Abstract
This review discusses the main developments in the systematics of coccoid green algae over the last three decades. The relationships of key groups of planktonic coccoid green algae are shown in the phylogenetic trees of Chlorophyceae and Trebouxiophyceae. The trees clearly show that the morphology of these algae do not adequately reflect their phylogenetic position. Different phylogenetic species can be hidden under one and the same morphotype. As most of the genera have a polyphyletic origin, they are in need of a systematic re-evaluation. Species classification using the phylogenetic species concept resulted in the establishment of new genera with smaller numbers of species and the description of new species that are not distinguishable by light microscopy. An overview of genera is given in tables and the revised designations of species as contained in the harmonized taxon list of the European Water Framework Directive lists is provided. In this transitional phase from an artificial to a more natural systematics of algae, field biologists and ecologists as well as molecular biologists must strengthen their interdisciplinary cooperation. The alignment of eco-functional groups of algae with true species identities using the barcoding conception will provide a better understanding of the interaction between organisms and their environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The classification of algae is presently going through an extremely interesting stage. The old, artificial classification system is being replaced by a new, more natural, phylogenetic system. This is especially the case for the coccoid green algae, mainly because numerous organisms in this morphological group do not propagate sexually. Hence, phenotypic criteria have to a large extent been used in the establishment of taxonomic clades. Initially, the introduction of molecular phylogenetic methods into the systematics of green algae led to a fundamental revision of the concepts of higher taxonomic lineages such as divisions, classes and orders (Melkonian & Surek, 1995; Friedl, 1997; Chapman et al., 1998; Leliaert et al., 2012). Following these new phylogenetic conceptions the orders that contain coccoid green algae have been considerably changed. The systematics of lower taxonomic levels such as families and genera remain provisional because detailed taxon sampling for molecular sequence analyses have not been carried out for the taxa. Nevertheless, key groups of planktonic coccoid greens have in the recent past been studied by molecular methods, and as such a picture of their phylogenetic designation has started to take shape.
The central unit for biological classification is the species. The most established species concept is the biological species (Mayr, 1942), which refers to groups of interbreeding natural populations that are reproductively isolated. Because of its restriction to sexually reproducing organisms, this concept cannot be applied to most of the coccoid green algae. In an effort to deal with this limitation, limnologists adopted the morphological species concept using morphology-based diacritical characteristics. The climax of the morphological species concept for the coccoid green algae was reached following the publication of the famous handbook on “Chlorococcales” by Komárek & Fott (1983). Approximately 1,200 taxa from water, soil, and other habitats were compiled in this comprehensive work. The works of Hindák entitled “Studies on the Chlorococcal Algae (Chlorophyceae) I–V” (1977, 1980, 1984, 1988, 1990), which were published partly before and after the handbook of Komárek & Fott, have inspired many phytoplankton researchers. Combining a sharp eye for detail and experience, Hindák identified several new morphospecies. His observations triggered lively discussions on diacritic morphological characteristics such as presence or absence of pyrenoids, cell wall incrustations and mucilaginous envelopes.
Running parallel to these traditional approaches, an ultrastructural concept to classify green algae based on anatomy of flagellated cells and cytokinesis during mitosis was initiated by Melkonian (1982, 1984) and Mattox & Stewart (1984). Based on the orientation of the basal bodies of the flagellar apparatus, three main types have been suggested: counter clockwise orientation (CCW, 11–5 o’clock), clockwise orientation (CW, 1–7 o’clock), and directly opposite orientation (DO, 12–6 o’clock). Later studies on molecular characteristics by Lewis et al. (1992) revealed that the relationships established on the basis of ultrastructural data were closely supported by molecular data.
As discussions on the relations between the various concepts of classifying green algae continued, the phylogenetic concept, which utilizes molecular markers to delineate taxa, steadily made its way into the daily routine of phycologists. The evidence that has so far accumulated clearly suggests that biological and morphological conceptions cannot solve the main questions on natural grouping of coccoid green algae. A polyphasic approach combining morphological, ecophysiological and molecular phylogenetic methods should contribute to modern systematics (Pröschold & Leliaert, 2007). In this review, we focus on the main developments in the systematics of coccoid green algae following the landmarks set by the works of Komárek & Fott, Hindák, and Mattox & Stewart in the middle of the 1980s.
Higher taxonomic lineages which contain coccoid green algae
The green algae evolved about 1,500 million years ago (Yoon et al., 2004) into two large lineages (divisions), the Chlorophyta and the Charophyta (Lewis & McCourt, 2004). In this review, we follow the work of Lewis & McCourt (2004) who suggested a “working classification of green algae and land plants” (Table 1).
The Charophyta has often been labeled the Streptophyta by Bremer et al. (1987). This division contains seven classes that include the Charophyceae (stoneworts), the Embryophyceae (higher land plants), the Zygnemophyceae (conjugates), and the Chlorokybophyceae (has only one species Chlorokybus atmophyticus, an aerophytic coccoid green alga). The Zygnemophyceae has within the order Desmidiales, a special type of coccoid green algae with a striking morphology characterized by two symmetrical halves (semicells). The majority of coccoid green algae considered here occur in several orders of Chlorophyceae, Trebouxiophyceae, and Prasinophyceae within the division Chlorophyta (Melkonian, 1990a; Fawley et al., 2000; Krienitz et al., 2003). Conventionally, these coccoid taxa were lumped in the order Chlorococcales sensu lato (s.l.) by Komárek & Fott (1983) and represented one of the most diverse groups of photoautotrophic cryptogams. However, this classical approach based on Pascher’s (1918) idea of establishing orders according to life forms has not been supported by phylogenetic methods and is therefore not applicable in modern systematic considerations of green algae. Consequently, most of the taxa that do not belong to the Chlorococcales s.l. have been transferred to other orders.
In the division Chlorophyta, coccoid taxa occur in three different classes; the Chlorophyceae, Trebouxiophyceae, and Prasinophyceae (Table 1). In the Chlorophyceae, the coccoid taxa occur in two orders; the Chlorococcales sensu stricto (s.str.) and Sphaeropleales. The only remaining taxa in the order Chlorococcales s.str. are those related to the polyphyletic genus Chlorococcum and some other genera that all are still under revision. It has also become evident that some taxa of Chlamydomonadales, Dunaliellales, and Volvocales occur in the order Chlorococcales s.str. (Nakayama et al., 1996; Booton et al., 1998; Chapman et al., 1998; Pröschold et al., 2001). The ultrastructures of all these taxa are characterized by a CW orientation of the basal apparatus of the flagella. Apart from the Chlorococcales s.str. and Sphaeropleales, several other lineages (incertae sedis) which do not correspond to the named clades have coccoid taxa. Future revisions of the CW-group of Chlorophyceae may probably lead to the establishment of several new orders. Furthermore, the content of ambiguous orders such as Actinochloridales, Chlorosarcinales, and Tetrasporales must be emendated.
A majority of chlorophycean members of Chlorophyta are placed in the order Sphaeropleales (Fig. 1). Some members of these taxa such as Sphaeropleaceae, Hydrodictyaceae, and Bracteacoccus produce zoospores with DO-orientation of the flagellar apparatus (Lewis, 1997; Buchheim et al., 2001; Wolf et al., 2002a; Shoup & Lewis, 2003). However, many non motile unicellular or colonial taxa such as Selenastraceae and Mychonastes are also included in this order (Krienitz et al., 2001, 2003, 2011a, b, 2012). The Scenedesmaceae also belong to this order. Members of this family are commonly non motile. However, presence of zoospores in cultures of Acutodesmus has been reported (Trainor, 1963). Keller et al. (2008) confirmed the monophyly of Sphaeropleales; however, from several other studies the relations between the clade containing the type genus Sphaeroplea (Sphaeropleales s.str.) and the other clades (e.g., Hydrodictyon-clade, Scenedesmus-clade, Ankistrodesmus-clade, Bracteacoccus-clade) of the Sphaeropleales s.l. remain ambiguous (Fawley et al., 2005a; Pröschold & Leliaert, 2007; Krienitz et al., 2011a). Better-supported conclusions will require more data, based on sequences of a larger number of molecular markers.
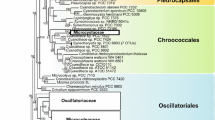
Molecular phylogeny of the Chlorophyceae based on SSU rRNA gene sequence comparisons. The phylogenetic tree shown was inferred using the maximum likelihood (ML) method (with substitution model: J1 [Optimum, Empirical]:G [Optimum]:5), based on 1558 aligned positions of 87 taxa using Treefinder (Jobb, 2008). Bayesian values (>0.95) (MB) were calculated by MrBayes 3.1 using GTR settings (Ronquist & Huelsenbeck, 2003; Posada & Buckley, 2004). The stationary distribution was assumed after 4 million generations when the average standard deviations of split frequencies between two runs was lower than 0.01. To test the tree confidence, bootstrap values (>50%) ML (1,000 replicates), neighbor-joining (NJ) (1,000 replicates; calculated using Paup 4.0), and maximum parsimony (MP) [1,000 replicates; calculated using Paup 4.0 (Swofford, 2002)] were determined. Support values are shown at the branches in the order: MB, ML, MP, NJ. Scale bar indicates substitutions per site. The sequences were obtained from Genbank [National Center for Biotechnology Information (NCBI)]. For each taxon, the NCBI accession number is given in brackets
Two major orders of coccoid taxa can be identified in the class Trebouxiophyceae; the Trebouxiales and Chlorellales (Fig. 2). Several other clades such as the Choricystis-clade and Oocystis-clade are distinctive and may probably be established as an independent order in future. Other clades (incertae sedis) hosting Coccomyxa, Botryococcus, Coenocystis and Pseudochlorella await delineation into higher taxonomic ranks. The Trebouxiales mainly comprises taxa from edaphic and aerophytic habitats as well as endosymbionts of lichens (Ettl & Gärtner, 1995; Friedl, 1995). Within Chlorellales (Fig. 3) are numerous new genera and species that were recently described from freshwaters (Fawley et al., 2005b; Bock et al., 2010, 2011a, b, c; Pröschold et al., 2010).
Molecular phylogeny of the Trebouxiophyceae based on SSU rRNA gene sequence comparisons. The phylogenetic tree shown was inferred using the maximum likelihood (ML) method (with substitution model: J1 [Optimum, Empirical]:G [Optimum]:5), based on 1,429 aligned positions of 58 taxa using Treefinder (Jobb, 2008). Bayesian values (>0.95) (MB) were calculated by MrBayes 3.1 using GTR settings (Ronquist & Huelsenbeck, 2003; Posada & Buckley, 2004). The stationary distribution was assumed after 4 million generations when the average standard deviations of split frequencies between two runs was lower than 0.01. To test the tree confidence, bootstrap values (>50%) for ML (1,000 replicates), NJ (1,000 replicates; calculated using Paup 4.0), and MP [1,000 replicates; calculated using Paup 4.0 (Swofford, 2002)] were calculated. Support values are shown at the branches in the order: MB, ML, MP, and NJ. Scale bar indicates substitutions per site. The sequences were obtained from Genbank [National Center for Biotechnology Information (NCBI)]. For each taxon, the NCBI accession number is given in brackets
Molecular phylogeny of the Chlorellaceae based on a partitioned dataset of SSU, ITS1, 5.8S, and ITS2 gene sequences. The phylogenetic tree shown is based on 2,536 manually aligned base positions of 50 taxa, calculated by Treefinder (Jobb, 2008) using the maximum likelihood (ML) method under different substitutional models for each partition. The substitution models were as follows: 18S (1,686 bases) J2 [Optimum, Empirical]:GI [Optimum]:5; ITS1 (388 bases) J1 [Optimum, Empirical]:G [Optimum]:5; 5.8S (137 bases) HKY [{3,1,1,1,1,3}, Empirical]:G [Optimum]:5; and ITS2 (325 bases) GTR [Optimum, Empirical]:G [Optimum]:5. The Bayesian values (>0.95) (MB) were calculated by MrBayes 3.1. A general time reversible model with gamma shape parameter and proportion of invariable sites (GTR+I+G) was applied to each partition. The parameters were unlinked and allowed to vary across the partitions (Ronquist & Huelsenbeck, 2003; Posada & Buckley, 2004). The stationary distribution was assumed after 4 million generations when the average standard deviations of split frequencies between two runs was lower than 0.01. To test the tree confidence, bootstrap values (>50%) for ML (1,000 replicates), NJ (1,000 replicates; using Paup 4.0), and MP (1,000 replicates; using Paup 4.0 (Swofford, 2002) were calculated. Support values are shown at the branches in the order: MB, ML, MP, and NJ. Scale bar indicates substitutions per site. The sequences were obtained from Genbank [National Center for Biotechnology Information (NCBI)]. For each taxon, the NCBI accession number is given in brackets
The class Prasinophyceae contains small flagellated and scaled green algae. A few of them have lost one or both the flagella and scales and now have a coccoid phenotype. Coccoid prasinophytes occur in different orders of the class Prasinophyceae (Table 1). It has been shown that this class is an artificial taxonomic lineage because these organisms have a paraphyletic origin; hence should better be named as prasinophytes (Steinkötter et al., 1994). The prasinophytes evolved in several independent lineages which probably represent independent classes. A report by Guillou et al. (2004) identifies seven different clades of the prasinophytes. Initial taxonomic revision of some of these clades has resulted in the description of the classes Nephroselmidophyceae (Cavalier-Smith, 1993), Chlorodendrophyceae (Masjuk, 2006), and Mamiellophyceae (Marin & Melkonian, 2010). The coccoid prasinophytes are restricted to marine habitats or saline inland waters (Guillou et al., 2004; Krienitz et al., 2012).
