Abstract
This study presents a complementary approach for the evaluation of water quality in a river basin by employing active and passive sampling. Thirty-eight hydrophilic organic compounds (HpOCs) (organohalogen herbicides, organophosphorous pesticides, carbamate, triazine, urea, pharmaceuticals, phenols, and industrial chemicals) were studied in grab water samples and in passive samplers POCIS collected along Strymonas River, Northern Greece, at three sampling campaigns during the year 2013. Almost all the target compounds were detected at the periods of high rainfall intensity and/or low flow rate. The most frequently detected compounds were aminocarb, carbaryl, chlorfenviphos, chloropropham, 2,4-D, diflubenzuron, diuron, isoproturon, metolachlor, and salicylic acid. Bisphenol A and nonylphenol were also occasionally detected. The use of POCIS allowed the detection of more micropollutants than active sampling. Low discrepancy between the concentrations obtained from both samplings was observed, at least for compounds with >50 % detection frequency; thus, POCIS could be a valuable tool for the selection and monitoring of the most relevant HpOCs in the river basin. Results showed relatively low risk from the presence of HpOCs; however, the potential risk associated with micropollutants such as carbaryl, dinoseb, diuron, fenthion, isoproturon, metolachlor, nonylphenol, and salicylic acid should not be neglected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The European Water Framework Directive 2000/60/EC (WFD) sets up environmental objectives to achieve “good water status” for all European waters by 2015 and establishes a clear framework to enable these objectives to be achieved (European Commission 2000). WFD introduces a list of 33 priority substances, which have to be monitored by member states. This list is accompanied with defined environmental quality standards (EQS) for these compounds in Directive 2008/105/EC (European Commission 2008). Recently, the Directive 2013/39/EC, amending the previous directives, introduces additional priority substances and their respective EQS values (European Commission 2013). Moreover, there is also concern for other hazardous compounds that could be released in the aquatic environment (European Commission 1976; European Commission 2006a). For this reason, specific pollutants in a river basin have to be identified regarding possible pollution sources and activities taking place in the area.
The concentrations of organic compounds such as pharmaceuticals and pesticides in surface waters are usually low (<1 μg/L), and there is a need of sensitive methods with very low detection limits. The concentration and variability of pharmaceutical compounds in the aquatic environment depend on their use pattern, the amounts discharged, and their persistence. On the other hand, pesticide loads are strongly connected to the application time and surface runoff. Due to the variability of micropollutants in the aquatic environment, there is a need for frequent sampling. Among other available monitoring methods, passive sampling presents a promising environmental monitoring tool recommended in the European Commission Guidance on surface water monitoring and in the Directive 2013/39 (European Commission Guidance No. 19 2009; European Commission 2013). Passive sampling offers a sensitive and time integrative measurement of free dissolved (i.e., bioavailable) concentrations of micropollutants in water, usually over a period of few weeks. This overcomes the problem of measurements below the detection limit often observed with conventional grab sampling techniques. Furthermore, different sources of variance and uncertainty can be much better controlled, which in turn results in reduction of the required number of analyzed samples to obtain results with comparable statistical power (Poulier et al. 2014). Passive samplers represent a well-defined sampling medium with a known uptake capacity which is the major advantage over alternative media such as sediments or biota used for trend monitoring. Moreover, passive samplers could be used to further evaluate the toxicity or estrogenicity of waters by employing various bioassays (Creusot et al. 2013; Jalova et al. 2013).
Polar organic chemical integrative samplers (POCIS) have been used for hydrophilic organic compounds, from polar to moderate polar micropollutants with logKow ≤4 such as pesticides, pharmaceuticals, ingredients from personal care and consumer products, natural and synthetic hormones, and industrial compounds (Arditsoglou and Voutsa 2008; Assoumani et al. 2013; Berho et al. 2013; Dalton et al. 2014; Ibrahim et al. 2012; Jalova et al. 2013; Lissalde et al. 2011; Lohmann et al. 2012). Two types of absorbents are used in POCIS. POCIS-Pest preferentially concentrates waterborne HpOCs such as polar pesticides and natural and synthetic hormones whereas POCIS-Pharm concentrates polar pharmaceuticals, endocrine-disrupting compounds (EDCs), ingredients from personal care and consumer products (PPCPs), and other wastewater-related contaminants (Arditsoglou and Voutsa 2008; Coes et al. 2014; Creusot et al. 2013; Jarosova et al. 2012; Munaron et al. 2012; Thomatou et al. 2011).
This study aims at the integrate assessment of 38 organic micropollutants (priority and other pollutants of emerging concern) in the basin of Strymonas River, Northern Greece, by employing active and passive sampling. Various groups of pollutants such as carbamate pesticides, organohalogen herbicides, organophosphorous pesticides, triazine pesticides, urea pesticides, pharmaceuticals, phenols, and industrial chemicals have been determined. Water samples have been collected along the river during three sampling campaigns in 2013 and analyzed by employing GC-MS/MS. At the same period, passive sampling was also conducted by employing POCIS. In this article, the dominant micropollutants in the river basin as well as their temporal and spatial variation are presented. Results from passive sampling (detection frequency of micropollutants and concentrations) are discussed, regarding the possible application of POCIS as complementary tool in water monitoring. Moreover, the possible ecotoxicological risk from the presence of organic micropollutants is estimated according to the risk quotient approach.
Materials and methods
Study area
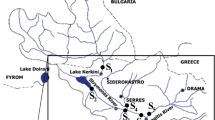
The examined area is the basin of Strymonas River. Strymonas is a transboundary river in the Balkan Peninsula (runs through Bulgaria, Serbia, FYROM, and Greece). Detailed information for the study area have been previously presented (Terzopoulou and Voutsa, Quality assessment of surface water at the basin of Strymonas river, Northern Greece, Fresen Environ Bull, accepted). Briefly, the river drains approximately 17,152 km2 and outflows to the Strymonic Gulf. Significant wetlands (Kerkini Lake, river estuaries) in the river basin offer a variety of habitats for birds and animals. Due to the ecological importance, these systems are included in the EU “Natura 2000” network and are protected through various conventions and regulations.
