Abstract
Disinfection by-products (DBPs) are a concern due to their presence in chlorinated wastewater, sewage treatment plant discharge, and surface water, and their potential for environmental toxicity. Despite some attention to their ecotoxicity, little is known about the phytotoxicity of DBPs. This study aimed to evaluate the individual and combined phytotoxicity of four trihalomethanes (THMs: trichloromethane (TCM), bromodichloromethane (BDCM), dibromochloromethane (DBCM), and tribromomethane (TBM) and their mixture (THM4)), and trichloroacetic acid (TCAA) using genotoxic and cytotoxic assays. The analysis included seed germination tests using Vigna radiata and root growth tests, mitosis studies, oxidative stress response, chromosomal aberrations (CA), and DNA laddering using Allium cepa. The results showed a progressive increase in root growth inhibition for both plant species as the concentration of DBPs increased. High concentrations of mixtures of four THMs resulted in significant (p < 0.05) antagonistic interactions. The effective concentration (EC50) value for V. radiata was 5655, 3145, 2690, 1465, 3570, and 725 mg/L for TCM, BDCM, DBCM, TBM, THM4, and TCAA, respectively. For A. cepa, the EC50 for the same contaminants was 700, 400, 350, 250, 450, and 105 mg/L, respectively. DBP cytotoxicity was observed through CAs, including C-metaphase, unseparated anaphase, lagging chromosome, sticky metaphase, and bridging. Mitotic depression (MD) increased with dose, reaching up to 54.4% for TCAA (50–500 mg/L). The electrophoresis assay showed DNA fragmentation and shearing, suggesting genotoxicity for some DBPs. The order of phytotoxicity for the tested DBPs was TCAA > TBM > DBCM > BDCM > THM4 > TCM. These findings underscore the need for further research on the phytotoxicity of DBPs, especially given their common use in agricultural practices such as irrigation and the use of sludge as manure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reuse of treated wastewater for irrigation is a common practice. Wastewater is often disinfected with chlorine before it is discharged into surface water bodies or land for irrigation. However, chlorination generates disinfection by-products (DBPs) due to the high organic concentrations in wastewater. The two primary groups of DBPs formed after chlorination are trihalomethanes (THMs) and haloacetic acids (HAAs). DBP concentration of 4.12 to 2337.2 µg/L was observed in treated wastewater around the world (Heng et al. 2021; Albolafio et al. 2022; Wang et al. 2022a). The toxic effects of DBPs on microbes and humans have been extensively documented in previous literature, with some of these DBPs being proven or probable carcinogens (Kali et al. 2021; Kumar et al. 2023). Despite this, the impact of DBPs on plants and aquatic organisms has not been adequately studied.
Recently, the ecotoxicity of DBPs to aquatic organisms has become a concern due to the widespread use of chlorine for disinfection, particularly during the COVID-19 pandemic (Parveen et al. 2022a). Elevated levels of DBPs have been found in many surface water bodies after the widespread use of chlorinated disinfectants, leading to concerns about ecotoxicity (Chu et al. 2021; Liu et al. 2022). For instance, Cui et al. (2021) evaluated the toxicity of seventeen DBPs to freshwater organisms at three trophic levels and found that phytoplankton was more sensitive to HAAs, zooplankton was sensitive to THMs and haloacetonitriles, and DBPs caused abnormalities in the embryo development of Danio rerio (zebrafish). Similarly, Liu and Zhang (2014) reported a median effective concentration of 9.12 µM for 2,5-dibromohydroquinone (an emerging DBP) to cause toxicity in Platynereis dumerilii, a marine worm, and Gordon et al. (2015) found the median effective concentration of tribromoacetic acid to microalgae to be in the range of 194—240 mg/L (Liu and Zhang 2014; Gordon et al. 2015). These studies suggest that DBPs have the potential for acute toxicity to aquatic organisms.
Although the effects of DBPs on animals and algae have been studied, their impact on plants has been largely overlooked. Since chlorinated reclaimed water and DBP-containing surface water are commonly used for irrigation, the possibility of adverse effects on plants is high. Additionally, the use of chlorinated sewage sludge as fertilizer can also contribute to the problem. Ranjan et al. (2019, 2021) studied the physiological impacts of trichloromethane (TCM), tribromomethane (TBM), and trichloroacetic acid (TCAA) on plants and observed significant phytotoxic effects. Akande et al. (2016) found a decrease in biomass gain in Solanum lycopersicum (tomato) seedlings with higher degrees of bromination in THMs and an increase in oxidative damage and a decrease in essential nutrient concentration with higher degrees of chlorination in THMs. However, currently, there are no studies on the relative and combined effects of all four THMs (TCM, bromodichloromethane (BDCM), dibromochloromethane (DBCM), and TBM).
Since the 1940s, A. cepa and V. radiata have been utilized as model plant species in toxicity studies (Grant 1982). These species have gained popularity due to their year-round availability, cost-effectiveness, straightforward testing procedures, and ability to easily detect chromosomal aberrations (CA) owing to their size. A. cepa bulbs have been extensively used in genotoxic and cytotoxic assays since 1938. CA in A. cepa cells has become the primary indicator of cytotoxicity. Similarly, certain nuclear abnormalities and mitotic indices are utilized to evaluate cytotoxicity, while the presence or absence of micronuclei serves to determine the mutagenicity of compounds (Leme and Marin-Morales 2009).
Seed germination tests are widely employed for evaluating phytotoxicity, particularly concerning inhibition of germination and root growth, caused by individual contaminants or multi-compound matrices such as compost. For instance, Lin and Xing (2007) utilized rapeseed, ryegrass, corn, lettuce, and cucumber seeds to investigate the phytotoxicity of zinc and aluminum nanoparticles, dispersed separately in deionized water (Lin and Xing 2007). Such an approach proves useful for understanding the toxicity of individual compounds. However, in the case of multi-compound matrices, evaluating the toxicity of each compound present in compost on plants is a time-consuming, expensive, and impractical process. Furthermore, assessing their combined effect on plants via individual compound analysis is not feasible. As a result, seed germination bioassays using these combined matrices have become the preferred solution for researchers (Luo et al. 2018). Therefore, in the present study, we assessed the phytotoxicity of four THMs individually and in combination, as well as of one HAA, using two plant model systems. The specific objectives were to (i) understand the impact of DBPs on seed germination using V. radiata, (ii) study the effect of DBPs on root growth using V. radiata and A. cepa, and (iii) investigate oxidative stress and abnormalities during mitosis in A. cepa roots due to exposure to DBPs.
Materials and methods
Experimental setup
Four THMs, namely, TCM, BDCM, DBCM, and TBM, along with one HAA, namely, TCAA were selected for the study. TCAA was selected as it is one of the most frequent HAA in water along with dichloroacetic acid (DCAA) (Albolafio et al. 2022; Carter et al. 2019; Dong et al. 2023) and the most frequent HAA in wastewater (Li et al. 2021). To prepare the treatment doses for the experiment, concentrations ranging from 100–10,000 mg/L of TCM, BDCM, DBCM, TBM, THM4 (a mixture of the four THMs in equal proportion), and TCAA were prepared in ultra-pure water. Although the highest doses of the sample treatment are unlikely to be found in the real world, they were utilized to assess the model plant species' tolerance to disinfection by-products (DBPs). Previous studies have employed similar doses, as described by Ranjan et al. (2021). To estimate variability in test results, each treatment dose was replicated three times.
Vigna radiata
Locally available V. radiata (green grams or mung beans) seeds were washed with 0.1% sodium hypochlorite (NaOCl; Merck Life Sciences) solution followed by rinsing with ultra-pure water. The germination protocol was based on past studies that employed V. radiata for bioassays (Banerjee et al. 2014; Aziz et al. 2020; Ranjan et al. 2021; Vanapalli et al. 2021). To initiate the germination process, 18 V. radiata seeds were evenly spaced between two germination sheets (20 cm × 30 cm) and wetted with equal volumes of treatment doses (0 – 10,000 mg/L). These sheets were then rolled and placed in a dark incubator at 25°C for five days. Control sheets were prepared in the same manner but without DBP dosing, utilizing ultra-pure water. Both control and treatment sheets were triplicated to ensure accuracy. To maintain adequate moisture levels, the sheets were rewetted every other day. After five days, the lengths of the roots were measured using thread and scale. The seed radicles were weighed after being dried for 48 h in an oven at 80°C.
