Abstract
A study of pesticides in the Bizerte lagoon watershed on the Mediterranean coast of Tunisia showed that herbicides and fungicides are the most commonly used compounds. A survey was made of selected farmers. Pesticide contamination was monitored in the water column and sediments at four selected sampling sites (lagoon (A) and in three oueds—Chegui (B), Garaa (C), and Tinja (D)). Polar organic chemical integrative samplers (POCIS) were used to assess pesticide contamination. Thirty-two pesticides were investigated; the total concentration of active ingredients ranged from 35.9 ng L−1 in Tinja oued to 1246 ng L−1 in Chegui oued. In the lagoon, the total concentration of pesticides was 67.7 ng L−1. In the sediments, the highest concentration was measured in Chegui oued in the spring (31 ng g−1 dw). The main compounds found in the analyzed sediments were prosulfocarb and tebuconazole molecules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impact of agricultural activities on the environments has strongly increased in the last decade. The use of pesticides improves crop productivity by reducing the adverse effects of pathogens (Salem 2017), but their excess can be a major source of ecosystem pollution (Arellano-Aguilar et al. 2017). Monitoring of the contamination of water and sediments by pesticides is thus needed to evaluate the impact of human activities on ecosystems (Board-ESF 2011).
Pesticides can reach aquatic ecosystems via direct applications, spray drift, aerial spraying, atmospheric fallout, soil erosion and runoff from agricultural land, discharge of industrial and domestic sewage, leaching, careless disposal of empty containers, and equipment washing (Kaushik et al. 2010). Many of these compounds have been detected in different matrices, including surface water (De Gerónimo et al. 2014; Palma et al. 2014), sediments (Guo et al. 2014; Li et al. 2014), fish pulp (Zhang et al. 2012; Yohannes et al. 2014), and bivalves (Boonyatumanond et al. 2002; Khaled et al. 2004; Carro et al. 2014; Herceg-Romanić et al. 2014). Guidelines for concentrations of pesticides in waters are published by the International Union of Pure and Applied Chemistry (IUPAC) (Hamilton et al. 2003) and permissible limits are laid down in the European reports: 2008/105/EC (EU 2008) and 2013/39/EU (EU 2013).
In Tunisia, the use of pesticides has increased in recent years due to the expansion of agricultural activities. Although national legislation on their uses is scarce, their concentrations are regulated by the Stockholm convention (2004), with serious restrictions on the use of several compounds, including aldrin, chlordane, dieldrin, endrin, heptachlor, hexachlorobenzene (HCB), mirex, and toxaphene molecules (Salem et al. 2016). In 2016, 668 commercial pesticides were approved for use by Tunisian legislation (Mezghani et al. 2016); these products have to be homologated before being sold to ensure that they do not pose a risk to human health and the environment. Pesticides comprise 31% insecticides, 39% fungicides, 20% herbicides, and 8% other products (rodenticides, nematicides, etc.) (Mezghani et al. 2016).
The Bizerte lagoon is one of the most important economic areas in Tunisia with farming, fishing, ceramics, metallurgy, oil refineries, and tire production (Barhoumi et al. 2014). Agriculture represents a major activity in the zone with cereal crops accounting for 7800 ha, vegetables for 3400 ha, and tree crops for 500 ha (Barhoumi 2014). Large quantities of fertilizers and pesticides are used to increase productivity (Necibi et al. 2015), and as a consequence, the quality of the lagoon water might be affected by the discharge of effluents (Macdonald et al. 2005; Botta et al. 2009). This environmental pollution is not only a concern in Tunisia but it has been the subject of studies worldwide in recent years (Comoretto and Chiron 2005; Vryzas et al. 2009; Momplaisir et al. 2010; Palma et al. 2014). The protection of coastal wetlands, including lagoons, in the Mediterranean region has become a priority in resource conservation policies. Like other countries around the world, Tunisia reacted to increasing anthropogenic pressures by developing several national regulations and participating in several international Conventions; e.g., Ramsar Convention (FAO 2015) and Stockholm Convention (UNEP 2001). In previous studies on the Bizerte lagoon, Barhoumi et al. (2013) and Salem et al. (2016) detected organochlorine pesticides (OCPs) in sediments with total concentrations of 11.5 ng g−1 dw and 574 ng g−1 dw, respectively. Several other authors also reported the presence of pollutants in the Bizerte lagoon, including inorganic mercury, methyl-mercury (Mzoughi et al. 2002), organotin (Mzoughi et al. 2005), and polycyclic aromatic hydrocarbons (Mzoughi et al. 2002; Barhoumi et al. 2016). Necibi et al. (2015) found OCPs and polychlorinated-biphenyl (PCBs) in water samples collected from the lagoon.
However, there is no published literature on the pollution of the lagoon ecosystem by polar pesticides, mainly herbicides and fungicides. Water quality is usually monitored by spot (grab) sampling, but due to the low concentrations of micropollutants (trace level), a large volume has to be collected to enable detection of these molecules (Poulier et al. 2014), and this sampling has to be repeated frequently to obtain a diagnosis of contamination over time (Greenwood et al. 2007). Thanks to its ability to accumulate pollutants through time-weighted average concentration (TWAC), in recent decades passive sampling has been widely used to increase the representativeness of the contamination of different water bodies (surface water, lakes, marine water, etc.) (Ahrens et al. 2015). Polar organic chemical integrative samplers (POCIS) are widely used to investigate hydrophilic contaminants such as pesticides (Ibrahim et al. 2013a; Lissalde et al. 2014; Poulier et al. 2014; Desgranges 2015). Due to its effective exposed surface area, POCIS is a particularly useful tool to determine the concentrations of trace compounds (Liess et al. 1999; Miège et al. 2013; Martínez Bueno et al. 2014; Poulier et al. 2015; Terzopoulou and Voutsa 2016).
The main goal of this work was to identify and quantify the common pesticides present in the water and sediments of Bizerte lagoon by combining a survey of farmers in the region and monitoring of water pollution using passive samplers. Both spot and POCIS sampling techniques were used. This is the first time these techniques have been used in Tunisia to identify contamination by polar pesticides.
Materials and methods
Study area
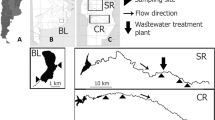
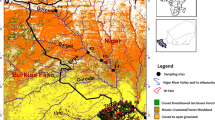
The Bizerte lagoon is located in the southwestern Mediterranean Sea, on the northern coast of Tunisia (37°8′–37°14′ N, 9°46′–9°56′ E; Fig. 1). Its surface area is 128 km2 and its average depth is 7 m (Béjaoui et al. 2008). A 7-km-long channel (300 m wide and 12 m deep) connects the lagoon to the Mediterranean Sea. The lagoon is supplied with fresh water from the surrounding 384 km2 watershed through seven oueds (M’razig, Garaa, Guenine, Ben Hassine, Chegui, Gharek, and Tinja) (Fig. 1). By definition, an oued is a stream whose size depends on the quantity of rainfall. It can be a big river or a small stream (Salem 2017). The watershed receives waste from several anthropogenic activities (aquaculture waste, industrial sewage, fertilizers, and pesticides) (Ben Said et al. 2010). The Bizerte lagoon watershed is surrounded by agricultural zones (Tinja, Menzel Bourguiba, and South of Bizerte) (Fig. 1).
The smallest agricultural areas are located in the Tinja region, particularly around Tinja oued (D). The area represents 39% of the total area. The majority of crops planted there are cereals and fodder crops. One oued was selected in each of the three areas for sampling: Chegui oued (B) (Menzel Bourguiba), Garaa oued (C) (South of Bizerte), and Tinja oued (D) (Tinja) (Fig. 1). The Chegui oued (B) is located in the downstream part of the Bizerte lagoon watershed, with a high percentage of arable land. The two other oueds are situated in the upstream part of the catchment with a smaller percentage of arable land. These surrounding oueds discharge their effluents into the lagoon, and for this reason, a lagoon sampling site (A) was chosen to study the impact of agricultural pesticides on this aquatic ecosystem. To combat fungal diseases and weeds, farmers apply different pesticides depending on the season. Hence, samples were collected in three sampling campaigns in October 2015 (autumn), March 2016 (spring), and June 2016 (summer).