Recent systematics of key groups of coccoid green algae of inland waters
The ranking of the groups discussed in this section follow the topology in the phylogenetic trees (Figs. 1, 2, 3). These trees are based on a selection of sequences obtained from Genbank [National Center for Biotechnology Information (NCBI) http://www.ncbi.nlm.nih.gov/]. The accession numbers of these sequences are indicated in Figs. 1, 2, and 3).
Chlorophyceae
Selenastraceae
Morphologically, this group comprises of “needles and capricorns.” Members of this family are very common in freshwaters and exhibit a high morphological diversity. They exclusively propagate by autospores. Discussions on the diacritical features of this group have been very intense. Marvan et al. (1984) investigated 18 genera of the Selenastraceae by means of numerical evaluation of morphological and ontogenetic characteristics. The shape of the cells or colonies, the arrangement of autospores inside the mother cell, the development of mucilage and incrustations on the cell wall, and the presence, number, and type of pyrenoids were used to differentiate the genera. According to this conception, several genera differ from each other only by one of the above characters. For example, Selenastrum differs from Ankistrodesmus by the curvature of the cells (Komárek & Comas, 1982), Raphidocelis differs from Kirchneriella by cell wall incrustations (Hindák, 1977), and Chlorolobion differs from Monoraphidium by the presence of a pyrenoid with starch envelope (Komárek, 1979; Heynig & Krienitz, 1982). Fawley et al. (2005a) correlated the morphological features of several morphospecies of Selenastraceae with their molecular characteristics and found that one morphotype can cover different phylotypes. The data suggested that a broad morphospecies concept would result in a substantial underestimation of species diversity. Subsequent molecular studies have confirmed the existence of “small” genera in Selenastraceae containing only a few species (Krienitz et al., 2001, 2011b). From the molecular studies, 10 different genera have been confirmed in this family (Table 2). Several other genera are yet to be subjected to detailed molecular investigation the outcome of which may be the description of new genera. This is especially the case for the relatives of the needle-shaped Ankistrodesmus s.l. and Monoraphidium s.l. which are found in nine different clades and probably represent different genera (Krienitz et al., 2011b). However, further taxon sampling is essential before conclusions on the taxonomic identities of these clades can be drawn.
The adjustment of the diacritical value of the main morphological characteristics in line with the molecular findings has revealed the following: the solitary versus colonial life form, the general shape of cells and colonies are relatively good criteria. Although changes in environmental conditions can result in some colonies disintegrating, the arrangement of autospores in the mother cells can be used for the differentiation of genera. The establishment of mucilage and incrustations are of limited taxonomic value because of the wide variability. Similarly, views on the value of starch formation and the presence of pyrenoids have changed over time. Traditionally, most of the genera were thought to be devoid of pyrenoids. However, this position was later, revised following the observation of pyrenoids in some genera. The first report on the presence of pyrenoids was made by Eloranta (1979), who used the TEM to observe pyrenoids in Monoraphidium griffithii. This finding was later confirmed for other species (Krienitz et al., 1985, 2001; Krienitz & Scheffler, 1994). In subsequent studies, pyrenoids were detected in all taxa studied; some exhibited a naked matrix, while others were equipped with starch grains covering the pyrenoid matrix (Krienitz et al., 2011b). In aerated cultures, it was observed that the concentration of CO2 influenced pyrenoid formation (Miyachi et al., 1986). In Monoraphidium terreste, pyrenoids were well developed under normal air conditions. However, they were found to disappear under CO2 enrichment (Krienitz & Klein, 1988). Hence the presence or absence of pyrenoids cannot be used as a basis for the differentiation of members of the Selenastraceae family.
Based on molecular phylogeny, several taxa traditionally included in the Selenastraceae were removed from this family. Choricystis minor is member of a picoplanktonic lineage in the Trebouxiophyceae (Krienitz et al., 1996a; Darienko et al., 2010). Keratococcus bicaudatus and Pseudococcomyxa simplex are closely related to Choricystis (Friedl, 1996). The authentic strain of P. simplex was recovered in the Elliptochloris-clade and found to be closely related to other strains of Coccomyxa (Pröschold et al., 2011). Consequently, the taxon Coccomyxa simplex has been re-established. Hence, other members of Pseudococcomyxa need a taxonomic revision. Dicloster acuatus is a needle-shaped coenobial member of Chlorellaceae (Hegewald & Hanagata, 2000). Closteriopsis acicularis is a solitary, needle-shaped member of the Chlorellaceae and is closely related to Dicloster (Ustinova et al., 2001). The ultrastructure of its pyrenoid is comparable to that of Chlorella and other Chlorellaceae (Hegewald & Schnepf, 1986). The apochloric Hyaloraphidium curvatum is a member of the lower fungi (Ustinova et al., 2000).
Scenedesmaceae
This is the largest group of coccoid green algae in freshwater ecosystems. Among the genera, the genus Scenedesmus s.l. with its extremely wide morphological variability is a nightmare for field ecologists who wish to determine a taxon in a fixed sample under the inverted microscope. The “Annotated catalogue of Scenedesmus and nomenclaturally related genera,” published by Hegewald & Silva (1988) is a milestone in the history of conventional systematics of green algae. More than 800 taxa were included in details that provide a clear testimony of how the game of nature creates a multitude of morphospecies. Although this catalogue is a good guide for microscopists, at the end of the day it is difficult to decide on the species delineation in Scenedesmaceae. Several publications, each justifying the different views of the authors, have been written.
According to Hegewald (1997), the great morphological variability of Scenedesmaceae can be attributed to the strictly non-sexual propagation. This argument may also be valid for many other coccoid green algae such as the Selenastraceae. The simple reproduction by means of autospores results in a situation whereby all mutations occurring, which do not significantly influence growth and ability of the mutants to compete, remain and are not lost by genetic processes. This discussion leads to the old question on the possible occurrence of flagellated stages in Scenedesmus, first reported by a Ukrainian phycologist J. J. Valz more than 130 years ago (according to Hegewald, 1997). Trainor (1963) recorded the presence of flagellates in cultures of Scenedesmus, while Lukavský (1991) and Cepák (1993) found them in outdoor mass cultures. Trainor (1996) has described the production and germination of zygospores. However, the majority of these algae propagate asexually by autospores, and the reasons given by Hegewald to explain the wide morphological variability remain valid.
Revisions of the class followed the introduction of molecular methods. First, it became evident that the morphologically differentiated subgenera should be upgraded to generic status. Consequently, the genus Desmodesmus was separated from Scenedesmus (An et al., 1999). Whereas Desmodesmus comprises species characterized by many substructures on the cell wall and are equipped with teeth, rosettes, warts and spines, the species of Scenedesmus have a smooth, non-ornamented cell wall (Hegewald, 2000). The genus Acutodesmus, which comprises of more or less ellipsoidal, spindle-shaped taxa that show longitudinal ridges under the TEM, was established by Tsarenko & Petlevanny (2001). Later, Pectinodesmus was erected for taxa with similar morphology as Acutodesmus but differing in molecular phylogeny (Hegewald et al., 2010). Several genera which are presently monotypic such as Hylodesmus and Comasiella have recently been established (Eliáš et al., 2010; Hegewald et al., 2010).
A major surprise resulting from the molecular studies is the clustering of Coelastrum-morphotypes with Scenedesmus suggesting that the flat coenobia with cells arranged in one or two rows of Scenedesmus-relatives are phylogenetically closely related to the spherical coenobia of Coelastrum and allied species (Fig. 1) (Hegewald et al., 2010). Following confirmation through molecular studies of the separate position of Coelastrum reticulatum, the old name Hariotina was reintroduced (Hegewald et al., 2002).
At present, 13 genera of Scenedesmaceae are phylogenetically and morphologically well defined (Table 3). Several other genera of this family, such as all the crucigenoid groups, are yet to be revised.
Hydrodictyaceae
The Hydrodictyaceae comprises microscopic colonies of Pediastrum, Euastropsis, and Sorastrum as well as macroscopic colonies of Hydrodictyon. The most recent review reduced the number of Pediastrum-species to 24 (Komárek & Jankovská, 2001). Hydrodictyaceae exhibit distinct reproduction strategies: they produce asexually inside the parental cells through biflagellated zoospores that aggregate after swarming to daughter colonies which are released from the mother-sporangiae in a gelatinous bubble. Sexually, Hydrodictyaceae reproduce by isogametes. The ultrastructure of the flagellar apparatus is characterized by a directly opposite (DO) configuration in Hydrodictyon and Pediastrum (Wilcox & Floyd, 1988).
Phylogenetic analysis of Hydrodictyon, Pediastrum, and Sorastrum has revealed a pattern of colony-form evolution within the family from two-dimensionality to three-dimensionality (McManus & Lewis, 2005). Another molecular phylogenetic study of 28 hydrodictyacean strains revealed polyphyly in Pediastrum and resulted in taxonomic conclusions (Buchheim et al., 2005). Beside Pediastrum, the genera Monactinus, Parapediastrum, Pseudopediastrum, and Stauridium were delineated. It is interesting to note that Pediastrum duplex with a complex morphology of colonies evolved polyphyletically (McManus & Lewis, 2011). Consequently, a new genus Lacunastrum was erected (McManus et al., 2011). It has also been shown that members of the genus Tetraedron evolved as a sister clade to the Hydrodictyaceae. However, the evolutionary link between the tetraedric unicells of autosporic Tetraedron, zoosporic Chlorotetraedron, and the zoosporic colonial Hydrodictyaceae remains obscure (McEntee et al., 1977; Komárek & Kováčik, 1985; Hegewald et al., 2001; Buchheim et al., 2005). Presently, the Hydrodictyaceae has 10 genera as confirmed by morphological and molecular analyses (Table 4).
Sphaeropleaceae
This group provides a good example for demonstrating that life form sensu Pascher (1918) is not suitable for the natural grouping of algae. Two different life forms in this family, the filamentous (Sphaeroplea) and the coccoid (Ankyra) green algae cluster together (Fig. 1). Sphaeroplea is a filamentous green algal genus with multinucleate (coenocytic) cells (Buchheim et al., 2001). Its propagation is by asexual division of the cells in the unbranched filaments and oogamous sexual reproduction. Actractomorpha produces extremely long needle-shaped solitary cells that are coenocytic in character and can propagate asexually by zoospores and sexually by anisogamy or seldom by oogamy (Hoffman, 1983). Normally, this alga occurs mostly in soil. However, it has also been observed in freshwaters (Schmidt & Fehér, 1999–2000). Freshwater forms are not always correctly designated (e.g., as Closteriopsis longissima f. gigantea Heynig, 1980). Such planktonic cells can grow to lengths of 1,000–1,900 μm. The most frequently observed members of Sphaeropleaceae in the plankton are species of Ankyra, which produce spindle-shaped heteropolar cells with an anchor (Reymond & Hegewald, 1988). Species of Ankyra dominate in the clear water stages of stagnant waters (Barone & Naselli Flores, 1994). Although Ankyra mainly propagate asexually by zoospores, propagation in the genus needs more detailed investigation. Several types of unidentified aplanospores or resting stages have been observed in Ankyra cultures and field samples (Fott, 1971; Krienitz & Heynig, 1982).
Other clades
The Mychonastes-clade contains tiny, mostly spherical or oval cells of small size that occur as solitary cells or in colonies previously considered as two separate genera, Mychonastes and Pseudodictyosphaerium. Members of these genera belong to the most common pico- or small nanoplankton green algae in fresh or brackish waters. Molecular phylogenetic analyses have shown that both genera are mixed in the same clade (Krienitz et al., 1999, 2011a). Most species were previously described under the generic name of Pseudodictyosphaerium (Hindák, 1978a, b, 1988). However, since the genus Mychonastes was described four month earlier in 1978 (Simpson & Van Valkenburg, 1978), it therefore has nomenclatural priority. Consequently, the species of Pseudodictyosphaerium have been transferred to Mychonastes.
The Bracteacoccus-clade comprises mainly of soil algae. However, Planktosphaeria gelatinosa, which is a freshwater plankton commonly present, has been found to be a close relative of Bracteacoccus and Radiococcus (Wolf et al., 2003b). Unfortunately, this morphotype has been under-represented in recent taxon samplings for molecular considerations and needs further investigation. The edaphic “Mychonastes” zofingiensis does not belong to the true Mychonastes genus, and it is very likely that a new generic designation will be necessary for this taxon (Krienitz et al., 2011a).