Allium cepa
A. cepa (red onion) bulbs were collected from local vendors and cleaned with water and 0.1% NaOCl like that in the case of V. radiata. They were sprouted in ultra-pure water for 24 h, then exposed to treatment doses of 0 – 1000 mg/L of the same DBPs used in the V. radiata experiments for 5 days. The outer shell of the onion bulb was removed as they are highly antioxidants and can limit the toxic effects and change the properties of the germination mixture (Sidhu et al. 2019; Kaya et al. 2023; Vujčić Bok et al. 2023). Control samples were treated with ultra-pure water. The treatment doses and protocols followed those of previous studies (Kumari et al. 2009; Banerjee et al. 2014; Ranjan et al. 2019; Aziz et al. 2020). Each dose was tested with three bulbs, and the treatment solution was replaced daily to maintain sufficient DBP concentration. After 5 days of exposure, root lengths were measured using thread and scale, and the samples were prepared for further analysis.
Analytical methods
Phytotoxicity
Various growth indices and effective concentration at 50% root length inhibition (EC50) were calculated using the measured root lengths of A. cepa and V. radiata. Tolerance index (TI), relative seed germination (RSG), relative radicle growth (RRG), and germination index (GI) were calculated for V. radiata, and EC50 was determined for both species as follows (Luo et al. 2018; Sardar et al. 2022).
Mitotic index and abnormalities
A. cepa root tips harvested on the 5th day of growth were firmed in glacial acetic acid and ethanol (Merck Life Sciences) solution (1:3) for at least 3 h and subsequently were stored at 4°C (Nefic et al. 2013; Sabeen et al. 2020). Six root tips (two from triplicate bulbs) were used per treatment dose and control. Fixed root tips were hydrolyzed in 1N hydrochloric acid (Merck Life Sciences) for 20 min at 60°C prior to squashing. The squash method prescribed by (Sharma and Sharma 1980) was adopted after staining the root tips with 10% acetocarmine for 30 min. Temporary microscopic slides were prepared by sealing the squashed cells with clear nail polish and they were observed under the microscope (× 100). A total of 2500 cells were counted for mitotic endpoint evaluation and the cells with various chromosomal and cytological abnormalities were noted and photographed.
The mitotic index (MI) is characteristic of the total number of dividing cells in the cell cycle. Therefore, it has been an indicator of cell cytotoxicity by various agents. The DBP dosage for MI analysis was selected as 100—6000 mg/L (for THMs), and 10 – 1000 mg/L (for HAAs) based on the results of root growth analysis. MI values of A. cepa root cells were calculated using Eq. 5 and percentage aberrant cells were calculated in subjects with total no. of total cells (Sabeen et al. 2020).
DNA laddering
DNA laddering is a widely used assay for detecting apoptosis and genotoxicity caused by chemicals or pathogens (Ghosh et al. 2010, 2015; Petriccione et al. 2013). In DNA laddering assay, DNA fragments of varying sizes are separated based on their molecular weight by gel electrophoresis, with smaller fragments migrating farther than larger ones.
For analysis, A. cepa root tips were processed by cetyl trimethylammonium bromide (CTAB) method using a plant DNA isolation kit (MB 502, Himedia). DNA sample preparation procedure followed previous studies (Ranjan et al. 2019, 2021). Root trips were ground using a mortar and pestle. 9 ml of prewarmed CTAB extraction buffer was added to 300–400 mg of the lyophilized ground tissue in a sterile centrifuge tube. The mixture was gently mixed by inversion. It was ensured that CTAB powder and β-mercaptoethanol were added to CTAB extraction buffer prior to use. The samples were incubated for 60–90 min at 65°C, with occasional inversion, and subsequently cooled at room temperature (15–25°C) for 5 min. Following this, 5 ml of chloroform: octanol (24:1) was added to each tube, and the samples were gently mixed by inverting the tubes for 5 min. The samples were then centrifuged at 850 × g (≈ 3000 rpm) for 2 min at room temperature (15–25°C). The top aqueous layer was transferred into a fresh tube, and 25 µl of RNase A (RNase A is added to remove RNA since the extract contains DNA and RNA; 20 mg/ml) was added. The sample was gently mixed by inversion and incubated for 30 min at room temperature. 6 ml of isopropanol was added to each tube, with gentle mixing until a white fluffy DNA precipitate appeared. Subsequently, the samples were centrifuged at 850 × g (≈ 3000 rpm) for 5 min to pellet the DNA, and the supernatant was discarded. The pellet was resuspended in 8 ml of cold diluted CTAB wash buffer. The resuspended mixture was incubated at room temperature for 20 min and then centrifuged at 850 × g (≈ 3000 rpm) for 5 min. To further purify the DNA, 8 ml of cold 70% ethanol was added to the tube containing DNA and centrifuged at 850 × g (≈ 3000 rpm) for 5 min.
The DNA pellet was air-dried to eliminate any residual traces of ethanol. Finally, the DNA sample was transferred to a tube containing 1 ml of elution buffer, and the pellet was gently dissolved by pipetting (Southern 1975; Saghai-Maroof et al. 1984; Tai and Tanksley 1990; Williams et al. 1990; Guillemaut and Maréchal-Drouard 1992; Narzary et al. 2015; Gray et al. 2023; Sumi et al. 2023; You et al. 2023).
The extracted DNA samples were kept at -20°C till the electrophoresis analysis. Purity of the DNA samples was assessed by calculating the ratio of absorbance at 260 nm to 280 nm using a NanoDrop™ One/OneC Microvolume UV–Vis spectrophotometer. For loading, 1% agarose gel prepared in 1 × tris acetate ethylenediaminetetraacetic acid (EDTA) buffer containing 2 µl ethidium bromide (EtBr; 10mg/ml) was used. Electrophoresis was carried out at 100 V at room temperature for a duration of 45 min (Green and Sambrook 2012; Lee et al. 2012). A 1kb DNA ladder (ProxiO Ladder Plus) was loaded as reference. DNA ladders were visualised using a gel imaging system (Bio Rad ChemiGel Doc™ Touch).
Hydrogen peroxide content
Generation of hydrogen peroxide (H2O2) in plants is a symptom of oxidative stress due to cellular injury (Wahid et al. 2007). A. cepa roots (100 mg) were ground to paste in 0.1% trichloroacetic acid (Merck Life Sciences) in a water bath under cold conditions. Following Ranjan et al. (2019), this mixture was homogenized and centrifuged for 20 min at 10,000 rpm and 4°C. The supernatant (0.5 mL) was mixed with 1 mL 1M potassium iodide (Sigma Aldrich) and 0.5 ml phosphate buffer (SRL Chemicals) to maintain pH at 7. Absorption at 390 nm was determined using a spectrophotometer (Mortars India) and the H2O2 concentration was calculated by dividing the absorption value by the extinction coefficient of 0.28 µm−1.cm−1 (Singh et al. 2007).
Statistical analysis
All the data in the present research work were represented as mean ± standard deviation (SD). Moreover, 2 sample t-test (p < 0.05) was performed to compare the mean values for determining the significance of the differences using Minitab 19.1.
Results and discussion
Germination and root growth inhibition
The presence of DBPs in the treatment water had a visible impact on the germination of V. radiata seeds. The length of the radicles in treated seeds was noticeably shorter, thinner, and, in some cases, appeared yellowish compared to control seeds (Fig. 1a-i). Furthermore, the roots of treated seeds were observed to be more folded than those of control seeds. Several treatments at different doses displayed the development of root hairs. At higher doses of DBPs, the epidermis (the outermost layer of the root) was appeared to be thicker than in the control group. This suggests that water uptake in the plant was hindered since the epidermis in healthy plants is thin and semipermeable to allow for the movement of water.
a Root length of control V. radiata seed; b non-germinated V. radiata seed in treatment; c – h V. radiata seeds treated with 4000 mg/L of TCM, BDCM, DBCM, TBM, THM4, and TCAA; i Root length of V. radiata increasing dose of TCAA from 0 mg/L – 6000 mg/L; and (j) A. cepa roots at increasing dose of TCAA from 0 mg/L – 1000 mg/L
In the present study, A. cepa bulbs were initially placed in deionized water before being transferred to the desired DBP doses to assess changes in root growth compared to control bulbs. Similar to V. radiata, the roots of A. cepa also exhibited characteristics such as thickness, shortness, and discoloration at higher doses of DBPs (Fig. 1j). The root tips of A. cepa bulbs treated with higher DBP doses, especially of TBM, required additional heating time while preparing microscopic slides for mitosis analysis. Further, as the DBP doses increased, the root length of both plant species tested was significantly reduced. At elevated doses, A. cepa exhibited the development of C-tumour roots and stunted root growth, similar to the response observed in A. cepa exposed to bilge water containing heavy metals, ammonia, and nitrates (Olorunfemi et al. 2015). Interestingly, individual THMs were found to have a greater impact on germination and root length than the THM mixture for both plant species treated. The seeds and bulbs treated with TCM and THM4 were similar in appearance.
