Abstract
In this study, the passive sampling strategy was evaluated for its ability to improve water quality monitoring in terms of concentrations and frequencies of quantification of pesticides, with a focus on flux calculation. Polar Organic Chemical Integrative Samplers (POCIS) were successively exposed and renewed at three sampling sites of an extensive French multi-agricultural watershed from January to September 2012. Grab water samples were recovered every 14 days during the same period and an automated sampler collected composite water samples from April to July 2012. Thirty-nine compounds (pesticides and metabolites) were analysed. DEA, diuron and atrazine (banned in France for many years) likely arrived via groundwater whereas dimethanamid, imidacloprid and acetochlor (all still in use) were probably transported via leaching. The comparison of the three sampling strategies showed that the POCIS offers lower detection limits, resulting in the quantification of trace levels of compounds (acetochlor, diuron and desethylatrazine (DEA)) that could not be measured in grab and composite water samples. As a consequence, the frequencies of occurrence were dramatically enhanced with the POCIS compared to spot sample data. Moreover, the integration of flood events led to a better temporal representation of the fluxes when calculated with the POCIS compared to the bimonthly grab sampling strategy. We conclude that the POCIS could be an advantageous alternative to spot sampling, offering better performance in terms of quantification limits and more representative data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intensive use of pesticides in agriculture and urban activities since the 1950s has led to diffuse contamination of environmental compartments (air, soil, water) (Loos et al. 2009; Jaward et al. 2004). Pesticides are driven from the application area to water bodies via different hydrological processes including runoff, leaching and transport with soil particles (Lennartz et al. 1997; Louchart et al. 2001). The presence of these molecules in natural water can lead to toxic effects for biota (Mason et al. 2003; Solomon et al. 1996; DeLorenzo et al. 2001), but can also have effects on humans, as pesticides are sometimes quantified in drinking water. In the past decades, European governments realized the necessity of controlling water quality. The Water Framework Directive (WFD), implemented in 2000 by the European Union (2000/60/EC 2000), requires member states to reach good chemical and ecological status for all water bodies by 2015. In this context, Environmental Quality Standards (EQS) have been determined for 41 priority compounds, including 14 pesticides (2008/105/EC 2008). Water quality must be monitored at least once a month and compared to the EQS in order to determine the water body’s chemical quality status. Today, the only sampling strategy authorized is grab sampling. However, despite the simplicity of this strategy, numerous limitations have been noted.
The first is the sampling frequency. Indeed, pesticide concentrations can rapidly increase after rainfall, or during a flood event (Taghavi et al. 2011). In a recent study conducted in the stream of a small vineyard catchment, Rabiet et al. (2010) observed that the dissolved diuron concentration could be multiplied by a factor of 50 in only 5 days. This high reactivity was related to the rapid transfer of pesticides via runoff after a storm event. Moreover, this transfer can be strongly enhanced when rainfall occurs just after pesticide application. These examples show that the sampling frequency must be adapted to the temporal variation of pesticide concentrations, which is not the case with grab samples taken once a month on planned dates in regulatory monitoring programmes. Another disadvantage of the grab sampling strategy is its high limit of quantification. Indeed, some hydrophobic compounds have very low EQS (e.g. 0.01 μg L−1 for endosulfan) because they can accumulate in biota, leading to toxic effects even at low water concentrations. Such a low EQS may be difficult to identify with conventional analytical methods and sample preparation procedures.
When a water body fails to achieve “good status”, remediation actions may be implemented. The reduction of pesticide use is encouraged, and landscape design can be modified to reduce transfer (e.g. grass strips bordering streams, hedgerow construction). The best way to evaluate the efficiency of these remediation strategies required by the WFD is to monitor pesticide fluxes. However, depending on the water flow, high fluxes can be related to very low concentrations of pesticides because of a dilution effect. When these low concentrations are below the limit of detection, fluxes cannot be calculated although they can be high. But flux information could benefit regulatory investigative and operational monitoring network, resulting in better understanding of the global contamination of a watershed, and in the implementation of more accurate remediation strategies.
The passive sampling strategy developed over the past 20 years could overcome some of the problems related to grab sampling. Passive sampling theory and modelling are well described elsewhere (Huckins et al. 1993; Vrana et al. 2005; Stuer-Lauridsen 2005; Alvarez et al. 2004). Briefly, the strategy consists of an integrating device composed of a receiving phase (liquid or solid) exposed in the water body for a defined period (from a few hours to several weeks) that continuously accumulates contaminants. After retrieval and analysis of the receiving phase, a time-weighted average concentration (TWAC) can be calculated for the period of exposure. Passive sampling offers significant benefits compared to grab sampling. On one hand, the in situ accumulation of pesticides in the device allows quantification at lower limits of detection without an additional sample pre-concentration step (Lissalde et al. 2011). On the other hand, and as a consequence of sampling duration, potential pesticide peaks occurring after flood events are integrated (Allan et al. 2007), and the representativeness of the data collected is enhanced, especially regarding chronic exposures.