The Golenkinia-clade is a member of the Chlorococcales s.str. (Fig. 1). The morphological and ontogenetic peculiarities, especially the CW basal body orientation shown by Hegewald & Schnepf (1984), have been supported by molecular analyses (Wolf et al., 2003a). Members of the polyphyletic genus Chlorococcum mostly occur in soils. However, after a heavy downpour, they can be washed out and transported into water bodies where they are able to propagate as observed in the case of Chlorococcum robustum (Krienitz et al., 1997).
Trebouxiophyceae
Chlorellaceae
This clade contains the classical “green balls.” Following the description of the archetypical form of coccoid green algae, Chlorella vulgaris by Beijerinck (1890), more than 100 species of this genus have been described from freshwater, marine, and soil habitats. However, most of them need to be revised and transferred to other genera and other families. It has been shown that Chlorella-like green spheres evolved independently in different evolutionary lineages of Chlorophyceae, Trebouxiophyceae, and prasinophytes (Friedl, 1997; Chapman et al., 1998; Pröschold & Leliaert, 2007; Darienko et al., 2010). Based on the examination of biochemical and molecular data, Huss et al. (1999) have reduced the Chlorella genus to four species.
In the year 2000, a new development followed the work of Hegewald & Hanagata (2000) who found out that the coenobial ellipsoid D. acuatus, formerly classified in Scenedesmaceae, was closely related to Chlorella kessleri. The needle-shaped C. acicularis, traditionally considered as member of Selenastraceae, was also found to cluster in this lineage (Ustinova et al., 2001). Actinastrum hantzschii, a coenobial taxon formerly in the Coelastraceae, has also been transferred to Chlorellaceae (Wolf et al., 2002b). Krienitz et al. (2004) have identified two separate lineages within Chlorellaceae, designated as a Chlorella-clade and a Parachlorella-clade. Presently, these two clades have both old and new genera (Table 5; Figs. 2, 3).
The Chlorella-clade has eight different lineages that form clusters designated as genera. The scope of this genus has been extended to include 14 species. Among these species are solitary cells with or without mucilage and colonial forms that exhibit a morphology resembling Dictyosphaerium (Bock et al., 2011a). Chlorella-species occur in freshwater, soil and as endosymbionts (Pröschold et al., 2011). Fawley et al. (2005b) found Meyerella, a tiny sphere without pyrenoid, within the Chlorella-clade. According to Luo et al. (2010), the genera Actinastrum, Didymogenes, Hegewaldia and Micractinium also belong to this clade. Hegewaldia comprises taxa with facultative bristle production and oogamy (Pröschold et al., 2010). Micractinium usually occur in colonies and produce bristles. However, the colonies can disintegrate to form single cells without bristles. The formation of bristles can be triggered by substances produced by grazers such as the rotifer Brachionus (Luo et al., 2006). In addition to the several species of Chlorella, the Chlorella-clade has two other lineages with a colonial morphology similar to that of Dictyosphaerium. The two lineages belong to the genera Heynigia and Hindakia (Bock et al., 2010).
Six genera occur in the Parachlorella-clade. The genus Parachlorella has three species that are solitary or colonial, and covered by a mucilaginous envelope (Krienitz et al., 2004; Bock et al., 2011b). According to Krienitz et al. (2010), the Dictyosphaerium-morphotype evolved independently in different lineages of Chlorellaceae. The scope of the genus Dictyosphaerium has been reduced to three species, the type species D. ehrenbergianum (Bock et al., 2011c) and two new taxa. The genus Mucidosphaerium was established based on differences in its molecular phylogeny from that of Dictyosphaerium. Mucidosphaerium contains two former Dictyosphaerium-species, D. pulchellum, and D. sphagnale, as well as two new species. The taxa of the genera Dictyosphaerium and Mucidosphaerium are of great importance as they play an important role of establishing plankton communities. The spindle- to-needle-shaped genera Closteriopsis and Dicloster, as well as the marine, spherical Marinichlorella also belong to the Parachlorella-clade (Aslam et al., 2007).
Oocystaceae
The family of Oocystaceae is a natural lineage in Trebouxiophyceae confirmed by ultrastructural and molecular criteria. Many members of this group are very common in the plankton of stagnant and flowing waters. The extended cell wall is multilayered and constructed from crystalline cellulose fibers. Molecular phylogenetic data have revealed the monophyly of Oocystaceae. However, the species concept in this group has not been confirmed (Hepperle et al., 2000; Pažoutová et al., 2010; Krienitz & Bock, 2011). Because of the reduced taxon availability in strain collections and remarkable uncertainties on the concept of the type genus Oocystis, the circumscription of the genera of Oocystaceae has remained obscure. Hindák (1988) re-applied Lemmermanns (1903) criteria for distinguishing between Oocystis (without pyrenoids) and Oocystella (with pyrenoids). Hence, the taxonomic relevance of pyrenoids in Oocystaceae must be resolved. Several genera are characterized by incrustations (Amphikrikos, Granulocystis, Granulocystopsis, and Siderocelis); however, their origins and taxonomical values are still the subject of ongoing discussions.
Other clades
Choricystis and Botryococcus. Choricystis-species exhibit tiny bean-shaped, solitary cells and were traditionally assigned to the Chlorophyceae family. However molecular data have revealed that they have a close affiliation to the Trebouxiophyceae (Krienitz et al., 1996a, 1999). Surprisingly, the colonial and oil-producing alga Botryococcus braunii clusters close to Choricystis (Senousy et al., 2004). Komárek & Marvan (1992) established a multitude of Botryococcus species based on morphology. However, according to Plain et al. (1993) the morphological features of B. braunii vary depending on growth conditions hence it is not reliable to define more species. The figures provided by Plain et al. (1993) were exclusively those of B. braunii. This study did not include any of the new species described by Komárek & Marvan (1992) such as B. terribilis that has been shown to be very common in freshwater phytoplankton of diverse climatic regions (Krienitz et al., 1996b; Ballot et al., 2009; Fanés Treviño et al., 2009). The conversion of the braunii-morphotype into the terribilis-morphotype and vice versa was never demonstrated. It is therefore, important to as a matter of urgency include more Botryococcus-taxa into molecular studies. From an ecological point of view, the alliance of the picoplanktonic, invasive Choricystis, and the microplanktonic, attuning Botryococcus shown in Fig. 2, is not explainable. Padisák et al. (2009, 2010) grouped Choricystis into the functional group X1 and Botryococcus in group F. Later considerations will probably reveal the existence of some undiscovered lineages that have evolved between these two clades. Henley et al. (2004) documented a close relationship between C. minor and Paradoxia multiseta based on 18S rRNA phylogeny. However, according to the micrograph given (showing globular cells) in the catalogue of the UTEX strain collection, the identity of the Paradoxia strain investigated is doubtful.
Between the Choricystis-clade and the Chlorellaceae, several clades of green coccoid soil and aerophytic algae, such as Chloroidium, Watanabea, Dictyochloris, and the lichen symbionts of Trebouxiales have evolved (Friedl, 1995; Darienko et al., 2010; Rindi et al., 2010). These algae occasionally occur in the plankton of freshwaters. However, if a sufficient starting inoculum of these air- and soil-born algae is available and if the algae are able to propagate under the new conditions, then colonization of standing water bodies can occur rapidly (Happy-Wood, 1988).
Evolution of morphological peculiarities of coccoid green algae and their possible ecological functions
One of the most striking peculiarities of numerous coccoid green algae is the development of a uniform morphology hidden in the picoplankton size group which is nearly unidentifiable by LM. Convergent evolution resulted in a tiny, more or less spherical morphotype which covers an extremely high diversity with regard to phylogeny and physiology (Potter et al., 1997; Hepperle & Krienitz, 2001; Krienitz et al. 2011a). Based on its fast growth and high rates of reproduction and primary production, picophytoplankton can play a key role in food webs of freshwater, marine, and saline habitats (Stockner & Antia, 1986; Raven, 1999). Picoplankton may establish large populations under nearly all levels of trophy (Stockner, 1991; Weisse, 1993; Padisák et al., 1997; Hehmann et al., 2001; Hepperle & Krienitz, 2001). Even under very saline conditions they can dominate all the succession stages of eukaryotic primary producers (Henley et al., 2004; Somogyi et al., 2011; Krienitz et al., 2012).
Picoplanktons evolved as adaptive strategies in all the classes considered here. In the Chlorophyceae, the Mychonastes-clade contains several species of picoplankton (Krienitz et al., 2011a). In the Trebouxiophyceae, a number of lineages, which include Picochloron and Chloroparva from salt pans (Henley et al., 2004; Somogyi et al., 2011), Choricystis, and Nannochloris from freshwaters (Krienitz et al., 1996a; Yamamoto et al., 2003), have picoplankton species. Marvania is a picoplankton species from different inland waters characterized by vegetative propagation through budding (Hindák, 1976; Reymond et al., 1986). Among the prasinophytes, there are many picoplankton lineages which are usually abundant in the oceans (Guillou et al., 2004; Leliaert et al. 2012). Extreme saline inland waters have one lineage with Picocystis (Lewin et al., 2000; Hollibaugh et al., 2001; Roesler et al., 2002). Picocystis salinarum from saline inland waters represents a link between picoplankton from marine and freshwater habitats and is therefore of great ecological and phylogenetic interest (Krienitz & Kotut, 2010; Krienitz et al., 2012).
Comparison of the fatty acid's contents of green (Choricystis, Mychonastes) and eustigmatophycean (Nannochloropsis) freshwater picoplankton revealed surprising differences in the composition of polyunsaturated fatty acids. The sums of n-6 and n-3 fatty acids are ten times higher in Nannochloropsis limnetica than in the green picoplankton (Krienitz et al., 2000; Krienitz & Wirth, 2006). Hence, the eustigmatophycean picoplankton has a higher nutritional value for grazers than green algal picoplankton. This raises the question on how the grazers can differentiate between the nutritious eustigmatophycean and the less nutritious green algae. As a follow-up to Hartmann & Kunkel’s (1991) statement “The paradigm of invariate, nonselective feeding by zooplankton is rejected,” limnologists have come up with possible mechanism of food selection that include: chemosensory, electrical charge, surface hydrophobicity, and chemical cues (Weisse, 2004). All these interactions between green algae and possible grazers need to be further elucidated.
Incrustations on the surface of coccoid green algae are common across the whole range of systematic groups of green algae. For example, they have been observed in Selenastraceae (Raphidocelis), Scenedesmaceae (Scenedesmus), Oocystaceae (Amphikrikos, Siderocelis), and Radiococcaceae (Coenochloris) (Hindák, 1977; Crawford, 1978; Krienitz, 1986; Vanormelingen et al., 2007). These incrustations are precipitates of ferric and manganic hydroxides and are of crystalline or amorphous nature (Crawford & Heap, 1978). The genesis and structure of this cell wall deposits varies with taxon suggesting a genetic influence and give the impression that this process is under the control of the cell (Crawford & Heap, 1978). So far, no vesicle driven expression through pores from inner to outer cell wall has been observed. The ecological function of these incrustations remains a subject of discussion. From our field observation, it was apparent that numerous algae tend to develop incrustations under highly disturbed conditions in rivers (Krienitz, 1990, 1998). It can therefore be hypothesized that the incrustations increase the weight of the cells, and this allows the cells to sink to the lower less disturbed habitats, which are conducive for propagation.
Spines and bristles of the plankton algae are adaptive features that promote buoyancy and reduce grazing pressure (Van den Hoek et al., 1995). A large number of publications have documented the interaction of kairomon-producing grazers and spine- and bristle-formations by coccoid green algae (summarized by Van Donk, 2005). Spines and bristles have different origins and compositions. Scenedesmaceae produce rigid, tube-like spines as elaborated parts of the outer sporopollenin-like cell wall layer (Schnepf et al., 1980). In contrast to spines, bristles develop after cell wall formation and lack cellulosic fibers and algaenan substances (Schnepf et al., 1980; Hegewald & Schnepf, 1984). Didymogenes and Micractinium produce bristles of the same type (Schnepf & Hegewald, 1993). The spines of Golenkinia contain cellulosic fibers and are produced after cell wall formation (Hegewald & Schnepf, 1984). The morphological and biochemical differentiation of spines and bristles in the various groups of coccoid green algae closely agree with their differences based on molecular data. The spine producing Scenedesmaceae belong to the Chlorophyceae (Hegewald, 1997). The close relationship between Didymogenes and Micractinium as members of Trebouxiophyceae was demonstrated by Luo et al. (2006). The polyphyletic origin of bristles within the Trebouxiophyceae was confirmed by Pröschold et al. (2010). The separate position of Golenkinia as a member of Chlorococcales s.l. was revealed by Wolf et al. (2003a).
