Abstract
The organic toxicants formed in chlorinated water cause potential harm to human beings, and it is extensively concentrated all over the world. Various disinfection by-products (DBPs) occur in chlorinated water are genotoxic and carcinogenic. The toxicity is major concern for chlorinated DBPs which has been present more in potable water. The purpose of the work was to evaluate genotoxic properties of DBPs in Allium cepa as a plant model system. The chromosomal aberration and DNA laddering assays were performed to examine the genotoxic effect of trichloroacetic acid (TCAA), trichloromethane (TCM), and tribromomethane (TBM) in a plant system with distinct concentrations, using ethyl methanesulfonate (EMS) as positive control and tap water as negative control. In Allium cepa root growth inhibition test, the inhibition was concentration dependent, and EC50 values for trichloroacetic acid (TCAA), trichloromethane (TCM), and tribromomethane (TBM) were 100 mg/L, 160 mg/L, and 120 mg/L respectively. In the chromosome aberration assay, root tip cells were investigated after 120 h exposure. The bridge formation, sticky chromosomes, vagrant chromosomes, fragmented chromosome, c-anaphase, and multipolarity chromosomal aberrations were seen in anaphase–telophase cells. It was noticed that with enhanced concentrations of DBPs, the total chromosomal aberrations were more frequent. The DNA damage was analyzed in roots of Allium cepa exposed with DBPs (TCAA, TCM, TBM) by DNA laddering. The biochemical assays such as lipid peroxidation, H2O2 content, ascorbate peroxidase, guaiacol peroxidase, and catalase were concentration dependent. The DNA interaction studies were performed to examine binding mode of TCAA, TCM, and TBM with DNAs. The DNA interaction was evaluated by spectrophotometric and spectrofluorometric studies which revealed that TCAA, TCM, and TBM might interact with Calf thymus DNA (CT- DNA) by non-traditional intercalation manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The chlorination of potable water has principal part in minimizing mortality and morbidity rate confederated with waterborne diseases. Rook (1974) identified trihalomethanes as first chlorinated by-products in drinking water. Among all the trihalomethanes, chloroform (trichloromethane) was the main focus of many genotoxic and carcinogenic studies (Pentamwa et al. 2013). It is broadly believed that principally many disinfection by-products were occurred through the reaction of chlorine and organic substances inherently present in water just as fulvic as well as humic acid. One of the strong disinfecting agents is chlorine, which has the capability to functionally inactivate the waterborne pathogens, but it also reacts with natural organic substance form a range of toxic disinfection by-products (DBPs). Diverting from chlorination to chloramination is a deal to observe with modulations which limit the concentrations of some carbonaceous DBPs in drinking water, like trihalomethanes (THMs) and haloacetic acids (HAA). Identified DBPs in chlorinated waters contain haloamines, THM, HAA, haloacetonitriles (HAN), halodiacids, haloaldehydes, haloketones (HK), haloamides, halophenols, halobenzoquinones, and N-nitrosamines (Richardson et al. 2010; Chowdhury et al. 2014; Teo et al. 2015). Manasfi et al. 2016 identified disinfection by-products in freshwater and seawater swimming pools and evaluated its genotoxicity. Persistent contact to excessive extents of DBPs related to harmful health issues includes problems to oculus, cutis, nostril and throat (Fantuzzi et al. 2010), dreadful reproductive issues (Hinckley et al. 2005), and bladder carcinoma (Villanueva et al. 2007). Sapone et al. 2016 has reported perturbation of xenobiotic metabolism in Dreissena polymorpha model exposed in situ to surface water (Lake Trasimene) purified with various disinfectants (Sapone et al. 2016).
HAA as well as THMS are randomly formed components in high concentration among all the disinfection by-products and are also major concern because of its strong impact on human health. Existence of vital THMs like trichloromethane, tribromomethane, dichlorobromomethane, and chlorodibromomethane in potable water is studied to be carcinogen (Plewa et al. 2012). DBPs when discharged into environment by domestic waste and swimming pools may hamper the microhabitat of soil or even water. These may cause transformation to food cycle and distort productivity of flora by damaging nitrogen catabolism and vitality and even harm marine creature (Oberdorster et al. 2007). The responsive effects of known DBPs accompany to center of attention to biological evaluation on carcinogenicity and mutagenecity in earlier reports (Richardson et al. 2007; Plewa et al. 2012). Kogevinas et al. 2010 reported genotoxic effects in swimmers exposed to disinfection by-products in indoor swimming pools. In general, pollutants contaminate soil as well as water, so leading a significant warning to flora and fauna in environment. Plants have improved well-ordered scavenging machinery including antioxidant enzymes as well as antioxidant compounds which act as a defender by providing ammunition towards heavy metal-influenced injuries (Grata¨o et al. 2005). It has been reported that the metabolism of xenobiotic effected in Cyprinus carpio exposed with chlorinated drinking water samples collected from two different Italian municipal treatment plants (Cirillo et al. 2016). Canistro et al. 2012 has also reported modulation of cytochrome P450 and induction of DNA damage in Cyprinus carpio exposed in situ to surface water treated with chlorine or alternative disinfectants in different seasons.
The genotoxic effect, indifferent cytotoxic effect, and oxidative pressure were also observed as a good signal for the generation of DBPs (Neale et al. 2012; Farre´ et al. 2013). Plewa et al. (2011) explored the cytotoxicity of pool water samples which showed more harmful effects than disinfected tap water samples in mammalian cells. There were more than 600 DBP components identified by 2006, formed not only from chlorine, but also from other disinfectants like chlorine dioxide, chloramines, and ozone (Richardson et al. 2007). Even so, among all the identified DBPs, only small proportions are regulated in drinking water broadly. The USEPA (United States Environmental Protection Agency) has limit for total THMs at 80 μg/L and for HAAs at 60 μg/L). The effect of chlorinated acetic acids in pine needles has been reported via reactions of peroxidases and glutathione S-transferase activity (Schroder and Gotzberger 1997). However, genotoxic effect of disinfection by-products generated from drinking water has been done by using Vicia faba bioassay (Hu et al. 2017).
