Abstract
Urban agriculture is crucial to local populations, but the risk of it contaminating water has rarely been documented. The aim of this study was to assess pesticide contamination of surface waters from the Méfou watershed (Yaoundé, Cameroon) by 32 selected herbicides, fungicides, and insecticides (mainly polar) according to their local application, using both grab sampling and polar organic compounds integrative samplers (POCIS). Three sampling campaigns were conducted in the March/April and October/November 2015 and June/July 2016 rainy seasons in urban and peri-urban areas. The majority of the targeted compounds were detected. The quantification frequencies of eight pesticides were more than 20% with both POCIS and grab sampling, and that of diuron and atrazine reached 100%. Spatial differences in contamination were evidenced with higher contamination in urban than peri-urban rivers. In particular, diuron was identified as an urban contaminant of concern because its concentrations frequently exceeded the European water quality guideline of 0.200 μg/L in freshwater and may thus represent an ecological risk due to a risk quotient > 1 for algae observed in 94% of grab samples. This study raises concerns about the impacts of urban agriculture on the quality of water resources and to a larger extent on the health of the inhabitants of cities in developing countries.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban populations are increasing rapidly, and by 2025, 52% of the world’s population is predicted to be living in cities. Africa has the highest population growth rate in the world at 2.55% annually from 2010 to 2015, and the continent is expected to be home to one quarter of the world population (1.3 billion people) by 2050 (AFD 2014).
Among other developments taking place in cities, urban agriculture could be a key response to the food security challenge (Hamilton et al. 2014). Urban agriculture is defined as crop production and livestock farming within the city and in its outskirts (Mougeot 2000). From a theoretical point of view, urban agriculture has been integrated in the “urban metabolism” as its inputs including wastewaters, and recycling of domestic wastes, come from the city and its production is mainly intended for sale on local markets (Zeeuw et al. 2011). In developing countries, unlike the recreational, pedagogical, and environmental functions urban agriculture fulfills in the highest income countries (Aubry and Pourias 2013; White and Bunn 2017), this kind of agriculture is indispensable to feed the inhabitants cities (Hamilton et al. 2014) and provides additional benefits ranging from job creation and related services to social interactions and landscape improvement (Aubry et al. 2012; Mougeot 2000). The risks related to the contamination of plant products not only by fecal coliforms have mainly been evaluated in the context of the lack of sanitation but also by chemical pollutants in urban discharges (Abass et al. 2016; Binns et al. 2003; Huang et al. 2018; Padgham et al. 2015).
Urban agriculture uses synthetic herbicides, insecticides, and fungicides to reduce crop losses caused by pests and to improve the yields of cash and food crops (Oerke 2006). Monitoring studies across the five continents (Sousa et al. 2018) have drawn attention to the risk of pesticides contaminating natural water resources (Elfman et al. 2011; Herrero-Hernández et al. 2017; Mac Loughlin et al. 2017; Rippy et al. 2017; Teklu et al. 2016). Several studies in sub-Saharan Africa have reported contamination of water by pesticides in rural areas where cash crops are produced in intense agricultural systems. A wide range of insecticides, fungicides, and herbicides and in particular organochlorine pesticides has been monitored. The latter were shown to reach concentrations up to several micrograms per liter (Adeyemi et al. 2011; Berthe Dem et al. 2007; Dalvie et al. 2003; Elibariki and Maguta 2017).
However, no data are currently available concerning pesticide contamination of urban surface waters due to their proximity to urban agriculture in sub-Saharan Africa, raising key questions with respect to the increase in urban agriculture in parallel with increased urbanization.
The capital city of Cameroon, Yaoundé, is a good example of the combination of both population growth and urban and peri-urban agriculture practices that involve the massive use of pesticides (Gockowski and Ndoumbé 1999; Temple et al. 2009). The aim of this study was thus to monitor the contamination of surface water by pesticides in the specific context of urban and peri-urban sites impacted by agricultural practices. In Yaoundé, 21 herbicides, 10 fungicides, and one insecticide were selected based on their potential application on local crops (PPDB 2011) according to their homologation in Cameroon by the Sahelian Pesticide Committee (CSPFootnote 1) and the Cameroonian Ministry of Agriculture and Rural Development (MINADERFootnote 2) (CSP INSAH 2015; Ministère de l’agriculture et du développement rural (Cameroun) 2013). The log Kow of the selected pesticides ranges from − 0.06 to 4.48, so they are rather polar and tend to be transferred to surface waters. Sampling was performed using complementary tools, grab, and passive sampling (polar organic chemical integrative samplers, POCIS). Ten plots distributed across specific urban (Mfoundi River) and peri-urban (Méfou River) areas were monitored during the 2-year study with three campaigns conducted in March/April, October/November 2015, and June/July 2016.
Our specific objectives were (i) to evaluate the occurrence of polar pesticides (mainly herbicides and fungicides) in surface waters in the Méfou watershed, (ii) to quantify pesticide contamination in urban and peri-urban areas, and (iii) to assess the potential risks for ecosystems. This study is a step toward a better understanding of the contamination and fate of pesticides in the Méfou basin and in sub-Saharan Africa cities linked to urban agriculture.
Materials and methods
Study area and sampling sites
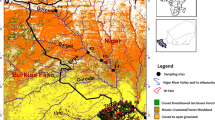
Yaoundé, located in the Center Region, Cameroon, counted 1.9 million inhabitants in 2005 with an annual growth rate of 5.7% per year between 1987 and 2005 according to the Central Office of Cameroonian Population Study and Census. The Méfou River and its main tributary the Mfoundi River drain Yaoundé and are particularly concerned by agricultural inputs in addition to inputs of wastewater and industrial wastes (Endamana et al. 2003). The Méfou watershed (Fig. 1) is located in the Center Region of Cameroon (latitude 3° 30′ to 3° 58′; longitude 11° 20′ to 11° 40′) and covers an area of 840 km2 (Olivry 1986). The source of the Méfou River is 40 km northwest of Yaoundé, in the Mbam Minkom Mountains (high point of 1245 m asl), and joins the Nyong River on its right side 121 km downstream in the northwest-southeast direction. The region has a Guinean climate characterized by two rainy seasons (March–June and September–November) and two dry seasons (December–February and July–August); the annual rainfall is 1600 mm.
The watershed is hilly with submersible swampy valleys. Soils are ferralitic on the top of the hills and on the slopes. The soils in the lowlands are hydromorphic and originate from slope colluvium and river alluvium (Bachelier 1959).
It is a mixed land use basin, and three main areas can be distinguished. The northwest part is constituted of humid semi-deciduous tropical forest anthropized mostly by farmlands. The northeast part is occupied by the Mfoundi River sub-watershed. The southern part is made up of a mildly deteriorated forest and swampy area, with more and more habitations (Lieunang Letche et al. 2009). Yaoundé’s raw water resource is surface water. A catchment station is situated at the Méfou artificial lake on the upper part of the Méfou basin surrounded by peri-urban area. A second water catchment is located downstream the confluence between the Méfou and Nyong rivers.
The Mfoundi River is one of the Méfou’s major tributaries and drains the city of Yaoundé (latitude 3° 52; longitude 11° 31). It stretches on 180 km2 and ranges in altitude between 630 and 1100 m. The Mfoundi River receives 12 secondary tributaries including the Biyeme River, which is its main tributary and drains the western part of the basin. Land use is 40% of urbanization, 28.8% of intermediate areas (beaten soil), 17% of forests, 6% of green areas, and 0.2% of surface waters. Agricultural activities account for 8% of the area (Open Street Map 2016 data).
Sampling stations were selected for their spatial distribution across the hydrographic network. They are distributed over the upper Méfou sub-watershed and the Mfoundi basin (Fig. 1). Five sites are situated on the Méfou River, before and after its confluence with the Mfoundi River, in a peri-urban environment. Five other stations are situated in the urban Mfoundi basin, with four sites on the Mfoundi River and one site on the Biyeme River. They are numbered from 1 to n according to their location from upstream to downstream.
Sampling procedure
Samples were collected in the rainy seasons in 2015 (March/April and October/November) and at the end of the short rainy season in 2016 (June/July) to better assess contamination in the same hydrological context (Table 1). Even though no clear pattern of the use of pesticides was identified, crops appeared to be treated more frequently in the rainy seasons (Isogai et al. 2016). Both passive and grab samplings were performed at the Méfou and Mfoundi sites (Fig. 1; Table 1). POCIS were deployed in duplicate for a mean period of 20 ± 3 days and at a depth of 1 to 2 m according to guidelines in the literature (Alvarez et al. 2008; Ibrahim et al. 2013). At the same time, POCIS were deployed, and grab samples were retrieved at a depth of about 1 m with a 1-L glass bottle previously cleaned with a potassium hydroxide solution (Neodisher® LM3 from Dr. Weigert, France) and Milli-Q water to avoid cross contamination. After sampling, POCIS samples and the glass bottles were stored at 4 °C until treatment within 24 h.
Chemicals and materials
The 32 targeted products (21 herbicides including 7 degradation products, 10 fungicides, and 1 insecticide) are listed in Table 2. Of the targeted compounds, 32% are homologated in Cameroon by the Ministry of Agriculture and Rural Development (MINADER) (Ministère de l’agriculture et du développement rural (Cameroun) 2013). Five of the pesticides are also on the list of pesticides homologated by the Sahelian Pesticide Committee (CSP) (CSP INSAH 2015) and are thus likely to be applied to local crops (Table 2). To date, very few data are available on the occurrence of polar pesticides in surface waters in sub-Saharan Africa, but atrazine and its degradation products desethyl atrazine (DEA) and desisopropyl atrazine (DIA), carbendazim, imidacloprid, and metalaxyl have been identified in waters in Burkina Faso and Ethiopia (Jansen and Harmsen 2011; Lehmann et al. 2017) reinforcing our choice to include them in the present study.
