Abstract
Industrial effluents contain hazardous substances that can be a serious threat to the agriculture and human health. In the present study, the cytotoxic and genotoxic impacts of agricultural soil from the industrial area of Dera Bassi (Punjab, India) have been evaluated. Assays such as defects in DNA repair in K-12 mutants of Escherichia coli and chromosomal aberrations in Allium cepa were used to estimate the acute toxicity and chromosomal mutagenesis, respectively. Atomic absorption spectrometry and GC-MS analysis revealed contamination of the soil with high concentrations of heavy metals and organic compounds, respectively. Dichloromethane extract of site I soil sample caused maximum damage to 40 μL mL−1 DNA repair defective mutants and showed 38 and 49% survival in lexA and recA mutants, respectively, which was least among all the sites. In A. cepa test, an inverse relationship between soil extract concentration and the mitotic index was observed. Exposure of growing roots of A. cepa to soil extracts induced chromosomal abnormalities and alterations in mitotic phases in root tip cells. The study concludes that agricultural sites near the industrial area were contaminated with genotoxic and mutagenic compounds. Hence, adequate measures should be taken to reduce the toxicity of industrial effluents discharged onto the agricultural fields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increased industrialization has escalated the volume and frequency of wastewater discharge into the ecosystem. Owing to inefficient treatment and improper waste disposal, several toxic compounds including heavy metals and polyaromatic hydrocarbons are released into the soil by industrial activities and modern agricultural practices (Ho et al., 2012; Anh et al., 2019). These hazardous substances pose a serious threat to soil biota, plants, and animals including humans, thereby affecting the health of the receiving ecosystem (FAO & UNEP, 2021). Exposure to heavy metals impedes proper functioning of brain, kidney, liver, and lungs causing muscular and mental degenerative diseases in humans (Reddy & Osborne, 2020). Overexposure to toxic organic compounds leads to lung problems, weakness, and damaged immune system, and it can be fatal even at low concentrations (Raza et al., 2019). Agricultural lands adjacent to industries have higher risk of accumulation of toxic compounds through direct and indirect ways, from where these pollutants can enter the food chain (Datta et al., 2018; Husejnovic et al., 2018). In fact, world’s soil is under great pressure due to pollution (FAO & UNEP, 2021). It is thus pertinent to undertake genotoxic risk assessment of agricultural soil contaminated due to industrial pollution (Xiao et al., 2006; FAO & UNEP, 2021). The effect of pollutants in the soil varies due to synergistic and antagonist actions; it is, therefore, not advisable to undertake physicochemical methods for risk assessment of contaminated soil (Jensen & Pedersen, 2006). The toxicity of industrial waste could be evaluated using in vivo assays, but these are limited due to economical, temporal, and ethical factors. Therefore, in vitro studies employing both prokaryotic and eukaryotic systems can overcome these limitations as it detects DNA damage from point mutations to chromosomal alterations (Khan et al., 2019). Fernandez et al. (2005) opined that bioassay involving biological organisms should be used along with chemical analysis for genotoxic risk assessment of soil contaminants.
Among the various bacterial genotoxicity methods, DNA repair assays are the most widely used ones (Maslowska et al., 2019). These generally determine the level of DNA damage, thus approximating the genotoxic/mutagenic potential of a compound or test sample. Induction of SOS repair system in bacteria and exposure to DNA damaging agents is another test for genotoxicity (Simmons et al., 2008). There is a high degree of correlation in DNA repair system in bacteria with higher organisms (Ronen & Glickman, 2001). Escherichia coli has been employed as a model organism to study DNA repair genes in eukaryotes (Augusto-Pinto et al., 2003). SOS response system in E. coli involves a set of about 50 co-regulated genes (Maslowska et al., 2019), which are induced in response to the loss of genetic integrity due to DNA damaging agents and inhibition of replication. SOS response is initiated by single-stranded DNA and involves two genes, repressor recA and an inducer lexA protein, which play a key role in repair of DNA (Maslowska et al., 2019). However, the mutant strains lacking SOS response are overly sensitive to DNA damage by mutagenic chemicals (Kuzminov, 1999).
