Abstract
To assess the status of polycyclic aromatic hydrocarbon (PAH) contamination in sediments from the Bizerte Lagoon (northern Tunisia), 18 surface sediment samples were collected in March 2011 and analyzed for 14 US Environmental Protection Agency priority PAHs by high-performance liquid chromatography. The total concentrations of the 14 PAHs (ΣPAHs) ranged from 16.9 to 394.1 ng g−1 dry weight (dw) with a mean concentration of 85.5 ng g−1 dw. Compared with other lagoons, coasts, and bays in the world, the concentrations of PAHs in surface sediments of the Bizerte Lagoon are low to moderate. The PAHs’ composition pattern was dominated by the presence of four-ring PAHs (45.8 %) followed by five-ring (26.8 %) and three-ring PAHs (12.7 %). The PAH source analysis suggested that the main origin of PAHs in the sediments of the lagoon was mainly from pyrolytic sources. According to the numerical effect-based sediment quality guidelines of the USA, the levels of PAHs in the Bizerte Lagoon should not exert adverse biological effects. The total benzo[a]pyrene toxicity equivalent values calculated for the samples varied from 3.1 to 53.7 ng g−1 dw with an average of 10.6 ng g−1 dw.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) with two or more fused rings are an important class of persistent organic pollutants and are ubiquitous in the environment. PAHs are introduced into the environment mainly via anthropogenic inputs and the biological conversion of biogenic precursors (Means et al. 1980). Anthropogenic input was regarded as the main source for the elevated concentration of PAHs in environmental matrices including fossil fuel combustion, refuse incineration, coal gasification and liquefaction processes, petroleum cracking, and the production of coke, carbon black, coal tar pitch, and asphalt (Countway et al. 2003). Industrial wastewater, sewage, road runoff/street dust, and petroleum-related activities are other important sources of PAHs. A minor portion of PAHs also originates from the discharges of non-combusted fossil fuel products (Bouloubassi and Saliot 1991; Näf et al. 1992; Manoli et al. 2000). Because of their low solubility and high hydrophobicity, PAHs entering the aquatic environment preferentially adsorb onto particulates and finally accumulate in the sediments (Karickhoff 1981; Chiou et al. 1998). They are therefore considered a potentially dangerous group of chemicals and a threat to the environment and its bioresources, especially those adjacent to major urban centers with high PAH inputs. The recognition of these facts prompted the US Environmental Protection Agency (USEPA) to include 16 PAHs within the priority pollutants list (Manoli et al. 2000). Due to their persistent, toxic, mutagenic, and carcinogenic characteristics (NRC 1983; Connel et al. 1997), the sources and distribution of PAHs have been the focus of numerous investigations in coastal/estuarine regions in industrialized countries in areas with anthropogenic impact all over the world (Kennicutt et al. 1994; Budzinski et al. 1997; Fernandes et al. 1997; Baumard et al. 1998a, b; Zakaria et al. 2002; Hartmann et al. 2004; Cachot et al. 2006; Fang et al. 2007; Yan et al. 2009).
In the last few decades, progressive economic and demographic development at the vicinity of the Mediterranean coastline and lagoons has resulted in substantial changes taking place in northern Tunisia. Industrialization has increased, which results in that the lagoon basins and streams have unfortunately became open-dumping disposal sites, as was the case of the Bizerte Lagoon in Tunisia. In this area, several studies have investigated the application of biochemical tools for pollution biomonitoring (Dellali et al. 2001, 2004; Khessiba et al. 2001, 2005; Roméo et al. 2006; Mahmoud et al. 2010). However, little information is available on the sources and distribution of PAHs in surface sediments of Bizerte Lagoon (Mzoughi et al. 2002; Trabelsi and Driss 2005). In light of this data, our present work aims to (1) investigate the spatial distribution of PAHs at a relatively large scale through the analysis of 18 surface sediment samples from the Bizerte Lagoon, (2) distinguish the possible sources of PAHs using diagnostic ratios of PAHs, and (3) assess the potential ecological risk of benthic organisms residing in this area.
Materials and methods
Study area
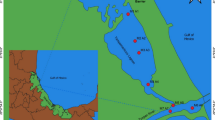
The Bizerte Lagoon is a Mediterranean lagoon located in the northern coast of Tunisia (latitude, 37°80′–37°14′ N; longitude, 9°46′–9°56′ E). It covers an area of 128 km2 and has a mean depth of 7 m. It communicates, in the north, with the Mediterranean Sea by a 7-km-long canal and in the south with the Ichkeul Lake by the Tinja River. The main tributaries of this lagoon are the rivers Tinja, Mrezig, Garek, Ben Hassine, and Gueniche (Fig. 1). The lagoon is subjected to the influence of several physical factors that strongly fluctuate during the year. In winter, winds induce a vertical mixture of the water column, rains are strong, and the freshwater flow coming from the Ichkeul Lake is important. On the other hand, in summer, inputs of seawater into the lagoon are important and the water column warming can induce its stratification (Sakka Hlaili et al. 2003). The human population around the lagoon is estimated at 163,000 inhabitants (2004 census) of which approximately 70 % are concentrated in the city of Bizerte. The other main important towns bordering the lagoon are Menzel Bourguiba (which has a naval port and a metal factory), Menzel Abderrahman, and Menzel Jemil. Some other industries (an iron and steel plant, a cement factory, and a refinery) are established nearby (Essid and Aissa 2002). The majority of these surrounding towns and industries discharge their waste into the lagoon. The exchange of water between the Mediterranean and the lake determines the salinity of the lagoon, which varies between 32.5 and 38.5 ‰. The water temperature range between 10 °C during winter (wet season) and 29 °C in summer time (dry season). The residence time of water bodies and contaminants in the Bizerte Lagoon is governed by several factors such as tide, wind, atmospheric pressure, and temperature. A recent study, based on numerical modelling, was performed by Harzallah (2003) focusing on water and heat exchange between the lagoon and the Mediterranean Sea. This model showed that the residence time of the contaminants is about 7 months. Actually, urban and industrial discharges in the Bizerte Lagoon take about 10 days to reach the canal entrance but several months to reach the Mediterranean Sea. This relatively high output time is due to the length of the canal which is about 7 km long.
Sample collections, storage, and preparation
Surface sediments were collected at 18 locations in March 2011 (Fig. 1). The selection of the sampling sites was based on different criteria related to the geomorphology of the lagoon, the hydrological regime, and the localization of the urban and industrial discharges. Water depth at the sampling sites ranged from 2.5 to 12.1 m, with a mean value of 6.7 m. The top 0–10 cm of sediment was collected using a stainless steel grab sampler. Individual sediment samples were mixed well and immediately frozen at −20 °C in pre-cleaned glass jars on board the boat used for sampling. In the laboratory, samples were defrosted and freeze dried, and the sediments were then passed through a stainless steel sieve (2-mm mesh) and stored at 4 °C until their analysis.