Analytical standards (purity > 99%) were purchased from Cluzeau Info Labo (Sainte-Foy-la-Grande, France). Deuterated labeled compounds used were atrazine-d5 (CAS: 163165-75-1) as recovery control and simazine-d5 (CAS: 220621-41-0) as internal standard. Acetonitrile, the high-performance liquid chromatography (HPLC) grade solvent, was purchased from Biosolve (Dieuze, France). Formic acid purchased from Carlo Erba Reagents (Peypin, France) was added to the acetonitrile for the HPLC gradient. Milli-Q water was generated by a MilliPore Synergy UV water purification system from Merck Millipore (Billerica, USA). Glass-fiber filters (GF/F) (with 0.7 μm pore size) purchased from Whatman (Maidstone, UK) were used to filter water samples. Oasis HLB cartridges (60 μm, 6 cm3, 500 mg) were purchased from Waters Corporation (Milford, USA), and a Visiprep Solid Phase Extraction (SPE) vacuum manifold from Supelco (Bellefonte, USA) was used for solid phase extraction. POCIS were purchased from ExposMeter AB Company (Tavelsjö, Sweden) with approximately 230 mg of the solid adsorbent N-vinylpyrrolidone-divinylbenzene (Oasis HLB). The POCIS sampling area is 41 cm2. The 3 mL polypropylene cartridges used to recover POCIS receiving phases were purchased from Supelco (Bellefonte, USA).
Pesticide analysis
Sample treatment
Receiving phases of the POCIS were transferred into 3 mL SPE cartridges, spiked with 50 μL of atrazine-d5 (1 mg/L acetone), and then eluted with 8 mL of acetonitrile. Filtered water samples were extracted using Oasis HLB cartridges previously conditioned with 5 mL acetonitrile, 5 mL methanol, and then 5 mL of ultrapure water at 5 mL/min. Prior to extraction, each water sample was fortified with 50 μL of atrazine-d5 (1 mg/L acetone). The samples were passed through the cartridges. Before elution, the cartridges were dried under vacuum for 1 h. Analytes were recovered by eluting the cartridges with 8 mL of acetonitrile at a flow rate of 3 mL/min. After concentration under a gentle stream of nitrogen, final extracts (1.5 mL) were spiked with 150 μL of the internal standard simazine d-5 (1 mg/L acetonitrile) and analyzed by HPLC-MS/MS.
Instrumentation and analysis
Extracts were analyzed with an Alliance HPLC system (Waters Series 2695). The stationary phase is a Kinetex reverse phase C18 (100 mm × 4.6 I.D × 260 Å, Phenomenex). The mobile phase is composed of Milli-Q and acetonitrile, both with 0.05% formic acid, at a constant flow rate of 0.4 mL/min. The gradient was programmed so the proportion of acetonitrile increased from 60 to 100% in 10 min followed by stabilization for 2 min, before returning to initial conditions. This system was coupled with a triple quadrupole mass spectrometer Micromass Quattro micro API (Waters) fitted with an ESI source operating in positive ion mode. Argon was used as collision gas. The multiple reaction monitoring mode was then used for the ion specific acquisition.
Analytical results were exploited with MassLynx software from Waters. Its Quantlynx interface enables the quantification of the targeted substances. Analytical parameters are presented in Supplementary Material.
Quality assurance/quality control
The linearity, LOQs and LODs, precision, and accuracy of the analytical methods were carefully validated. The LODs were calculated as 3 × Sy/x/b and the LOQs as 10 × Sy/x/b, where Sy/x is the residual standard deviation and b is the slope of the matrix calibration curves. The LODs and LOQs obtained by HPLC/MS (analytical LOD and LOQ expressed on μg/L) as well as LOD and LOQ in grab water sample (ng/L) are listed in Table 2. LODs and LOQs for the calibration curves of all selected pesticides were prepared for each analytical campaign obtaining R2 > 0.99.
The mean recovery rate in the synthetic water solution spiked with the 32 compounds under study (Table 2) was 69%, with the highest recovery for simazine (101%) and the lowest for DCPMU (19%). Flazasulfuron, terbuthylazine hydroxy, and simazine hydroxy were not recovered.
A certificated reference material (WaR™ Pollution Nitrogen Pesticides, Lot No. P246-674) from the ERA Waters Company (Golden, USA) was used to determine the pesticide recoveries in the water samples. The reference material was a water solution composed of 24 pesticides including the 6 pesticides selected in our study (alachlor, atrazine, DEA, DIA, metolachlor, and simazine). The concentrations of pesticides in the reference material ranged from 3.37 to 16.80 μg/L. The recovery yields and variation coefficients were 108 ± 4% (atrazine), 78 ± 4% (alachlor), 61 ± 3% (DEA), 58 ± 3% (DIA), 110 ± 2% (metolachlor), and 79 ± 2 (simazine) after solid-phase extraction (HLB) and HPLC/MS analysis (n = 9) of the water sample of the reference material using our laboratory method.
The mean extraction recoveries with atrazine-d5 were 85 ± 10% for the grab samples (n = 70 samples) and 90 ± 11% for the passive samples (n = 32 samples). The mean variation coefficient with the internal standard simazine-d5 was 17 ± 5% for all the HPLC/MS injections (n = 305 injections).
Concentration of pesticides in the water over the POCIS deployment period
Accumulation of contaminants by passive samplers typically follows first-order kinetics which includes an initial integrative phase followed by curvilinear and equilibrium-partitioning phases. In the linear region of POCIS uptake, the amount of a chemical accumulated in the sampler (M) is described by Eq. (1):
where Rs is the sampling rate (L/day), Cw is the concentration of the compound in water (ng/L), and t the exposure time (days).
The time-weighted averaged (TWA) concentration in water is calculated with the Eq. (2):
with
- Cw:
the concentration of pesticide in water (ng L−1)
- Md:
mass of accumulated pesticides (ng)
- Mo:
mass of compounds in initial receiving phase (ng)
- Rs:
sampling rate (L day−1) (Table 2)
- t:
duration of POCIS deployment (days)
POCIS sampling rates (Table 2) were determined in laboratory conditions for each compound (Ibrahim et al. 2012, 2013). The quantities of pesticides that accumulated in POCIS were converted into nanograms per liter with pesticide sampling rates. For each pesticide, the sampling rate was determined by dividing the slope of the linear regression curve by the mean aqueous concentration of the selected compounds over a 15-day period of exposure. For the compounds whose sampling rates were not determinate in our laboratory, Rs values (also determined in laboratory conditions) were taken from the literature (Ahrens et al. 2015; Desgranges 2015; Greenwood et al. 2007; Poulier et al. 2014). As they depend on environmental conditions such as flow, temperature, pH, organic matter, and biofouling (Charlestra et al. 2012; Yabuki et al. 2016), Rs makes it possible to calculate semi-quantitative concentrations of pesticides in water and to compare the relative levels of contamination between sites.
Physical-chemical measurements
The general water quality parameters (pH, conductivity, dissolved oxygen and temperature) were measured in situ with Hach HQ40d multi-parameter probes during the March/April 2015 campaign and with YSI Professional Plus in the October/November 2015 and June/July 2016 campaigns at the same time as each grab sampling of water for pesticide analysis.
Statistical analyses
R software (R Core Team 2017, “https://www.r-project.org/”) was used for Spearman’s rank-order correlation with a p value < 0.05.
Ecological risk assessment
The ecological risk quotient (RQ) was calculated according to OCDE and European guidelines for diuron and atrazine. The RQ was calculated as a chronic toxicity test on three representative trophic levels: algae (Scenedesmus quadricauda and green algae), an aquatic invertebrate (Daphnia magna) and a fish species (Oncorhynchus mykiss). The non-observed effect concentration (NOEC) of each pesticide considered was obtained from the Pesticide Properties Database of the University of Hertfordshire (https://sitem.herts.ac.uk/aeru/ppdb/en/index.htm). NOEC corresponded to chronic 21 days exposure for D. magna and O. mykiss, and the median efficient concentration (EC50) of an acute 72 h exposure was used for algae. The effects studied were growth inhibition (algae), immobilization (D. magna) and survival (O. mykiss). A safety factor of 1000 was applied to calculate the predicted non-effect concentration (PNEC) in order to consider the inherent uncertainty in the obtained laboratory toxicity data (Eq. 3)
The RQ was calculated using Eq. 4 with the measured environmental concentration (MEC) of the grab samples (μg/L). For each sample, the highest concentration measured during the considered campaign defined the MEC (worst-case scenario).
Ecological risk assessment (ERA) was conducted at the urban sites sampled throughout the three campaigns (Mfoundi1, Mfoundi2, Mfoundi3, Mfoundi4, and Biyeme1) and at the peri-urban site Méfou4 that corresponds to the outlet of the upstream Méfou sub-watershed. Atrazine and diuron were the two herbicides selected for the ERA because they were the main pesticides identified by the contamination diagnosis. Their maximum concentrations in each campaign determined the measured environmental concentration (MEC), thus representing a worst-case scenario.
Results and discussion
Contamination of surface waters of the Méfou watershed by herbicides, fungicides, and insecticide
Herbicides, fungicides, and insecticide in the water samples
Taken together (all sites and all campaigns), a total of 30 pesticides (94%) were at least detected (Fig. 2a). Only the herbicides DET and terbuthylazine were never detected. Twenty-four pesticides (75%) were quantified at least once. The herbicides DEA, flazasulfuron, propyzamide, simazine hydroxy (degradation product of simazine), terbuthylazine hydroxy (degradation product of terbuthylazine), and the fungicide prochloraze were never quantified (Fig. 2b).
