Abstract
Phytoplankton and associated environmental factors were collected fortnightly during a 1-year cycle in the upper and lower reaches of the River Adige (northeastern Italy). The river has a typical Alpine flow, with the period of high flow and flooding occurring in the spring and summer months. Phytoplankton biomass was constrained by physical variables, mainly water discharge and associated variables directly linked to water fluxes. These factors acted negatively and synchronously by diluting phytoplankton cells and worsening the light regime. Nutrient concentrations did not appear to limit phytoplankton growth. Compared to many other central European rivers, the very low maximum algal biomasses supported by River Adige (Chl a < 7 μg l−1) are due to the Alpine flow regime, which is characterised by higher flow during the warmer months, when conditions for algal development are more favourable. Hydrology and flow regime, along with the channelisation of the river, caused development of a simplified phytoplankton community, which was almost exclusively composed of diatoms. Moreover, these factors contributed significantly to the lack of ordered and cyclic temporal patterns in phytoplankton dynamics. In fact, the gradient of species composition showed a strong association with hydrological factors. If the scenarios predicting increase of atmospheric temperatures and decrease of atmospheric precipitations and water availability in the regions south of the Alps are realistic, algal biomasses may rise and be associated with an increase of groups other than diatoms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The factors controlling growth and loss processes in phytoplankton communities in marine and inland waters have been extensively studied (Harris, 1986; Reynolds, 2006). These factors include physical drivers (hydraulic discharge and renewal time, changes in solar radiation, thermal and light regime), chemical characteristics (salinity and availability of nutrients), and biotic interactions (competition, grazing, parasitism). In inland waters, the relative importance of these factors in promoting or depressing phytoplankton growth is strongly dependent on the typology of the water body under consideration. In lacustrine environments, the stability of the water column is considered a key factor in the control of the structure of phytoplankton assemblages (Harris, 1983). On a temporal scale of months, the passage from non-stratified, turbulent water column to conditions of strong stratification and water column stability is instrumental for the vertical distribution and availability of nutrients, the control of algal growth and selection of phytoplankters with different life strategies, and the control of zooplankton and grazing pressure (Sommer et al., 1986).
The effects of the physical stability of the water column and of the seasonality of nutrients and grazing pressure on the temporal development of plankton communities are clearly manifest in the largest and deepest lakes, which operate as large inertial systems that minimise the effect of external disturbances (such as water renewal and flushing, and meteorological events). At the opposite extreme, planktonic communities in large rivers are harshly constrained by water discharge and other variables related to water flow, primarily transport of suspended solids, light attenuation and sedimentation (Reynolds & Descy, 1996; Wehr & Descy, 1998). Nutrients in rivers are generally present at high concentrations and in excess of algal requirements: therefore they are not generally considered an important controlling factor for algal growth (Kelly & Whitton, 1998). With a few exceptions (e.g., Sellers & Bukaveckas, 2003), the same applies to silica. Analogously, the control of phytoplankton by grazers is difficult due to the insufficient growth rates of zooplankton, which are not high enough to compensate for losses, including washout downstream (Reynolds & Descy, 1996). However, during periods of lower discharge and higher temperature, physical constraints are reduced, determining more favourable conditions for phytoplankton growth and, at times, a major control of algal development by zooplankton grazing (Thorp et al., 1994; Ferrari et al., 2006). These aspects are further complicated by the recognition that significant volumes of water in rivers may be very slow or not moving at all. Of these, a fraction is localised in storage zones connected with the main flow. In these backwaters, plankton production may be greatly enhanced, thus contributing significantly to the maintenance of quantifiable pelagic biomass (Reynolds, 2006; Casper & Thorp, 2007). Hydrological isolation is instrumental for the development of a richer and more diversified plankton community (Lewis et al., 2000; Havel et al., 2009) and for the regulation of nutrient fluxes (Richardson et al., 2004). Larger biodiversity is also favoured by greater habitat heterogeneity and by the presence of macrophytes (Stoyneva, 1994).