Chemicals
Standards (solids) of 14 USEPA priority PAHs [naphthalene (Nap), acenaphthene (Ace), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), dibenzo[a,h]anthracene (DahA), and benzo[g,h,i]perylene (BgP)] and a certified PAH standard mixture containing all 14 PAHs (PAH-Mix 45 at 10 μg mL−1 in cyclohexane) were purchased from Supelco (Bellefonte, PA, USA) and Dr. Ehrenstorfer (Germany), respectively. The purity of individual standards was higher than 96 %. The first standard of PAHs was dissolved in acetonitrile (ACN) to prepare calibration solutions in the range 15–400 ng mL−1, and the second solution was diluted with isooctane for the standard addition method. All solvents used for sample processing and analyses (dichloromethane (DCM) and acetonitrile) were of high-performance liquid chromatography (HPLC) grade and were purchased from Fisher (UK). The water used was from a MilliQ system (Milford, MA, USA). Glass pearls (Roth) were used to fill the headspace of the pressurized fluid extraction cells (Dionex). A marine sediment certified reference material (EC-7, National Water Research Institute, Canada) was used in the evaluation of the analytical method.
Sample extraction
An ASE 200 accelerated solvent extractor (Dionex, France) with 22 mL extraction cells was used to perform the extraction of samples. Five grams of dry sediment was accurately weighed into the stainless steel extraction cell that had been prefitted with a filter paper. The headspace was filled with glass pearls. The cell was placed in the carousel and the extraction was carried out using DCM. The parameters used during the extraction procedure are listed in Table 1. When the extraction was complete, the extract was completely evaporated under a gentle nitrogen stream and dissolved in 0.5 mL of acetonitrile. The final acetonitrile extracts were then filtered through a 0.45-μm PTFE syringe filter before being analyzed.
HPLC analysis
Quantitative analysis of PAHs was carried out using an analytical HPLC unit (JASCO, Japan) equipped with a JASCO PU-2089 HPLC pump, a type 7125 Rheodyne injector (with a 20 μL loop), and a fluorescence detector (FP-2020) with excitation and emission wavelengths that could be varied throughout the analysis. The optimized parameters of the fluorescence detector for the determination of each PAH compound are illustrated in Table 2. Chromatographic separation and resolution were best achieved by using a SUPELCOSIL LC-PAH (Supelco, Inc. Bellefonte, PA) reverse-phase C18 column (4.6 × 250 mm, 5 μm particle size) specific for PAH analysis. Data acquisition and processing were controlled by Chrom NAV (JASCO) software. The mobile phase was acetonitrile/water in gradient mode at a flow rate of 1.5 mL min−1 with the column thermostated at 22.0 ± 0.5 °C. The gradient elution program started with an initial mobile phase at 50:50 ACN/water (v/v), changing linearly to 100 % ACN in 20 min and, after 10 min, changing back to the initial phase (50:50). Total run time of an analysis was 40 min. The injection volume was 20 μL.
Quality assurance and quality control
PAHs were identified by comparison of their retention time with those of the authentic standards; quantification was accomplished by using the standard addition method from an average sample obtained from a mixture of all the samples analyzed. The method is described in detail elsewhere (Bader 1980). The principle is presented as follows: a calibration curve is obtained by the addition of a known amount of analytes to the unknown specimen. Serial concentrations of the analyte are added to the sample; the intensity measured is then plotted versus the concentration added. The intercept point of the regression line and the concentration axis equals the amount of analyte in the original sample. In this work, the standard addition graph for all PAHs in sediments extracted was linear in the concentration range 1.97–28.75 ng mL−1 (r 2 > 0.99).
To perform the quality control, procedural blanks were analyzed and any analyte of interest was detected. The external standard multipoint calibration technique (in the range 15−400 ng mL−1) was used to determine the linear response interval of the detector, and in all cases, regression coefficients were higher than 0.996 for all the analytes. Detection limits (signal-to-noise ratio greater than 3) and quantification limits (estimated for a signal-to-noise ratio of 10) for each PAH ranged from 0.01 to 0.54 ng mL−1 and 0.04 to 4.65 ng g−1 dry weight (dw), respectively. The whole analytical procedure was validated by analyzing the EC-7 certified reference material (CRM) from the National Water Research Institute (Canada). For all the studied PAHs, recoveries of higher than 80 % were obtained for the CRM.
Other analyses
Total organic carbon (TOC) contents of sediments were determined by combustion in an LECO CS 125 analyzer (Etcheber et al. 2007). The dried and homogenized sediment samples were acidified in crucibles with 2 N HCl to destroy carbonates, then dried at 60 °C to remove inorganic C and most of the remaining acid and water. The analyses were performed by direct combustion in an induction furnace, and the CO2 formed was determined quantitatively by infrared absorption. TOC contents are expressed as a percentage of the dw of the sediment, abbreviated as TOC (%).
The percentage of finer grain size fractions (% < 63 μm) of each sediment sample was determined gravimetrically after wet sieving (Savinov et al. 2000).
Results and discussion
PAH concentrations
Surface sediments can reflect the current sediment contaminant status. All sediment samples (n = 18) of the Bizerte Lagoon contained detectable amounts of PAHs. The individual PAH concentrations (on a dry weight basis) were measured at each station. Table 3 showed that Ace was only found in the sites S1, S2, and S5; DahA was not found in the sites S17 and S18. The other priority PAHs were found at most sampling sites. Indeed, the total concentration of 14 USEPA PAHs (∑PAHs) in surface sediments varied from 16.9 to 394.1 ng g−1 dw with a mean concentration of 85.5 ng g−1 dw. The largest amounts of PAHs were found along the channel (between the narrow part and the arsenal of Menzel Bourguiba), the path most frequented by boats. Maximum PAH levels were recorded in site S1 (394.1 ng g−1 dw), followed by sites S2 (160.2 ng g−1) and S9 (102.8 ng g−1). Stations S1 and S2 are located along the channel connecting the lagoon with the Mediterranean Sea. These areas are highly populated and characterized by industrial activities including lead and cement manufacturing. In addition, S1 is located near the Bizerte Harbor where heavy traffic of tankers and commercial cargo boats is common. PAH inputs could also be attributed to the Merazig River and the incineration landfill located close to S2. In fact, the Merazig River, compared to the other rivers opening on the Bizerte Lagoon, receives the largest amounts of urban and industrial effluent discharges (Harzallah 2003). High concentrations of PAHs were also observed at station S9, probably due to the closeness of the mouth of the Tinja River which is affected by agricultural inputs. Site 4 is located near an urban area (Menzel Abderrahman City) and receives direct inputs of untreated urban sewage. S14 and S15 are located near the city of Menzel Bourguiba with intensive industrial activity: metallurgic industry, naval construction, and tire production. In all the other stations, the PAH levels ranged from 16.9 to 73.3 ng g−1 dw, with a mean value of 53.6 ng g−1 dw. The highest concentration of 73.3 ng g−1 dw was recorded at S6, located near an urban and an industrial zone (Menzel Jemil City). However, the lowest concentration, 16.9 ng g−1 dw, was found at S17. The geochemical composition (%TOC) of this last site contributes to this observation. The distribution of PAHs in terms of the physical and chemical properties of sediments will be discussed later.
The comparison of ∑PAH concentrations with those carried out in other coastal areas is difficult due to the geological characteristics of the sampling areas, the number of samples taken, and the number of PAH compounds analyzed in the different studies, which may not be the same. However, a general idea of the pollutant levels in other coastal areas could be useful. Baumard et al. (1998b) classified four pollution levels of sediment: low, 0–100 ng g−1 dw; moderate, 100–1,000 ng g−1; high, 1,000–5,000 ng g−1; and very high, >5,000 ng g−1. Compared to other coasts, bays, and lagoons in the world, the pollution levels of the PAHs in all the sediment samples from the Bizerte Lagoon (16.9–394.1 ng g−1 dw) are low to moderate (Table 4).