The mean discharge of Strymonas River at the entrance of Greek territory is 83 m3/s. The rainfall in the area ranged from 50 mm in summer up to 85 mm in winter period (data for the period 2006–2010) and temperature between 4.12 °C in January to 26.7 °C in July. Agricultural, urban, and industrial activities are the main pressures in the river basin. Wheat and irrigated crops (cotton, maize, lucerne, sugar beet, tomato, tobacco, olive, grape, and rice) are cultivated in the area. Industrial activity includes mainly food processing plants of the primary sector (milk, meat, sugar, and olive). Finally, treated as well as raw sewage effluents are discharged in the river.
Sampling
Water samples
Water samples were collected in amber glass bottles, previously washed with hexane, acetone, and ultrapure water and heated at 250 °C for at least 6 h. Water samples were collected at seven sites along Strymonas River (Fig. 1). Three sampling campaigns (June/July, August/September, and October/November) were conducted in the year 2013 in order to cover variation of hydrological conditions (high-low flow rate) as well as possible impacts from seasonally dependent inputs (i.e., agricultural runoff). The samples were kept in a portable refrigerator (4 °C) during the transportation to the laboratory.
Sampling details and water physicochemical parameters are shown in Table 1. The concentrations of suspended particulate matter (SPM) determined after filtration through 0.45 μm, whereas dissolved organic carbon (DOC) was measured in filtered samples with the combustion catalytic oxidation/NDIR method using a TOC-Vcsh analyzer (SHIMADZU). The physicochemical characteristics of water have been previously discussed in details (Terzopoulou and Voutsa, Quality assessment of surface water at the basin of Strymonas river, Northern Greece, Fresen Environ Bull, accepted).
Passive sampling
Two types of passive samplers, POCIS-Pest and POCIS-Pharm (Exposmeter, Sweden), were used in this study. The passive sampler POCIS-Pest consists of 200 mg triphasic sorbent mixture 80:20 (w/w) Isolute ΕΝV+: Ambersorb 1500 dispersed on S-X3 Βio Beads which is enclosed between two PES membranes (0.1-μm pore size) and held together with compression between two stainless steel washers (5.1 cm ID, 8.9 cm OD). The passive samplers POCIS-Pharm consists of 200 mg Oasis HLB (pοly[divylbenzene]-cο-Ν-vinylpyrrolidone) which is enclosed between two PES membranes (0.1-μm pore size) and held together with compression between two stainless steel washers (5.1 cm ID, 8.9 cm OD).
Before their use, they were stored in gas tight metal containers at −20 °C. POCIS were exposed at four sites along Strymonas River during three sampling campaigns (June/July as first campaign, August/September as second campaign, and October/November as third campaign). The exposure time was 18 days. Sampling details are shown in Table 1. At the end of exposure period, each sampler was rinsed with ultrapure water, wrapped in aluminum foil, and kept in a portable refrigerator (4 °C) during the transportation to the laboratory. POCIS were stored at −25 °C until analysis. The first and the last day of the exposure of POCIS grab water samples were also collected and analyzed.
Studied micropollutants
Samples were analyzed for 38 HpOCs, including carbamate pesticides, organohalogen herbicides, organophosphorous pesticides, triazine pesticides, urea pesticides, pharmaceuticals, phenols, and industrial chemicals. The compounds are listed in Table S1. Eleven compounds (alachlor, atrazine, bisphenol A, chlorfenviphos, chlorpyrifos, diuron, isoproturon, mecoprop, nonylphenol, simazine, and terbutryn) are priority substances according to the EU (2008 and 2013). The choice of target compounds was based on their possible occurrence in the river basin in relation to the current agricultural, urban, and industrial activities in the area.
Chemicals and reagents
For the needs of this study a number of standards as mixtures or single compounds have been purchased. Chlorinated pesticides (4-chloro-o-tolyoxyacetic acid, mecoprop, dalapon, dichlorprop, dinoseb, dicamba, 2,4-D, silvex), triazine pesticides (atraton, terbuthylazine, propazine, atrazine, simazine, prometryne, ametryne, simetryn, terbutryne), organophosphorous pesticides (fenthion, chlorfenviphos, chlorpyrifos), carbamate and urea pesticides (swep, aminocarb, carbaryl, methiocarb, chloropropham, carbofuran, diuron), alachlor, metolachlor, isoproturon, and diflubenzuron were of analytical grade and purchased from Chem Lab (Zedelgem, Belgium). 1H-benzotriazole, salicylic acid, nitrophenol, and 4-tert-octylphenol were obtained from Fluka (Buchs, Switzerland). Caffeine and nonylphenol were purchased from Sigma (Sigma-Aldrich, Steinhem, Germany) and bisphenol A from Ceriliant (Round Rock, TX, USA). A mixture of tetrachloro-m-xylene and decachlorobiphenyl as surrogate standard and a mixture of isotopically labeled PAHs (acenapthene-d10, phenanthrene-d10, chrysene-d12, perylene-d10) as internal standards were obtained from Chem Lab (Zedelgem, Belgium).
Methanol (HPLC grade), dichloromethane (pesticide grade), ethylacetate (HPLC grade), and hexane (95 % HPLC grade) were purchased from Chem Lab (Zedelgem, Belgium). Toluene (GR for analysis) was purchased from Merck (Darmstadt, Germany). Ultrapure water was produced with a Pure Lab system (Sation 9000, Spain). Oasis HLB (200 mg, 6 mL) were obtained from Waters (Milford, MA, USA). Passive samplers, POCIS-Pest and POCIS-Pharm, were obtained from Exposmeter SA (Tavelsjo, Sweden).