Considering these points, it appears that the monitoring of pesticide concentrations in water, and the calculation of pesticide fluxes could be improved using a passive sampling strategy.
The main objective of this study was to verify the application of passive sampling compared to grab sampling to measure pesticide concentrations in a context of low contamination levels. The Auvézère catchment (France) was specifically selected due to its low level contamination with a wide range of pesticides. Polar Organic Chemical Integrative Samplers (POCIS) were exposed from January to September 2012 in the Auvèzère River and one of its small tributaries, the Arnac River. TWACs were then used to calculate fluxes in both rivers and to determine the contribution of the Arnac tributary to the contamination of the Auvézère River.
Materials and methods
Study area
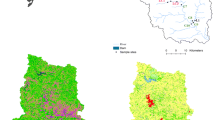
The Auvézère river is located in southwest France and flows 112 km between the regions of Limousin (Corrèze) and Aquitaine (Dordogne), draining a catchment of about 900 km2 (Fig. 1). The geological substratum is mostly metamorphic rocks, created from granitic material (gneiss) in the Limousin region. The climate is characterised as Atlantic oceanic, with mild and wet seasons. Seventy-four percent of the Auvézère catchment is dedicated to agricultural activities (Fig 1), mainly grasslands (50 %) for raising Limousin cattle, and cereal crops (23 %). Maize, wheat and sunflowers make the use of herbicides by farmers very common in this area. Insecticides are also in use, due to the importance of orchards (23 %). Three sampling stations were chosen on the watershed. The first one, located at Quatre-Moulins, is used to produce drinking water and was therefore selected due to its low pesticide contamination. Two other sampling points were located in the Arnac stream, a small tributary of the Auvézère River. These stations were chosen due to the small surface area of the catchment drained by the Arnac River which allows better identification of flood phenomena and the methods of pesticide transfer to the stream.
Selection of the studied molecules
Two criteria were used to select the studied compounds. The contamination history of the two rivers was analysed through regulatory monitoring data (years 2009, 2010 and 2011, Water Agency Adour-Garonne) and the most frequently detected compounds were selected. Then, only the molecules that could be sampled by the POCIS device, i.e. neutral and moderately polar, were chosen. The 39 selected compounds, including herbicides, insecticides, fungicides and metabolites are listed in Table 1.
Chemicals
Ammonium acetate was purchased from Fluka (Sigma-Aldricht, Schnelldorf, Germany). Methanol, acetonitrile and ethyl acetate were obtained from Sharlau (HPLC grade, AtlanticLabo, Bruges, France). Ultra pure water (UPW, resistivity >18 MΩ) was produced with a Synergy UV system from Millipore (Billerica, MA, USA). Analytical standards (Table 1), surrogates (simazine-d5, prometyn-d6 and monuron-d6) and internal standards (atrazine-d5, carbaryl-d3, carbendazim-d4, DEA-d6, diuron-d6, methomyl-d3, metolachlor-d6 and pyrimicarb-d6) were purchased from Dr Erhenstorfer GmbH (Augsburg, Germany). Monomolecular stock solutions were prepared in acetonitrile (100 μg mL−1) and stored at −18 °C for no more than 6 months. Working solutions (1 μg mL−1) of pesticide standards, internal standards and surrogates were also prepared in acetonitrile and stored at −6 °C for 6 months.
Water samples
Grab water samples were retrieved every 14 days (unless stated) from January 16th to September 17th 2012. An automated sampler 6712 (Teledyne ISCO, USA), which collected a 50-mL water sample every hour, was installed for 3 months (from April to July 2012) at the Quatre-Moulins station. Fifty millilitre-water samples were transferred and mixed in a glass bottle (10 L) recovered once a week. Automated and grab water samples were transported in a cool box from the field to the laboratory and stored at 4 °C until extraction (less than 24 h).
Before analysis, pre-concentration of the analytes was performed using solid-phase extraction (SPE) with Oasis HLB cartridges (Waters), according to the method described by Lissalde et al. (2011). Prior to SPE, 100 mL water samples (pH adjusted to 7.0 ± 0.1 with NaOH or H3PO4 0.1 N) were filtered using GF/F glass microfiber filters (0.7 μm pore size). Afterwards, 10 μL of a stock solution (acetonitrile) containing 20, 1 and 10 ng μL−1 of simazine-d5, prometyn-d6 and monuron-d6 (surrogates), respectively, was added to the water samples, resulting in a fortification level of 100 ng L−1. SPE was conducted using a Visiprep 12-port manifold (Supelco, France). The conditioning, extraction and rinsing steps were carried out under a 53.33 kPa vaccum. The SPE cartridges were successively washed with 5 mL of methanol, conditioned with 5 mL of UPW, loaded with 100 mL water samples, then rinsed with 5 mL of UPW containing 15 % HPLC-grade methanol. Cartridges were then dried under a nitrogen stream for 30 min. Elutions were achieved with 3 mL of methanol, followed by 3 mL of a mix of methanol: ethyl acetate (75:25 v/v). The 6-mL extracts were blown under a gentle stream of nitrogen and dissolved in 990 μL of UPW containing 10 % HPLC-grade acetonitrile. Ten microliters of a solution of eight internal standards (10 ng μL−1) was then added to the sample, prior to analysis. The final concentrations of the internal standards were about 100 μg L−1 in the final sample.