With the above mentioned deleterious impact of various DBPs studied so far, as well as their availability in freshwater, has made an important issue to deal. Human beings are directly being exposed to chlorinated water as a source of potable water and in pool water also. Due to these disinfection by-products presences in fresh water, the agriculture productivity may get impaired as well. Scanty reports are there for study on agricultural crops. The environmental risks associated with various hazardous chemicals are generally studied using plants as model system as they are complex higher eukaryotic system. So, this study focuses on the evaluation of effect of DBPs on plant growth and its mechanism of action using model plant system Allium cepa. In this test, the screening of cytotoxic potential is determined by the alterations of mitotic index (MI), while the evaluation of chromosomal aberrations (CA) has been used as a parameter to detect potentially genotoxic agents. Even plants contain huge part of our biosphere and constitute a vital link in the food chain. Vegetables are the staple food for most of the world and play an important role in human diet also. Among all the vegetables, onions (Allium cepa) are of great importance and a natural part of daily diet for most of the world’s population. Ordinary red onion (Allium cepa) is a vegetable of substantial productive significance grown worldwide (Mogren et al. 2007). Allium test has been established to have excessive link with another system for test like MIT-217 cell test with rats, mice, or humans in vivo, and, even in toxicological research, it can be used as a substitute to laboratory animals (Fiskesjo and Levan 1993). During the last years, the utilization of higher plant in assays to evaluate the genotoxic, cytotoxic, and mutagenic effects of environmental pollutants has been increased (Lutterbeck et al. 2015). The DNA damaging effects were also examined. These risk assessments improve studies on plant system as well our knowledge about the risks to the environment and human health from exposure to these chemicals.
Materials and methods
Chemicals
Ethidium bromide (EtBr) and cetyltrimethylammonium bromide (CTAB) were bought from Himedia Ltd. (Mumbai, India). Normal melting agarose (NMPA), ethylene diamine tetra acetic acid (EDTA), and calf thymus DNA were supplied from Sigma-Aldrich Co. (USA). Trichloromethane and tribromomethane were purchased from Merck India Pvt. Ltd. All other chemicals like trichloroacetic acid and thiobarbituric acid (TBAR), glacial acetic acid, carmine, isoamyl alcohol, ethanol, and sodium chloride were of analytical grade and procured from reputed companies. Bulbs of onions (Allium cepa) were purchased from local market.
Root growth inhibition test and evaluation of EC50
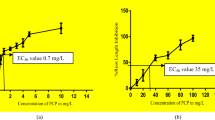
Equal-sized and equal-weighed bulbs of Allium cepa were allowed to sprout in tap water for 48 h as described by Fiskesjö 1987 with slight modifications in plant growth chamber at 23–25 °C under proper dark and light phase. Then A.cepa balls were treated with distinct concentrations of TCAA, TCM, and TBM solutions, with three bulbs for each and every concentration. After the 5th day of exposure period, the roots were used for different assays, and EC50 (50% retardation in root length) was resulted from graph for different concentration of test compounds (TCAA, TCM, and TBM) versus length of root showed as percentage of control. Tap water and ethyl methane sulfonate (0.2%) were used as control.
Chromosomal aberration and mitotic index (MI)
The sprouted onion bulbs were treated with different concentrations of TCAA, TCM, and TBM solutions. At 120 h, root meristems were examined for chromosomal aberration and MI. The roots were cut and firmed for 3 h in acetic acid and ethanol solution in ratio of 1:3. The fixed root tips were stained in 45% aceto-carmine solution. The slides were prepared with the help of squash method by Sharma and Sharma (1980). Minimum of 2500 cells per exposed concentration were counted, and mean values for mitotic index, micronuclei, and chromosomal aberrations were calculated. The remaining roots were utilized for the other assays used in this study.
DNA isolation and laddering using A.cepa
DNA was extricated from A. cepa roots after exposure time using slightly improved CTAB technique using Himedia DNA isolation kit. The onion roots were homogenized in extraction buffer provided by Himedia DNA isolation kit. The extracted suspension was kept at 65 °C for 60 min by random mixing. The uniform quantity of chloroform:octanol (24:1) was mixed to suspension. The suspension was centrifuged for 5 min at 2300 rpm. The top aqueous layer was transferred to a tube that contained chilled isopropanol and mixed by gentle inversion until white fluffy DNA precipitate appeared then centrifuged at 2300 rpm for 5 min. The soup collected was mixed with CTAB wash buffer and incubated at 15–25 °C for 20 min. The DNA was recovered by centrifugation at 2300 rpm for 5 min. The collected pellet was rinsed with 70% ethanol. The pellet obtained was dehydrated in air and dissolved in elution buffer (0.1 M Tris-Cl, pH- 8.5) at room temperature and kept at 4 °C till used. RNA was removed by treatment with RNase A solution (20 mg/mL) for 30 min at room temperature. Pure DNA was estimated by recording the absorbance of isolated DNA sample at wavelength of 260 and 280 nm. The extracted DNA solution from each exposed test compounds was analyzed on 2% agarose gel in 1X TAE buffer (Tris–acetate–EDTA) at 50 V, at room temperature with 100 bp ladder as reference. EtBr solution was used to stain DNA and UV transilluminator was used to visualize the DNA and for photography also.
Biochemical assays
Lipid peroxidation
Oxidative deterioration to lipids was estimated by lipid peroxidation as malondialdehyde (MDA) followed by Çelik et al. 2008 with modifications. The experiments were conducted with three clones. 0.2 g roots from control and exposed plants were grated in 5% trichloroacetic acid (TCA) solution (5% w/v) and centrifuged the mixture for 15 min at 12,000 rpm at room temperature. The same volume of soup and 0.5% thiobarbituric acid (TBAR) solution in 20% of freshly prepared TCA solution were mixed with incubation time of 25 min at 96 °C. The solution was cooled on ice and centrifuged for 5 min at 10,000 rpm. The absorbance of soup was measured at 532 nm with correction of non-specific absorbance at wavelength of 600 nm. Malondialdehyde (MDA) concentration was calculated by using the extinction coefficient of 155 mM−1 cm−1.
