Abstract
In Brazil impounded vehicle scrapyards (IVS) are often overcrowded and may pose a source of potentially toxic elements (PTEs). In this study, PTEs content in soil cores and groundwater of an IVS located at a municipality of the São Paulo metropolitan region was assessed. INAA, XRF and ICP-MS were the analytical techniques employed. PTEs results and statistical approaches indicated that As, Pb, Ni, Cu and Nb are mostly anthropic. Pb, Cu, Ni and Nb mass fraction increased with depth indicating some downward mobility. Arsenic may represent a moderate to very high potential ecological risk. PTEs groundwater levels were bellow drinking water recommendation limits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban sprawl has become a challenge all over the world, especially in developing countries. Thus, the introduction of toxic substances or poor land management systems in the urban environment and its consequent soil quality deterioration can affect food security, groundwater quality and human health in general.
In 2010, around to 85% of Brazilian population lived in urban areas [1] with multiple management and environmental problems that end up compromising water supply, sewage treatment, air and soil quality, as well as, its inhabitant’s quality of life. São Paulo Metropolitan Region (SPMR) is the largest urban concentration in South America, with more than 21 million people in a surface area of 8000 km2 approximately [2].
From 2014 to 2015, preservation awareness over groundwater quality was intensified in SPMR due to a drought that severely restricted water supply. Many aspects compromised the city regular water supply such as the lack of rainfall coupled with poor supply and distribution planning and with irregular and/or disorderly springs occupation resulted in a water crisis and severe reduction from main water supply systems [3].
In this scenario of uncontrolled urbanization and increased number of motor vehicles with more than 7 million cars in SPMR [2], local authorities are challenged to plan adequately to meet land use and vehicle related management needs. Usually, environmental contaminants from a variety of sources and vehicular emission tend to accumulate in urban soils already composed of a heterogeneous mixture of geogenic and anthropogenic materials.
Vehicle-related facilities such as dismantlers, mechanic workshops, parking-lots, discarded and impounded vehicle scrapyard (IVS) have been increasingly sources of environmental contamination [4,5,6,7]. Most of these areas are unpaved, and vehicles are parked directly on topsoil. Thus, soil and groundwater quality is continually threatened by emissions resulting from tire wear off, break lining, wear of individual vehicular components such as the car body, clutch or motor parts, oil leaking or spill from engine and hydraulic systems, and fuel additives [8]. These emissions may contain Potentially Toxic Elements (PTEs), such as As, Ba, Cd, Cr, Cu, Ni, Pb and V and alloy elements, such as Fe and Mn [4, 7].
Contamination severity depends on several factors, such as vehicle integrity, parking time, vehicle type, weathering, soil properties and management practices. Potentially toxic metals mobility in soils depend on the binding state and metal properties, environmental factors and soil properties, such as pH, soil organic matter (SOM) and redox conditions [9]. In urban soils, surface runoff is the main water flow mechanism because percolation is significantly reduced by compactation, by contamination derived from deposition of wastes and atmospheric particles, by loss of organic matter and buffering capacity and changes in the structure [10]. Such changes affect soil properties culminating with failure in the ability of retaining contaminants by sorption processes. As a consequence, trace metals solubilization can increase, favouring their bioavailability and percolation through the soil profile.
Studies concerning PTEs mobility in vehicle related facilities soils have already been reported [4, 5], however in Brazil they are scarce, specially concerning IVS areas.
Recently Lange et al. [7] have evaluated the PTEs mass fractions on topsoil (0.2 m) in an IVS at Ribeirao Pires city, a municipality of the SPMR, surrounded by protected watersheds that play an essential role in major streams headwaters protection and for drinking water supply. Hot spots found for most PTEs suggest vehicular source. The IVS is operational since the 90s, and no PTEs evaluation in soil distribution by depth was performed, nor in its groundwater. Therefore, many doubts concerning PTEs downward mobility persist in Riberão Pires IVS site. The present study aims to evaluate how PTEs are distributed over IVS depths in an apparently very degraded area and if/how car components layed over this IVS terrain affected soil and groundwater quality.
Experimental
Sampling site
The sampling site was an IVS located in the city of Ribeirão Pires, a municipality in the SPMR, Brazil with around 15,000 m2 area. According to the IVS administration, only since 2013 information about receives and releases of vehicles in the area were available, reporting around 700 receives and 350 releases per year, remaining an average of 1000 vehicles per year. From 1936 until early 90s the study area was a former pesticide-manufacturing site. The site’s neighbor sidewalk stores some oil filled stone base tanks. More information about the site was published elsewhere [7].