Impact on germination of V. radiata
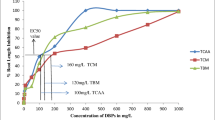
TI is the measure of tolerance of a plant towards a contaminant. It is important to note that the same plant species may exhibit different levels of tolerance to different contaminants based on their toxicity. In the case of V. radiata seed germination, it was evident that the tolerance varied for different DBPs. The order of TI for the various DBPs tested was TCM > THM4 > BDCM > DBCM > TBM > TCAA (Fig. 2).
The RSG, which indicates the relative number of seeds germinated in treatments compared to control, was evaluated. Control seeds showed an average of 18 ± 0.5 germinated seeds from triplicate sheets containing 20 seeds each. TCM and BDCM treatments at 100 mg/L showed similar no. of seed germination (Fig. 2). Notably, individual THMs showed lower RSGs than THM4 for the same dose. TCAA exhibited the highest toxicity to the seeds, with a significant decrease in RSG to 33.3% at a dose of 1000 mg/L, in contrast to other DBPs that maintained at least 50% relative germination at the same dose. The significant reduction in RSG observed for TCM and TBM beyond the 6000 mg/L threshold may be attributed to several factors, such as the potential toxicity exceeding the tolerance of the seeds, a depletion of oxygen in the reaction mixture due to the high toxin concentration, potential interference with enzymes in the seeds, or the formation of a barrier hindering water uptake due to toxin development (Bewley 1997). The drop in TI for TBM beyond 6000 mg/L aligns with this observation, indicating a sudden decrease in tolerance. However, it's worth noting that the TI of TCM did not exhibit a similar pattern. Consequently, the specific cause of the sharp decline in RSG for TCM warrants further investigation, and additional research is needed to confirm the validity of these potential reasons.
Since no pH adjustments were made to the treatment solutions, the TCAA solutions were acidic (pH ranging from 4.1 ± 0.5 to 6.5 ± 0.4) compared to the near-neutral or slightly alkaline pH (6.7 ± 0.4 to 8.3 ± 0.2) of the other DBPs. Acidic pH is known to negatively affect V. radiata seed germination (Kannan and Upreti 2008), and the adverse effects of TCAA on germination may be attributed to the low pH as well.
Among TI, RSG, RRG, and GI, GI is commonly used to evaluate the phytotoxicity of chemical compounds and composts (Wang et al. 2022b). Alternatively, some studies use RRG for the same application. However, since GI is a function of both RRG and RSG, it serves as a more comprehensive representation of phytotoxicity. Figure 2 presents the GI values for V. radiata seeds exposed to various DBPs. A higher GI value represents better germination and, hence, low toxicity. GI above 80% is indicative of a healthy plant environment with no phytotoxicity due to the exposed substances. In contrast, GI values below 80% indicate that the compound is phytotoxic to seed germination and growth (Yuan et al. 2016; Luo et al. 2018). While initial doses of TCM, BDCM, and THM4 did not cause phytotoxicity, the GI decreased significantly with increasing DBP concentrations. Moreover, the GI values for DBCM, TBM, and TCAA were below 80% at the initial dose of 100 mg/L, suggesting that these compounds are phytotoxic to V. radiata seeds and adversely affect germination and growth. The order of GI for the tested DBPs was TCM > THM4 > BDCM > DBCM > TBM > TCAA, with TCAA, TBM, and DBCM being the three most phytotoxic DBPs. These results are consistent with those reported by Ranjan et al. (2021), who found that the order of phytotoxicity for the tested DBPs was TCAA > TBM > TCM. However, no other studies have investigated the phytotoxicity of BDCM, DBCM, and THM4 to date.
Studying the nature of interactions between contaminants and their impact on plant physiology is crucial as contaminants can induce stress in plants. The classification of these interactions is determined by the plant's response to multiple stressors, with interactions being categorized as either synergistic or antagonistic. If the combined effect of stressors is greater than the additive sum of the individual stressors acting independently, then the interaction is considered synergistic. Conversely, if the combined effect is less than the additive sum, then the interaction is considered antagonistic. The chemical interactions between individual THMs and THM4 were analysed based on their GI values, which are reported in Table 1.
The observed GIs for THM4 values represent the combined effect, while the calculated GIs for THM4 values represent the arithmetic average of GIs for TCM, BDCM, DBCM, and TBM. Therefore, the calculated GIs values depict the phytotoxic effects of different THMs without considering the interactions between them. The arithmetic difference between the observed and calculated GIs for THMs was calculated to understand the interactive effects of individual THMs. A negative difference between the calculated and observed GIs for THMs depict synergistic interactions between the individual THMs, while the positive difference between them indicates antagonistic effects. Moreover, the statistical significance of the differences between the means of calculated and observed THM4 was evaluated through 2 sample t-test (p < 0.05) using Minitab 19.1. The statistical analysis was performed considering each GI value for the corresponding concentration of calculated and observed THM4 as an independent variable. At low tested concentrations (100, 500 mg/L), there was no significant difference between the mean values of observed and calculated THMs (p > 0.05), indicating no additional stress to the plants. However, as the THM dose increased, an antagonistic effect was observed with a significant difference between the means (p < 0.05), suggesting that at higher doses of mixtures, THMs reduced each other's effects. With further increases in dosage to greater than 2000 mg/L, the rate of antagonistic effect significantly decreased, likely due to low germination at higher doses of individual brominated THMs. The statistical significance of these differences indicates the prominence of the synergistic or antagonistic phytotoxic effects of THMs at higher concentrations (≥ 1000 mg/L) on germination of V. radiata.
Plant stressors can either have antagonistic or synergistic effects and often interact with other abiotic and biotic factors. Understanding these interactions is gaining more attention nowadays. The use of transcriptomics, metagenomics, and metabolomics data has proven pivotal in addressing challenges in plant-stress interaction research (Nadeem et al. 2023). The results of a metadata analysis of 286 freshwater ecosystems for plant diversity and biomass revealed that the net effect of stressors was frequently more antagonistic (41%) than synergistic (28%), additive (16%), or reversed (15%) (Jackson et al. 2016). Similar to the findings of the current study, the interactions between two herbicides were observed to be antagonistic on plant health (Zhang et al. 1995). Further, the plant stressors that activate systemic acquired resistance (SAR; a potent innate immunity system in plants that provides protection against a broad range of pathogens) as well as many abiotic plant stressors were antagonistic than synergistic (Yasuda et al. 2008; Bansal et al. 2013).
Zandalinas and Mittler (2022) found that simultaneous or sequential abiotic and biotic stressors significantly impair plant growth, crop yields, and overall plant health. Interestingly, when multiple stressors affecting a plant increase in number and complexity, the plant's growth and survival decline significantly, even if each individual stressor is considered low impact. Studies on interactions between various plant stressors and nutrients have shown that the antagonistic effect of one stressor/nutrient on another can be stronger than the reverse in root absorption and transport within plants (Rietra et al. 2017; Aboyeji et al. 2020). For instance, the antagonistic effect of potassium (K) on magnesium (Mg) was stronger than that of Mg on K in higher plants (Xie et al. 2021). Therefore, further research is needed to understand the impacts of individual THMs on each other. Recently, biochemometric approaches, which combine the biochemistry of various substances with chemometric models, have been employed as effective tools for investigating synergy (Ma et al. 2018).
