Abstract
Lake water level fluctuations (WLF) are an important factor driving the selection and seasonal dynamics of phytoplankton and potentially toxigenic cyanobacteria. Nevertheless, the relative importance of environmental drivers connected to WLF may be completely different, depending on the typology and use of waterbodies, latitude and climatic regimes. In this study, we investigated the impact of WLF in a large subtropical reservoir in south-eastern China (Hongfeng Reservoir, Guizhou Province). The study was based on monthly samplings carried out in 2014 in six stations. The strong increase in the water level observed in early summer caused a radical shift in the phytoplankton community. While in the pre-flooding period phytoplankton was composed of large diatoms, chrysophytes and Oscillatoriales (mostly Limnothrix sp.), the post-flooding period showed an increase in smaller and more competitive chlorophytes, smaller diatoms and cryptophytes better adapted to a fast colonisation of new and nutrient-rich environments. The environmental drivers that drove the change were dilution, flushing and interference with the seasonal water stratification processes. We concluded that, because WLF represents a complex variable integrating different physical effects in one explanatory descriptor, its value as a predictor of phytoplankton and cyanobacteria dynamics in lake ecosystems is difficult to generalise and needs to be investigated on a case-by-case basis. For this reason, considering the year-to-year hydrological variability that potentially characterise reservoirs, definite indications for management should be outlined considering more than 1-year study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common strategy to decrease phytoplankton biomass and improve water quality and usability in freshwater ecosystems relies on the control and abatement of algal nutrient loads. The first fertilisation experiments performed at the basin level during the 1960s demonstrated a clear link between phosphorus and the development of huge algal biomasses (Schindler 2006). These studies promoted the development of strategies aimed at the bottom–up control of phytoplankton based on the reduction of P-loads within watersheds and waterbodies (Ryding and Rast 1989; Ansari et al. 2011). Besides the reduction of nutrient loads originating from point and nonpoint source pollution (Chorus and Schauser 2011; Jochimsen et al. 2013; Hamilton et al. 2016), the decrease of nutrients can rely on a wide range of actions, including hypolimnetic oxygenation, hypolimnetic withdrawal and sediment dredging (Bormans et al. 2016). These measures can be accompanied by the adoption of a wide array of measures based on the top–down control of phytoplankton, including removing of planktivorous fish (Mehner et al. 2002) and phytoremediation (Mahujchariyawong and Ikeda 2001). Considering the strong link between eutrophication and the development of toxigenic cyanobacteria (Smith 2003; Sukenik et al. 2015; Meriluoto et al. 2017), the above actions were generally proved efficient also in the control or mitigation of cyanobacterial harmful algal blooms (CyanoHABs). Owing to the peculiarity of this group of phytoplankters, specific strategies were developed (Ibelings et al. 2016), including artificial mixing (which eliminates the advantage of gas-vacuolated cyanobacteria to move to the euphotic layers) (Visser et al. 2016) and selective suppression of cyanobacteria in entire lakes with H2O2 (Matthijs et al. 2012).
The selection and adoption of the above measures are severely dependent on the climatic conditions and on the morphometric and hydrological characteristics of waterbodies. Many of the mitigation and restoration actions such as sediment removal, artificial mixing or phytoremediation are simply not feasible in large lakes, whereas lakes with different hydrology rarely develop the same algal assemblages or react in the same way to changes in nutrient loads (Salmaso et al. 2003). A paradigmatic example is represented by the large impounded lakes, where biogeochemical processes and the control of phytoplankton development are severely controlled by hydrodynamic processes. Moreover, compared with natural lakes in temperate climates, top–down control of phytoplankton in tropical regions tends to be less important due to a generally smaller size of zooplankton and to the high predation exerted by continuously reproducing omnivorous fish (Kosten et al. 2009). Water level fluctuations (WLF) in reservoirs are much more ample than in natural lakes. Besides the regional rainfall pattern, lake levels are controlled by the artificial regulation of water discharges, which depend on the human water demand. Changes in WLF can therefore occur at temporal frequencies spanning from seasons to weeks, strongly affecting mixing and nutrient cycling processes, with important consequences on the aquatic biota and phytoplankton dynamics (Zohary et al. 2009; Zohary and Ostrovsky 2011; Bakker and Hilt 2015). Given these conditions, the biological cycle of riparian vegetation and macrophytes can be severely impaired due to extensive drying up and rewetting of shores. Periods of low water level, renewal and water turbulence, coupled with high availability of nutrients, could represent a serious hazard in large and deep reservoirs, favouring the potential development of nuisance species belonging to cyanobacteria and compromising the exploitation of the reservoirs. These changes widely contrast with the positive effects on macrophytes and shift in tropic status caused by the lowering of water levels and improvement of light regime in natural shallow lakes (Coops and Hosper 2002). Actually, as it was recently demonstrated in the review by Bakker and Hilt (2015), the effects of WLF on cyanobacteria cannot be generalised, depending on a wide range of lake ecosystem properties, which include morphometry, sediment type, hydrology, quality of inlet water, submerged vegetation, fish and climate. Nevertheless, despite its crucial importance for the determination of water quality and the range of utilisation, the response of aquatic ecosystems, particularly deep lakes, to fluctuations in water level still remains an understudied field (Zohary and Ostrovsky 2011). This is particularly true considering the relatively scarce knowledge on the effects of WLF in the large subtropical reservoirs (Bakker and Hilt 2015; Yang et al. 2016).
In this contribution, we will evaluate the influence of water level fluctuations, temperature, stratification and nutrient temporal dynamics on the development of phytoplankton and cyanobacteria in the large subtropical Hongfeng Reservoir (Southwestern China, Guizhou Province, 26.5° N). The Hongfeng Reservoir is an essential strategic water resource which, like many other waterbodies in China (Lv et al. 2014), is principally used for drinking purposes. The exploitation of this reservoir therefore relies not only on water availability but also on water quality. The Hongfeng Reservoir is generally characterised by a strong increase in water levels after April/May. Our general hypothesis is that the seasonal phytoplankton dynamic and selection of life strategies are principally controlled by changes induced on the lake by WLF. Specific objectives of this work are as follows: (i) the identification of the dominant phytoplankton species and principal life strategies associated with the periods of low and high lake water levels; (ii) the evaluation of the tolerance of potential cyanobacteria to changes in WLF; and (iii) the implications due to WLF changes in the management of large subtropical reservoirs.