Geology
Ribeirão Pires lies at the Paulistano plateau formation, south of the geomorphological compartment corresponding to Atlantic Plateau province [11]. The Plateau contains a diverse set of metamorphic and igneous rocks from the Precambrian and Eopaleozoic ages, with various forms of topography, characterized by relatively low to moderate relief with rolling hills and some ridges with altitudes varying between 715 and 900 m [12]. Some small sedimentary basins characterize the Basin of São Paulo. The Quaternary deposits are colluvial and alluvial materials [13]. The site is located on a sedimentary plain spot on steep hillsides made up of residual soils with a silt–clay to fine-sand texture. The IVS is operational since the 90s. Eight years ago this yard was covered by a 0.5 m layer of a mixture of soil with demolition waste, such as brick, tiles, steel, wood, plastic, glass, asphalt fragments, rubbers and also auto parts pieces.
Hydrogeochemistry
Ribeirao Pires is located at the hydro stratigraphic unity named Center South Crystalline Aquifer (Escudo Oriental Sudeste), a crystalline Pre-Cambrian formation with a potential water flow of 1–23 m3 h−1 [14], with average production of 10 m3 h−1 and good quality. Geochemical data of the pristine aquifer was provided by CETESB (São Paulo State Environmental Agency) groundwater monitoring data available for Ribeirao Pires Area from 1998 to 2000 [15] and from 2004 to 2006 [16]. The aquifer is unconfined with shallow waters and most of the recharge occurs by vertical infiltration of rainwater (over 70%). Discharge happens mostly by evapotranspiration and in small, but growing amount, by groundwater extraction. In sandy shallow layers, high hydraulic conductivity is expected with lateral water movement following the drainage canal topography. Groundwater table varies from 0.5 to 3 m. Total dissolved solids (TDS) are lower than 300 mg L−1 with a mean concentration of 140 mg L−1; pH ranges from 4.7 to 9.1 with a mean temperature of 22.4 °C. Overall anion chemistry has bicarbonate as the dominant species, with low sulfate and chloride. Calcium is the dominant cation ranging from 0.25 to 85 mg L−1, with mean value of 11.3 mg L−1. Following cations were Na, Mg and K [16]. The groundwater is dominated by bicarbonate over carbonate alkalinity. The area presents a good water quality with low metal content frequently below detection limit of 0.1 µg L−1 to Hg, of 10–20 µg L−1 to Cu, Cr, Ni, Zn, of 50–100 µg L−1 Ba and Al.
Soil sampling and analysis
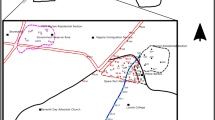
Soil sampling was performed at three different spots inside the IVS, at the same locations of the monitoring wells, as following: PM1 located at a spot where vehicles were not parked, PM2 and PM3 (Fig. 1).
Soil samples were collected at vertical direction using a Geoprobe direct push system (Geoprobe model 7822DT) equipped with a macro core soil sampling system by using plastic liners catchers (Geoprobe systems, KS, USA). The cores were subsampled by soil texture. Nine samples were collected: four in PM1 (PM1S1, PM1S2, PM1S3 and PM1S4), three in PM2 (PM2S1, PM2S2 and PM2S3) and two in PM3 (PM3S1 and PM3S2), as schematically presented in Fig. 2. Some oil amount was observed in soil samples PM2S2 and PM3S2. Samples were dried at 40 °C, sieved (< 2 mm) and quartered. Each soil subsample was characterized. Soil characterization analysis, namely, pH, cation exchange capacity (pH 7), sum of bases (Ca2+, K+, Mg2+), potential acidity (H + Al), organic matter and soil texture, was performed in Campinas Agronomic Institute (IAC), São Paulo state, according to Camargo et al. [17]. Organic matter (OM) content was measured by oxidation with potassium dichromate and sulfuric acid [17]. Clay, silt and sand percentage was measured by the hydrometer method [17].
Monitoring wells
For this study, three tubular PE water sampling wells with 2 inches diameter and total depth of 3.5–4 m were installed at the sampling site, as presented in Fig. 2. The wells were constructed over a crystalline shallow unconfined aquifer that presents mostly granitic rocks covered with soil, a shallow fine sandy layer and later silt-clayed layer. Filtering elements stood from water level to the first fine permeable sandy loam layer just before residual soil, in order to collect percolation waters from topsoil as well as waters from aquifer flow. Local groundwater flow was estimated from Southeast to Northwest towards a drainage valley that forms a temporary tributary that ends at Northeast of Billings Dam.