POCIS
Preparation
The samplers used were handmade “pharmaceutical” POCIS (Alvarez et al. 2004) containing 200 mg of Oasis HLB sorbent enclosed between two polyethersulfone (PES) membranes (0.1 μm pore size). The membrane-sorbent-membrane layers were compressed between two holder washers. The total exchange surface area of the membrane (both sides) was approximately 41 cm2 and the surface area per mass of sorbent ratio was approximately 200 cm2 g−1.
Field deployment and elution
POCIS (one per sampling station) were successively exposed in the rivers from January 16th to September 17th 2012. Moreover, in spring (from April to July 2012), POCIS were exposed in triplicates at the Quatre-Moulins station. Since the position of the POCIS can affect the Rs value, passive samplers were systematically exposed vertically, face to the stream flow (they were also exposed this way during the calibration step). After 14 days of exposure, POCIS were retrieved and each device was opened. The Oasis HLB sorbent was recovered with 2 × 5 mL of UPW, directly transferred into a 1 mL empty SPE cartridge with a polyethylene (PE) frit and packed under vacuum using a Visiprep SPE manifold. Lissalde et al. (2011) published tap water extraction recoveries in Oasis HLB higher than 70 % for our compounds. So, we assume that important losses of analytes do not occur during the POCIS sorbent recovery with UPW. Afterwards, another PE frit was added to the top of the SPE tube and all cartridges were dried under a nitrogen stream for 30 min. Afterwards, cartridges were weighted to measure the exact mass of adsorbent recovered. Elutions were achieved with 3 mL of methanol followed by 3 mL of a mix of methanol: ethyl acetate (75:25 v/v). The 6-mL extracts were blown under a gentle stream of nitrogen and dissolved in 1 mL of acetonitrile. To avoid matrix interferences, samples were diluted by a factor 10 prior to analysis (Lissalde et al. 2011).
Calculation of the TWAC
When the receiving phase is acting as an infinite sink, and assuming the accumulation of analytes is linear with time, the TWA concentrations Cw (μg L−1) of the contaminants can be estimated from the amount of these analytes within the POCIS using (Eq. 1) (Alvarez et al. 2004; Huckins et al. 1993):
where NPOCIS is the mass (μg) of the analyte in the sorbent, t the time of exposure (days) and Rs the sampling rate constant (L day−1). This relationship is valid for the chosen compounds because their uptake remains linear over the time of exposure (14 days) (Mazzella et al. 2007; Lissalde et al. 2011). The Rs constants (Table 1) were determined for each analyte with a laboratory calibration according to the protocol described by Fauvelle et al. (2012), with a continuous spiking and renewing of water.
Sampling rate constants depend on environmental conditions, particularly temperature, water flow rate and biofouling (Harman et al. 2008; Li et al. 2010; Togola and Budzinski 2007; Mazzella et al. 2008; Alvarez et al. 2004). As a consequence, the Rs constants determined during laboratory calibration can be different from the field uptake rates, resulting in semi-quantitative data for the POCIS. However, previous studies showed that the variation of Rs constants with environmental conditions are generally less than twofold (Harman et al. 2012; Morin et al. 2012) which is quite moderate and really acceptable for our applications such as studying frequencies of detection or occurrence.
Analysis
High Performance Liquid Chromatography (HPLC) and electrospray tandem mass spectrometry (ESI-MS/MS) analysis
Pesticide analyses were performed by HPLC (HPLC Ultimate 3000 apparatus from Dionex) and ESI-MS/MS detection (API 2000 triple quadrupole apparatus from AB SCIEX). The procedures used are fully described by Lissalde et al. (2011). The limit of quantification (LQ) associated with water samples was 0.020 μg L−1. For passive samplers, LQs depend on the analytical procedure, but also on the Rs constants and the time of exposure. For the studied compounds, and for an exposure of the POCIS of 14 days, estimated LQs ranged from 0.024 (DIA) to 0.001 μg L−1 (terbuthylazine) with a median value of 0.002 μg L−1. To simplify data interpretation while maintaining a safety factor on the basis of the median value, we decided to fix a threshold value of 0.005 μg L−1 for the POCIS.
The instrumental quantification limit (IQL) associated with the apparatus was about 500 fg injected onto the column for all analytes except for diuron and its metabolites (IQL = 1 pg on column). These IQLs correspond to 20 ng POCIS−1 for diuron and its metabolites and 10 ng POCIS−1 for other analytes. For water samples, these IQLs are 0.002 and 0.001 μg L−1, respectively.