A comparison of the temporal of PAH contamination in the Bizerte Lagoon with those in the past was also made to show a decrease of PAH concentrations (Table 5). The ∑PAH concentration in the present study was approximately three times lower than those in sediments collected in 2001 (Trabelsi and Driss 2005). The difference in pollutant levels may reflect differences in the sampling periods. Trabelsi and Driss collected sediments in December (winter period), while in the present study, sediments were collected in March (beginning of the spring period). The higher concentration of PAHs in December could be attributed to three main factors including the increasing use of domestic heating systems, lower microbial degradation, and photolytic decomposition during winter. The biodegradation of organic compounds is facilitated by the presence of environmental conditions which favor microorganism metabolic activity (Cerniglia 1993; Hinga 2003). Quan et al. (2009) demonstrated that the metabolic activity of microorganisms was inhibited at low temperatures. Furthermore, temperature could potentially affect the sorption rate of pollutants onto particle and organic matter (Wu and Gschwend 1986). When temperatures decrease, PAH solubility subsequently decreases and conversely PAH bioavailability and biodegradation decrease (Whitehouse 1984; Eriksson et al. 2003). During summer time, increasing temperatures and light intensity may favor biodegradation and photodegradation of PAHs. Interestingly, several PAH-degrading bacterial strains were recently isolated from Bizerta Lagoon sediments (Ben Said et al. 2008), which demonstrated the high PAH biodegradation capabilities of sediment microflora in this lagoon. As shown in Table 5, the ∑PAH concentration is only slightly different between 2006 (Louiz et al. 2008) and 2011 (this study), indicating that the net balance between the input and the degradation of PAHs have not changed significantly over this time period.
Indeed, there are many other factors affecting temporal variations of PAH levels in sediments, such as the physical and chemical characteristics of the PAH, the composition and chemical characteristics of the sediment, the depositional patterns, and the partitioning processes.
Sediment geochemistry and PAH accumulation
TOC and fine fraction percentages in surface sediments from the Bizerte Lagoon ranged from 0.44 to 3.85 % and from 11.32 to 95.28 %, respectively (Table 2). The S1 and S3 stations showed the highest content of TOC: 3.85 and 2.16 %, respectively. The high levels of organic carbon recorded at these two stations are related to the high occurrence of urban and industrial wastewater discharges, which are rich in organic matter. For grain size analysis, with the exception of stations S2, S6, S17, and S18 that show a predominantly sand composition, all other stations were composed of particles below 63 μm in size. Station S2 is located near a naval base and a solid waste landfill. Stations S6, S17, and S18 are located near the lagoon bank in an area characterized by sandy sediments (Mouldi et al. 2008). The abundance of fine particles was assumed to come from anthropogenic inputs associated with the erosion of surrounding agricultural areas (Ben Hassine River, Garek River, Gueniche River, Tinja River, Merazig River). This result was consistent with the findings of Mouldi et al. (2008) and Boufahja (2010) who showed that the surface sediments of the Bizerte Lagoon are mostly composed of silt and clay fractions.
It is generally stated that the environmental fate and behavior of hydrophobic organic compounds are ultimately determined by the physicochemical properties of both compound and sediments, namely, sediment organic matter content, size particle distribution, PAH octanol–water partition coefficients (K ow), and water salinity (Baker et al. 1991; Doong and Lin 2004). Previous studies have indicated that sediments with high organic carbon content generally contained high PAH concentrations. On the contrary, the sediments with low organic carbon content generally had low levels of PAHs. Furthermore, PAHs are mainly adsorbed onto fine particles (clay) because of their higher specific surface area (Xia and Wang 2008). In the present study, data from stations S1 and S17 are distinguished from the others and were excluded of the data set. Indeed, the highest and the lowest PAH concentration in these sites correspond to the highest and the lowest TOC content, respectively (Table 3). Regression analysis was carried out to investigate the relationship between the concentration of ∑PAHs and the percentage of TOC and fine fraction. No significant correlation was found between ∑14PAHs and %TOC (r = −0.330, P = 0.212, n = 16). This suggests that distribution and concentrations of PAHs are more influenced by direct inputs rather than by organic matter content in the sediments. Several other studies reported similar results indicating no significant correlation between PAHs and %TOC (Mostafa et al. 2009; Arias et al. 2010). Simpson et al. (1996) suggested that PAH concentrations and organic carbon (OC) content in sediments are significantly correlated only for highly contaminated sites where total PAH concentrations exceed 2,000 ng g−1. In the present study, total PAH concentrations of all sampled sediments did not exceed 2,000 ng g−1 (Table 3). Hence, it can be concluded that the distributions and concentrations of PAHs in sediments of the Bizerte Lagoon are not controlled by the sediment OC content. In our study, the homogeneity of the grain size composition between sediments made any correlation analysis between fine fraction and PAH concentrations difficult.
Although PAHs and TOC were correlated fairly well for the highly contaminated site (S1), it cannot be assumed that the observed positive correlation represents preferential partitioning of PAHs onto sediment according to the TOC percentages. As shown in Table 3, in most of the studied locations of the Bizerte Lagoon, high PAH concentrations are not associated with high TOC and fine particulate contents. These preliminary results reveal a complex relationship between PAH concentrations in sediments and some physicochemical parameters of sediments of the Bizerte Lagoon, thus suggesting involvement of other factors such as sources, transport, mixing, and deposition (Mai et al. 2005).
PAH composition
According to the number of aromatic rings, the 14 PAHs were divided into five groups representing two-ring, three-ring, four-ring, five-ring, and six-ring PAHs. The relative concentrations of PAHs by aromatic groups in surface sediments from the Bizerte Lagoon are shown in Fig. 2. As illustrated, four-ring PAHs (46 % of total PAHs, on average) were the most abundant followed by five-ring PAHs which accounted for 27 % of the total PAHs. Two-ring, three-ring, and six-ring PAHs accounted for about 5, 13, and 9 % of the total PAHs, respectively. Similar contributions of four-ring PAHs (49–56 %) were found in the same region in 2001 and 2006 by Trabelsi and Driss (2005) and Louiz et al. (2008), respectively (Fig. 3).
In general, high molecular weight (HMW) four-ring to six-ring PAHs were prevalent (82 %) in the Bizerte Lagoon sediments. Usually, HMW PAHs predominated in sediment samples. The low molecular weight (LMW) two-ring and three-ring PAHs, such as Nap, Ant, and Phe, are more labile and are expected to degrade faster than larger ones (Fernandes et al. 1997; Macias-Zamora et al. 2002). The higher concentration of HMW PAHs compared to those of LMW PAHs has been commonly observed in sediments from rivers, lagoons, and marine environments (Culotta et al. 2006; Berto et al. 2009; Mostafa et al. 2009). The sources of PAHs will be further discussed with other evidence in the following section.
Sources of PAHs
Table 6 shows correlation (Spearman’s) values among the 14 individual PAHs and ∑PAHs. All PAHs (except Nap, Fl, and BgP) show a good to very good correlation (r = 0.601–0.969) with the ∑PAH values. Such result suggests that the compositions of the major PAHs are fairly constant at different sampling sites and may come from similar sources.