Survey of pesticides used: methodology
Three zones (South of Bizerte, Tinja, and Menzel Bourguiba) were chosen to investigate the pesticides used. Data was first collected from the Regional Commissioner for Agricultural Development (RCAD) and their representatives in delegations called Extension Territorial Cells (ETC), to better understand the characteristics of the watershed. Representative samples of the general population of farmers were interviewed (50 people), all of whom cultivate land around the Bizerte lagoon (Fig. 1). The farmers who were selected for the survey were those whose land was located closest to the lagoon and to the oueds that feed the watershed. The interview was semi-directive.
The interviews were structured in two parts: the first part collected general information (age, gender, level of education), while the second part focused on farming (extent of arable land, type of crops cultivated, disease frequency, pesticides used, period and frequency of treatments). The field survey was carried out in February and March 2015.
A list of commercial pesticides with numerous active ingredients was drawn up based on the data and on the information collected in the survey. For each commercial pesticide, the suppliers provided the dose per gram and per hectare, and the dose of active ingredients per hectare was determined according to the pesticide formula. The concentration of active ingredients used by farmers was calculated based on the dilution and the volume of water sprayed per hectare (applied dose g ha−1). A list of active ingredients was selected and ranked according to this dose (g ha−1).
Water sampling procedures
Both passive and grab sampling was performed at the four sites: the lagoon ((A); 37°10.957′N 9°51.355′E), Chegui oued ((B); 37°9′20″ N and 9°54′13″ E), Garaa oued ((C); 37°13′34″ N and 9°44′29″ E), and Tinja oued ((D); 37°11′18″ N and 9°46′54″ E) (Fig. 1). Three sampling campaigns were conducted in October 2015 (autumn), March 2016 (spring), and June 2016 (summer). At each site, POCIS were deployed in triplicate (n = 3) for a mean period of 21 days (Ibrahim et al. 2013b). They were placed in cages and submerged vertically in the water column. As quality control, a field blank was transported to the site and exposed to the air each time the immersed samplers were retrieved from the water (Ibrahim et al. 2013b). On the days of the deployment and retrieval of the POCIS samplers, grab water samples were collected in clean amber glass bottles at the spot where each cage was immersed. The retrieved POCIS were rinsed with ultrapure water, wrapped in aluminum foil and placed in a plastic bag. Both grab and POCIS samples were stored in cool conditions during transport to the laboratory and until extraction. During each sampling campaign, the physical parameters (water column temperature, pH, dissolved oxygen, and salinity) were determined using field multi-parameter sensors.
Sediment sampling procedure
Sediment samples were collected at the four selected sites in the Bizerte lagoon watershed (the lagoon (A), Chegui oued (B), Garaa oued (C), and Tinja oued (D)) (Fig. 1), at three sampling campaigns (autumn, spring, summer). Sediments were sampled using plexiglass cylindrical cores (30 cm long, 3.6 cm diameter). Only the superficial sediment layer (less than 10 cm) was sampled. Three replicates were collected per sampling site then transferred to an icebox in the dark. In the laboratory, the samples were kept in the freezer until extraction. Before extraction, frozen samples were freeze-dried then passed through a stainless-steel sieve (200-μm mesh) and stored at 4 °C until analysis (Barhoumi et al. 2013).
Analytical procedures
Chemicals and materials
Thirty-two targeted compounds, mainly herbicides and fungicides, were analyzed (Table 1). The compounds included 25 pesticides (acetochlor, alachlor, atrazine, azoxystrobin, carbendazim, chlortoluron, dimethomorph, diuron, epoxiconazole, flazasulfuron, imidachloprid, isoproturon, linuron, metalaxyl, metholachlor, oxadixyl, penconazole, prochloraz, propyzamide, prosulfocarb, pyrimethanil, simazine, tebuconazole, terbuthylazine, tetraconazole) and seven degradation products (DCPMU, DCPU, DEA, DET, DIA, simazine hydroxy, terbuthylazine hydroxy).
Analytical standards (purity > 99%) were purchased from Cluzeau Info Labo (Sainte Foy la Grande, France). Deuterated-labeled compounds were atrazine-d5 (CAS 163165-75-1) as recovery control and simazine-d5 (CAS 220621-41-0) as internal standard. High-performance liquid chromatography (HPLC) grade solvents used were acetonitrile from Biosolve (Dieuze, France) and formic acid from Carlo Erba Reagents (Peypin, France). Ultrapure water was generated with a MilliPore Synergy UV water purification system from Merck Millipore (Billerica, US). Glass-fiber filters (GF/F) (0.7 μm pore size) purchased from Whatman (Maidstone, UK) were used to filter the water samples. Oasis HLB® cartridges (60 μm, 6 cm3, 500 mg) were obtained from Waters Corporation (Milford, USA) and a Visiprep Solid Phase Extraction (SPE) vacuum manifold from Supelco (Bellefonte, USA) was used for SPE. POCIS were purchased from Expos Meter AB Company (Tavelsjö, Sweden) with the pharmaceutical receiving phase composed of approximately 230 mg of the solid adsorbent N-vinylpyrrolidone-divinylbenzene (Oasis HLB®). The sampling area of the POCIS device was 41 cm2. The 3-mL polypropylene cartridges used to recover the POCIS receiving phases were from Supelco (Bellefonte, USA).
POCIS and water sample extraction
Grab samples (500 mL) were filtered through GF/F filters to eliminate suspended matter, spiked with 50 μL of atrazine d5 (1 ng μL−1 acetone) and extracted by solid phase extraction (SPE) using Oasis HLB® cartridges. Prior to extraction, the Oasis HLB® cartridges were activated with 5 mL of acetonitrile under vacuum, followed by 5 mL of methanol (MeOH) and 5 mL of ultrapure water (Ibrahim et al. 2013b). Before elution, they were dried under vacuum for 1 h. Analytes were recovered by eluting the cartridges with 8 mL of acetonitrile at a flow rate of 1 mL min−1.
After they were retrieved, the receiving phases of the POCIS were transferred into an empty solid-phase extraction tube packed with polyethylene (SPE) frit, 20 μm porosity. The sorbents were spiked with 50 μL of atrazine d5 (1 ng μL−1 acetone) then eluted with 8 mL of acetonitrile.
Both for Oasis HLB® cartridges and POCIS receiving phase elution, the extracts were concentrated under a gentle stream of nitrogen to obtain a final extract of 1.5 mL. This final extract were then spiked with 150 μL of the internal standard simazine d5 (1 ng μL−1 acetonitrile) and analyzed by HPLC-MS/MS (Ibrahim 2013).
Sediment extraction
The extraction was carried out using an Accelerated Solvent Extraction (ASE) system (Dionex®, France) with a hexane/acetone mixture of solvents (50:50, v/v). The parameters used during the extraction procedure were as follows: temperature 120 °C, static time 5 min, pressure 1500 psi, heating time 6 min, flush volume 60%, and purge time 100 s. Atrazine d5 recovery control (1 ng μL−1 acetonitrile) was added to the sediment prior to the ASE step and the resulting extracts (hexane/acetone) were cleaned on a Strata SAX® (8B-S008-JCH). To recover analytes, elution was carried out with 3 mL of MeOH and 3 mL of dichloromethane (CH2Cl2), respectively. Purified extracts were completely evaporated under a gentle stream of nitrogen and then dissolved in 1.5 mL of acetonitrile (Barhoumi et al. 2013). Before analysis, all sample extracts were spiked with 120 μL of the deuterated internal standard simazine d5 (1 ng μL−1 acetonitrile) and then analyzed by HPLC-MS/MS.
The percentage of finer grain size fractions (< 63 μm) of each sediment sample was determined gravimetrically after wet sieving (Savinov 2000). Total organic carbon (TOC) content was measured using the coulometry method in a 702 Coulomat, after decarbonization of sediments with 2 N HCl at 60 °C overnight (Ouertani et al. 2006).