Flux calculation
Auvézère’s water flow
Auvézère’s daily water flow was taken from the national database “Banque Hydro” at Bénayès and Lubersac (Fig 1). However, as the sampling point Quatre-Moulins is located at several kilometres from these two cities, the river discharge measurements taken from the database could not be used as is. The data were therefore combined to calculate Auvézère’s water flow rate (L s−1) at the Quatre-Moulins station (QQM) using (Eq. 2), based on the assumption that the water flow rate is proportional to the surface of the catchment drained:
where QBénayès and QLubersac are the daily water flow rate (L s−1) measured respectively at Bénayès and Lubersac, SW QM is the surface of the Auvézère’s watershed at the Quatre-Moulins station (90.2 km2), and SW Bénayès and SW Lubersac are the surfaces of the Auvézère’s watershed at Bénayès (23.4 km2) and Lubersac (112 km2), respectively.
Arnac’s water flow
The same procedure was used to calculate Arnac downstream water flow (L s−1), with (Eq. 3). SW Arnac downstream is the area of the Arnac’s downstream watershed (9 km2).
Additional spot measurements were made every 14 days at the Arnac downstream station and used to check the reliability of the water flow calculated with (Eq. 3). The resulting factor of correction was about 1.74 and the Arnac downstream water flow (L s−1) was calculated using (Eq. 4).
Arnac upstream water flow was then calculated using (Eq. 5), with SW Arnac upstream the surface of the upstream watershed (1.5 km2).
Fluxes
Fluxes were calculated in two different ways, depending on the sampling strategy used.
-
POCIS
The daily average fluxes of pesticides (FPpesticide) (g day−1) over the successive periods of POCIS exposure (n = 18) were calculated for each analyte and for each sampling station using (Eq. 6):
where Cpocis (g L−1) is the TWAC measured with the POCIS, \( \overline{ Qsamplingstation} \) is the average daily water flow (L day−1) calculated over the time of POCIS exposure, and (i) are the successive periods of exposure (n = 18). Fluxes were set to be null when the TWAC was below the LQ.
-
Water samples
For comparison purposes with the passive sampler data, daily average fluxes of pesticides (FWpesticide) were calculated from water samples data using (Eq. 7):
where FWpesticide is the average daily flux of analyte (g day−1), \( \overline{ Qsamplingstation} \) is the average daily water flow (L day−1), and \( \overline{ Cw} \) (g L−1) is the mean of the concentrations measured in the two grab samples or composite samples taken during the period of exposure (i) of the corresponding POCIS. Values below the LQ were set to be null for the calculation of \( \overline{ Cw} \).
Meteorological data
Daily rainfall data were purchased from Meteo France, at the Lubersac station (n°19121002).
Results and discussion
Watershed hydrology
Daily rainfall and water flow measured on the watershed are presented in Fig 2. The total amount of precipitation was 616 mm (from January to September 2012), which is close to the normal values registered during the past 20 years. However, the winter season was very dry (106 mm, 62 % less than the mean value) whereas spring was especially wet. 197 mm of rainfall were registered in April alone, which was almost threefold the mean value. The highest water flows were registered at the same period at the three sampling stations (at the beginning of May) and were directly related to this high rainfall volume. Wet conditions are known to favour both herbicide and fungicide use by farmers (to avoid crop diseases) and increased pesticide transfer via leaching and runoff. As a consequence, pesticide contamination of the rivers may be intensified at these times.
Comparison of the POCIS and spot sampling strategies for pesticide concentration measurements
Pesticide concentrations measured with the POCIS and in grab and automated samples for two representative periods are reported in Fig 3. Composite sample concentrations were calculated as the mean of the concentrations measured in the two weekly samples collected with an automated sampler. These composite samples were collected during deployment of the corresponding POCIS. First and second grab samples were respectively recovered at deployment and retrieval of the corresponding POCIS. During the first period (Fig 3a), no pesticides were quantified in the composite samples whereas three different compounds were quantified with the POCIS (i.e. acetochlor, alachlor, diuron). The measured concentrations ranged from 0.005 to 0.013 μg L−1 for alachlor and diuron, respectively, which are below the quantification limit associated with water extracts (0.020 μg L−1). This explains why these pesticides could not be quantified in the composite samples. In comparison, the analyte pre-concentration in the POCIS offers a limit of quantification fourfold lower (0.005 μg L−1), which permits the quantification of compounds at very low concentrations. Lissalde et al. (2011) also showed this advantage for the passive sampling of polar pesticides in a previous study comparing automated grab sample concentrations with POCIS measurements. The concentration in acetochlor measured in the second grab sample was almost sevenfold higher than the concentration measured with the POCIS (Fig 3a). This spot sample may have been collected just at the peak of contamination so that a high concentration was measured, whereas the first spot sample may have been recovered before the peak resulting in no quantification of acetochlor. These results show the variability associated with spot sampling, and highlight the risk of missing contamination peaks that may be very short. Conversely, the POCIS tends to smooth the contamination curve by integrating both decreases and rises.
During the second period (Fig 3b), only acetochlor was quantified. The first grab sample in Fig 3b was collected on 22nd May and is thus the same as the second grab sample presented in (Fig 3a). The fact that acetochlor was not quantified in the grab sample taken on 4th June shows that the contamination peak lasted less than 14 days. Interestingly, there was no significant difference between the concentration measured in the automated sampler and in the POCIS, which shows the relative reliability of the POCIS data.