At the community level, the difficulty to identify general models of phytoplankton development in rivers is even more exacerbated. The identification of regular and characteristic taxa, and the recognition of temporal dynamic patterns of phytoplankton successions have been only rarely attempted (e.g., Reynolds & Descy, 1996). The consolidated picture is that of an algal community composed of small species with high potential growth rates, mainly belonging to diatoms and chlorophytes. As underlined by Wehr & Descy (1998), more comprehensive studies are needed to elucidate the general patterns of phytoplankton succession in relation to different river types, hydrological regimes and seasonal variability in physical, chemical and biotic drivers.
The general aim of this paper is to identify the principal factors driving seasonal phytoplankton changes in two stations located in the middle and lower reaches of a river characterised by a typical Alpine flow regime (River Adige, North Eastern Italy). We hypothesised that phytoplankton composition, maximum biomass, and temporal dynamics are fully constrained by physical drivers, i.e., hydrological characteristics and patterns of river discharge. More specifically, we want to determine if the same set of factors act homogeneously along the ‘pelagic’ stations of the river, with particular focus on the overall production of algal biomass and the composition and temporal development of the main algal groups.
Study site
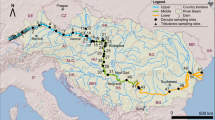
The River Adige originates in the Eastern Alps at 1,550 m a.s.l., and flows into the Adriatic Sea (Fig. 1). It is the second largest river in Italy, after the Po River. The river is 409-km long and has a catchment area of 12,100 km2. The hydrographic basin extends to Albaredo (SE of Verona), but the major tributaries are located between the provinces of Bolzano and Trento (Rivers Isarco, Noce, Avisio and Fersina). Similarly to many other rivers studied in northern Europe, the River Adige has a typically Alpine flow regime, with high flow and river flooding occurring in spring and summer, in connection with the thawing of snow and ice, and low water period occurring in late autumn and winter (Kristensen & Hansen, 1994). The mountain zone of the hydrographic basin contains more than 30 dams with an overall storage of > 570 × 106 m3. The largest reservoirs are Lake Resia (upstream of both stations; V = 122 × 106 m3), and Lake S. Giustina (upstream of Boara Pisani; V = 183 × 106 m3). In the lowland area, water is used also for drinking purposes (ca. 2.3 m3 s−1), but the prevalent use between the provinces of Verona and Venezia is the irrigation of agricultural fields (up to over 150 m3 s−1 during the vegetation season, from May to August). In the two reaches considered in this research, the fluvial course is regulated by river banks, and riparian zones are generally limited to very restricted areas.
Materials and methods
Samplings were carried out in two stations representative of the middle and lower reaches of the river, i.e., Cortina all’Adige (CA, ca. 25 km SSW of Bolzano) and Boara Pisani (BP, 4 km north of Rovigo) (Fig. 1). The northern station (CA) is located after the entry of River Isarco and before the entries of the rivers Noce, Avisio and Fersina in the main river. It has torrential characteristics and the maximum height of waters generally range between 1.5 and 3 m. The southern station (BP) is located after the closing of the hydrographic basin; it has more potamal characteristics, with a water height generally ranging between 4.5 and 7 m. Hydrological data in the two stations were obtained from the ‘Servizio Opere Idrauliche’ of the Autonomous Province of Trento, and by the ‘Direzione Difesa Suolo e Protezione Civile’ of the Veneto Region.
The two sites were sampled fortnightly from March 2007 to February 2008. Water was collected from bridges using a rinsed plastic bucket. The representativeness of the samples was tested in the frame of previous investigations; Salmaso & Braioni (2008) found comparable temporal developments of both abiotic and biotic variables in three sampling stations along a 26-km stretch of the lowland course of the river. Water temperature was measured immediately after the sampling. Total phosphorus (TP) was measured on unfiltered samples. Soluble reactive phosphorus (SRP), nitrate (NO3–N), nitrite (NO2–N) and ammonium (NH4–N) were determined after sample filtration through 0.45-μm cellulose acetate filters (Sartorius). Chemical analyses were carried out by the Agency for Environmental Protection of Trento following the standard methods described by APHA et al. (1995).