Hydrogen peroxide (H2O2) content
Hydrogen peroxide content was estimated using the method of Singh et al. 2007 with little modifications. One hundred milligrams of treated roots was ground in 5 mL of 0.1% TCA (w/v) in ice cold condition. The homogenate was centrifuged for 15 min at 7000 rpm at 4 °C. The H2O2 content was estimated by measuring absorbance at wavelength of 390 nm and determined using extinction coefficient (ɛ) of 0.28 μM−1 cm−1. The reaction mixture contained 0.5 mL of supernatant, phosphate buffer solution (pH 7.0) 0.5 mL, and potassium iodide (1 M) 1 mL.
Antioxidant enzyme assays
The enzyme extraction and antioxidant enzymatic assays of roots treated with DBPs were done using the Singh et al. 2007 method with little modifications. Two hundred fifty milligrams of treated roots of plant was ground in 10.0 mL of cooled 100 mM phosphate buffer (pH 7.0) in cold conditions. The suspension collected was centrifuged at 10,000 rpm for 30 min. The soup collected was kept at 4 °C until used for enzyme activities assays.
The ascorbate peroxidase (APX) action was estimated by producing absorbance at wavelength of 290 nm (ɛ = 2.8 mM−1 cm−1) caused by oxidation of ascorbic acid to dehydroascorbate. The solution (2.0 mL) contained 0.2 mL extract of enzyme, 0.1 mM EDTA, 25 mM phosphate buffer (pH 7.0), 1.0 mM H2O2, and 0.25 mM ascorbic acid. For catalase (CAT) activity, the solution (2.0 mL) included 0.2 mL of extract of enzyme, 25 mM phosphate buffer (pH 7.0), 10 mM H2O2, and the activity of enzyme was evaluated as the loss of H2O2 at wavelength of 240 nm (ɛ = 39.4 mM−1 cm−1). For action of guaiacol peroxidase (GPX), the solution 2.0 mL included 0.2 mL extract of enzyme, 25 mM phosphate buffer (pH 7.0), 1.0 mM H2O2, 0.05% guaiacol (w/v), 0.1 mM EDTA, and the activity of enzyme was examined as recording absorbance at wavelength of 470 nm (ɛ = 26.6 mM−1 cm−1) because of guaiacol oxidation.
UV-visible and fluorescence spectral analysis for DNA-DBPs interaction
The calf thymus DNA stock was prepared in 100 mM Tris buffer (pH 7.2) and kept for 24 h. A freshly prepared working solution with concentration of 60 μM of DNA was used every day using Tris buffer and used for different studies. The UV-vis spectra for TCAA, TCM, and TBM–DNA interactions were obtained by using Agilent spectrophotometer at room temperature after incubation of 10 min at 37 °C where 1.0 mL of solution contained 1, 2.5, 5, 7.5, 10 mg/L of selected DBPs and calf thymus DNA solution with a concentration of 60 μM was placed.
The fluorescence analysis was done using an F4-2500 fluorescence spectrophotometer with ethidium bromide (EB) as fluorescent probe. The spectra were measured at room temperature after 10 min incubation at 37 °C where 3.0 mL of solution containing calf thymus DNA solutions with a concentration of 60 μM. When the first absorption spectrum was reached, DBPs solutions were added of different concentrations, i.e., 1, 2.5, 5, 7.5, 10 mg/L, which followed in a new spectral addition. This process permitted us to raise the concentration of DBPs with every addition without changing the total volume, so that the DNA concentration remained same. Thus, acquired characteristics for absorption spectra above the entire succession of bands were comparable.
Statistical analysis
The data obtained in the present research work were considered as the mean ± standard deviation (SD). The data were reviewed by one-way ANOVA followed by the comparison of mean values using post-hoc Tukey’s test at P ≤ 0.05.
Result and discussion
Phytotoxicity assessment of DBPs
The EC50 value of TCAA, TCM, and TBM as observed from inhibition of root growth for A.cepa was 100 mg/L, 160 mg/L, and 120 mg/L respectively. Figure 1 showed consequential depletion in root growth elongation at growing concentration of DBPs as compared to control set in plant system. The observed results of DBPs on root growth appears similar to the effects of various other compounds like arsenic and titanium dioxide as reported earlier in A.cepa and V.radiata (Kapustka et al. 1995; Hartley-Whitaker et al. 2001; Ghoshal et al. 2010). However, among all the three selected DBPs, TCAA showed more toxicity than TCM and TBM in A.cepa. In the current research, according to root growth inhibition test, the selected DBP compounds showed toxicity in this manner TCAA > TBM > TCM. Further, the possible reason behind root growth inhibition was evaluated by chromosomal aberration, DNA laddering, lipid peroxidation, and biochemical assays.
Chromosomal aberration and mitotic index (MI)
A. cepa test system was evaluated as the most efficacious, practically viable, and inexpensive technique in determining toxicity, cytotoxicity, and genotoxicity of pollutants in the marine environmental systems (Leme and Marin-Morales 2009). All the three DBPs tested revealed that mitotic indices were markedly lesser in the tips of A. cepa root and percentage of chromosome aberration was higher as compared to control set (Table 1). The most frequent cytological abnormalities in mitotic cells were sticky metaphase, unseparated anaphase, unorganized metaphase, fragmented anaphase, bridge chromosome in anaphase, disturbed spindle fibers in anaphase, late anaphase, binucleated chromosome, unseparated chromatids, and nuclear disintegration formation as shown in Fig. 2. DBP compounds showed a dose-dependent raise in chromosomal aberrations as compared to control set, and in our study, among the three selected DBP compounds, TCAA was observed to be more toxic than TBM and TCM. Although retardation in growth of root and depression of mitotic index in meristematic root cells reveal cytotoxic effect while enhancements in chromosomal abnormalities, micronuclei in the root cells reveal genotoxic effect. Appearance of micronuclei can be a good indicator to validate mutagenic effects (Leme and Marin-Morales 2009). The appearance of micronuclei was not properly visible in our study.