Analytical procedure
Soil samples were ground using a mechanic agate ball mill in order to obtain a fine and homogenous powder. Mass fraction of several elements in soil samples were determined by Instrumental Neutron Activation Analysis (INAA) and X-ray Fluorescence (XRF), as has been shown elsewhere [18, 7]. Al2O3, CaO, Fe2O3, K2O, MgO, MnO, Na2O, P2O5, SiO2, Cu, Ni, Pb, V and Y were determined by XRF. The homogenity of the samples was verified by three replicates of half of the analysed samples by INAA. The relative standard deviation for each three replicates was less than 8%.
Water sampling
Water sampling was performed by using a stainless steel submersible purging pump at discharge rates between 0.02 and 0.06 L s−1. The pumping system was rinsed between samplings with distilled water. Samples were collected after the stabilization of pH, and electric conductivity (EC). Samples were collected at the groundwater flow zones with the help of EC readings and local geology information to assure that the water collected corresponded to the local flow at the site and not to a longtime trapped water inside the well. Water and well depth readings were recorded to provide the water column at each well.
Measurements of the physical and chemical parameters (temperature, electric conductivity (EC), pH, Eh, and dissolved oxygen) were made in situ. The groundwater samples were collected in acid precleaned low-density polyethylene bottles and were filtered in situ using 60 mL plastic syringes coupled with 0.45 μm polyvinylidene fluoride (PVDF). The samples were stored at 4 °C until analysis for major, minor, and trace elements. For cations analysis, the samples were acidified to pH 2 with ultrapure HNO3. Eh was corrected against Standard Hydrogen Electrode (SHE). Analyses were carried out at IPEN and UNICAMP laboratories for the following parameters: (i) seven anions (F−, Cl−, SO42−, PO43−, Br−, NO3− and NO2−) by ion chromatography (Dionex, ICS 2500), (ii) and trace elements by inductively coupled plasma mass spectrometry (ICP-MS, Thermo X Series II). Alkalinity was titrated within 12 h after sample collection according to APHA [19]. At each well, field blanks were prepared using deionized water and duplicate analysis were performed, to check the reliability of the measurement procedure. During each analytical run, the standard reference material (CRM) of river water SLRS-5 (NRC, Canada) was analyzed to evaluate the analytical procedure recovery.
Soil contamination and human health risk assessment indices
Soil contamination by some PTEs was assessed by using the Geoaccumulation Index, Igeo (Eq. 1), described by Müller [20] and the single element contamination factor, Ci (Eq. 2), and single element potential ecological risk, Eir (Eq. 3), described by Håkanson [21].
where Cn is the total mass fraction of the PTE in the tested soil and Bn is the respective elemental mass fraction on the Earth’s crust [22]. The factor 1.5 compensates for fluctuation of natural and minor anthropogenic origin. \(C_{\text{f}}^{\text{i}}\) is the contamination factor, \(C_{\text{n}}^{\text{i}}\) is the reference value Earth’s crust [22], \(C_{0 - 1}^{\text{i}}\) is the mean content of the PTE in question. \(E_{\text{r}}^{\text{i}}\) is the ecological risk factor for the given PTE and T ir is the toxic response factor for the given PTE (Ba = Mn = Zn = 1 < Cr = V = 2 < Co = Cu = Ni = Pb = 5 < As = 10) [21, 23].
Statistical analysis
Person correlations and principal component analysis (PCA), a multivariate statistical method based on principal component scores, was performed to evaluate multivariate relationships using Statistica 7.0 software program [24].
Results and discussion
Soil properties
Table 1 presents the analytical results of the soil core scrapyard physicochemical properties and major elements content.
Soil cores acidity increases with depth. Most of the soils are moderately to slightly acid (pH 4.0–4.7) as a result of the influence of the geological parent material of the soil (granite rocks), which was attenuated by the presence of construction waste materials on the shallow soil of landfill (pH 5.1–7.1). OM content varied greatly in soils from PM2 (6–27 g kg−1) to PM3 (9–30 g kg−1), the highest values were found at PM2S2 and PM3S2 where some oil was observed. No significant variation of OM was observed in PM1 soil.