Quantification frequencies differed between grab sampling and POCIS (Fig. 2b). To demonstrate the complementarity of the sampling tools, we selected sites sampled in all the three campaigns using both grab and passive methods: the outlet of Mfoundi basin (Mfoundi4), Biyeme River site (Biyeme1), and the outlet of the upper Méfou River (Méfou4). POCIS allowed quantification of more compounds than grab sampling: the herbicides oxadixyl, linuron, prosulfocarb and DIA, and the fungicides tebuconazole and azoxystrobin. These compounds were not detected by grab sampling. The fungicide dimetomorph was quantified by grab sampling but not with POCIS (Fig. 2b).
Quantified, detected, and undetected pesticide frequencies differed according to the sampling period and type (Table 3). In all the campaigns, POCIS always enabled quantification of more compounds than grab sampling, with quantification percentages ranging from 28 to 72% versus 9 to 53% for grab sampling. The highest proportion of compounds was quantified in the March/April 2015 campaign (quantification frequencies 53–72%). Smaller proportions of compounds were quantified in the two other campaigns (quantification frequencies 9–31%).
Grab and POCIS sampling were complementary tools to monitor contamination of surface water by pesticides. Passive sampling is recommended water policy in the European Commission Guidance Document (EC Guidance document no. 19) and in Directive 2013/39/EU (EU 2013) for priority substances as a complementary method to improve the quality of the assessment. According to previous studies, for a representative diagnosis of pesticide occurrence, they should be used as a complement to grab sampling (Dworak et al. 2005; Miège et al. 2015; Poulier et al. 2014).
POCIS concentrations were in good agreement with average concentrations of pesticides in water (Criquet et al. 2017). The mean Rs of the compounds targeted in the present study was 0.265 L/day. Several pesticides have a lower Rs, ranging from 0.133 L/day (DEA) to 0.195 L/day (propyzamide). Four compounds in particular have a very low Rs (DIA 0.068 L/day, prochloraze 0.080 L/day, prosulfocarb 0.071 L/day), so their concentrations (DIA, prosulfocarb) may have been underestimated. On the contrary, dimetomorph has a high Rs (0.395 L/day) and was not quantified by POCIS but was quantified with grab sampling.
The use of POCIS made it possible to compare the pesticide TWA concentration between sampling sites in an urban environment in tropical conditions (median water temperature 22–27 °C and intense rainfall) and between periods whereas grab sampling in nanograms per liter makes it possible to acquire “snapshots” of pesticide contamination between sampling periods and to compare these values with data in the literature and with water quality norms.
Levels of pesticide contamination of water samples
The pesticides with quantification frequencies greater than 20% in both passive and grab sampling were the four herbicides diuron (89–100%), atrazine (28–100%), acetochlor (22–100%), and metolachlor (22–89%) and the four fungicides metalaxyl (28–100%), carbendazim (22–78%), tetraconazole (22–33%), and penconazole (22%) (Fig. 2b). Of these, diuron and atrazine had the highest concentrations (Tables 4 and 5). In grab samples, diuron concentrations ranged from 8 ± 2 to 13,893 ± 3209 ng/L and atrazine concentrations from 9 ± 1 to 461 ± 157 ng/L. DCPMU and DCPU degradation products of diuron were also quantified (17–78 and 11–33%, respectively) with concentrations ranging from 5 ± 2 to 11 ± 5 ng/L. Detection frequencies of DEA and DIA, two degradation products of atrazine, ranged from 11 to 33%. Mean concentrations of the other pesticides in each campaign reached a maximum of 159 ng/L: acetochlor (30 ± 7 ng/L), metolachlor (17 ± 4 ng/L), carbendazim (19 ± 13 ng/L), metalaxyl (16 ± 1 ng/L), penconazole (159 ± 151 ng/L), and tetraconazole (5 ± 2 ng/L).
With grab sampling, the highest concentrations of the two main pesticides (atrazine and diuron) were measured in the urban area on March 11 (Fig. 3). Water sampled at the same sites 3 weeks later (on March 31) had lower concentrations of diuron, which ranged from 77 ± 13 to 213 ± 20 ng/L, and of atrazine, which ranged from 19 ± 3 to 42 ± 9 ng/L. In the other campaigns, diuron concentrations ranged from 50 ± 8 to 874 ± 54 ng/L in October/November 2015 and from 29 ± 4 to 141 ± 7 ng/L in June/July 2016. Atrazine concentrations ranged from the detection limit to 27 ± 2 ng/L in October/November 2015 and ranged from the LOD to 9 ± 1 ng/L in June/July 2016. These two campaigns were conducted during the long rainy season and at the end of the short rainy season/beginning of the short dry season. However, two high peaks of diuron were measured at Mfoundi2 (809 ± 70 ng/L) and Mfoundi4 (874 ± 54 ng/L) that could be linked to point contamination.
The same observations were made concerning POCIS TWA concentrations. Diuron reached 369 ± 169 ng/L with a mean concentration of 145 ± 10 ng/L in March/April 2015. During the two other campaigns, mean TWA concentrations were 9 ± 12 ng/L (peri-urban area) and 52 ± 10 ng/L (urban area) in October/November 2015; mean TWA concentrations were 24 ± 29 ng/L (peri-urban area) and 63 ± 29 ng/L (urban area) in June/July 2016. Atrazine reached 38 ± 13 ng/L with a mean of 14 ± 7 ng/L in March/April 2015. The mean concentration of atrazine in the other campaigns ranged from 3 ± 1 to 6 ± 0.5 ng/L.
Variations in pesticide contamination
Instream pesticide concentrations can be influenced by the period and intensity of the rainfall. Heavy rainfall can drain pesticides from where they are applied and cause a first flush effect i.e., a peak of contamination. After this phenomenon, concentrations may be diluted by an increase in flow (Palma et al. 2004). In the present study, samples were collected under rainy climatic conditions. Daily rain data are currently available for the year 2015. Rainfall events occurred 3 days before the March 11 samplings, when the recorded rainfall for the 3 days was 27.2, 1, and 3.4 mm, respectively (Fig. 3). These rainfall events followed 8 days without rain at the end of the long dry season that lasts from December to February. No rain fell in January 2015, but nine rainfall events occurred in February 2015, with a maximum of 39.8 mm. These rainfall events marked the beginning of the short rainy season that lasts from March to May. This rain may have drained pollutants from crops and soils in the form of runoff and could explain why the highest pesticide concentrations were found on samples taken on March 11. The next rainfall event lasted for several days between March 11 and March 31 and could have diluted pollutants instream (Masiá et al. 2013).
The ubiquity and the high concentrations of diuron are evidence for diffuse contamination of the Mfoundi River. The highest peak (13,894 ± 3209 μg/L) measured in one sample in March 2015 might correspond to point contamination, compared to the other lower concentrations. More frequent supplementary sampling is needed for a complete understanding of variations in pollutants over time. Atmospheric monitoring of pesticides by Isogai et al. (2016) demonstrated higher organochlorine pesticide concentrations in the rainy season (June–August 2012) in Cameroon and in particular in Yaoundé. These authors’ hypothesis suggests that pesticides inputs are higher in the rainy season. However, with grab sampling, the highest concentrations were measured at the beginning of the short rainy season (March) and later diluted in streams following heavier rainfall.
Water pesticide contamination in the international context
Because of their biological effect on living organisms and their potential impacts on ecosystems, pesticides have been classified as hazards by the WHO (Organization and Safety 2010). Environmental concentrations of pesticides are consequently monitored and regulated in several countries across the world (IUPC 2003). Among the eight pesticides selected in this study, the herbicides (acetochlor, atrazine, diuron, metolachlor) and one fungicide (penconzaole) are classified as slightly hazardous (class III). Carbendazim is classified as “unlikely to present acute hazard in normal use” (class U). Tetraconazole is classified as moderately hazardous (class II). Cameroon has no specific guidelines on pesticide concentrations, so national and international guidelines on water quality standards can be used as references. Atrazine and metolachlor do not exceed the maximum acceptable concentration of 1.8 and 7.8 μg/L respectively in Canada (CCME 2014). In the European Water Framework guidelines, the European Environmental Quality Standard Annual Mean for freshwater is 0.600 μg/L for atrazine and 0.200 μg/L for diuron. Diuron exceeded these guidelines in 60% of the grab samples in March/April 2015 and in 30% of grab samples in October/November 2015.