After removing of larger particles with a 200 μm mesh plankton net, seston dry weight (total suspended solids, 105°C) and ash-free seston dry weight (550°C) were determined by filtering water samples on previously combusted (550°C) and weighed Whatman GF-C filters (APHA et al., 1995). Water turbidity (NTU, Nephelometric Turbidity Units) was estimated by a turbidimeter Hach 2100N. Light attenuation coefficients (K d) at the station of Boara Pisani were estimated from irradiance profiles obtained with a submersible irradiance sensor, LiCor 192SA. The euphotic depth (Z eu) was estimated from K d values, \( Z_{\text{eu}} = \ln (100)/K_{\text{d}} \) (Kirk, 1994).
Chlorophyll a (Chl a) was determined by spectrophotometry after filtration on Whatman GF-C glass-fibre filters, disruption of the filters with a grinder and 24 h extraction in 90% acetone. Phytoplankton analysis was carried out on subsamples preserved in acetic Lugol’s solution. Algal cells were counted under Leica and Zeiss inverted microscopes. Species with variable cell sizes (particularly diatoms) were counted in size classes. Algal biovolumes were calculated from recorded abundances and specific biovolumes approximated to simple geometrical solids. Biological methods used in the field and laboratory have been described in detail by Salmaso (2003) and Rott et al. (2007). Species identification followed the more recent monographs of the series Süßwasserflora von Mitteleuropa, established by A. Pascher (Gustav Fisher Verlag, and Elsevier, Spectrum Akademischer Verlag), and specific manuals of the series Das Phytoplankton des Süßwassers, established by G. Huber-Pestalozzi (Komárek & Fott, 1983).
Correlations between environmental and biotic variables were computed using the product-moment correlation coefficient on both original and log-transformed data (Green, 1979; Sokal & Rohlf, 1995). Phytoplankton communities of the two stations were analysed by Nonmetric Multidimensional Scaling (NMDS) applied to Bray and Curtis’ dissimilarity matrices (Legendre & Legendre, 1998) computed on species biovolume values. Unidentified cells <4 μm length and rare species found in less than three occasions and with maximum biovolumes <20 mm3 m−3 were neglected. Before computation, the data were transformed by double square root to reduce the weight of the more abundant taxa/groups. Environmental variables were related to the strongest gradients in species composition by fitting environmental vectors to the NMDS configuration. Vector fitting finds the maximum correlation of the single variables with a set of samples in ordination space. A fitted-vector points to the direction of most rapid change in the environmental variable, whereas its length is proportional to the correlation between the environmental variable and ordination. The significances of vectors were based on 1000 random permutations of the data. NMDS analysis and vector fitting were carried out with Systat™ 10.2, and the vegan (Oksanen et al., 2008) and ecodist (Goslee & Urban, 2007) packages in R (R Development Core Team, 2008).
Results
Physical variables
Water discharge showed an increase during spring and summer due to snowmelt and contribution of the glacial melt to runoff (Fig. 2). A decrease during the winter months was more apparent at Cortina all’Adige. From June to August, the southern station (BP) in many occasions showed lower discharge values compared with the station upstream due to the subtraction of water for agricultural irrigation. In both stations, the small weekly oscillations (20–40 m3 s−1), particularly noticeable during low discharge, were caused by the release of water from the reservoirs located in the Alpine region of the hydrographic basin. The peak in December at BP was due to the rivers located downstream of CA.
Water temperatures in the two stations showed a similar temporal development (r = 0.98, P < 0.01), but different amplitudes, with ranges between 0.4–15.0°C (CA) and 2.8–22.4°C (BP) (Fig. 3a).
Dry weight showed the higher values (16–44 mg l−1) between June and September, with differences in the timing of maximum values in the two stations (Fig. 3b), but with a comparable temporal development over the study period (r = 0.46, P < 0.05). Dry weight was mainly composed by inorganic mineral particles (1.7–40.1 mg l−1; from 61 to 91% of the total dry weight), with only a minor proportion of organic particles (0.7–5.6 mg l−1).
Apart from an exceptional peak measured on 8 August at Cortina all’Adige soon after a period of heavy rain (318 NTU), and a secondary peak at Boara Pisani (69 NTU), turbidity varied between 3 and 37 NTU (Fig. 3c); excluding the two higher peaks, NTU values showed a high temporal correlation in the two stations (r = 0.80, P < 0.01).