The production of mucilage by the cells is one of the most readily observed phenomena in coccoid green algae. It is commonly observed in all groups under discussion and has a polyphyletic origin. The production of mucilage largely depends on environmental influences and interaction with other species such as grazing pressure and resource competition (Reynolds, 2007). It may protect the algae from ingestion or, even if ingested, against digestion while passing through the intestinal tract of zooplankton (Porter, 1973). On the other side, the mucilaginous envelope can act as microhabitat for bacterial flora that produces substances with a nutritional value or have stimulatory effects (Cole, 1982). The mucilage can also act as a depository of nutrients (Decho, 1990). Furthermore, mucilage affects the buoyancy of the phytoplankton (Boney, 1981). The multitude of ecological functions makes it understandable that morphotypes characterized by mucilage evolved in different lineages and at different times as a response to the diverse interactions. This observation has clearly been demonstrated in the Dictyosphaerium-morphotype (Bock et al., 2010, 2011c; Krienitz et al., 2010). Even in one and the same genus such as Chlorella or Mychonastes mucilage possession has appeared and disappeared several times (Bock et al., 2011a; Krienitz et al., 2011a). Hence, the question on which of the contrasting features, “with mucilage” or “without mucilage,” is more ancestral remains open.
A very complicated case is the elucidation of the origin of the radiococcacean morphotype. Kostikov et al. (2002) revised this “family” based on a detailed examination of morphology and ontogeny. However, a few molecular phylogenetic studies have indicated a polyphyletic evolution of members of this family (Wolf et al., 2003b; Bock et al., 2011b). The study of this group has been hampered by scarcity of cultures, because of their poor growth performance in culture. Future studies subjecting single colonies to PCR can help to shed some light on the phylogeny of this “slimy colonial green spheres.”
Coccoid green algae represent the most diverse group among plankton algae. It is one of the groups well suitable for cultivation and therefore provides a wide range of experimental opportunities to study the genesis and ecological advantages of these organisms. Based on autecological features and functional characteristics, Reynolds et al. (2002) and Padisák et al. (2009) have placed most of the coccoid green algae in codon X1 (shallow mixed layers in enriched conditions), and some of them (Botryococcus, Dictyosphaerium, and Oocystis) in codon F (clear epilimnia), and others (Coelastrum, Pediastrum, and Scenedesmus) in codon J (shallow enriched lakes, ponds, and rivers). Recent observations on species characterisics that include drastic changes in size through colony disintegration, and the periodic appearance and disappearance of features, such as mucilage, incrustations, and spines depending on interactions with abiotic and biotic environments suggest that a species can possibly deviate from this ecofunctional classification.
The phylogenetic species concept
The debate on the “right” species concept is certainly old and ongoing. In the post-Darwinian time, numerous concepts were proposed with different criteria for species delineation (e.g., Mayr, 1942; Henning, 1966; de Queiroz, 1998). As the biological (or reproductive) species concept of reproductive isolation (Mayr, 1942) is not applicable to asexually reproducing taxa (which is the case for many protists lineages, and especially the coccoid green algae), and the morphological species concept is very subjective, the phylogenetic species concept (or diagnostic concept sensu Mallet (2006)) has gained a lot of ground (Cracraft, 1989). This concept is based on genetic markers and recognizes the smallest monophyletic clusters of taxa worthy of taxonomic recognition as individual species. The populations/strains must share the same ancestor and all its descendants to be considered as individual species (Johansen & Casamatta, 2005; Mallet, 2006). This concept allows the delimitation of species by specific data like base changes in gene sequences. Hence, the number of recognized species largely depends on the chosen marker; the more conserved the analyzed region, the fewer the number of species recognized and vice versa (Hoef-Emden, 2007; Rindi et al., 2009). The same applies to the chosen threshold of genetic divergence between sequences. A higher threshold for the congruence of sequences (e.g., 98% congruence) results in more species recognized than a lower threshold (e.g., 94%). The small subunit (SSU) of the ribosomal rRNA (18S) gene sequence is conventionally used as marker in phylogenetic studies (Chapman et al., 1998; Huss et al., 1999). The 18S rRNA is a universal gene and plays a major role in protein translation. It contains highly conserved regions which favors the development of universal primers and simplifies sequence alignment of distant taxa (Long & David, 1980; Sogin et al., 1986). As it is present in numerous copies within the genome, it is easy to amplify during a PCR. These criteria makes the 18S rDNA a useful tool for the phylogenetic resolution of higher algal ranks such as classes and orders (Friedl, 1995; Krienitz et al., 2003, 2011a). Exhaustive studies combining molecular and morphological data have shown that the 18S is too conserved to separate closely related species in coccoid green algal lineages that are clearly distinguishable by morphological and/or ecological factors (e.g., Krienitz et al., 2004; Bock et al., 2010; Darienko et al., 2010).
New insights and methodological opportunities in the molecular techniques have shifted the focus to the internal transcribed spacer 2 (ITS2). The ITS2 is a fast evolving marker region, situated between the 5.8S and the 28S rRNA on the ribosomal gene. To obtain a mature rRNA, the ITS2 needs to be excised during the maturing stage so that it folds up into a characteristic secondary structure. The primary sequence of the ITS2 is highly variable, even between closely related taxa. However, the ITS2 secondary structure motive seems to be extremely conserved in all eukaryotes (Mai & Coleman, 1997; Coleman, 2003, 2007; Schultz et al., 2005). Indeed the ITS2 folds normally in four helices, with helix III being the longest and the most conserved (Coleman, 2007). Due to this specific folding pattern, it is possible to align closely related sequences unambiguously.
The Compensatory Base pair Changes (CBC) concept refers to base changes within the secondary structure where a matching pair of bases in a double stranded section of the structure in one taxon is exchanged by a different matching pair in a second taxon (Gutell, 1994). Mating experiments in sexual protist lineages in combination with CBCs within the ITS2 have pushed the discussion toward concepts merging Mayr’s biological species concept with molecular data (Coleman, 2000; Denboh et al., 2003; Behnke et al., 2004; Hoef-Emden, 2007). In protist lineages with at least occasional sexual reproduction, the occurrence of CBCs in conserved regions of the ITS coincides with the sexual incompatibility between species (Mai & Coleman, 1997; Müller et al., 2007; Coleman, 2009). As a consequence, the presence of CBCs or hemi-CBCs (only one-sided base changes) is often used for species delineation in morphological difficult groups or when only asexual reproduction is known (Krienitz et al., 2004; Hoef-Emden, 2007). Additionally, the CBC concept includes the step of calculating the secondary structure. Software for secondary structure prediction can help to facilitate the procedure but are not reliable if the entire ITS2 sequence is directly submitted to a RNA folding server. The sequences have to be submitted in small pieces which correspond to the different expected helices to get consistent results (Zuker, 2003; Hoef-Emden, 2007; Schultz & Wolf, 2009).
Apart from the nuclear regions (18S, ITS, 28S), a variety of markers have been used for species delineation. Among these markers is the chloroplast encoded large subunit of the ribulose-bisphosphate carboxylase (rbcL) gene. This protein coding region results in a straightforward alignment and tends to be more variable than the 18S but not as variable as the ITS regions. Another advantage of this marker over the nuclear markers is that since it is a plastid gene, the risk of amplifying contaminants like fungi is reduced. As a protein-coding gene, the rbcL sequence can be partitioned into codons with first, the second, and the third base positions. The third base (wobble) position has a higher evolution rate than the first or the second base positions because of its substitutional saturation (Rindi et al., 2009). However, the usually applied rbcL sequence has been criticized because it is not variable enough for the different substitution rates for the wobble position may cause problems within the phylogeny. Several other markers have been tested on closely related algae in an effort to separate species but none has proved to be exhaustive for a large number of groups. Examples are the trnGucc intron sequences for Desmidiales (Neustupa et al., 2010; Nemjova et al., 2011), actin I locus sequences for Asterochloris (Škaloud & Peksa, 2010); the psbA/rbcL spacer in combination with the rbcL gene for the Tribonemataceae (Rybalka et al., 2009) and many more.
The recent discussion proposing the use of a “barcode” to facilitate the identification of taxa has resulted in the recognition of the need to identify a specific DNA region for species delineation again (Hebert et al., 2003). The ideal barcode should be a short sequence, easy to amplify by universal primers and with the power to resolve organisms at species level (e.g., Hebert et al., 2003; Zimmermann et al., 2011). Under this context, various barcodes have been proposed for different organisms. For animals, part of the mitochondrial cytochrome oxidase c subunit I gene (COI, cox1) has been proposed and is now widely used (Hajibabaei et al., 2007). Evans et al. (2007) have proposed the cox1 as barcode for diatoms. The V4 subregion on the 18S rRNA gene was proposed by Zimmermann et al. (2011) for diatoms. Moniz & Kaczmarska (2010) have suggested use of the 5.8S and part of the ITS2 for diatoms, and the same was also successfully tested on Chlorella-related strains (Bock et al., 2011a). The ITS2 is favored by many scientists because of its variability combined with the conserved folding pattern (Buchheim et al., 2011). As the ribosomal operon may contain several versions of ITS2, indels of numerous nucleotides may be present in the less-conserved parts of the different ITS2 versions, which prevents direct sequencing and requires cloning or the use of specific primers (Pröschold et al., 2005). Hence, the establishment of a barcode conception for algae is of great practical importance. Unambiguous designation of species is essential for water quality studies. Barcodes can be used for the identification of standard organisms because morphological concepts often fail to provide accurate identification of algae (Zimmermann et al., 2011). It is especially important to identify barcodes for indicator species similar to the functional group conception established by Reynolds et al. (2002). There are many other indices in different parts of the world. However, it is important that whichever index is used, it should give a correct species identification.
The role of the field workers and experts of phenotypic and ecological characterization of algae
In the assessment of the quality of inland waters, phytoplankton community structure provides a useful indicator tool (Salmaso et al., 2006; Pearl et al., 2007). This is due to the following important attributes:
-
being the main pelagic primary producers the phytoplankton play a key role in the functioning of standing water bodies,
-
as a consequence of its high reproduction rates, phytoplankton gives a rapid response to changes in environmental conditions,
-
phytoplankton communities are generally more diverse than other eukaryotic populations within aquatic food webs,
-
species composition of the phytoplankton community, i.e., its biodiversity, has a critical influence on many kinds of water-utilization by man.
Hence phytoplanktologists worldwide contribute to the establishment of water quality assessment methods. In Europe, the Water Framework Directive is the key approach to the evaluation and protection of the inland surface waters (Padisák et al., 2006; Anneville et al., 2008). To support the practical field work, harmonized taxon lists have been established. For the phytoplankton, such a list was developed by Mischke (2006) and Mischke & Nixdorf (2008). About 300 taxa of coccoid green algae are included in this list. However, the list is under continuous improvement, and there are initiatives to assist the field-workers with information systems on taxonomy of algae such as AlgaTerra (Jahn & Kusber, 2006), and AlgaeBase (Guiry & Guiry, 2011). The value of ecological data is to a large extent dependent on the correct identification of organisms as any incorrectly identified samples cannot be improved by any statistical treatment or other sophisticated methods (Kotut & Krienitz, 2011). In this paper (Table 6), we have included 84 coccoid green algae from the harmonized taxon list, which have already been subjected to molecular phylogenetic examinations, and provided their old and new taxonomic designations. It has become evident that many taxa are still missing, and joint research activities are necessary to facilitate the acquisition of more cultures from field samples that can be included in the morphological, ecophysiological, and molecular analyses.
There is no doubt that there are difficulties in the microscopic identification of many species of coccoid green algae. Different phylogenetic species can be hidden under one and the same morphotype (Potter et al., 1997; Šlapeta et al., 2005). On the other hand, one and the same genotype can exhibit different morphological peculiarities during its ontogeny or as result of interactions with the environment (Lürling & Beekman, 1999; Verschoor et al., 2004). Currently, molecular biologists are screening the contents of public strain collections (Day et al., 2004). However, these collections are not representative of the diversity of taxa in the field. Similarly, the designations of many taxa in culture collections are doubtful (Hegewald, 1989). Many algae are difficult to maintain in cultures and are not available for the sequencing work.
In the present situation, workers of both the types, the traditional phenotypic ones and those with a modern genotypic approach, should come together and forge interdisciplinary collaborations. Field limnologists have the privilege of working directly in ecosystems where evolution takes place. Organisms in their natural habitats are constantly interacting with each other and with their environment, while the natural selection process is acting on them. It is therefore so difficult to interpret the organismic structure of the ecosystem using the old phenotypic taxonomic units created by man using an artificial system. They do not adequately reflect how nature works. For ecologists, the genetic diversity within taxa is of major interest because of possible genetic shifts in populations as an adaptive response to a changing environment (Lynch et al., 1991; Wood & Leatham, 1992). Discussions on species concepts should in addition to the phenotype take into consideration intraspecific genetic variation. For species with well-studied phenotypic population characteristics from field observations, the description should be completed by genetic characteristics, especially for those cultured strains that conform to the original type description (Wood & Leatham, 1992). Limnologist should therefore support the efforts of molecular biologists to circumscribe phylogenetic species. Alongside this, large-scale ecophysiological experiments focusing on autecological features, physiological, and biochemical characteristics should be correlated to morphological variability of cultures of these phylospecies. Limnologists can also support an extensive taxon sampling. Through this exercise, they can advise on interesting algae, which need taxonomic revision and provide fresh samples, and isolate interesting strains for the public strain collections. On the other hand, molecular biologists have the basic tools for supporting the natural system of organisms. Their work should include developing methods for evaluating species composition in field (environmental) samples and comparing them with microscopic findings (Fawley et al., 2004; Richards et al., 2005; Medinger et al., 2010; Luo et al., 2011). A good example is the multiphased study of freshwater samples from México by Tavera & Diéz (2009).
