state, Brazil, and finally to serve as the basis for several countries that produce these types of rock aggregate by-product. The crops considered were selected because they are widely cultivated in Brazil. Another crop that shows great potential for the application of the dacite rock by-product would be soybean, which is considered as the largest consumer of fertilizers in Brazil. This study presents a viable sustainable alternative that could replace soluble fertilizers adding value to the investigated rock by-product.
Materials and Methods
Soil, By-Product, and Seeds Samples
Soil was sampled in June 2013 from several locations in the experimental site, in the Nova Santa Rita city (29°52′12″S; 51°15′28″W, South American Datum 1969), before re-mineralization treatments application. Soil samples were collected at depths of 0–20 cm (A horizon) to evaluate the soil fertility, mineralogy, and particle size distribution. For soil classification, samples were collected at depths of 20–40 cm (Bw horizon).
Soil of 1300 kg was collected at depths of 0–20 cm for the greenhouse experiment. This material was air-dried, homogenized, sieved at 4-mm mesh, and then finally quartered. For fertility analysis, an amount of approximately 500 g of soil was used. The experimental overview is shown in Figure 1.
Twenty kg of by-product powder for particle size classification and chemical characterization and 1 kg of bedrock for petrographical description and chemical composition by scanning electron microscope were used in this investigation. The samples were obtained from the quarry Sindicato da Indústria de Extração de Pedreiras de Nova Prata in Southern Brazil (28°46′27.37″S, 51°38′16.61′W, South American Datum 1969). The distance between the quarry and the experimental site is 172.6 km.
For the cultivation of black oats and maize, seeds of black oats (Campo Bello—Controlled by the State Commission of Seeds and Seedlings—CESM/RS) and maize (cultivar HIB ITAP 700—Seed ISA 509) were purchased in a local store.
Soil and By-Product Particle Size Distribution
The particle size distribution of soil and by-product powder was determined according to the methodology for Brazilian norm, NBR-7181 (ABNT 1986). This methodology is relevant because the smaller the size of the mineral particles, the greater will be the surface area exposed to weathering, favoring the alteration of the material (Priyono and Gilkes 2008). For this analysis, 120 g of each sample was oven dried for 24 h at 105 °C. The samples were then split into different particles sizes using sieves of different apertures (ASTM 4—4.8 mm, ASTM 7—2.8 mm, ASTM 10—2.0 mm, ASTM 20—0.84 mm, and ASTM 50—0.3 mm). The sieves were stacked on a mechanical shaker (Produtest®) for 15 min. After this, the particles retained per size were weighed on a semi-analytical scale (Gehaka ®, BG 1000, accuracy of ± 0.01 g). The percentages of particles sizes were determined from weights materials passing through each mesh size.
Analytical Procedures
By-Product Petrography
Thin-section petrographical observations were made to identify the dacite mineral phases. The samples were analyzed on a Nikon Eclipse 50iPOL optical microscope (OM) under natural (NL) and polarized reflected light (PL).
By-Product and Soil Mineralogy
X-ray diffraction (XRD) technique was employed to characterize the mineralogical composition of the by-product and soil samples using a Philips X-ray diffractometer, model X’Pert MPD, equipped with a curved graphite monochromator and fixed copper anode, operating at 40 kV and 40 mA. The angle range analyzed was from 5° to 75°. The step size used was 5°/1 s. Cu Kα radiation (1.54184 Å), Kα1 (1.54056 Å), Kα2 (1.54439 Å), and Kβ (1.39222 Å). The mineral identification from XRD data was done using the X’pert High Score Software.
By-Product and Soil Chemical Composition
Chemical analyses of the by-product and soil were performed in triplicate, after manual milling using a porcelain mortar and pestle to obtain particles less than 0.074 mm. Chemical composition in percentage weight of oxides was determined by X-ray fluorescence (XRF) using a Panalytical brand MagiX equipment (DY1583) after digestion of 2 g of the sample by total fusion in automatic machine with lithium tetraborate (Silva et al. 2011).
The by-product sample was digested with four acids (HCl:HNO3:HF:HClO4) in a microwave for 1 h (Querol et al. 1997) to determine a potentially toxic elements (PTEs), As, Cd, Hg, and Pb composition. The analysis was performed at the Institute of Environmental Assessment and Water Research (Spain) by inductively coupled plasma-mass spectroscopy (ICP-MS).
Soil fertility was determined according to Tedesco et al. (1995), before application of the treatments and after harvesting of black oats and maize crops. This analysis included P and K (Mehlich 1), Ca, Al, and Mg (KCl 1 mol l−1), and pH in water (1:1).
The micromorphology, texture, and chemical composition of the by-product were investigated on a polished thin section. The soil sample was adhered to stub using carbon tape. Then, the materials were gold-coated using a sputter coating device (BAL-TEC, SCD 005) for 3 min at 30 mA. The analyses were performed using a Zeiss EVO MA10 scanning electron microscope equipped with an energy-dispersive X-ray spectrometer (EDS). The mineral identifications were made using a back-scattered electron mode to produce images displaying high resolution.
Location and Site Preparation
The experimental site was in a greenhouse located in Nova Santa Rita city in the state of Rio Grande do Sul, Brazil. The work started in June 2013. The re-mineralization treatments were defined based on fertilization practices used in the region according to the Brazilian Society of Soil Science (SBCS 2004). Most of the tropical soils are acid and have low fertility, with very low P and K contents (Rabel et al. 2018). For this reason, the treatments were dimensioned from the K (62 mg l−1) concentration in the experimental soil and on the K2O concentration of by-product. This is because 3.31% K2O concentration in the by-product is higher than that of CaO (3.06%), MgO (2.27%), and P2O5 (0.26%).
Before planting, limestone, triple superphosphate (TSP), and by-product doses were thoroughly mixed in the soil. The re-mineralization treatments are shown in Table 1.