Methods
Study site
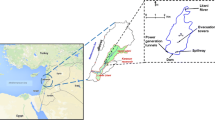
The Hongfeng Reservoir was built in 1960 by damming the Maotiao River. The reservoir is located near Qingzhen City, a suburb of Guiyang City, in the Guizhou Province, South-western China. It has a surface area of 57.2 km2, a full volume of 6.01 × 108 m3 and a maximum depth (z max) of approximately 45 m. A volume-water level curve over a range of 20 m (1220–1240 m, Wusong Elevation System) was reported in Wu et al. (2016). Precipitation maximum in the catchment occurs between April and September with monthly values ranging from1600 to1900 mm; annual average precipitation is 1198 mm, whereas the mean water retention time is 0.76 year. Water levels are typically lowest in spring/early summer and highest in early winter. Other characteristics of the reservoir are resumed in Table 1. The Hongfeng Reservoir may be divided into a south and north basin (Fig. 1). The main inflow is the Yangchang River, whereas the Maotiao River is the only outlet of Hongfeng Reservoir. The lake is an important drinking water source for Guiyang City. Since 2000, its major function changed from electricity generation to drinking water supply. At present, other secondary functions include hydroelectric power generation, flood control, recreation and fishery production.
Sampling and laboratory methods
The limnological investigations were carried out at six stations located downstream of the confluence of the Yangchang River (YCH), along the thalweg (NHZ, HW, HYD, BHZ), and near the dam (Dam-DB) (Fig. 1). YHC, NHZ and HW stations (z max < 20 m) are located in the southern basin, whereas HYD, BHZ and DB (z max around or greater than 20 m) are located in the northern basin. Field measurements and samplings were carried out at monthly frequency from January to December 2014. Vertical profiles of water temperature, electrical conductivity (at 25 °C) and pH were measured using a portable multiparameter probe (YSI-6600v2). Water transparency (zSD) was estimated with a Secchi disk from June to December. The euphotic depth (z eu) was estimated using the ratio z eu/z SD = 2.7 (Kirk 2011). The lake water levels were measured and provided by the administration of Hongfeng, Baihua and Aha reservoirs. The collection of samples for the chemical and phytoplankton analyses along the water column was carried out at intervals of 2 m. Chemical analyses are available for the YCH and the Dam station (DB). Further samples for the qualitative analyses of phytoplankton were taken with a 30-μm-mesh plankton net. Before arrival to the laboratory, all the samples were kept in the dark and refrigerated. In this work, we will consider the samples collected between the surface and 6 m. Based on preliminary investigations, the 0–6 m layer can be considered representative of the euphotic trophogenic layer (cf. Fig. 2a, b). Water stability was estimated by the relative thermal resistance (RTR6), which was computed by the density difference in the layer 0–6 m compared to the density difference between 4 and 5 °C (Wetzel 2001; Kallf 2002); water densities were estimated from temperature values.
Temporal development of isotherms (°C) in the a HYD and b DB stations. The dashed line indicates the depth of the euphotic zone (z eu). c Water level changes and relative thermal resistance values (RTR6) in the stations HYD and DB between 0 and 6 m. d Mean total phosphorus concentrations in the stations YCH and DB between 0 and 6 m
In the laboratory, total phosphorus (TP) was analysed according to the Chinese standard methods for water quality analysis, GB3838-2002 (Li et al. 2013). Phytoplankton was counted after sedimentation of 1.5 L of samples fixed with 1% formaldehyde solution in a graduated flask. After 48~72 h, the supernatant was syphoned off with a 2-mm-diameter hose until obtaining 35 mL of residual concentrated sample. Two 0.1 mL replicates from the homogeneous concentrated sample were allowed to settle in a Sedgewick-Rafter chamber. Taxonomical determination and counting were carried out at × 400 magnifications using an upright microscope (Olympus-BX41). For every single taxon, phytoplankton biovolumes (PB, mm3 m−3) were calculated from abundances (cells mL−1) and specific biovolumes (μm3) estimated by approximating the phytoplankton shapes to simple geometrical solids. Specific biovolumes, estimated from direct linear measurements, were further verified by using standard references (Druart and Rimet 2008), especially in the case of more complicated shapes. The size of abundant taxa was checked regularly (generally over ten linear measurements), whereas only a few measurements were carried out for the less abundant taxa. Phytoplankton was classified at the lowest taxonomical rank possible. Species names and the definition of higher taxonomic groups of eukaryotic phytoplankton and cyanobacteria were based on the most recent literature (e.g. Krienitz and Bock 2012; Guiry and Guiry 2016). Taxonomic determinations made on the fixed samples were confirmed by the analysis of qualitative samples collected with the 30-μm-mesh net. Species were grouped into morpho-functional groups (MFGs) following the updated classification in Salmaso et al. (2015).
Data analysis
The effects of RTR6, water temperature and (for the only YCH and DB stations) TP on the total phytoplankton and cyanobacteria biovolumes and including, as categorical variables, the water level periods before (months 1–5; low level, WLPL) and during/after (6–12; high level, WLPH) the main flooding event and stations were evaluated by applying models testing both main effects and interaction effects (ANCOVA tests; Crawley 2005; Kéry 2010). Models were tested and verified also using water levels in place of WLP. Since RTR6 was derived from water temperature values, the effects of these two variables were tested independently. In the different models tested, different intercepts and/or slopes were included only if significant. Before computation, total phytoplankton and cyanobacteria biovolumes were loge-transformed. Selection of models was evaluated based on the AIC (Akaike Information Criterion) values and ANOVA tests (Zuur et al. 2009). The independence of residuals in regression analyses was confirmed by computing the Durbin–Watson test (5% level).