Water turbidity showed a strong dependence on the average discharge values (D 3d) recorded during the 72 h before the sampling operations in both stations (Table 1). The same dependence from D 3d was confirmed, for the station of Cortina all’Adige, also for the suspended solids (Table 1).
Euphotic depths estimated from K d values measured at Boara Pisani are reported in Fig. 3d (black triangles). Z eu showed a strong negative and nonlinear relationship with water turbidity \( Z_{\text{eu}} = 21.3 \times {\text{NTU}}^{ - 0.70} \) (r 2 = 0.81, n = 8, P < 0.01). This relationship was used to estimate Z eu values at the station of Cortina all’Adige and missing recordings at Boara Pisani (Fig. 3d). Similarly, Z eu (obtained from K d measured values) showed a negative, nonlinear dependence also from water discharge, D 3d (r 2 = 0.65, n = 8, P < 0.05) and dry weight (r 2 = 0.49, n = 8, P = 0.05).
Phosphorus, nitrogen and silica
SRP showed a comparable temporal development in the two stations (r = 0.67, P < 0.01), though with different concentrations (annual averages, mean ± SD, equal to 12 ± 7 μg P l−1, CA, and 37 ± 12 μg P l−1, BP) (Fig. 4a). SRP concentrations at Cortina all’Adige were below 5 μg l−1 in the second half of June, and between September and the first half of November. At Boara Pisani, SRP concentrations never went below 20 μg P l−1. Total phosphorus concentrations were always above 20 μg P l−1 (Fig. 4b), with annual mean concentrations of 35 ± 10 μg P l−1 (CA) and 75 ± 24 μg P l−1 (BP), and with only a weak temporal correlation in the two stations (r = 0.38, P < 0.10).
Dissolved inorganic nitrogen concentrations (DIN: NO3–N + NO2–N + NH4–N) ranged between 0.5 and 2 mg N l−1, with a comparable temporal development in the two stations (r = 0.81, P < 0.01) and annual mean concentrations equal to 0.74 ± 0.16 mg N l−1 (CA) and 1.12 ± 0.31 mg N l−1 (BP) (Fig. 4c). The dominant nitrogen compound was nitrate (0.5–1.8 mg N l−1). Ammonium nitrogen was always below 0.2 mg N l−1, whereas nitrites were present with negligible concentrations (<0.04 mg N l−1). In the two stations, N:P (DIN/RP) mass ratios ranged between 24 and over 200.
As DIN, reactive silica showed a comparable temporal development in the two stations (r = 0.74, P < 0.01). Generally, concentrations were well above 1.5 mg Si l−1, with two minima measured on 18th April and 8th August at Boara Pisani (Fig. 4d), and annual mean concentrations equal to 2.3 ± 0.3 mg Si l−1 (CA) and 2.2 ± 0.4 mg Si l−1 (BP).
Phytoplankton biomass
Temporal variations of Chl a and total phytoplankton biovolume showed a close correlation both at Cortina all’Adige (r = 0.79, P < 0.01) and Boara Pisani (r = 0.93, P < 0.01) (Fig. 5a, b). The two estimates of algal biomass did not show any correlation (P > 0.10) in the two sampling stations. Maximum values of Chl a and biovolume were 5.7 μg l−1 and 2,356 mm3 m−3 (CA), and 6.9 μg l−1 and 3,210 mm3 m−3 (BP), respectively. Average concentrations of Chl a and total biovolume over the whole period (mean ± SD) were 2.1 ± 1.7 μg l−1, and 583 ± 557 mm3 m−3 (CA), and 2.3 ± 1.6 μg l−1, and 785 ± 740 mm3 m−3 (BP). Both Chl a and total biovolume showed a strong negative, nonlinear dependence on water discharge, D 3d (Fig. 6; Table 1). However, the relationships hold true also considering linear models (Table 1). The northern station was characterised by larger discharges and lower biomasses during spring and summer (Fig. 6a, b). In particular, on 8th August, soon after a period of heavy rain, the phytoplankton samples contained only very sporadic algal cells. Data from October to March, though characterised by a much lower range of water discharge, showed a larger biomass variation. Conversely, at Boara Pisani, higher biomasses were reached also during the warmer period, during low discharge, as in the case of the peak measured on 8th August (Figs. 5a, b, 6c, d).