Samples had from 100 to 278 g kg−1 of clay, with high sand content between 500 and 798 g kg−1 and, in general, contained average silt that varied from 102 to 306 g kg−1. The texture range varied from loam to sandy-clay-loam, with a predominance of sandy-loam soils. The CEC, which expresses capacity of the soil to retain cations, varied greatly from 30.2 to 155.4 mmolc dm−3. The same occurs to base saturation (V %), which ranged between 5 and 91%. Fe2O3 content ranged from 0.59 to 2.94% in PM1 and PM2 soils. The highest values of pH, OM and V %, and the highest contents of Fe2O3 and CEC were observed at PM3, and can be attributed mainly to the massive presence of an oiled residue at PM3. Although the IVS small area (15,000 m2), sampled soil cores presented very heterogeneous characteristics. Therefore, there is a strong potential for marked changes in the soil PTEs mass fraction between samples of close proximity.
Total PTEs concentration in soil cores
Previous topsoil analysis (0–20 cm), performed by Lange et al. [7], showed few hotspots for some PTEs on the site of the IVS. This study of three soil cores and groundwater from the same IVS investigates extension of the contamination.
Figure 3 presents the analytical results obtained for the measured PTEs of the IVS soil cores and groundwater.
Results indicate significant data scatter for most PTEs, especially in soil samples, that reflect complex diffuse inputs from distinct sources across IVS area, such as vehicles, rubble and some passive waste from the former chemical company like the oiled residue.
For all PTEs, except As, the mass fractions obtained in soil cores samples were lower than mass fractions ranges reported previously for topsoil samples, which varied from 3.1 to 19.1 mg kg−1 [7].
For Cr and V, the mass fraction decreases with depth. Chromium content varied from 9.6 to 68 mg kg−1. The highest value was close to the reported mean value (67 mg kg−1) obtained in topsoils [7]. The highest Cr mass fraction found at PM3 shallow soil could be correlated with the distinct pH value (pH 7.1) of this soil. Generally, in soils, chromate is pH-dependent with other forms of Cr6+ such as HCrO4− and dichromate (Cr2O72−), with CrO42− being the predominant form at pH > 6. Cr-III is much less mobile and adsorbs to particulates more strongly. The solubility of Cr3+ decreases above pH 4 and above pH 5.5 complete precipitation occurs [25].
Vanadium content varied from 5 to 110 mg kg−1 and reasonably matched the mass fractions of topsoil, which varied from 14 to 153 mg kg−1 [7]. The highest V mass fraction was found at PM3 shallow soil.
Vanadium distribution in PM3 soil core differed from that of PM1 and PM2 cores, because V mass fraction dropped dramatically with depth at PM3. A plausible explanation for this could be a reflection of the highest content of exchangeable Ca2+, Fe2O3 and pH at PM3S1. Vanadium in soil can be present in several oxidation states forming insoluble vanadates of Cu, Fe, Mn, Ca and sorbs to Fe- and Mn-oxides, clay and organic matter [26]. It should be noticed that V is usually associated to fossil derivatives and also to metallic alloys.
Copper, Nb and Ni mass fractions varied essentially following the same pattern in each soil core. No significant variation with depth was observed for PM1 samples, but a small decrease appears for PM2 and an increase with depth at PM3. Therefore, assuming that contamination was surface deposited, the enhanced of these elements accumulation may have some influence of the oiled residue presence at PM3S2, since the OM content can immobilize greatly these elements [25]. Nevertheless, the highest mass fraction observed for Cu (61 mg kg−1) and Ni (36 mg kg−1) at PM3S2 matched with the highest values observed in some hotspots at topsoil, Cu (73 mg kg−1) and Ni (38 mg kg−1) [7].
Oxides of Fe and Mn are, in general, significantly correlated with all the PTEs and play an important role in these elements mobility in tropical soils [27]. Pearson correlations showed that in the studied soil cores they indeed played an important role, since Fe and Mn content correlated positively with Ba, Co, Cu, Ni, Pb and V (r = 0.70–0.90, p = 0.05). Manganese highest contents were found in the shallow soils of the three soil cores and these values (240–480 mg kg−1) reasonably matched with the mean value reported for topsoil (356 mg kg−1) [7].