PAHs are introduced into the environment mainly via industrial discharge, fossil fuel combustion, petroleum spills, and automobile exhausts, as well as through nonpoint sources, such as urban runoff and atmospheric fallout (Countway et al. 2003; Liu et al. 2008).
Identifying the possible sources of PAHs is very important to understand the fate of PAHs in the environment. The PAHs’ congener distribution varied with the source as well as the composition and combustion temperature of the organic material. Molecular indices based on ratios of selected PAHs may be used to differentiate PAHs from pyrogenic and petrogenic origins. Pyrogenic sources (combustion processes) are enriched in HMW PAHs, whereas petrogenic sources, such as the releasing of fuel oil or light refined petroleum products, are dominated by LMW PAHs. Thus, the LMW PAHs/HMW PAHs (LMW/HMW) ratio may be used to determine the petrogenic and pyrogenic sources of PAHs in sediments (Budzinski et al. 1997; Soclo et al. 2000; Rocher et al. 2004). LMW/HMW ratio values which are lower than 1 indicate pyrolytic origin pollution, while ratio values higher than 1 indicate that the contaminations are mainly controlled by petrogenic PAHs (Soclo et al. 2000). To estimate the origin of the PAHs in sediment samples from the Bizerte Lagoon, the LMW/HMW ratios were determined and the results were listed in Table 7. LMW/HMW ratios at all sampling sites were lower than one (<1) suggesting the predominance of pyrolytic origin. In addition, ratios of specific PAH compounds including Phe/Ant, Flu/Pyr, Chr/BaA, Ant/(Ant + Phe), BaA/(BaA + Chr), and Flu/(Flu + Pyr) were also used to differentiate PAHs from pyrogenic, and petrogenic origins (Budzinski et al. 1997; Zeng and Vista 1997; Baumard et al. 1998a; Yunker et al. 2002; Doong and Lin 2004; Liu et al. 2008) were also determined in this study. Table 7 showed that all of the Phe/Ant values were lower than 10 and all of the Flu/Pyr values were higher than 1, indicating that pyrogenic inputs could be the main source of PAHs in surface sediments of the study area. The Chr/BaA ratios obtained ranged from 1.10 to 1.61, indicating a petrogenic source of contamination of the sediment. This index is based in part on the concentrations of Chr; however, in the literature, opinions on the sources of chrysene are divided. Rocher and Moilleron (2007) favored a pyrolytic origin, while Doong and Lin (2004) classified this PAH as the marker of petrogenic origin. The isomer pair ratios in our study, such as Ant/(Ant + Phe) and BaA/(BaA + Chr), ranged from 0.13 to 0.24 and from 0.38 to 0.48, respectively, indicating that the main origin of PAHs in sediments in this lagoon was from a pyrolytic source (Table 7). Plots of isomeric ratios BaA/(BaA + Chr) and Ant/(Ant + Phe) versus Flu/(Flu + Pyr) (Fig. 4) confirmed this predominant source and showed that the pyrogenic PAHs originated from the incomplete combustion of coal, grass, or wood, for all the samples, except S6 that had the combustion of petroleum as a source. This station is located close to the motorway, in front of the municipal draining discharge for the city of Menzel Jemil that is a highly urbanized region. Thus, the hypothesis of a contamination by the road at this station is likely, but remains to be confirmed.
Predominance of pyrolytic PAHs in the studied area could be explained by the high number of factories using coal as a fuel. In fact, since the 1950s, about 100 industries settled in the vicinity of the lagoon, the most important being the cement factory (1950) and the steel complex “El Fouledh” (1965). These industrial units are responsible for various pollutant discharges reaching the sediments as a result of atmospheric deposition and urban runoff (Stout et al. 2004; Van Metre and Mahler 2005). Altogether, industries generate a daily average flow of wastewater of about 31,600 m3/day in the Bizerte Lagoon. These wastewater discharges account for 3,139 kg/day of PAH inputs into the lagoon. In addition, urban sources generate a daily average flow of wastewater of about 20,000 m3/day, which corresponds to 23.5 kg/day of hydrocarbons, while harbor activities provide only 0.11 kg/day of hydrocarbons (MAERH 2003). Open burning of biomass is a common procedure for crop and forest residue disposal and land preparation. Combustion by-products are transported to the marine environment by dry and/or wet depositions from the atmosphere (Wu et al. 2001). In addition, multiple other sources of PAHs such as transport, heating systems, waste incinerators, municipal waste, etc. can explain the pyrolytic origin of the PAH contamination in the area. Dominance of pyrolytic PAHs in marine sediments has been found in most Mediterranean countries in the vicinity of industrialized or urbanized areas (Van Metre and Mahler 2005; Barra et al. 2009; Pietzsch et al. 2010; Viñas et al. 2010; Alebic-Juretic 2011; Hu et al. 2011; Hung et al. 2011; Perra et al. 2011). Despite the presence of an oil refinery in the vicinity of stations S1 and S2, petrogenic PAHs were not predominant in our work. This result agrees with the results obtained in the Galician coast (Franco et al. 2006) and in the case of the Erika accident (Tronczynsky et al. 2004).
Although distinct sources may be inferred from PAH isomer pair ratios, it is well documented that the fingerprint of PAHs may be altered by biological (e.g., bacterial activity), chemical (e.g., oxidation and reduction), and/or physical (e.g., air mass mixing and sediment resuspension) processes during transport and after deposition in marine sediments (Tolosa et al. 1996). The importance of using a battery of different ratios is highlighted here; when only considering the Chr/BaA ratio, the conclusion about the sources of PAHs would have been different. The ratios used, except this one, converge to the same conclusion: PAHs in surface sediments in the Bizerte Lagoon are mainly from pyrolytic sources.
Assessment of sediment quality and toxicity
PAHs are ubiquitously distributed in diverse environmental matrices such as soil and sediment and deserve increasing attention due to their high stability, low solubility, and toxic, carcinogenic, and mutagenic effects. Sediment quality guidelines (SQGs) are useful tools for the assessment of contamination in marine and estuarine sediments (Long et al. 1995; Qiao et al. 2006; Quiroz et al. 2010). Long et al. (1995) developed two guideline values, an effects range low (ERL) and an effects range median (ERM), to assess the sediment quality using a ranking of low to high impact values. The measured concentrations of PAHs were compared with the ERL and ERM values (Table 8). Results obtained in this study showed that the average total PAH concentrations at all sites were below the ERL and ERM. For individual components, this result is also valid, except in the case of Ace and Fl (with a slightly higher concentration than the ERL value). Thus, concentrations of PAHs found in this study are not expected to be a threat to benthic marine organisms along the Bizerte Lagoon.
Some PAHs, and especially their metabolic products, are of great concern due to their documented carcinogenicity. These potentially carcinogenic PAHs (CPAHs) include BaA, BaP, BbF, BkF, DahA, and InP (Savinov et al. 2003). The InP was not investigated in this study. The total concentrations of CPAHs in sediments from the Bizerte Lagoon varied from 6.6 to 120.8 ng g−1 dw (with a mean concentration of 29.1 ng g−1 dw) and accounted for 30.1–39.8 % of the total PAH concentrations.