Experimental Design
The experiment was carried out using a randomized block design with five treatments replicated seven times, and each experimental unit consisted of three pots. Generally, in greenhouse experiments, containers are small (usually with a capacity of around 10 kg of soil), which do not allow the development of plants until the physiological maturation stage of the cultivated species (usually more than 110 cycle days, depending of the species). Thus, black oats were produced in the first 70 days and in sequence maize crops were also cultivated for 70 days. For this, nine seeds of black oats were planted on the drilled pots in the background, which contained 10 dm3 of soil with treatments, and only three plants were grown for 70 days. Plants were harvested, washed with de-ionized water, and separated into leaves, stems, and roots. The material was dried at 40 °C until weight stabilization, weighed and then finally milled. According to Jones et al. (1991), after digestion in concentrated H2SO4 and H2O2 at 390 °C, concentrations of Ca, K, Mg, and P in the leaves were determined using an atomic absorption spectrophotometer. According to Tedesco et al. (1995), for soil fertility analysis, approximately 500 cm3 of soil was collected from each treatment.
Thereafter, the remaining soil samples (approximately 9.5 dm3) were homogenized and passed through a 4 mm mesh sieve and repotted. Then, in the same soil samples, nine seeds of maize were planted, and only three plants were cultivated for 70 days. Plant and soil samples were processed as described above for black oats.
The least significant difference (LSD) test was used to examine the results. Regression models were used to analyze the effects of treatments on dry matter production and nutrient accumulation in plant leaves. To determine the relationship between the mean accumulated concentrations of nutrients in the leaves and the averages of dry matter yield of black oats and maize crops at 5% probability, the Pearson’s linear correlation was applied using the t test. The BioEstat Statistics software (free distribution copy), version 5.3, was used to run all tests.
Results and Discussion
By-Product and Soil Particle Size Distribution
The particle size distribution of the by-product and soil, obtained by sieving, is shown in Tables 2 and 3, respectively.
The size distribution analysis showed that 100% of the particles of the by-product measure less than 2 m. This result meets the granulometric guarantee established by IN no 5 (Brazil 2016). The lower size distribution in the by-product may increase nutrients release. This interpretation was proven by the investigations of Priyono and Gilkes (2008), who studied the dissolution kinetics of silicate rock with particles size below 0.25 mm and 0.15 mm in an organic acid solution. These authors showed that the rate of cations dissolution increases with finer particle size.
The soil was classified as a typical Hapludox (HSoil), according to the US Department of Agriculture soil classification (USDA 1999), with 21% of clay in A horizon and 13% in Bw horizon. The soil particle size results (Table 3) confirm that the soil is a typical Hapludox. Briefly, the soil is composed of medium K (62 mg dm−3), low P (6.2 mg dm−3), and Al (0.3 cmolc dm−3) concentrations. Soil has 6.9 cmolc dm−3 cation exchange capacity (CEC) with pHH2O of 5.2, Ca and Mg concentrations of 2.6 and 0.7 cmolc dm−3, respectively, 50% base saturation (V), and 7.8% of Al saturation indicator (m).
By-Product Petrography
Optical microscope observations revealed that the dacite has an acid composition, featuring a micro-crystalline to glassy matrix (amorphous) with porphyritic to micro-porphyritic texture of granulation less than 0.25 mm (Fig. 2a, b). Phenocrysts, micro-phenocrysts (less than 10% of the volume of the rock) and a significant number of vesicles were observed (Fig. 2a, b). Plagioclase micro-phenocrysts appear isolated and comprise 67% of the volume of the rock with grains size of 0.5–1.5 mm (Fig. 2c). The micro-phenocrysts of clinopyroxene are less than 1.8 mm length (Fig. 2d, e). The minerals identified in the matrix were plagioclase (albite), clinopyroxene (augite), quartz, and opaque (hematite) minerals with apatite as accessory mineral. The quartz is colorless and anhedral, with diameter less than 0.35 mm, and occupies interstitial spaces (Fig. 2d). Apatite crystals have needle shapes of grains less than 0.15 mm (Fig. 2f).
Photomicrographs of dacite rock. (a) and (b) plagioclase (pl) and clinopyroxene (px) phenocrysts under NL and PL; (c) plagioclase (pl) phenocrysts under NL; (d) clinopyroxene (px) microphenocryst and quartz (qz) under PL; (e) clinopyroxene (px) phenocryst under NL; (f) apatite (apt) crystals under PL
By-Product and Soil Mineralogy
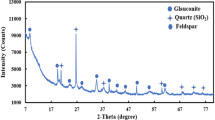
The presence of montmorillonite, saponite, and hematite (Fig. 3) shows that the by-product has been altered by a hydrothermal agent. Hydrothermal alteration of volcanic rock produces high proportions of amorphous material, which presents low mineralogical stability when exposed to exogenous conditions (Ridley 2012). This makes the rock interesting for use in soil re-mineralization (Ramos et al. 2017). The by-product contains an undetermined proportion of glassy mass, undetectable by X-ray diffraction analysis, which may supply macro-nutrients such as Ca, K, and Mg to soil (Deer et al. 2013). The weathering of the glassy matrix, together with the weathering of sanidine, albite, and augite, in addition to clay minerals that fill the vesicles and occur in the rock matrix, indicates hydrothermal alteration. This process changes the mineral phases, increasing the cations release potential, which may contribute to the soil re-mineralization (Korchagin et al. 2019). The dominant minerals observed in the by-product were plagioclase albite and pyroxene augite (Fig. 3) are the most common volcanic rock-forming minerals (Deer et al. 2013).
X-ray diffraction and SEM/EDS analysis show the occurrence of sanidine in the by-product (Figs. 3 and 4A). This mineral belongs to the group of K-feldspars, and when added to soil can release its elements more easily due to the action of weathering (Manning et al. 2017). K-feldspar is an important source of K to the soils and may be the largest reservoir of K (Ciceri et al. 2017). The presence of smectites (montmorillonite and saponite) as clay minerals in the by-product was observed in Fig. 3. Smectites have a relevance in soils due to their high cation exchange capacity (Huggett 2015).