Aberrations induced by disinfection by-products (DPBs) (TCAA, trichloroacetic acid (50, 100, and 200 mg/L); TCM, trichloromethane (50, 160, and 800 mg/L); TBM, tribromomethane (50, 120, and 400 mg/L) in Allium cepa root tips: i normal interphase, ii normal metaphase, iii normal anaphase, iv normal telophase, v unseparated anaphase, vi sticky metaphase, vii binucleated chromosome, viii unorganized metaphase, ix fragmented anaphase, x unequal telophase, xi unseparated chromatids, xii bridged chromosome in anaphase, xiii disturbed spindle fibers in anaphase, xiv late anaphase, and xv nuclear disintegration
Mitotic depletion can damage the development and growth of treated organisms (Leme and Marin-Morales 2009). The growth inhibition test evaluated the toxicity of DBPs, and chromosomal aberration signified mutagenicity of theses DBPs.
DNA damaging analysis of DBPs by DNA laddering
The genotoxic effect of DBPs in A.cepa was qualitatively affirmed by applying DNA laddering. At higher TCAA concentration (Fig. 3 i, lane 6, 200 mg/L) the nuclear DNA of A.cepa appears as smear of fragmented DNA which specified random DNA fragmentation as compared to control (Fig. 3 i lane 3). DNA fragmentation obtained in this manner TCM < TBM < TCAA even at higher concentration in isolated DNA of Allium cepa roots examined. The concentration selected for DNA laddering study for three DBP compounds were as per percentage of root length inhibition of 10, 50, and 80% in A. cepa. DNA fragmentation is concentration dependent in all DBPs tested. In Fig. 3 i, lanes 4 to 6 show increasing concentration of TCAA (50, 100, and 200 mg/L), lane 1 DNA ladder, lane 2 positive control, lane 3 negative control; ii, lanes 4 to 6 show increasing concentration of TCM(50, 160, and 800 mg/L), lane 1 DNA ladder, lane 2 positive control, lane 3 negative control; and iii, lanes 4 to 6 also show increasing concentration of TBM (50, 120, 400 mg/L), lane 1 DNA ladder, lane 2 positive control, lane 3 negative control respectively. Therefore, treatment of A. cepa cells with DBPs leads to DNA fragmentation. The present study shows greater amount of shearing of DNA at higher concentration of TCAA than in TCM and TBM in A.cepa. The DNA laddering assay showed a good correlation with chromosomal aberration assay in this study as showed in previous results of Ghoshal et al. 2010.
DNA laddering of iAllium cepa roots treated with different concentration of TCAA, trichloroacetic acid in lane 4,5,6 (50,100,200 mg/L) and lane 1 DNA ladder, lane 2 positive control (EMS), lane 3 negative control (tap water); iiAllium cepa roots treated with different concentration of TCM, trichloromethane in lane 4,5,6 (50, 160, 800 mg/L) and lane 1 DNA ladder, lane 2 positive control (EMS), lane 3 negative control (tap water); iiiAllium cepa roots treated with different concentration of TBM, tribromomethane in lane 4,5,6 (50, 120, 400 mg/L) and lane 1 DNA ladder, lane 2 positive control (EMS, ethyl methanesulphonate), lane 3 negative control (tap water) showing shearing of DNA
Effect of DBPs on lipid peroxidation of A.cepa
DNA laddering study with DBPs on A.cepa indicated that there could be certain impact on DNA by DBPs. However, the reason is not yet well understood. To understand the mechanism of DNA cleavage lipid peroxidation, examination was done. The formation of MDA is a common indicator of lipid peroxidation by oxidative stress. The MDA concentration in Allium cepa roots by 12, 27, and 48% in 50, 100, and 200 mg/L of TCAA; 15, 25, and 56% in 50, 160, and 800 mg/L in TCM; and 33, 41, and 71% in 50, 120, and 400 mg/L in TBM increased over control as shown in Table 2. An increased MDA level signifies damage of membrane caused by polyunsaturated fatty acids peroxidation, which results in accumulation of reactive oxygen species (ROS) and oxidative stress (Montillet et al. 2005). These indicated DBP components incidentally guide to immoderate production of superoxide radicals which result to raised lipid peroxidation and oxidative pressure. This trend is similar with reported article published that arsenic and chlorophenols originate acute lipid peroxidation in Holcus lanatus, wheat and reed canary grass (Hartley-Whitaker et al. 2001; Michałowicz et al. 2009; Michalowicz et al. 2010), T.pratense (Mascher et al. 2002), P.vulgaris (Stoeva et al. 2005), and Brake (Srivastava et al. 2005; Singh et al. 2006). Protonation of O2 radicals creates the hydroperoxyl radical (OH, H2O2), which transformed fatty acids to injurious lipid peroxides, which may tear down biological laminates (Zhang et al. 2005). From preceding articles, in mung bean, it is perceivable that arsenic provoked enormous number of hydroxyl free radical by that ruling to DNA degeneration (Reeves et al. 2007; Zhu et al. 2008). Either directly or indirectly, abiotic stress might result in DNA destruction to plant cells (Zhang et al. 2005; Kumari et al. 2009). The function of numerous cellular molecules containing DNA can be disrupted through ROS generation by lead directly or indirectly (Kumar and Majeti 2014; Malar et al. 2014). The increased level of MDA indicates the occurrence of membrane damage due to peroxidation of polyunsaturated fatty acids, resulting in the generation of ROS and subsequent oxidative stress, and excessive generation of MDA may be responsible for the higher DNA damage in the treated root cells as stated in different studies (Montillet et al. 2005 and Arya and Mukherjee 2014). DBPs increased the MDA content in Allium cepa roots, reflecting intensified lipid peroxidation. In the current study, MDA contents of A. cepa roots increased after exposure with DBP components in concentration dependent manner.