Among all the known potentially carcinogenic PAHs, BaP is the only PAH for which toxicological data is sufficient to derive a carcinogenic potency factor (Peters et al. 1999; Bowman et al. 2002). Toxic equivalence factors (TEFs) were applied to quantify the carcinogenicity of other PAHs relative to BaP and to estimate benzo(a)pyrene-equivalent doses (BaPeq dose). Calculated TEFs for BaA, BaP, BbF, BkF, DahA, and Chr were 0.1, 1, 0.1, 0.01, 0.1, 1, and 0.001, respectively, according to the USEPA. In this study, we converted the abovementioned six PAH concentrations into one toxic concentration for each site using the corresponding TEFs. According to IARC (1987), the total toxic benzo(a)pyrene equivalent (TEQ) of all these PAHs is:
Total TEQ values calculated for samples from the Bizerte Lagoon varied from 3.1 to 53.7 ng g−1 dw with a mean value of 10.6 ng g−1. The maximum value of total TEQ was found at station S1 situated right next to the Bizerte commercial harbor indicating a local source of contamination. Mean values of relative BaPeq doses in total TEQ, decreased in the following order: BaP (65.4 %) > DahA (18.7 %) > BbF (10.4 %) > BaA (7 %) > BkF (0.4 %) > Chr (0.1 %) (Fig. 5). Similar contributions of BaP (43.6–70 %) were found in other studies in different environments (Savinov et al. 2003; Qiao et al. 2006; Ünlü et al. 2009), confirming the importance of using BaP as an indicator of carcinogenic PAHs in sediments.
In comparison to other studies, the total TEQ values in sediments of the Bizerte Lagoon were lower than the values detected in the Coastal lagoon, Northern Adriatic, Italy (Guerra 2012) and close to the ones found at the coastal lagoons in central Vietnam (Giuliani et al. 2008).
Conclusions
In the present study, analysis of surface sediment samples from 18 sites along the Bizerte Lagoon showed that the highest levels of PAHs were found at station S1. Four- to five-ring PAHs were predominant in sediments. Comparison of the concentration range with a worldwide survey of PAH concentrations in sediments ranked PAH contamination in Bizerte Lagoon ’s sediments as poorly to moderately polluted. PAH source identification showed that grass, wood, and coal combustion were the primary PAH sources in most sediments of the Bizerte Lagoon. The sediment quality guidelines assessed the surface sediment of the Bizerte Lagoon to be within minimal effect ranges for benthic organisms. To better understand the importance of organic matter and particle geochemistry in the distribution of PAHs in sediments, further detailed work is required. The results of this study would be helpful for understanding the levels, distribution, biological effects, and sources of PAHs in Bizerte Lagoon sediments, which can provide information for protecting water resources and human health of lagoon and/or coastal areas in Tunisia.
References
Alebic-Juretic, A. (2011). Polycyclic aromatic hydrocarbons in marine sediments from the Rijeka Bay area, Northern Adriatic, Croatia, 1998–2006. Marine Pollution Bulletin, 62, 863–869.
Arias, A. H., Vazquez-Botello, A., Tombesi, N., Ponce-Vélez, G., Freije, H., & Marcovecchio, J. (2010). Presence, distribution, and origins of polycyclic aromatic hydrocarbons (PAHs) in sediments from Bahía Blanca estuary, Argentina. Environmental Monitoring and Assessment, 160, 301–314.
Bader, M. (1980). A systematic approach to standard addition methods in instrumental analysis. Journal of Chemical Education, 57, 703–706.
Baker, J. E., Eisenreich, S. J., & Eadie, B. J. (1991). Sediment trap fluxes and benthic recycling of organic carbon, polycyclic aromatic hydrocarbons and polychlorobiphenyl congeners in Lake Superior. Environmental Science & Technology, 18, 838–849.
Barra, R., Quiroz, R., Saez, K., Araneda, A., Urrutia, R., & Popp, P. (2009). Sources of polycyclic aromatic hydrocarbons (PAHs) in sediments of the Biobio River in south central Chile. Environmental Chemistry Letters, 7, 133–139.
Baumard, P., Budzinski, H., & Garrigues, P. (1998a). PAHs in Arcachon Bay, France: origin and biomonitoring with caged organisms. Marine Pollution Bulletin, 36, 577–586.
Baumard, P., Budzinski, H., & Garrigues, P. (1998b). Polycyclic aromatic hydrocarbons in sediments and mussels of the western Mediterranean Sea. Environmental Toxicology and Chemistry, 17, 765–776.
Ben Ameur, W., Trabelsi, S., & Driss, M. R. (2010). Polycyclic aromatic hydrocarbons in superficial sediments from Ghar El Melh Lagoon, Tunisia. Bulletin of Environmental Contamination and Toxicology, 85, 184–189.
Ben Said, O., Goñi-Urriza, M. S., El Bour, M., Dellali, M., Aissa, P., & Duran, R. (2008). Characterization of aerobic polycyclic aromatic hydrocarbon degrading bacteria from Bizerte lagoon sediments, Tunisia. Journal of Applied Microbiology, 104, 987–97.
Berto, D., Ausili, A., Sunseri, G., Bellucci, L. G., Frignani, M., Albertazzi, S., & Giani, M. (2009). Polycyclic aromatic hydrocarbons (PAHs) from diffuse sources in coastal sediments of a not industrialized Mediterranean island. Water, Air, and Soil Pollution, 200, 199–209.
Boufahja, F. (2010). Approches communautaires et populationnelles de biosurveillance du milieu marin chez les nématodes libres (lagune et baie de Bizerte, Tunisie). Thèse de Doctorat, Université du 7 Novembre à Carthage, Tunisie.
Bouloubassi, I., & Saliot, A. (1991). Sources and transport of hydrocarbons in the Rhone delta sediments (North western Mediterranean). Fresenius Journal of Analytical Chemistry, 339, 765–771.
Bowman, J. C., Zhou, J. L., & Readman, J. W. (2002). Sorption and desorption of benzo(a)pyrene in aquatic systems. Journal of Environmental Monitoring, 4, 761–766.
Budzinski, H., Jones, I., Bellocq, J., Pierrad, C., & Garrigues, P. (1997). Evaluation of sediment contamination by polycyclic aromatic hydrocarbons in the Gironde estuary. Marine Chemistry, 58, 85–97.
Cachot, J., Geffard, O., Augagneur, S., Lacroix, S., Le Menach, K., Peluhet, L., Couteau, J., Denier, X., Devier, M. H., Pottier, D., & Budzinski, H. (2006). Evidence of genotoxicity related to high PAH content of sediments in the upper part of the Seine estuary (Normandy, France). Aquatic Toxicology, 79, 257–267.
Cerniglia, C. E. (1993). Biodegradation of polycyclic aromatic hydrocarbons. Current Opinion in Biotechnology, 4, 331–338.
Cheng, J. O., Ko, F. C., Li, J. J., Chen, T. H., Cheng, Y. M., & Lee, C. L. (2011). Concentrations of polycyclic aromatic hydrocarbon in the surface sediments from inter-tidal areas of Kenting coast, Taiwan. Environmental Monitoring and Assessment, 184, 3481–3490.
Chiou, C. T., McGroddy, S. E., & Kile, D. E. (1998). Partition characteristics of polycyclic aromatic hydrocarbons on soils and sediments. Environmental Science & Technology, 32, 264–269.
Connel, D. W., Hawker, D. W., Warne, M. J., & Vowles, P. P. (1997). Polycyclic aromatic hydrocarbons (PAHs). In K. McCombs & A. W. Starkweather (Eds.), Introduction into environmental chemistry (pp. 205–217). Boca Raton: CRC.