Apatite is a widespread accessory phosphate mineral that occurs in almost all igneous rocks and is known to be partially resistant to weathering (Piccoli and Candela, 2002). This suggests slow dissolution and P release. This interpretation is contradictory to studies of Ramos et al. (2015), which showed recovery up to 93% of the P in leaching tests using dacite rock powder from the same region of this study. The presence of apatite is a good indication that by-product can release P to the soils.
Figure 5 shows the appearance and composition of the mineral particles of HSoil. The soil consists predominantly of quartz, kaolinite, and goethite.
By-Product and Soil Chemical Composition
The data in Table 4 indicate the predominant presence of Si, Al, Fe, and Ti oxides in decreasing order, and, in smaller proportions, oxides of Mg, Mn, P, Ca, K, and Na in the HSoil sample. The by-product contains different chemical elements, expressed as oxides (Table 4). These results concur with those obtained by Nunes et al. (2014), who characterized four similar rocks and their application as a soil re-mineralizer. The results of XRD (Fig. 5) and XRF, with respect to Al, Si, Fe, and Ti oxides, support the presence of quartz, kaolinite, and goethite (Fig. 6a, a, c).
In terms of chemical composition expressed as major and minor elements, it can be stated that the studied rock is dacite (Streckeisen 1976). In terms of potential (based on content) for the macro-nutrient K, the by-product could be indicated as a soil re-mineralizer according to the Brazilian IN no 5 (Brazil 2016). The by-product presents the sum of chemical compounds (CaO + MgO + K2O) higher than 9% and K2O content higher than 1%, in compliance with the Brazilian IN no 5 (Brazil 2016). According to Ramos et al. (2017), most of the Brazilian volcanic rocks contain a sum of chemical compounds with K2O content higher than the values established by Brazilian IN no 5. From these results, the criteria for a re-mineralizer are satisfied by the by-product studied here. This is a positive characteristic that represents good potential for agricultural use, especially in nutrient-poor soil such as HSoil (Table 4).
The average phosphorus concentration of the Earth’s upper crust is approximately 0.1% P2O5 (Cordell and White 2011). According to Table 4, the P2O5 content of the by-product is almost three times above the crustal average. This result may be attributed to the occurrence of apatite in the by-product, because this mineral was identified by OM (Fig. 2f) and by SEM/EDS (Fig. 4b). This finding concurs with Ptáček (2016) that the apatite is the most abundant phosphate mineral, which accounts for more than 95% of all phosphorus in the Earth’s crust and is found as an accessory mineral in volcanic rocks.
According to data presented in Table 4, the by-product is characterized by primary macro-nutrients such as K and P. Phosphorus and K are important nutrients to obtain high productivity in several crops; for example, in tropical soils (Carvalho et al. 2018). Calcium and Mg appear as secondary macro-nutrients. According to Gilliham et al. (2011), calcium serves vital physiological functions in plants. Magnesium is involved in chlorophyll pigments, in photosynthesis, and aids in phosphate metabolism, plant respiration, and the activation of many enzyme systems (Guo et al. 2016). Sodium is a common constituent in plants, but it is not an essential element, although for some plants, such as sugar beet, it is an important element to achieve high yields (Nieves-Cordones et al. 2016). The high Al content in the by-products is not concerning because Al precipitates in environments with pH above 5 and will not be available for soil solution and consequently for plants (Ramos et al. 2017). This is environmentally relevant because it supports the sustainable agricultural use of the by-products and hardly causes Al toxicity to plants. High content of Na in tissues is critical to ionic toxicity in plants, because, besides interfering with the proper homeostasis of K, Na reduces the availability, translocation, and mobilization of Ca to the growing regions, which affects vegetative growth and production (Cabot et al. 2014). Silicon is a micro-nutrient considered beneficial (Haynes 2014) but not essential to plants. This nutrient is extremely important for the development and crops protection (Keeping 2017; Beerling et al. 2018).
The maximum limits of PTEs in soil re-mineralizers allowed by Brazilian normative instruction are As (15 ppm), Cd (10 ppm), Hg (0.1 ppm), and Pb (200 ppm) (Brazil 2016). The results of analysis by ICP-MS demonstrate that the by-product sample has low concentrations of PTEs As (3 ppm), Cd (< 0.01 ppm), Hg (< 0.01 ppm), and (Pb 19 ppm), which do not represent an environmental risk.
Agronomic Performance of the By-Product
Soil Composition
Figure 7 shows that, after the soil treatments, soil fertility attributes such as base saturation (V), Al saturation (m), Al concentration, and CEC varied significantly when analyzed 140 days after maize crops were harvested. The application of all by-product doses (treatments T2–T5) significantly reduced the soil Al levels after 140 days. This result conforms to the observations of Ramos et al. (2017), which also supported the agricultural use of the volcanic mining by-products. Aluminum is toxic to plants, acidifies soil, and causes nutritional deficiency. The reduction in Al levels in soils with treatments T2–T5 is extremely important because most agricultural plant species do not achieve maximum production potential when produced on highly acid soils (Sade et al. 2016; Barbosa et al. 2017). Although the by-product has high levels of Al2O3 (13.8%), the toxicity by its dissolution is not considered to be an environmental concern. Aluminum released during dissolution in soils with pH above 5 is generally precipitated as secondary aluminosilicates or as oxides and hydroxides (Lindsay 1979; Faquin 1982).
As illustrated in Figure 7, the pH of the soils was not increased significantly (p = 0.3394). The highest pH value was found in treatment T5, which was measured at the end of maize cultivation. However, the pH value was significantly higher (p = 0.0053) in treatment T5 as compared to the treatment T1 that received only limestone (at 3.03 t ha−1) and triple superphosphate 0.238 t ha−1 after maize cultivation. According to Fageria (1998), it is necessary to apply approximately 12 t ha−1 of limestone in order to increase the pH value of the HSoil to 6.0.