Effect of DBPs on hydrogen peroxide (H2O2) content
Lipid peroxidation is mainly assessed as biochemical marker for ROS-conciliated damage or stress indicator. In contrast to lipid peroxidation, DBPs caused accumulation of H2O2. The H2O2 revealed a similar trend like lipid peroxidation with a significant elevation at increasing concentrations of DBPs. The reactive oxygen species and H2O2 level can be increased either by declined activity of defense mechanism or by their increased production (Bela et al. 2015). Reactive oxygen species (ROS) are partially reduced forms of atmospheric oxygen (O2). ROS mainly generate from the excitation of O2 to form singlet oxygen (O21) or from the transfer of one, two, or three electrons to O2 to form, respectively, a superoxide radical (O2 −), hydrogen peroxide (H2O2), or a hydroxyl radical (HO−). In contrast to atmospheric oxygen, ROS are capable of unrestricted oxidation of various cellular components and can lead to the oxidative destruction of the cell (Asada and Takahashi 1987; Dat 2000; Hammond-Kosack and Jones 1996). So, DBPs are responsible for higher H2O2 content which caused ROS generation and resulted cellular damage in A.cepa (Fig. 4).
Hydrogen peroxide (H2O2) content of Allium cepa roots exposed with different DBPs at different concentrations against control (without DBPs). i Trichloroacetic acid (TCAA) with concentrations of 50, 100, and 200 mg/L. ii Trichloromethane (TCM) with concentrations of 50, 160, and 800 mg/L. iii Tribromomethane (TBM) with concentrations of 50, 120, and 400 mg/L
Effect of DBPs to instigate antioxidant enzyme activities
The increased lipid peroxidation level indicates accumulation of ROS by DBPs in plant systems influenced the investigation of role of scavenging enzymes also. The maintenance is necessary within the particular rummaging enzymes to detoxicate reactive oxygen species in cells. If this stability is disorganized, a remunerative system is procured that inclined the actions of another enzyme. It has been also reported that chlorophenolic compounds induce lipid peroxidation, and antioxidant parameter has been changed in leaves of wheat (Michałowicz et al. 2009). In the current analysis, it was observed that DBP components remarkably amend the actions of rummaging enzymes alike CAT, APX, and GPX in Allium cepa roots. The concentration of DBP compounds selected for this study was based on percentage of root length inhibition of 10, 50, and 80%. Moreover, it was remarkably increased in the actions of CAT, APX, and GPX according to increasing concentrations of DBPs in Allium cepa roots. In contrast with control, the action of CAT increased in TCAA and TBM with increasing concentrations of DBPs whereas in TCM with increasing concentrations, there was slight decrease in enzyme activity but increased as correlated with control in Allium cepa roots as shown in Table 2. Despite a notable increase in CAT enzyme action, as perceived in this evaluation, this study illustrates that this enzyme participated in contributing ammunition across DBP compounds toxic effects in A.cepa. Table 2 showed APX activity increased in A.cepa with increasing concentration of TCAA, TCM, and TBM. APX is situated in chloroplasts and some deface to chloroplast affects its action. Therefore, increase in APX action at a higher concentration of DBP components, as obtained in this study, is not surprising. Ascorbate peroxidase and catalase are capable to destroy hydrogen peroxidase in the cells (Rhizsky et al. 2002; Ammar et al. 2008). The increased actions of CAT and APX confirmed their activation owing to DBP-induced oxidative stress and vice versa. The antioxidant enzymes activity is consonant with prior article in Vigna radiata (Singh et al. 2007). Perhaps, too much H2O2 was detoxicated by peroxidases, especially GPX, that one was consequentially up-regulated in respond to DBP compounds. GPX has important role in scavenging of H2O2 in the cytoplasmic chamber (Drotar et al. 1985). In present study, in the case of A. cepa, GPX action was inclined in TCM over control and declined in TCAA and TBM as compared to control which showed in Table 2. A similar trend in activity of GPX exposed to chlorinated xenobiotic compound has been detected in plant system (Roy et al. 1992). Decreased GPX activity signifies that it is not taking part in maintaining ammunition towards oxidative injury through H2O2 while increased activity indicates its involvement against oxidative injury through H2O2. Additionally, intensified action of GPX might be coordinated by consequential retarted growth, as adapt to tension by phenolic compounds (Pasqualini et al. 2003). Michalowicz et al. 2010 reported the similar trend of antioxidant enzymes activity (CAT, GPX, and APX) in the leaves of reed canary grass. In the current analysis, the overall actions of distinct rummaging enzymes in unpurified material were evaluated. However, a confined raise in reactive oxygen species adjust the oxidoreduction stability in the entire plant proportion due to their immeasurable complex of protection arrangement, with interference within different chamber that might be tripped by reactive oxygen species (Foyer and Noctor 2005). The oxidative damage can induce different types of adverse reactions like membrane peroxidation, ions loss, protein scission, and even DNA strand breakage (Collins and Harrington 2002; Mittler 2002). It is observed that raised origination of reactive oxygen species owing to DBPs exposure was also merged to increase the actions of scavenging enzymes, just as GPX, APX as well as CAT.
DNA interaction studies
UV-visible absorbance is a potential approach to determine the pattern of DNA binding to metal complexes (Son et al. 1998). As a result, the spectroscopic titration of DNA with DBP compounds has been carried out to contribute authentication for prospect of calf thymus DNA binding to DBP components. The interaction mechanism of DBP components to CT-DNA was explored at molecular level by UV-Vis and fluorescence spectroscopic techniques to understand the DNA damaging effect and toxicity of DBP compounds. The UV-visible absorbance of binding between DNA and DBPs has been noted for persistent DNA concentration. Figure 5 showed increased UV-vis spectra of DNA with distinct concentrations of DBP compounds at 37 °C for 10 min. Traditionally, hyperchromaticity is the spectral attribute of DNA double helical structure which signifies secondary structure deterioration of DNA, whereas hypochromaticity signifies the binding pattern of DNA to complex is electrostatic or intercalating that may maintain the DNA duplex, at the same time appearance of red shift indicates the maintenance of DNA duplex (Kelly et al. 1985). As there is no any aromatic ring present in DBPs to expedite intercalating, classical intercalative interaction was prevented (Chowdhury et al. 2005). Moreover, the DBP compounds are small molecules, so the minor groove binding is favorable. The differences noticed in the absorption spectrum of CT-DNA in the presence of increasing concentration of TCAA, TCM, and TBM compounds, indicates increase in intensity at 258 nm, which reflects hyperchromic effect in absorption spectra as compared to control. The hyperchromic effect indicates conformational and structural changes of DNA due to DBP-DNA interaction. In addition, the hyperchromic effect may appear due to charged ions which can bind to DNA by electrostatic interaction to phosphate group of DNA spinal column and so that promoting convulsion and altogether destruction to the secondary conformation of DNA (Arjmand and Jamsheera 2011). The slightly conformational changes of DNA may be due to these kinds of bindings (Kelly et al. 1985). The data were treated for weak bonding relation using Eq. (1) (Shahabadi and Hadidi 2012),
Where A is absorbance at different concentrations of DBPs, A0 is the absorbance of DNA without DBPs, and A∞ is the final absorbance of the DBPs–DNA. The bonding constant (K) values were summarized in Table 3 which were 4.2 × 104, 3.7 × 104, 3.9 × 104 L mol−1 for TCAA, TCM, and TBM respectively.