Individual stock solutions (1 mg/mL) were prepared in methanol and stored at −20 °C. Multi-compound methanolic stock standard solution was prepared and stored at −20 °C in the dark. Working solutions were prepared by appropriate dilution of the stock standard solutions with methanol and were stored at 4 °C in the dark for a maximum period of 6 months.
Instrumentation
Target compounds were determined by GC-MS/MS using a Trace GC gas chromatograph equipped with a mass selective detector Polaris Q (ThermoFinnigan, Waltham, MA, USA), a Tri Plus Autosampler, and a Dell computer system with Xcalibur data acquisition software (ThermoElectron, San Jose, CA, USA) according to a multi-residue method described in details in a previous article (Terzopoulou et al. 2015). The TR-5MS analytical column (30 m, 0.25 mm ID, 0.25 μm df, ThermoElectron) was used. The oven temperature program was as follows: the initial temperature was set at 60 °C, held for 2 min and then increased at a rate 3 °C/min to 175 °C, held for 4 min. Then, temperature was increased at a rate 3 °C/min to 240 °C and held for 10 min. Finally, temperature was increased to 320 °C at a rate 7 °C/min and held for 24 min. The total run time was 100 min. The temperature of ion source and transfer line was set at 200 and 275 °C, respectively. Helium, carrier gas, was maintained at a flow rate of 1.0 mL/min. EI in positive ionization mode was employed. The excitation energy was set at 0.45 V.
Analytical procedure
Water sample preparation
The target compounds were recovered from water samples by employing SPE through OASIS HLB cartridges according to a previously proposed protocol (Terzopoulou et al. 2015). Briefly, cartridges were conditioned by 5 mL mixture dichloromethane/ethylacetate (50:50, v/v), 5 mL hexane/dichloromethane (50:50, v/v), 1 mL methanol, and 1 mL of ultrapure water. The filtered water sample (1 L), without any pH adjustment, spiked with decachlorobiphenyl and tetrachloro-m-xylene, was percolated through cartridges at a flow rate of 6 mL/min. Afterward, cartridges were rinsed with 2 mL ultrapure water to remove matrix interferences and dried under vacuum for 30 min to ensure that no residual water would be eluted with the final extract. The analytes were eluted with 5 mL of hexane/dichloromethane (50:50, v/v) and 3 mL of dichloromethane/ethylacetate (50:50, v/v) at a rate of 1 mL/min followed by 2 mL of pushing air. The final extract was evaporated to dryness under a gentle stream of nitrogen at 35 °C, redissolved in methanol, spiked with the mixture of acenapthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d10 and transferred to an injection vial.
Passive samplers preparation
POCIS samplers were cleaned from debris and mud with ultrapure water. Empty polypropylene SPE tubes (1 mL) with polyethylene (PE) frits were packed with 200 mg of triphasic sorbent admixture from POCIS-Pest and with 200 mg of OASIS HLB bulk sorbent from POCIS-Pharm, respectively. Afterward, cartridges were rinsed with 20 mL ultrapure water to remove matrix interferences and dried under vacuum for 30 min to ensure that no residual water would be eluted with the final extract. Cartridges were spiked with decachlorobiphenyl and tetrachloro-m-xylene. The analytes from POCIS-Pest were eluted with 5 mL of methanol/toluene/dichloromethane (1:1:8, v/v/v) at a rate of 1 mL/min followed by 2 mL of pushing air. The analytes from POCIS-Pharm were eluted with 5 mL of hexane/dichloromethane (50:50, v/v) and 3 mL of dichloromethane/ethylacetate (50:50, v/v) at a rate of 1 mL/min followed by 2 mL of pushing air. The final extract was evaporated to dryness under a gentle stream of nitrogen at 35 °C, redissolved in 0.5 mL methanol, spiked with the mixture of acenapthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d10 and transferred to an injection vial.
Quality assurance–quality control
Procedural and field POCIS-Pest and POCIS-Pharm blank samples were extracted and analyzed, according to treatment procedure previously described, in each experimental set to check for any possible background concentration and/or cross contamination during sampling, handling, and experimental procedure. The examined compounds in these samples were not found at detectable levels. Moreover, a solvent blank was injected at regular intervals to check for a potential contamination of analytical system. No carry over contamination was observed. Positive detection of target compounds in samples was confirmed by comparing retention time, selected monitoring ions and their relative intensities between calibration solutions and samples. GC-MS/MS measurements were estimated on calibration curves for each compound by standard solutions. The performance characteristics of analytical method have been reported in a previous article (Terzopoulou et al. 2015) and summarized in Table S2. For this purpose, a validation protocol was employed based on the Decision 2002/657/EC validation guidelines and ISO 5725-3 on accuracy, trueness, and precision. Linearity, accuracy, precision, and uncertainty of the method were evaluated. Because of the diversity of the target compounds with very different chemical and physical properties, internal and surrogate standards showed retention times at the beginning, middle, and end of the chromatogram. For the evaluation of the recovery, two experiments were performed, according to the Decision 2002/657/EC (European Commission 2002). Most compounds exhibited recoveries ranged from 60 to 110 %. The coefficient of variation (CV) ranged from 0.84 to 23.8 %. LOD and LOQ ranged from 6.4 to 40.1 ng/L and from 21.5 to 134 ng/L, respectively. The expanded uncertainties (U %) ranged from 5.98 to 36.98 %.