The results presented in Figure 7 proved that the by-product studied has potential for increasing the soil pH because it acted as correctives of acidity in the short term (140 days). The results conform to those of Dumitru et al. (1999) whereby the application of rock dust slowly increases soil pH. One of the factors that considerably restrict the productivity of different crops in various parts of the world, including Brazil, is soil acidity. For the incorporation of these soils into the productive process, the use of materials that have the potential to increase pH is indispensable (Fageria 2009). The soil–plant system is dynamic, and it is difficult to define the optimal pH for several annual crops. According to Fageria (1998), most crops can produce well in soil with pH of around 6 (Fageria 1998).
With increase in the by-product doses, the CEC of the soils also increased; thus indicating the interaction of this effect with the period of the experiment (Fig. 7). Especially after maize cultivation, the tested doses provided a linear increase in CEC. However, the increases in CEC obtained in soils did not to reach 16.2 cmolc dm−3, which is the minimum considered adequate by SBCS (2004).
Before treatment T1 of the HSoil, the Al saturation indicator (m) was 7.8%. This decreased to 2.0% after black oat cultivation (Fig. 7), which could possibly be due to the application of limestone. However, the values were significantly lower, between 1.8% and 0% after maize cultivation in the treatments T2 to T5, respectively. This shows that the decrease in the Al saturation indicator was caused by increased by-product doses.
The data in Figure 8 show that the by-product application did not significantly change the concentrations of Ca, K, Mg, and P in the soil after 70 days of black oat cultivation (p > 0.05). However, a significant (p < 0.05) increase in the availability of Ca, K and P in the soil was observed after maize cultivation (140 days). Though the Mg did not increase significantly, its contents reached high levels in the soils for all the treatments. Soils with Ca contents between 2.1 and 4.0 cmolc l−1 and Mg contents between 0.6 and 1.0 cmolc l−1 are considered satisfactory according to SBCS (2004). The Ca and Mg contents reached above satisfactory levels. This result can be attributed primarily to the presence of albite and augite minerals in by-product, which were able to release Ca and Mg when added to the soils. In addition, the Ca and Mg contents were well below the levels added via by-product, which showed that these materials have the potential for the immediate release of these nutrients.
Generally, tropical soils have low available K concentration (Zhang et al. 2017), but it is not so low as P. After P, K is the second most consumed nutrient in Brazilian agriculture (Coelho and França 1995; Nowaki et al. 2017). In this study, the available K concentrations were observed to be high (91 and 98 mg l−1; SBCS 2004), which correspond to 0.23 and 0.25 cmolc l−1 in the treatments T4 and T5, respectively, after maize cultivation. This can be explained by the presence of sanidine mineral in the by-product, which was detected via XRD and SEM/EDS analyses (Fig. 3 and 4a) and is directly associated with the release of K. This result is very important and unusual in the existing literature. As an example, we can cite the study of Bakken et al. (2000), who investigated the release of K by crushed rocks and mining tailings by cultivating ryegrass for 6 months. The authors showed that K was sparsely available to plants, regardless of rock doses. Santos et al. (2016) determined the release of K in soils by the verdete rock with 77 g kg−1 of K and verified that soil K levels were decreased after grass, maize, and eucalyptus plantations. In soils treated with doses up to 50 t ha−1 of ground basalt, the low release of K was also observed by Motta and Feiden (1992). In the present study, the availability of K obtained, indicated that the dacite powder has potential for soil re-mineralization.
Typically, tropical soils have low concentrations of available P and high potential for the “fixation” of P applied by using soluble fertilizers. This factor rated P, along with N, as one of the most limiting nutrients in crop production in Brazil (Fageria 2009). The pH value of soil, as an isolated factor, is the most important factor that affects the availability of P in soil, which promoted the greater availability of P in the soil solution with pH of 5.7 (Fig. 7), and consequently, it showed higher uptake by maize plants cultivated in treatment T5 (Fig. 9).
A gradual increase in the available P in soils with the by-product dose increase (Fig. 8) showed that such a source may be considered good as a useful fertilizer. According to SBCS (2004), these doses were enough to alter the level of P in the soils, because P concentration without by-product (treatment T1) was low (5.40 mg dm−3 corresponding to 0.052 cmolc l−1) and the P concentration became average (11.0–14.0 mg dm−3 corresponding to 0.1-0.13 cmolc l−1) after maize cultivation.
The results obtained in this study conform to those of Theodoro et al. (2010) that rocks can provide nutrients that are important for crops. Considering the fact that the experimental time was only 70 days for each crop, the by-product of this study presented notable reactivity in soils of treatments T3–T4.
Growth and Nutrient Uptake by Black Oats and Maize Crops
The growth and Ca, K, Mg, and P uptake by black oats and maize leaves were significantly affected by the by-product doses (Fig. 9). The reference values for black oats and maize leaves contents, according to Pauletti (2004), are shown in Table 5. These results indicate that these nutrients were released rapidly from the by-product, suggesting that the by-product had an immediate effect of release nutrients onto soil.
According to Figure 9, the K concentration in treatment T1 was below the critical value in maize leaves (15.9 g kg−1), and in leaves of black oats it was at the limit of critical value (15.2 g kg−1). It was possible, as evidenced in Figure 9, that with increasing by-product dose, the K content of leaves of both crops increased linearly. In treatments T2–T5, the levels of K in black oat leaves were within the appropriate range, whereas K in maize leaves was well above the range (Table 5).
The K content in the foliar tissues of black oats cultivated in treatment T5 was two times greater than in treatment T1. In maize leaves, it was four times greater than treatment T1. Curi et al. (2005) stated that the release of K in soils by K-feldspars can supply the K demand of plants for some cycles. During these vegetative cycles, monitoring should be done so that the plants do not present deficiency of this nutrient.