Root length inhibition and effective concentration
The concentration that causes a 50% reduction in root length, known as the EC50, is typically expressed as mg/L of toxin. Figure 3 presents the EC50 of TCM, BDCM, DBCM, TBM, THM4, and TCAA for V. radiata and A. cepa. The order of EC50 was TCM > THM4 > BDCM > DBCM > TBM > TCAA for both plant species. Plants can take up soluble toxic contaminants along with water at all stages of their growth cycle. If the plant fails to degrade the contaminant, it can adversely affect the plant directly and/or indirectly. During germination, direct effects can include disturbed water balance, inhibition of cytoplasmic enzymes, and damage to cell structure resulting from oxidative stress. Indirect effects can include the replacement of nutrients with toxins (Bansal 2018). Both direct and indirect effects can negatively impact the germination and growth process.
The lower EC50 values for A. cepa indicate relatively higher sensitivity to DBPs compared to V. radiata. This tendency was observed in two previous studies that assessed the EC50 of TCM, TBM, and TCAA on A. cepa and V. radiata roots (Ranjan et al. 2019, 2021). These studies reported that A. cepa was relatively more sensitive to these three DBPs by 37.5, 33.3, and 18.5 times, respectively.
As there is no available literature on the phytotoxicity of DBPs and their interactions with plant enzymes, heavy metal(loid)s were used as a reference to understand the direct and indirect effects of toxins on germination and root growth. The EC50 of lead, copper, arsenate (AsV), and cadmium were 924.8 mg/kg of soil (on Hordeum vulgare), 28.9 µM (on Zostera marina), 18.8 µM (on Triticum aestivum), and 2268.4 µM (on Zostera marina), respectively (Liu et al. 2021; Li et al. 2022; Qin et al. 2022; Qiao et al. 2022). Similarly, the EC50 of ionic silver (Ag+) was 0.27 µM (on Triticum aestivum) and that of hydrogen sulphide was 500 µM (on Arabidopsis) (Zhang et al. 2017; Luo et al. 2021).
Previous studies reported an EC50 of 160 mg/L for TCM on A. cepa and 6000 mg/L on V. radiata. Similarly, TBM exhibited an EC50 of 120 mg/L on A. cepa and 4000 mg/L on V. radiata and TCAA impacted A. cepa at EC50 = 100 mg/L and V. radiata at EC50 = 1850 mg/L (Ranjan et al. 2021, 2019). Although heavy metals and metal ions have lower EC50 values compared to DBPs, indicating their higher toxicity, their toxin pathways were assumed to be similar. For example, Elsholtzia argyi and Triticum aestivum seeds and A. cepa roots exposed to lead (Pb) exhibited cell wall thickening, folds, protuberances, and nicks, similar to the observations in the current study (Fahr et al. 2013). Additionally, cytotoxicity and morphological effects were reported in Oryza sativa L. seedlings treated with arsenic (As) and cadmium (Cd), especially in lateral root development (Ronzan et al. 2018). Roots treated with As or Cd or both showed severe damage to the lateral root primordia (the nascent developing unit of lateral roots) resulting in incorrect evolution of them to lateral roots, which was comparable to the irregular patterns of lateral roots observed in the current study, despite the difference in the root development period (10 days vs 5 days in the present study) and the plant species.
The phytotoxicity pathway of Pb involves its binding with the physical barriers of roots such as the cell wall or callose in higher plants, followed by the breaking of this barrier. At higher doses, Pb enters the cells through ion transport. Inside the cell, Pb forms complex compounds by bonding with phytochelatins, which are cysteine-rich peptides. These complexes are transported into the vacuoles, which are the main storage site for toxins in plants. Vacuoles in plant cells play an essential role in maintaining cell acidity and turgor pressure, regulating the storage and transport of substances, controlling the transport and localization of key proteins, and responding to biotic and abiotic stresses. Therefore, the presence of a toxin-bound compound in the vacuole can disrupt cell function. Since the effect of DBPs on A. cepa roots showed similar morphological and physical changes as Pb, the pathway of the toxin may follow the same stages.
Mitotic abnormalities to Allium cepa
Mitotic index and depression
The stained and sealed slides of A. cepa root tip cells from control and treatment groups were examined under a microscope to assess cell division. The control roots grown in ultra-pure water had a MI of 14.1 ± 0.2%, which decreased significantly for the various DBP treatments (Table 2). A lower MI value suggests a slower rate of mitosis, and a significantly lower MI than that of the control indicates alterations in the growth and development of the organism due to the contaminant exposure. The MI of control A. cepa roots was reported to be 60.3% in tap water and 8.6%, 8.8%, and 7.3% in de-ionized water in previous studies, which is in accordance with the current study (Kumari et al. 2009; Ranjan et al. 2019; Kuloğlu et al. 2022; Macar et al. 2022).
The MI values for 6000 mg/L of TCM, BDCM, and THM4 were 64.5%, 65.9%, and 65.8% lower than the MI of control roots, respectively. Similarly, DBCM (4000 mg/L), TBM (4000 mg/L), and TCAA (500 mg/L) resulted in 68.5%, 72.5%, and 84.7% lower MI than that of the control MI, respectively. The cytotoxic effects of TCM and BDCM were similar, while DBCM and TBM exhibited much lower MI values than the control, suggesting that increased bromination increases cytotoxicity. This trend is similar to the cancer slope factors (CSF) of THMs that are derived from dose–response animal studies and epidemiology. As per the Integrated Risk Information System (IRIS) by US Environmental Protection Agency (USEPA), the oral CSF of TCM, BDCM, DBCM, and TBM are 1 × 10–02, 6.21 × 10–02, 8.4 × 10–02, and 7.9 × 10–03 (mg/kg-day)−1, respectively (Parveen et al. 2022b). The corresponding value for TCAA is 7.2 × 10–02 (mg/kg-day)−1. Although TCAA has a higher CSF than TBM, the drastic change in pH due to the introduction of TCAA may contribute to its higher phytotoxicity.
Mitotic inhibition or depression (MD) is a valuable indicator of cytotoxicity, providing insights into the extent to which a toxin slows down mitosis in treated roots compared to untreated ones. The mathematical formula for MD is (MI of control—MI of treatment) / MI of control, expressed as a percentage (Alaguprathana et al. 2022). The MD of DBPs was observed to increase with dose (Table 2). Notably, the MD of BDCM was higher than that of TCM for the same increase in dose from 100 mg/L to 2000 mg/L. However, with a further increase from 2000 mg/L to 6000 mg/L, TCM-treated roots showed higher inhibition than BDCM-treated ones. In addition, the same increase in DBCM and TBM dose resulted in slightly higher inhibition of DBCM than TBM. This observation is supported by the MD of these two compounds, where DBCM exhibited a 38.5% to 56.6% increase in inhibition from 100 mg/L to 2000 mg/L (an 18.1% increase), while TBM showed a 49.8% to 65.1% increase in inhibition for the same dosage (a 15.7% increase). It is worth noting that the MD of TCAA was the highest of all DBPs, with even lower doses of TCAA than THMs resulting in the highest MD among all.
Previous research reported low MI for A. cepa root cells at lower dose of TCM (2.64 ± 0.006 at 800 mg/L), TBM (4.92 ± 1.08 at 400 mg/L), and TCAA (2.48 ± 0.01 at 200 mg/L) than what observed in the current study (Ranjan et al. 2019). The difference in MI between the previous and current studies suggest further exploration on this topic. Based on the MI values observed for several other environmental pollutants such as herbicides like quizalofop-p-ethyl (MI = 9.6 ± 0.6 at 15,000 mg/L, control = 30.2 ± 1.8), cycloxydim (MI = 15.9 ± 0.3 at 15,000 mg/L, control = 34.1 ± 1.9), and atrazine at 3 mg/L (MI showed no significant difference from the control cells), di(2-ethylhexyl)phthalate (MI = 68.47 ± 13.00 at ≈200 mg/L, control = 100.00), triclosan (MI = 1.46 ± 20.14 at ≈150 mg/L, control = 100.00), propylparaben (MI = 43.50 ± 13.08 at ≈6 mg/L, control = 100.00), bilge water containing various heavy metals, ammonia, and nitrate (MI ≈ 2 at 50% volume of bilge water, control ≈ 14), and heavy metals such as chromium (MI = 36.66 ± 1.44), copper (MI = 46.66 ± 1.44), lead (MI = 32.50 ± 0.00), and zinc (MI = 50.00 ± 0.00) at 200 mg/L (control = 60.00), indicates that the toxicity of DBPs is similar to certain herbicides and notably lower than that of heavy metals (Herrero et al. 2012; Olorunfemi et al. 2015; Abubacker and Sathya 2017; Silveira et al. 2017; Rosculete et al. 2019).