This result shows the usefulness of POCIS for monitoring water quality in mildly contaminated media, in which some compounds could not be quantified with traditional spot sampling. Indeed, very few compounds were quantified in the whole grab samples (n = 18) (acetochlor, DEA, imidacloprid) and even less in the automated samples (n = 12) (acetochlor only) (data not shown). Each one was quantified only once over the whole studied period. On the contrary, seven molecules were quantified in the POCIS samples. To go further, the POCIS data were used to calculate frequencies of occurrence for each molecule and each sampling station.
Pesticide concentrations and frequencies of occurrence measured with POCIS
Here, the word “occurrence” means that the molecule was present in the POCIS. More precisely, the frequencies of occurrence (FO) of the molecules in the three sampling stations were calculated on the basis of the minimum amount of analyte that could be quantified in the POCIS sorbent (NPOCIS), which corresponds to the IQL. FOs were preferred to frequencies of quantification because, contrary to the LQ, the IQL does not depend on the Rs (sampling rate constant, which varies with environmental conditions and with the time of exposure). Data are reported in Fig 4.
Five pesticides and two metabolites were present in the Auvézère River at the Quatre-Moulins station. DEA, a metabolite of atrazine, was the compound most frequently present (FO = 90 %), followed by acectochlor (FO = 33 %). For other compounds, FOs were lower, less than 10 %.
The maximum concentrations recorded for each compound over the entire study period ranged from 0.022 μg L−1 for acetochlor to less than the LQ for DCPMU and metolachlor.
The Arnac stream was more contaminated than the Auvézère River. Seven pesticides and three metabolites were detected at the upstream station. The compounds most frequently present were DEA, atrazine and diuron with FOs ranging from 60 to 100 %. DEA was the most frequently detected compound (FO = 100 %) and also the most concentrated with 0.047 μg L−1 (sampling period from August 20th to September 3rd).
At the downstream station, 14 pesticides and 4 metabolites were detected. The most frequently detected compounds were the same as at the upstream station. However, the maximum concentration was recorded for simazine (0.125 μg L−1, sampling period from September 3rd to September 17th). These results show that the downstream part of the Arnac River was more contaminated than the upstream part both in terms of number of compounds present and in terms of maximum concentrations recorded. However, some molecules were detected at the upstream station and were not recovered downstream (i.e. metoxuron and carbendazim). This could be due to degradation of the molecules between the two sampling stations (metoxuron is classified as very instable in water (Table 1)), and/or to a dilution effect, as water flow was almost twice as fast at the downstream station (Fig 2). Sorption processes on sediments and suspended matter could also be involved: metoxuron and carbendazim have a slight tendency to adsorb on soil particles as their sorption coefficients (Koc) are 120 and 227 L kg−1, respectively (Table 1). On the contrary, some compounds were detected only at the downstream station. Their presence could be due to (1) application to the agricultural parcels bordering the stream, (2) contribution from the two small streams that flow into the Arnac stream just between the two sampling stations (Fig 1).
Comparison of the POCIS and spot sampling strategies for pesticides flux calculation
Pesticide fluxes were calculated from the grab and composite sample data (Eq. 7 ) and from the POCIS data (Eq. 6 ) in order to compare the three sampling strategies. Results are listed in Table 2 and dates correspond to the retrieval of the corresponding POCIS. We hypothesized that the composite sample data were the most reliable because (1) they are not impacted by environmental variations as it is the case for POCIS concentrations and (2) the temporal representativeness is better with hourly composite water samples than with bimonthly grab samples. On the 9th May and 18th June, the calculation of fluxes for acetochlor, DEA and dimethanamid was possible only with the passive sampling strategy. As previously noted in section 3.2, this result is directly linked to the ability of the POCIS to detect trace levels of compounds, which cannot be quantified in water samples. The fluxes calculated on the 22nd May and 4th June are related to the concentration data presented in Fig 3. The high concentrations of acetochlor measured in the grab samples (Fig 3) led to high flux estimates, but an overestimation by a factor of 3 to 5 is likely, taking the composite sample value as a reference. This result shows that the bimonthly grab sampling strategy fails to give reliable data and is not suitable for flux calculation purposes, particularly in this context of low contamination levels. On the contrary, the flux calculated with the POCIS data for acetochlor on the 4th June is very close to the reference (2.9 and 2.8 g day−1, respectively).