To screen and monitor the toxicity of environmental pollutants, plant bioassays are also well acknowledged (de Souza et al., 2016). Among them, the Allium cepa (onion) is used widely to study the genotoxicity because of its easy handling, low cost, greater sensitivity, reliability, and high correlation with other test systems (Pasha et al., 2015). A. cepa bioassay has been regarded as a bioindicator of pollutants and genotoxicity (Tedesco & Laughinghouse, 2012; de Souza et al., 2016).
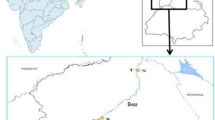
Dera Bassi (Mohali, Punjab, India) is an industrial town located in foothills of Shiwaliks in north India, with humid sub-tropical climate, and has an average elevation of 321 m above mean sea level (Sharma et al., 2021). It houses around 300 industries including chemical, pharmaceutical, electroplating, paper mills, textile, dyeing, brewery, paint, dairy, and meat etc. There have been reports of discharge of untreated industrial effluents directly onto the nearby drains/rivers and agricultural soils, which prompted National Green Tribunal (NGT) of India to shut down several industries (Dogra, 2018). Despite the release of harmful effluents from the industries in the region, no study has undertaken the cyto- and genotoxic risk assessment of the polluted agricultural soils. We, therefore, conducted a study to investigate the cytotoxicity and genotoxicity of contaminated agricultural soils from this region. The toxicity was evaluated using bacterial genotoxicity assays in terms of survivability of SOS defective K-12 mutants of E. coli and chromosomal aberrations in A. cepa root tips.
Materials and methods
Soil collection and analyses
The study was conducted on the soils collected from agricultural fields around the industrial hub, Dera Bassi (30.58° N 76.84° E; Punjab, India). Soil samples were collected at 0−15 cm depth from four sites (sites I, II, III, and IV). The soil samples were analyzed for physicochemical parameters and heavy metal content. Soil properties such as pH (1:2 soil/water, v/v), NH4+-N (kg ha−1), organic carbon (%), phosphates (kg ha−1), NO3‒-N (kg ha−1), and available K (kg ha−1) were determined as per the methods given by Tandon (1993). To evaluate the metal content in collected soil, samples were dried in an oven at 40 °C, grounded, sieved (0.1 mm), and converted into ash at 450 °C. A slurry was prepared by adding 1 mL of distilled water to 1 g of ash followed by the addition of HCl and HNO3 acid (3:1, v/v). The samples were digested by heating the flask until the solution became clear. The clear solution was concentrated to 1 mL, and volume was made up to 100 mL using double-distilled water. Then, it was filtered successively through Whatman no. 1 and no. 42 filters. The digested samples were examined through atomic absorption spectroscopy (GBC 932 Plus, Australia), and various heavy metals were quantified.
Extraction of soil samples
Solvent extraction of soil samples with dichloromethane (DCM; HPLC grade) was carried out as per Knize et al. (1987). For this, 10 mL of DCM was added to 10 g of soil, and the mixture was centrifuged (at 5000 g, for 10 min). The supernatant was collected and evaporated completely. The residues were dissolved in 1 mL of dimethyl sulfoxide. The solution was filtered through 0.45 µm nylon filter and stored at −20 °C for analysis.
GC-MS analysis
Soil extracts were analyzed for the presence of organic compounds using GC-MS (Thermo Trace 1300 GC with Triple Quadrupole MS, Thermo Fischer Scientific, USA) fitted with TG 5 column (30 m × 0.25 mm × 25 µm). The operating conditions were 250 °C injector temperature, sample injection at 1:15 split ratio, Helium as carrier gas at 1 mL min‒1, initial oven set at 60 °C for 2 min, increased to 280 °C at 10 °C min‒1, and held for another 10 min. The mass spectra were obtained in the range of m/z 50−600 amu. The fragmentation patterns of the major peaks were compared with National Institute of Standards and Technology library (NIST 2.0) to identify the compounds present in the different soil extracts.