For P, behavior similar to K was observed. The concentration of P in treatment T1 was below the critical value in maize leaves (1.01 g kg−1) and in black oats (0.98 g kg−1) (Fig. 9). With the increase in the application of by-product, the concentrations of P in the leaves also increased linearly (Fig. 9). In treatment T5, the P content in black oat leaves reached the appropriate range, whereas in maize leaves it was well above of appropriate range (Table 5). This behavior agrees with the finding of Ros et al. (2017) that P exhibits great mobility in young maize plants.
As can be observed from Figure 9, the treatments T2–T5 had the highest concentrations of P and Mg after maize harvesting. Phosphorus is significantly influenced by the synergistic concentration of Mg in the soil (Ribes et al. 2012). Foliar Ca content of black oats and maize crops presented a linear effect as a function of by-product doses and was approximately 1.6-times higher in treatment T5 than in treatment T1 for both crops. This may have occurred because Ca was added to the soil via limestone in treatment T1, but in treatment T5 it was released via weathering of the albite and augite present in by-product. These minerals were detected by XRD (Fig. 3).
As illustrated in Figure 9, it was observed that the Mg contents in black oats leaves were not significant in relation to the by-product doses. In contrast, Mg levels were significantly increased in maize leaves, probably due to limestone in treatments T1 and T2. In treatments T3–T5, such increase in Mg can be attributed to the weathering of the augite mineral present in the by-product.
The dry matter production of black oats increased linearly (Fig. 10), but statistically there was no significant difference (p = 0.246). A quadratic response in the dry matter production of maize was observed, with statistically significant difference (p = 0.010) between treatments, which according to the regression model indicated efficient maximum values at 5000 kg ha−1 (Fig. 10). The dry matter yield of black oats obtained in this study showed increases of 11% and 19% in treatments T4 and T5, respectively. Evans (1947) used 247 and 497 t ha−1 of basalt powder and obtained increases of 33.7% and 56.7% in dry matter production with potted oats. If the same doses suggested by Evans (1947) were applied in the present study, the increases would be 754% and 1311%.
The above results agree to those obtained by Theodoro and Leonardos (2006), who concluded that the use of the rock powder increased the production of maize, rice, cassava, sugar cane, vegetables, and fruits compared to soluble fertilization.
Nutrients Accumulation by Black Oat and Maize Crops
A linear increase in the accumulation of Ca and K in the leaves of both crops as a function of the addition rates of the by-product is presented in Figure 11. In black oat leaves, the same results were observed for Mg and P. According to the regression model, a quadratic response was observed for Mg and P accumulation in maize leaves, which indicates maximum efficiency at 5416 kg ha−1 and 5800, respectively (Fig. 11).
Treatment T5 presented a significantly higher value (p < 0.01) than the other treatments for the accumulation of K by maize leaves (Fig. 11). This was possibly because the leaves were collected at 70 days, which coincides with the recommended time for leaf sampling (SBCS 2004). This result suggests that the plants absorbed the amount of K required for their complete development (Table 6).
Most of the linear correlation coefficients between the mineral nutrition elements of black oats and maize crops were found by the t test to be significant with 95% confidence (Table 2). As observed in Table 2 for black oats and maize crops, the positive correlations of P with K and Ca indicate that the greater P content in plant is the better the root system growth of the plants is thus allowing the plant to explore a larger volume of the soil (Malavolta et al. 1997). It was observed that K correlated positively with Ca, and the synergistic effect between K and Ca was evidenced in maize leaves due to the high correlation between them.
Considering the presented results, we can infer that the application of the by-product was satisfactory because it positively altered the soil fertility attributes and it met the demand for Ca, K, Mg, and P by maize crops and black oat plants in a short term. All parameters evaluated in this study met the requirements established by IN no 5 (Brazil 2016). These results suggest that this technique can and should be adopted at a global level, especially in countries that have rock mining activity. It is important to note that several studies have shown that other rock types, such as metasedimentary rocks (e.g., verdete; Santos et al. 2016), plutonic rocks (e.g., nepheline syenite; Nascimento and Loureiro 2004), and metamorphic rocks (e.g., shale; Silveira et al. 2010), are composed of minerals that are rich in nutrients, particularly K.
Conclusions
This work evaluated the ability of a by-product of volcanic rock mining to positively alter the agronomic properties of the soil and the development and productivity of black oat and maize plants. The purpose was achieved by using the optical microscopy, XRD, XRF, SEM/EDS, and ICP-MS for determining the petrographical, mineralogical, and chemical composition of the by-product.
The safe use of the by-product evaluated here requires an evaluation of the PTEs composition, to ensure the quality of the inputs added to the soil, which will result in better food nutritional quality and environmental protection. The by-product contains macro-nutrients such as Ca, K, Mg, and P, besides low PTEs concentrations, which do not represent environmental risk, indicating good potential to be used as soil re-mineralizer. This study demonstrated that the by-product positively influenced the nutrition and development of black oats and maize grown in treatments T3–T5. These results suggest that this by-product could replace soluble fertilizers. In addition, it does require chemical processing and can be used in natura.
The increase in pH of soil treated with 7251 kg ha−1 of the by-product was considered significant when compared to the treatment with zero-dose of the by-product. In treatments T3–T5, relevant reductions in exchangeable Al and Al saturation were observed, which show the residual effect due to large reserves of Ca and Mg that were slowly released by the by-product. The application of by-product doses in treatments T3–T5 increased the plant growth, reduced Al toxicity in the soil, and amplified the levels of P, Ca, and Mg in the soil and the levels of Ca and Mg in plants. The application of the by-product studied here is a sustainable technology for soil re-mineralization. The use of this by-product in agriculture may be suitable for solving the problem of by-products deposited outside the mines and decrease the consumption of soluble fertilizers.