To our knowledge, POCIS concentrations have never been used for flux calculations. Our results show that this technique could be an advantageous alternative to the spot sampling strategy, making flux calculations possible for low concentration compounds while reducing the number of samples. It should be pointed out that the TWAC measured with POCIS (and therefore the calculated fluxes) may be over or underestimated by a factor of 2 because of varying environmental conditions affecting the sampling rate constants (Eq. 1). It is also important to be aware that the POCIS (and passive samplers in general) have a latency period going from a few hours to one or several days (Mazzella et al. 2008). This means that very fleeting peaks of contamination (typical for little watersheds) could not be integrated. However, Rabiet et al. (2010) showed in a recent study that monthly fluxes of diuron calculated with weekly grab samples could lead to underestimation by a factor of 5 (i.e. 400 % inaccuracy) (when compared to composite weekly sampling results taken as a reference value). This underestimation is essentially due to the lack of representativeness of the grab sampling strategy in which most flood events are not accounted for. Therefore, although the accuracy of the POCIS measurement is only about 50 or 100 %, the fluxes calculated with the TWAC should still be more reliable than those calculated with grab samples taken once a month as is generally the case in regulatory monitoring programmes. According to Harman et al. (2012) and Morin et al. (2012), the variation of Rs constants with environmental conditions are generally less than twofold. Therefore, a confidence interval taking into account the maximum variation of the Rs could be calculated by dividing, then multiplying the POCIS TWAC by a factor of 2 in the calculation of fluxes. The minimum and maximum fluxes generated this way would give an estimate of the range of possible flux value.
Interpretation of the fluxes estimated with the POCIS
Since previous results showed that POCIS had a lower limit of detection compared to spot samples, daily average fluxes of pesticides were estimated using the semi-quantitative TWACs (dissolved fraction) determined with POCIS during the exposure period (Eq. 6).
Five compounds were quantified in the Auvézère river at the Quatre-Moulins station (Fig 5a). Diuron, alachlor and dimethanamid were quantified only once (between May and June), with fluxes of about 2.2, 0.9 and 1.2 g day−1, respectively. The highest flux of acetochlor was also recorded during this period (3.6 g day−1). DEA appeared several times during the study period, with fluxes varying from 0 to 2.7 g day−1.
Nine compounds were quantified in the Arnac stream. The downstream part (Fig 5c) was more contaminated than the upstream (Fig 5b) both in terms of number of compounds (five at the upstream station versus nine at the downstream) and in terms of intensity of the fluxes. The maximum fluxes calculated for DEA and diuron were indeed threefold higher downstream than upstream.
For most compounds, the highest fluxes were recorded during spring (carbaryl, diuron, linuron, DCPU), but for atrazine and its metabolites (DEA and DIA), the highest fluxes were measured at the beginning of winter.
Most pesticides quantified at the three sampling stations are banned in France, except acetochlor, imidacloprid, dimethanamid and linuron. Acetochlor, dimethanamid and imidacloprid are used for herbicide and insecticide treatments of maize and orchards, respectively. These crops are very common in the studied area and the peaks of contamination coincide well with the application periods of these products. So, the presence of these compounds in the river could be related to recent applications on crops and transport via leaching (Rabiet et al. 2010). Conversely, the crops on which linuron is authorized are not very common in the watershed. Its presence in the Arnac stream may be due to domestic uses, for example on carrot or celery plants in vegetable gardens. Other compounds (atrazine, diuron, carbaryl) have been banned in France for many years (Table 1) so it is unlikely that their presence in the river is due to recent application. Atrazine and diuron show very low degradation in water (Table 1), resulting in possible accumulation in aquifers. Landry et al. (2006) and Morvan et al. (2006) observed that atrazine was still systematically quantified at the outlet of a spring draining an agricultural watershed 5 years after application ended , with concentrations up to 0.43 μg L−1. Similar studies conducted on diuron (Gooddy et al. 2002; Landry et al. 2006) clearly showed that it has potential to accumulate in aquifers. So, these pesticides may come from groundwater stocks. On the contrary, carbaryl degrades rapidly in water but has a moderate tendency to adsorb on soil particles (Table 1). In a previous study conducted on agricultural soil, Ahmad et al. (2004) showed that 50 % of the total carbaryl in soil was not bioavailable and therefore was not degraded by soil organisms. So, the presence of carbaryl in the stream may be due to slow desorption of the fraction accumulated in soil.
Except in winter, the increases in pesticide fluxes for all compounds were during rainy periods and were related to high water flow (Fig 2). The level of rainfall was quite low in January when the highest peaks of atrazine, its metabolites and diuron occurred. However, December 2011 was extremely wet (about 150 mm of rainfalls), so this high flux might have been a consequence of a higher peak that occurred in December.
To go further, the specific input from the upstream and the downstream parts of the Arnac stream, and their contribution to the Auvézère contamination were assessed. This part of the study aimed to exemplify possible applications of the passive sampling strategy. Although the data obtained with the POCIS are only semi-quantitative, they could offer valuable information about major sources of contamination in a watershed.
The surface of the Arnac’s downstream watershed (9 km2) is very much higher than the surface of the upstream watershed (1.5 km2). To overcome the influence of this parameter and to access the specific contribution of the downstream watershed to the global contamination of the river, the pesticide fluxes (Eq. 6) were weighted with the surface of each watershed. More precisely, the surface of the upstream watershed was subtracted from the surface of the downstream watershed and, in the same way, the upstream pesticide fluxes were subtracted from the downstream fluxes. The sum of the weighted fluxes was then calculated for each molecule over the entire study period. Data are reported in Fig 6.
Atrazine, its metabolites DEA and DIA, and diuron mainly came from the upstream part of the river. This tends to confirm that these molecules were partly driven to the water body via groundwater.