Exposure of E. coli mutants
SOS response of DNA repair mechanism was studied using recA and lexA mutants of E. coli. The mutants and the isogenic wild-type strains were collected from an overnight-grown culture having 100−300 × 106 cells mL−1. The bacterial pellets were dissolved in a solution of 10 mM MgSO4. Then, 40 µL of soil extract was added. Samples were obtained at 2-, 4-, and 6-h intervals. These were diluted, and their CFA (colony-forming ability) was assayed in an incubator set at 37 °C for overnight. A parallel control, with solvent only, was maintained simultaneously.
Allium cepa anaphase-telophase test
Cytotoxicity of the soil samples was tested in terms of chromosomal aberrations (CAs) and mitotic index (MI) using squash technique (Chandel et al., 2019). Onion bulbs (ϕ=1.5–2.0 cm) were procured from the local market. Prior to use, their outer scales were removed. The basal ends of the bulbs were immersed in distilled water in beakers at 25 ± 2 °C. Emerging roots (~ 1–2 cm) were harvested for determining the toxicity of soil samples. The soil extracts were mixed with distilled water (1:10, w/v) and shaken on a rotary shaker for 24 h at 25 °C (Cotelle et al., 1999). These were diluted to get working concentrations of 5%, 10%, 25%, 50%, and 100%. Parallel set-ups with distilled water served as negative control, whereas methyl methane sulfonate (MMS, 10 mg mL−1) was used as positive control. After 72 h of treatment, root apices were removed, fixed in Clarke’s solution (75 mL ethanol/25 mL acetic acid glacial) for 12 h at 4 °C. After fixation, root apices were washed in distilled water and stained with aceto-carmine.
For each soil extract, 3–4 root tips per slide were used, and three such slides were prepared as replicates. A total of 3000 cells (1000 per slide) for each treatment were analyzed under light microscope (model 66475; Getner, India) to determine MI. However, 300 dividing cells per treatment (100 per slide) were analyzed for determining CAs. According to the mechanism of action, CAs were classified as aneugenic (chromosomal breaks and bridges) resulting from spindle disturbance or clastogenic (chromosomal adherence, losses, and C-metaphases).
Statistical analysis
The CAs and MI data were expressed as percent. The data were analyzed by one-way ANOVA followed by the comparison of mean values at P≤0.05 using post hoc Duncan’s multiple range test. The statistical analyses were done using SPSS ver. 16.
Results
Soil samples were neutral in nature with pH ranging from 7.2 to 7.5 (Table 1). The soil organic carbon (0.6%) was found to be least for site III. Nitrate-nitrogen (NO3-N) of the soil samples ranged from 3.0 to 9.0 kg/ha, whereas the amount of ammonium-nitrogen (NH4-N) was highest in soil collected from site I and least in soil sample from site IV. The amount of available P and available K was in the range of 18–60 kg ha−1 and 90–112 kg ha−1, respectively (Table 1). The amount of Cr, Cu, Cd, Ni, and Pb ranged from 19.18 to 28.23 mg per kg, 18.6 to 30.5 mg kg−1, 0.1 to 0.3 mg kg−1, 24.5 to 45.9 mg kg−1, and 34.8 to 42.9 mg kg−1, respectively (Fig. 1). Soil extracts contained various organic compounds as revealed by GC-MS (Table 2).
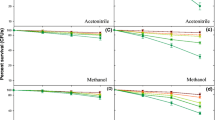
The percent survivability of E. coli mutant strains after treatment with soil extracts is presented in Fig. 2. Soil extract of site I exhibited maximum decrease in survival compared with samples from sites II, III, and IV. After 6 h of treatment with soil extracts, the survival in lexA mutants was 38% for site I extract, which was least, while survival was 41, 43, and 47% in sites II, III, and IV, respectively, as compared with isogenic wild type. For recA mutants, the highest survival (60%) was noticed in site IV soil extract followed by 54% survival in site III extract. There was not much difference in the survivability of recA mutants in soil extract of site I and site II when compared with the isogenic wild type (Fig. 2).