Results and discussion
Active sampling
The concentrations (min, max, median, mean, standard deviation) and frequency of detection of target analytes during the three sampling campaigns are shown in Table 2. Twenty-three compounds were found at detectable concentrations in water samples. The higher detection frequencies as well as the higher median concentrations were observed for aminocarb (54.6 %, 0.234 μg/L), caffeine (63.6 %, 0.094 μg/L), carbaryl (90.9 %, 0.337 μg/L), chlorfenviphos (100 %, 0.878 μg/L), chloropropham (63.6 %, 0.230 μg/L), diflubenzuron (72.7 %, 0.114 μg/L), diuron (100 %, 0.420 μg/L), isoproturon (90.9 %, 0.856 μg/L), metolachlor (100 %, 2.303 μg/L), nonylphenol (100 %, 0.049 μg/L), and salicylic acid (100 %, 3.14 μg/L).
During the period June–July, the lowest concentrations of micropollutants were found. At the second sampling campaign (August–September), the highest detection frequencies for most of the micropollutants as well as the highest concentrations were observed (Table 2). This could be attributed to the possible surface runoff of pesticides applied in agricultural fields as well as to the lower water flow rate. Furthermore, during the third sampling campaign (October–November), maximum concentrations were observed for pesticides (aminocarb, dicamba, and metolachlor) and for salicylic acid. This could be attributed to the higher leaching of pesticides due to abundant rain waters in this period as well as to the lower degradation rates in autumn compared to warm period. Atrazine was also detected in the water, although its use has been banned in Europe since 2004. The presence of atrazine in surface waters in Europe has also been reported by other investigators (Jablonowski et al. 2011; Loos et al. 2009; Nödler et al. 2013; Nödler et al. 2014). Moreover, this compound has constantly detected in the coastal area of Northern Greece (Nödler et al. 2013, 2014). Significant amounts of this compound have been applied in agricultural areas in Greece before its prohibition. Due to its persistency, leaching from old inventories in the soil and/or in the sediments is possible. Moreover, illegal use of this compound cannot be excluded. The sampling sites S1, S4, S5, and S7 (Fig. 1) were the most polluted to target compounds particularly during the second and third sampling campaigns. Maximum concentrations of chlorfenviphos, diuron, and isoproturon above the MAC EU limits were determined (2008/105/EC; 2013/39/EC).
A previous study in the catchment area of the Strymonas River completed 15 years ago (October 1998–September 1999) reported 44 micropollutants with organohalogen herbicides, organophosphorous pesticides, and triazines at highest detection frequencies (Lekkas et al. 2004). Moreover, similar compounds have been frequently detected in other surface waters in Greece (Kalogridi et al. 2014; Stamatis et al. 2013; Vryzas et al. 2011). Phenols and pharmaceutical compounds, which were detected in the basin of the river Strymonas, have also been detected in various surface waters in Greece (Arditsoglou and Voutsa 2008; Kosma et al. 2014; Thomaidi et al. 2015; Vryzas et al. 2009).
Furthermore, similar compounds have also been frequently detected in surface waters in Europe. Phenylurea, diuron, and isoproturon were among the most frequently detectable organic compounds in rivers of 27 European countries (Loos et al. 2009). Diuron also was recorded with high frequency of detection in the rivers Llobregat and Guadalquivir, in Spain (Kock-Schulmeyer et al. 2012; Robles-Molina et al. 2014). Alachlor, atrazine, bisphenol A, chlorpyrifos, metolachlor, nonylphenol, octylphenol, propazine, simazine, terbuthylazine, and terbutryn were detected in the catchment area of Ebro river in Spain (Gorga et al. 2014; Hilderbrandt et al. 2008; Navarro et al. 2010). Chlropyrifos and endosulfan sulfate were the most frequently detectable pesticides in the basin of the River Alqueva in Portugal (Palma et al. 2010). Brown and Beinum (2009) reported isoproturon and metolachlor runoff from soil after application, especially during spring and summer under conditions of rainfall and irrigation. Alachlor, atrazine, chloropropham, chlorpyrifos, 2,4-D, dicamba, dichlorprop, diuron, isoproturon, mecoprop, metolachlor, nonylphenol, octylphenol, and simazine were detected at similar concentrations in basins of rivers Orge and Seine and the Rhone-Alpes region in France (Barrek et al. 2009; Botta et al. 2012; Gasperi et al. 2009; Lissalde et al. 2011). Finally, caffeine and salicylic acid are often detected in surface water impacted by effluents, thus are considered as indicators of wastewaters (Robles-Molina et al. 2014; Tran et al. 2014).
Passive sampling
POCIS were exposed along Strymonas river at four sites (S1, S4, S5, and S7) during the three sampling campaigns. The levels of hydrophilic organic compounds in POCIS and their frequency of detection are shown in Table 3. Thirty-five organic compounds were detected in the river basin. In comparison to active sampling, additional 12 compounds have been detected by passive sampling (35 vs 23, respectively). Aminocarb, carbaryl, and chloropropham were the dominant carbamates; diflubenzuron and diuron were the dominant urea pesticides. Metolachlor and 2,4-D were the most frequently detected organochlorine pesticides and chlorfenviphos from organophosphates. Salicylic acid, 1H-benzotriazole, and bisphenol A were the main pharmaceutical and industrial compounds. The presence of methiocarb (66.7%) and dichlorprop (33.3 %), although they have been banned, could be attributed to possible leaching from soil and sediments or illegal use of these compounds. Jalova et al. (2013) identified 13 target hydrophilic pesticides at similar concentrations (0.279–2.836 μg/POCIS) in surface waters of the Czech Republic. Dalton et al. (2014) identified atrazine to similar concentrations (2.393 μg/POCIS) in surface waters of Canada.