Liquid chromatography–mass spectrometry (HPLC-MS/MS) analysis
The pesticide analysis was performed by HPLC-MS/MS using an Alliance HPLC system (Waters Series 2695). The HPLC is equipped with a quaternary pump, a vacuum degasser, and an autosampler. Analytic separation was achieved with a Kinetex C18 analytical column (100 mm × 4.6 I.D. × 260 Å; Phenomenex). The volume injected was 25 μL. Acetonitrile (A) and ultrapure water (B), both with 0.05% formic acid, were used as mobile phases at a constant flow rate of 0.4 mL min−1. The linear gradient was started at 40% for 0.2 min, ramped to 80% for 8 min, then to 100% for 1 min, and finally back to the initial conditions for 2 min. A triple quadrupole mass spectrometer (Micromass Quatromicro TM; Waters) equipped with an electrospray ionization source (ESI) was used as the detector device. The spectrometer operated in positive ESI mode under the following conditions: capillary voltage (3.5 kV), source temperature (120 °C), desolvation temperature (300 °C), drying (600 L h−1), and nebulization gas (N2) flow (30 L h−1). Argon was used as the collision gas. For each compound, acquisition was performed in the multiple reaction-monitoring modes (MRM). Two transitions were retained: one was used for quantification and the other for confirmation (Table S1—Supplementary materials).
Concentrations of pesticides in the water during POCIS deployment
The accumulation of contaminants by passive samplers typically follows first-order kinetics, which includes an initial integrative phase, followed by curvilinear and equilibrium-partitioning phases. In the linear region of the POCIS uptake, the amount of a chemical accumulated in the sampler (M) is described by Eq. (1):
where Rs is the sampling rate (L day−1), Cw is the time-weighted concentration of the compound in water (ng L−1), and t is the exposure time (days).
For each pesticide, the sampling rate was determined by dividing the slope of the linear regression curve by the mean aqueous concentration of the selected compounds over the 15 days of exposure time (Ibrahim et al. 2013b). POCIS sampling rates (Table 1) were determined in laboratory conditions for each compound (Ibrahim et al. 2013b). Given that they depend on environmental conditions such as flow, temperature, pH, organic matter, and biofouling (Charlestra et al. 2012; Yabuki et al. 2016), they allow the calculation of semi-quantitative concentrations of pesticides in water and the comparison of the relative levels of contamination between sites (Ibrahim et al. 2013b). Time-weighted averaged concentrations (TWAC) in water were calculated with the equation proposed by Miège et al. 2013 (2):
where Cwater is the mean concentration of the contaminant (over the sampling period) in the ambient water (μg L−1); Cpocis is the concentration in the POCIS (μg g−1); Mpocis is the mass of adsorbent phase in the POCIS (g); Rs is the sampling rate (L day−1), which corresponds to the volume of water purified per unit of time; and t is the total exposure time (days).
Among the 40 compounds inventoried in the survey, 15 were selected (Table 2) as being the most representative, according to (1) the use rate per hectare; (2) the type of crops grown in the zone; (3) the concentration of active ingredient in the commercial pesticide. For analytical reasons, not all the selected active ingredients could be analyzed by the HPLC-MS/MS method developed in our laboratory. In particular, glyphosate and 2,4 D were not included in the final list of targeted pesticides. The final list of active ingredients comprised 32 compounds analyzed by liquid chromatography–mass spectrometry (Table 1).
Quality assurance/control
The linearity, limits of detection (LODs) and limits of quantification (LOQs), precision, and accuracy of the analytical methods were carefully checked. The LODs were calculated as 3Sy/x/b and the LOQs as 10Sy/x/b, where Sy/x is the residual standard deviation and b is the slope of the matrix calibration curves. The LODs and LOQs obtained by HPLC-MS/MS (analytical LOD and LOQ expressed in μg L−1) are listed in Table 1. The LODs and LOQs of all the selected pesticides were determined from the calibration curves for each analytical campaign in which R2 > 0.98. The analytical limits of detection ranged from 0.10 to 2.20 μg L−1 and the limits of quantification from 0.40 to 7.50 μg L−1. The LOQs of the POCIS (which were calculated from the equation of Poulier et al. 2014), water, and sediment samples are also listed in Table 1.
Mean recovery from the synthetic water solution spiked with the 32 compounds was 63%, the highest recovery being for simazine (101%) and the lowest for DCPU (8%). Flazasulfuron, hydroxy-terbuthylazine, and hydroxy-simazine were not recovered (Table 1).
A certificated reference material (WaRTM Pollution Nitrogen Pesticides, Lot No. P246-674) purchased from the ERA Waters Company (Golden, USA) was used to determine pesticide recoveries in water samples. The reference material was a water solution composed of 24 pesticides including six of the pesticides selected for our study (alachlor, atrazine, DEA, DIA, metolachlor, and simazine). The concentrations of pesticide in the reference material ranged between 16.80 and 3.37 μg L−1. The global recovery yields were for atrazine (108 ± 4%), for alachlor (78 ± 4%), for DEA (61 ± 3%), for DIA (58 ± 3%), for metolachlor (110 ± 2%), and for simazine (79 ± 2%) after solid phase extraction (Oasis HLB) and HPLC-MS/MS analysis (n = 9) with our laboratory method. The mean extraction recoveries with atrazine-d5 were 98 ± 11% and 92 ± 12% for the passive and grab samples, respectively (n = 36 samples). The relative standard deviation (RSD) obtained for the analysis of the internal standard simazine-d5 was 17 ± 6% for all the HPLC-MS/MS injections (n = 108 injections).
Statistical analyses
Triplicate analytical measurements were made for all the analyses and the data are reported as mean ± standard deviation. Pearson’s correlation coefficient was calculated to investigate possible relationships between parameters. Statistical significance was set as p < 0.05. The statistical treatment of the data was performed using the STATISTICA 6.0 Analysis System version 5.
Results and discussion
Survey results
According to the farmers and engineers of Regional Commissioner for Agricultural Development (RCAD), field crops (wheat, legumes, and oats) occupy the largest percentage of cultivated fields. Wheat accounts for 58% (i.e., 3.153 ha) of the total cultivable area, and legumes and oats for 13% and 10%, respectively. The dominance of this type of crop could be related to economic factors. Indeed, since the 1970s, Tunisia and other North African countries have benefited from the support of ICARDA (International Center for Agricultural Research in Arid Zones) to develop their cultivar production programs, with the objective of intensifying the amount of cereal crops and legumes (Nefzaoui et al. 2012). This result is consistent with those obtained in an investigation commissioned by the Tunisian Ministry of Agriculture (DGEDA 2006), which reported that the areas treated in Tunisia correspond to low crops, which represent 73% of all cereals grown.
Our survey results showed that farmers use 47 commercial pesticides, mainly fungicides (42.5%), herbicides (42.5%), and insecticides (15.0%). The prospected regions are wetlands, where fungal diseases (septoria, rust, and mildew) develop easily, hence, intensive use of fungicides by farmers was observed. Fungicides are used twice a year (at the end of March and May) as preventive treatments. They are also used as a curative treatment for “rust” whenever there is an outbreak of the disease. The survey also revealed high use of herbicides to control the weeds that compete with crops for access to water, light and soil nutrients. Herbicides are usually applied twice a year, in autumn (October) and spring (March).
Table 2 lists the most frequently used active ingredients based on our inventory of commercial pesticides in the regions around the Bizerte lagoon surveyed. The active ingredients are classified first according to the type of pesticide (herbicides, fungicides, and insecticides) then by the dose of the active ingredients applied per hectare (calculated according to the dilution and the water volume sprayed per hectare) and by target crop, mainly field crops (wheat, legumes, oats). The list contains 40 active ingredients (Table 2). Among these compounds, 87.5% are approved for use in Tunisia (Mezghani et al. 2016). Our list of active ingredients is in agreement with the results of a study by Salem et al. (2016), who used another approach to calculate the total quantities of active ingredients, i.e., the percentage use of active ingredients was calculated by dividing the number of farmers who used the active ingredient concerned by the total number of farmers surveyed (× 100). Finally, the amount of each active ingredient (Qsubstance) is the product of the cultivated area (ha) divided by the percentage of pesticide used by the assay of the active ingredient in the pesticide. Despite the different methods of calculation used in the two studies, some of the ingredients we found were also found by Salem et al. (2016) including herbicides (e.g., iodosulfuron, mesosulfuron, 2,4-dichlorophenoxyacetic acid (2,4 D), glyphosate, and fenoxaprops) and fungicides (e.g., tebuconazole, epoxiconazole, azoxystrobin). The study by Salem et al. (2016) revealed that insecticides are the least used, and only two pyrethroids were inventoried (deltamethrin and bifenthrin).