The effect of soil extracts on the changes in MI and frequency of mitotic phases in root meristem of A. cepa is presented in Fig. 3 and Table 3, respectively. MI was the minimum in samples exposed to MMS (positive control) and exhibited the lowest average (8.0%), while the highest MI (28.5%) was observed in negative control (distilled water). MI significantly declined in a concentration-dependent manner in all the test soil extracts compared with negative and positive controls (Fig. 3). For site I, the MI was 9.5% (P≤0.05) in response to 100% soil extracts, whereas, at the same concentration, sites II, III, and IV showed MI (P≤0.05) of 10.3, 12.4, and 13.9% respectively (Fig. 3). All the soil extracts affected the rate of each mitotic stage in A. cepa with an increase in prophase stage and a consistent decrease in the anaphase and telophase stages, thereby suggesting an antiproliferative activity of the compound (Table 3).
Changes in mitotic index in root meristems of Allium cepa, measured after 72 h of treatment with extracts of soil collected from agricultural fields near industrial hub Dera Bassi (Punjab, India). PC positive control, NC negative control. Values represented as mean ± standard deviation. Different lowercase letters represent significant difference at P≤ 0.05 applying Duncan’s multiple range test
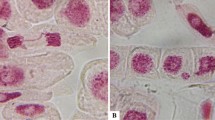
In chromosomal aberration assay, cells in anaphase-telophase of the positive control (MMS) and the treatments showed high abnormalities (P≤0.05) as compared to the negative control (Table 4). Total CAs (%) were observed to be higher in site I than that of other sites (Fig. 4). Treatment of soil extracts induced CAs such as C-mitosis, sticky chromosomes, and laggard chromosomes (Fig. 5), and the effect was concentration-dependent (Table 4).
Aberrant cells (%) induced in root meristems of Allium cepa by extracts of soil collected from agricultural fields near Dera Bassi (Punjab, India). PC positive control, NC negative control. Values represented as mean ± standard deviation. Different lowercase letters represent significant difference at P≤ 0.05 applying Duncan multiple range test
Chromosomal abnormalities in Allium cepa root-tips caused by extracts of soil collected from agricultural fields near Dera Bassi (Punjab, India). a Spindle disturbance. b Sticky chromosomes. c Laggard, d Sticky chromosomes. e Mitotic bridge. f Vagrant chromosome. g Diagonal spindle. h c-mitosis. i Morphological changes. All micrographs have the same magnification (bar = 50 μm)
Discussion
Soil is the most important non-renewable biological resource that supports plant life and is essential for the subsistence of humans. However, the increasing population coupled with rapid industrialization has put it under tremendous pressure (FAO & UNEP, 2021). In the present study, the pH of soil samples was found to be nearly neutral. Effluents with too high or low pH upon entering the soil change soil pH, hence making soil infertile. Changed pH of soil can alter the solubility of the pollutants, influence the availability of micro-nutrients, and can enhance their toxicity level (Odjadjare & Okoh, 2010). High levels of organic carbon, phosphate, NH3-N, NO3-N, and available K were observed for site I and site II soil samples, which could be due to the high volume of wastewater received by these sites when compared with site III and site IV (Osakwe, 2012).
Industrial waste generally contains hazardous heavy metals and organic compounds. High concentration of heavy metals such as Cr and Cd can cause serious morbidity and mortality. Cu and Cd are used in corrosion prevention, galvanizing iron and steel, metal plating, and motors and generators. Hence, the presence of such metals in soil may be ascribed to the untreated effluents discharged by the industries using these metals (Singh et al., 2009). The presence of Cr, Ni, Co, Cu, and Cd in the agricultural soil observed during the current investigation agreed with the earlier reports from the northwestern regions of India (Dheri et al., 2007). In the present study, GC-MS analysis of soil samples revealed the presence of esters, acids, ketones, and phenols of benzene, hexadecane, dodecane, pentadecane, pyrimidine, and hexadecanoic and octadecanoic acid. The findings corroborate previous reports, which identified similar compounds in industrial wastewater (Liang et al., 2018; Yadav et al., 2019). The derivatives of phthalate and benzene are known for carcinogenicity (Dixit et al., 2015), phenolic compounds are endocrine disruptors (Kumari et al., 2016), and hexadecanoic and octadecanoic acid are probable carcinogen and mutagens (EPA, 2004). From agriculture lands, these contaminants may enter the food chain and bioaccumulate. Abegunrin et al. (2016) argued that irrigation using wastewater could be valuable as it increases nutrients and soil fertility, which in turn enhance crop growth. However, it should be reused with caution to protect soil quality and crop yield. Keeping in mind the possible adverse effects of industrial wastewater used for irrigation, Central Pollution Control Board (CPCB, India) has advised to use treated effluents for irrigation with caution (Aggarwal, 2019).