Pesticides have been monitored all over the world (Sousa et al. 2018), and herbicides are the main compounds detected. In particular, urea (diuron and its degradation products) and triazine (atrazine) herbicides are frequently quantified (Herrero-Hernández et al. 2017; Mac Loughlin et al. 2017; Ryberg and Gilliom 2015). In Africa, levels of pesticide contamination reported in previous research are in the same range as those found in the present study and mainly focused on non-polar compounds (log Kow of 3 up to 6.91) in the agricultural environment. Water organochlorine pesticides were monitored in Botswana (Mmualefe et al. 2009), Ethiopia (Teklu et al. 2016), Mali (Berthe Dem et al. 2007), Nigeria (Okoya et al. 2013), and South Africa (Dalvie et al. 2003) in agricultural environments. The main pesticide concentrations in surface waters in Botswana ranged from 2.4 μg/L (4,4′-DDD) to 61.4 μg/L (HCB) (Mmualefe et al. 2009). In irrigated vegetable crop systems and floriculture areas in Ethiopia, the majority of organochlorine pesticides searched for were not detected in surface waters, except heptachlor epoxide B (115 ng/L) and a-chlordane (192 ng/L) in river samples and g-chlordane (10,100 ng/L) and β-HCB (2720 ng/L) in downstream irrigation systems (Teklu et al. 2016). In cotton-growing areas in Mali, lindane, endosulfan I, endosulfan II, endosulfan sulfate, dieldrin and p,p′-DDD, p,p′-DDE were detected and atrazine was quantified (1400 ng/L) in surface waters (Berthe Dem et al. 2007). In the cocoa producing area of Nigeria, dieldrin ranged from nd to 1510 ng/L (Okoya et al. 2013). Also, in Nigeria, higher concentrations of organochlorine pesticides were measured in agricultural land in the vicinity of Lagos Lagoon (chlordane, endosulfan, HCB, dieldrin, aldrin; range 1–966 ng/L) than in rivers in urban Lagos (Adeyemi et al. 2011). In South Africa, endosulfan regularly exceeded the European Drinking Water Standard of 0.1 μg/L in sub-surface drain and dam sites (Dalvie et al. 2003). The same conclusion was reached for organochlorine pesticide contamination of the Kikavu River in Tanzania (Hellar-Kihampa 2011).
In a previous study, Anderson et al. (2014) also employed passive samplers made of low-density polyethylene tubing to monitor a wide range of non-polar pesticides but not polar compounds which was the case in our study. The concentrations reported by these authors were in the range of few nanograms per liter for the non-polar compounds, mostly DDT and permethrins, in the agricultural watersheds of the Niger and Senegal rivers. However, the methods often used to assess pesticide concentrations only measure freely dissolved chemicals, whereas—depending on their octanol-water partition coefficient—some of the compounds are bound on soil and sediment particles.
This is thus one of the first studies to highlight the occurrence of polar to moderate polar pesticides (log Kow comprised between − 0.06 and 4.14) in an urban and peri-urban environment in sub-Saharan Africa (Sousa et al. 2018).
Urban agriculture as the origin of pesticide contamination
A proportion of applied pesticides can be transferred to other environmental compartments, i.e., the air, soil, and water via processes such as volatilization, wet and dry deposition, and runoff. Depending on the land use, and on soil and pesticide properties, pesticides can be adsorbed by soil particles then transported to surface waters by runoff (Chen et al. 2017; Fairbairn et al. 2016; Jorgenson et al. 2013; Mast et al. 2007; Smernik and Kookana 2015). Once in the water column, pesticide properties (solubility, volatilization, polarity, adsorption, rate and mode of degradation) interact with abiotic and biotic conditions that thus influence their environmental distribution and fate (Dumas et al. 2017; Huang et al. 2016). According to their octanol/water partition coefficient (Kow), the pesticides selected in our study are rather polar, that is, ranging from 0.57 (imidacloprid) to 3.70 (tebuconazole), except for acetochlor (4.14) and are thus more likely to occur in the water phase than to accumulate in the sediment. Persistence in the environment of each pesticides is a function of their susceptibility to degradation, either physical-chemical or biological and to sequestration in other compartments (i.e., adsorption to suspended matters). Some pesticides present low decay rates and can thus be found in the water column several weeks after discharge, while others present a relatively rapid decay. For example, atrazine has a half-life comprised between 335 and 742 days through photolysis and hydrolysis (Toxicology Data Network of the U.S. National Library of Medicine) and degrades into DIA and DEA which were detected in samples (detection range 11–33%).
Except for terbuthylazine, the pesticides included in the homologation list in Cameroon were all detected in the samples. Of the eight pesticides with quantification frequencies higher than 20%, atrazine, diuron, metolachlor, carbendazim, and metalaxyl are homologated in Cameroon. Acetochlor, penconazole, and tetraconazole are not homologated in Cameroon or by the Sahelian Pesticide Committee. The presence of these forbidden pesticides in samples could nevertheless be explained by their application on local crops. Acetochlor can be used on vegetables and fruits; penconazole can be applied on fruits and tomatoes in urban and peri-urban agriculture in Yaoundé (PPDB 2011). Moreover, certain practices might lead to over-treatment of crops that then become sources of contamination. Farmers are not always trained in the correct use of pesticides including preparation, handling, spreading, and storage (Mattah et al. 2015; Williamson et al. 2008). Some pesticides may be spread too liberally or disposed of inappropriately thereby creating hazards for both the environment and for consumers. In addition, pesticides forbidden in industrialized countries because of their hazardousness are still circulating. Some are obsolete or their labels do not correspond to the actual active substances inside the packaging (Mansour 2004; Mattah et al. 2015; Mengistie et al. 2015; Ntow et al. 2006; Williamson et al. 2008, Wodageneh 1997). Large amounts of unauthorized pesticides may thus be spread on local crops, thereby explaining their occurrence in surface waters in Yaoundé.
Characterization of pesticide contamination in urban and peri-urban areas
The sum of TWA pesticide concentrations in the urban POCIS samples ranged from 57 ng/L (Biyeme1, June/July 2016) to 467 ng/L (Mfoundi4, March/April 2015) and from 9 ng/L (Méfou1, June/July 2016) to 101 ng/L (Méfou5, June/July 2016) in the peri-urban POCIS samples (Table 6). Thus, urban sites were always more contaminated by pesticides than peri-urban sites with a factor of about four between the maximum concentrations. The urban Mfoundi4 site was the most contaminated by pesticides with TWA concentrations > 100 ng/L. The Méfou River was progressively more contaminated upstream to downstream with a particularly high concentration at Méfou5 where the level was the same as at urban sites (Table 6; Fig. 4). POCIS samples were mainly contaminated by herbicides (45–96%). A higher proportion of fungicides was quantified in peri-urban areas (13–55%) compared to urban area (3–26%). The main fungicides were metalaxyl and carbendazim. In the urban sites, contamination was mainly due to the level of concentration of the herbicides diuron and atrazine. These results suggest a difference in the type of pesticide used in the Mfoundi basin and in the upper Méfou basin.
Medians of in situ physical-chemical measures on the whole sites for the three campaigns and pesticides quantified at least in 40% of POCIS samples (acetochlor, atrazine, carbendazime, diuron, imidacloprid, metalaxyl, metolachlor and tebuconazole) and also the degradation products of diuron (DCPMU, DCPU) were included to characterize the 18 water samples (Fig. 5). No significant correlations were found between oxygen, pH, temperature, and pesticides. However, conductivity values are positively correlated to pesticide amounts (Rs = 0.72, p value < 0.05).
Distribution of urban (triangles) and peri-urban (squares) sampling sites according to their TWA pesticide concentrations (ng/L) and their conductivity (a) and oxygen (b) medians. Sampling sites are labeled according to their sampling campaign (A: March/April 2015, B: October/November 2015, C: June/July 2016). Triangles and squares correspond to urban and peri-urban sites, respectively. Pesticides with quantification frequencies greater than 40% with POCIS and diuron degradation products (DCPMU, DCPU) were selected (Fig. 2b)
The distribution of sites according to pesticides and physical-chemical measures pointed out a difference in the chemical signature of urban and peri-urban site contamination. Urban sites (triangles) were characterized by an important pesticide contamination and greater values of conductivity and oxygen depletion. The Mfoundi4_A sample was an outlier due to its high sum of TWA pesticide concentration on POCIS (467 ng/L), in particular for diuron (369 ± 169 ng/L). Other Mfoundi sites had sum of TWA pesticide concentration ranging from 57 ng/L (Biyeme1_C) to 150 ng/L (Biyeme1_A). Both pesticide concentrations, conductivity, and oxygen saturation values illustrate the anthropogenic contamination of urban sites. In fact, the Mfoundi urban sites had a greater conductivity (range 215–401 μS/cm) than the Méfou peri-urban sites (range 30–166 μS/cm) with the exception of Méfou3 with 234 μS/cm (Fig. 5a). The Mfoundi conductivity values were in the range of those reported in a previous study conducted in the same basin. The reported increase in conductivity from upstream to downstream was attributed to agricultural (corn, market gardening, fish breeding, pig farming, and slaughterhouses) and domestic (drains, household refuse, laundry) sources of contamination (Nougang et al. 2011). Oxygen saturation was under 50% at the main urban sites (range 18–41%) exept for Mfoundi2_C (55%). Increasing conductivity and oxygen depletion were reported to be indicators of mineral and organic pollution, respectively, through anthropogenic inputs in the form of urban wastewater (Hayzoun et al. 2015).
Pesticide TWA concentrations at the Méfou sites ranged from 9 ng/L (Méfou1_C) to 101 ng/L (Méfou5_C). The majority of peri-urban sites on the Méfou River had the lowest conductivity values, between 28 μS/cm (Méfou1_B) and 166 μS/cm (Méfou5_C). Méfou3_C differed from the other Méfou sites with a measured conductivity of 234 μS/cm, in the same range as urban sites. Measured oxygen saturation (Fig. 5b) ranged from 7% (Méfou4_B) to 89% (Méfou1_C) indicating relatively good water quality.