UV-visible spectra of DNA in the presence of the increasing DBPs (disinfection by-products) concentrations. i Calf thymus DNA (60 μm) + TCAA, trichloroacetic acid (1, 2.5, 5, 7.5, 10 mg/L). ii Calf thymus DNA (60 μm) + TCM, trichloromethane (1, 2.5, 5, 7.5, 10 mg/L). iii Calf thymus DNA (60 μm) + TBM, tribromomethane (1, 2.5, 5, 7.5, 10 mg/L)
Fluorescent titration of suspension consisting of DNA including ethidium bromide (EtBr) with DBPs has been explored. The fluorescent intensity of EtBr increases during it passes from polar medium to nonpolar medium due to decrease in the intersystem interchanging durations (Rahban et al. 2010). The conventional method to investigate DNA bonding factors is the rearrangement of DNA interpolated with EtBr through groove binding elements (Sambrook et al. 1989). The fluorophore EtBr configure solvable compounds for nucleic acids and transmit enormous fluorescent when DNA is present because interpolation of homogenous phenenthridinium rings within adjoining base pairs on double helical EtBr (Butour and Macquet 1977). Previously, the two binding points had been revealed for EtBr on DNA: first position is interpolation between base pairs and second position is electrostatic within cationic EtBr on surface of DNA’s anionic phosphate groups. As shown in Fig. 6, by addition of each DBPs concentration to the DNA-EtBr solutions, the fluorescent intensity was expanded. The expansion in fluorescent emission indicates that EtBr might be released after addition of each DBP compound in solutions and the accumulation of DBPs–DNA composite avert EtBr bonding with DNA. The constant for formation of DBP-DNA was thus estimated by produced fluorescent data using improved Eq. (2) (Shahabadi and Hadidi 2012):
Where F is absorbance at different concentrations of DBPs, F0 is the absorbance of DNA without DBPs, and F∞ is the final absorbance of the DBPs–DNA. The formation constant (K) values were summarized in Table 3 which were 6.8 × 103, 6.5 × 103, 6.6 × 103 L mol−1 for TCAA, TCM, and TBM respectively.
Fluorescence spectra for DNA-DBPs (disinfection by-products) binding study. i Calf thymus DNA (60 μm) + TCAA, trichloroacetic acid (1,2.5,5,7.5,10 mg/L). ii Calf thymus DNA (60 μm) + TCM, trichloromethane (1,2.5,5,7.5,10 mg/L). iii Calf thymus DNA (60 μm) + TBM, tribromomethane (1,2.5,5,7.5,10 mg/L)
To understand the thermodynamics of interaction between DBPs and DNA, the contributions of entropy and enthalpy of interaction is helpful. The forces of interaction between drug and biomolecule assumed hydrogen bond, electrostatic force, hydrophobic force, Van der Waals force, etc. (Ross and Subramanian 1981). So according to entropy (▲S) and enthalpy (▲H) data, the interaction concluded: (i) enthalpy (▲H) > 0 and entropy (▲S) > 0, hydrophobic interactions; (ii) enthalpy (▲H) < 0 and entropy (▲S) < 0, Vander Waals force and hydrogen bond; (iii) enthalpy (▲H) < 0 and entropy (▲S) > 0, electrostatic force (Shahabadi and Fatahi 2010). The entropy (▲S) and enthalpy (▲H) can be estimated from van’t Hoff equation: (3)

Where K is binding constant and R is gas constant, and ▲S is entropy and ▲H is enthalpy can be acquired by plotting lnK vs 1/T. The values of entropy (▲S) and enthalpy (▲H) in both cases can be seen positive from the data listed in Table 3. The positive values of ▲S and ▲H revealed that hydrophobic forces have important role in DBPs–DNA binding.
From the mentioned outcome, it is assumed that binding of disinfection by-products to DNA showed significant changes in the structure by deterioration of secondary configuration of DNA and conformation of DNA through non-traditional intercalation fashion with DBP components. The changes in DNA due to disinfection by-products binding acquired here may contribute functional outcomes, particularly to evaluate the DNA damaging effect and toxicity of these emerging compounds as pollutants.
Conclusion
Many studies have been conducted in order to validate the use of disinfectant in water treatment plant to disinfect water. However, not many studies have been done to appraise the toxic effects of these associations on the biota. The study disseminates the genotoxic prospects of DBPs in plant model system, A.cepa. To the best of our knowledge, these results represent the first dataset confining genotoxic and clastogenic effects for DBPs assessed in vivo in plant model system. Results from studies demonstrate that disinfection by-products were genotoxic to the plant model system and human beings also due to direct vital link in the food chain. The genotoxicity and cytotoxicity rank order of disinfection by-products in Allium cepa were TCAA > TBM > TCM. As a measure of clastogenic effects, all the three DBP components induced an increase in chromosomal aberration frequency. DNA laddering was used as a DNA fragmentation marker for genotoxicity determination. The feasible reasoning for genotoxic potential of DBP components is due to higher H2O2 and lipid peroxidation (oxidative stress). Allium test has been found to have a high correlation with other test system so considering the myriad of endpoints screened, DBPs has been found to be genotoxic and cytotoxic to the plant, human system, and also the environment.