SOS response in bacteria is a DNA repair process that is induced in response to mutagenic/DNA-damaging chemicals. Though plants do not have any SOS repair mechanism, there exist homologies in the proteins (enzymes and amino acids) involved in DNA repair in eukaryotes (Janion, 2008). Accordingly, it has been used as an important test to evaluate genotoxic impacts of mutagenic and DNA-damaging chemicals, including pollutants (Kenyon & Walker, 1981). In the present investigation, the importance of recA+ and lexA+ genes to control the harmful effects of pollutants was studied, and DNA damage in the exposed cell revealed the sensitivity of recA and lexA mutants to the test samples. In general, the substances which induce SOS response in bacteria are genotoxic to higher eukaryotes, and this is the ground for various bacterial assays for determining genotoxic and carcinogenic potential (Maron & Ames, 1983).
We evaluated both cyto- and genotoxicity in terms of several CAs including breaks, bridge formation, sticky chromosomes, and MI. Extracts of polluted soil from all the four sites significantly (P≤0.05) declined MI in a dose-response pattern. According to Tedesco and Laughinghouse (2012), chromosomal assay provides an easy, good, and reliable tool to determine cytotoxicity of a pollutant/chemical. Occurrence of a cytotoxic effect was concluded based on the change in the proportion of mitotic phases and a decline in the mitotic activity after the treatment. Numerous checkpoints in the mitotic cycle make sure of the proper distribution of genetic material. A decline in mitotic activity may either be due to DNA damage or inhibition of DNA synthesis resulting from G2 phase blocking in cells (Selmi et al., 2014). Increase in prophase might be due to the occlusion of the dividing cells at checkpoint between prophase and metaphase if microtubule structure is altered (Scolnick & Halazonetis, 2000).
Based on the results of the A. cepa test, we suggest that the soil samples contained chemical substances with cytotoxic, aneugenic, and/or clastogenic potential. Stickiness and anaphasic bridges and c-metaphase were the most frequent CAs observed in the study. These suggest chromatin dysfunction (thus disruption of chromatin organization) or spindle failure. The formation of bridges during anaphase and telophase is indicative of clastogenic effects, whereas c-metaphase suggests enhanced aneuploidy (Chandel et al., 2019). Our findings are corroborated by previous studies documenting mutagenic/genotoxic effects of soil collected from residential areas (Watanabe et al., 2008), biodiesel-polluted soil (Leme et al., 2012), pesticide-polluted soil (Datta et al., 2018), and fields with sugarcane vinasse (da Silva Souza et al., 2013).
In summary, our study reveals that the agricultural site near the industrial hub, Dera Bassi, is contaminated with genotoxic and mutagenic compounds, as indicated using different bioassays such as E. coli SOS response and A. cepa root tip assay. Further, genotoxic and chemical tests are required to evaluate toxicity profile of soil samples from agricultural sites in this region. Suitable measures should be taken to prevent the discharge of untreated wastewater in the agricultural fields or its use for irrigation purposes to avoid the entry of contaminants into the food chain.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abegunrin, T. P., Awe, G. O., Idowu, D. O., & Adejumobi, M. A. (2016). Impact of wastewater irrigation on soil physico-chemical properties, growth and water use pattern of two indigenous vegetables in southwest Nigeria. Catena, 139, 167–178. https://doi.org/10.1016/j.catena.2015.12.014
Aggarwal, M. (2019). Use treated effluents for irrigation with caution, advises CPCB. Mongabay. https://india.mongabay.com/2019/11/use-treated-effluents-for-irrigation-with-caution-advises-cpcb/. Assessed 1 June 2021.