Cobalt distribution pattern throughout the cores was very similar to Mn distribution. Cobalt can be adsorbed onto Mn minerals and then become more strongly bound with time as it is oxidized and replaces Mn in the crystal structure [25]. Cobalt content varied from 1 to 15 mg kg−1 in soil cores and these values were lower than the range observed to topsoil (2–30 mg kg−1) [7].
Barium content observed in soil cores (157–390) was lower than the mean value observed in topsoil (434 mg kg−1) [7]. Barium distribution was very similar to Mn distribution along the cores, since this element presented the highest correlation with Mn (0.90, p = 0.05). Barium highest contents were found in the shallow soils of the three soil cores.
Iron content distribution pattern varied widely in each soil core (Fig. 3). Iron highest contents were observed in PM3 soils (4.0–4.7%) and were close to the mean value reported to topsoil (4.0%) [7]. The same distribution was observed for Pb and Zn along the three soil cores and the range content of these elements were lower than observed for topsoil, varying from 19.7 to 50 and 22.7 to 89 mg kg−1, respectively.
A remarkable hotspot was observed for As in PM2S1 (91 mg kg−1). The presence of an oil spill residue could explain this anomalous content of As. Arsenic content in the other soil core samples was lower (0.4–7.8 mg kg−1) than the mean value observed for topsoil samples (8.5 mg kg−1) [7]. In a previous study [28], lower values of As content (< 2.8 mg kg−1) in soils from the vicinity of an automobile mechanic’s workshop from different soil depths (0–45 cm) were reported.
Soil contamination and human health risk assessment indices results
Ribeirão Pires IVS soil can be classified as moderately to extremely pollute for As in most soil cores, mainly in PM2, according to Igeo results (Table 2). Arsenic may represent a moderate to very high potential ecological risk. This PTE can be found in car batteries, fuel, glass and alloys. Igeo results indicated also a moderate accumulation of Cr, Cu, Ni, Pb in PM2 and PM3 soil cores (Table 2).
Principal component analysis
Given the varying temporal scale of distinct inputs in the studied area and their complex spatial distribution, it is difficult to clearly define dominant or discrete sources of PTEs inputs in soil core samples. Physical and chemistry results from the three soil cores were evaluated by Principal Component Analysis (PCA) multivariate statistics. PCA has been useful to identify natural geochemical signatures and anthropogenic sources [29]. The dataset was composed of a matrix with 9 cases (3 soil cores with 2–4 layers each) and 54 variables that corresponded to measured parameters. Data was normalized and rotated (varimax normalized), grouping parameters that could explain total matrix variability with less components. Only eigenvalues > 1 were considered in this study. Group loading values > 0.600 were considered with high or strong correlation. Scores for each layer from each individual core were calculated to identify PCA factors. Six principal components were found that explained around 92.5% of total variance (Table 3). Parameters with high covariance were linked under the same principal component and this covariance could be explained by similar chemical behavior or process. PCA revealed groups of geochemical variables which show similar variation patterns in Ribeirão Pires´s soil cores, for a possible interpretation in terms of source apportionment (Table 3).
The first three factors represent 65% of total data variability and are most probably related to lithological occurrence, since they involve conservative elements, such as some REEs, Hf, Y, U, Sc, Rb and Zr, insoluble oxides of Fe, Mn and Ti and soluble and easily exchangeable elements from natural occurrence (Na, K, Rb, Cs, Mg, Ca, Ba and P). Cobalt, Cr and Zn content are associated with exchangeable elements and are also most probably related to geogenic occurrence, except to Pb.
Factor 4, accounting for 12.9% of total variability, grouped Cu > Nb > Ni followed by OM > Sr > CEC > P>K and with lower correlation to Ca2+ and P-ex opposed by Si > Al > Ga > V. This factor identifies a very tight probable anthropogenic cluster of Cu, Nb and Ni that had affinity to OM, P and apparently compete and exchange with natural occurring Si, Al, Ga and V binding sites. Copper and Ni are applied in several auto-parts, especially in break pads [30]. Niobium can be used as alloying element with applications in automotive cylinder heads, piston rings, exhaust system and truck brakes [31].
Factor 5, accounting for 7.8% of total variability, had high correlation loading with Sand > Si > Th > As, lower correlation with K and Na also with opposed strong correlation to Clay, Silt and Sc and opposed lower correlation to Al. This suggests that As content is not related to the genesis of clay minerals or silt fraction and this element occurrence is most probable from anthropogenic sources. As mentioned previously, the IVS site was a former pesticide manufacture. The employment of arsenic oxides in pesticides is well known [32] and this arsenic high content may be due to a former contamination from past activities.