Differences in urban and peri-urban area pesticide contamination profiles
We propose several hypotheses to explain the spatial difference between urban and peri-urban pesticide contamination and are linked to human river management. First, agricultural practices differ in the urban and peri-urban basin and could impact pesticide transfer into the environment. In the urban Mfoundi basin, intense market gardening along the waterways means that pesticides are used very close to the stream and may be directly transferred into surface waters. Reports in the literature refer to large amounts of pesticide used in intensive urban agriculture (Gockowski and Ndoumbé 1999). In contrast to the peri-urban Méfou basin, the lowlands are better preserved and the crops are grown on the hills, where the cropping system is more extensive and pesticides may be used in smaller quantities (Gockowski et al. 2003; Lieunang Letche et al. 2009; Prain et al. 2010). We hypothesize that part of pesticides applied are retained by the vegetation along riverbanks unlike along the urban riverbanks (McCutcheon and Schnoor 2004; Stehle et al. 2016; Tien et al. 2013; Zogning Moffo et al. 2016). In support of this hypothesis, large pineapple plots located on the hills overhanging the Méfou1 peri-urban sampling site were seen to be frequently treated with diuron, but only small quantities of diuron were measured at this point (range from 0.6 ± 0.3 to 3 ± 0.3 ng/L in POCIS). As at this point, the Méfou River has natural vegetation along its banks, so further studies would enable us to understand if they play a role in the environmental fate of diuron.
To summarize, the hypotheses proposed to explain the higher level of contamination of the urban Mfoundi basin compared to the Méfou peri-urban basin are (i) the difference in agricultural systems between the Méfou and Mfoundi basins and (ii) modifications of the riverbank due to human activities in the inner city. Hydrological, hydrogeological, and geochemistry studies of these watersheds are needed to better understand their functioning and to improve pollution management.
Contribution of urban sites to surface water contamination
The relatively preserved environment in the vicinity of the upper Méfou compared to that downstream could explain the lower pesticide contamination of the Méfou1 and Méfou2 sites. Méfou surface water is polluted by pesticides starting from Méfou3 site. Méfou3 is surrounded by market gardening where we saw pesticide wrappings on the ground, in addition to various experimental crops (tobacco, rice). However, the Méfou4 site, which is considered as the outlet of the upper Méfou basin, is relatively preserved from pesticide contamination compared to Méfou3 and Méfou5. The pesticide and physical-chemical measurements were close to the levels of those measured at urban sites, suggesting the influence of land use and local discharges in the vicinity of the sampling sites on water quality. Comparison of pesticide contamination sampled with POCIS between Méfou4 and Méfou5 suggests that pollutants in the Mfoundi River are discharged into the Méfou River and impact its quality (Fig. 4).
High levels of pollution downstream from the urban area have been reported elsewhere (Perrin et al. 2014). The Mfoundi2 and Biyeme1 sites, which are situated in an urban area surrounded by domestic activities and catering, as well as Mfoundi4, which is situated in a swampy area at the outlet of the basin where people collect oil palm wine, can be considered as “pollution hotspots.” Added to this contamination, these urban sites are also at the heart of human activities (i.e., water uses, collection of oil palm) or situated near homes, raising concerns about the relation between this hazardous environment and human health (Shirangi et al. 2011). The growth of Yaoundé urban area toward the south of the Méfou watershed and related impacts on water quality question the sustainability of the downstream part of the Méfou River, which is currently preserved by wetland areas (Lieunang Letche et al. 2009). In particular, the Nyong water catchment located downstream from the confluence between the Méfou River and the Nyong River may be threatened in the long term.
Other worldwide studies report pesticide contamination of urban watersheds in which the herbicide diuron is often reported to be the main contaminant (Kaonga et al. 2015; Rippy et al. 2017). Blanchoud et al. (2004, 2007) argued that even if pesticide amounts are higher in rural areas, transfer and contamination are at the same level in urban areas. They distinguished urban pesticide use (gardens, railways, roads and street maintenance, cemeteries, etc.) as a major contributor to stream contamination. Pesticides are applied on hard inorganic surfaces that may be completely impervious or more or less permeable thereby allowing both pesticide infiltration and runoff. In the city of Yaoundé, pesticide contamination is mainly attributed to specific agricultural activities, but “urban pesticides” can also be taken into consideration in addition to accidental releases.
Depending on their level of urbanization, the contamination profiles of the Méfou River sub-watersheds are a regional specificity that could be generalized to other urban watersheds with the same context. Indeed, the need for food resulting from urban expansion in developing countries, particularly in sub-Saharan Africa (Cohen 2006), will likely be filled by urban agriculture (Bakker et al. 2000; Mackay 2018; Robineau and Dugué 2018). There is a substantial body of literature on the multi-functionality of urban agriculture, its implications for urban development, and the challenges it involves in capital cities or intermediate-sized cities (Bellwood-Howard et al. 2018; Smart et al. 2015). It has been predicted that urban agriculture will have to face urban growth, poverty alleviation, and urban water resource management (Lee-Smith 2010; Padgham et al. 2015), making the regulation and use of pesticides topical environmental issues (Galt 2008; Zeeuw et al. 2011).
Potential risks for ecosystems related to pesticide contamination of surface waters
The ecotoxicological assessment revealed chronic toxicity caused mainly by diuron at the three trophic levels but particularly for algae. The RQ > 1 were mainly found in the urban sites. Among these sites, Mfoundi4 and Mfoundi2 had a more frequent RQ > 1 (89%) for diuron (Table 7).
Urban surface waters are more contaminated by pesticides than waters in the peri-urban area, and the concentrations of the herbicides atrazine and diuron measured in the urban grab water samples could pose an ecological risk for non-targeted organisms. The peri-urban Méfou4 site represents a lower ecological risk. This ERA thus confirms the difference between the urban and the peri-urban watersheds in terms of ecological risks.
Algae in particular might be more sensitive to the toxicological effect of these herbicides because the endpoint (inhibition of growth) corresponds to the direct biological effect of atrazine and diuron. These inhibit photosynthesis by modifying the photosystem II and inhibiting the photosynthetic electron transport chain. Algae are primary producers and provide oxygen and biomass to aquatic ecosystems. Selective herbicide pressure and their site specific contamination history may facilitate the development of more herbicide resistant species, changes in algae community structure and hence in river biofilm communities (Ricart et al. 2009; Wood et al. 2017).
The ecological effect of atrazine and diuron on aquatic invertebrate and vertebrate trophic levels at the individual and community scale has been widely reported. Atrazine affects the reproduction, and behavior of zooplankton and modifies community biodiversity. It reduces the growth and behavior of fish (Graymore et al. 2001), and recent evidence shows that it acts as a chemical barrier to the migration of fish populations (Araújo et al. 2018). Diuron was identified as being slightly toxic to aquatic organisms but its metabolites, and in particular 3,4-dichloroaniline (DCA), are persistent in the environment and toxic to a wide range of organisms (Giacomazzi and Cochet 2004). Special attention should thus be paid to diuron and its metabolites (DCPMU, DCA) whose endocrine effects cause both anti-androgenic and estrogenic disruptions (Boscolo Pereira et al. 2016; Pereira et al. 2015) and to their consequences for fish behavior (Boscolo et al. 2018).
However, our ERA only focused on two compounds and their precise individual endpoints in three trophic organisms. As we observed, and as reported in the literature, pesticides are present in mixtures in streams (Schreiner et al. 2016). Their effects on aquatic organisms have been attributed to their individual action, but combined effects have also been evidenced. The effects of pesticide mixtures can lead to more sensitive organisms (Ccanccapa et al. 2016; Hasenbein et al. 2017; He et al. 2012) raising the question of the toxic effects of the whole pesticide cocktail in aquatic environments.
Given the threat represented by pesticide use and environmental contamination, further monitoring and risk assessment is thus required in sub-Saharan Africa (Jepson et al. 2014). As humans interact with their environment, potential exposure to pesticides has been demonstrated in several populations worldwide (Alavanja and Bonner 2012; Donald et al. 2016; Negatu et al. 2017). Ecological risks are related to the question of human health, an issue highlighted in a review by Gwenzi and Chaukura (2018), warning against possible pathways transfers, and the fate and risks involved in the “aquatic-human system” in Africa.
Conclusion
The contamination diagnosis conducted in this study demonstrated the presence of pesticides in samples of water in the Méfou and the Mfoundi Rivers. POCIS was shown to be a suitable tool to monitor polar pesticide contamination in a tropical urban environment thereby complementing the snapshot results provided by grab samples.
A total of 94% of targeted pesticides were detected in the samples with eight main pesticides frequently quantified both with grab and passive sampling. The measured herbicides, fungicides, and insecticide can be transferred from agricultural plots to surface waters in runoff, underlining the contribution of urban agriculture to the deterioration of river quality in the watersheds studied here. Two herbicides, diuron and atrazine, were the main pesticides identified in samples whose concentrations reached up to several micrograms per liter. Diuron in particular is an urban contaminant of concern, whose concentrations frequently exceeded the European environmental guideline of 0.200 μg/L for freshwater.
Spatial differences were observed in the contamination profiles of the urban Mfoundi and the peri-urban Méfou watersheds. Urban rivers were more contaminated than peri-urban rivers whatever the sampling campaign. Anthropogenic management of the area, agricultural practices, and riverbank modifications could be at the origin of these differences and could modify transfers of the contaminant in the watersheds. The potential discharge of pollution from the urban Mfoundi River to the peri-urban Méfou River after their confluence raises concerns about the sustainability of regional water resources. These spatial differences were confirmed by an ERA of the main polluted sites, which confirm that measured concentrations of diuron and atrazine pose a risk to aquatic organisms.
In a wider context, pesticide contamination in urban and peri-urban areas concerns many cities in developing countries as they face significant growth and related challenges. The approach described in the present paper could be used in other sub-Saharan capitals or middle-sized cities to better understand the sources, fate, and impacts of pesticides on urban basins. The proximity of a population to a polluted water resource is a crucial health issue in this context.