Anh, H. Q., Tomioka, K., Tue, N. M., Tuyen, L. H., Chi, N. K., Minh, T. B., Viet, P. H., & Takahashi, S. (2019). A preliminary investigation of 942 organic micro-pollutants in the atmosphere in waste processing and urban areas, northern Vietnam: levels, potential sources, and risk assessment. Ecotoxicology and Environmental Safety, 167, 354–364.
Augusto-Pinto, L., da Silva, C., Lopes, D., Machado-Silva, A., & Machado, C. (2003). Escherichia coli as a model system to study DNA repair genes of eukaryotic organisms. Genetics and Molecular Research, 2, 77–91.
Chandel, S., Kaur, S., Issa, M., Singh, H. P., Batish, D. R., & Kohli, R. K. (2019). Exposure to mobile phone radiations at 2350 MHz incites cyto- and genotoxic effects in root meristems of Allium cepa. Journal of Environmental Health Science and Engineering, 17, 97–104. https://doi.org/10.1007/s40201-018-00330-1
Cotelle, S., Masfaraud, J. F., & Ferard, J. F. (1999). Assessment of the genotoxicity of contaminated soil with the Allium/Vicia-micronucleus and the Tradescantia-micronucleus assays. Mutatation Research, 426, 167–171. https://doi.org/10.1016/S0027-5107(99)00063-9
Datta, S., Singh, J., Singh, J., Singh, S., & Singh, S. (2018). Assessment of genotoxic effects of pesticide and vermicompost treated soil with Allium cepa test. Sustainable Environment Research, 28, 171–178. https://doi.org/10.1016/j.serj.2018.01.005
da Silva Souza, T., Hencklein, F. A., de Angelis, D. D. F., & Fontanetti, C. S. (2013). Clastogenicity of land farming soil treated with sugar cane vinasse. Environmental Monitoring and Assessment, 185, 1627–1636. https://doi.org/10.1007/s10661-012-2656-3
de Souza, C. P., Guedes Tde, A., & Fontanetti, C. S. (2016). Evaluation of herbicides action on plant bioindicators by genetic biomarkers: a review. Environmental Monitoring and Assessment, 188, 694. https://doi.org/10.1007/s10661-016-5702-8
Dheri, G. S., Brar, M. S., & Malhi, S. S. (2007). Heavy-metal concentration of sewage-contaminated water and its impact on underground water, soil, and crop plants in alluvial soils of northwestern India. Communications in Soil Science and Plant Analysis, 38, 1353–1370. https://doi.org/10.1080/00103620701328743
Dixit, S., Yadav, A., Dwivedi, P. D., & Das, M. (2015). Toxic hazards of leather industry and technologies to combat threat: a review. Journal of Cleaner Production, 87, 39–49. https://doi.org/10.1016/j.jclepro.2014.10.017
Dogra, S. (2018). National Green Tribunal orders shut down of 57 factories in Dera Bassi. Hindustan Times, Mohali. https://bit.ly/3fivQTQ. Accessed 22 June 2021
EPA. (2004). Guidelines for water reuse. United States Environmental Protection Agency. Office of Wastewater Management Municipal Support Division, National Risk Management Research Laboratory (US). Technology Transfer and Support Division.
FAO & UNEP. (2021). Global assessment of soil pollution: Report. Italy.