Factor 6, accounting for 7.2% of total variability, grouped with high correlation loadings to Pb and with lower correlation loadings to CEC opposed by high correlation to Nd > Sm > La > Eu > Tb > Ce > K and with lower correlation to Yb, Y and Na. This suggests that part of Pb content is not related to REEs and its occurrence is most probable from anthropogenic activities.
Sodium, K, Ca, Fe, Mn, Si, V, BS, Yb and Y presented small correlation loadings to more than one factor as well as Pb. This behavior could be related to more than one source or to more than one chemical mechanism in soil core samples.
Groundwater
For all analyzed PTEs the concentrations observed in Ribeirão Pires IVS (Fig. 3) groundwater were bellow the drinking water guiding values US EPA [33].
The ionic balance ranged from 0.78 to 1.18, which is acceptable to low ionic content waters (< 2 mEq L−1) assuring analytical consistency. The only well with an anion deficit was PM3 that also had the highest conductivity (691 µS cm−1), the highest ionic content, and the lowest redox potential (197.7 mV) among other monitored wells in this study and the aquifer reference data provided by CETESB [16]. However, PM3 lower relative anion to cation ratio (ionic balance of 0.78) could be explained by unaccounted organic ions. No NO2−–N, Br− and PO43−-P were found in all monitoring wells nor occurred in aquifer.
An increase on redox-sensitive elements such as Fe, Mn, Cr, V, Ce, As, U was identified at PM1 and PM3, when compared with pristine water and PM2, as presented in Table 4. Groundwater with concentrations of Fe > 100 µg L−1, Mn > 50 µg L−1 and NO3−–N < 0.5 mg L−1 such as found in PM3 are considered to be anoxic [34]. PM1 presented Mn > 50 µg L−1 but Fe < 100 µg L−1, which indicates smaller sub oxic condition than PM3. The reduction starts by consuming dissolved oxygen (O2) that produces most energy per mole of oxidized organic carbon than any other electron acceptor. The next electron acceptor is NO3− followed by Mn(IV), Fe(III), SO42− and CO2 [34]. Considered a system under transient conditions, groundwater is affected both by the aquifer water flow and by the water level. Therefore, when Mn2+ and Fe2+ (soluble species) are found in groundwater, dissolved oxygen and nitrate had been already depleted, as observed at PM3, but not completely at PM1. Manganese starts to be released into the water only after NO3− is below 0.5 mg L−1, and Fe2+ tends to appear 4.5 m below Mn2+ occurrence. In a relative small study area and in shallow wells, groundwater samples indicated heterogeneous redox condition. Especially in shallow wells at unconfined aquifers, such as in Ribeirao Pires case, oxic conditions with presence of NO3− are expected. Anthropogenic contributions tend to disrupt the smooth gradient under these conditions.
Arsenic did not occur naturally in Ribeirao Pires aquifer [15, 16]. An As increase with the reducing potential was observed in PM2 to PM3. All measured values are below 10 ug L−1 US EPA drinking water recommendation limit [33]. Arsenic is reported to occur both in reducing and oxidizing conditions, but an enrichment is associated with reducing conditions before Fe release from soil into water [35], that seems to occur also in IVS groundwater.
Uranium under oxic conditions is conservative [35] and under reducing conditions is removed by precipitation, however in IVS groundwater an increase with reducing conditions was observed.
The redox conditions of PM3 imply in hidrogeochemical changes that result in increase of some elements concentrations, such as As, Fe, Mn and U. In urban environment, main anthropogenic changes occur due to land use changes and chemical spills that affect water recharge (residence time) and electron/acceptor availability, i.e., fuel spills, fertilizer infiltration or septic tanks leakage. These could be a plausible explanation for the difference between pristine water and IVS groundwater NO3− and SO −24 concentrations.
Rare earth elements distribution
REEs are believed to be potential tracers for groundwater aquifer-rocks and aquifer-solid phase interactions due to their conservative chemical behavior [35]. Redox changes alter REEs concentration and REEs fractioning pattern.