Chromosomal aberrations
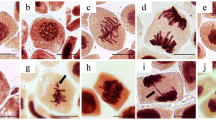
The cell cycle comprises various stages such as interphase, prophase, metaphase, anaphase, and telophase. Interphase involves growth and DNA replication, which is followed by prophase which initiates mitosis with condensed chromosomes, metaphase aligns chromosomes for cytogenesis and toxicity assessments, anaphase sees chromatids split and move to poles, and telophase clusters chromosomes, forms nuclear membranes, and initiates cytokinesis, yielding two identical cells (Boden and Ziebold 2006; Qi and Zhang 2020). The chromosomes temporarily stop moving at metaphase and only correctly aligned chromosomes enter the next stage, anaphase (Registre and Proudlock 2016). At anaphase, the non-aligned chromosomes fail to get separated properly and end up in various aberrations (Potapova and Gorbsky 2017). The normal stages of cell division in control onion cells are displayed in Fig. 4a.
a Photomicrograph of mitosis of control onion cells with normal mitotic stages; b C—metaphase; c unseparated anaphase; d lagging chromosome; e sticky metaphase; f unoriented chromosomes and bridging; g irregular anaphase; h sticky metaphase; i binucleated and extended cell; j unaligned metaphase; k unoriented chromosomes; l sticky anaphase; m prolonged prophase; n faulty polarization in telophase; o lobulated nucleus; p multipolar anaphase
Any alterations in the normal stages of mitosis are considered CA. The extent of CA was determined for A. cepa roots treated with various DBPs and control roots as a percentage of abnormal cells to the total number of cells counted.
In control roots, CA was relatively low compared to treated roots (Table 2). Most number of the CAs observed were in metaphase followed by anaphase and telophase. Different CAs included C—metaphase, sticky metaphase, unaligned metaphase, unseparated anaphase, lagging chromosome and bridging in anaphase, un-oriented chromosomes in anaphase, irregular anaphase, sticky anaphase, multipolar anaphase, binucleated and extended cell, prolonged prophase and unseparated chromatids, and unequal and faulty polarized telophase (Fig. 4). The findings in the current study align with those of Ranjan et al. (2019) and affirm that the majority of the CAs were observed in the metaphase. Additionally, their study indicated that the Cas of TCAA surpassed that of TBM and TCM similar to what observed in the present study.
DBP doses for CA analysis were decided based on the EC50 values with an increase in DBP concentration, CA was found to increase differently for different compounds. For example, the CA observed for 4000 mg/L of DBCM was higher than the CA for 6000 mg/L of TCM, BDCM, and THM4. This is even higher for TBM and TCAA (Table 2). CA was calculated as the percentage of cells with CA out of the total number of cells (Eq. 6). CA can happen only to the cells that are under mitosis. For higher doses of TBM and TCAA, the mitosis was very less (Table 2). Since there were very few cells that were dividing, the cells with CA were very less in number and hence the numerator in Eq. 6 was low compared to the rest of the doses. This explains the low CA for high doses of TBM and TCAA.
To understand the extent of aberrations, CA was further calculated against the number of cells dividing instead of the total number of cells (i.e., in Eq. 6, the denominator was ‘number of dividing cells’). In this case, the CA (%) for the tested DBP doses (doses are the same as noted in Table 2) are as follows: TCM (22.4 ± 1.0, 48.8 ± 1.2, 83.2 ± 1.8), BDCM (25.7 ± 0.6, 54.9 ± 0.6, 87.1 ± 2.7), DBCM (32.0 ± 1.0, 66.9 ± 0.5, 95.8 ± 1.3), TBM (48.4 ± 1.1, 85.7 ± 1.7, 95.3 ± 1.2), THM4 (21.6 ± 0.9, 44.9 ± 1.5, 84.8 ± 2.0), and TCAA (47.5 ± 0.8, 81.0 ± 4, 87.9 ± 6.7).
DNA laddering assay of Allium cepa
Figure 5 displays the DNA ladder of A. cepa root cells that are subjected to increasing concentrations of DBPs, harvested from specific doses based on the EC50. The brightest bands in the ladder correspond to the highest concentration of a particular DNA fragment size. In the control cells, the bright, thick fragment at the top of the agarose gel indicates intact DNA. DNA shearing was observed for root tip cells treated with several DBPs, indicating genotoxicity, DNA fragmentation, and cell death. Among the five DBPs tested, TBM-treated roots exhibited the darkest (completely smeared) ladders. A bright patch at the bottom of lane 5 in Fig. 5b indicates fragmentation into smaller pieces in roots treated with 100 mg/L TBM, which disappears in lane 6 which represent roots treated with 500 mg/L. The DNA ladders of BDCM (Fig. 5a, Lane 5 – 7) and DBCM (Fig. 5b, Lane 2,3) were visually similar indicating similarity in genotoxicity. These findings are consistent with previous studies demonstrating DNA fragmentation caused by TCM, TBM, and TCAA (Ranjan et al. 2019).
The DNA laddering assay of DBPs showed the genotoxicity and subsequent apoptosis cell death in A. cepa roots. Apoptosis and necrosis are two different types of cell deaths that have fundamental differences in their mechanisms and consequences. Apoptosis is an active, programmed process that avoids inflammation, while necrosis is a passive, accidental cell death that results in the uncontrolled release of inflammatory cellular contents (Bortner et al. 1995). Cells undergoing apoptosis undergo non-random DNA cleavage, resulting in the characteristic ladder pattern of fragmented DNA, whereas non-apoptotic cells exhibit a single, large DNA fragment (Fink and Cookson 2005). Similarly, cells undergoing necrosis have random and general cleavage as they are completely digested and appears smeared in agarose gel electrophoresis (Maines 2002). However, according to Fink and Cookson (2005), necrosis pertains to the observable features in cells once they have perished and stabilized in their environment, and apoptotic bodies may lose their structure and progress to a secondary stage known as apoptotic necrosis. Even these cells are characterized by a smeared DNA ladder. Therefore, in the case of TBM, the smeared ladder might be representing either direct and sudden cell death or apoptotic necrosis. The presence of bright patch at the end of Lane 5 in Fig. 5b suggest towards the second possibility as the cells were fragmented (apoptosis) first and were then digested (apoptotic necrosis). In this context, it could be understood that the genotoxicity and cell death due to TBM occurred faster than the other DBPs as TBM-treated cells underwent apoptosis and necrosis while the others are still at the apoptosis stage.
Hydrogen peroxide content in Allium cepa roots
Plants may generate H2O2 as a result of various stressors such as UV stress, pathogen invasion, toxic contaminants, or oxygen shortage, leading to oxidative stress and cellular damage (Blokhina et al. 2003; Singh et al. 2007). At lower concentrations, H2O2 acts as a signaling molecule to help plants acclimatize to abiotic stressors, while at higher concentrations, it can be fatal, causing cell damage and death. Figure 6 shows the accumulation of H2O2 in A. cepa roots at different doses of DBPs. The results of this study align with previous findings by Ranjan et al. (2019) which revealed a progressive increase in H2O2 concentration with an increase in plant stressor (DBP) doses. Conversely, Singh et al. (2007) observed no significant change in the H2O2 concentration of V. radiata cells when exposed to arsenic. This indicates the potential for H2O2 to function as a signal molecule, triggering the defense response in plants to avoid cell damage and reducing further H2O2 production. Therefore, the increasing H2O2 concentration in the current study indicates cell damage due to DBP-induced H2O2 production. Understanding the significance of H2O2 in plant growth and the cell cycle requires clarification of its production in plant cells under abiotic stress.
Plants produce reactive oxygen species, also known as reactive oxygen intermediates (ROIs), when under abiotic stress. ROIs are partially reduced forms of atmospheric oxygen that are traditionally believed to be highly reactive, toxic byproducts of aerobic metabolism, and can exist as free radical (O2¯·, OH·) or non-radical molecular (H2O2, 1O2) forms. Unlike molecular oxygen, ROIs are highly oxidative and can damage various cellular components, including proteins, lipids, carbohydrates, and DNA (Mittler 2002; Gill and Tuteja 2010). Recent studies confirm the role of RIOs as signaling molecules in controlling programmed cell death, pathogen defense, and abiotic stress response. Thus, RIOs serve as dual-purpose cellular indicators of stress and secondary messengers in stress-response signal transduction pathways (Mittler 2002). To serve these roles, cells must maintain a minimum ROI concentration. Plants use at least two different scavenging mechanisms to achieve this balance: maintaining low levels of ROIs for signalling applications and eliminating excess ROIs for cellular detoxification. Balancing these mechanisms determines the level of ROIs, especially with H2O2, which serves as the primary scavenging ROI in plant cells. Therefore, any increase in its concentration disrupts cellular components (Polle 2001; Asada 2006).