References
Abass K, Ganle JK, Adaborna E (2016) Coliform contamination of peri-urban grown vegetables and potential public health risks: evidence from Kumasi, Ghana. J Community Health 41:392–397. https://doi.org/10.1007/s10900-015-0109-y
Adeyemi D, Anyakora C, Ukpo G, Adedayo A, Darko G (2011) Evaluation of the levels of organochlorine pesticide residues in water samples of Lagos Lagoon using solid phase extraction method. J Environ Chem Ecotoxicol 3:160–166
AFD (2014) Afrique subsaharienne (Cadre d’Intervention Régional 2014–2016)
Ahrens L, Daneshvar A, Lau AE, Kreuger J (2015) Characterization of five passive sampling devices for monitoring of pesticides in water. J Chromatogr A 1405:1–11. https://doi.org/10.1016/j.chroma.2015.05.044
Alavanja MCR, Bonner MR (2012) Occupational pesticide exposures and cancer risk: a review. J Toxicol Environ Health B Crit Rev 15:238–263. https://doi.org/10.1080/10937404.2012.632358
Alvarez DA, Cranor WL, Perkins SD, Clark RC, Smith SB (2008) Chemical and toxicologic assessment of organic contaminants in surface water using passive samplers. J Environ Qual 37:1024–1033. https://doi.org/10.2134/jeq2006.0463
Anderson KA, Seck D, Hobbie KA, Traore AN, McCartney MA, Ndaye A, Forsberg ND, Haigh TA, Sower GJ (2014) Passive sampling devices enable capacity building and characterization of bioavailable pesticide along the Niger, Senegal and Bani Rivers of Africa. Philos Trans R Soc B Biol Sci 369:20130110. https://doi.org/10.1098/rstb.2013.0110
Araújo CVM, Silva DCVR, Gomes LET, Acayaba RD, Montagner CC, Moreira-Santos M, Ribeiro R, Pompêo MLM (2018) Habitat fragmentation caused by contaminants: atrazine as a chemical barrier isolating fish populations. Chemosphere 193:24–31. https://doi.org/10.1016/j.chemosphere.2017.11.014
Aubry C, Pourias J (2013) L’agriculture urbaine fait déjà partie du “métabolisme urbain.” Déméter 2013 21
Aubry C, Ramamonjisoa J, Dabat M-H, Rakotoarisoa J, Rakotondraibe J, Rabeharisoa L (2012) Urban agriculture and land use in cities: an approach with the multi-functionality and sustainability concepts in the case of Antananarivo (Madagascar). Land Use Policy 29:429–439. https://doi.org/10.1016/j.landusepol.2011.08.009
Bachelier G 1959 Etude pédologique des sols de Yaoundé: contribution à l’étude de la pédologie des sols ferralitiques. Agron Trop 14:279–305
Bakker, N., Dubbeling, M., Guendel, S., Sabel-Koschella, U., de Zeeuw, H. (Eds.), 2000. Growing cities, growing food: urban agriculture on the policy agenda; a reader on urban agriculture. ZEL, Feldafing
Bellwood-Howard I, Shakya M, Korbeogo G, Schlesinger J (2018) The role of backyard farms in two West African urban landscapes. Landsc Urban Plan 170:34–47. https://doi.org/10.1016/j.landurbplan.2017.09.026
Berthe Dem S, Cobb JM, Mullins DE (2007) Pesticide residues in soil and water from four cotton growing areas of Mali, West Africa. J Agric Food Environ Sci 1:1–12
Binns JA, Maconachie RA, Tanko AI (2003) Water, land and health in urban and peri-urban food production: the case of Kano, Nigeria. Land Degrad Dev 14:431–444. https://doi.org/10.1002/ldr.571
Blanchoud H, Farrugia F, Mouchel JM (2004) Pesticide uses and transfers in urbanised catchments. Chemosphere 55:905–913. https://doi.org/10.1016/j.chemosphere.2003.11.061
Blanchoud H, Moreau-Guigon E, Farrugia F, Chevreuil M, Mouchel JM (2007) Contribution by urban and agricultural pesticide uses to water contamination at the scale of the Marne watershed. Sci Total Environ 375:168–179. https://doi.org/10.1016/j.scitotenv.2006.12.009
Boscolo Pereira TS, Pereira Boscolo CN, Felício AA, Batlouni SR, Schlenk D, Alves de Almeida E (2016) Estrogenic activities of diuron metabolites in female Nile tilapia (Oreochromis niloticus). Chemosphere 146:497–502. https://doi.org/10.1016/j.chemosphere.2015.12.073
Boscolo CNP, Pereira TSB, Batalhão IG, Dourado PLR, Schlenk D, de Almeida EA (2018) Diuron metabolites act as endocrine disruptors and alter aggressive behavior in Nile tilapia (Oreochromis niloticus). Chemosphere 191:832–838. https://doi.org/10.1016/j.chemosphere.2017.10.009
Ccanccapa A, Masiá A, Navarro-Ortega A, Picó Y, Barceló D (2016) Pesticides in the Ebro River basin: occurrence and risk assessment. Environ Pollut 211:414–424. https://doi.org/10.1016/j.envpol.2015.12.059
CCME 2014 Canadian environmental quality guidelines [WWW document]. Can. Counc. Minist. Environ. URL http://ceqg-rcqe.ccme.ca/en/index.html (accessed 1.8.18)
Charlestra L, Amirbahman A, Courtemanch DL, Alvarez DA, Patterson H (2012) Estimating pesticide sampling rates by the polar organic chemical integrative sampler (POCIS) in the presence of natural organic matter and varying hydrodynamic conditions. Environ Pollut 169:98–104 https://doi.org/10.1016/j.envpol.2012.05.001
Chen L, Feng Q, He Q, Huang Y, Zhang Y, Jiang G, Zhao W, Gao B, Lin K, Xu Z (2017) Sources, atmospheric transport and deposition mechanism of organochlorine pesticides in soils of the Tibetan Plateau. Sci Total Environ 577:405–412. https://doi.org/10.1016/j.scitotenv.2016.10.227
Cohen B (2006) Urbanization in developing countries: current trends, future projections, and key challenges for sustainability. Technol Soc Sustain Cities 28:63–80. https://doi.org/10.1016/j.techsoc.2005.10.005
Criquet J, Dumoulin D, Howsam M, Mondamert L, Goossens J-F, Prygiel J, Billon G (2017) Comparison of POCIS passive samplers vs. composite water sampling: a case study. Sci Total Environ 609:982–991. https://doi.org/10.1016/j.scitotenv.2017.07.227
CSP INSAH 2015 Liste globale des pesticides autorisés par le CSP. Version de Mai
Dalvie MA, Cairncross E, Solomon A, London L (2003) Contamination of rural surface and ground water by endosulfan in farming areas of the Western Cape, South Africa. Environ Health 2:1. https://doi.org/10.1186/1476-069X-2-1
Desgranges N (2015) Développement d’échantillonneurs passifs de type POCIS pour l’évaluation de la contamination en pesticides des eaux de bassins versants languedociens. Université de Bordeaux, Bordeaux
Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC, Anderson KA (2016) Silicone wristbands detect individuals’ pesticide exposures in West Africa. R Soc Open Sci 3:160433. https://doi.org/10.1098/rsos.160433
Dumas E, Giraudo M, Goujon E, Halma M, Knhili E, Stauffert M, Batisson I, Besse-Hoggan P, Bohatier J, Bouchard P, Celle-Jeanton H, Costa Gomes M, Delbac F, Forano C, Goupil P, Guix N, Husson P, Ledoigt G, Mallet C, Mousty C, Prévot V, Richard C, Sarraute S (2017) Fate and ecotoxicological impact of new generation herbicides from the triketone family: an overview to assess the environmental risks. J Hazard Mater 325:136–156. https://doi.org/10.1016/j.jhazmat.2016.11.059
Dworak T, Gonzalez C, Laaser C, Interwies E (2005) The need for new monitoring tools to implement the WFD. Environ Sci Policy 8:301–306. https://doi.org/10.1016/j.envsci.2005.03.007
Elfman L, Tooke NE, Patring JDM (2011) Detection of pesticides used in rice cultivation in streams on the island of Leyte in the Philippines. Agric Water Manag 101:81–87. https://doi.org/10.1016/j.agwat.2011.09.005
Elibariki R, Maguta MM (2017) Status of pesticides pollution in Tanzania—a review. Chemosphere 178:154–164. https://doi.org/10.1016/j.chemosphere.2017.03.036
Endamana D, Kengne IM, Gockowski J, Nya J, Wandji D, Nyemeck J, Soua NN, Bakwowi JN 2003 Wastewater reuse for urban and periurban agriculture in Yaoundé (Cameroon): opportunities and constraints. Presented at the International Symposium on Water, Poverty and Productive uses of Water at the Household Level, Muldersdrift
EU (2013) Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy
Fairbairn DJ, Karpuzcu ME, Arnold WA, Barber BL, Kaufenberg EF, Koskinen WC, Novak PJ, Rice PJ, Swackhamer DL (2016) Sources and transport of contaminants of emerging concern: a two-year study of occurrence and spatiotemporal variation in a mixed land use watershed. Sci Total Environ 551–552:605–613. https://doi.org/10.1016/j.scitotenv.2016.02.056
Galt RE (2008) Beyond the circle of poison: significant shifts in the global pesticide complex, 1976–2008. Glob Environ Chang 18:786–799. https://doi.org/10.1016/j.gloenvcha.2008.07.003
Giacomazzi S, Cochet N (2004) Environmental impact of diuron transformation: a review. Chemosphere 56:1021–1032. https://doi.org/10.1016/j.chemosphere.2004.04.061