Relationships between algal biomasses (chlorophyll a and total biovolumes) and average values of water discharge recorded during the 72 h before the sampling operations (D 3d) at a, b Cortina all’Adige and c, d Boara Pisani. In (a), ‘asterisk’ was excluded from the computation. The graphs highlight the samples of the colder (open circles: October–March) and warmer season (grey circles: April–September) (cf. Fig. 3a)
Chl a and algal biovolume showed a negative, nonlinear dependence (0.01 < P < 0.05) on water turbidity and suspended solids only in the northern station (Table 1).
In the northern station (CA), Chl a and phytoplankton biovolume showed a significant (at least P < 0.05) and positive correlation with SRP, TP, DIN and silica (0.42 ≤ r ≤ 0.60; untransformed data). In the southern station (BP), a positive (at least P < 0.05) correlation was noticed only between biovolume and TP, whereas both Chl a and biovolume showed a strong negative correlation with silica (Table 1). This was confirmed also considering the correlation between the biomass of diatoms and silica (r = −0.78, P < 0.01).
Community variations
Phytoplankton community was almost exclusively dominated by diatoms, but with a different proportion of the two orders in the two stations (Fig. 5c, d). At Cortina all’Adige, Pennales were the dominant order, whereas at Boara Pisani, Centrales (mainly Cyclotella spp., followed by Stephanodiscus spp.) represented a consistent fraction of the diatoms in spring and during the highest biomass peak (8th August). In the northern station, pennate diatoms were composed of meroplanktonic and tychoplanktonic species mainly belonging to Cymbella spp., Diatoma spp. (mainly D. ehrenbergii), followed by Fragilaria spp. (mainly F. arcus, F. crotonensis and F. ulna), Gomphonema sp., Cocconeis sp., Navicula spp., Didymosphenia geminata and Nitzschia spp. The same species were also present at Boara Pisani, but with a minor contribution of Cymbella spp. and Diatoma spp., and a major presence of Fragilaria spp., Navicula spp. and Didymosphaenia geminata.
At Cortina all’Adige, the biovolume of both Pennales and Centrales showed a negative, nonlinear dependence on water discharge, D 3d (r = −0.48, P < 0.05; r = −0.74, P < 0.01, respectively). At Boara Pisani, the negative impact of water flow was particularly clear for the biovolume of centric diatoms (r = −0.56, P < 0.01).
Orders other than diatoms showed very small cumulative biovolumes, always below 50 mm3 m−3 at Cortina all’Adige, and 250 mm3 m−3 at Boara Pisani (Fig. 5e, f). With a few exceptions, cumulative percentage biomasses of these minor groups were always below 15% (CA) and 30% (BP). The overall abundance of the algal orders other than diatoms showed a temporal development comparable with pennate and centric diatoms (r = 0.51, P < 0.05, and r = 0.53, P < 0.01) only at Boara Pisani, and a negative, nonlinear dependence on water discharge, D 3d, in both stations (r = −0.56, P < 0.01, CA; r = −0.49, P < 0.05, BP).
The differences in the temporal development of the phytoplankton community in the two sampling stations were clearly evident in the results of the NMDS (Fig. 7; stress = 0.20). As for Cortina all’Adige, the configuration does not include 8th August, when the sample was practically free of phytoplankton. The ordinations in Fig. 7a, b were obtained from a single NMDS analysis (cf. Fig. 7c); but, to better highlight the temporal dynamics of the different samples, the results are presented separately. Even excluding the two cases characterised by a very dilute phytoplankton community at Cortina all’Adige (Fig. 7a), the two groups of samples belonging to CA and BP were placed in two different areas of the configuration. Moreover, the temporal dynamics showed different paths. The couples of coordinates of the two stations were uncorrelated (P > 0.1) both along the first and the second axis. Conversely, a common characteristic was the apparent random temporal arrangement of the samples. The fitting of environmental variables onto the configuration allowed us to disentangle the temporal dynamics (Fig. 7c). The gradient of species composition was strongly associated to water discharge and suspended materials, and, in the opposite direction, to phytoplankton biomass and light availability. Moreover, the samples of the two stations were separated along a gradient strongly associated with phosphorus and DIN. The overall picture essentially did not change when we analysed data separately for the two stations, or when we excluded the two separate points of the northern station (Fig. 7a) from the computations.