For our study, based on the calculations of the dispersed dose (g ha−1), the inventoried active ingredients were ranked to obtain a list of 40 targeted compounds (Table 2). The survey revealed that most of the pesticides were used on wheat, legumes, and oats, which are the main crops grown in the regions surveyed. A restricted list is thus proposed containing 15 active ingredients of fungicides (azoxytrobin, chlorothalonil, prochloraz, tebuconazole, fluzilazole, flutriafol, propiconazole, carbendazim, prothioconazole, and epoxiconazole) and herbicides (glyphosate, simazine, bentazone, prosulfocarb, 2,4D). The estimated concentrations of these compounds ranged from 200 g ha−1 for azoxystrobin to 0.47 g h−1 for epoxiconazole. Despite their high dose per hectare, some pesticides were not included in this list because they are not used to treat the field crops grown in the survey area, mainly wheat. This is the case of difenoconazole, mancozeb, maneb, zineb, and boscalid, which are used to protect vines.
Surface water quality
Occurrence of pesticide in water
To study the occurrence of target pesticides, detection and quantification frequencies were calculated, based on the results obtained by the two sampling techniques (grab and passive sampler). By definition, the frequency of detection and quantification campaigns correspond to the ratio of the number of times in which one compound was detected and quantified divided by the total number of sites analyzed in the campaigns, respectively.
Of the 32 pesticides studied (Table 1), 23 were detected using passive sampling technique (Fig. 2a), which represents 72% of the total studied pesticides. Whereas, 11 compounds were quantified (34%) with the same sampling technique (POCIS): simazine, chlortoluron, DIA, acetochlor, alachlor, DCPU, isoproturon, DET, epoxiconazole, tebuconazole, and azoxystrobine (Fig. 2a). Among the detected compounds using POCIS, two fungicides (epoxiconazole and tebuconazole) and one herbicide (simazine) were detected at a frequency of 100%. The accumulation of these compounds in the POCIS receiving phase (OASIS HLB) is favored by their log Kow values (3.30 for epoxiconazole, 2.18 for simazine, 3.70 for tebuconazole) (Hijosa-Valsero et al. 2016; Zhang et al. 2016). Grab sampling allowed the detection of 44% of all target pesticides and the quantification of 31% (Fig. 2b).
Acetochlor and alachlor were only quantified by POCIS. Conversely, prosulfocarb was not quantified by POCIS, which can be attributed to the low sampling rate (Rs = 0.071 L day−1) (Fig. 2a). Retention of prosulfocarb by POCIS is not favored and the concentration obtained was lower than the analytical LOQ. It should be noted that DET, by-products of terbuthylazine, was quantified only by the POCIS (5.00 ng L−1, Table 3). Thus, passive samplers could lead to a more representative level of contamination than that provided by grab sampling and allowed us to measure low concentrations of pesticides (Lissalde et al. 2011; Poulier et al. 2015; Van Metre et al. 2017).
The most frequently detected and quantified compounds at all the study sites were two herbicides (simazine, chlortoluron) and two fungicides (epoxiconazole, tebuconazole) regardless of the sampling technique (Fig. 2a, b). The high level of detection and quantification of these compounds at the different sites by passive sampling (Fig. 3) can be explained by their intensive use in the treatment of crops in the Bizerte lagoon watershed. Indeed, simazine is the active ingredient of “Agzozine,” which is a pesticide sold in Tunisia to treat weeds and field crops, particularly legumes, one of the main crops cultivated in our survey areas. Epoxiconazole and tebuconazole are the active ingredients most widely used by farmers to treat crops. Both are applied to wheat, barley, and oats. They are used to control powdery mildew, helminthosporiasis, septoria, and brown rust. Even if chlortoluron is not inventoried in the list of active ingredients used for crops in the watershed, this substance was one of the major compounds detected during the sampling campaigns (up to 60%). This compound is one of the active ingredients of two commercial products, “Dicuran 500” and “Tolurex 50,” that are used to treat weeds and wild oats in wheat and barley fields; probably, it was used by farmers who were not interviewed in our survey.
Space and time variations in concentrations of pesticides in water
The total amount of pesticides (∑pesticides), obtained by passive sampling and present at each sampling site, was used to assess the impact of the agricultural activity. The sum of pesticides (∑pesticides) measured for all the seasons vary according to sites (Table 3). The highest concentration of ∑pesticides was measured in Chegui oued (B): 1246 ng L−1, and the lowest in Tinja oued (D): 35.9 ng L−1 (Table 3). The total concentrations of pollutants in Garaa oued (C) were close to 202.6 ng L−1. Among the oued sites, the least impacted sites were Tinja (D) and Garaa (C) oueds. This may be due to the fact they are located in the downstream part of the Bizerte lagoon watershed, and to the lesser presence of large agricultural areas around them. Total pesticide concentrations in the lagoon itself were only 68 ng L−1; this low concentration compared to the concentration in Chegui oued can be explained by the dilution of the oued water in the lagoon (Carafa et al. 2007).
The intensity of agricultural activities is another argument to justify the high concentration of target compounds in Chegui oued. According to the survey, wheat accounts for 60% of crops grown at this site and legumes for 65%. These crops are treated with herbicides and fungicides, mainly simazine and tebuconazole. Our results are in agreement with the results of previous studies showing that the highest concentrations of pesticides are generally measured at sampling sites located in areas with a high proportion of arable land (Zhang et al. 2016).
Variations over time showed that the concentrations of ∑pesticides vary with the season (Fig. 4). The high concentrations measured in the Chegui oued (B) compared to concentrations at the other sites can be explained by its geographical and hydrological properties. Chegui oued is located on the upstream side of the Bizerte lagoon catchment, and the highest concentrations were found in Chegui oued in summer (B) (832.5 ng L−1); this could be due to the transport of pollutants from agricultural lands into the oued because of the rains, which drain the pesticides from crops to the sampling sites. Whereas, value of 413.8 ng L−1 was found in the spring season (Fig. 4). Concerning Garaa oued (C), the highest concentration was observed in spring (194.1 ng L−1). For sites (A) and (D), the ∑pesticides was very low regardless the season (e.g., concentrations were 20.8 ng L−1 in the spring in sites (A) and (D)). These results can be explained by the environmental conditions in these areas (solar radiation and water temperatures) (Bondarenko et al. 2004; Navarro et al. 2004; Carafa et al. 2007; Carvalho et al. 2009). Temperature can affect the sorption rate of pollutants onto suspended matter and organic matter (Wu and Gschwend 1986). When temperatures increase, the solubility of the compounds increases, and biodegradation and photodegradation are favored (Eriksson et al. 2003).
In the Mediterranean basin, the annual average global solar radiation reaching the surface is 168 W/m2 (Chelbi et al. 2015). In Bizerte city (located in the study area), the annual average global solar radiation is 208 W/m2 (Ben Othman et al. 2018).
The most frequently measured compounds with high concentrations were simazine and tebuconazole in the spring and summer in Chegui oued (B) (Table 3). This result is not surprising as these pesticides are mostly used to treat the wheat and legumes grown around this study site. The concentrations of simazine found in spring and summer by passive sampling were 168 and 431.6 ng L−1, respectively (Table 3). The concentrations of Tebuconazole were 142 and 190.5 ng L−1 in spring and summer (Table 3). Moreover, the significant concentrations of fungicides (tebuconazole, epoxiconazole) in the spring can be explained by their application in April and May to protect wheat against fungal diseases such as septoria and rust.
The results suggest significant variations in concentrations as a function of the agricultural seasons. For example, herbicides are widely applied to field crops, particularly legumes and wheat, in spring and autumn. This explains the abundance of herbicides at the study sites during these periods and a few months after they were sprayed in the fields (Zhang et al. 2016).