There is a lot of discussion on the possible use of passive sampling as an alternative sampling tool for monitoring of water quality. Besides the qualitative information for spatial and temporal variation, the estimation of water concentrations is a challenge (Miége et al. 2015; Poulier et al. 2014). The time-weighted average concentrations (TWA, μg/L) can be calculated from the levels in POCIS during the linear phase of uptake, by accumulated mass of target compound in the sampler (Ms, μg), sampling rate (Rs, L/day) and deployment time (t):
The sampling rates are usually obtained by calibration in laboratory under static or dynamic conditions. Additionally, field calibration is also encouraged because environmental factors such as temperature, hydrodynamics (flow rate, turbulence), exposure time, water type, and biofouling may affect Rs values (Jalova et al. 2013; Lissalde et al. 2014). Moreover, the use of performance reference compounds (PRCs) for calibration of POCIS has been discussed (Lissalde et al. 2014). All the calibration methods have their limitations, and several shortfalls have to be overcome. However, the uncertainty related to Rs values, and therefore TWA calculation, is counterbalanced by the reduced uncertainty related to the sampling. Consequently, POCIS could be applied in monitoring networks in order to study detection frequencies and occurrence of micropollutants in the aquatic environment (Ibrahim et al. 2012; Liu et al. 2013; Morin et al. 2012; Moschet et al. 2015; Munaron et al. 2012; Miége et al. 2015; Poulier et al. 2014).
In this study, the Rs values reported by other investigators in similar water types and conditions for approximately the same exposure period (Table 4) were used for the estimation of TWA water concentrations. The higher median TWA water concentrations were observed for metolachlor (0.932 μg/L), isoproturon (0.443 μg/L), chlorfenviphos (0.303 μg/L), 2,4-D (0.151 μg/L), diuron (0.101 μg/L), carbaryl (0.066 μg/L), atrazine (0.064 μg/L), caffeine (0.053 μg/L), nonylphenol (0.016 μg/L), terbutryn (0.012 μg/L), chlorpyrifos (0.009 μg/L), and bisphenol A (0.007 μg/L). Arditsoglou and Voutsa (2008) identified the group of alkylphenols (nd–3.76 μg/L) and bisphenol A (nd–0.9 μg/L) by applying POCIS to surface waters in Northern Greece. Also, Coes et al. (2014) identified 4-tert-octylphenol by applying POCIS-Pharm in the basin of the river Santa Cruz, AZ, USA.
The concentrations (mean ± SD) resulting from active and passive sampling in Strymonas River are illustrated in Fig. 2. Only the compounds exhibited detectable concentrations (higher than LOD) at least for 50 % of samples (alachlor, atrazine, carbaryl, chlorfenviphos, 2,4-D, dinoseb, diuron, isoproturon, metolachlor, simazine, bisphenol A, caffeine, nonylphenol) are presented. The discrepancy between the two measurements was relatively low. The ratios of concentrations obtained from active to passive sampling were 1–3 for most compounds whereas lower ratios (<0.2–0.4) were observed for atrazine and 2,4-D. This discrepancy may be due to the characteristics inherent in two sampling approaches. Grab samples could represent peak contamination at the time of collection; in contrast, TWA is averaged over the period of the deployment of POCIS. Moreover, uptake rates from literature usually varied (< twofold) under field environmental conditions (Harman et al. 2012; Morin et al. 2012; Poulier et al. 2014). Nevertheless, it seems that these differences are within the acceptable uncertainties as reported by Poulier et al. (2014; Poulier et al. 2014). These findings suggest that the application of POCIS could be a valuable tool at least for investigative and operational monitoring of water quality. However, an extended survey is needed to obtain Rs values under different field conditions in order to be used in monitoring networks (Moschet et al. 2015). The difference between concentrations obtained from active and passive sampling (logCact − logCpas) in relation to Kow values are shown in Fig. 3. As shown, this difference is increased toward higher Kow, suggesting that the really dissolved fraction in passive sampling decreased slightly with increasing the hydrophobicity of the compounds.
Environmental risk assessment
The ecotoxicological risk for the aquatic organisms in an ecosystem can be estimated by risk assessment approach (Barrek et al. 2009; Gotz et al. 2010; European Commission Guidance No 27 2011; McKinlay et al. 2008; Scott et al. 2014) based on EU Technical Guidance Document (2003) and REACH (European Commission Regulation 2006b).
In this study, the environmental risk assessment was performed according to the risk quotients following the method described in previous studies (Cho et al. 2014; Kosma et al. 2014; Palma et al. 2014; Stamatis et al. 2013; Thomatou et al. 2013). The risk quotient (RQ) comprises a useful tool in order to characterize potential ecological risk of many micropollutants in aquatic ecosystems (ECOFRAM 1999; Von der Ohe et al. 2011). Based on EC Technical Guidance Document on Risk Assessment, RQ was calculated as the ratio of measured environmental concentration (MEC) and predicted no-effect concentration (PNEC) using median concentrations or maximum concentrations for worst case scenario. PNEC was estimated using the lowest values of acute EC50 or LC50 or the chronic NOEC for the target compound, divided by a default assessment factor (AF), including algae, bacteria, invertebrates, and fish (European Chemical Agency 2008). For risk analysis, a commonly used risk ranking criterion was applied. When RQ ≥ 1 an ecological “high risk” is suspected, when 0.1 ≤ RQ ≤ 1 “medium risk” and for RQ ≤ 0.1 “low risk” is suggested.
According to Von der Ohe et al. (2011), the relative ranking of the resulting hazard ratio (RQ = MEC/lowest PNEC) was assigned 0 points for compounds with RQs from 0 to 1, 0.1 points for 1 < RQ < 10, 0.2 points for 10 < RQ < 100, 0.5 points for 100 < RQ < 1000, and 1.0 point for exceedances >1000.