Ordination of phytoplankton samples by Nonmetric Multidimensional Scaling (NMDS). a Cortina all’Adige, b Boara Pisani; the Arabic numbers indicate the month of sampling, from March 2007 (highlighted in grey) to February 2008 (highlighted in black). c Vector fitting of environmental variables on the NMDS configuration (CA Cortina all’Adige, BP Boara Pisani). Vectors are significant at least at P < 0.05, excluding Zeu (P < 0.10)
Discussion
Algal nutrients
Nutrient levels in the southern reaches of River Adige did not represent an important factor regulating phytoplankton growth. In the northern station, in a few occasions SRP showed concentrations below 5 μg P l−1, suggesting the existence of potential P-deficiency for phytoplankton replication. The onset of regulation, below which phytoplankters may be generally controlled by P supply, generally occurs at around 3 μg P l−1 (Reynolds, 2006). Other species are considered to have higher P requirements. One of these is Cyclotella, for which half-saturation growth constant for phosphorus of ≤10 μg P l−1 has been reported (Wehr and Descy, 1998). Pelagic, small centric diatoms were abundant in the southern station, where SRP never fell below 20 μg P l−1, but constituted only a tiny fraction in the northern station. Here, the occasional low concentrations of RP could have enhanced the constraints imposed on the small centric diatoms by the hydrological factors and by the torrential conditions which characterise the middle reaches of the river. Conversely, TP was always present with large concentrations in both stations. Similarly, minimum DIN concentrations in the two stations were around 15–30 times greater than the limits below which phytoplankters may experience problems in obtaining sufficient N to half-saturate growth (i.e., 15–30 μg N l−1; Reynolds, 2006). In addition, N:P ratios were always much higher than 7–15, which is the range routinely considered for assessing potential N or P limitation (OECD, 1982; but see Reynolds, 1999).
Silica variations in the southern station showed a strong negative link with diatom biomass. The largest reduction of Si in August (with a decrease to over 50%; Fig. 4d) coincided with the maximum development of pelagic small Cyclotella species during periods of low discharge and nutrient replenishment (Fig. 5d). However, even in this case, the concentrations (1.1 mg Si l−1) never went below the growth-limiting values encountered in most lacustrine environments (<0.5–0.1 mg Si l−1; Reynolds, 2006). These manifest reductions indicate in Si a potential limiting factor imposing lower limits on the supportive capacity of the river. The lack of a negative relationship between diatoms and Si in the northern station reflects the absence of a peak in diatom biomass during summer and, possibly, a major availability of Si coming from the hydrographic basin.
Constraints to phytoplankton development
The river discharge and the variables directly linked to water fluxes (turbidity and, partly, dry weight) had a significant impact on the development of phytoplankton biomass, particularly during the periods more favourable for algal growth, i.e., during spring and summer, when better conditions of temperature and light can be found. A greater water flux has negative effects on the algal development due to dilution and algae not having sufficient time to increase their populations whilst moving downstream (Salmaso & Braioni, 2008). The low values of Z eu (<2–3 m) during periods of high water discharge (Figs. 2, 3d) could have depressed phytoplankton growth, particularly in the southern station, where the depth of the river channel during high flow can reach and exceed 7 m. The largest peaks of algal biomass in the lowland station were determined by a favourable combination of higher temperatures, low discharge and more favourable light. In the northern station, the largest biomass values were observed also during the autumnal and winter months, where temperatures reached as low as 2.4°C, but always during periods of low discharge. Owing to the lower depth of the river channel in this station (1.5–3 m), the higher biomasses during the colder months were determined also by a substantial fraction of epilithic and meroplanktonic organisms, as suggested by the dominance of pennate diatoms belonging to Cymbella and Diatoma. On the contrary, collapse of the phytoplankton or the maintenance of very scarce populations was always associated with high D 3d values and, in the ‘deeper’ station (BP), lower light availability.