In the near future, molecular tools may be used to identify individual filaments, colonies or cells picked from samples. It may also be possible to identify individuals directly within the samples. Promising results have already been published on cyanobacteria (Hayes et al., 2002), large sulfur bacteria (Salman et al., 2011), dinoflagellates (Ki & Han, 2005) and chrysophytes (Jost et al., 2010). Once the tools are fully developed, specialists with a good knowledge on morphospecies will have a good opportunity to align these individual phenotypes with the molecular findings (Auinger et al., 2008). However, presently we have to content with the use of two different and parallel systems. Although field biologists have no other option than to apply the traditional approach, they should be careful to provide very detailed documentation of their findings. The handbook by Komárek & Fott (1983) and the five volumes of Hindák’s studies (1977, 1980, 1984, 1988, 1990) can be of great help in the determination of morphospecies of coccoid green algae. In the near future, the establishment of molecular signatures, the barcoding conception, will allow an unambiguous designation of organisms. The alignment of eco-functional groups of algae with true species identities using the barcoding conception will provide a better understanding of the interaction between organisms and their environment. The reasons why and how an individual species adapts to different environmental conditions by producing spines, mucilage or incrustations, or losing them, establishing colonies or disintegrating them, exhibiting striking colonial appearances or returning to the simple green ball habit as a survival tactic will become evident.
References
An, S. S., T. Friedl & E. Hegewald, 1999. Phylogenetic relationships of Scenedesmus and Scenedesmus-like coccoid green algae as inferred from ITS-2 rDNA sequence comparisons. Plant Biology 1: 418–428.
Anneville, O., C. Kaiblinger, R. D. Tadonléké, J.-C. Druart & M. T. Dokulil, 2008. Contribution of long-term monitoring to the European Water Framework Directive implementation. In Sengupta, M. & R. Dalwani (eds), Proceedings of Taal2007: The 12th World Lake Conference, Jaipur: 1122–1131.
Aslam, Z., W. Shin, M. K. Kim, W.-T. Im & S.-T. Lee, 2007. Marinichlorella kaistiae gen. et sp. nov. (Trebouxiophyceae, Chlorophyta) based on polyphasic taxonomy. Journal of Phycology 43: 576–584.
Auinger, B. M., K. Pfandl & J. Boenigk, 2008. An improved methodology for the identification of protists and microalgae from plankton samples preserved in Lugol’s iodine solution: combining microscopic analysis with single cell PCR. Applied Environmental Microbiology 74: 2505–2510.
Ballot, A., K. Kotut, E. Novelo & L. Krienitz, 2009. Changes of phytoplankton communities in Lakes Naivasha and Oloidien, examples of degradation and salinization of lakes in the Kenyan Rift Valley. Hydrobiologia 632: 359–363.
Barone, R. & L. Naselli Flores, 1994. Phytoplankton dynamics in a shallow, hypertrophic reservoir (Lake Arancio, Sicily). Hydrobiologia 289: 199–214.
Behnke, A., T. Friedl, V. A. Chepurnov & D. G. Mann, 2004. Reproductive compatibility and rDNA sequence analyses in the Sellaphora pupula species complex (Bacillariophyta). Journal of Phycology 40: 193–208.
Beijerinck, M. W., 1890. Culturversuche mit Zoochlorellen, Lichenengoniden und anderen niederen Algen I-III. Botanische Zeitung 48: 726–740.
Bock, C., T. Pröschold & L. Krienitz, 2010. Two new Dictyosphaerium-morphotype lineages of the Chlorellaceae (Trebouxiophyceae): Heynigia gen. nov. and Hindakia gen. nov. European Journal of Phycology 45: 267–277.
Bock, C., L. Krienitz & T. Pröschold, 2011a. Taxonomic reassessment of the genus Chlorella (Trebouxiophyceae) using molecular signatures (barcodes), including description of seven new species. Fottea 11: 293–312.
Bock, C., M. Pažoutová & L. Krienitz, 2011b. Phylogenetic relationship of Coronastrum ellipsoideum and description of Parachlorella hussii sp. nov. Biologia 66: 585–594.
Bock, C., T. Pröschold & L. Krienitz, 2011c. Updating the genus Dictyosphaerium and description of Mucidosphaera gen. nov. (Trebouxiophyceae) based on morphological and molecular data. Journal of Phycology 47: 638–652.
Boney, A. D., 1981. Mucilage: the ubiquitous algal attribute. British Phycological Journal 16: 115–132.
Booton, G. C., G. L. Floyd & P. A. Fuerst, 1998. Polyphyly of tetrasporalean green algae inferred from nuclear small-subunit ribosomal DNA. Journal of Phycology 34: 306–311.
Bremer, K., C. J. Humphries, B. D. Mishler & S. P. Churchill, 1987. On cladistic relationships in green plants. Taxon 36: 339–349.
Buchheim, M. A., E. A. Michalopulos & J. A. Buchheim, 2001. Phylogeny of the Chlorophyceae with special reference to the Sphaeropleales: a study of 18S and 26S rDNA data. Journal of Phycology 37: 819–835.
Buchheim, M. A., J. Buchheim, T. Carlson, A. Braband, D. Hepperle, L. Krienitz, M. Wolf & E. Hegewald, 2005. Phylogeny of the Hydrodictyaceae (Chlorophyceae): inferences from rDNA data. Journal of Phycology 41: 1039–1054.
Buchheim, M. A., A. Keller, C. Koetschan, F. Förster, B. Merget & M. Wolf, 2011. Internal Transcribed Spacer 2 (nu ITS2 rRNA) sequence-structure phylogenetics: towards an automated reconstruction of the green algal tree of life. PLoS ONE 6: e16931.
Cavalier-Smith, T., 1993. The origin, losses and gains of chloroplasts. In Lewin, R. A. (ed.), Origin of Plastids: Symbiogenesis, Prochlorophytes and the Origins of Chloroplasts. Chapman and Hall, New York: 291–348.
Cepák, V., 1993. Scenedesmus obliquus zoospores in outdoor mass culture – reality or artefact? Algological Studies 71: 37–42.
Chapman, R. L., M. A. Buchheim, C. F. Delwiche, T. Friedl, V. A. R. Huss, K. G. Karol, L. A. Lewis, J. Manhart, R. M. McCourt, J. L. Olsen & D. A. Waters, 1998. Molecular systematics of the green algae. In Soltis, D. E., P. S. Soltis & J. J. Doyle (eds), Molecular Systematics of Plants II. DNA Sequencing. Kluwer Academic Publishers, Boston, Dordrecht, London: 508–540.
Cole, J. J., 1982. Interactions between bacteria and algae in aquatic ecosystems. Annual Review of Ecology Evolution and Systematics 13: 291–314.
Coleman, A. W., 2000. The significance of a coincidence between evolutionary landmarks found in mating affinity and a DNA sequence. Protist 151: 1–9.
Coleman, A. W., 2003. ITS2 is a double-edged tool for eukaryote evolutionary comparisons. Trends in Genetics 19: 370–375.
Coleman, A. W., 2007. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Research 35(10): 3322–3329.
Coleman, A. W., 2009. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Molecular Phylogenetics and Evolution 50: 197–203.
Cracraft, J., 1989. Speciation and its ontology: the empirical consequences of alternative species concepts for understanding patterns and processes of differentiation. In Otte, D. & J. Endler (eds), Speciation and Its Consequences. Sinauer Associates, Sunderland, MA: 28–59.
Crawford, R. M., 1978. A new forma and a new species of the genera Scenedesmus and Siderocelis (Chlorophyceae) from the nannoplankton of a brackish-water coastal pool. Protoplasma 96: 351–360.
Crawford, R. M. & P. F. Heap, 1978. Transmission electron microscopy X-ray microanalysis of two algae of the genera Scenedesmus and Siderocelis. Protoplasma 96: 361–367.
Darienko, T., L. Gustavs, O. Mudimu, C. Rad Menendez, R. Schumann, U. Karsten, T. Friedl & T. Pröschold, 2010. Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta). European Journal of Phycology 45: 79–95.
Day, J. G., J. Lukavský, M. Lorenz, T. Friedl, J. Elster, C. N. Cambell & J. J. Brand, 2004. Pringsheim’s living legacy: CAUP, CCALA, CCAP, SAG and UTEX culture collections of algae. Nova Hedwigia 79: 27–37.
de Queiroz, K., 1998. The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. In Howard, D. J. & S. H. Berlocher (eds), Endless Forms: Species and Speciation. Oxford University Press, Oxford, England: 57–75.
Decho, A. W., 1990. Microbial exopolymer secretions in ocean environments – their role(s) in food webs and marine processes. Oceanography and Marine Biology 28: 73–153.
Denboh, T., T. Ichimura, D. Hendrayanti & A. W. Coleman, 2003. Closterium moniliferum-ehrenbergii (Charophyceae; Chorophyta) Specie complex viewed from 1506 group I intron and nuclear rDNA. Journal of Phycology 39: 960–977.
Eliáš, M., Y. Němková, P. Škaloud, J. Neustupa, V. Kaufnerová & L. Šejnohová, 2010. Hylodesmus singaporensis gen. et sp. nov., a new autosporic subaerial green alga (Scenedesmaceae, Chlorophyta) from Singapore. International Journal of Systematic and Evolutionary Microbiology 60: 1224–1235.
Eloranta, V., 1979. The compound internal pyrenoid in cultured cells of the green alga Monoraphidium griffithii (Berkel.) Komár.-Legner. Protoplasma 99: 229–235.
Ettl, H. & G. Gärtner, 1995. Syllabus der Boden-, Luft- und Flechtenalgen. Gustav Fischer, Stuttgart.
Evans, K. M., A. H. Wortley & D. G. Mann, 2007. An assessment of potential diatom “barcode” genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta). Protist 158: 349–364.
Fanés Treviño, I., P. Sánchez-Castillo & A. Comas González, 2009. Contribution to the taxonomic study of the family Botryococcaceae (Trebouxiophyceae, Chlorophyta) in southern Spain. Cryptogamie, Algologie 30: 17–30.
Fawley, M. W., Y. Yun & M. Qin, 2000. Phylogenetic analyses of 18S rDNA sequences reveal a new coccoid lineage of the Prasinophyceae (Chlorophyta). Journal of Phycology 36: 387–393.
Fawley, M. W., K. P. Fawley & M. A. Buchheim, 2004. Molecular diversity among communities of freshwater microchlorophytes. Microbial Ecology 48: 489–499.
Fawley, M. W., M. L. Dean, S. K. Dimmer & K. P. Fawley, 2005a. Evaluating the morphospecies concept in the Selenastraceae (Chlorophyceae, Chlorophyta). Journal of Phycology 42: 142–154.
Fawley, M. W., K. P. Fawley & H. A. Owen, 2005b. Diversity and ecology of small coccoid green algae from Lake Itasca, Minnesota, USA, including Meyerella planktonica, gen. et sp. nov. Phycologia 44: 35–48.
Fott, B., 1971. Algenkunde. VEB Gustav Fischer Verlag, Jena: 581 pp.
Friedl, T., 1995. Inferring taxonomic positions and testing genus level assignments in coccoid green lichen algae: a phylogenetic analysis of 18S ribosomal RNA sequences from Dictyochloropsis reticulata and from members of the genus Myrmecia (Chlorophyta, Trebouxiophyceae cl. nov.). Journal of Phycology 31: 630–637.
Friedl, T., 1996. Systematik und Phylogenie terrestrischer Grünalgen unter besonderer Berücksichtigung von Flechtenalgen: molekulare und morphologische Merkmalsanalysen. University of Bayreuth, Habilitation: 122 pp.
Friedl, T., 1997. The evolution of the green algae. Plant Systematics and Evolution 11: 87–101.
Guillou, L., W. Eikrem, M.-J. Chrétiennot-Dinet, F. Le Gall, R. Massana, K. Romari, C. Pedrós-Alió & D. Vaulot, 2004. Diversity of picoplanktonic prasinophytes assessed by direct nuclear SSU rDNA sequencing of environmental samples and novel isolates retrieved from oceanic and coastal marine ecosystems. Protist 155: 193–214.
Guiry, M. D. & G. M. Guiry, 2011. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway [available on internet at http://www.algaebase.org]; searched on 28 June 2011.