Pesticide concentrations (obtained by grab sampling) (Table 4) are in agreement with those obtained by POCIS (Table 3). Indeed, the highest concentration of DIA, simazine, tebuconazole, and epoxiconazole were observed in spring even for Chegui oued (B) and Garaa oued (C).
Simazine was mainly found in spring in Chegui oued (B), Garaa oued (C), and Tinja oued (D) with concentrations of 730.9, 56.9, and 32.5 ng L−1, respectively (Table 4). These values are below than those of the existing Quality Standards in Directive 2013/39/EU of the European Parliament and the Council of 12 August 2013 (1 μg L−1) (EU 2013). The concentrations of tebuconazole in the same season and at the same sites were 500.3, 178.4, and 56.5 ng L−1, respectively (Table 4). According to previous studies, for a representative diagnosis of pesticide occurrence, POCIS and spot sampling should be used as a complement to grab sampling (Poulier et al. 2014; Miège et al. 2015; Branchet et al. 2018). Passive sampling is recommended by water policy in the European Commission Guidance Document (EC Guidance document no. 19) and in Directive 2013/39/EU (EU 2013).
Additionally, the results of monitoring of the water column by passive and grab sampling confirmed those of the survey (Tables 3 and 4). Indeed, screening revealed some pesticides identified during the survey, including simazine, tebuconazole, prosulfocarb, and epoxiconazole. These are among the compounds most widely used by farmers to treat wheat.
Occurrence and variations in pesticide concentrations in sediments in space and over time
Occurrence of pesticides in sediments
Studying the composition of coastal sediments is one of the main ways used to identify the level and source of contamination of marine ecosystems (Zaghden et al. 2017). Indeed, sediments are known to trap hydrophobic contaminants, particularly non-polar pesticides like chlorinated compounds (Meakins et al. 1995). Consequently, sediments were also investigated to complete the diagnosis of the contamination of the Bizerte lagoon watershed by agricultural pesticides. The detection and quantification frequencies of the targeted compounds during the three campaigns and at the four study sites are summarized in Fig. 5. No compounds were quantified in Tinja oued (D). The most impacted sediments were those of Chegui oued (B) and Garaa oued (C). The majority of the targeted compounds, e.g., alachlor, acetochlor, and metolachlor, were detected at a frequency of 34%. Prosulfocarb, epoxiconazole, and tebuconazole were detected at a frequency of 100% in the Chegui and Garaa oueds. These compounds were also quantified at these two sites (B and C). Indeed, prosulfocarb was quantified at frequencies of 100% and 34% in Garaa oued (C) and Chegui oued (B), respectively. However, tebuconazole and epoxiconazole were quantified at frequencies of 100% and 68% in Chegui oued (B) and Garaa oued (C), respectively.
Space and time variations in the concentration of pesticide in sediments
The sum of pesticides (∑pesticides) in the sediments of the watershed of the Bizerte lagoon ranged from 9.8 ng g−1 dw in the lagoon (A) to 53 ng g−1 dw in the Chegui oued (C) (Table 5). Particle size is an important parameter to consider when analyzing sediment samples (Ackermann 2008). In the present study, the percentage of fine particles (< 63 μm) in the sediments differed at each site, varying from 12% to 69% in the lagoon (A), from 62% to 77% in Chegui oued (B), from 94% to 100% in Garaa oued, and from 11% to 57% in Tinja oued (D) (Table 5). In spring season, the higher total concentration of pesticides in Chegui oued sediments (31.1 ng g −1) can be explained by the higher content of fine particles; the large surface area available (per unit of mass) of the small particles increases the adsorption of pollutants onto the grains (Gao et al. 1998). Additionally, fine sediment particles may accumulate pesticides due to their mineral compounds (e.g, silicate, goethite and hematite, montmorillonite, iron oxides, manganese oxides, illite, etc.). No apparent relationship was found between TOC and grain size (< 63 μm) at the sites analyzed (p > 0.05, n = 12). In addition, no significant correlations were found between TOC content, grain size (< 63 μm) and the majority of compounds analyzed. This implies that the distributions and concentrations of pesticides are not only determined by sedimentary characteristics such as TOC and grain size (Mai et al. 2005) but probably by other factors such as sources, transport, mixing, and deposition that were not analyzed in this study.
Table 5 reports the individual concentration of pesticides; the highest concentration of tebuconazole was found in Chegui oued (B) in spring (31.1 ng g−1 dw) (Table 5). The highest concentration of prosulfocarb (6.3 ng g−1 dw) was measured in Garaa oued (C) in autumn. A relatively high log Kow (octanol/water coefficient) means that pollutants can be strongly adsorbed onto sediment particles and organic matter (PAPP 2010). For tebuconazole and prosulfocarb, log Kow values were 3.70 and 4.48, respectively (INERIS 2011, 2013), these values indicate that tebuconazole and prosulfocarb are sorbed to sediments (Chamberlain et al. 1996; Čadková et al. 2013).
Space–time variations in pesticide concentrations in the Bizerte lagoon watershed
These results reveal a space–time variation in pesticide concentrations in the water column and in the sediments in the Bizerte lagoon watershed. These results show that among the pesticides found in the sediments and water, 60% are approved for use in Tunisia (20% herbicides, 36% fungicides, 4% insecticides).
These results are in agreement with those of Jiménez et al. (1999), who showed that pesticide concentrations in water and sediments were subject to seasonal variations in 27 Mediterranean lakes. Moreover, the presence and distribution of pesticides in the water and/or in the sediments depends on the physical–chemical properties of the compounds (log Kow and water solubility). Some of the pesticides (e.g, atrazine, diuron) found in the water at the sites we investigated are not yet approved for use in Tunisia. These herbicides (atrazine, diuron) pose a serious risk for aquatic ecosystems as well as for the environment (Moncada 2004; Ralston-Hooper et al. 2009).
Literature on levels of polar pesticides in samples of water is scarce (in particular on fungicides and herbicides), making it difficult to compare our results with those obtained elsewhere in the world. In sediments, the studied pesticides are mainly the organochlorines (Barhoumi 2014; Salem et al. 2016). The concentrations of total pesticides in water and sediments in the lagoon and oueds were lower than those measured in Mondego River Estuary in Portugal (Cruzeiro et al. 2016), Ria Formosa lagoon in Portugal (Cruzeiro et al. 2015), Tagus River basin in Greece (Papadakis et al. 2015), in a river basin in Costa Rica (Carazo-Rojas et al. 2018), and in Méfou watershed in Cameroon (Branchet et al. 2018). However, the concentrations we measured were higher than those measured in the Arade River estuary in Portugal (Gonzalez-Rey et al. 2015), in the River Ugie in Scotland (Zhang et al. 2016), and in the Marque River in France (Criquet et al. 2017).
The concentrations of total pesticides in the lagoon and oued sediments studied here are lower than those reported in Mediterranean coastal lagoons in SE Spain (Moreno-González and León 2017).
Conclusion
This study highlights a worrying issue that affects the majority of Mediterranean lagoons: human pressure, especially agricultural pollution. The Bizerte lagoon is one of the most affected by this anthropization in particular by agriculture activities, justifying our investigation of the occurrence of herbicides and fungicides. This study confirmed contamination by polar pesticides (herbicides and fungicides) in the watershed and in the lagoon ecosystem. Analyses of samples from the four sampling sites revealed the highest contaminations in the water and sediments of Chegui oued. The results of our analyses are in agreement with survey data: several molecules (including simazine, tebuconazole, and prosulfocarb) were found at the target sites. Simazine and tebuconazole molecules, the most significant species present in the water samples, were identified by passive sampling at a maximum concentration of 431.6 and 190.5 ng L−1 in the Chegui oued, respectively. The molecule found at the highest concentrations in the sediments was tebuconazole (31.1 ng g−1 dw) and although prosulfocarb was detected in trace amounts in the water samples, a concentration of 6.3 ng g−1 dw was found in the sediments of the Garaa oued. The POCIS technique was used for the first time in Tunisia and was shown to improve the analysis of the trace concentrations of polar pollutants. The results of this work support the hypothesis that the ability of passive samplers (POCIS) to integrate the contaminant concentrations over a period of exposure enables better quantification of contamination at low concentrations.