The temporal changes in the phytoplankton assemblages were analysed by nonmetric multidimensional scaling (NMDS) applied to a chord distance matrix computed on transformed square root phytoplankton biovolumes. The results are equivalent to the application of NMDS to a Hellinger dissimilarity matrix distance (Legendre and Gallagher 2001). Rarer species found in less than three occasions over the whole study period were not included in the analyses. NMDS was started with different random starting coordinates using the metaMDS function in the package vegan (Oksanen et al. 2016) in R (R Core Team 2017). The final solution was rotated by PCA so that the variance of site scores was maximised on the first axis. The correlation of selected variables with the samples in the NMDS configuration was analysed with vector fitting. Vector fitting maximises the correlation of single variables with a set of samples in the ordination space. The significance of the single vectors was estimated by computing 1000 random permutations of the data. In this regard, vector fitting was also used as a mere descriptive tool to visualise the link between phytoplankton phyla and dominant MFGs (mean biovolume > 40 mm3 m−3) with the NMDS configuration. Relationships were further analysed by relating selected environmental variables to the gradients in species composition by surface fitting. This technique fits smooth surfaces for each variable and plots the results on the ordination diagrams (Oksanen et al. 2016). The significance of selected groups of samples was tested using PERMANOVA (Anderson 2001) applied to the distance matrix used as input for the NMDS ordination of samples (function adonis in the package vegan; Oksanen et al. 2016).
All the statistical analyses and statistical graphs were calculated by using the free software R 3.4.1 (R Core Team 2017).
Results
Physical variables and nutrients
In the study period, water temperatures in the water column ranged between 8 and 25 °C. The isotherms in the two deepest stations (HYD and DB) showed either a persistent condition of homogeneous temperatures and complete mixing along the water column or the presence of a well-mixed epilimnion (Fig. 2a, b). The RTR6 values between the surface and 6 m indicated the existence of thermal gradients from April to July (Fig. 2c). From January to February/March and after July, the differences in water temperatures between the surface and the bottom layers were always low, generally < 2–3 °C. The stability of the sampled layer showed a clear drop during the flooding period, with isothermal conditions and RTR6 values around zero after September (Fig. 2c; see below). In the six stations, the euphotic depth between June and December ranged generally between 3 and 8 m (Fig. 2a, b). After August, the mixing depths well exceeded the corresponding euphotic depths.
Lake water levels showed a swift increase between April/May and June (Fig. 2c), in coincidence with the start of the seasonal precipitations in April. Measurements allowed to clearly separate two periods of low (around 1233 m) and high (around 1239 m) water levels (WLPL and WLPH, respectively) between January and May and July and December, respectively. Based on the data in Wu et al. (2016), these two water levels roughly corresponded to water volumes around 50 and 90% of maximum storage capacity, respectively. After an initial increase up to 65 μg L−1 (YCH) and 40 μg L−1 (DB), the high influx of water was followed by a general decrease in TP concentrations up to 10–45 μg L−1 (YCH) and 10–20 μg L−1 (DB) (Fig. 2d). The concentrations of TP in the main affluent (Yangchang River) were generally a little bit higher than those in the lake (between 40 and 90 μg L−1), with peaks until 140 μg L−1 in summer.
Mean values of pH and conductivity in the sampled layers (0–6 m) of the six stations were between 7.0 and 8.2 and 200 and 400 μS cm−1, respectively.
Total phytoplankton biovolume and cyanobacteria
Phytoplankton biovolumes showed wide seasonal changes (Fig. 3a, b). The lower abundances between January and March were soon followed by a consistent increase caused by the development of Ochrophyta (Dinobryon divergens) and large diatoms. After the increase of the water levels, in June, total biovolumes showed an initial minor increase followed by high biovolume values between July and September/October (southern basin; Fig. 3a) or a continuous gradual decrease (northern basin; Fig. 3b). The seasonal development of cyanobacteria generally followed the same patterns of total biovolumes, but with a few important differences. In the two stations near the dam (BHZ and DB), the spring peak of cyanobacteria was anticipated to March; moreover, after the increase of the lake water level, the development of this group was generally low in the whole lake (Fig. 3c, d).
The mean annual values of phytoplankton biovolumes showed a slight tendency to decrease from the southern stations (annual averages around 3700 mm3 m−3) to the northern stations (3000–3400 mm3 m−3). Conversely, cyanobacteria showed a higher development in the two stations near the dam (BHZ and DB) (Fig. 4a).
a Annual average biovolumes of phytoplankton and cyanobacteria in the six stations, from south to north (0–6 m layer). b Average values of phytoplankton biovolume and water transparency between June and December in the six stations. c Relationship between water transparency and total phytoplankton biovolumes between June and December in the six stations; the data have been fitted by a power regression; d the same relationship, integrated by annual average values computed for a group of large clear lakes south of the Alps (DSL Deep Southern Subalpine Lakes; Salmaso et al. 2003)
The increase of the mean values of transparency from south to north between June and December (i.e. when the measurements of Secchi disk were available) followed an opposite pattern compared to the corresponding (June–December) biovolume values (Fig. 4b). It is worth underlining that the mean June–December biovolumes at the six stations differed more than the corresponding annual means (Fig. 4a). The power regression between the two average estimates (z SD = 7494 × PB−1.034) was highly significant (p < 0.01; Fig. 4c). The consistency of these estimates was compared with a small set of annual averages of z SD and PB recorded in a group of deep and clear lakes south of the Alps (Salmaso et al. 2003) (DSL; Fig. 4d). The data recorded in the Hongfeng Reservoir fitted and completed the data recorded in the clear lakes (z SD = 32705 × PB −1.215; p < 0.01).
The selection of ANCOVA models based on AIC and ANOVA tests allowed confirming the general positive effect of RTR6 and water temperature in the determination of total phytoplankton biomasses and a greater positive response of phytoplankton to RTR6 and water temperature in the period before the flooding (see the negative terms RTR6 × WLPH and Temp × WLPH) (Table 2 (a, b)). Though significant, the relationship between cyanobacteria and RTR6 and WLP was characterised by a low percentage of explained variance (Table 2 (c)); similarly to phytoplankton, cyanobacteria showed a positive response to RTR6 in the period before the flooding and no relationship (slope around zero) at high water level (negative term RTR6 × WLPH). The relationship between cyanobacteria and water temperature and WLP was better represented by a main effects model (i.e. without interaction) (Table 2 (d)); the higher absolute value of the negative term WLPH compared to the baseline intercept indicates a lower response of cyanobacteria to water temperature at high water levels and is fully coherent with the lower development of cyanobacteria after flooding.