Gockowski J, Ndoumbé M 1999 An analysis of horticultural production and marketing systems in the forest margins ecoregional benchmark of southern Cameroon: RCMP Research monograph, No. 27, RCMP Research monograph, No. 27. International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria
Gockowski J, Mbazo’o J, Mbah G, Fouda Moulende T (2003) African traditional leafy vegetables and the urban and peri-urban poor. Food Policy 28:221–235. https://doi.org/10.1016/S0306-9192(03)00029-0
Graymore M, Stagnitti F, Allinson G (2001) Impacts of atrazine in aquatic ecosystems. Environ Int 26:483–495. https://doi.org/10.1016/S0160-4120(01)00031-9
Greenwood R, Mills G, Vrana B (2007) Passive sampling techniques in environmental monitoring. Elsevier, Amsterdam
Gwenzi W, Chaukura N (2018) Organic contaminants in African aquatic systems: current knowledge, health risks, and future research directions. Sci Total Environ 619–620:1493–1514. https://doi.org/10.1016/j.scitotenv.2017.11.121
Hamilton AJ, Burry K, Mok H-F, Barker SF, Grove JR, Williamson VG (2014) Give peas a chance? Urban agriculture in developing countries. A review. Agron Sustain Dev 34:45–73. https://doi.org/10.1007/s13593-013-0155-8
Hasenbein S, Peralta J, Lawler SP, Connon RE (2017) Environmentally relevant concentrations of herbicides impact non-target species at multiple sublethal endpoints. Sci Total Environ 607–608:733–743. https://doi.org/10.1016/j.scitotenv.2017.06.270
Hayzoun H, Garnier C, Durrieu G, Lenoble V, Le Poupon C, Angeletti B, Ouammou A, Mounier S (2015) Organic carbon, and major and trace element dynamic and fate in a large river subjected to poorly-regulated urban and industrial pressures (Sebou River, Morocco). Sci Total Environ 502:296–308. https://doi.org/10.1016/j.scitotenv.2014.09.014
He H, Yu J, Chen G, Li W, He J, Li H (2012) Acute toxicity of butachlor and atrazine to freshwater green alga Scenedesmus obliquus and cladoceran Daphnia carinata. Ecotoxicol Environ Saf 80:91–96. https://doi.org/10.1016/j.ecoenv.2012.02.009
Hellar-Kihampa H (2011) Pesticide residues in four rivers running through an intensive agricultural area, Kilimanjaro, Tanzania. J Appl Sci Environ Manag 15:307–316
Herrero-Hernández E, Rodríguez-Cruz MS, Pose-Juan E, Sánchez-González S, Andrades MS, Sánchez-Martín MJ (2017) Seasonal distribution of herbicide and insecticide residues in the water resources of the vineyard region of La Rioja (Spain). Sci Total Environ 609:161–171. https://doi.org/10.1016/j.scitotenv.2017.07.113
Huang X, He J, Yan X, Hong Q, Chen K, He Q, Zhang L, Liu X, Chuang S, Li S, Jiang J (2016) Microbial catabolism of chemical herbicides: microbial resources, metabolic pathways and catabolic genes. Pestic Biochem Physiol 143:272–297. https://doi.org/10.1016/j.pestbp.2016.11.010
Huang Y, Chen Q, Deng M, Japenga J, Li T, Yang X, He Z (2018) Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in Southeast China. J Environ Manag 207:159–168. https://doi.org/10.1016/j.jenvman.2017.10.072
Ibrahim I, Togola A, Gonzalez C (2012) Polar organic chemical integrative sampler (POCIS) uptake rates for 17 polar pesticides and degradation products: laboratory calibration. Environ Sci Pollut Res 20:3679–3687. https://doi.org/10.1007/s11356-012-1284-3
Ibrahim I, Togola A, Gonzalez C (2013) In-situ calibration of POCIS for the sampling of polar pesticides and metabolites in surface water. Talanta 116:495–500. https://doi.org/10.1016/j.talanta.2013.07.028
Isogai N, Hogarh JN, Seike N, Kobara Y, Oyediran F, Wirmvem MJ, Ayonghe SN, Fobil J, Masunaga S (2016) Atmospheric monitoring of organochlorine pesticides across some West African countries. Environ Sci Pollut Res Int. https://doi.org/10.1007/s11356-016-7284-y
IUPC (2003) Regulatory limits for pesticide residues in water. Pure Appl Chem 75:1123–1155
Jansen HC, Harmsen J (2011) Pesticide monitoring in the Central Rift Valley 2009–2010: ecosystems for water in Ethiopa (no. 2083). Wageningen Environmental Research (Alterra), Wageningue
Jepson PC, Guzy M, Blaustein K, Sow M, Sarr M, Mineau P, Kegley S (2014) Measuring pesticide ecological and health risks in West African agriculture to establish an enabling environment for sustainable intensification. Philos Trans R Soc B 369:20130491. https://doi.org/10.1098/rstb.2013.0491
Jorgenson B, Fleishman E, Macneale KH, Schlenk D, Scholz NL, Spromberg JA, Werner I, Weston DP, Xiao Q, Young TM, Zhang M (2013) Predicted transport of pyrethroid insecticides from an urban landscape to surface water. Environ Toxicol Chem 32:2469–2477. https://doi.org/10.1002/etc.2352
Kaonga CC, Takeda K, Sakugawa H (2015) Diuron, irgarol 1051 and fenitrothion contamination for a river passing through an agricultural and urban area in Higashi Hiroshima City, Japan. Sci Total Environ 518–519:450–458. https://doi.org/10.1016/j.scitotenv.2015.03.022
Lee-Smith D (2010) Cities feeding people: an update on urban agriculture in equatorial Africa. Environ Urban 22:483–499. https://doi.org/10.1177/0956247810377383
Lehmann E, Fargues M, Nfon Dibié J-J, Konaté Y, de Alencastro LF (2017) Assessment of water resource contamination by pesticides in vegetable-producing areas in Burkina Faso. Environ Sci Pollut Res Int 25:3681–3694. https://doi.org/10.1007/s11356-017-0665-z
Lieunang Letche AR, Fonteh MF, Ndam Ngoupayou JR 2009 La Gestion Intégrée des Ressources en Eau (GIRE) au Cameroun: Préoccupations environnementales affectant la gestion des ressources en eau dans le bassin versant de la Méfou. Editions Universitaires Européennes
Mac Loughlin TM, Peluso L, Marino DJG (2017) Pesticide impact study in the peri-urban horticultural area of Gran La Plata, Argentina. Sci Total Environ 598:572–580. https://doi.org/10.1016/j.scitotenv.2017.04.116
Mackay H (2018) Mapping and characterising the urban agricultural landscape of two intermediate-sized Ghanaian cities. Land Use Policy 70:182–197. https://doi.org/10.1016/j.landusepol.2017.10.031
Mansour SA (2004) Pesticide exposure—Egyptian scene. Toxicology 198:91–115. https://doi.org/10.1016/j.tox.2004.01.036
Masiá A, Campo J, Vázquez-Roig P, Blasco C, Picó Y (2013) Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). J Hazard Mater 263(Part 1):95–104 https://doi.org/10.1016/j.jhazmat.2013.09.035
Mast MA, Foreman WT, Skaates SV (2007) Current-use pesticides and organochlorine compounds in precipitation and lake sediment from two high-elevation national parks in the Western United States. Arch Environ Contam Toxicol 52:294–305. https://doi.org/10.1007/s00244-006-0096-1
Mattah MM, Mattah PAD, Futagbi G (2015) Pesticide application among farmers in the catchment of Ashaiman irrigation scheme of Ghana: health implications. J Environ Public Health 2015:1–7. https://doi.org/10.1155/2015/547272
McCutcheon SC, Schnoor JL 2004 Phytoremediation: transformation and control of contaminants. Wiley
Mengistie BT, Mol APJ, Oosterveer P (2015) Pesticide use practices among smallholder vegetable farmers in Ethiopian Central Rift Valley. Environ Dev Sustain 19:1–24. https://doi.org/10.1007/s10668-015-9728-9
Miège C, Mazzella N, Allan I, Dulio V, Smedes F, Tixier C, Vermeirssen E, Brant J, O’Toole S, Budzinski H, Ghestem J-P, Staub P-F, Lardy-Fontan S, Gonzalez J-L, Coquery M, Vrana B (2015) Position paper on passive sampling techniques for the monitoring of contaminants in the aquatic environment—achievements to date and perspectives. Trends Environ Anal Chem 8:20–26. https://doi.org/10.1016/j.teac.2015.07.001
Ministère de l’agriculture et du développement rural (Cameroun) (2013) Liste des pesticides homologués au Cameroun au 31 juillet 2013
Mmualefe LC, Torto N, Huntsman-Mapila P, Mbongwe B (2009) Headspace solid phase microextraction in the determination of pesticides in water samples from the Okavango Delta with gas chromatography-electron capture detection and time-of-flight mass spectrometry. Microchem J 91:239–244. https://doi.org/10.1016/j.microc.2008.12.005
Mougeot 2000 Urban agriculture: definition, presence, potentials and risks, and policy challenges (no. 31), Cities feeding people series. International Development Research Centre (IDRC)
Negatu B, Kromhout H, Mekonnen Y, Vermeulen R (2017) Occupational pesticide exposure and respiratory health: a large-scale cross-sectional study in three commercial farming systems in Ethiopia. Thorax 72:498–499. https://doi.org/10.1136/thoraxjnl-2016-208924
Nougang ME, Nola M, Ateba Bessa H, Tamatcho Kweyang BP, Noah Ewoti OV, Moungang LM (2011) Prevalence of pathogenic strains of Escherichia coli in urban streams in the equatorial region of Cameroon (Central Africa). J Appl Biosci 48:3293–3305