To confirm the use feasibility of volcanic rock mining by-products in agriculture, it will be necessary to carry out experiments in field conditions. For this, it is suggested that the experiments are monitored for at least 2 years, with summer and winter crops. Special attention should be given to experimental sites, to obtain a correct control of the variations of soil types, fertility levels and conditions of excess and/or lack of humidity. Generally, experimental plots in the field have variable dimensions but a suitable size can be 5.0 × 5.0 m (25 m2), which allows the useful area to provide adequate amount of grains or phytomass for the nutritional evaluations in leaves and grains. Evaluations of the response variables should consist of soil analysis, leaf tissue analysis, shoot dry mass, and production/productivity. Soil analyses can only be done at the end of the last successive crop, but it is preferable that they are carried out at the end of the cycle of each crop.
References
ABNT. (1986). NBR 7181 Amostragem de solo: Preparação para ensaios de compactação e ensaios de caracterização. São Paulo: Associação Brasileira de Normas Técnicas (ABNT).
Bakken, A. K., Gautneb, H., Sveistrup, T., & Myhr, K. (2000). Crushed rocks and mine tailings applied as K fertilizers on grassland. Nutrient Cycling in Agroecosystems,56(1), 53–57.
Barbosa, J. Z., Motta, A. C. V., Consalter, R., & Pauletti, V. (2017). Wheat (Triticum aestivum L.) response to boron in contrasting soil acidity conditions. Revista Brasileira de Ciências Agrárias,12, 148–157.
Beerling, D. J., Leake, J. R., Long, S. P., Scholes, J. D., Ton, J., Nelson, P. N., et al. (2018). Farming with crops and rocks to address global climate, food and soil security. Nature Plants,4(3), 138.
Bergmann, M., Juchen, P. L., Petroli, L., & Sander, A. (2017). Caracterização litoquímica e petrográfica de riodacitos vítreos mineralizados com ametista no RS: Possíveis fontes de potássio e multinutrientes para remineralização de solos. In M. Donato, L. C. Duarte, & F. S. Vilasboas (Eds.), Ações Aplicadas à cadeia produtiva de Gemas e Joias do Rio Grande do Sul (pp. 26–35). Porto Alegre: IGEO/UFRGS.
Brazil. (2013). Lei 12.890/2013 de 10 de dezembro de 2013—Altera a Lei no 6.894, de 16 de dezembro de 1980. https://www.planalto.gov.br/ccivil_03/_ato2011-2014/2013/lei/l12890.htm. Accessed May 28, 2019.
Brazil. (2016). Instrução Normativa Nº 05 de 10 de março de 2016. http://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-agricolas/fertilizantes/legislacao/in-5-de-10-3-16-remineralizadores-e-substratos-para-plantas.pdf. Accessed May 28, 2019.
Cabot, C., Sibole, J. V., Barceló, J., & Poschenrieder, C. (2014). Lessons from crop plants struggling with salinity. Plant Science,226, 2–13.
Carvalho, M. D., Nascente, A. S., Ferreira, G. B., Mutadiua, C. A., & Denardin, J. E. (2018). Phosphorus and potassium fertilization increase common bean grain yield in Mozambique. Revista Brasileira de Engenharia Agrícola e Ambiental,22(5), 308–314.
Ciceri, D., Oliveira, M., & Allanore, A. (2017). Potassium fertilizer via hydrothermal alteration of K-feldspar ore. Green Chemistry. https://doi.org/10.1039/C7GC02633A.
Coelho, A. M., & França, G. E. (1995). Seja doutor do seu milho: nutrição e adubação. Piracicaba, SP: Potafos.
Cordell, D., & White, S. (2011). Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability. https://doi.org/10.3390/su3102027.
Curi, N., Kampf, N., & Marques, J. J. (2005). Mineralogia e formas de potássio em solos brasileiros—Potássio na agricultura brasileira. Piracicaba, SP: Instituto da Potassa e do Fosfato.
Deer, W. A., Howie, R. A., & Zussman, J. (2013). An introduction to the rock-forming minerals (3rd ed.). London: The Mineralogical Society.
Dumitru, I., Zdrilic, A., & Azzopardi, A. (1999). Soil Remineralisation with basaltic rock dust in Australia. http://cinderite.com/wp-content/uploads/2018/05/basaltic_rock_dust_paper_1-3.pdf. Accessed November 19, 2018.
Escosteguy, P. A. V., & Klamt, E. (1998). Ground basalt as nutrient source. Revista Brasileira de Ciência do Solo. https://doi.org/10.1590/S0100-06831998000100002.
Evans, H. (1947). Annual report. In: Investigations on the fertilizer value os crushed basaltic rock. Mauritius Sugar Cane Research Station, 18, 227.
Fageria, N. K. (1998). Optimizing nutrient use efficiency in crop production. Revista Brasileira de Engenharia Agricola e Ambiental,122, 122. https://doi.org/10.1590/1807-1929/agriambi.v02n01p6-16.
Fageria, N. K. (2009). The use of nutrients in crop plants. Boca Raton, FL: CRC Press.
Faquin, V. (1982). Efeito do tratamento térmico do sienito nefelínico adicionado de calcário dolomítico, na disponibilidade de potássio ao milho (Zea mays L.), em casa de vegetação. Tese de Mestrado. Escola Superior de Agricultura “Luiz de Queiroz”. Piracicaba, SP.
Ferrari, V., Taffarel, S. R., Espinosa-Fuentes, E., Oliveira, M. L., Saikia, B. K., & Oliveira, L. F. (2019). Chemical evaluation of by-products of the grape industry as potential agricultural fertilizers. Journal of Cleaner Production,208, 297–306.
Gilliham, M., Dayod, M., Hocking, B. J., Xu, B., Conn, S. J., Kaiser, B. N., et al. (2011). Calcium delivery and storage in plant leaves: Exploring the link with water flow. Journal of Experimental Botany,62(7), 2233–2250.
Gillman, G. P., Burkett, D. C., & Coventry, R. J. (2001). A laboratory study of application of basalt dust to highly weathered soils: Effect on soil cation chemistry. Soil Research,39(4), 799–811.