References
Ammar WB, Nouairi I, Zarrouk M, Ghorrbel M, Jemal F (2008) Antioxidative response to cadmium in roots and leaves of tomato plants. Biol Plant 52:727–731
Arjmand F, Jamsheera A (2011) DNA binding studies of new valine derived chiral complexes of tin (iv) and zirconium (iv). Spectrochim Acta A 78:45–51
Arya SK, Mukherjee A (2014) Sensitivity of Allium cepa and Vicia faba towards cadmium toxicity. Jour Soil Sci Plant Nutri 14(2):447–458
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Photoinhibition, pp 227–287
Bela K, Horvath E, Galle A, Szabados L, Tari I, Csiszar J (2015) Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. Jour Plant Physio 176:192–197
Butour JL, Macquet JP (1977) Differentiation of DNA-platinum complexes by fluorescence. The use of an intercalating dye as a probe. Eur J Biochem 78:455–463
Canistro D, Melega S, Ranieri D, Sapone A, Gustavino B, Monfrinotti M, Rizzoni M, Paolini M (2012) Modulation of cytochrome P450 and induction of DNA damage in Cyprinus carpio exposed in situ to surface water treated with chlorine or alternative disinfectants in different seasons. Mutat Res 729(1–2):81–89
Çelik A, Ünyayar S, Çekiç FO, Güzel A (2008) Micronucleus frequency and lipid peroxidation in Allium sativum root tip cells treated with gibberellic acid and cadmium. Cell Biol Toxicol 24:159–164
Chowdhury S, Alhooshani K, Karanfil T (2014) Disinfection byproducts in swimming pool: occurrences, implications and future needs. Water Res 53:68–109
Chowdhury SR, Mukherjee KK, Bhattacharyya R (2005) Biophisical and biochemical investigation on the binding of the manganese - cyanonitosyl complex with DNA. Trans Met Chem 30:601-604
Cirillo S, Canistro D, Vivarelli F, Paolini M (2016) Effects of chlorinated drinking water on the xenobiotic metabolism in Cyprinus carpio treated with samples from two Italian municipal networks. Environ Sci Pollut Res 23:18777–18788
Collins A, Harrington V (2002) Repair of oxidative DNA damage: assessing its contribution to cancer prevention. Mutagenesis 17:489–493
Dat J (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci 42:35–40
Fantuzzi G, Righi E, Predieri G, Giacobazzi P, Mastroianni K, Aggazzotti G (2010) Prevalence of ocular, respiratory and cutaneous symptoms in indoor swimming pool workers and exposure to disinfection by-products (DBPs). Int J Environ Res Public Health 7:1379–1391
Farre´ MJ, Day S, Neale PA, Stalter D, Tang JYM, Escher BI (2013) Bioanalytical and chemical assessment of the disinfection by-product formation potential: role of organic matter. Water Res 47(14):5409–5542
Fiskesjö G (1987) The Allium test as a standard in environmental monitoring. Hereditas 102:99–112
Fiskesjo G, Levan A (1993) Evaluation of the first ten MEIC chemicals in the Allium test. ATLA 21:139–149
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Ghoshal S, Bandyopadhyay A, Mukherjee A (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two tropic levels: plant and human lymphocytes. Chemosphere 81:253–1262
Grata¨o PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8:1773–1791
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:13–22
Hinckley AF, Bachand AM, Reif JS (2005) Late pregnancy exposures to disinfection byproducts and growth-related birth outcomes. Environ Health Perspect 113:1808–1813
Hu Y, Tan L, Zhang S, Zuo Y, Han X, Liu N, Lu W, Liu A (2017) Detection of genotoxic effects of drinking water disinfection by- products using Vicia faba bioassay. Environ Sci Pollut Res 24:1509–1517
Kapustka LA, Lipton J, Galbraith H, Cacela D, Lejeune K (1995) Metallic and arsenic impacts to soils, vegetation communities and wildlife habitat in southwest Montana uplands contained by smelter emissions: II. Laboratory phytotoxicity studies. Environ Toxicol Chem 14:1905–1912
Kelly TM, Tossi AB, McConnel DJ, Streakas TC (1985) A study of the interactions of some polypyridylruthenium (II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res 13:6017–6034
Kogevinas M, Villanueva CM, Font-Ribera L, Liviac D, Bustamante M, Espinoza F, Nieuwenhuijsen MJ, Espinosa A, Fernandez P, DeMarini DM, Grimalt JO, Grummt T, Marcos R (2010) Genotoxic effects in swimmers exposed to disinfection by-products in indoor swimming pools. Environ Health Perspect 118(11):1531–1537
Kumar A, Majeti NVP (2014) Proteomic responses to lead-induced oxidative stress in Talinum triangulare Jacq. (Willd.) roots: identification of key biomarkers related to glutathione metabolisms. Environ Sci Pollut Res 21:8750–8764
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Lutterbeck CA, Kem DI, Machado ÊL, Kümmerer K (2015) Evaluation of the toxic effects of four anti-cancer drugs in plant bioassays and its potency for screening in the context of waste water reuse for irrigation. Chemosphere 135:403–410
Malar S, Manikandan R, Favas PJC, Vikram Sahi S, Venkatachalam P (2014) Effect of lead on phytotoxicity, growth, biochemical alterations and its role on genomic template stability in Sesbania grandiflora: a potential plant for phytoremediation. Ecotoxicol Environ Saf 108:249–257
Manasfi T, De Méo M, Coulomb B, Di Giorgio C, Boudenne JL (2016) Identification of disinfection by-products in freshwater and seawater swimming pools and evaluation of genotoxicity. Environ Int 88:94–102
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Michałowicz J, Posmyk M, Duda W (2009) Chlorophenols induce lipid peroxidation and change antioxidant parameters in the leaves of wheat (Triticum aestivum L.). J Plant Physiol 166:559–568
Michalowicz J, Urbanek H, Bukowska, Duda W (2010) The effect of 2,4-dichlorophenol and pentachlorophenol on antioxidant system in the leaves of Phalaris arudinacea. Biol Plant 54(3):597–600
Mittler R (2002) Oxidative stress, antioxidant and stress tolerance. Trends Plant Sci 7:405–410