Although the tested doses of DBPs may not occur naturally in the environment, there is a potential for unusually high concentrations of these compounds to form in highly chlorinated reclaimed water and discharges from wastewater treatment plants, which are often used for irrigation (Munasinghe-Arachchige et al. 2021; Hong et al. 2022; Liang et al. 2022; Souza-Chaves et al. 2022; Xiao et al. 2023). Consequently, plants grown in such conditions should be monitored for phytotoxicity. In addition, it is important to investigate the potential accumulation of DBPs in plants, particularly in edible plant parts. Plants grown with reclaimed water need to be assessed for potential risks associated with the ingestion of these compounds.
Conclusions
The present study established the phytotoxicity of TCM, BDCM, DBCM, TBM, and their mixture, as well as of TCAA. High doses of DBPs negatively impacted seed germination and root growth of V. radiata and A. cepa. The observed increasing toxicity with higher DBP concentrations emphasizes the need for careful monitoring of DBP concentrations in the environment to prevent potential harm to plants and ecosystems. The presence of CAs in onion root cells indicated cytotoxicity and the rising H2O2 concentration suggested oxidative stress and cell disruption. The individual THMs posed higher toxicity to both plant species than their mixture. Based on the root inhibition assay and mitosis assay results, TCAA was found to be the most toxic of all five DBPs tested, followed by TBM, DBCM, BDCM, and TCM. This implies that brominated THMs were more toxic to plants than TCM. The study provided suggestive evidence of the genotoxicity of various DBPs, particularly TBM and TCAA. Although the tested DBP doses are unlikely to occur in the natural environment, this study offers some insights into the phytotoxicity of DBPs and enables a comparative assessment of individual THMs and their phytotoxicity.
Data availability
All data used while preparing this manuscript is available on request.
References
Aboyeji CM, Dunsin O, Adekiya AO, Suleiman KO, Chinedum C, Okunlola FO, Joseph A, Ejue SW, Adesola OO, Olofintoye TAJ, Owolabi IO (2020) Synergistic and antagonistic effects of soil applied P and Zn fertilizers on the performance, minerals and heavy metal composition of groundnut. Open Agric 5:1–9. https://doi.org/10.1515/opag-2020-0002
Abubacker MN, Sathya C (2017) Genotoxic effect of heavy metals Cr, Cu, Pb and Zn using Allium cepa L. Biosci Biotechnol Res Asia 14:1181–1186. https://doi.org/10.13005/bbra/2559
Akande B, Fatoki O, Odendaal J, Marnewick J, Ndakidemi P (2016) The halogen effects of disinfectant by-products on nutrient concentration, oxidative stress, fatty acids and α-tocopherol concentrations in membrane lipids of two Solanum lycopersicum cultivars. Theor Exp Plant Physiol 28:255–271. https://doi.org/10.1007/s40626-016-0060-z
Alaguprathana M, Poonkothai M, Al-Ansari MM, Al-Humaid L, Kim W (2022) Cytogenotoxicity assessment in Allium cepa roots exposed to methyl orange treated with Oedogonium subplagiostomum AP1. Environ Res 213:113612. https://doi.org/10.1016/j.envres.2022.113612
Albolafio S, Marín A, Allende A, García F, Simón-Andreu PJ, Soler MA, Gil MI (2022) Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 288:132583. https://doi.org/10.1016/j.chemosphere.2021.132583
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Aziz AA, Lim KB, Rahman EKA, Nurmawati MH, Zuruzi AS (2020) Agar with embedded channels to study root growth. Sci Rep 10:14231. https://doi.org/10.1038/s41598-020-71076-w
Banerjee P, Satapathy M, Mukhopahayay A, Das P (2014) Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess 1:3. https://doi.org/10.1186/s40643-014-0003-y
Bansal OP (2018) The influence of potentially toxic elements on soil biological and chemical properties, metals in soil - contamination and remediation. IntechOpen. https://doi.org/10.5772/intechopen.81348
Bansal S, Hallsby G, Löfvenius MO, Nilsson M-C (2013) Synergistic, additive and antagonistic impacts of drought and herbivory on Pinus sylvestris: leaf, tissue and whole-plant responses and recovery. Tree Physiol 33:451–463. https://doi.org/10.1093/treephys/tpt019
Bewley J (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Blokhina O, Virolanen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194. https://doi.org/10.1093/aob/mcf118
Boden C, Ziebold U (2006) Cell division. In: Encyclopedic reference of genomics and proteomics in molecular medicine. Springer, Berlin, Heidelberg, pp 233–236. https://doi.org/10.1007/3-540-29623-9_5690
Bortner CD, Oldenburg NB, Cidlowski JA (1995) The role of DNA fragmentation in apoptosis. Trends Cell Biol 5:21–26. https://doi.org/10.1016/s0962-8924(00)88932-1
Carter RAA, Allard S, Croué J-P, Joll CA (2019) 500 days of swimmers: the chemical water quality of swimming pool waters from the beginning. Environ Sci Pollut Res 26:29110–29126. https://doi.org/10.1007/s11356-019-05861-0
Chu W, Fang C, Deng Y, Xu Z (2021) Intensified disinfection amid COVID-19 pandemic poses potential risks to water quality and safety. Environ Sci Technol 55:4084–4086. https://doi.org/10.1021/acs.est.0c04394
Cui H, Chen B, Jiang Y, Tao Y, Zhu X, Cai Z (2021) Toxicity of 17 disinfection by-products to different trophic levels of aquatic organisms: ecological risks and mechanisms. Environ Sci Technol 55:10534–10541. https://doi.org/10.1021/acs.est.0c08796
Dong F, Zhu J, Li J, Fu C, He G, Lin Q, Li C, Song S (2023) The occurrence, formation and transformation of disinfection byproducts in the water distribution system: a review. Sci Total Environ 867:161497. https://doi.org/10.1016/j.scitotenv.2023.161497
Fahr M, Laplaze L, Bendaou N, Hocher V, El Mzibri M, Bogusz D, Smouni A (2013) Effect of lead on root growth. Front Plant Sci 4:7. https://doi.org/10.3389/fpls.2013.00175
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907–1916. https://doi.org/10.1128/IAI.73.4.1907-1916.2005
Ghosh M, Bandyopadhyay M, Mukherjee A (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere 81:1253–1262. https://doi.org/10.1016/j.chemosphere.2010.09.022
Ghosh M, Bhadra S, Adegoke A, Bandyopadhyay M, Mukherjee A (2015) MWCNT uptake in Allium cepa root cells induces cytotoxic and genotoxic responses and results in DNA hyper-methylation. Mutat Res Mol Mech Mutagen 774:49–58. https://doi.org/10.1016/j.mrfmmm.2015.03.004
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gordon AR, Richardson TL, Pinckney JL (2015) Ecotoxicology of bromoacetic acid on estuarine phytoplankton. Environ Pollut 206:369–375. https://doi.org/10.1016/j.envpol.2015.07.014
Grant WF (1982) Chromosome aberration assays in allium: a report of the U.S. environmental protection agency gene-tox program. Mutat Res Genet Toxicol 99:273–291. https://doi.org/10.1016/0165-1110(82)90046-X
Gray A, Kuffel A, Daeid NN (2023) An improved rapid method for DNA recovery from cotton swabs. Forensic Sci Int Genet 64:102848. https://doi.org/10.1016/j.fsigen.2023.102848
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Guillemaut P, Maréchal-Drouard L (1992) Isolation of plant DNA: a fast, inexpensive, and reliable method. Plant Mol Biol Report 10:60–65. https://doi.org/10.1007/BF02669265
Herrero O, Pérez Martín JM, Fernández Freire P, Carvajal López L, Peropadre A, Hazen MJ (2012) Toxicological evaluation of three contaminants of emerging concern by use of the Allium cepa test. Mutat Res Toxicol Environ Mutagen 743:20–24. https://doi.org/10.1016/j.mrgentox.2011.12.028