Gutell, R. R., 1994. Collection of small-subunit (16S- and 16S-like) ribosomal-RNA structures. Nucleic Acids Research 22: 3502–3507.
Hajibabaei, M., G. A. C. Singer, P. D. N. Hebert & D. A. Hickey, 2007. DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends in Genetics 23: 167–172.
Happy-Wood, C. M., 1988. Ecology of freshwater planktonic green algae. In Sandgren, C. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 175–226.
Hartmann, H. J. & D. D. Kunkel, 1991. Mechanisms of food selection in Daphnia. Hydrobiologia 225: 129–154.
Hayes, P. K., G. L. A. Barker, J. Batley, S. J. Beard, B. A. Handley, P. Vacharapiyasophon & A. E. Walsby, 2002. Genetic diversity within populations of cyanobacteria assessed by analysis of single filaments. Antonie van Leeuwenhoek 81: 197–202.
Hebert, P. D. N., A. Cywinska, S. L. Ball & J. R. DeWaard, 2003. Biological identifications through DNA barcodes. Proceedings of the Royal Society of London. Series B: Biological Sciences 270: 313–321.
Hegewald, E., 1989. The Scenedesmus strains of the culture collection at Austin, Texas (UTEX). Archiv für Hydrobiologie, Supplement 82, Algological Studies 55: 153–189.
Hegewald, E., 1997. Taxonomy and phylogeny of Scenedesmus. Algae (The Korean Journal of Phycology) 12: 235–246.
Hegewald, E., 2000. New combinations in the genus Desmodesmus (Chlorophyceae, Scenedesmaceae). Algological Studies 96: 1–18.
Hegewald, E. & N. Hanagata, 2000. Phylogenetic studies on Scenedesmaceae (Chlorophyta). Archiv für Hydrobiologie, Supplement 136, Algological Studies 100: 29–49.
Hegewald, E. & E. Schnepf, 1984. Zur Struktur und Taxonomie bestachelter Chlorococcales (Micractiniaceae, Golenkiniaceae, Siderocystopsis). Nova Hedwigia 39: 297–383.
Hegewald, E. & E. Schnepf, 1986. Zur Struktur und Taxonomie spindelförmiger Chlorellales (Chlorophyta): Schroederia, Pseudoschroederia gen. nov., Closteriopsis. Archiv für Hydrobiologie, Supplement 73, 1, Algological Studies 42: 21–48.
Hegewald, E. & P. C. Silva, 1988. Annotated Catalogue of Scenedesmus and nomenclaturally related genera, including original descriptions and figures. Bibliotheca Phycologica 80: 1–587.
Hegewald, E. & M. Wolf, 2003. Phylogenetic relationships of Scenedesmus and Acutodesmus (Chlorophyta, Chlorophyceae) as inferred from 18S rDNA and ITS-2 sequence comparisons. Plant Systematics and Evolution 241: 185–191.
Hegewald, E., D. Hepperle, M. Wolf & L. Krienitz, 2001. Phylogenetic placement of Chlorotetraedron incus, C. polymorphum and Polyedriopsis spinulosa (Neochloridaceae, Chlorophyceae). Phycologia 40: 399–402.
Hegewald, E., P. F. M. Coesel & P. Hegewald, 2002. A phytoplankton collection from Bali, with the description of a new Desmodesmus species (Chlorophyta, Scenedesmaceae). Algological Studies 105: 51–78.
Hegewald, E., A. Schmidt, A. Braband & P. Tsarenko, 2005. Revision of the Desmodesmus (Sphaeropleales, Scenedesmaceae) species with lateral spines. 2. The multi-spined to spineless taxa. Archiv für Hydrobiologie, Supplement 157, Algological Studies 116: 1–38.
Hegewald, E., M. Wolf, A. Keller, T. Friedl & L. Krienitz, 2010. ITS2 sequence-structure phylogeny in the Scenedesmaceae with special reference to Coelastrum (Chlorophyta, Chlorophyceae), including the new genera Comasiella and Pectinodesmus. Phycologia 49: 325–335.
Hehmann, A., L. Krienitz & R. Koschel, 2001. Long-term phytoplankton changes in an artificially divided top-down manipulated humic lake. Hydrobiologia 448: 83–96.
Henley, W. J., J. L. Hironaka, L. Guillou, M. A. Buchheim, J. A. Buchheim, M. W. Fawley & K. P. Fawley, 2004. Phylogenetic analysis of the ‘Nannochloris-like’ algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta). Phycologia 43: 641–652.
Henning, W., 1966. Phylogenetic Systematics. University of Illinois Press, Urbana.
Hepperle, D. & L. Krienitz, 2001. Systematics and ecology of chlorophyte picoplankton in German inland waters along a nutrient gradient. International Revue of Hydrobiology 86: 269–284.
Hepperle, D., E. Hegewald & L. Krienitz, 2000. Phylogenetic position of the Oocystaceae (Chlorophyta). Journal of Phycology 36: 590–595.
Heynig, H., 1980. Interessante Phytoplankter aus Gewässern des Bezirks Halle (DDR). III. Archiv für Protistenkunde 123: 349–357.
Heynig, H. & L. Krienitz, 1982. Monoraphidium neglectum n.sp. sowie einige Bemerkungen zu den Gattungen Monoraphidium, Chlorolobion und Keratoccus (Chlorococclaes). Archiv für Protistenkunde 125: 335–344.
Hindák, F., 1976. Marvania geminata gen. nov. et sp. nov., a new green alga. Algological Studies 16: 261–270.
Hindák, F., 1977. Studies on the chlorococcal algae (Chlorophyceae). I. Biologické Práce 28(4): 1–263.
Hindák, F., 1978a. New taxa and reclassifications in the Chlorococcales (Chlorophyceae). Preslia 50: 97–109.
Hindák, F., 1978b. The genus Lagerheimia Chod. and Lagerheimia-like unicells in the genus Scenedesmus Meyen (Chlorophyceae). Biológia 33: 795–808.
Hindák, F., 1980. Studies on the chlorococcal algae (Chlorophyceae). II. Biologické Práce 26(6): 1–195.
Hindák, F., 1984. Studies on the chlorococcal algae (Chlorophyceae). III. Biologické Práce 30(1): 1–308.
Hindák, F., 1988. Studies on the chlorococcal algae (Chlorophyceae). IV. Biologické Práce 34: 1–263.
Hindák, F., 1990. Studies on the chlorococcal algae (Chlorophyceae). V. Biologické Práce 36: 1–225.
Hoef-Emden, K., 2007. Revision of the genus Cryptomonas (Cryptophyceae) II: incongruences between the classical morphospecies concept and molecular phylogeny in smaller pyrenoid-less cells. Phycologia 46: 402–428.
Hoffman, L. R., 1983. Atractomorpha echinata gen. et sp. nov., a new anisogamous member of the Sphaeropleaceae (Chlorophyceae). Journal of Phycology 19: 76–86.
Hollibaugh, J. T., P. S. Wong, N. Bano, S. K. Pak, E. M. Prager & C. Orrego, 2001. Stratification of microbial assemblages in Mono Lake, California, and response to a mixing event. Hydrobiologia 466: 45–60.
Huss, V. A. R., C. Frank, E. C. Hartmann, M. Hirmer, A. Kloboucek, B. M. Seidel, P. Wenzeler & E. Kessler, 1999. Biochemical taxonomy and molecular phylogeny of the genus Chlorella sensu lato (Chlorophyta). Journal of Phycology 35: 587–598.
Jahn, R. & W. H. Kusber (eds), 2006. AlgaTerra Information System (online). Botanic Garden and Botanical Museum Berlin-Dahlem, FU-Berlin [available on internet at http://www.algaterra.orgS].
Jobb, G., 2008. Treefinder, version of October 2008. Munich, Germany. Distributed by the author at www.treefinder.de.
Johansen, J. R. & D. A. Casamatta, 2005. Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algological Studies 116: 71–93.
Jost, S., R. Medinger & J. Boenigk, 2010. Cultivation-independent species identification of Dinobryon species (Chrysophyceae) by means of multiplex single-cell PCR. Journal of Phycology 46: 901–906.
Keller, A., T. Schleicher, F. Förster, B. Ruderisch, T. Dandekar, T. Müller & M. Wolf, 2008. ITS2 data corroborate a monophyletic chlorophycean DO-group (Sphaeropleales). BMC Evolutionary Biology 8: 218.
Kessler, E., M. Schäfer, C. Hümmer, A. Kloboucek & V.A.R. Huss, 1997. Physiological, biochemical, and molecular characters for the taxonomy of the subgenera of Scenedesmus (Chlorococcales, Chlorophyta). Botanica Acta 110: 244–250
Ki, J.-S. & M.-S. Han, 2005. Sequence-based diagnostics and phylogenetic approach of uncultured freshwater dinoflagellate Peridinium (Dinophyceae) species, based on single-cell sequencing of rDNA. Journal of Applied Phycology 17: 147–153.
Komárek, J., 1979. Änderungen in der Taxonomie der chlorococcalen Algen. Algological Studies 24: 239–263.
Komárek, J. & B. Fott, 1983. Chlorophyceae (Grünalgen) Ordnung: Chlorococcales. In: Huber-Pestalozzi, G. (ed.), Das Phytoplankton des Süßwassers 7. Teil, 1. Hälfte. Schweizerbart’sche Verlagsbuchhandlung (Nägele u. Obermiller), Stuttgart.
Komárek, J. & A. G. Comas, 1982. Taxonomical definition of the genera and several species of Ankistrodesmus and Selenastrum (Chlorococcales). Archiv für Hydrobiologie, Supplement 63.3, Algological Studies 32: 259–277.
Komárek, J. & V. Jankovská, 2001. Review of the green algal genus Pediastrum; implication for pollen-analytical research. Bibliotheca Phycologica 108: 1–127.
Komárek, J. & L. Kováčik, 1985. The genus Chlorotetraedron McEntee et al. (Protosiphonales, Chlorophyceae). Preslia 57: 289–297.
Komárek, J. & P. Marvan, 1992. Morphological differences in natural populations of the genus Botryococcus (Chlorophyceae). Archiv für Protistenkunde 141: 65–100.
Kostikov, I., T. Darienko, A. Lukešová & L. Hoffmann, 2002. Revision of the classification system of Radiococcaceae Fott ex Komárek (except the subfamily Dictyochlorelloideae) (Chlorophyta). Algological Studies 104: 23–58.
Kotut, K. & L. Krienitz, 2011. Does the potentially toxic cyanobacterium Microcystis exist in the soda lakes of East Africa? Hydrobiologia 664: 219–225.
Krienitz, L., 1986. Drei neue Arten coccaler Grünalgen (Chlorellales) aus dem Plankton der Elbe. Archiv für Protistenkunde 132: 299–311.
Krienitz, L., 1990. Coccale Grünalgen der mittleren Elbe. Limnologica 21: 165–231.
Krienitz, L., 1998. Amphikrikos variabilis sp. nov. (Chlorophyta), a common species of inland waters of Namibia. Archiv für Hydrobiologie, Supplement 126, Algological Studies 91: 1–10.
Krienitz, L. & C. Bock, 2011. Elongatocystis ecballocystiformis gen. et comb. nov., and some reflections on systematics of Oocystaceae (Trebouxiophyceae, Chlorophyta). Fottea 11: 271–278.
Krienitz, L. & H. Heynig, 1982. Beobachtungen an Ankyra lanceolata (Kors. 1953) Fott 1957 und Ankyra spatulifera (Kors. 1953) Fott 1957 (Chlorococcales) im Freiland. Archiv für Protistenkunde 126: 265–271.
Krienitz, L. & G. Klein, 1988. Morphologie und Ultrastruktur einiger Arten der Gattung Monoraphidium (Chlorellales) III. Monoraphidium terrestre (Bristol) nov. comb. Algological Studies 49: 447–463.
Krienitz, L. & K. Kotut, 2010. Fluctuating algal food populations and the occurrence of Lesser Flamingos (Phoeniconaias minor) in three Kenyan Rift Valley lakes. Journal of Phycology 46: 1088–1096.
Krienitz, L. & W. Scheffler, 1994. The Selenastraceae of the oligotrophic Lake Stechlin (Brandenburg, Germany). Biologia 49: 463–471.
Krienitz, L. & M. Wirth, 2006. The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 36: 204–210.
Krienitz, L., G. Klein & H. Böhm, 1985. Zur Morphologie und Ultrastruktur von Selenastrum gracile Reinsch 1867 (Chlorellales). Archiv für Protistenkunde 130: 79–92.
Krienitz, L., V. A. R. Huss & C. Hümmer, 1996a. Picoplanktonic Choricystis species (Chlorococcales, Chlorophyta) and problems surrounding the morphologically similar ‘Nannochloris-like algae’. Phycologia 35: 332–341.
Krienitz, L., P. Kasprzak & R. Koschel, 1996b. Long term study on the influence of eutrophication, restoration and biomanipulation on the structure and development of phytoplankton communities in Feldberger Haussee (Baltic Lake District, Germany). Hydrobiologia 330: 89–110.