Countway, R. E., Dickhut, R. M., & Canuel, E. A. (2003). Polycyclic aromatic hydrocarbon (PAH) distributions and associations with organic matter in surface waters of the York River, VA Estuary. Organic Geochemistry, 34, 209–224.
Culotta, L., De Stefano, C., Gianguzza, A., Mannino, M. R., & Orecchio, S. (2006). The PAH composition of surface sediments from Stagnone coastal lagoon, Marsala (Italy). Marine Chemistry, 99, 117–127.
Dellali, M., Gnassia Barelli, M., Romeo, M., & Aissa, P. (2001). The use of acetylcholinesterase activity in Ruditapes decussatus and Mytilus galloprovincialis in the biomonitoring of Bizerta lagoon. Comparative Biochemistry and Physiology Part C, 130, 227–235.
Dellali, M., Romeo, M., Gnassia-Barelli, M., & Aïssa, P. (2004). A multivariate data analysis of the clam Ruditapes decussatus as sentinel organism of the Bizerta lagoon (Tunisia). Water, Air, and Soil Pollution, 156, 131–144.
Doong, R., & Lin, Y. (2004). Characterization and distribution of polycyclic aromatic hydrocarbon contaminations in surface sediment and water from Gao-ping River, Taiwan. Water Research, 38, 1733–1744.
Eriksson, M., Sodersten, E., Yu, Z., Dalhammar, G., & Mohn, W. W. (2003). Degradation of polycyclic aromatic hydrocarbons at low temperature under aerobic and nitrate-reducing conditions in enrichment cultures from northern soils. Applied and Environmental Microbiology, 69, 275–284.
Essid, N., & Aissa, P. (2002). Etude quantitative des nématodes libres des secteurs Nord et Est de la lagune de Bizerte (Tunisie). Bulletin de l’Institut National des Sciences et Technologies de la Mer de Salammbô, Tunisia, 29, 53–63.
Etcheber, H., Taillez, A., Abril, G., Garnier, J., Servais, P., Moatar, F., & Commarieu, M. V. (2007). Particulate organic carbon in the estuarine turbidity maxima of the Gironde, Loire and Seine estuaries: origin and lability. Hydrobiologia, 588, 245–259.
Fang, M., Hsieh, P. C., Ko, F. C., Baker, J. E., & Lee, C. L. (2007). Sources and distribution of polycyclic aromatic hydrocarbons in the sediments of Kaoping river and submarine canyon system, Taiwan. Marine Pollution Bulletin, 54, 1179–1189.
Fernandes, M. B., Sicre, M. A., Boireau, A., & Tronszynski, J. (1997). Polyaromatic hydrocarbon (PAH) distributions in the Seine River and its estuary. Marine Pollution Bulletin, 34, 857–867.
Forster, G. D., & Wright, D. A. (1988). Unsubstituted polynuclear aromatic hydrocarbons in sediments, clams, and clam worms from Chesapeake Bay. Marine Pollution Bulletin, 19, 459–465.
Franco, M. A., Viñas, L., Soriano, J. A., de Armas, D., González, J. J., Beiras, R., Salas, N., Bayona, J. M., & Albaigés, J. (2006). Spatial distribution and ecotoxicity of petroleum hydrocarbons in sediments from the Galicia continental shelf (NW Spain) after the Prestige oil spill. Marine Pollution Bulletin, 53, 260–271.
Geptner, A. R., Richter, B., Pikovskii, Y. I., Chernyansky, S. S., & Alekseeva, T. A. (2006). Hydrothermal polycyclic aromatic hydrocarbons in marine and lagoon sediments at the intersection between Tjörnes Fracture Zone and recent rift zone (Skjálfandi and Öxarfjörður bays), Iceland. Marine Chemistry, 101, 153–165.
Giuliani, S., Sprovieri, M., Frignani, M., Cu, N. H., Mugnai, C., Bellocci, L. G., Albertazzia, S., Romanoa, S., Luisa Feob, M., Marsellab, E., & Hoai Nhonc, D. (2008). Presence and origin of polycyclic aromatic hydrocarbon in sediments of nine coastal lagoons in central Vietnam. Marine Pollution Bulletin, 56, 1486–1512.
Guerra, R. (2012). Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and trace metals in sediments from a coastal lagoon (Northern Adriatic, Italy). Water, Air, and Soil Pollution, 223, 85–98.
Hartmann, P. C., Quinn, J. G., Cairns, R. W., & King, J. W. (2004). The distribution and sources of polycyclic aromatic hydrocarbons in Narragansett Bay surface sediments. Marine Pollution Bulletin, 48, 351–358.
Harzallah, A. (2003). Transport de polluants dans la lagune de Bizerte simulé par un modèle de circulation de l’eau. Bulletin de l’Institut National des Sciences et Technologies de la Mer de Salammbô, Tunisia, 30, 121–133.
Hinga, K. R. (2003). Degradation rates of low molecular weight PAH correlate with sediment TOC in marine subtidal sediments. Marine Pollution Bulletin, 46, 466–474.
Hu, N., Shi, X., Huang, P., Mao, J., Liu, J., Liu, Y., & Ma, D. (2011). Polycyclic aromatic hydrocarbons (PAHs) in surface sediments of Liaodong Bay, Bohai Sea, China. Environmental Science and Pollution Research, 18, 163–172.
Hung, C. C., Gong, G. C., Ko, F. C., Lee, H. J., Chen, H. Y., Wu, J. M., Hsu, M. L., Peng, S. C., Nan, F. H., & Santschi, P. H. (2011). Polycyclic aromatic hydrocarbons in surface sediments of the East China Sea and their relationship with carbonaceous materials. Marine Pollution Bulletin, 63, 464–470.
IARC. (1987). IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Overall evaluation of carcinogenicity: an updating of IAPC monographs (vol. 1–42). Lyon: International Agency for Research on Cancer. Suppl. 7.
Kanzari, F., Syakti, A. D., Asia, L., Malleret, L., Mille, G., Jamoussi, B., Abderrabba, M., & Doumenq, P. (2012). Aliphatic hydrocarbons, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, organochlorine, and organophosphorous pesticides in surface sediments from the Arc river and the Berre lagoon, France. Environmental Science and Pollution Research, 19, 559–576.
Karickhoff, S. (1981). Semiempirical estimation of sorption of hydrophobic pollutants on natural sediments and soils. Chemosphere, 10, 833–846.
Kennicutt, M. C., Wade, T. L., Presley, B. J., Requejo, A. G., Brooks, J. M., & Denoux, D. J. (1994). Sediment contaminants in Casco Bay, Maine: inventories, sources, and potential for biological impact. Environmental Science & Technology, 28, 1–15.
Khessiba, A., Hoarau, P., Gnassia-Barelli, M., Aissa, P., & Roméo, M. (2001). Biochemical response of the mussel Mytilus galloprovincialis from Bizerta (Tunisia) to chemical pollutant exposure. Archives of Environmental Contamination and Toxicology, 40, 222–229.
Khessiba, A., Roméo, M., & Aïssa, P. (2005). Effects of some environmental parameters on catalase activity measured in the mussel (Mytilus galloprovincialis) exposed to lindane. Environmental Pollution, 133, 275–281.
Leaute, F. (2008). Biogéochimie des contaminants organiques HAP, PCB et Pesticides organochlorés dans les sediments de l’étang de Thau (France). Thèse de Doctorat, Université de Pierre et Marie Curie, France.