Hong N, Li Y, Liu J, Yang M, Liu A (2022) A snapshot on trihalomethanes formation in urban stormwater: implications for its adequacy as an alternative water resource. J Environ Chem Eng 10:107180. https://doi.org/10.1016/j.jece.2022.107180
Heng B, Zhang R, Wang Z, Zhang Y, Wang Y, Song Z, Liu C, Sun D, Qi F (2021) Occurrence and risk assessment of volatile halogenated disinfection by-products in an urban river supplied by reclaimed wastewater. Ecotoxicol Environ Saf 211:111912. https://doi.org/10.1016/j.ecoenv.2021.111912
Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT (2016) Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Chang Biol 22:180–189. https://doi.org/10.1111/gcb.13028
Kali S, Khan M, Ghaffar MS, Rasheed S, Waseem A, Iqbal MM, BilalKhanNiazi M, Zafar MI (2021) Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: a comprehensive review. Environ Pollut 281:116950. https://doi.org/10.1016/j.envpol.2021.116950
Kannan A, Upreti RK (2008) Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J Hazard Mater 153:609–615. https://doi.org/10.1016/j.jhazmat.2007.09.004
Kaya M, Çavuşoğlu K, Yalçin E, Acar A (2023) DNA fragmentation and multifaceted toxicity induced by high-dose vanadium exposure determined by the bioindicator Allium test. Sci Rep 13:8493. https://doi.org/10.1038/s41598-023-35783-4
Kuloğlu SS, Yalçin E, Çavuşoğlu K, Acar A (2022) Dose-dependent toxicity profile and genotoxicity mechanism of lithium carbonate. Sci Rep 12:13504. https://doi.org/10.1038/s41598-022-17838-0
Kumar M, Shekhar S, Kumar R, Kumar P, Govarthanan M, Chaminda T (2023) Drinking water treatment and associated toxic byproducts: concurrence and urgence. Environ Pollut 320:121009. https://doi.org/10.1016/j.envpol.2023.121009
Kumari M, Mukherjee A, Chandrasekaran N (2009) Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ 407:5243–5246. https://doi.org/10.1016/j.scitotenv.2009.06.024
Lee PY, Costumbrado J, Hsu C-Y, Kim YH (2012) Agarose gel electrophoresis for the separation of DNA fragments. JoVE J Vis Exp:e3923. https://doi.org/10.3791/3923
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res Mutat Res 682:71–81. https://doi.org/10.1016/j.mrrev.2009.06.002
Li M, Song N, Song X, Liu J, Su B, Chen X, Guo X, Li M, Zong Q (2022) Investigating and modeling the toxicity of arsenate on wheat root elongation: assessing the effects of pH, sulfate and phosphate. Ecotoxicol Environ Saf 239:113633. https://doi.org/10.1016/j.ecoenv.2022.113633
Li Z, Song G, Bi Y, Gao W, He A, Lu Y, Wang Y, Jiang G (2021) Occurrence and distribution of disinfection byproducts in domestic wastewater effluent, tap water, and surface water during the SARS-CoV-2 pandemic in China. Environ Sci Technol 55:4103–4114. https://doi.org/10.1021/acs.est.0c06856
Liang J-K, Lu Y, Song Z-M, Ye B, Wu Q-Y, Hu H-Y (2022) Effects of chlorine dose on the composition and characteristics of chlorinated disinfection byproducts in reclaimed water. Sci Total Environ 824:153739. https://doi.org/10.1016/j.scitotenv.2022.153739
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250. https://doi.org/10.1016/j.envpol.2007.01.016
Liu J, Hu L-X, Deng W-J, Ying G-G, Hong H, Tsang EPK, Barceló D (2022) Pilot study of pollution characteristics and ecological risk of disinfection byproducts in natural waters in Hong Kong. Environ Toxicol Chem 41:2613–2621. https://doi.org/10.1002/etc.5449
Liu J, Zhang X (2014) Comparative toxicity of new halophenolic DBPs in chlorinated saline wastewater effluents against a marine alga: halophenolic DBPs are generally more toxic than haloaliphatic ones. Water Res 65:64–72. https://doi.org/10.1016/j.watres.2014.07.024
Liu Z, Bai Y, Luo L, Wan J, Wang W, Zhao G (2021) Effects of high dose copper on plant growth and mineral nutrient (Zn, Fe, Mg, K, Ca) uptake in spinach. Environ Sci Pollut Res 28:37471–37481. https://doi.org/10.1007/s11356-021-13395-7
Luo X, Wang X, Tang Y, Liu Y, Wang Y (2021) Using quantitative ion character–activity relationship (QICAR) method in evaluation of metal toxicity toward wheat. Ecotoxicol Environ Saf 221:112443. https://doi.org/10.1016/j.ecoenv.2021.112443
Luo Y, Liang J, Zeng G, Chen M, Mo D, Li G, Zhang D (2018) Seed germination test for toxicity evaluation of compost: its roles, problems and prospects. Waste Manag 71:109–114. https://doi.org/10.1016/j.wasman.2017.09.023
Ma H, Pineda A, van der Wurff AWG, Bezemer TM (2018) Synergistic and antagonistic effects of mixing monospecific soils on plant-soil feedbacks. Plant Soil 429:271–279. https://doi.org/10.1007/s11104-018-3694-6
Macar O, KalefetoğluMacar T, Yalçın E, Çavuşoğlu K (2022) Acute multiple toxic effects of Trifloxystrobin fungicide on Allium cepa L. Sci Rep 12:15216. https://doi.org/10.1038/s41598-022-19571-0
Maines MD (2002) [32] - heme oxygenase 1 Transgenic mice as a model to study neuroprotection. In: Sen CK, Packer L (eds) Methods in enzymology, redox cell biology and genetics part B. Academic Press, pp 374–388. https://doi.org/10.1016/S0076-6879(02)53062-7
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Munasinghe-Arachchige SP, Sparks KM, Ahmed A, Delanka-Pedige HMK, Abeysiriwardana-Arachchige ISA, Schaub T, Trainor PJ, Nirmalakhandan N (2021) Algal-based wastewater treatment: evaluation of nitrogenous byproducts in chlorinated effluent. Algal Res 59:102443. https://doi.org/10.1016/j.algal.2021.102443
Nadeem H, Khan A, Gupta R, Hashem M, Alamri S, Siddiqui MA, Ahmad F (2023) Stress combination: when two negatives may become antagonistic, synergistic or additive for plants? Pedosphere 33:287–300. https://doi.org/10.1016/j.pedsph.2022.06.031
Narzary D, Verma S, Mahar KS, Rana TS (2015) A rapid and effective method for isolation of genomic DNA from small amount of silica-dried leaf tissues. Natl Acad Sci Lett 38:441–444. https://doi.org/10.1007/s40009-015-0357-5
Nefic H, Musanovic J, Metovic A, Kurteshi K (2013) Chromosomal and nuclear alterations in root tip cells of Allium cepa L. induced by Alprazolam. Med Arch 67:388–392. https://doi.org/10.5455/medarh.2013.67.388-392
Olorunfemi DI, Duru L, Olorunfemi OP (2015) Genotoxic effects of bilge water on mitotic activity in Allium cepa L. Caryologia 68:265–271. https://doi.org/10.1080/00087114.2015.1032574
Parveen N, Chowdhury S, Goel S (2022a) Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-18316-2
Parveen N, Ranjan VP, Chowdhury S, Goel S (2022b) Occurrence and potential health risks due to trihalomethanes and microplastics in bottled water. Environ Eng Sci. https://doi.org/10.1089/ees.2021.0295
Petriccione M, Forte V, Valente D, Ciniglia C (2013) DNA integrity of onion root cells under catechol influence. Environ Sci Pollut Res 20:4859–4871. https://doi.org/10.1007/s11356-012-1422-y
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. computer simulations as a step towards flux analysis. Plant Physiol 126:445–462
Potapova T, Gorbsky GJ (2017) The consequences of chromosome segregation errors in mitosis and meiosis. Biology 6:12. https://doi.org/10.3390/biology6010012
Qi F, Zhang F (2020) Cell cycle regulation in the plant response to stress. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01765
Qiao Y, Zhang Y, Xu S, Yue S, Zhang X, Liu M, Sun L, Jia X, Zhou Y (2022) Multi-leveled insights into the response of the eelgrass Zostera marina L to Cu than Cd exposure. Sci Total Environ 845:157057. https://doi.org/10.1016/j.scitotenv.2022.157057