The ecological risk due to the presence of organic micropollutants in Strymonas River is shown in Table 5. RQ values were calculated for each sampling campaign, and on yearly basis from results of both passive and active sampling. The higher risk was observed during second sampling campaign (1.30 points). High risk were found for diuron and salicylic acid as their yearly RQs were higher than 1. High risks were also suspected for fenthion and isoproturon during the second sampling campaign. Medium risk were suspected for carbaryl and metolachlor on a yearly basis. Due to the fact that during active sampling most of the compounds were found at concentrations < LODs, the TWA water concentrations calculated from passive sampling with POCIS were employed. The higher risk was observed during second sampling campaign. High risk was found for dinoseb as its RQ was higher than 1.0 during the second sampling campaign. Medium risk were also suspected for carbaryl, diuron, and metolachlor on a yearly basis. Medium risk was also considered for isoproturon during the second sampling campaign. Our results are in agreement with those reported from Papadakis et al. (2015), Palma et al. (2014), Thomatou et al. (2013), and Vryzas et al. (2009) for surface waters.
The application of RQ method in the present study showed that although concentrations of most micropollutants complied with the European EQS, the potential risk associated with compounds such as carbaryl, dinoseb, diuron, fenthion, isoproturon, metolachlor, and salicylic acid should not be neglected. Furthermore, sampling site S1 presents high risk for diuron (RQ>1 at this site, Fig. 4); therefore, a regular monitoring of the transboundary sampling station is suggested.
Conclusions
A complementary approach by active and passive sampling employing POCIS was used to assess hydrophilic organic micropollutants in the basin of Strymonas River, Northern Greece. Pesticides, pharmaceutical and industrial compounds were determined along the river in three sampling campaigns in 2013. Almost all the target compounds were detected by passive sampling. Temporal and spatial variation was observed, with higher concentrations at the periods of high rainfall intensity and/or low flow rate at sites that are mainly affected by agricultural and urban activities. The most frequently detected compounds were chlorfenviphos, diuron, and isoproturon at concentrations that occasionally exceed the maximum acceptable criteria. Moreover, compounds such as atrazine, dichlorprop, and methiocarb that are banned in Europe were also detected. Carbamate and urea compounds (aminocarb, carbaryl, chloropropham, diflubenzuron, diuron, isoproturon), organophosphorous pesticides (chlorfenviphos, fenthion), certain organohalogen herbicides ( 2,4-D, dinoseb, metolachlor), and salicylic acid must be under regular monitoring since these compounds were frequently detected. POCIS deployed in Strymonas River exhibited higher frequency of detection for hydrophilic micropollutants. Low discrepancy between concentrations obtained from active and passive sampling was observed. Therefore, POCIS could be a valuable tool for the selection and monitoring of the most relevant micropollutants in the river basin at least for investigative and operational monitoring of water quality in order to complete data obtained with current grab sampling. However, an extended survey is needed for further evaluation of passive samplers with calibration under a variety of field conditions. Environmental risk was also estimated based on both active and passive sampling. Results showed relatively low risk in the river basin; however, the potential risk associated with micropollutants such as carbaryl, dinoseb, diuron, fenthion, isoproturon, metolachlor, nonylphenol, and salicylic acid should not be neglected.
References
Alvarez DA, Huckins JN, Petty JD, Jones-Lepp T, Stuer-Lauridsen F., Getting DT, Goddard JP, Gravell A (2007) Tool for monitoring hydrophilic contaminants in water: polar chemical integrative sampler (POCIS). Compreh Anal Chem 48:171–197
Arditsoglou A, Voutsa D (2008) Passive sampling of selected endocrine disrupting compounds using polar organic chemical integrative samplers. Environ Pollut 156:316–324
Assoumani A, Lissalde S, Margoum C, Mazzelle N, Coquery M (2013) In situ application of stir bar sorptive extraction as a passive sampling technique for the monitoring of agricultural pesticides in surface waters. Sci Total Environ 463–464:829–835
Barrek S, Cren-Olive C, Wiest L, Baudot R, Arnaudguilhem C, Grenier-Loustalot MF (2009) Multi-residue analysis and ultra-trace quantification of 36 priority substances from the European Water Framework Directive by GC-MS and LC-FLD-MS/MS in surface waters. Talanta 79:712–722
Berho C, Togola A, Coureau C, Ghestem JP, Amalric L (2013) Applicability of polar organic compound intergative samplers for monitoring pesticides in groundwater. Environ Sci Pollut Res 20:5220–5228
Botta F, Fauchon N, Blanchoud H, Chevreuil M, Guery B (2012) Phyt’Eaux Cites: application and validation of a programme to reduce surface water contamination with urban pesticides. Chemosphere 86:166–176
Brown CD, Beinum W (2009) Pesticide transport via sub-surface drains in Europe. Environ Pollut 1–11. doi: 10.1016/j.envpol.2009.06.029.
Cho E, Khim J, Chung S, Seo D, Son Y (2014) Occurrence of micropollutants in four major rivers in Korea. Sci Total Environ 491–492:138–147
Coes A, Paretti N, Foreman W, Iverson J, Alvarez D (2014) Sampling trace organic compounds in water: a comparison of a continuous active sampler to continuous passive and discrete sampling methods. Sci Total Environ 473–474:731–741
Creusot N, Tapie N, Piccini B, Balaguer P, Porcher JM, Budzinski H, Aissa SA (2013) Distribution of steroid- and dioxin-like activities between sediments, POCIS and SPMD in a French river subject to mixed pressures. Environ Sci Pollut Res 20:2784–2794
Dalton R, Pick FR, Boutin C, Saleem A (2014) Atrazine contamination at the watershed scale and environmental factors affecting sampling rates of the polar organic chemical integrative sampler (POCIS). Environ Pollut 189:134–142
ECOFRAM (Ecological committee on FIFRA Risk 3 Assessment methods) (1999) ECOFRAM aquatic report. www.epa.gov/oppefed1/ecorisk/index.htm
European Commission (2011) Guidance Document No. 27. Technical Guidance for deriving Environmental Quality Standards. Technical Report – 2011 – 05.