The REEs concentrations in groundwater are presented in Table 5 and the determined values are in the range of expected REEs values in groundwater, considering physicochemical and sampling conditions. However, it was observed an alteration of the distribution of Heavy (Y, Tb, Dy, Ho, Er, Tm, Yb, e Lu) and light (Sc, La, Ce, Pr, Nd, Pm, Sm, Eu e Gd) REEs between the wells as following: PM1 21.6% HREE and 78.4% LREE, PM2 27.0% HREE and 73.0% LREE, and, PM3 7.8% HREE e 92.2% LREE. The sub-oxic conditions observed mainly at PM3 influenced the REEs distribution by increasing HREEs concentration in groundwater.
Conclusions
The findings herein indicated that although Ribeirão Pires IVS is a small area (15,000 m2), it has heterogeneous characteristics. By soil cores exhumation and groundwater sampling it was possible to observe a landfill layer with rubber and an oiled residue from a former pesticide manufacturer, in addition to vehicles parked on topsoil. The presence of this oiled residue in underground environment was not observed in a previous study performed in the same IVS topsoil [7] and the results herein showed that it plays an important role in the PTEs distribution.
Soil cores analysis and multivariate statistics have indicated that As, Pb, Ni, Cu and Nb content are mostly from anthropic sources, such as vehicle derivate particulate or fuel/oil leakage. Pb, Cu, Ni and Nb mass fraction increased with depth indicating some downward mobility.
Although most PTEs loading from the diverse sources are presently low in groundwater, a redox condition change along groundwater flow path was identified by Eh and other Redox sensitive parameters (Fe, Mn, As, U, Cu, Ce) transition from oxic to sub-oxic conditions. LREEs/REEs increased with reducing conditions.
The levels of most of PTEs in soil are not greatly enriched, however the Igeo showed that As content in soil cores classified the area as moderately to extremely polluted. Igeo results also indicated a moderate accumulation of Cr, Cu, Ni, Pb in soil cores. The potential ecological risk index adopted for this IVS conditions presents satisfactory results for most elements, except for As, which may represent a moderate to very high potential ecological risk.
To conclude, in spite of the apparently degraded conditions of the studied IVS, at this moment, it was not observed a soil and groundwater severe contamination. Some PTEs hotspots were observed, as well it was previously reported for the topsoil [7]. However, it should be noticed that this very complex environment, with such a lot of anthropic inputs might represent a potential risk for the soil and groundwater quality of this municipality, which plays an important role into water supply to SPMR.
References
IBGE (2010) Instituto Brasileiro de Geografia e Estatística (Brazilian Institute of Geography and Statistics). Censo Demográfico 2010 http://7a12.ibge.gov.br/vamos-conhecer-o-brasil/nosso-povo/caracteristicas-da-populacao.html (accessed 21 Jul 17)
IBGE (2016) Instituto Brasileiro de Geografia e Estatística (Brazilian Institute of Geography and Statistics). Cidades http://cidades.ibge.gov.br/xtras/perfil.php?codmun=3550308 (accessed 21 Jul 17)
Soriano E et al (2016) Water crisis in São Paulo evaluated under the disaster’s point of view. Ambiente Soc 19(1):21–42
Nwachukwu MA, Feng H, Alinnor J (2011) Trace metal deposition in soil from auto-mechanic village to urban residential areas in Owerri, Nigeria. Proc Environ Sci 4:310–322
Doumett S, Lamperi L, Checchini L, Azzarello E, Mugnai S, Mancuso S, Petruzzelli G, Del Bubba M (2008) Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: influence of different complexing agents. Chemosphere 72:1481–1490
Chokor AA (2016) Soil profile distribution of heavy metals in automobile workshops in Sapele, Nigeria. Int J Basic Sci Technol 2(1):30–38
Lange CN, Figueiredo AMG, Enzweiler J, Castro L (2017) Trace elements status in the terrain of an impounded vehicle scrapyard. Radioanal Nucl Chem 311(2):1323–1332
Werkenthin M, Kluge B, Wessolek G (2014) Metals in European roadside soils and soil solution—a review. Environ Pollut 189:98–110
Teng Y, Feng D, Wu J, Zuo R, Song L, Wang J (2015) Distribution, bioavailability, and potential ecological risk of Cu, Pb and Zn in soil in a potential groundwater source area. Environ Monit Assess 187:293–306
Imperato M, Adamo P, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy). Eviron Pollut 124:247–256