Qin L, Wang L, Sun X, Yu L, Wang M, Chen S (2022) Ecological toxicity (ECx) of Pb and its prediction models in Chinese soils with different physiochemical properties. Sci Total Environ 853:158769. https://doi.org/10.1016/j.scitotenv.2022.158769
Ranjan J, Mandal T, Mandal DD (2021) Mechanistic insight for DBP induced growth inhibition in Vigna radiata via oxidative stress and DNA damage. Chemosphere 263:128062. https://doi.org/10.1016/j.chemosphere.2020.128062
Ranjan J, Mandal T, Mandal DD (2019) Environmental risk appraisement of disinfection by-products (DBPs) in plant model system: Allium cepa. Environ Sci Pollut Res 26:8609–8622. https://doi.org/10.1007/s11356-019-04262-7
Registre M, Proudlock R (2016) Chapter 7 - the in vitro chromosome aberration test. In: Proudlock R (ed) Genetic toxicology testing. Academic Press, Boston, pp 207–267. https://doi.org/10.1016/B978-0-12-800764-8.00007-0
Rietra RPJJ, Heinen M, Dimkpa CO, Bindraban PS (2017) Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun Soil Sci Plant Anal 48:1895–1920. https://doi.org/10.1080/00103624.2017.1407429
Ronzan M, Piacentini D, Fattorini L, Della Rovere F, Eiche E, Riemann M, Altamura MM, Falasca G (2018) Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ Exp Bot 151:64–75. https://doi.org/10.1016/j.envexpbot.2018.04.008
Rosculete CA, Bonciu E, Rosculete E, Olaru LA (2019) Determination of the environmental pollution potential of some herbicides by the assessment of cytotoxic and genotoxic effects on Allium cepa. Int J Environ Res Public Health 16:75. https://doi.org/10.3390/ijerph16010075
Sabeen M, Mahmood Q, Ahmad Bhatti Z, Faridullah, Irshad M, Bilal M, Hayat MT, Irshad U, Ali Akbar T, Arslan M, Shahid N (2020) Allium cepa assay based comparative study of selected vegetables and the chromosomal aberrations due to heavy metal accumulation. Saudi J Biol Sci 27:1368–1374. https://doi.org/10.1016/j.sjbs.2019.12.011
Sardar R, Ahmed S, Yasin NA (2022) Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ Pollut 292:118373. https://doi.org/10.1016/j.envpol.2021.118373
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci 81:8014–8018. https://doi.org/10.1073/pnas.81.24.8014
Sharma AK, Sharma A (1980) Chromosome techniques, 3rd edn. Butterworths, London
Sidhu JS, Ali M, Al-Rashdan A, Ahmed N (2019) Onion (Allium cepa L.) is potentially a good source of important antioxidants. J Food Sci Technol 56:1811–1819. https://doi.org/10.1007/s13197-019-03625-9
Silveira GL, Lima MGF, dos Reis GB, Palmieri MJ, Andrade-Vieria LF (2017) Toxic effects of environmental pollutants: comparative investigation using Allium cepa L. and Lactuca sativa L. Chemosphere 178:359–367. https://doi.org/10.1016/j.chemosphere.2017.03.048
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73. https://doi.org/10.1007/s10725-007-9205-z
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517. https://doi.org/10.1016/S0022-2836(75)80083-0
Souza-Chaves BM, Alhussaini MA, Felix V, Presson LK, Betancourt WQ, Hickenbottom KL, Achilli A (2022) Extending the life of water reuse reverse osmosis membranes using chlorination. J Membr Sci 642:119897. https://doi.org/10.1016/j.memsci.2021.119897
Sumi M, Nevaditha NT, Sindhu Kumari B (2023) Synthesis, spectroscopic investigation and bioactivities of metal complexes from curcuma longa derivative. Inorganica Chim Acta 549:121397. https://doi.org/10.1016/j.ica.2023.121397
Tai TH, Tanksley SD (1990) A rapid and inexpensive method for isolation of total DNA from dehydrated plant tissue. Plant Mol Biol Report 8:297–303. https://doi.org/10.1007/BF02668766
Vanapalli KR, Bhattacharya J, Samal B, Chandra S, Medha I, Dubey BK (2021) Single-use LDPE - Eucalyptus biomass char composite produced from co-pyrolysis has the properties to improve the soil quality. Process Saf Environ Prot 149:185–198. https://doi.org/10.1016/j.psep.2020.10.051
Vujčić Bok V, Gerić M, Gajski G, Gagić S, Domijan A-M (2023) Phytotoxicity of bisphenol a to allium cepa root cells is mediated through growth hormone gibberellic acid and reactive oxygen species. Molecules 28:2046. https://doi.org/10.3390/molecules28052046
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. https://doi.org/10.1016/j.envexpbot.2007.05.011
Wang L, Song S, Xu L, Graham NJD, Yu W (2022a) Beneficial role of pre- and post-ozonation in a low rate biofiltration-ultrafiltration process treating reclaimed water. Water Res 226:119284. https://doi.org/10.1016/j.watres.2022.119284
Wang G, Yang Y, Kong Y, Ma R, Yuan J, Li G (2022b) Key factors affecting seed germination in phytotoxicity tests during sheep manure composting with carbon additives. J Hazard Mater 421:126809. https://doi.org/10.1016/j.jhazmat.2021.126809
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535. https://doi.org/10.1093/nar/18.22.6531
Xiao R, Ou T, Ding S, Fang C, Xu Z, Chu W (2023) Disinfection by-products as environmental contaminants of emerging concern: a review on their occurrence, fate and removal in the urban water cycle. Crit Rev Environ Sci Technol 53:19–46. https://doi.org/10.1080/10643389.2022.2043101
Xie K, Cakmak I, Wang S, Zhang F, Guo S (2021) Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J 9:249–256. https://doi.org/10.1016/j.cj.2020.10.005
Yasuda M, Ishikawa A, Jikumaru Y, Seki M, Umezawa T, Asami T, Maruyama-Nakashita A, Kudo T, Shinozaki K, Yoshida S, Nakashita H (2008) Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in arabidopsis. Plant Cell 20:1678–1692. https://doi.org/10.1105/tpc.107.054296
You J, Park H, Lee H, Jang K, Park J, Na S (2023) Sensitive and selective DNA detecting electrochemical sensor via double cleaving CRISPR Cas12a and dual polymerization on hyperbranched rolling circle amplification. Biosens Bioelectron 224:115078. https://doi.org/10.1016/j.bios.2023.115078
Yuan J, Chadwick D, Zhang D, Li G, Chen S, Luo W, Du L, He S, Peng S (2016) Effects of aeration rate on maturity and gaseous emissions during sewage sludge composting. Waste Manag 56:403–410. https://doi.org/10.1016/j.wasman.2016.07.017
Zandalinas SI, Mittler R (2022) Plant responses to multifactorial stress combination. New Phytol 234:1161–1167. https://doi.org/10.1111/nph.18087
Zhang J, Hamill AS, Weaver SE (1995) Antagonism and synergism between herbicides: trends from previous studies. Weed Technol 9:86–90. https://doi.org/10.1017/S0890037X00023009
Zhang P, Luo Q, Wang R, Xu J (2017) Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci Rep 7:868. https://doi.org/10.1038/s41598-017-01046-2
Acknowledgements
We thank Anuja Joseph and Aishwarya Rastogi for their help during experiments.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Naseeba Parveen: Conceptualization, Methodology, Investigation, Writing- Original draft preparation, Visualisation. Papiya Mondal: Methodology, Investigation, Validation. Kumar Raja Vanapalli: Methodology, Formal analysis, Resources. Abhijit Das: Resources. Supervision. Sudha Goel: Methodology. Formal analysis. Supervision. Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Informed consent
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Gangrong Shi
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parveen, N., Mondal, P., Vanapalli, K.R. et al. Phytotoxicity of trihalomethanes and trichloroacetic acid on Vigna radiata and Allium cepa plant models. Environ Sci Pollut Res 31, 5100–5115 (2024). https://doi.org/10.1007/s11356-023-31419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-31419-2