Li, Q., Zhang, X., & Yan, C. (2010). Polycyclic aromatic hydrocarbon contamination of recent sediments and marine organisms from Xiamen Bay, China. Archives of Environmental Contamination and Toxicology, 58, 711–721.
Liu, Y., Ling, C. N., Zhao, J. F., Huang, Q. H., Zhu, Z. L., & Gao, H. W. (2008). Distribution and sources of polycyclic aromatic hydrocarbons in surface sediments of rivers and an estuary in Shanghai, China. Environmental Pollution, 154, 298–305.
Long, E. R., MacDonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects with ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Louiz, I., Kinani, S., Gouze, M. E., Ben-Attia, M., Menif, D., Bouchonnet, S., Porcher, J. M., Ben-Hassine, O. K., & Aït-Aïssa, S. (2008). Monitoring of dioxin-like, estrogenic and anti-androgenic activities in sediments of the Bizerta lagoon (Tunisia) by means of in vitro cell-based bioassays: contribution of low concentrations of polynuclear aromatic hydrocarbons (PAHs). The Science of the Total Environment, 402, 318–329.
Macias-Zamora, J. V., Mendoza-Vega, E., & Villaescusa-Celaya, J. A. (2002). PAHs composition of surface marine sediments: a comparison to potential local sources in Todos Santos Bay, B.C., Mexico. Chemosphere, 46:459−468.
MAERH, (2003). Etude sur la dépollution industrielle dans le bassin versant du lac de Bizerte. Rapport, Ministère de l’Agriculture, de l’Environnement et des Ressources Hydrauliques, Tunis, 182 pp.
Mahmoud, N., Dellali, M., Bour, M. E., Aissa, P., & Mahmoudi, E. (2010). The use of Fulvia fragilis (Mollusca: Cardiidae) in the biomonitoring of Bizerta lagoon: a multimarkers approach. Ecological Indicators, 10, 696–702.
Mai, B., Chen, S., Luo, X., Chen, L., Yang, Q., Sheng, G., Peng, P., Fu, J., & Zeng, E. Y. (2005). Distribution of polybrominated diphenyl ethers in sediments of the Pearl River Delta and adjacent South China Sea. Environmental Science & Technology, 39, 3521–3527.
Manoli, E., Samara, C., Konstantinou, I., & Albanis, T. (2000). Polycyclic aromatic hydrocarbons in the bulk precipitation and surface waters of Northern Greece. Chemosphere, 41, 1845–1855.
Means, J. C., Wood, S. G., Hassett, J. J., & Banward, W. L. (1980). Sorption of polynuclear aromatic hydrocarbons by sediments and soils. Environmental Science & Technology, 14, 1524–1528.
Medeiros, P. M., Caruso Bícego, M., Menezes Castelao, R., Del Rosso, C., Fillmann, G., & Josemar Zamboni, A. (2005). Natural and anthropogenic hydrocarbon inputs to sediments of Patos Lagoon Estuary, Brazil. Environmental International, 31, 77–87.
Mostafa, A. R., Wade, T. L., Sweet, S. W., Al-Alimi, A. K. A., & Barakat, A. O. (2009). Distribution and characteristics of polycyclic aromatic hydrocarbons (PAHs) in sediments of Hadhramout coastal area, Gulf of Aden, Yemen. Journal of Marine Systems, 78, 1–8.
Mouldi, B., Bejaoui, B., & Atoui, A. (2008). Etude de l’hydrodynamique sédimentaire de la lagune de Bizerte. Bulletin de l’Institut National des Sciences et Technologies de la Mer de Salammbô, Tunisia, 35, 149–160.
Mzoughi, N., Hellal, F., Dachraoui, M., Villeneuve, J. P., Cattini, C., De Mora, S. J., & El Abed, A. (2002). Méthodologie de l’extraction des hydrocarbures aromatiques polycycliques. Application à des sédiments de la lagune de Bizerte (Tunisie). Comptes Rendus-Geoscience, 334, 893−901.
Näf, C., Broman, D., Pettersen, H., Rolff, C., & Zebühr, Y. (1992). Flux estimates and pattern recognition of particulate polycyclic aromatic hydrocarbons, polychlorinated dibenzo-p-dioxins, and dibenzofurans in the waters outside various emission sources on the Swedish Baltic coast. Environmental Science & Technology, 26, 1444–1457.
NRC. (1983). Polycyclic aromatic hydrocarbons: evaluation of sources and effects. Washington, DC: National Academy Press.
Pereira, W. E., Hostettler, F. D., & Rapp, J. B. (1996). Distributions and fate of chlorinated pesticides, biomarkers and polycyclic aromatic hydrocarbons in sediments along a contamination gradient from a point source in San Francisco Bay, California. Marine Environmental Research, 41, 299–314.
Perra, G., Pozo, K., Guerranti, C., Lazzeri, D., Volpi, V., Corsolini, S., & Focardi, S. (2011). Levels and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) in superficial sediment from 15 Italian marine protected areas (MPA). Marine Pollution Bulletin, 62, 874–877.
Peters, C. A., Knightes, C. D., & Brown, D. G. (1999). Long-term composition dynamics of PAH-containing NAPLs and implications for risk assessment. Environmental Science & Technology, 33, 4499–4507.
Piazza, R., Ruiz-Fernández, A. C., Frignani, M., Zangrando, R., Bellucci, L. G., Moret, I., & Páez-Osuna, F. (2008). PCBs and PAHs in surficial sediments from aquatic environments of Mexico City and the coastal states of Sonora, Sinaloa, Oaxaca and Veracruz (Mexico). Environmetal Geology, 54, 1537–1545.
Pietzsch, R., Patchineelam, S. R., & Torres, J. P. M. (2010). Polycyclic aromatic hydrocarbons in recent sediments from a subtropical estuary in Brazil. Marine Chemistry, 118, 56–66.
Qiao, M., Wang, C. X., Huang, S. B., Wang, D. H., & Wang, Z. J. (2006). Composition, sources, and potential toxicological significance of PAHs in the surface sediments of Meiliang Bay, Taihu Lake, China. Environmental International, 32, 28–33.
Quan, X., Tang, Q., He, M., Yang, Z., Lin, C., & Guo, W. (2009). Biodegradation of polycyclic aromatic hydrocarbons in sediments from the Daliao river watershed. China. Journal of Environmental Sciences, 21, 865–871.
Quiroz, R., Grimalt, J. O., & Fernández, P. (2010). Toxicity assessment of polycyclic aromatic hydrocarbons in sediments from European high mountain lakes. Ecotoxicology and Environmental Safety, 73, 559–564.
Rocher, V., Azimi, S., Moilleron, R., & Chebbo, G. (2004). Hydrocarbons and heavy metals in the different sewer deposits in the Le Marais’ catchment (Paris, France): stocks, distributions and origins. The Science of the Total Environment, 323, 107–122.
Rocher, V., & Moilleron, R. (2007). Identification des sources d’hydrocarbures en milieu urbain: approche automatisée. http://www.sisyphe.jussieu.fr/internet/piren/rapports/archives/2000/Theme_5/th5_rocher.pdf. Site consulté le: 12 février 2007.