Similar significance levels were obtained with regressions including water levels instead of WLP in the model selection, with determination coefficients (r 2) equal to 0.41, 0.60, 0.19 and 0.34 in models of Table 2 (a, b, c, d), respectively.
The same models including stations (as a factor) were not significant, indicating a homogeneous impact of the explanatory variables RTR6 and WLP at the lake basin scale. This was confirmed also by the application of a simpler model relating phytoplankton and cyanobacteria biovolumes and stations as a categorical variable (p > 0.1). Similarly, the inclusion of TP (stations YCH and DB) did not improve the models.
Development of the phytoplankton community and MFGs
The main pattern suggested by the ordination of samples based on their phytoplankton composition was a clear separation of two temporal periods, WLPL and WLPH, namely before and during/after the increase of the lake water level (Fig. 5a). The separation of these two periods was confirmed by the results of the PERMANOVA (p < 0.001). Conversely, the application of the same analysis to check differences in the NMDS configurations among the single stations did not provide significant results (PERMANOVA, p > 0.5) to indicate a similar development of phytoplankton species at the basin scale. The two WLP periods (low and high water level) were characterised by different phytoplankton taxonomic groups and MFGs (Fig. 5b, c; Table 3). Before flooding, the main phytoplankton phyla were cyanobacteria (mostly Limnothrix sp., Oscillatoria sp. and Pseudanabaena sp.), Ochrophyta (D. divergens), Bacillariophyta (Synedra sp.) and Euglenophyta. Conversely, after flooding, the main groups were Cryptophyta (Cryptomonas sp.) and Chlorophyta (many Chlorococcales). In terms of functional groups, the picture was further clarified, showing, along with a general decrease of biovolumes, especially in the northern basin (Fig. 3), a shift from cyanobacteria (mostly Oscillatoriales), and large diatoms, conjugatophytes and chrysophytes to specific MFGs belonging to small chlorophytes (small naked and gelatinous chlorophytes, mostly Tetraedron spp., Scenedesmus spp., Monactinus simplex, Coelastrum microporum, Hariotina reticulata), small centric diatoms (Cyclotella sp.) and cryptophytes (Cryptomonas spp.) (Table 3). The application of the vector and surface fitting analysis to two stations representative of the southern (NHZ) and northern (BHZ) basins showed a clear and quasi-linear fitting of lake water levels with the NMDS phytoplankton configuration (Fig. 5d, e). Conversely, the vector fitting of RTR6 to the NMDS configuration did not provide significant results; vector fitting of water temperature, on the two selected stations, was at the border of significance (p = 0.09) in the BHZ station. Contrary to the station near the inlet (YCH), the configuration of the station DB showed a significant relationship with TP concentrations (Fig. 5f).
a NMDS ordination of monthly phytoplankton samples of the six stations; the symbols indicate the months before (< 6), during (6) and after (> 6) the flooding event, in June. b Vectors of significant (p < 0.05) algal orders. c Vectors of significant (p < 0.05) morpho-functional groups (LargeChry 1a-large Chrysophytes/Haptophytes, FilaCyano 5a-thin filaments (Oscillatoriales), LUniPenn 6b2-large unicellular pennates, FilaConj 10b-filaments–conjugatophytes, LargeDino 1b-large dinophytes, NakeChlor 11a-Chlorococcales-naked colonies, SmallCent 7a-small centrics, SmallChlor 9b-small unicells-Chlorococcales, Crypto 2d-Cryptophytes, GelaChlor 11b-Chlorococcales-gelatinous colonies). Surface and vector fitting of lake water levels in two selected stations in the d southern (NHZ) and e northern (BHZ) basins; significance of vector fitting, p = 0.06 and p = 0.001, respectively; approximate significance of surface smooth terms, p = 0.07 and p < 0.001, respectively. f Surface (p < 0.01) and vector (p < 0.05) fitting of TP in the DB station
Discussion
The quick and important changes of water level in the Hongfeng Reservoir were proven to have a significant impact on the phytoplankton biovolumes and dynamics. The most apparent change was a strong shift from large phytoplankters and filamentous cyanobacteria to different functional groups characterised by individuals of smaller size belonging to chlorophytes (mostly Chlorococcales, sensu Komárek and Fott 1983) and cryptophytes.
In a recent review, Bakker and Hilt (2015) found a wide range of effects caused by WLF on the development of cyanobacteria and phytoplankton in lakes and reservoirs of different size/depth and hydrology. Generally, a drawdown in summer was shown to stimulate growth and abundance of cyanobacteria and eukaryotic phytoplankters, though these effects could be counteracted by the development and competition by submerged macrophytes, which are favoured by the lowering of water depth and improving light regime (e.g. Nõges and Nõges 1999; Beklioglu and Tan 2008). The effects triggered by water level rise due to flooding had however less predictable effects on the abundance of cyanobacteria (Bakker and Hilt 2015). Extended flooding in Lake Kinneret caused an increase in diazotrophic cyanobacteria (Aphanizomenon ovalisporum and Cylindrospermopsis raciborskii) possibly due to fish population explosion and zooplankton decline (Zohary and Ostrovsky 2011). Similarly, flooding in Lake Peipsi (Kangur et al. 2003) and in the lower Dutch river Rhine (Van den Brink et al. 1992) triggered an expansion of Aphanizomenon flos-aquae caused by a reduction in light availability and macrophytes growth and higher nutrient loads, respectively. Conversely, in the shallow Lake Sakadas and in the Arancio reservoir, water level rise connected to flooding caused a reduction in the biomass of filamentous cyanobacteria associated to a greater growth of macrophytes (Mihaljević et al. 2010) and a dilution of Microcystis aeruginosa colonies (Naselli-Flores et al. 2007), respectively. The results of these observations highlight that very different processes may be involved in the control of phytoplankton and cyanobacteria as response to WLF and other physical constraints (Mantzouki et al. 2016). The difficulty to generalise depends on the different responses of lakes characterised by different physiographic characteristics, the balance between inflows and outflows, trophic state and trophic web linkages and light regime in the water column and lake bottom. Nevertheless, the ecological processes in deeper waterbodies appeared more circumscribed to the effects caused by retention time (dilution of algal cell densities and effects on nutrients), disturbance or disruption of thermal stability and, possibly, modification of trophic webs due to changes in zooplankton grazing pressure (e.g. Zohary and Ostrovsky 2011). In this regard, WLF might represent a complex variable able to integrate different effects in one explanatory descriptor.