Krienitz, L., A. Hehmann & S. J. Casper, 1997. The unique phytoplankton community of a highly acidic bog lake in Germany. Nova Hedwigia 65: 411–430.
Krienitz, L., H. Takeda & D. Hepperle, 1999. Ultrastructure, cell wall composition, and phylogenetic position of Pseudodictyosphaerium jurisii (Chlorococcales, Chlorophyta) including a comparison with other picoplanktonic green algae. Phycologia 38: 100–107.
Krienitz, L., D. Hepperle, H.-B. Stich & W. Weiler, 2000. Nannochloropsis limnetica (Eustigmatophyceae), a new species of picoplankton from freshwater. Phycologia 39: 219–227.
Krienitz, L., I. Ustinova, T. Friedl & V. A. R. Huss, 2001. Traditional generic concepts versus 18S rRNA gene phylogeny in the green algal family Selenastraceae (Chlorophyceae, Chlorophyta). Journal of Phycology 37: 852–865.
Krienitz, L., E. Hegewald, D. Hepperle & M. Wolf, 2003. The systematics of coccoid green algae: 18S rRNA gene sequence data versus morphology. Biologia 58: 437–446.
Krienitz, L., E. Hegewald, D. Hepperle, V. A. R. Huss, T. Rohr & M. Wolf, 2004. Phylogenetic relationship of Chlorella and Parachlorella gen. nov. (Chlorophyta, Trebouxiophyceae). Phycologia 43: 529–542.
Krienitz, L., C. Bock, W. Luo & T. Pröschold, 2010. Polyphyletic origin of the Dictyosphaerium-morphotype within Chlorellaceae (Trebouxiophyceae). Journal of Phycology 46: 559–563.
Krienitz, L., C. Bock, P. K. Dadheech & T. Pröschold, 2011a. Taxonomic reassessment of the genus Mychonastes (Chlorophyceae, Chlorophyta) including the description of eight new species. Phycologia 50: 89–106.
Krienitz, L., C. Bock, H. Nozaki & M. Wolf, 2011b. SSU rRNA gene phylogeny of morphospecies affiliated to the bioassay alga “Selenastrum capricornutum” recovered the polyphyletic origin of crescent-shaped chlorophyta. Journal of Phycology 47: 880–893.
Krienitz, L., C. Bock, K. Kotut & W. Luo, 2012. Picocystis salinarum (Chlorophyta) in saline lakes and hot springs of East Africa. Phycologia 51: 22–32.
Leliaert, F., D. R. Smith, H. Moreau, M. D. Herron, H. Verbruggen, C. F. Delwiche & O. De Clerck, 2012. Phylogeny and molecular evolution of the green algae. Critical Reviews in Plant Sciences 31: 1–46.
Lemmermann, E., 1903. Brandenburgische Algen. Zeitschrift für Fischerei 11: 73–123.
Lewin, R. A., L. Krienitz, R. Goericke, H. Takeda & D. Hepperle, 2000. Picocystis salinarum gen. et sp. nov. (Chlorophyta) – a new picoplanktonic green alga. Phycologia 39: 560–565.
Lewis, L. A., 1997. Diversity and phylogenetic placement of Bracteacoccus Tereg (Chlorophyceae, Chlorophyta) based on 18S ribosomal RNA gene sequence data. Journal of Phycology 33: 279–285.
Lewis, L. A. & R. M. McCourt, 2004. Green algae and the origin of land plants. American Journal of Botany 91: 1535–1556.
Lewis, L. A., L. W. Wilcox, P. A. Fuerst & G. L. Floyd, 1992. Concordance of molecular and ultrastructural data in the study of zoosporic chlorococcalean green algae. Journal of Phycology 28: 375–380.
Long, E. O. & I. B. David, 1980. Repeated genes in eukaryotes. Annual Review Biochemistry 49: 727–764.
Lukavský, J., 1991. Motile cells in Scenedesmus obliquus in outdoor mass culture. Archiv für Protistenkunde 140: 345–348.
Luo, W., S. Pflugmacher, T. Pröschold, N. Walz & L. Krienitz, 2006. Genotype versus phenotype variability in Chlorella and Micractinium (Chlorophyta, Trebouxiophyceae). Protist 157: 315–333.
Luo, W., T. Pröschold, C. Bock & L. Krienitz, 2010. Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae). Plant Biology 12: 545–553.
Luo, W., C. Bock, H. R. Li, J. Padisák & L. Krienitz, 2011. Molecular and microscopic diversity of planktonic eukaryotes in the oligotrophic Lake Stechlin (Germany). Hydrobiologia 661: 133–143.
Lürling, M. & W. Beekman, 1999. Grazer induced defenses in Scenedesmus (Chlorococclaes; Chlorophyceae): coenobium and spine formation. Phycologia 38: 368–376.
Lynch, M., W. Gabriel & A. M. Wood, 1991. Adaptive and demographic responses of plankton populations to environmental change. Limnology and Oceanography 36: 1301–1312.
Mai, J. C. & A. W. Coleman, 1997. The internal transcribed spacer 2 exhibits a common secondary structure in green algae and flowering plants. Journal of Molecular Evolution 44: 258–271.
Mallet, J., 2006. Species concepts. In Fox, C. W. J. (ed.), Evolutionary Genetics: Concepts and Case Studies. Oxford University Press, Oxford: 367–373.
Marin, B. & M. Melkonian, 2010. Molecular phylogeny and classification of the Mamiellophyceae class. nov. (Chlorophyta) based on sequence comparisons of the nuclear- and plastid-encoded rRNA operons. Protist 161: 304–336.
Marvan, P., J. Komárek & A. Comas, 1984. Weighting and scaling of features in numerical evaluation of coccal green algae (genera of the Selenastraceae). Archiv für Hydrobiologie, Supplement 67, Algological Studies 37: 363–399.
Masjuk, N. P., 2006. Chlorodendrophyceae class nov. (Chlorophyta, Viridiplantae) in the Ukrainian flora: I. Phylogenetic relations and taxonomical status. Ukrainian Botanical Journal 63: 601–614. (in Ukrainian).
Mattox, K. R. & K. D. Stewart, 1984. Classification of the green algae: a concept based on comparative cytology. In Irvine, D. E. G. & D. M. John (eds), Systematics of the green algae. The Systematics Association, Special Volumes, No. 27. Academic Press, London, Orlando: 29–72.
Mayr, E., 1942. Systematics and the Origin of Species from the Viewpoint of a Zoologist. Harvard University Press, Cambridge, MA: 1–372.
McEntee, F. J., H. C. Bold & P. A. Archibald, 1977. Notes on some edaphic algae of the South Pacific and Malaysian areas, with special reference to Pseudotetraedron polymorphum gen. et spec. nov. Soil Science 124: 161–166.
McManus, H. A. & L. A. Lewis, 2005. Molecular phylogenetics, morphological variation and colony-form evolution in the family Hydrodictyaceae (Sphaeropleales, Chlorophyta). Phycologia 44: 582–595.
McManus, H. A. & L. A. Lewis, 2011. Molecular phylogenetic relationships in the freshwater family Hydrodictyaceae (Sphaeropleales, Chlorophyceae), with an emphasis on Pediastrum duplex. Journal of Phycology 47: 152–163.
McManus, H. A., L. A. Lewis & E. T. Schultz, 2011. Distinguishing multiple lineages of Pediastrum duplex with morphometrics and a proposal for Lacunastrum gen. nov. Journal of Phycolgy 47: 123–130.
Medinger, R., V. Nolte, R. V. Pandey, S. Jost, B. Ottenwaelder, C. Schlötterer & J. Boenigk, 2010. Diversity in a hidden world: potential and limitation of next generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Molecular Ecology 19: 32–40.
Melkonian, M., 1982. Structural and evolutionary aspects of the flagellar apparatus in green algae and land plants. Taxon 31: 255–265.
Melkonian, M., 1984. Flagellar apparatus ultrastructure in relation to green algal classification. In Irvine, D. E. G. & D. M. John (eds), Systematics of the green algae. The Systematics Association, Special Volumes, No. 27. Academic Press, London, Orlando: 73–120.
Melkonian, M., 1990a. Phylum Chlorophyta. Class Prasiniphyceae. In Margulis, L., et al. (eds), Handbook of Protoctista. Jones and Bartlett, Boston, MA: 600–607.
Melkonian, M., 1990b. Phylum Chlorophyta. Class Chlorophyceae. In Margulis, L., et al. (eds), Handbook of Protoctista. Jones and Bartlett, Boston, MA: 608–616.
Melkonian, M. & B. Surek, 1995. Phylogeny of the Chlorophyta – congruence between ultrastructural and molecular evidence. Bulletin de la Societe Zoologique de France 120: 191–208.
Mischke, U., 2006. Downloads zum Bewertungsverfahren Phytoplankton nach WRRL. Leibniz-Institut für Gewässerökologie und Binnenfischerei [available on internet at http://www.igb-berlin.de/abt2/mitarbeiter/mischke/].
Mischke, U. & B. Nixdorf (eds), 2008. Bewertung von Seen mittels Phytoplankton zur Umsetzung der EU-Wasserrahmenrichtlinie, BTUC-AR 2/2008: 266 pp. ISBN 978-3-940471-06-2 ISSN 1434-6834.
Miyachi, S., M. Tsuzuki, I. Mruyama, M. Gantar, S. Miyachi & H. Matsushima, 1986. Effects of CO2 concentration during growth on the intracellular structure of Chlorella and Scenedesmus (Chlorophyta). Journal of Phycology 22: 313–319.
Moniz, M. B. J. & I. Kaczmarska, 2010. Barcoding of diatoms: nuclear encoded ITS revisited. Protist 161: 7–34.
Müller, T., N. Philippi, T. Dandekar, J. Schultz & M. Wolf, 2007. Distinguishing species. RNA 13: 1469–1472.
Nakayama, T., S. Watanabe, K. Mitsui, H. Uchida & I. Inouye, 1996. The phylogenetic relationship between Chlamydomonadales and Chlorococcales inferred from 18S rDNA sequence data. Phycological Research 44: 47–55.
Nemjova, K., J. Neustupa, J. St’astny, P. Škaloud & J. Vesela, 2011. Species concept and morphological differentiation of strains traditionally assigned to Micrasterias truncata. Phycological Research 59: 208–220.
Neustupa, J., P. Škaloud & J. St’astny, 2010. The molecular phylogenetic and geometric morphometric evaluation of Micrasterias Crux-Melitensis/M-Radians species complex1. Journal of Phycology 46: 703–714.
Padisák, J., L. Krienitz, R. Koschel & J. Nedoma, 1997. Deep layer autotrophic picophytoplankton maximum in the oligotrophic Stechlinsee, Germany: origin, activity, development and erosion. European Journal of Phycology 32: 403–416.
Padisák, J., G. Borics, I. Grigorszky & E. Soroczki-Pinter, 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: the assemblage index. Hydrobiologia 553: 1–14.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Padisák, J., É. Hajnal, L. Krienitz, J. Lakner & V. Üveges, 2010. Rarity, ecological memory, rate of floral change in phytoplankton – and the mystery of the Red Cock. Hydrobiologia 653: 45–64.
Pascher, A., 1918. Von einer allen Algenreihen gemeinsamen Entwicklungsregel. Berichte der Deutschen Botanischen Gesellschaft 36: 390–409.
Pažoutová, M., P. Škaloud & K. Nemjová, 2010. Phylogenetic position of Ooplanctella planoconvexa, gen. et comb. nova and Echinocoleum elegans (Oocystaceae, Trebouxiophyceae, Chlorophyta). Fottea 10: 75–83.
Pearl, H. W., L. M. Valdes, A. R. Joyner & V. Winkelmann, 2007. Phytoplankton indicators of ecological change in the eutrophying Pamlico Sound system, North Carolina. Ecological Applications 17: S88–S101.
Plain, N., C. Largeau, S. Derenne & A. Couté, 1993. Variabilité morphologique de Botryococcus braunii (Chlorococcales, Chlorophyta): corrélations avec les conditions de croissance et la teneur en lipides. Phycologia 32: 259–265.
Porter, K. G., 1973. Selective grazing and differential digestion of algae by zooplankton. Nature 244: 179–180.
Posada, D. & T. R. Buckley, 2004. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. System Biology 53: 793–808.
Potter, D., T. C. Lajeunesse, G. W. Saunders & R. A. Anderson, 1997. Convergent evolution masks extensive biodiversity among marine coccoid picoplankton. Biodiversity and Conservation 6: 99–107.
Pröschold, T. & F. Leliaert, 2007. Systematics of the green algae: conflict of classic and modern approaches. In Brodie, J. & J. Lewis (eds), Unravelling the Algae: The Past, Present, and Future of the Algae. Taylor and Francis, London: 123–153.