Mogren LM, Olssen ME, Gertsson UE (2007) Effects of cultivar, lifting time and nitrogen fertilizer level on quercetin content in onion (Allium cepa L.) at lifting. J Sci Food Agri 87:470–476
Montillet J-L, Chamnongpol S, Ruste’rucci C, Dat J, Van de Cotte B, Agnel J-P, Battesti C, Inze’ D, Van Breusegem F, Triantaphylide’s C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526
Neale Pa, Antony A, Bartkow ME, Farré MJ, Heitz A, Kristiana I, Tang JYM, Escher BI (2012) Bioanalytical assessment of the formation of disinfection byproducts in a drinking water treatment plant. Environ Sci Technol 46:10317–10325
Oberdorster G, Stone V, Donaldson K (2007) Toxicology of nanoparticles: a historical perspective. Nanotoxicology 1:2–25
Pasqualini V, Robles C, Garcino S, Greff S, Bousquet- Melou A, Bonin G (2003) Phenolic compounds content in Pinus halepensis mill. Needles: a bioindicator of air pollution. Chemosphere 52:239–248
Pentamwa P, Sukton B, Wongklom T, Pentamwa S (2013) Cancer risk assessment from trihalomethanes in community water supply at northeastern Thailand. Int Jour of Environ Sci Develop 5:538–544
Plewa MJ, Wagner ED, Mitch WA (2011) Comparative mammalian cell cytotoxicity of water concentrates from disinfected recreational pools. Environ. Sci. Technol. 45(9):4159–4165
Plewa MJ, Wagner ED, Metz DH, Kashinkunti R, Jamriska K, Meyer M (2012) Differential toxicity of drinking water disinfected with combinations of ultraviolet radiation and chlorine. Environ. Sci. Technol. 46(14):7811–7817
Rahban M, Divsalar A, Saboury AA, Golestani A (2010) Nanotoxicity and spectroscopy studies of silver nanoparticle: calf thymus DNA and K562 as targets. J Phys Chem C 114:5798–5803
Reeves JF, Davies SJ, Dodd NJF, Jha AN (2007) Hydroxyl radicals (-OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytoxicity and oxidative DNA damage in fish cells. Mutat Res 640:113–122
Rhizsky L, Hallak-Herr E, Van Breusegen F, Rachmilevich S, Barr J, Rodermel S, Inze S, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase and catalase. Planta 32:329–342
Richardson SD, Plewa MJ, Wagner ED, Schoeny R, DeMarini DM (2007) Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection byproducts in drinking water: a review and roadmap for research. Mutat Res Rev Mutat Res 636(1–3):178–242
Richardson SD, DeMarini DM, Kogevinas M, Fernandez P, Marco E, Lourencetti C, Balleste C, Heederik D, Meliefste K, McKague AB, Marcos R, Font-Ribera L, Grimalt JO, Villanueva CM (2010) What’s in the pool? A comprehensive identification of disinfection by-products and assessment of mutagenicity of chlorinated and brominated swimming pool water. Environ Health Perspect 118(11):1523–1530
Rook JJ (1974) Formation of haloforms during chlorination of natural waters. J Water Treat Examin 23:234–243
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. J Biochem 20:3096–3102
Roy S, Ihantola R, Hanninen O (1992) Peroxidase activity in lake macrophytes and its relation to pollution tolerance. Environ Exp Bot 32:457–464
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, vol 9, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, Chapter, pp 14–23
Sapone A, Canistro D, Vivarelli F, Paolini M (2016) Perturbation of xenobiotic metabolism in Dreissena polymorpha model exposed in situ to surface water (Lake Trasimene) purified with various disinfectants. Chemosphere 144:548–554
Schroder P, Gotzberger C (1997) Partial purification and characterization of glutathione S- trasferase isozymes from the leaves of Juniperus communis, Larix deciduas and Taxus baccata. Appl Botany 71:31–37
Shahabadi N, Fatahi A (2010) Multispectroscopic DNA-binding studies of a tris-chelate nickel (II) complex containing 4,7-diphenyl 1,10-phenanthroline ligands. J Mol Struct 970(1–3):90–95
Shahabadi N, Hadidi S (2012) Spectroscopic studies on the interaction of calf thymus DNA with the drug levetiracetam. Spectrochim Acta A Mol Biomol Spectrosc 96:278–2833
Sharma AK, Sharma A (1980) Chromosome Techniques – Theory and Practice, third edn. Butterworths, London, p 711
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Singh H, Batish D, Kohli R, Arora K (2007) Arsenic-induced root growth inhibition in mung bean ( Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhance lipid peroxidation. Plant Growth Regul 53:65–73
Son GS, Yeo JA, Kim JM, Kim SK, Moon HR, Nam W (1998) Base specific complex formation of norfloxacin with DNA. Biophys Chem 74:225–236
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J Exp Bot 56:1335–1342
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Teo TLL, Coleman HM, Khan SJ (2015) Chemical contaminants in swimming pools: occurrence, implications and control. Environ Int 76:16–31
Villanueva CM, Cantor KP, Grimalt JO, Malats N, Silverman D, Tardon A, Garcia-Closas R, Serra C, Carrato A, Castaño-Vinyals G, Marcos R, Rothman N, Real FX, Dosemeci M, Kogevinas M (2007) Bladder cancer and exposure to water disinfection by-products through ingestion, bathing, showering, and swimming in pools. Am J Epidemiol 165:148–156
Zhang H, Jiang Y, He Z, Ma M (2005) Cadmium accumulation and oxidative burst in garlic (Allium sativum). J Plant Physiol 162:977–984
Zhu R, Wang S, Chao J, Sun X, Yao S (2008) Oxidative and binding effect of nano- TiO2 on plasmid DNA and pepsin. In: the 2nd international conference on bioinformatics and Biomed Eng, pp. 1013–1016
Acknowledgments
The authors would like to thank TEQIP II and the National Institute of Technology, Durgapur, for providing good research lab facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjan, J., Mandal, T. & Mandal, D.D. Environmental risk appraisement of disinfection by-products (DBPs) in plant model system: Allium cepa. Environ Sci Pollut Res 26, 8609–8622 (2019). https://doi.org/10.1007/s11356-019-04262-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04262-7