In the Hongfeng Reservoir, high total biovolume values were found both before and—though with general minor values at the lake basin scale—after the highest influx of water. The sharp decrease in total biomass and cyanobacteria in May could have been favoured by the incipient and rapid change in the hydrological conditions, which caused the increase of the lake level by ca. 1 m, just before the full flooding event. After the flooding, the higher biovolumes observed in the stations nearer to the tributary (YCH, NHZ) may have been possibly favoured by a general higher availability of nutrients in the Yangchang River, as suggested by the higher TP concentrations recorded in the southern YCH station. Considering that a fraction of TP also includes phytoplankton biomass, its concentrations should be regarded as a potential availability of phosphorus (as well as other nutrients). Nevertheless, also considering the exclusive relationship with the NMDS phytoplankton configurations in the station near the dam, further attempts to generalise the role of TP over the entire reservoir are quite difficult. The biovolume of phytoplankton and cyanobacteria showed a significant relationship with the stability of the water column and water temperature. Although there are differences at species level, an increase in these two factors are in general instrumental for the development of cyanobacteria (Paerl and Otten 2013; Paerl et al. 2016). Nevertheless, water level fluctuations (described by both water levels and WLP) were proved to have a major and significant impact on the biovolumes, composition, structure and dynamic of the phytoplankton community. Models in Table 2 showed a decreased or null (in the case of cyanobacteria) effect of water temperature and RTR6 on phytoplankton and cyanobacteria during and after the flooding event. Overall, the increasing water influx caused a dilution and removal of the extant populations, whereas the shift to a higher water level state favoured the expansion of new populations composed by competitive species with smaller dimensions. Phytoplankton structure shifted from the dominance of large diatoms, large chrysophytes and filamentous cyanobacteria (i.e. mostly species with strategy R-ruderals) to small chlorophytes (naked and gelatinous chlorophytes), small centric diatoms and cryptophytes. Most of the new MFGs that established during and after the flooding were typical C-competitive strategists characterised by high surface to volume ratios adapted to exploit nutrient pulses and with relatively high metabolic activity and replication rates over a wide range of temperatures (Reynolds 1988; Padisák 2004). In this regard, the experimental work by Lürling et al. (2013) demonstrated that, while cyanobacteria and chlorophytes possessed a large coincidence in the optimum temperatures for growth, many chlorophytes showed higher growth rates than cyanobacteria at higher temperatures. This functional group after October/November, with the complete isotherm and mixing of the lake and decrease of water temperatures, phytoplankton almost completely collapsed to the same biovolume values recorded between January and February.
The decrease of cyanobacteria in the Hongfeng Reservoir after the large flooding event is compatible with the low tolerance of this group of prokaryotic algae to high water loads (Salmaso and Zignin 2010; Salmaso et al. 2015; Visser et al. 2016). The existence of rapid renewal times over the year was considered instrumental for the almost complete absence of cyanobacteria from the high flushed meso-eutrophic reservoirs in NE, Italy (Moccia et al. 2000; Tolotti et al. 2010). For these reasons, compared with the concentrations of TP, the biovolumes of cyanobacteria in the Hongfeng Reservoir could be considered as underrepresented, especially after the flooding event. In this respect, one of the most notable facts was the permanently very low biovolume of large gas-vacuolated filamentous Nostocales (< 100 mm3 m−3) and colonial Chroococcales (< 50 mm3 m−3) in the analysed samples both before and after flooding. Among cyanobacteria, these groups are particularly sensitive to the deterioration of the thermal regime and entrainment and disentrainment by mixing (Wu et al. 2013). In well mixed lakes with low renewal times, large gas-vacuolated cyanobacteria lose the capability to control the vertical movement and therefore to exploit vertical resource gradients (Reynolds 2006; Visser et al. 2016). Owing to their smaller sizes and linear shapes, the thinner filaments of the larger planktonic Oscillatoriales (e.g. Planktothrix, former “Oscillatoria”) are able to reach lower maximum vertical velocities along the water column, typically 5–10 μm s−1, compared to the larger colonies of Nostocales and Chroococcales (up to well over 1000 μm s−1) (Padisák 2004; Oliver et al. 2012). The ability of the thin shade-tolerant Oscillatoriales to control vertical positioning is actually utilised to exploit specific microhabitats along the water column, such as the metalimnetic layers by Planktothrix rubescens in the deep lakes (Walsby et al. 2006; Akçaalan Albay et al. 2014). In shallower lakes, Planktothrix agardhii can dominate for most of the year in turbid mixed environments (Kokociński et al. 2009); in species like this, the presence of gas vesicles may become important to avoid fast sinking during lower turbulence periods. The thinner filaments of Limnothrix sp. accounts for their relative very low velocity compared with that of Planktothrix, i.e. < 1 μm s−1 (Walsby 2005). Limnothrix sp. is therefore more adapted to exploit mixed water columns, and, sharing with the other pelagic Oscillatoriales the ability to tolerate shaded environments (with daily irradiances < 1.5 mol photons m−2 day−1; Reynolds et al. 2002), it can also tolerate variable periods characterised by mixing depths greater that the corresponding euphotic depths. Overall, the significant dilution of the population of Limnothrix sp. in the Hongfeng Reservoir during and after the filling period is fully consistent with the high sensitivity of pelagic Oscillatoriales to flushing events (Reynolds et al. 2002; Bormans et al. 2004). These considerations are valid also for the other two Oscillatoriales identified in the Hongfeng Reservoir, i.e. Pseudanabaena sp. (which can develop without or with gas vesicles) and other Oscillatoriales (“Oscillatoria” sp.). Nevertheless, since no information on z eu is available before the flooding event, a complete quantitative evaluation of the effects of light climate on the Oscillatoriales and other algal groups is no further possible. The findings in this research are consistent also with the previous work by Yang et al. (2016), who showed an increase in the relative contribution of cyanobacteria (though with different species compared with those in the Hongfeng Reservoir) after a decline in water level in a group of subtropical reservoirs. Nevertheless, considering that WLF can control cyanobacteria directly or indirectly (e.g. Fadel et al. 2015) by influencing light, nutrients and other limnological variables, Yang et al. (2016) concluded that WLF could not be necessarily considered a strong predictor of cyanobacteria biomass in subtropical reservoirs.