The main objective of the Arnac River monitoring was to assess its contribution to the Auvézère contamination. To do this, total masses of pesticides flowing in the two rivers (the Auvézère at the Quatre-Moulins station and the Arnac at the downstream station) were calculated by adding the fluxes recorded during the entire study period. For this purpose, fluxes were not weighted with the watershed surface areas. DEA and diuron were the only compounds quantified both in the Auvézère and Arnac Rivers, so the percentage contribution could be calculated only for these two molecules. We hypothesized that there was no degradation and no addition of the molecules between the Quatre-Moulins sampling station and the Arnac downstream sampling station. Data are reported in Table 3.
The contribution of the Arnac River to the Auvézère's contamination was very similar for DEA and diuron (16 and 18 %, respectively). Taking into account the fact that the Arnac’s watershed represents less than 10 % of the surface of the Auvézère watershed, we can say that the Arnac contribution was more than 1.5 fold higher than expected. This result may indicate that the sources of pesticide contamination were not homogeneously distributed on the watershed.
Conclusion
The main conclusion of this study is that passive sampling is an efficient way to improve the representativeness of pesticide monitoring data. POCIS was shown to be a very useful tool to monitor pesticide concentrations in low contamination contexts. Indeed, the passive sampler gave better results in terms of limits of quantification and frequencies of occurrence compared to spot sampling. In the Quatre-Moulins station, three pesticides were quantified with POCIS during spring, whereas only acetochlor was measured in grab samples. DEA was the most frequently measured compound in the studied watershed with almost 100 % occurrence. Although the data are semi-quantitative, the ability of POCIS to detect many compounds at trace levels made the calculation of pesticide fluxes in the Auvézère River possible even when concentrations were too low to be quantified in composite water samples. Additionally, the technical constraints associated with automated sampler maintenance (necessity for electricity, protection against potential vandalism, possible degradation of analytes in the bottle…) can be easily avoided with the use of POCIS. This feature can be useful for the monitoring of several sampling stations in the same or different watersheds, especially in remote areas, etc. Using passive sampler data to assess the specific input from small watersheds and determine their contribution to the contamination of the main river is very original and has never before been implemented. Used with several passive samplers dedicated to different types of compounds, such as semi-permeable membrane devices or Chemcatcher® for hydrophobic molecules, this strategy could permit targeting areas with higher contamination contributions. Flux information could benefit regulatory investigative and operational monitoring network, resulting in better understanding of the global contamination of a watershed, and in the implementation of more accurate remediation strategies.
References
2000/60/EC (2000) Directive 2000/60/EC of The European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. . OJ L 327, 22.12.2000:1–73
2008/105/EC (2008) 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy. OJ L 348 24.12.2008:84–97
Ahmad R, Kookana RS, Megharaj M, Alston AM (2004) Aging reduces the bioavailability of even a weakly sorbed pesticide (carbaryl) in soil. Environ Toxicol Chem 23(9):2084–2089. doi:10.1897/03-569
Allan IJ, Knutsson J, Guigues N, Mills GA, Fouillac AM, Greenwood R (2007) Evaluation of the Chemcatcher and DGT passive samplers for monitoring metals with highly fluctuating water concentrations. J Environ Monit 9(7):672–681. doi:10.1039/b701616f
Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE (2004) Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem 23(7):1640–1648. doi:10.1897/03-603
DeLorenzo ME, Scott GI, Ross PE (2001) Toxicity of pesticides to aquatic microorganisms: a review. Environ Toxicol Chem 20(1):84–98. doi:10.1002/etc.5620200108
Fauvelle V, Mazzella N, Delmas F, Madarassou K, Eon M, Budzinski H (2012) Use of mixed-mode ion exchange sorbent for the passive sampling of organic acids by Polar Organic Chemical Integrative Sampler (POCIS). Environmental Science & Technology 46(24):13344–13353. doi:10.1021/es3035279
Gooddy DC, Chilton PJ, Harrison I (2002) A field study to assess the degradation and transport of diuron and its metabolites in a calcareous soil. Sci Total Environ 297(1–3):67–83. doi:10.1016/s0048-9697(02)00079-7
Harman C, Tollefsen KE, Bøyum O, Thomas K, Grung M (2008) Uptake rates of alkylphenols, PAHs and carbazoles in semipermeable membrane devices (SPMDs) and polar organic chemical integrative samplers (POCIS). Chemosphere 72(10):1510–1516
Harman C, Allan IJ, Vermeirssen ELM (2012) Calibration and use of the polar organic chemical integrative sampler—a critical review. Environ Toxicol Chem 31(12):2724–2738