Maximum values of Chl a in the two stations (<7 μg l−1) were around one order of magnitude lower than maxima recorded in other European rivers, e.g., Danube, Thames, Elbe and Meuse (150–285 μg l−1; Bothár & Kiss, 1990; Ruse & Hutchings, 1996; Desortová et al., 1996; Gosselain et al., 1998; Young et al., 1999). This aspect was underscored in previous research, when in three stations along a 26-km stretch of the lowland course of the River Adige, Salmaso and Braioni (2008) recorded maximum concentrations of Chl a ranging from 23 to 31 μg l−1. In the examples of European rivers reported above, waters are characterised by a higher content of phosphorus (with annual average concentrations ranging from 180 to over 500 μg P l−1) compared to River Adige (35 and 75 μg P l−1 in the two stations). With the exception of large rivers in Russia and the Nordic countries, the annual mean phosphorus concentrations in the downstream reaches in all of the largest rivers in Europe generally exceed 100 μg P l−1, with only 10–20% of the rivers having P concentrations below 50 μg P l−1 (Kristensen & Hansen, 1994). The relatively unpolluted rivers are generally situated in catchments in mountainous and forested regions.
These observations agree with the results reported in a growing number of articles about the existence of a positive relationship between phosphorus concentrations and algal biomasses in rivers (Basu & Pick, 1996; Van Nieuwenhuyse & Jones, 1996; Heiskary & Markus, 2001; Chételat et al., 2006). It is clear that the maximum supportable biomass cannot exceed the capacity of the scarcest resource relative to demand (see Reynolds, 2006). Therefore, these relationships could be related to the consumption of nutrients during periods more favourable for phytoplankton replication (growing season and low discharge) and, possibly, to the existence of negative seasonal relationships between assimilable phosphorus and phytoplankton abundance in the single rivers. This was for example demonstrated by Rossetti et al. (2008), who showed that, from May to October, Chl a in the River Po was inversely related to SRP concentrations, evidencing a potential causal relationship between phosphorus availability and phytoplankton abundance. As in previous investigations (Salmaso & Braioni, 2008), negative relationships between SRP and algal biomass were never ascertained during this research in River Adige (Table 1). This again suggests that, in this river, the observed algal biomasses are below the limits imposed by nutrient availability and far from the maximum supportable biomass, especially in the southern station.
These considerations are further confirmed when the phytoplankton development of River Adige is evaluated with the available general models linking phytoplankton abundance and nutrients. Basu & Pick (1996) and van Nieuwenhuyse & Jones (1996) found significant and comparable relationships between Chl a and TP measured in rivers during the growing period, generally between May and September. If these models are used to estimate algal biomasses of River Adige using TP concentrations measured for the same period, then the results are considerably higher than those observed in the field (Fig. 8). As highlighted by Lewis & Wurtsbaugh (2008), both phosphorus and chlorophyll are essential components of phytoplankton biomass. For this reason, models relating TP and Chl a should not be used to diagnose the mechanism of limitation, but rather to find the most powerful predictor of chlorophyll development. The discrepancies emerging in the application of the models (Fig. 8) underline the existence of other variables able to better describe the algal development in River Adige. The results indicate a greater proportion of TP not included in the algal matter of River Adige and possibly a lower ability of phytoplankton to utilise available resources compared with the other rivers considered in the models.
Relationship between chlorophyll a concentrations and total phosphorus in temperate rivers. Model 1, Van Nieuwenhuyse & Jones (1996); model 2, Basu & Pick (1996). Model 3 reports the same relationship for shallow and high alkalinity lakes (Phillips et al., 2008). As for rivers, models were derived from arithmetic mean of samples collected periodically (with frequencies ranging from monthly to biweekly or weekly) over a given summer (May–September, Model 1), and from single summer observations (July, Model 2). Single records from the two stations of the River Adige from May to September are indicated with open circles (Cortina all’Adige) and open triangles (Boara Pisani); black symbols indicate the average values of the considered periods in the two stations
Water discharge and flow velocity certainly represent important constraints for the attainment of higher biomasses, imposing a lower limit on the supportive capacity of the river. In the Garonne, despite high availability of nutrients, Améziane et al. (2003) measured Chl a concentrations between <5 μg l−1 up to around 20 μg l−1. These authors stated that flow velocities around 0.5 m s−1 (which represent the minimum velocities measured in the lowland course of River Adige; Egiatti & Cremonese, 2006) could approximate the limit above which phytoplankton might experience lower development. Due to the position of the river in the alpine region, a further constraint is due to the particular flow regime, which is comparable with that of rivers in northern Europe. Higher water discharge and river flooding are expected during the period more favourable for the algal growth, i.e., spring and summer. In the temperate lowlands in southern Europe, the rivers are primarily fed by rainfall and, therefore, high water fluxes occur during late autumn or winter. Many lowland temperate rivers in Europe have a less fluctuating regime, although flow is generally highest during the winter half-year and lowest during the summer half-year (e.g., Elbe, Thames, Meuse) (cf. Kristensen & Hansen, 1994).