Pröschold, T., B. Marin, U. G. Schlösser & M. Melkonian, 2001. Molecular phylogeny and taxonomic revision of Chlamydomonas (Chlorophyta). I. Emendation of Chlamydomonas Ehrenberg and Chloromonas Gobi, and description of Oogamochlamys gen. nov. and Lobochlamys gen. nov. Protist 152: 265–300.
Pröschold, T., E. H. Harris & A. W. Coleman, 2005. Portrait of a species: Chlamydomonas reinhardtii. Genetics 170: 1601–1610.
Pröschold, T., C. Bock, W. Luo & L. Krienitz, 2010. Polyphyletic distribution of bristle formation in Chlorellaceae: Micractinium, Diacanthos, Didymogenes and Hegewaldia gen. nov. (Trebouxiophyceae, Chlorophyta). Phycological Research 58: 1–8.
Pröschold, T., T. Darienko, P. C. Silva, W. Reisser & L. Krienitz, 2011. The systematics of “Zoochlorella” revisited employing an integrative approach. Environmental Microbiology 13: 350–364.
Raven, J. A., 1999. Picophytoplankton. In Round, F. E. & D. J. Chapman (eds), Progress in phycological research, Vol. 13. Biopress Ltd, Bristol: 33–105.
Reymond, O. L. & E. Hegewald, 1988. A morphological and cytological assessment of Paradoxia and Ankyra (Chlorococcales, Chlorophyceae). Archiv für Protistenkunde 135: 167–172.
Reymond, O. L., F. Hindák & H. J. Sluiman, 1986. Morphologie cellulaire et cycle de développement chez l’algue verte Marvania geminata Hindák. Archives des Sciences, Genève 39: 243–255.
Reynolds, C. S., 2007. Variability in the provision and function of mucilage in phytoplankton: facultative responses to the environment. Hydrobiologia 578: 37–45.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Richards, T. A., A. A. Vepritskiy, D. E. Gouliamova & S. A. Nierzwicki-Bauer, 2005. The molecular diversity of freshwater picoeukaryotes from an oligotrophic lake reveals diverse, distinctive and globally dispersed lineages. Environmental Microbiology 7: 1413–1425.
Rindi, F., D. W. Lam & J. M. Lopez-Bautista, 2009. Phylogenetic relationships and species circumscription in Trentepohlia and Printzina (Trentepohliales, Chlorophyta). Molecular Phylogenetics and Evolution 52: 329–339.
Rindi, F., H. A. Allali, D. W. Lam & J. M. López-Bautista, 2010. An overview of the biodiversity and biogeography of terrestrial green algae. In Rescigno, V. & S. Maletta (eds), Biodiversity Hotspots. Nova Science Publishers, New York: 105–122.
Roesler, C. S., C. W. Culbertson, S. M. Etheridge, R. Goericke, R. P. Kiene, L. G. Miller & R. S. Oremland, 2002. Distribution, production, and ecophysiology of Picocystis strain ML in Mono Lake, California. Limnology and Oceanography 47: 440–452.
Ronquist, F. & J. P. Huelsenbeck, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574.
Rybalka, N., R. A. Andersen, I. Kostikov, K. I. Mohr, A. Massalski, M. Olech & T. Friedl, 2009. Testing for endemism, genotypic diversity and species concepts in Antarctic terrestrial microalgae of the Tribonemataceae (Stramenopiles, Xanthophyceae). Environmental Microbiology 11: 554–565.
Salman, V., R. Amann, A.-C. Girnth, L. Polerecky, J. V. Bailey, S. Hegslund, G. Jessen, S. Par & H. N. Schulz-Vogt, 2011. A single-cell sequencing approach to the classification of large, vacuolated sulfur bacteria. Systematic and Applied Microbiology 34: 243–259.
Salmaso, N., G. Morabito, F. Buzzi, L. Garibaldi, M. Simona & R. Mosello, 2006. Phytoplankton as an indicator of the water quality of the deep lakes south of the Alps. Hydrobiologia 563: 167–187.
Schmidt, A. & G. Fehér, 1999–2000. Adatol dél-Magyarországi Vizek Algáinal ismeretéhez IV. Botanikai Közlemények 86–87: 95–105.
Schnepf, E. & E. Hegewald, 1993. Didymogenes palatina Schmidle and Didymogenes anomala (G.M. Smith) Hind. (Chlorococcales): Taxonomy, ultrastructure, autosporogenesis and autospore wall assembly. Archiv für Protistenkunde 143: 41–53.
Schnepf, E., G. Deichgräber, M. Glaab & E. Hegewald, 1980. Bristles and spikes in Chlorococcales: ultrastructural studies in Acanthosphaera, Micractinium, Pediastrum, Polyedriopsis, Scenedesmus, and Siderocystopsis. Journal of Ultrastructure Research 72: 367–379.
Schultz, J. & M. Wolf, 2009. ITS2 sequence-structure analysis in phylogenetics: a how-to manual for molecular systematics. Molecular Phylogenetics and Evolution 52: 250–252.
Schultz, J., S. Maisel, D. Gerlach, T. Müller & M. Wolf, 2005. A common core of secondary structure of the internal transcribed spacer 2 (ITS2) throughout the Eukaryota. RNA 11: 361–364.
Senousy, H. H., G. W. Beakes & E. Hack, 2004. Phylogenetic placement of Botryococcus braunii (Trebouxiophyceae) and Botryococcus sudeticus isolate UTEX 1629 (Chlorophyceae). Journal of Phycology 40: 412–423.
Shoup, S. & L. A. Lewis, 2003. Polyphyletic origin of parallel basal bodies in swimming cells of chlorophycean green algae (Chlorophyta). Journal of Phycology 39: 789–796.
Simpson, P. D. & S. D. Van Valkenburg, 1978. The ultrastructure of Mychonastes ruminatus gen. et sp. nov., a new member of the Chlorophyceae isolated from brackish water. British Phycological Journal 13: 117–130.
Škaloud, P. & O. Peksa, 2010. Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta). Molecular Phylogenetics and Evolution 54: 36–46.
Šlapeta, J., D. Moreira & P. Lopez-Garcia, 2005. The extent of protist diversity: insights from molecular ecology of freshwater eukaryotes. Proceedings of the Royal Society of London. Series B: Biological Sciences 272: 2073–2081.
Sogin, M. L., M. T. Swanton, J. H. Gunderson & H. J. Elwood, 1986. Sequence of the small subunit ribosomal RNA gene from the hypotrichous ciliate Euplotes aediculatus. Journal of Protozoology 33: 26–29.
Somogyi, B., T. Felföldi, K. Solymosi, J. Makk, Z. G. Homonnay, G. Horváth, E. Turcsi, B. Böddi, K. Márialigeti & L. Vörös, 2011. Chloroparva pannonica gen. et sp. nov. (Trebouxiophyceae, Chlorophyta) – a new picoplanktonic green alga from a turbid, shallow soda pan. Phycologia 50: 1–10.
Steinkötter, J., D. Bhattacharya, I. Semmelroth, C. Bibeau & M. Melkonian, 1994. Prasinophytes form independent lineages within the Chlorophyta: evidence from ribosomal RNA sequence comparisons. Journal of Phycology 30: 340–345.
Stockner, J. G., 1991. Autotrophic picoplankton in freshwater ecosystems: the view from the summit. Internationale Revue der Gesamten Hydrobiologie 76: 483–492.
Stockner, J. G. & N. J. Antia, 1986. Algal picoplankton from marine and freshwater ecosystems: a multidisciplinary perspective. Canadian Journal of Fisheries and Aquatic Sciences 43: 2472–2503.
Swofford, D. L., 2002. Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4.0b 10. Sinauer Associates, Sunderland, MA.
Tavera, R. & B. Diéz, 2009. Multifaceted approach for the analysis of the phototrophic microbial community in a freshwater recreational area of Xochimilco, México. Hydrobiologia 636: 353–368.
Trainor, F. R., 1963. Zoospores in Scenedesmus obliquus. Science 142: 1673–1674.
Trainor, F. R., 1996. Reproduction in Scenedesmus. Algae (The Korean Journal of Phycology) 11: 183–201.
Tsarenko, P. M. & O. A. Petlevanny, 2001. Addition to the diversity of algae of Ukraine. Algologia, Supplement: 1–130.
Ustinova, I., L. Krienitz & V. A. R. Huss, 2000. Hyaloraphidium curvatum is not a green alga, but a lower fungus; Amoebidium parasiticum is not a fungus, but a member of the DRIPs. Protist 151: 253–262.
Ustinova, I., L. Krienitz & V. A. R. Huss, 2001. Closteriopsis acicularis (G.M. Smith) Belcher et Swale is a fusiform member of the genus Chlorella, closely related to C. kessleri (Chlorophyta, Trebouxiophyceae). European Journal of Phycology 36: 341–351.
Van den Hoek, C., D. G. Mann & H. G. Jahns, 1995. Algae: An Introduction to Phycology. Cambridge University Press, Cambridge: 627 pp.
Van Donk, E., 2005. Planktonic interactions: developments and perspectives. Verhandlungen der Internationalen Vereinigung für theoretische und angewandte Limnologie 29: 61–72.
Vanormelingen, P., E. Hegewald, A. Braband, M. Kitschke, T. Friedl, K. Sabbe & W. Vyverman, 2007. The systematics of a small spineless Desmodesmus taxon, D. costato-granulatus (Sphaeropleales, Chlorophyceae), based on ITS2rDNA sequence analyses and cell wall morphology. Journal of Phycology 43: 378–396.
Verschoor, A. M., I. Van Der Stap, N. R. Helmsing, M. Lürling & E. Van Donk, 2004. Inducible colony formation within the Scenedesmaceae: adaptive response to infochemicals from two different herbivore taxa. Journal of Phycology 40: 808–814.
Weisse, T., 1993. Dynamics of autotrophic picoplankton in marine and freshwater ecosystems. In Jones, G. (ed.), Advances in Microbial Ecology, Vol. 13. Plenum Press, New York: 327–370.
Weisse, T., 2004. Pelagic microbes – protozoa and the microbial food web. In O‘Sullivan, P. E. & C. S. Reynolds (eds), The Lakes Handbook, Vol. 1 Limnology and Limnetic Ecology. Blackwell Publishing, Malden, Oxford, Carlton: 417–460.
Wilcox, L. W. & G. L. Floyd, 1988. Ultrastructure of the gamete of Pediastrum duplex (Chlorophyceae). Journal of Phycology 24: 140–146.
Wolf, M., M. Buchheim, E. Hegewald, L. Krienitz & D. Hepperle, 2002a. Phylogenetic position of the Sphaeropleaceae (Chlorophyta). Plant Systematic and Evolution 230: 161–171.
Wolf, M., L. Krienitz, E. Hegewald & D. Hepperle, 2002b. Phylogenetic position of Actinastrum hantzschii (Chlorophyta, Trebouxiophyceae). Algological Studies 104: 59–67.
Wolf, M., E. Hegewald, D. Hepperle & L. Krienitz, 2003a. Phylogenetic position of the Golenkiniaceae (Chlorophyta) as inferred from 18S rDNA sequence data. Biologia 58: 433–436.
Wolf, M., D. Hepperle & L. Krienitz, 2003b. On the phylogeny of Radiococcus, Planktosphaeria and Schizochlamydella (Radiococcaceae, Chlorophyta). Biologia 58: 759–765.
Wood, M. A. & T. Leatham, 1992. The species concept in phytoplankton ecology. Journal of Phycology 28: 723–729.
Yamamoto, M., H. Nozaki, Y. Miyazawa, T. Koide & S. Kawano, 2003. Relationships between presence of a mother cell wall and speciation in the unicellular micro alga Nannochloris (Chlorophyta). Journal of Phycology 39: 172–284.
Yoon, H. S., J. Hackett, C. Ciniglia, G. Pinto & D. Bhattacharya, 2004. A molecular timeline for the origin of phytosynthetic eukaryotes. Molecular Biology and Evolution 21: 809–818.
Zimmermann, J., R. Jahn & B. Gemeinholzer, 2011. Barcoding diatoms: evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Organisms, Diversity and Evolution 11: 173–192.
Zuker, M., 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research 31: 3406–3415.
Acknowledgments
We thank Eva Willén for her helpful discussions and improvement of the manuscript. Kiplagat Kotut revised the text with the main focus of improving on the language. We thank Ute Mischke for providing information on the harmonized taxon list of the European Water Framework Directive. The E. Schweizerbart’sche Verlagsbuchhandlung (Nägele & Obermiller), Stuttgart (www.schweizerbart.de) provided permission to copy figures of algae in Tables 2, 3, 4, and 5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: N. Salmaso, L. Naselli-Flores, L. Cerasino, G. Flaim, M. Tolotti & J. Padisák / Phytoplankton responses to human impacts at different scales: 16th workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP)
Rights and permissions
About this article
Cite this article
Krienitz, L., Bock, C. Present state of the systematics of planktonic coccoid green algae of inland waters. Hydrobiologia 698, 295–326 (2012). https://doi.org/10.1007/s10750-012-1079-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1079-z