Fernandez, M. D., Cagigal, E., Vega, M. M., Urzelai, A., Babin, M., Pro, J., & Tarazona, J. V. (2005). Ecological risk assessment of contaminated soils through direct toxicity assessment. Ecotoxicology and Environmental Safety, 62, 174–184. https://doi.org/10.1016/j.ecoenv.2004.11.013
Ho, Y. C., Show, K. Y., Guo, X. X., Norli, I., Alkarkhi Abbas, F. M., & Morad, N. (2012). Industrial discharge and their effect to the environment. In K.-Y. Show (ed.) Industrial Waste. InTech, Shanghai, China. Available from: http://www.intechopen.com/books/industrial-waste/industrial-emissions-and-their-effect-on-the-environment
Husejnovic, M. S., Bergant, M., Jankovic, S., Zizek, S., Smajlovic, A., Softic, A., Music, O., & Antonijevic, B. (2018). Assessment of Pb, Cd and Hg soil contamination and its potential to cause cytotoxic and genotoxic effects in human cell lines (CaCo-2 and HaCaT). Environmental Geochemistry and Health, 40, 1557–1572. https://doi.org/10.1007/s10653-018-0071-6
Janion, C. (2008). Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli. International Journal of Biological Sciences, 4, 338–344. https://doi.org/10.7150/ijbs.4.338
Jensen, J., & Pedersen, M. B. (2006). Ecological risk assessment of contaminated soil. Reviews of Environmental Contamination and Toxicology, 186, 73–105. https://doi.org/10.1007/0-387-32883-1_3
Kenyon, C. J., & Walker, G. C. (1981). Expression of E. coli uvrA gene is inducible. Nature, 289, 808–810. https://doi.org/10.1038/289808a0
Khan, S., Anas, M., & Malik, A. (2019). Mutagenicity and genotoxicity evaluation of textile industry wastewater using bacterial and plant bioassays. Toxicology Reports, 6, 193–201. https://doi.org/10.1016/j.toxrep.2019.02.002
Knize, M. G., Takemoto, B. T., Lewis, P. R., & Felton, J. S. (1987). The characterization of the mutagenic activity of soil. Mutation Research, 192, 23–30. https://doi.org/10.1016/0165-7992(87)90121-7
Kumari, V., Yadav, A., Haq, I., Kumar, S., Bharagava, R. N., Singh, S. K., & Raj, A. (2016). Genotoxicity evaluation of tannery effluent treated with newly isolated hexavalent chromium reducing Bacillus cereus. Journal of Environmental Management, 183, 204–211. https://doi.org/10.1016/j.jenvman.2016.08.017
Kuzminov, A. (1999). Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiology and Molecular Biology Reviews, 63, 751–813. https://doi.org/10.1128/MMBR.63.4.751-813.1999
Leme, D. M., Grummt, T., Heinze, R., Sehr, A., Renz, S., Reinel, S., de Oliveirac, D. P., Ferraz, E. R., de Marchi, M. R., Machado, M. C., Zocolo, G. J., & Marin-Morales, M. A. (2012). An overview of biodiesel soil pollution: data based on cytotoxicity and genotoxicity assessments. Journal of Hazardous Materials, 199, 343–349. https://doi.org/10.1016/j.jhazmat.2011.11.026
Liang, J., Ning, X. A., Sun, J., Song, J., Lu, J., Cai, H., & Hong, Y. (2018). Toxicity evaluation of textile dyeing effluent and its possible relationship with chemical oxygen demand. Ecotoxicology and Environmental Safety, 166, 56–62. https://doi.org/10.1016/j.ecoenv.2018.08.106
Maron, D., & Ames, B. N. (1983). Revised methods for the Salmonella mutagenicity test. Mutation Research, 113, 173–215. https://doi.org/10.1016/0165-1161(83)90010-9
Masłowska, K., Makiela-Dzbenska, K., & Fijalkowska, I. (2019). The SOS system: a complex and tightly regulated response to DNA damage. Environmental and Molecular Mutagenesis, 60, 368–384. https://doi.org/10.1002/em.22267
Odjadjare, E. E., & Okoh, A. I. (2010). Physicochemical quality of an urban municipal wastewater effluent and its impact on the receiving environment. Environmental Monitoring and Assessment, 170, 383–394. https://doi.org/10.1007/s10661-009-1240-y
Osakwe, S. A. (2012). Effect of cassava processing mill effluent on physical and chemical properties of soils in Abraka and Environs, Delta State, Nigeria. Research Journal of Chemical Sciences, 2, 11.