Almeida FFM (1958) O Planalto Paulistano. In: Azevedo A (ed) A cidade de São Paulo. São Paulo, Associação dos Geógrafos Brasileiros, pp 113–167
Riccomini C, Coimbra AM (1992) Geologia da Bacia Sedimentar de São Paulo. In: Negro A, Ferreira AA, Alonso UR, Luz PAC (eds) Solos da cidade de São Paulo. ABMS-ABEF, São Paulo, pp 37–94
Crozera EH (2001) Identificação das áreas contaminadas no município de Ribeirão Pires—São Paulo. 205. Tese (Doutorado)—Instituto de Geociências, Universidade de São Paulo, São Paulo
Rocha G (ed) (2005) Mapa de Águas Subterrâneas do Estado de São Paulo, escala 1:1,000,000. CD-ROOM, São Paulo
CETESB (2016a) Companhia Ambiental do Estado de São Paulo. Relatório de qualidade de águas subterrâneas no Estado período de 1998–2000 http://aguassubterraneas.cetesb.sp.gov.br/wp-content/uploads/sites/13/2013/11/1998-2000-Relatorio-de-Qualidade-das-aguas-subterraneas.pdf (accessed 03 Mar 2016)
CETESB (2016b) Companhia Ambiental do Estado de São Paulo. Relatório de qualidade de águas subterrâneas no Estado período de 2004–2006 http://aguassubterraneas.cetesb.sp.gov.br/wp-content/uploads/sites/42/2013/11/qual_precambriano_2004_2006.pdf (accessed 03 Mar 2016)
Camargo OA, Moniz AC, Jorge JA, Valadares JMAS (2009) Métodos de Analise Química, Mineralógica e Física de Solos do Instituto Agronômico de Campinas. Campinas, Instituto Agronômico 77 (Boletim técnico, 106, Edição revista e atualizada)
Zambello F, Enzweiler J (2002) Multi-element analysis of soils and sediments by wavelength-dispersive X-ray fluorescence spectrometry. J Soils Sediment 2:29–36
APHA (1985) Standard methods for the examination of water and wastewater, 12th edn. American Public Health Assoc, New York
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. GeoJournal 2:108–118
Håkanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Taylor SR, McLennan SH (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Xu ZQ, Ni SJ, Tuo XG, Zhang CJ (2008) Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ Sci Technol 31(2):112–115
Statsoft (2005) Statistica 7.0 software. Tucksa
Alloway BJ (1995) Heavy metals in soils, 2nd edn. Blackie Academic & Professional, Great Britain
Guagliardi I, Cicchella D, de Rosa R, Ricca N, Buttafuoco N (2016) Geochemical souces of vanadium in soils: evidences in a Southern Italy área. J Geochem Explor. https://doi.org/10.1016/j.gexplo.2016.11.017
dos Santos-Araujo SN, Alleoni LRF (2016) Concentrations of potentially toxic elements in soils and vegetables from the macroregion of São Paulo, Brazil: availability for plant uptake. Environ Monit Assess 188:92
Oloye FF, Ololade IA, Oluwole OD, Bello MO, Olyuede OP, Ololade O (2014) Fate and potential mobility of arsenic (As) in the soil of mechanic workshops. Environ Pollut 3(4):70–78
Facchinelli A, Sacchi E, Mallen L (2001) Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ Pollut 114(3):313–324
Duong TTT, Lee BK (2001) Determining contamination level of heavy metals in road dust from busy traffic areas with different characteristics. J Environ Manage 92:554–562
CBMM (2017) Uses & End users of niobum http://www.cbmm.com.br/en/Pages/Uses-EndUsers-Niobium.aspx (accessed 23 Aug 17)
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58(1):201–235
US EPA (2001) National primary drinking water regulations: arsenic and clarifications to compliance and new source contaminants monitoring. In: Final Rule, Code of Federal Regulations 141–142
McMahon P, Chapelle F (2007) Redox processes and water quality of selected principal aquifer systems. Ground Water 46:259–271
Guo H, Zhang B, Wang G, Shen Z (2010) Geochemical controls on arsenic and rare earth elements approximately along a groundwaterflow path in the shallow aquifer of the Hetao Basin, Inner Mongolia. Chem Geol 270:112–117
Acknowledgements
We are grateful to Ribeirão Pires Municipality for permission to carry out this project and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for financial support. The author C.N. Lange thanks for the fellowship from the Brazilian Nuclear Energy Comission (CNEN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lange, C.N., Figueiredo, A.M.G., Enzweiler, J. et al. Potentially toxic elements downward mobility in an impounded vehicle scrapyard. J Radioanal Nucl Chem 316, 819–830 (2018). https://doi.org/10.1007/s10967-018-5729-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5729-0