References
Ackermann F (2008) A procedure for correcting the grain size effect in heavy metal analyses of estuarine and coastal sediments. Environ Technol Lett 1(11):518–527
Ahrens L, Daneshvar A, Lau AE, Kreuger J (2015) Characterization of five passive sampling devices for monitoring of pesticides in water. J Chromatogr A 1405:1–11. https://doi.org/10.1016/j.chroma.2015.05.044
Arellano-Aguilar O, Betancourt-Lozano M, Aguilar-Zárate G, Ponce de Leon-Hill C (2017) Agrochemical loading in drains and rivers and its connection with pollution in coastal lagoons of the Mexican Pacific. Environ Monit Assess 189:189–270. https://doi.org/10.1007/s10661-017-5981-8
Barhoumi B (2014) Biosurveillance de la pollution de la lagune de Bizerte (Tunisie) par l’analyse comparée des niveaux de contamination et de l’écotoxicité des sédiments et du biote (Doctoral dissertation, Bordeaux)
Barhoumi B, LeMenach K, Dévier MH, el megdiche Y, Hammami B, Ameur WB, Hassine SB, Cachot J, Budzinski H, Driss MR (2013) Distribution and ecological risk of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in surface sediments from the Bizerte lagoon, Tunisia. Environ Sci Pollut Res 21:6290–6302. https://doi.org/10.1007/s11356-013-1709-7
Barhoumi B, Lemenach K, Devier MH et al (2014) Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Bizerte lagoon, Tunisia: levels, sources, and toxicological significance. Environ Monit Assess 186:2653–2669. https://doi.org/10.1007/s10661-013-3569-5
Barhoumi B, El Megdiche Y, Clérandeau C et al (2016) Occurrence of polycyclic aromatic hydrocarbons (PAHs) in mussel (Mytilus galloprovincialis) and eel (Anguilla anguilla) from Bizerte lagoon, Tunisia, and associated human health risk assessment. Cont Shelf Res 124:104–116. https://doi.org/10.1016/j.csr.2016.05.012
Béjaoui B, Harzallah A, Moussa M, Chapelle A, Solidoro C (2008) Analysis of hydrobiological pattern in the Bizerte lagoon (Tunisia). Estuar Coast Shelf Sci 80:121–129. https://doi.org/10.1016/j.ecss.2008.07.011
Ben Othman A, Belkilani K, Besbes M (2018) Global solar radiation on tilted surfaces in Tunisia: measurement, estimation and gained energy assessments. Energy Reports 4:101–109. https://doi.org/10.1016/j.egyr.2017.10.003
Ben Said O, Goñi-Urriza M, El Bour M, Aissa P, Duran R (2010) Bacterial community structure of sediments of the Bizerte lagoon (Tunisia), a southern Mediterranean coastal anthropized lagoon. Microb Ecol 59:445–456
Board-ESF M (2011) A draft marine research plan for the European atlantic sea basin: discussion document
Bondarenko S, Gan J, Haver DL, Kabashima JN (2004) Persistence of selected organophosphate and carbamate insecticides in waters from a coastal watershed. Environ Toxicol Chem 23:2649–2654. https://doi.org/10.1897/03-436
Boonyatumanond R, Jaksakul A, Puncharoen P, Tabucanon MS (2002) Monitoring of organochlorine pesticides residues in green mussels (Perna viridis) from the coastal area of Thailand. Environ Pollut 119:245–252. https://doi.org/10.1016/S0269-7491(01)00329-3
Botta F, Lavison G, Couturier G, Alliot F, Moreau-Guigon E, Fauchon N, Guery B, Chevreuil M, Blanchoud H (2009) Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems. Chemosphere 77:133–139. https://doi.org/10.1016/j.chemosphere.2009.05.008
Branchet P, Cadot E, Fenet H, Sebag D, Ngatcha BN, Borrell-Estupina V, Ngoupayou JRN, Kengne I, Braun JJ, Gonzalez C (2018) Polar pesticide contamination of an urban and peri-urban tropical watershed affected by agricultural activities (Yaoundé, center region, Cameroon). Environ Sci Pollut Res 25:17690–17715. https://doi.org/10.1007/s11356-018-1798-4
Čadková E, Komárek M, Kaliszová R, Vaněk A, Balíková M (2013) Tebuconazole sorption in contrasting soil types. Soil Sediment Contam 22:404–414. https://doi.org/10.1080/15320383.2013.733448
Carafa R, Wollgast J, Canuti E, Ligthart J, Dueri S, Hanke G, Eisenreich SJ, Viaroli P, Zaldívar JM (2007) Seasonal variations of selected herbicides and related metabolites in water, sediment, seaweed and clams in the Sacca di Goro coastal lagoon (northern Adriatic). Chemosphere 69:1625–1637. https://doi.org/10.1016/j.chemosphere.2007.05.060
Carazo-Rojas E, Pérez-Rojas G, Pérez-Villanueva M, Chinchilla-Soto C, Chin-Pampillo JS, Aguilar-Mora P, Alpízar-Marín M, Masís-Mora M, Rodríguez-Rodríguez CE, Vryzas Z (2018) Pesticide monitoring and ecotoxicological risk assessment in surface water bodies and sediments of a tropical agro-ecosystem. Environ Pollut 241:800–809. https://doi.org/10.1016/j.envpol.2018.06.020
Carro N, Cobas J, García I, Ignacio M, Mouteira A (2014) Distribution and trend of organochlorine pesticides in Galicia coast using mussels as bioindicator organisms. Possible relationship to biological parameters. Chemosphere 102:37–47. https://doi.org/10.1016/j.chemosphere.2013.12.010
Carvalho FP, Villeneuve JP, Cattini C, Rendón J, Mota de Oliveira J (2009) Pesticide and PCB residues in the aquatic ecosystems of Laguna de Terminos, a protected area of the coast of Campeche, Mexico. Chemosphere 74:988–995. https://doi.org/10.1016/j.chemosphere.2008.09.092
Chamberlain K, Evans AA, Bromilow RH (1996) 1-Octanol/water partition coefficient (Kow) and pKa for ionisable pesticides measured by a pH-metric method. Pestic Sci 47:265–271. https://doi.org/10.1002/(SICI)1096-9063(199607)47:3<265::AID-PS416>3.0.CO;2-F
Charlestra L, Amirbahman A, Courtemanch DL, Alvarez DA, Patterson H (2012) Estimating pesticide sampling rates by the polar organic chemical integrative sampler (POCIS) in the presence of natural organic matter and varying hydrodynamic conditions. Environ Pollut 169:98–104. https://doi.org/10.1016/j.envpol.2012.05.001
Chelbi M, Gagnon Y, Waewsak J (2015) Solar radiation mapping using sunshine duration-based models and interpolation techniques: application to Tunisia. Energy Convers Manag 101:203–215. https://doi.org/10.1016/j.enconman.2015.04.052
Comoretto L, Chiron S (2005) Comparing pharmaceutical and pesticide loads into a small Mediterranean river. Sci Total Environ 349:201–210. https://doi.org/10.1016/j.scitotenv.2005.01.036
Criquet J, Dumoulin D, Howsam M, Mondamert L, Goossens JF, Prygiel J, Billon G (2017) Comparison of POCIS passive samplers vs. composite water sampling: a case study. Sci Total Environ 609:982–991. https://doi.org/10.1016/j.scitotenv.2017.07.227
Cruzeiro C, Pardal MÂ, Rocha E, Rocha MJ (2015) Occurrence and seasonal loads of pesticides in surface water and suspended particulate matter from a wetland of worldwide interest—the Ria Formosa Lagoon, Portugal. https://doi.org/10.1007/s10661-015-4824-8
Cruzeiro C, Rocha E, Ângelo M, João M (2016) Environmental assessment of pesticides in the Mondego River Estuary (Portugal). https://doi.org/10.1016/j.marpolbul.2015.12.013
De Gerónimo E, Aparicio VC, Bárbaro S et al (2014) Presence of pesticides in surface water from four sub-basins in Argentina. Chemosphere 107:423–431. https://doi.org/10.1016/j.chemosphere.2014.01.039
Desgranges N (2015) Développement d’échantillonneurs passifs de type POCIS pour l’évaluation de la contamination en pesticides des eaux de bassins versants languedociens. Université de Bordeaux