European Chemical Agency (E.C.A.) (2008) Characterization of dose [concentration]-response for environment. Guidance on Information Requirements and Chemical Safety Assessment
European Commission (1976) Directive 76/464/EC on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community. Off J Eur Commun L 129/23.
European Commission (2000) Directive 2000/60/EC establishing a framework for Community action in the field of water policy. Off J Eur Commun L 327/1.
European Commission (2002) European Commission Decision No 657/2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the intepretation of the results. Off J Eur Commun L 221/8.
European Commission (2003) Technical Guidance Document on Risk Assessment. Part II. Joint Research Centre, Italy
European Commission (2006a) Directive 2006/11/EC on pollution caused by certain dangerous substances discharged into the aquatic environment of the Community. Off J Eur Commun L 64/52.
European Commission (2006b) Regulation 1907/2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency. Off J Eur Commun L 396/1.
European Commission (2008) Directive 2008/105/EC on environmental quality standards in the field of water policy. Off J Eur Commun L 348/84.
European Commission (2009) Guidance Document No. 19. Guidance on surface water chemical monitoring under the Water Framework Directive, Technical Report – 025.
European Commission (2013) Directive 2013/39/EC of the European Parliament and of the Council as regards priority substances in the field of water policy. Off J Eur Commun L 226/1.
Gasperi J, Garnaud S, Rocher V, Moilleron R (2009) Priority pollutants in surface waters and settleable particles within a densely urbanised area: Case study of Paris (France). Sci Total Environ 407:2900–2908
Ginebreda A, Munoz I, Lopez de Alda M, Brix R, Lopez-Doval J, Barcelo D (2010) Environmental risk assessment of pharmaceuticals in rivers: relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat river (NE Spain). Environ Int 36:153–162
Gorga M, Insa S, Petrovic M, Barcelo D (2014) Occurrence and spatial distribution of EDCs and related compounds in waters and sediments of Iberian rivers. Sci Total Environ http://dx.doi.org/10.1016/j.scitoenv.2014.06.037.
Gotz CW, Stamm C, Fenner K, Singer H, Scharer M, Hollender J (2010) Targeting aquatic contaminants for monitoring: exposure categorization and application to the Swiss situation. Environ Sci Pollut Res 17:341–354
Harman C, Allan IJ, Vermeirssen ELM (2012) Calibration and use of the polar organic chemical integrative sampler-a critical review. Environ Toxicol Chem 31:2724–2738
Hilderbrandt A, Guillamon M, Lacorte S, Tauler R, Barcelo D (2008) Impact of pesticides used in agriculture and vineyards to surface and groundwater quality (North Spain). Water Res 42:3315–3326
Ibrahim I, Togola A, Gonzalez C (2012) Polar organic chemical integrative sampler (POCIS) uptake rates for 17 polar pesticides and degradation products: laboratory calibration. Environ Sci Pollut Res. doi:10.1007/s11356-012-1284-3
Jablonowski ND, Schaffer A, Burauel P (2011) Still present after all these years: persistence plus potential toxicity raise questions about the use of atrazine. Environ Sci Pollut Res 18:328–331
Jalova V, Jarosova B, Blaha L, Giesy JP, Ocelka T, Grabic R, Jurcikova J, Vrana B, Hilscherova K (2013) Estrogen-, androgen- and aryl hydrocarbon receptor mediated activities in passive and composite samples from municipal waste and surface waters. Environ Int 59:372–383
Jarosova B, Blaha L, Vrana B, Randak T, Grabic R, Giesy JP, Hilscherova K (2012) Changes in concentrations of hydrophilic organic contaminants and of endocrine-disrupting potential downstream of small communities located adjacent to headwaters. Environ Int 45:22–31
Kalogridi EC, Christophoridis C, Bizani E, Drimaropoulou G, Fytianos K (2014) Part I. Temporal and spatial distribution of multiclass pesticide residues in lake waters of Northern Greece: application of an optimized SPE-UPLC-MS/MS pretreatment and analytical method. Environ Sci Pollut Res 21:7239–7251
Kock-Schulmeyer M, Ginebreda A, Gonsalez S, Luis Cortina J, Lopez de Alda M, Barcelo D (2012) Analysis of the occurrence and risk assessment of polar pesticides in the Llobregat River Basin (NE Spain). Chemosphere 86:8–16
Kosma C, Lambropoulou D, Albanis T (2014) Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ 466–467:421–438
Lekkas T, Kolokythas G, Nikolaou A, Kostopoulou M, Kotrikla A, Gatidou G, Thomaidis NS et al (2004) Evaluation of the pollution of the surface waters of Greece from the priority compounds of List II, 76/464/EEC Directive, and other toxic compounds. Environ Int 30:995–1007
Lissalde S, Mazzella N, Fauvelle V, Delmas F, Mazellier P, Legube B (2011) Liquid chromatography coupled with tandem mass spectrometry method for thirty-three pesticides in natural water and comparison of performance between classical solid phase extraction and passive sampling approaches. J Chromatogr A 1218:1492–1502
Lissalde S, Mazzella N, Mazellier P (2014) Polar organic chemical integrative samplers for pesticides monitoring: impacts of field exposure conditions. Sci Total Environ 488–489:188–196
Liu HH, Wong CS, Zeng EY (2013) Recognizing the limitations of performance reference compound (PRC)—calibration technique in passive water sampling. Environ Sci Technol 47:10104–10105
Lohmann R, Booij K, Smedes F, Vrana B (2012) Use of passive sampling devices for monitoring and compliance checking of POP concentrations in water. Environ Sci Pollut Res 19:1885–1895