The seasonal development of phytoplankton had a strong impact on the transparency of the reservoir. Though the role of the inorganic sediment was not evaluated, the model relating water transparency and phytoplankton biovolumes was coherent with a small set of concurrent z SD and biovolume data recorded in a different group of deep and clear lakes south of the Alps, namely the lakes Garda, Maggiore, Como and Lugano. The overall model indicated that, to improve the mean annual values of transparency of the Hongfeng Reservoir to over 4 m, total biovolumes should decrease to values < 1500 mm3 m−3. At the same time, to maintain low phytoplankton biovolumes and the relative biomass of cyanobacteria below critical levels, besides low concentrations of nutrients, the water fluxes and water levels should be kept high, especially during the warmer months. Clearer water conditions and higher irradiance, coupled with low water renewal time and low WLF, could favour the development of other phytoplankton groups outcompeting shade-tolerant cyanobacteria (Nõges et al. 2003; Mihaljević et al. 2010).
A direct estimation of the concentrations of cyanotoxins associated with the development of cyanobacteria was not considered in this work. Nevertheless, this aspect, also considering that the lake is mainly used for drinking purposes, should not be overlooked. Cultured strains of Limnothrix sp., i.e. the taxon dominating cyanobacteria in the Hongfeng Reservoir, were reported to produce microcystins (Furtado et al. 2009), saxitoxins (Gkelis et al. 2005) as well as new cyanotoxins (“limnothrixin”; Humpage et al. 2012).
Owing to the large catchment areas drained by the rivers and the difficulty to control diffuse loads from agricultural practices and natural and anthropogenic habitats, the input of nutrients from the tributaries to reservoirs can be difficult to control. However, besides nutrient load reduction, which should be a priority and a long-term goal, the short-term management of reservoirs should not ignore the implications due to the effects of WLF on the development of phytoplankton and potentially toxigenic cyanobacteria and CyanoHABs.
Conclusions
The rise in water level in the Hongfeng Reservoir had a strong impact on the composition, structure and seasonal dynamic of phytoplankton and cyanobacteria. Before the wide flooding event experienced in early summer, phytoplankton was composed of large diatoms, chrysophytes and thin filamentous cyanobacteria. After the flooding, the larger species and cyanobacteria declined in favour, especially during summer and early autumn, of smaller chlorophytes and smaller diatoms better adapted to a fast colonisation of new and nutrient-rich environments. In winter, in high disturbed and almost complete mixing conditions, biovolumes were in any case very low. The general environmental drivers that drove the change were flushing, dilution and interference with the seasonal water stratification processes. The flooding appeared a negative factor particularly for the development of the thin Oscillatoriales dominating the cyanobacteria, i.e. Limnothrix sp. However, in presence of a favourable light regime favouring shade-tolerant species, the recovery of Limnothrix sp. after the flooding event was constrained by the higher development rate of the smaller chlorophytes and diatoms. This work contributed to demonstrate how the effects of WLF on lake ecosystems and water quality should be investigated on a case-by-case basis. By influencing different limnological variables such as water discharge, renewal time, dilution, thermal and light regime, nutrients and, in shallower basins, macrophytes growth and internal loads, the impact of WLF is difficult to generalise also considering more circumscribed typologies of lakes. Further, considering the year-to-year hydrological variability that characterise reservoirs, clear-cut recommendations for management should be verified and confirmed considering more than 1-year study. As a general indication, a growing body of evidence indicates that flooding and high water levels act as potent factors inhibiting the growth of cyanobacteria in subtropical reservoirs. This aspect should be taken into account in the management strategies aimed at preserving water quality especially in lakes also used for drinking purposes.
References
Akçaalan Albay R, Köker L, Gürevin C et al (2014) Planktothrix rubescens: a perennial presence and toxicity in Lake Sapanca. Turk J Botany 38:782–789
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Ansari AA, Singh Gill S, Lanza GR, Rast W (eds) (2011) Eutrophication: causes, consequences and control. Springer Netherlands, Dordrecht
Bakker ES, Hilt S (2015) Impact of water-level fluctuations on cyanobacterial blooms: options for management. Aquat Ecol. https://doi.org/10.1007/s10452-015-9556-x
Beklioglu M, Tan CO (2008) Restoration of a shallow Mediterranean lake by biomanipulation complicated by drought. Fundam Appl Limnol/Arch für Hydrobiol 171:105–118. https://doi.org/10.1127/1863-9135/2008/0171-0105
Bormans M, Ford PW, Fabbro L, Hancock G (2004) Onset and persistence of cyanobacterial blooms in a large impounded tropical river, Australia. Mar Freshw Res 55:1. https://doi.org/10.1071/MF03045
Bormans M, Maršálek B, Jančula D (2016) Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: a review. Aquat Ecol 50:407–422. https://doi.org/10.1007/s10452-015-9564-x
Chorus I, Schauser I (2011) Oligotrophication of Lake Tegel and Schlachtensee, Berlin. Analysis of system components, causalities and response thresholds compared to responses of other waterbodies. Umweltbundesamt, Berlin
Coops H, Hosper SH (2002) Water-level management as a tool for the restoration of shallow lakes in the Netherlands. Lake Reserv Manag 18:293–298
Crawley MJ (2005) Statistics: an introduction using R. John Wiley & Sons, Inc., Hoboken
Druart J-CJ-C, Rimet F (2008) Protocoles d’analyse du phytoplancton de l’INRA: Prélèvement, dénombrement et biovolumes. INRA-Thonon, Rapport SHL 283, Thonon