Guo, W., Nazim, H., Liang, Z., & Yang, D. (2016). Magnesium deficiency in plants: An urgent problem. The Crop Journal,4(2), 83–91.
Haynes, R. J. (2014). A contemporary overview of silicon availability in agricultural soils. Journal of Plant Nutrition and Soil Science,177, 831–844.
Huggett, J. M. (2015). Clay minerals, reference module in earth systems and environmental sciences. Elsevier. https://doi.org/10.1016/B978-0-12-409548-9.09519-1.
Jones, J. B., Wolf, B., & Mills, H. A. (1991). Plant analysis handbook: A practical sampling, preparation, analysis, and interpreting guide. Athens, GA: Micro-Macro Publishing.
Keeping, M. G. (2017). Uptake of silicon by sugarcane from applied sources may not reflect plant-available soil silicon and total silicon content of sources. Frontiers in Plant Science,8, 760.
Korchagin, J., Caner, L., & Bortoluzzi, E. C. (2019). Variability of amethyst mining waste: A mineralogical and geochemical approach to evaluate the potential use in agriculture. Journal of Cleaner Production,210, 749–758.
Kronberg, B. I., Leonardos, O. H., Fyfe, W. S., Mattoso, S. Q., & Santos, A. M. (1976). Alguns dados geoquímicos sobre solos do Brasil: Uso potencial do pó de pedreira como fonte de nutrientes críticos em solos altamente lixiviados – com atenção de geoquímica de alguns solos da Amazônia. Ouro Preto, MG: SBG.
Leonardos, O. H., Fyfe, W. S., & Kronberg, B. I. (1976). Rochagem O método de Aumento da Fertilidade em Solos Lixiviados e Arenosos. Belo Horizonte, MG: CBG.
Lindsay, W. L. (1979). Chemical equilibria in soils. New York: Wiley.
Machado, A. F, Lucena, G. N., Carneiro, J. S. S., Negreiros Neto, J. V., Santos, A. C., & Silva, R. R. (2014). Aproveitamento de rejeito de mineração na blendagem de calcário comercial para correção do solo. http://www.gurupi.uft.edu.br/amazonsoil/pdf/03.pdf. Accessed May 17, 2019.
Malavolta, E., Vitti, G. C., & Oliveira, S. A. (1997). Avaliação do estado nutricional das plantas: princípios e aplicações. Piracicaba, SP: Potafos.
Manning, D. A., Baptista, J., Limon, M. S., & Brandt, K. (2017). Testing the ability of plants to access potassium from framework silicate minerals. Science of the Total Environment,574, 476–481.
Manning, D. A., & Theodoro, S. H. (2018). Enabling food security through use of local rocks and minerals. The Extractive Industries and Society. Amsterdam: Elsevier. https://doi.org/10.1016/j.exis.2018.11.002.
Melfi, A., Cerri, C. C., Fritsch, E., & Formoso, M. L. L. (1999). Tropical soils: genesis, distribution and degradation of lateritic pedological systems. In Workshop on topical soils. Rio de Janeiro, RJ: Academia Brasileira de Ciências, pp. 9–30.
Meunier, A., Formoso, M. L. L., Patrier, P., & Chies, J. O. (1988). Altération hydrothermale de roches volcaniques liée à la genèse des améthystes-Bassin du Paraná-Sud du Brésil. Geochimica Brasiliensis,2(2), 127–142.
Motta, A. C. V., & Feiden, A. (1992). Avaliação do P em LE submetido a diferentes doses de basalto. Agrárias,12(47), 54.
Nascimento, M., & Loureiro, F. E. L. (2004). Fertilizantes e sustentabilidade: o potássio na agricultura brasileira, fontes e rotas alternativas. Série Estudos e Documentos 61. Rio de Janeiro, RJ: CETEM/MCT.
Nieves-Cordones, M., Al Shiblawi, F. R., & Sentenac, H. (2016). Roles and transport of sodium and potassium in plants. In A. Sigel, H. Sigel, & R. Sigel (Eds.), The alkali metal ions: Their role for life (pp. 291–324). Cham: Springer.
Nowaki, R. H., Parent, S. É., Cecílio Filho, A. B., Rozane, D. E., Meneses, N. B., Silva, J. A., et al. (2017). Phosphorus over-fertilization and nutrient misbalance of irrigated tomato crops in Brazil. Frontiers in Plant Science,8, 825.
Nunes, J. M. G., Kautzmann, R. M., & Oliveira, C. (2014). Evaluation of the natural fertilizing potential of basalt dust wastes from the mining district of Nova Prata (Brazil). Journal of Cleaner Production,84, 649–656.
Pauletti, V. (2004). Nutrientes: Teores e interpretações. Castro, PR: Fundação ABC para a Assistência e Divulgação Técnica Agropecuária.
Piccoli, P. M., & Candela, P. A. (2002). Apatite in igneous systems. Reviews in Mineralogy and Geochemistry,48(1), 255–292.
Priyono, J., & Gilkes, R. J. (2008). High-energy milling improves the effectiveness of silicate rock fertilizers: A glasshouse assessment. Communications in Soil Science and Plant Analysis,39(3–4), 358–369.
Ptáček, P. (2016). Apatites and their synthetic analogues: Synthesis, structure, properties and applications. BoD–Books on Demand. https://books.google.com.br/books?hl=pt-BR&lr=&id=dmqQDwAAQBAJ&oi=fnd&pg=PA1&dq=related:08xOVM0BtRnkgM:scholar.google.com/&ots=LohoL23lEw&sig=KWUlq2MFqVT-uaX3fDEXrffiL8I&redir_esc=y#v=onepage&q&f=false. Accessed July 17, 2019.
Querol, X., Whateley, M. K. G., Fernandez-Turiel, J. L., & Tuncali, E. (1997). Geological controls on the mineralogy and geochemistry of the Beypazari lignite, central Anatolia, Turkey. International Journal of Coal Geology,33(3), 255–271.