DGEDA (2006) Enquête sur les Structures des Exploitations Agricoles 2005. MARH
DIRECTIVE 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council
Eriksson M, Sodersten E, Yu Z et al (2003) Degradation of polycyclic aromatic hydrocarbons at low temperature under aerobic and nitrate-reducing conditions in enrichment cultures from northern soils. Society 69:275–284. https://doi.org/10.1128/AEM.69.1.275
EU (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy
FAO (2105) Mediterranean coastal lagoons: sustainable management and interactions among aquaculture, capture fisheries and the environment. General fisheries commission for the Mediterranean, Rome
Gao JP, Maguhn J, Spitzauer P, Kettrup A (1998) Sorption of pesticides in the sediment of the Teufelsweiher pond (southern Germany). I: Equilibrium assessments, effect of organic carbon content and pH. Water Res 32:1662–1672. https://doi.org/10.1016/S0043-1354(97)00377-1
Gonzalez-Rey M, Tapie N, Le Menach K et al (2015) Occurrence of pharmaceutical compounds and pesticides in aquatic systems. Mar Pollut Bull 96:384–400. https://doi.org/10.1016/j.marpolbul.2015.04.029
Greenwood R, Mills G, Vrana B (2007) Passive sampling techniques in environmental monitoring. Elsevier, Amsterdam; Oxford
Guo W, Zhang H, Huo S (2014) Organochlorine pesticides in aquatic hydrophyte tissues and surrounding sediments in Baiyangdian wetland, China. Ecol Eng 67:150–155. https://doi.org/10.1016/j.ecoleng.2014.03.047
Hamilton DJ, Ambrus Á, Dieterle RM, Felsot AS, Harris CA, Holland PT, Katayama A, Kurihara N, Linders J, Unsworth J, Wong SS (2003) Regulatory limits for pesticide residues in water (IUPAC technical report). Pure Appl Chem 75:1123–1155. https://doi.org/10.1351/pac200375081123
Herceg-Romanić S, Kljaković-Gašpic Z, Klinčić D, Ujević I (2014) Distribution of persistent organic pollutants (POPs) in cultured mussels from the Croatian coast of the Adriatic Sea. Chemosphere 114:69–75. https://doi.org/10.1016/j.chemosphere.2014.04.017
Hijosa-Valsero M, Bécares E, Fernández-Aláez C, Fernández-Aláez M, Mayo R, Jiménez JJ (2016) Chemical pollution in inland shallow lakes in the Mediterranean region (NW Spain): PAHs, insecticides and herbicides in water and sediments. Sci Total Environ 544:797–810. https://doi.org/10.1016/j.scitotenv.2015.11.160
Ibrahim I (2013) Etude de l’applicabilité des échantillonneurs passifs POCIS et Chemcatcher pour le suivi des pesticides en milieux aquatiques
Ibrahim I, Togola A, Gonzalez C (2013a) Polar organic chemical integrative sampler (POCIS) uptake rates for 17 polar pesticides and degradation products: laboratory calibration. Environ Sci Pollut Res 20:3679–3687. https://doi.org/10.1007/s11356-012-1284-3
Ibrahim I, Togola A, Gonzalez C (2013b) In-situ calibration of POCIS for the sampling of polar pesticides and metabolites in surface water. Talanta 116:495–500. https://doi.org/10.1016/j.talanta.2013.07.028
INERIS (2011) Normes de qualité environnementale du Tébuconazole n° CAS : 107534-96-3
INERIS (2013) Norme de qualité environnementale du Prosulfocarbe n° CAS: 52888-80-9
Jiménez JJ, Bernal JL, Rivera JM, Martin MT, Fernández M, Fernández C (1999) Seasonal changes in the contents of widely used pesticides in waters and sediments from small lakes in the province of Leon (Spain). Toxicol Environ Chem 70:57–66. https://doi.org/10.1080/02772249909358739
Kaushik A, Sharma HR, Jain S, Dawra J, Kaushik CP (2010) Pesticide pollution of river Ghaggar in Haryana, India. Environ Monit Assess 160:61–69. https://doi.org/10.1007/s10661-008-0657-z
Khaled A, El Nemr A, Said TO et al (2004) Polychlorinated biphenyls and chlorinated pesticides in mussels from the Egyptian Red Sea coast. Chemosphere 54:1407–1412. https://doi.org/10.1016/j.chemosphere.2003.10.042
Li Y, Niu J, Shen Z, Zhang C, Wang Z, He T (2014) Spatial and seasonal distribution of organochlorine pesticides in the sediments of the Yangtze estuary. Chemosphere 114:233–240. https://doi.org/10.1016/j.chemosphere.2014.04.112
Liess M, Schulz R, Liess MHD, Rother B, Kreuzig R (1999) Determination of insecticide contamination in agricultural headwater streams. Water Res 33:239–247. https://doi.org/10.1016/S0043-1354(98)00174-2
Lissalde S, Mazzella N, Fauvelle V, Delmas F, Mazellier P, Legube B (2011) Liquid chromatography coupled with tandem mass spectrometry method for thirty-three pesticides in natural water and comparison of performance between classical solid phase extraction and passive sampling approaches. J Chromatogr A 1218:1492–1502. https://doi.org/10.1016/j.chroma.2011.01.040
Lissalde S, Mazzella N, Mazellier P (2014) Polar organic chemical integrative samplers for pesticides monitoring: impacts of field exposure conditions. Sci Total Environ 488–489:188–196. https://doi.org/10.1016/j.scitotenv.2014.04.069
Macdonald RW, Harner T, Fyfe J (2005) Recent climate change in the Arctic and its impact on contaminant pathways and interpretation of temporal trend data. Sci Total Environ 342:5–86. https://doi.org/10.1016/j.scitotenv.2004.12.059
Mai B, Chen S, Luo X, Chen L, Yang Q, Sheng G, Peng P, Fu J, Zeng EY (2005) Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environ Sci Technol 39:3521–3527. https://doi.org/10.1021/es048083x
Martínez Bueno MJ, Herrera S, Munaron D, Boillot C, Fenet H, Chiron S, Gómez E (2014) POCIS passive samplers as a monitoring tool for pharmaceutical residues and their transformation products in marine environment. Environ Sci Pollut Res 23:5019–5029. https://doi.org/10.1007/s11356-014-3796-5
Meakins NC, Bubb JM, Lester JN (1995) The mobility, partitioning and degradation of atrazine and simazine in the salt marsh environment. Mar Pollut Bull 30:812–819. https://doi.org/10.1016/0025-326X(95)00074-W
Mezghani et al (2016) Liste des pesticides Homologués en Tunisie. https://www.neppo.org/wp-content/uploads/2017/08/liste-des-pesticides-homologues-2016.pdf
Miège C, Budzinski H, Jacquet R, Soulier C, Pelte T, Coquery M (2013) Polar organic chemical integrative sampler (POCIS): application for monitoring organic micropollutants in wastewater effluent and surface water. J Environ Monit 14:626–635. https://doi.org/10.1039/c1em10730e
Miège C, Mazzella N, Allan I, Dulio V, Smedes F, Tixier C, Vermeirssen E, Brant J, O’Toole S, Budzinski H, Ghestem JP, Staub PF, Lardy-Fontan S, Gonzalez JL, Coquery M, Vrana B (2015) Position paper on passive sampling techniques for the monitoring of contaminants in the aquatic environment—achievements to date and perspectives. Trends Environ Anal Chem 8:20–26. https://doi.org/10.1016/j.teac.2015.07.001
Momplaisir GM, Rosal CG, Heithmar EM, Varner KE, Riddick LA, Bradford DF, Tallent-Halsell NG (2010) Development of a solid phase extraction method for agricultural pesticides in large-volume water samples. Talanta 81:1380–1386. https://doi.org/10.1016/j.talanta.2010.02.038
Moncada A (2004) Environmental fate of Diuron. Environmental Monitoring Branch Department of Pesticide Regulation 1001 I Street Sacramento, CA 95812-4015