Loos R, Gawlik MB, Locoro G, Rimaviciute E, Contini S, Bidoglio G (2009) EU-wide survey of polar organic persistent pollutants in European river waters. Environ Pollut 157:561–568
McKinlay R, Plant JA, Bell JNB, Voulvounis N (2008) Endocrine disrupting pesticides: implications for risk assessment. Environ Int 34:168–183
Miége C, Mazzela N, Allan I, Dulio V, Smedes F (2015) Position paper on passive sampling techniques for the monitoring of contaminants in the aquatic environment—achievements to date and perspectives. TrAC 8:20–26
Morin N, Randon J, Coquery M (2012) Chemical calibration, performance, validation and applications of the polar organic chemical integrative sampler (POCIS) in aquatic environments. TrAC 36:114–175
Moschet C, Vermeirssen ELM, Singer H, Stamm C, Hollender J (2015) Evaluation of in-situ calibration of chemcatcher passive samplers for 322 micropollutants in agricultural and urban affected rivers. Water Res. doi:10.1016/j.watres.2014.12.043
Munaron D, Tapie N, Budzinski H, Andral B, Gonzalez JL (2012) Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: Results from a pilot survey using passive samplers. Estuar Coast Shelf Sci 114:82–92
Navarro A, Tauler R, Lacorte S, Barcelo D (2010) Occurrence and transport of pesticides and alkylphenols in water samples along the Ebro River Basin. J Hydrol 383:18–29
Nödler K, Licha T, Voutsa D (2013) Twenty years later-Atrazine concentrations in selected coastal waters of the Mediterranean and the Baltic Sea. Marin Pollut Bull 70:112–118
Nödler K, Licha T, Voutsa D (2014) Polar organic micropollutants in the coastal environment of different marine systems. Marin Pollut Bull 85:50–59
Palma P, Alvarenga P, Palma V, Matos C, Fernandes RM, Soares A, Barbosa IR (2010) Evaluation of surface water quality using an ecotoxicological approach: a case study of the Alqueva Reservoir (Portugal). Environ Sci Pollut Res 17:703–716
Palma P, Kock-Schulmeyer M, Alvarenga P, Ledo L, Barbosa IR, Lopez de Alda M, Barcelo D (2014) Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci Total Environ 488–489:208–219
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E (2015) A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf 116:1–9
Poulier G, Lissalde S, Charriau A, Buzier R, Delmas F, Gery K, Moreira A, Guibaud G, Mazzella N (2014) Can POCIS be used in Water Framework Directive (2000/60/EC) monitoring networks ? A study focusing on pesticides in a French agricultural watershed. Sci Total Environ 497–498:282–292
Robles-Molina J, Gilbert-Lopez B, Garcia-Reyes JF, Molina-Diaz A (2014) Monitoring of selected priority and emerging contaminants in the Guadalquivir river and other related surface waters in the province of Jaen, South East Spain. Sci Total Environ 479–480:247–257
RPA (2003) Interim risk reduction strategy and analysis of advantages and drawbacks for bisphenol-A. Risks and Policy Analysts Limited. Contract Ref: 1/41/34, 2003.
Scott PD, Bartkow M, Blockwell SJ, Coleman HM, Khan SJ, Lim R, McDonald JA, Nice H, Nugegoda D, Pettigrove V, Tremblay LA, Warne M, Leusch FDL (2014) An assessment of endocrine activity in Australian rivers using chemical and in vitro analyses. Environ Sci Pollut Res 21:12951–12967
Stamatis N, Hela D, Triantafyllidis V, Konstantinou I (2013) Spatiotemporal variation and risk assessment of pesticides in water of the Lower catchment basin of Acheloos river, Northern Greece. Scientific World Journal 231610:1–16
Terzopoulou E, Voutsa D, Kaklamanos G (2015) A multi-residue method for determination of 70 organic micropollutants in surface waters by solid-phase extraction followed by gas chromatography coupled to tandem mass spectrometry. Envir Sci Pollut Res 22:1095–1112
Thomaidi V, Stasinakis A, Borova V, Thomaidis N (2015) Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case study. J Hazard Mater 283:740–747
Thomatou AA, Zacharias I, Hela D, Konstantinou I (2011) Passive sampling of selected pesticides in aquatic environment using polar organic chemical integrative samplers. Environ Sci Pollut Res 18:1222–1233
Thomatou A, Zacharias I, Hela D, Konstantinou I (2013) Determination and risk assessment of pesticides residues in lake Amvrakia (W.Greece) after agricultural and use changes in the lakes’ drainage basin. Int Environ Anal Chem 93:780–799
Tran NH, Li J, Hu J, Ong SL (2014) Occurrence and suitability of pharmaceuticals and personal care products as molecular markers for raw wastewater contamination in surface and groundwater. Environ Sci Pollut Res 21:4727–4740
Von der Ohe PC, Dulio V, Slobodnik J, Deckere ED, Kuhne R, Ebert RU, Ginebreda A, Cooman WD, Schuurmann G, Brack W (2011) A new risk assessment approach for the prioritization of 500 classical and emerging organic contaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci Total Environ 409:2064–2077
Vryzas Z, Vassiliou G, Alexoudis C, Papadopoulou-Mourkidou E (2009) Spatial and temporal distribution of pesticide residues in surface waters in north-eastern Greece. Water Res 43:1–10
Vryzas Z, Alexoudis C, Vassiliou G, Galanis K, Papadopoulou-Mourkidou E (2011) Determination and aquatic risk assessment of pesticides in riparian drainage canals in northeastern Greece. Ecotoxicol Environ Saf 74:174–181
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Terzopoulou, E., Voutsa, D. Active and passive sampling for the assessment of hydrophilic organic contaminants in a river basin-ecotoxicological risk assessment. Environ Sci Pollut Res 23, 5577–5591 (2016). https://doi.org/10.1007/s11356-015-5760-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5760-4