Rabel, D. O., Motta, A. C. V., Barbosa, J. Z., Melo, V. F., & Prior, S. A. (2018). Depth distribution of exchangeable aluminum in acid soils: A study from subtropical Brazil. Acta Scientiarum. Agronomy. https://doi.org/10.4025/actasciagron.v40i1.39320.
Ramos, C. G., Querol, X., Dalmora, A. C., de Jesus Pires, K. C., Schneider, I. A. H., Oliveira, L. F. S., et al. (2017). Evaluation of the potential of volcanic rock waste from southern Brazil as a natural soil fertilizer. Journal of Cleaner Production,142, 2700–2706.
Ramos, C. G., Querol, X., Oliveira, M. L., Pires, K., Kautzmann, R. M., & Oliveira, L. F. (2015). A preliminary evaluation of volcanic rock powder for application in agriculture as soil a remineralizer. Science of the Total Environment,512, 371–380.
Ribes, R., Buss, R., Lazari, R., Potes, M., & Bamberg, A. (2012). Efeito de rochas moídas sobre a concentração de macronutrientes na parte áerea de plantas de milho. In Embrapa Clima Temperado. In Workshop Insumos Para Agricultura Sustentável, 2012, Pelotas. Anais… Pelotas: Embrapa Clima Temperado.
Ridley, W. I. (2012). Petrology of associated igneous rocks. In C. P. Shanks III, R. Thurston (Eds.), Volcanogenic massive sulfide occurrence model. Virginia, U.S: Geological Survey Scientific Investigations Report 2010–5070–C, Virginia.
Ros, C. O. D., Matsuoka, M., Silva, R. F. D., & Silva, V. R. D. (2017). Interference from the vertical variation of soil phosphorus and from water stress on growth in maize, the soybean and sunflower. Revista Ciência Agronômica,48(3), 419–427.
Rosenstengel, L. M., & Hartmann, L. A. (2012). Geochemical stratigraphy of lavas and fault-block structures in the Ametista do Sul geode mining district, Paraná volcanic province, southern Brazil. Ore Geology Reviews,48, 332–348.
Sade, H., Meriga, B., Surapu, V., Gadi, J., Sunita, M. S. L., Suravajhala, P., et al. (2016). Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. BioMetals,29(2), 187–210.
Sánchez-Peña, N. E., Narváez-Semanate, J. L., Pabón-Patiño, D., Fernández-Mera, J. E., Oliveira, M. L., da Boit, K., et al. (2018). Chemical and nano-mineralogical study for determining potential uses of legal Colombian gold mine sludge: Experimental evidence. Chemosphere,191, 1048–1055.
Santos, W. O., Mattiello, E. M., Vergutz, L., & Costa, R. F. (2016). Production and evaluation of potassium fertilizers from silicate rock. Journal of Plant Nutrition and Soil Science,179(4), 547–556.
Silva, R. C. (2016). Intemperismo de minerais de um remineralizador (p. 183). Tese (Doutorado), Piracicaba, SP: Escola Superior de Agricultura Luiz de Queiroz.
Silva, L. F., Izquierdo, M., Querol, X., Finkelman, R. B., Oliveira, M. L., Wollenschlager, M., et al. (2011). Leaching of potential hazardous elements of coal cleaning rejects. Environmental Monitoring and Assessment,175(1–4), 109–126.
Silveira, C. A. P., Ferreira, L. H. G., Pillon, C. N. Giacomini, S. J. E., & Santos, L. C. (2010). Efeito da combinação de calcário de xisto e calcário dolomítico sobre a produtividade de grãos de dois sistemas de rotação de culturas. Anais do I Congresso Brasileiro de Rochagem. Brasília. Embrapa. Brasília/DF: CBR.
Sociedade Brasileira de Ciência do Solo—SBCS. (2004). Manual de Adubação e de Calagem: para os estados do Rio Grande do Sul e Santa Catarina. Porto Alegre, RS: Comissão de Química e Fertilidade do Solo.
Streckeisen, A. (1976). To each plutonic rock its proper name. Earth-Science Reviews,12, 1–33.
Tedesco, M. J., Gianello, C., Bissani, C. A., Bohnen, H., & Volkweiss, S. J. (1995). Análise do solo plantas e outros materiais (2nd ed.). Porto Alegre, RS: Departamento de Solos da UFRGS.
Theodoro, S. H., & Leonardos, O. H. (2006). The use of rocks to improve family agriculture in Brazil. Anais da Academia Brasileira de Ciências,78(4), 721–730.
Theodoro, S. H., Leonardos, O. H., & de Almeida, E. (2010). Mecanismos para Disponibilização de Nutrientes Minerais a Partir de Processos Biológicos. Planaltina, DF: EMBRAPA.
United States Department of Agriculture—USDA. (1999). Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys (2nd ed.). Washington: Agriculture Handbook.
Van Straaten, P. (2007). Agrogeology: The use of rocks for crops (No. 631.811 S894a). Ontario, CA: Enviroquest.
Zhang, Y., Nachimuthu, G., Mason, S., McLaughlin, M. J., McNeill, A., & Bell, M. J. (2017). Comparison of soil analytical methods for estimating wheat potassium fertilizer requirements in response to contrasting plant K demand in the glasshouse. Scientific Reports,7(1), 11391.
Acknowledgments
The authors are thankful to Geologists Magda Bergmann and Andrea Sander by important contributions in this research; to Agronomic Engineer Carlos Augusto Posser Silveira for the agronomic orientations; to Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS), Edital 014/2012—BMT for financial support; to the Sindicato da Indústria de Extração de Pedreiras de Nova Prata for the supply of rock samples; and especially to James Hower for editing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramos, C.G., dos Santos de Medeiros, D., Gomez, L. et al. Evaluation of Soil Re-mineralizer from By-Product of Volcanic Rock Mining: Experimental Proof Using Black Oats and Maize Crops. Nat Resour Res 29, 1583–1600 (2020). https://doi.org/10.1007/s11053-019-09529-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-019-09529-x