Huckins JN, Manuweera GK, Petty JD, Mackay D, Lebo JA (1993) Lipid-containing semipermeable-membrane devices for monitoring organic contaminants in water. Environmental Science & Technology 27(12):2489–2496. doi:10.1021/es00048a028
Jaward FM, Farrar NJ, Harner T, Sweetman AJ, Jones KC (2004) Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environmental Science & Technology 38(1):34–41. doi:10.1021/es034705n
Landry D, Dousset S, Andreux F (2006) Leaching of oryzalin and diuron through undisturbed vineyard soil columns under outdoor conditions. Chemosphere 62(10):1736–1747. doi:10.1016/j.chemosphere.2005.06.024
Lennartz B, Louchart X, Voltz M, Andrieux P (1997) Diuron and simazine losses to runoff water in Mediterranean vineyards. J Environ Qual 26(6):1493–1502
Li H, Vermeirssen EL, Helm PA, Metcalfe CD (2010) Controlled field evaluation of water flow rate effects on sampling polar organic compounds using polar organic chemical integrative samplers. Environ Toxicol Chem 29(11):2461–2469
Lissalde S, Mazzella N, Fauvelle V, Delmas F, Mazellier P, Legube B (2011) Liquid chromatography coupled with tandem mass spectrometry method for thirty-three pesticides in natural water and comparison of performance between classical solid phase extraction and passive sampling approaches. J Chromatogr A 1218(11):1492–1502. doi:10.1016/j.chroma.2011.01.040
Loos R, Gawlik BM, Locoro G, Rimaviciute E, Contini S, Bidoglio G (2009) EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 157(2):561–568. doi:10.1016/j.envpol.2008.09.020
Louchart X, Voltz M, Andrieux P, Moussa R (2001) Herbicide transport to surface waters at field and watershed scales in a Mediterranean vineyard area. J Environ Qual 30(3):982–991
Mason CF, Underwood GJC, Baker NR, Davey PA, Davidson I, Hanlon G, Long SP, Oxborough K, Paterson DM, Watson A (2003) The role of herbicides in the erosion of salt marshes in eastern England. Environ Pollut 122(1):41–49. doi:10.1016/s0269-7491(02)00284-1
Mazzella N, Dubernet JF, Delmas F (2007) Determination of kinetic and equilibrium regimes in the operation of polar organic chemical integrative samplers—application to the passive sampling of the polar herbicides in aquatic environments.
Mazzella N, Debenest T, Delmas F (2008) Comparison between the polar organic chemical integrative sampler and the solid-phase extraction for estimating herbicide time-weighted average concentrations during a microcosm experiment. Chemosphere 73(4):545–550. doi:10.1016/j.chemosphere.2008.06.009
Morin N, Miège C, Coquery M, Randon J (2012) Chemical calibration, performance, validation and applications of the polar organic chemical integrative sampler (POCIS) in aquatic environments. TrAC Trends in Analytical Chemistry (0)
Morvan X, Mouvet C, Baran N, Gutierrez A (2006) Pesticides in the groundwater of a spring draining a sandy aquifer: temporal variability of concentrations and fluxes. J Contam Hydrol 87(3–4):176–190, doi:http://dx.doi.org/10.1016/j.jconhyd.2006.05.003
Rabiet M, Margoum C, Gouy V, Carluer N, Coquery M (2010) Assessing pesticide concentrations and fluxes in the stream of a small vineyard catchment—effect of sampling frequency. Environ Pollut 158(3):737–748, doi:http://dx.doi.org/10.1016/j.envpol.2009.10.014
Solomon KR, Baker DB, Richards RP, Dixon DR, Klaine SJ, LaPoint TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15(1):31–74, doi:10.1897/1551-5028(1996)015<0031:eraoai>2.3.co;2
Stuer-Lauridsen F (2005) Review of passive accumulation devices for monitoring organic micropollutants in the aquatic environment. Environ Pollut 136(3):503–524. doi:10.1016/j.envpol.2005.12.004
Taghavi L, Merlina G, Probst JL (2011) The role of storm flows in concentration of pesticides associated with particulate and dissolved fractions as a threat to aquatic ecosystems case study: the agricultural watershed of Save river (Southwest of France). Knowledge and Management of Aquatic Ecosystems (400). doi:06 10.1051/kmae/2011002
Togola A, Budzinski H (2007) Development of polar organic integrative samplers for analysis of pharmaceuticals in aquatic systems. Anal Chem 79(17):6734–6741
Vrana B, Mills GA, Allan IJ, Dominiak E, Svensson K, Knutsson J, Morrison G, Greenwood R (2005) Passive sampling techniques for monitoring pollutants in water. Trac-Trends in Analytical Chemistry 24(10):845–868. doi:10.1016/j.trac.2005.06.006
Acknowledgments
This study was financed by the French Water Agency Adour Garonne and the Région Limousin. The authors would like to thank Aurélie Moreira, Patrice Fondanèche, Julie Bonafos, Emma Bourjas and Chloé Nicolas for technical and field assistance and Katherine Flynn for English revision.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Laura McConnell
Rights and permissions
About this article
Cite this article
Poulier, G., Lissalde, S., Charriau, A. et al. Estimates of pesticide concentrations and fluxes in two rivers of an extensive French multi-agricultural watershed: application of the passive sampling strategy. Environ Sci Pollut Res 22, 8044–8057 (2015). https://doi.org/10.1007/s11356-014-2814-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-014-2814-y