Roméo, M., Gharbi-Bouraoui, S., Gnassia-Barelli, M., Dellali, M., & Aïssa, P. (2006). Responses of Hexaplex (Murex) trunculus to selected pollutants. The Science of the Total Environment, 359, 135–144.
Sakka Hlaili, A., Chikhaoui, M. A., El Grami, B., & Hadj Mabrouk, H. (2003). Variation hivernoestivale de la communauté phytoplanctonique de la lagune de Bizerte en milieux naturel et fertilisé en nutriments. Revue de la Faculté des Sciences de Bizerte, Tunisia, 2, 37–49.
Savinov, V. M., Savinova, T. N., Carroll, J., Mathisov, G. G., Dahle, S., & Naes, K. (2000). Polycyclic aromatic hydrocarbons (PAHs) in sediments of the White Sea, Russia. Marine Pollution Bulletin, 40, 807–818.
Savinov, V. M., Savinova, T. N., Matishova, G. G., Dahle, S., & Naes, K. (2003). Polycyclic aromatic hydrocarbons (PAHs) and organochlorines (OCs) in bottom sediments of the Guba Pechenga, Barents Sea, Russia. The Science of the Total Environment, 306, 39–56.
Secco, T., Pellizzato, F., Sfriso, A., & Pavoni, B. (2005). The changing state of contamination in the Lagoon of Venice. Part 1: organic pollutants. Chemosphere, 58, 279–290.
Simpson, C. D., Mosi, A. A., Cullen, W. R., & Reimer, K. J. (1996). Composition and distribution of polycyclic aromatic hydrocarbon contamination in surficial marine sediments from Kitimat Harbor, Canada. Science of The Total Environment, 181, 265–278.
Soclo, H. H., Garrigues, P. H., & Ewald, M. (2000). Origin of polycyclic aromatic hydrocarbons (PAHs) in coastal marine sediments: case studies in Cotonou (Benin) and Aquitaine (France) areas. Marine Pollution Bulletin, 40, 387–396.
Stefens, J., dos Santos, J. H., Filho, J. G., & Peralba, M. C. (2007). Polycyclic aromatic hydrocarbons in sediments from Rodrigo de Freitas Lagoon in the urban area of Rio de Janeiro, Brazil. Journal of Environmental Science and Health, Part A, 42, 399–404.
Stout, S. A., Uhler, A. D., & Emsbo-Mattingly, S. D. (2004). Comparative evaluation of background anthropogenic hydrocarbons in surficial sediments from mine urban waterways. Environmental Science & Technology, 38, 2987–2994.
Tolosa, I., Bayona, J. M., & Albaiges, J. (1996). Aliphatic and polycyclic aromatic hydrocarbons and sulfur/oxygen derivatives in northwestern Mediterranean sediments: spatial and temporal variability, fluxes, and budgets. Environmental Science & Technology, 30, 2495–2503.
Trabelsi, S., & Driss, M. R. (2005). Polycyclic aromatic hydrocarbons in superficial coastal sediments from Bizerte Lagoon, Tunisia. Marine Pollution Bulletin, 50, 344–359.
Tronczyński, J., Munschy, C., Héas-Moisan, K., Guiot, N., Truquet, I., Olivier, N., Men, S., & Furaut, A. (2004). Contamination of the Bay of Biscay by polycyclic aromatic hydrocarbons (PAHs) following the T/V “Erika” oil spill. Aquatic Living Resources, 17, 243–259.
Ünlü, S., & Alpar, B. (2009). Evolution of potential ecological impacts of the bottom sediment from the gulf of Gemlik; Marmara Sea, Turkey. Bulletin of Environmental Contamination and Toxicology, 83, 903–906.
Van Metre, P. C., & Mahler, B. J. (2005). Trends in hydrophobic organic contaminants in urban and reference lake sediments across the United States, 1970–2001. Environmental Science & Technology, 39, 5567–5574.
Viñas, L., Angeles Franco, M., Antonio Soriano, J., José González, J., Pon, J., & Albaigés, J. (2010). Sources and distribution of polycyclic aromatic hydrocarbons in sediments from the Spanish northern continental shelf. Assessment of spatial and temporal trends. Environmental Pollution, 158, 1551–1560.
Whitehouse, B. G. (1984). The effects of temperature and salinity on the aqueous solubility of polynuclear aromatic hydrocarbons. Marine Chemistry, 14, 319–332.
Wu, S., & Gschwend, P. M. (1986). Sorption kinetics of hydrophobic organic compounds to natural sediments and soils. Environmental Science & Technology, 20, 717–725.
Wu, Y., Zhang, J., Mi, T.-Z., & Li, B. (2001). Occurrence of n-alkanes and polycyclic aromatic hydrocarbons in the core sediments of the Yellow Sea. Marine Chemistry, 76, 1–15.
Xia, X., & Wang, R. (2008). Effect of sediment particle size on polycyclic aromatic hydrocarbon biodegradation: importance of the sediment-water interface. Environmental Toxicology and Chemistry, 27, 119–125.
Yan, W., Chi, J. S., Wang, Z. Y., Huang, W. X., & Zhang, G. (2009). Spatial and temporal distribution of polycyclic aromatic hydrocarbons (PAHs) in sediments from Daya Bay, South China. Environmental Pollution, 157, 1823–1830.
Yang, G. P. (2000). Polycyclic aromatic hydrocarbons in the sediments of the South China Sea. Environmental Pollution, 108, 163–171.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser river basin: a critical appraisal of PAH ratios as indicators of PAH sources and composition. Organic Geochemistry, 33, 489–515.
Zaghden, H., Kallel, M., Elleuch, B., Oudot, J., & Saliot, A. (2007). Sources and distribution of aliphatic and polyaromatic hydrocarbons in sediments of Sfax, Tunisia, Mediterranean Sea. Marine Chemistry, 105, 70–89.
Zakaria, M. P., Takada, H., Tsutsumi, S., Ohno, K., Yamada, J., Kouno, E., & Kumata, H. (2002). Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: a widespread input of petrogenic PAHs. Environmental Science & Technology, 36, 1907–1918.
Zeng, E. Y., & Vista, C. L. (1997). Organic pollutants in the coastal environment off San Diego, California. 1. Source identification and assessment by compositional indices of polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry, 16, 179–188.
Zonta, R., Botter, M., Cassin, D., Pini, R., Scattolin, M., & Zaggia, L. (2007). Sediment chemical contamination of a shallow water area close to the industrial zone of Porto Marghera (Venice Lagoon, Italy). Marine Pollution Bulletin, 55, 529–542.
Zrafi-Nouira, I., Khedir-Ghenim, Z., Zrafi, F., Bahri, R., Cheraeif, I., Rouabhia, M., & Saidane-Mosbahi, D. (2008). Hydrocarbon pollution in the sediment from the Jarzouna-Bizerte coastal area of Tunisia (Mediterranean Sea). Bulletin of Environmental Contamination and Toxicology, 80, 566–572.
Acknowledgments
This work was supported by the Ministry of Higher Education and Scientific Research of Tunisia. The author would also like to thank the language expert Nayua Abdelkefi for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barhoumi, B., LeMenach, K., Devier, MH. et al. Polycyclic aromatic hydrocarbons (PAHs) in surface sediments from the Bizerte Lagoon, Tunisia: levels, sources, and toxicological significance. Environ Monit Assess 186, 2653–2669 (2014). https://doi.org/10.1007/s10661-013-3569-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3569-5