Fadel A, Atoui A, Lemaire BJ et al (2015) Environmental factors associated with phytoplankton succession in a Mediterranean reservoir with a highly fluctuating water level. Environ Monit Assess 187:633. https://doi.org/10.1007/s10661-015-4852-4
Furtado ALFF, Calijuri M do C, Lorenzi AS et al (2009) Morphological and molecular characterization of cyanobacteria from a Brazilian facultative wastewater stabilization pond and evaluation of microcystin production. Hydrobiologia 627:195–209. https://doi.org/10.1007/s10750-009-9728-6
Gkelis S, Rajaniemi P, Vardaka E et al (2005) Limnothrix redekei (Van Goor) Meffert (Cyanobacteria) strains from Lake Kastoria, Greece form a separate phylogenetic group. Microb Ecol 49:176–182. https://doi.org/10.1007/s00248-003-2030-7
Guiry MD, Guiry GM (2016) AlgaeBase. World-wide electronic publication—National University of Ireland, Galway http://www.algaebase.org
Hamilton DP, Salmaso N, Paerl HW (2016) Mitigating harmful cyanobacterial blooms: strategies for control of nitrogen and phosphorus loads. Aquat Ecol 50:351–366. https://doi.org/10.1007/s10452-016-9594-z
Humpage A, Falconer I, Bernard C et al (2012) Toxicity of the cyanobacterium Limnothrix AC0243 to male Balb/c mice. Water Res 46:1576–1583. https://doi.org/10.1016/j.watres.2011.11.019
Ibelings BW, Bormans M, Fastner J, Visser PM (2016) CYANOCOST special issue on cyanobacterial blooms: synopsis—a critical review of the management options for their prevention, control and mitigation. Aquat Ecol 50:595–605. https://doi.org/10.1007/s10452-016-9596-x
Jochimsen MC, Kümmerlin R, Straile D (2013) Compensatory dynamics and the stability of phytoplankton biomass during four decades of eutrophication and oligotrophication. Ecol Lett 16:81–89. https://doi.org/10.1111/ele.12018
Kallf J (2002) Limnology. Prentice-Hall, Inc., Upper Saddle River, NJ
Kangur K, Möls T, Milius A, Laugaste R (2003) Phytoplankton response to changed nutrient level in Lake Peipsi (Estonia) in 1992–2001. Hydrobiologia 506–509:265–272. https://doi.org/10.1023/B:HYDR.0000008574.40590.8f
Kéry M (2010) Introduction to WinBUGS for ecologists: a Bayesian approach to regression, ANOVA, mixed models and related analyses, 1st edn. Academic Press, Elsevier
Kirk JTO (2011) Light and photosynthesis in aquatic ecosystems, 3rd edn. Cambridge University Press, Cambridge
Kokociński M, Dziga D, Spoof L et al (2009) First report of the cyanobacterial toxin cylindrospermopsin in the shallow, eutrophic lakes of western Poland. Chemosphere 74:669–675. https://doi.org/10.1016/j.chemosphere.2008.10.027
Komárek J, Fott B (1983) Chlorophyceae Chlorococcales. In: Huber-Pestalozzi G (ed) Das Phytoplankon des Süßwassers Systematik und Biologie. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, p 1043
Kosten S, Lacerot G, Jeppesen E et al (2009) Effects of submerged vegetation on water clarity across climates. Ecosystems 12:1117–1129. https://doi.org/10.1007/s10021-009-9277-x
Krienitz L, Bock C (2012) Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 698:295–326. https://doi.org/10.1007/s10750-012-1079-z
Legendre P, Gallagher E (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. https://doi.org/10.1007/s004420100716
Li Q, Chen L, Chen F et al (2013) Maixi River estuary to the Baihua Reservoir in the Maotiao River catchment: phytoplankton community and environmental factors. Chin J Oceanol Limnol 31:290–299. https://doi.org/10.1007/s00343-013-2111-5
Lürling M, Eshetu F, Faassen EJ et al (2013) Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw Biol 58:552–559. https://doi.org/10.1111/j.1365-2427.2012.02866.x
Lv H, Yang J, Liu L et al (2014) Temperature and nutrients are significant drivers of seasonal shift in phytoplankton community from a drinking water reservoir, subtropical China. Environ Sci Pollut Res Int 21:5917–5928. https://doi.org/10.1007/s11356-014-2534-3
Mahujchariyawong J, Ikeda S (2001) Modelling of environmental phytoremediation in eutrophic river—the case of water hyacinth harvest in Tha-chin River, Thailand. Ecol Model 142:121–134. https://doi.org/10.1016/S0304-3800(01)00283-6
Mantzouki E, Visser PM, Bormans M, Ibelings BW (2016) Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat Ecol. https://doi.org/10.1007/s10452-015-9526-3
Matthijs HCP, Visser PM, Reeze B et al (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res 46:1460–1472. https://doi.org/10.1016/j.watres.2011.11.016
Mehner T, Benndorf J, Kasprzak P, Koschel R (2002) Biomanipulation of lake ecosystems: successful applications and expanding complexity in the underlying science. Freshw Biol 47:2453–2465. https://doi.org/10.1046/j.1365-2427.2002.01003.x
Meriluoto J, Blaha L, Bojadzija G et al (2017) Toxic cyanobacteria and cyanotoxins in European waters—recent progress achieved through the CYANOCOST Action and challenges for further research. Adv Oceanogr Limnol 8:18. https://doi.org/10.4081/aiol.2017.6429
Mihaljević M, Špoljarić D, Stević F et al (2010) The influence of extreme floods from the River Danube in 2006 on phytoplankton communities in a floodplain lake: shift to a clear state. Limnol—Ecol Manag Inl Waters 40:260–268. https://doi.org/10.1016/j.limno.2009.09.001
Moccia A, Decet F, Salmaso N (2000) Plankton and nutrient evolution of a piedmont reservoir in Northern Italy (L. Santa Croce). Verh InternatVerein Limnol 27:2929–2933
Naselli-Flores L, Barone R, Chorus I, Kurmayer R (2007) Toxic cyanobacterial blooms in reservoirs under a semiarid Mediterranean climate: the magnification of a problem. Environ Toxicol 22:399–404. https://doi.org/10.1002/tox.20268
Nõges A, Nõges A (1999) The effect of extreme water level decrease on hydrochemistry and phytoplankton in a shallow eutrophic lake. Hydrobiologia 408–409:277–283. https://doi.org/10.1023/A:1017048329234
Nõges T, Nõges P, Laugaste R (2003) Water level as the mediator between climate change and phytoplankton composition in a large shallow temperate lake. Hydrobiologia 506–509:257–263. https://doi.org/10.1023/B:HYDR.0000008540.06592.48