Ntow WJ, Gijzen HJ, Kelderman P, Drechsel P (2006) Farmer perceptions and pesticide use practices in vegetable production in Ghana. Pest Manag Sci 62:356–365. https://doi.org/10.1002/ps.1178
Oerke E-C (2006) Crop losses to pests. J Agric Sci 144:31. https://doi.org/10.1017/S0021859605005708
Okoya AA, Ogunfowokan AO, Asubiojo OI, Torto N (2013) Organochlorine pesticide residues in sediments and waters from cocoa producing areas of Ondo State, Southwestern Nigeria. Int Sch Res Not 2013:e131647. https://doi.org/10.1155/2013/131647
Olivry J-C (1986) Fleuves et rivières du Cameroun, Monographies hydrologiques. MESRES-ORSTOM, Bondy
Organization W.H., Safety I.P. on C (2010) The WHO recommended classification of pesticides by hazard and guidelines to classification 2009. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification
Padgham J, Jabbour J, Dietrich K (2015) Managing change and building resilience: a multi-stressor analysis of urban and peri-urban agriculture in Africa and Asia. Urban Clim 12:183–204. https://doi.org/10.1016/j.uclim.2015.04.003
Palma G, Sánchez A, Olave Y, Encina F, Palma R, Barra R (2004) Pesticide levels in surface waters in an agricultural–forestry basin in Southern Chile. Chemosphere 57:763–770. https://doi.org/10.1016/j.chemosphere.2004.08.047
Pereira TSB, Boscolo CNP, da Silva DGH, Batlouni SR, Schlenk D, de Almeida EA (2015) Anti-androgenic activities of diuron and its metabolites in male Nile tilapia (Oreochromis niloticus). Aquat Toxicol Amst Neth 164:10–15. https://doi.org/10.1016/j.aquatox.2015.04.013
Perrin JL, Raïs N, Chahinian N, Moulin P, Ijjaali M (2014) Water quality assessment of highly polluted rivers in a semi-arid Mediterranean zone Oued Fez and Sebou River (Morocco). J Hydrol 510:26–34. https://doi.org/10.1016/j.jhydrol.2013.12.002
Poulier G, Lissalde S, Charriau A, Buzier R, Cleries K, Delmas F, Mazzella N, Guibaud G (2014) Estimates of pesticide concentrations and fluxes in two rivers of an extensive French multi-agricultural watershed: application of the passive sampling strategy. Environ Sci Pollut Res 22:8044–8057. https://doi.org/10.1007/s11356-014-2814-y
PPDB (2011) Pesticides Properties DataBase [WWW Document]. URL http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed 5.5.15)
Prain G, Lee-Smith D, Karanja N (eds) (2010) African urban harvest. Springer New York, New York, NY. https://doi.org/10.1007/978-1-4419-6250-8
Ricart M, Barceló D, Geiszinger A, Guasch H, de Alda ML, Romaní AM, Vidal G, Villagrasa M, Sabater S (2009) Effects of low concentrations of the phenylurea herbicide diuron on biofilm algae and bacteria. Chemosphere 76:1392–1401. https://doi.org/10.1016/j.chemosphere.2009.06.017
Rippy MA, Deletic A, Black J, Aryal R, Lampard J-L, Tang JY-M, McCarthy D, Kolotelo P, Sidhu J, Gernjak W (2017) Pesticide occurrence and spatio-temporal variability in urban run-off across Australia. Water Res 115:245–255. https://doi.org/10.1016/j.watres.2017.03.010
Robineau O, Dugué P (2018) A socio-geographical approach to the diversity of urban agriculture in a West African city. Landsc Urban Plan 170:48–58. https://doi.org/10.1016/j.landurbplan.2017.09.010
Ryberg KR, Gilliom RJ (2015) Trends in pesticide concentrations and use for major rivers of the United States. Sci Total Environ 538:431–444 https://doi.org/10.1016/j.scitotenv.2015.06.095
Schreiner VC, Szöcs E, Bhowmik AK, Vijver MG, Schäfer RB (2016) Pesticide mixtures in streams of several European countries and the USA. Sci Total Environ 573:680–689. https://doi.org/10.1016/j.scitotenv.2016.08.163
Shirangi A, Nieuwenhuijsen M, Vienneau D, Holman CDJ (2011) Living near agricultural pesticide applications and the risk of adverse reproductive outcomes: a review of the literature. Paediatr Perinat Epidemiol 25:172–191. https://doi.org/10.1111/j.1365-3016.2010.01165.x
Smart J, Nel E, Binns T (2015) Economic crisis and food security in Africa: exploring the significance of urban agriculture in Zambia’s Copperbelt province. Geoforum 65:37–45. https://doi.org/10.1016/j.geoforum.2015.07.009
Smernik RJ, Kookana RS (2015) The effects of organic matter-mineral interactions and organic matter chemistry on diuron sorption across a diverse range of soils. Chemosphere 119:99–104. https://doi.org/10.1016/j.chemosphere.2014.05.066
Sousa JCG, Ribeiro AR, Barbosa MO, Pereira MFR, Silva AMT (2018) A review on environmental monitoring of water organic pollutants identified by EU guidelines. J Hazard Mater 344:146–162. https://doi.org/10.1016/j.jhazmat.2017.09.058
Stehle S, Dabrowski JM, Bangert U, Schulz R (2016) Erosion rills offset the efficacy of vegetated buffer strips to mitigate pesticide exposure in surface waters. Sci Total Environ 545–546:171–183. https://doi.org/10.1016/j.scitotenv.2015.12.077
Teklu BM, Adriaanse PI, Van den Brink PJ (2016) Monitoring and risk assessment of pesticides in irrigation systems in Debra Zeit, Ethiopia. Chemosphere 161:280–291. https://doi.org/10.1016/j.chemosphere.2016.07.031
Temple L, Marquis S, David O, Simon S (2009) Le maraîchage périurbain à Yaoundé est-il un système de production localisé innovant?. Econ For Soc 2309–2328. https://www.hal.archives-ouvertes.fr/hal-00384377
Tien C-J, Lin M-C, Chiu W-H, Chen CS (2013) Biodegradation of carbamate pesticides by natural river biofilms in different seasons and their effects on biofilm community structure. Environ Pollut 179:95–104. https://doi.org/10.1016/j.envpol.2013.04.009
White JT, Bunn C (2017) Growing in Glasgow: innovative practices and emerging policy pathways for urban agriculture. Land Use Policy 68:334–344. https://doi.org/10.1016/j.landusepol.2017.07.056
Williamson S, Ball A, Pretty J (2008) Trends in pesticide use and drivers for safer pest management in four African countries. Crop Prot 27:1327–1334. https://doi.org/10.1016/j.cropro.2008.04.006
Wodageneh A 1997 Obsolete and unwanted pesticide stocks, in: Prevention and disposal of obsolete and unwanted pesticide stocks in Africa and the Near East. Presented at the Inter-Organization Programme for the Sound Management of Chemicals (IOMC), Bamako (Mali)
Wood RJ, Mitrovic SM, Lim RP, Kefford BJ (2017) Chronic effects of atrazine exposure and recovery in freshwater benthic diatoms from two communities with different pollution histories. Aquat Toxicol 189:200–208. https://doi.org/10.1016/j.aquatox.2017.06.013
Yabuki Y, Nagai T, Inao K, Ono J, Aiko N, Ohtsuka N, Tanaka H, Tanimori S (2016) Temperature dependence on the pesticide sampling rate of polar organic chemical integrative samplers (POCIS). Biosci Biotechnol Biochem 80:2069–2075. https://doi.org/10.1080/09168451.2016.1191329
Zeeuw HD, Veenhuizen RV, Dubbeling M (2011) The role of urban agriculture in building resilient cities in developing countries. J Agric Sci 149:153–163. https://doi.org/10.1017/S0021859610001279
Zogning Moffo MO, Tsalefac M, Ursu A, Iatu C (2016) Contribution of geographic information systems for the mapping of flooding factors in Yaoundé: the case study of Mfoundi upstream watershed. Present Environ Sustain Dev 10:217–234. https://doi.org/10.1515/pesd-2016-0019
Acknowledgements
The authors thank Moïse NOLA (University of Yaoundé I) for his advice and support, Jean-Claude NTONGA, and Henriette ATEBA MASSUSSI (Cameroonian Institute of Geological and Mineral Researches) for their welcome and support and Alain FEZEU and Sylvie SPINELLI for their technical and field assistance.
Funding
This study was financed by LMI PICASS’EAU, HydroSciences Montpellier Lab., Montpellier Institute of Water and Environment and Institut Mines Télécom Mines Alès.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Highlights
• Pesticides contaminate surface waters in urban and peri-urban areas (Yaoundé).
• Urban rivers are more polluted by pesticides than peri-urban rivers.
• Measured concentrations of the herbicide diuron reached 14 ± 3 μg/L in urban rivers.
• Measured concentrations of diuron represent a potential risk for aquatic organisms.
Electronic supplementary material
ESM 1
(XLSX 14 kb).
Rights and permissions
About this article
Cite this article
Branchet, P., Cadot, E., Fenet, H. et al. Polar pesticide contamination of an urban and peri-urban tropical watershed affected by agricultural activities (Yaoundé, Center Region, Cameroon). Environ Sci Pollut Res 25, 17690–17715 (2018). https://doi.org/10.1007/s11356-018-1798-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-1798-4