Moreno-González R, León VM (2017) Presence and distribution of current-use pesticides in surface marine sediments from a Mediterranean coastal lagoon (SE Spain). Environ Sci Pollut Res 24:8033–8048. https://doi.org/10.1007/s11356-017-8456-0
Mzoughi N, Hellal F, Dachraoui M, Villeneuve JP, Cattini C, de Mora SJ, el Abed A (2002) Méthodologie de l’extraction des hydrocarbures aromatiques polycycliques. Application à des sédiments de la lagune de Bizerte (Tunisie). Compt Rendus Geosci 334:893–901. https://doi.org/10.1016/S1631-0713(02)01827-8
Mzoughi N, Lespes G, Bravo M, Dachraoui M, Potin-Gautier M (2005) Organotin speciation in Bizerte lagoon (Tunisia). Sci Total Environ 349:211–222. https://doi.org/10.1016/j.scitotenv.2004.12.067
Navarro S, Vela N, José Giménez M, Navarro G (2004) Effect of temperature on the disappearance of four triazine herbicides in environmental waters. Chemosphere 57:51–59. https://doi.org/10.1016/j.chemosphere.2004.05.016
Necibi M, Mzoughi N, Néjib Daly Yahia M, Pringault O (2015) Distributions of organochlorine pesticides and polychlorinated biphenyl in surface water from Bizerte lagoon, Tunisia. Desalin Water Treat 56:2663–2671. https://doi.org/10.1080/19443994.2015.1024935
Nefzaoui A, Ketata H, El-Mourid M (2012) Changes in North Africa production systems to meet climate uncertainty and new socio-economic scenarios with a focus on dryland areas. Options Méditerranéennes : Série A. Séminaires Méditerranéens 421:403–421
Ouertani N, Hamouda R, Belayouni H (2006) Etude de la matière organique dans les sèdiments superficiels d’un système aquatique confinè anoxique èvoluant en milieu urbain: Cas du Lac sud de Tunis. Geo Eco Trop 30:21–34
Palma P, Köck-Schulmeyer M, Alvarenga P, Ledo L, Barbosa IR, López de Alda M, Barceló D (2014) Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci Total Environ 488–489:208–219. https://doi.org/10.1016/j.scitotenv.2014.04.088
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E (2015) Ecotoxicology and environmental safety a pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf 116:1–9. https://doi.org/10.1016/j.ecoenv.2015.02.033
PAPP R (2010) Les propriétés environnementales des substances Rév-Roger Rapp CNEEIC- Collège National d’Experts en Environnement de l’Industrie Chimique
Poulier G, Lissalde S, Charriau A, Buzier R, Delmas F, Gery K, Moreira A, Guibaud G, Mazzella N (2014) Can POCIS be used in water framework directive (2000/60/EC) monitoring networks? A study focusing on pesticides in a French agricultural watershed. Sci Total Environ 497–498:282–292. https://doi.org/10.1016/j.scitotenv.2014.08.001
Poulier G, Lissalde S, Charriau A, Buzier R, Cleries K, Delmas F, Mazzella N, Guibaud G (2015) Estimates of pesticide concentrations and fluxes in two rivers of an extensive French multi-agricultural watershed: application of the passive sampling strategy. Environ Sci Pollut Res 22:8044–8057. https://doi.org/10.1007/s11356-014-2814-y
Ralston-Hooper K, Hardy J, Hahn L, Ochoa-Acuña H, Lee LS, Mollenhauer R, Sepúlveda MS (2009) Acute and chronic toxicity of atrazine and its metabolites deethylatrazine and deisopropylatrazine on aquatic organisms. Ecotoxicology 18:899–905. https://doi.org/10.1007/s10646-009-0351-0
Salem FB (2017) First survey of agricultural pesticides used for crops in Ichkeul Lake–Bizerte lagoon watershed (Tunisia). Environ Sci Ind J 13:1–10
Salem FB, Said OB, Aissa P et al (2016) Pesticides in Ichkeul Lake–Bizerta lagoon watershed in Tunisia: use, occurrence, and effects on bacteria and free-living marine nematodes. Environ Sci Pollut Res 23:36–48. https://doi.org/10.1007/s11356-015-4991-8
Savinov V (2000) Polycyclic aromatic hydrocarbons (PAHs) in sediments of the White Sea, Russia. Mar Pollut Bull 40:807–818. https://doi.org/10.1016/S0025-326X(00)00004-7
Terzopoulou E, Voutsa D (2016) Active and passive sampling for the assessment of hydrophilic organic contaminants in a river basin-ecotoxicological risk assessment. Environ Sci Pollut Res 23:5577–5591. https://doi.org/10.1007/s11356-015-5760-4
UNEP (2001) Stockholm Convention on Persistent Organic Pollutants (POPs)
Van Metre PC, Alvarez DA, Mahler BJ et al (2017) Complex mixtures of pesticides in Midwest U.S. streams indicated by POCIS time-integrating samplers. Environ Pollut 220:431–440. https://doi.org/10.1016/j.envpol.2016.09.085
Vryzas Z, Vassiliou G, Alexoudis C, Papadopoulou-Mourkidou E (2009) Spatial and temporal distribution of pesticide residues in surface waters in northeastern Greece. Water Res 43:1–10. https://doi.org/10.1016/j.watres.2008.09.021
Wu SC, Gschwend PM (1986) Sorption kinetics of hydrophobic organic compounds to natural sediments and soils. Environ Sci Technol 20:717–725. https://doi.org/10.1021/es00149a011
Yabuki Y, Nagai T, Inao K, Ono J, Aiko N, Ohtsuka N, Tanaka H, Tanimori S (2016) Temperature dependence on the pesticide sampling rate of polar organic chemical integrative samplers (POCIS). Biosci Biotechnol Biochem 80:2069–2075. https://doi.org/10.1080/09168451.2016.1191329
Yohannes YB, Ikenaka Y, Saengtienchai A, Watanabe KP, Nakayama SMM, Ishizuka M (2014) Concentrations and human health risk assessment of organochlorine pesticides in edible fish species from a Rift Valley Lake—Lake Ziway, Ethiopia. Ecotoxicol Environ Saf 106:95–101. https://doi.org/10.1016/j.ecoenv.2014.04.014
Zaghden H, Tedetti M, Sayadi S, Serbaji MM, Elleuch B, Saliot A (2017) Origin and distribution of hydrocarbons and organic matter in the surficial sediments of the Sfax-Kerkennah channel (Tunisia, southern Mediterranean Sea). Mar Pollut Bull 117:414–428. https://doi.org/10.1016/j.marpolbul.2017.02.007
Zhang J, Liu F, Chen R, Feng T, Dong S, Shen H (2012) Levels of polychlorinated biphenyls and organochlorine pesticides in edible shellfish from Xiamen (China) and estimation of human dietary intake. Food Chem Toxicol 50:4285–4291. https://doi.org/10.1016/j.fct.2012.08.052
Zhang Z, Troldborg M, Yates K, Osprey M, Kerr C, Hallett PD, Baggaley N, Rhind SM, Dawson JJC, Hough RL (2016) Evaluation of spot and passive sampling for monitoring, flux estimation and risk assessment of pesticides within the constraints of a typical regulatory monitoring scheme. Sci Total Environ 569–570:1369–1379. https://doi.org/10.1016/j.scitotenv.2016.06.219
Acknowledgements
This study was financed by LMI Cosy-MED (IRD), Institut Mines Télécom Mines Alès, and the Tunisian Ministry of Higher Education and Scientific Research (Carthage University), The authors would like to thank the Tunisian farmers and fishermen of Bizerte for their kind assistance in the monitoring studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Electronic supplementary material
ESM 1
(DOC 65 kb)
Rights and permissions
About this article
Cite this article
Mhadhbi, T., Pringault, O., Nouri, H. et al. Evaluating polar pesticide pollution with a combined approach: a survey of agricultural practices and POCIS passive samplers in a Tunisian lagoon watershed. Environ Sci Pollut Res 26, 342–361 (2019). https://doi.org/10.1007/s11356-018-3552-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3552-3