Oksanen J, Blanchet FG, Friendly M et al (2016) vegan: Community Ecology Package https://cran.r-project.org/web/packages/vegan/index.html
Oliver RL, Hamilton DP, Brookes JD, Ganf GG (2012) Physiology, blooms and prediction of planktonic cyanobacteria. In: Whitton BA (ed) Ecology of cyanobacteria II their diversity in space and time. Springer, Dordrecht, pp 155–194
Padisák J (2004) Phytoplankton. In: O’Sullivan PE, Reynolds CS (eds) The lakes handbook. Volume 1. Limnology and limnetic ecology. Blackwell Science Ltd, pp 251–308
Paerl HW, Gardner WS, Havens KE et al (2016) Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted both by climate change and anthropogenic nutrients. Harmful Algae 54:213–222
Paerl HW, Otten TG (2013) Harmful Cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 65:995–1010. https://doi.org/10.1007/s00248-012-0159-y
R Core Team (2017) R: a language and environment for statistical computing (v. 3.4.1). R Foundation for Statistical Computing, Vienna URL https://www.R-project.org/
Reynolds CS (1988) Functional morphology and the adaptive strategies of freshwater phytoplankton. In: Sandgren CD (ed) Growth and reproductive strategies of freshwater phytoplankton. Cambridge University Press, Cambridge, pp 388–433
Reynolds CS (2006) The ecology of phytoplankton. Cambridge University Press, Cambridge
Reynolds CS, Huszar V, Kruk C et al (2002) Towards a functional classification of the freshwater phytoplankton. J Plankton Res 24:417–428. https://doi.org/10.1093/plankt/24.5.417
Ryding S-O, Rast W (1989) The control of eutrophication of lakes and reservoirs. UNESCO and The Parthenon Publishing Group, Paris
Salmaso N, Morabito G, Mosello R et al (2003) A synoptic study of phytoplankton in the deep lakes south of the Alps (lakes Garda, Iseo, Como, Lugano and Maggiore). J Limnol 62:207. https://doi.org/10.4081/jlimnol.2003.207
Salmaso N, Naselli-Flores L, Padisák J (2015) Functional classifications and their application in phytoplankton ecology. Freshw Biol 60:603–619. https://doi.org/10.1111/fwb.12520
Salmaso N, Zignin A (2010) At the extreme of physical gradients: phytoplankton in highly flushed, large rivers. Hydrobiologia 639:21–36. https://doi.org/10.1007/s10750-009-0018-0
Schindler DW (2006) Recent advances in the understanding and management of eutrophication. Limnol Oceanogr 51:356–363. https://doi.org/10.4319/lo.2006.51.1_part_2.0356
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ Sci Pollut Res 10:126–139. https://doi.org/10.1065/espr2002.12.142
Sukenik A, Quesada A, Salmaso N (2015) Global expansion of toxic and non-toxic cyanobacteria: effect on ecosystem functioning. Biodivers Conserv 24:889–908. https://doi.org/10.1007/s10531-015-0905-9
Tolotti M, Boscaini A, Salmaso N (2010) Comparative analysis of phytoplankton patterns in two modified lakes with contrasting hydrological features. Aquat Sci 72:213–226. https://doi.org/10.1007/s00027-009-0124-0
Van den Brink FWB, De Leeuw JPHM, Van Der Velde G, Verheggen GM (1992) Impact of hydrology on the chemistry and phytoplankton development in floodplain lakes along the Lower Rhine and Meuse. Biogeochemistry 19:103–128. https://doi.org/10.1007/BF00000798
Visser PM, Ibelings BW, Bormans M, Huisman J (2016) Artificial mixing to control cyanobacterial blooms: a review. Aquat Ecol 50:423–441. https://doi.org/10.1007/s10452-015-9537-0
Walsby AE (2005) Stratification by cyanobacteria in lakes: a dynamic buoyancy model indicates size limitations met by Planktothrix rubescens filaments. New Phytol 168:365–376. https://doi.org/10.1111/j.1469-8137.2005.01508.x
Walsby AE, Schanz F, Schmid M (2006) The Burgundy-blood phenomenon: a model of buoyancy change explains autumnal waterblooms by Planktothrix rubescens in Lake Zürich. New Phytol 169:109–122. https://doi.org/10.1111/j.1469-8137.2005.01567.x
Wetzel RG (2001) Limnology. Lake and river ecosystems. Academic Press, San Diego
Wu B, Wang G, Jiang H et al (2016) Impact of revised thermal stability on pollutant transport time in a deep reservoir. J Hydrol 535:671–687. https://doi.org/10.1016/j.jhydrol.2016.02.031
Wu T, Qin B, Zhu G et al (2013) Dynamics of cyanobacterial bloom formation during short-term hydrodynamic fluctuation in a large shallow, eutrophic, and wind-exposed Lake Taihu, China. Environ Sci Pollut Res Int 20:8546–8556. https://doi.org/10.1007/s11356-013-1812-9
Yang J, Lv H, Liu L et al (2016) Decline in water level boosts cyanobacteria dominance in subtropical reservoirs. Sci Total Environ 557–558:445–452. https://doi.org/10.1016/j.scitotenv.2016.03.094
Zohary T, Ostrovsky I (2011) Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inl Waters 1:47–59
Zohary T, Padisák J, Naselli-Flores L (2009) Phytoplankton in the physical environment: beyond nutrients, at the end, there is some light. Hydrobiologia 639:261–269. https://doi.org/10.1007/s10750-009-0032-2
Zuur A, Ieno EN, Walker N et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
The data on the lake level fluctuations were provided by the administration of Hongfeng, Baihua and Aha reservoirs.
Funding
This work was financed by the Natural Science Foundation of China (41163005), the Department of Science and Technology of Guizhou Province ([2015]2001, [2015]10, [2014]7001, [2014]7058), and the Water Resources Department of Guizhou Province [KT201401]. The first author received a scholarship from the China Scholarship Council to fund a research visit to Fondazione E. Mach—Istituto Agrario di S. Michele all’Adige.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Li, Q., Xiao, J., Ou, T. et al. Impact of water level fluctuations on the development of phytoplankton in a large subtropical reservoir: implications for the management of cyanobacteria. Environ Sci Pollut Res 25, 1306–1318 (2018). https://doi.org/10.1007/s11356-017-0502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-0502-4