Pasha, M. K., Aarey, A., Tamkeen, S., & Jahan, P. (2015). Forskolin: genotoxicity assessment in Allium cepa. Mutation Research, 777, 29–32. https://doi.org/10.1016/j.mrgentox.2014.11.005
Raza, W., Lee, J., Raza, N., LuoY, Kim KH., & Yang, J. (2019). Removal of phenolic compounds from industrial wastewater based on membrane-based technologies. Journal of Industrial and Engineering Chemistry, 71, 1–18. https://doi.org/10.1016/j.jiec.2018.11.024
Reddy, S., & Osborne, W. J. (2020). Heavy metal determination and aquatic toxicity evaluation of textile dyes and effluents using Artemia salina. Biocatalysis and Agricultural Biotechnology, 25, 101574. https://doi.org/10.1016/j.bcab.2020.101574
Ronen, A., & Glickman, B. W. (2001). Human DNA repair genes. Environmental and Molecular Mutagenesis, 37, 241–283. https://doi.org/10.1002/em.1033
Scolnick, D. M., & Halazonetis, T. D. (2000). Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature, 406, 430–435. https://doi.org/10.1038/35019108
Selmi, S. S., Abdelfattah, T. S., & Mostafa, F. D. (2014). Deregulation of mitosis progression and cytotoxic effect triggered in Allium cepa L. roots by Rubus sancatus Schreber extract. Journal of Life Science, 11, 1047–1058.
Sharma, N., Vaid, U., & Sharma, S. K. (2021). Assessment of groundwater quality for drinking and irrigation purpose using hydrochemical studies in Dera Bassi town and its surrounding agricultural area of Dera Bassi Tehsil of Punjab, India. SN Applied Sciences, 3, 245. https://doi.org/10.1007/s42452-021-04199-y
Simmons, L. A., Foti, J. J., Cohen, C. E., Walker, G. C. (2008). The SOS regulatory network. EcoSal Plus, 3. https://doi.org/10.1128/ecosalplus.5.4.3
Singh, A., Sharma, R. K., Agrawal, M., & Marshall, F. (2009). Effects of wastewater irrigation on physicochemical properties of soil and availability of heavy metals in soil and vegetables. Communications in Soil Science and Plant Analysis, 40, 3469–3490. https://doi.org/10.1080/00103620903327543
Tandon, H. L. S. (1993). Methods of analysis of soils, plants, waters, and fertilizers. Fertilizer Development and Consultation Organization, New Delhi, India.
Tedesco, S. B., & Laughinghouse, H. D., IV. (2012). Bioindicator of genotoxicity: the Allium cepa test. In J. K. Srivastava (Ed.), Environmental Contamination (pp. 137–156). InTech Publisher.
Watanabe, T., Takahashi, K., Konishi, E., Hoshino, Y., Hasei, T., Asanoma, M., Hirayama, T., & Wakabayashi, K. (2008). Mutagenicity of surface soil from residential areas in Kyoto city, Japan, and identification of major mutagens. Mutation Research, 649, 201–212. https://doi.org/10.1016/j.mrgentox.2007.09.002
Xiao, R. Y., Wang, Z., Wang, C. X., Yu, G., & Zhu, Y. G. (2006). Genotoxic risk identification of soil contamination at a major industrialized city in northeast China by a combination ofin vitro and in vivo bioassays. Environmental Science and Technology, 40, 6170–6175. https://doi.org/10.1021/es0607335
Yadav, A., Raj, A., Purchase, D., Ferreira, L. F. R., Saratale, G. D., & Bharagava, R. N. (2019). Phytotoxicity, cytotoxicity and genotoxicity evaluation of organic and inorganic pollutants rich tannery wastewater from a common effluent treatment plant (CETP) in Unnao district, India using Vigna radiata and Allium cepa. Chemosphere, 224, 324–332. https://doi.org/10.1016/j.chemosphere.2019.02.124
Acknowledgements
For the GC-MS analysis in the current study, the authors would like to acknowledge Sophisticated Analytical Instrument Facility (SAIF), Panjab University.
Funding
The funds for undertaking this research were provided by University Grants Commission, New Delhi, India (FM and RP).
Author information
Authors and Affiliations
Contributions
HPS, DRB, and RKK conceived the idea and designed this study. FM, RP, and ASG collected the data. RP and FM did the analysis. FM wrote the first draft. RP and SK edited the earlier versions of the manuscript. HPS, DRB, and RKK edited the final versions of the manuscript. All the authors contributed to the final draft of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masood, F., Pandey, R., Singh, H.P. et al. Cytotoxic and genotoxic assessment of agricultural soils from an industrial region. Environ Monit Assess 193, 526 (2021). https://doi.org/10.1007/s10661-021-09289-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09289-3