If compared with many other rivers (Chételat et al., 2006), the contributions of algal groups other than diatoms are very low, particularly at the northern station. Here, the only algae able to survive in flowing waters were pennate meroplanktonic and tychoplanktonic diatoms originating from the riverbed. A significant increase of reproducing small centric diatoms, followed by an increase of true potamoplankters other than diatoms, was demonstrated only in the lowland reaches of the river. Small unicellular centric diatoms are r-selected opportunistic species with high surface-to-volume ratios, characters that render them superior competitors in terms of light interception and settling velocities (Reynolds, 1988). A further factor responsible for the presence of a simplified community is due to the channelisation of the river and to the strong reduction of dead zones which constitute an important source of diverse planktonic organisms.
With conditions of very low river flow or stagnant water, phosphorus concentrations such as those measured in the River Adige could promote a sustained growth of the more eutrophic groups, particularly cyanobacteria and chlorophytes (Downing et al., 2001; Jeppesen et al., 2005; Phillips et al., 2008). In the Australian rivers, conditions of high nutrient concentrations associated with the stability of the water column can lead to huge populations of buoyant cyanobacteria (Sherman et al., 1998; Mitrovic et al., 2001).
During the past 80 years, the River Adige has experienced a significant decrease of water discharge, at a yearly average rate of around 1 m3 s−1 year−1 in the lowland reaches. This decrease was mainly due to a high utilisation of water for irrigation, but also to climatic changes in the hydrographic basin (cf. Bellin & Zardi, 2004; Rossi & Veltri, 2007). If the scenarios predicting increase of atmospheric temperatures and decrease of atmospheric precipitations and water availability in the regions south of the Alps in the next decades are realistic (IPCC, 2007), parallel increases in algal biomass may occur in groups other than diatoms.
Control of phytoplankton biomass by zooplankton grazing in rivers can be observed only for short period, during the growing season and conditions of low flow (e.g., Garnier et al., 1995).The studies currently carried out in the river are showing the existence of a poorly diversified community, developing during periods of lower discharge with maximum densities of rotifers and microcrustaceans in the two stations below 15–45 ind. l−1 and 5–10 ind. l−1, respectively (unpublished data).
Phytoplankton seasonal dynamics
The apparent casual temporal development of the phytoplankton community is both due to the strong control operated by the hydrological regime and to its very simple structure. The unpredictable temporal dynamics characterised both synthetic variables (total biovolume and Chl a) and algal groups. More regular temporal patterns in total algal biomass are expected when physical disturbances represented by higher discharges are occurring during the coldest months, at the time of minimum phytoplankton growth. For example, in the River Trent (UK) Skidmore et al. (1998) found a regular, cyclic development of phytoplankton biomass, with larger populations occurring when discharge was relatively low and light availability was high (spring and early-mid summer). Successive decreases in late summer, with low flow conditions, were linked to the increase rates of particle sedimentation and, possibly, grazing by zooplankton and benthic filter feeders.
The irregular temporal pattern of phytoplankton development was almost completely controlled by water discharge and, partly, to the variables directly linked to water fluxes (turbidity, suspended solids). Owing to the absence of a well-structured algal community, changes were almost all due to the modifications of the biovolumes of a few dominant diatom species. The presence of richer and more structured communities and the action of a more balanced set of environmental factors are of key importance in shaping a regular, cyclic development of phytoplankton in large, inertial systems such as the deep lakes (Salmaso & Padisák, 2007). In these large water bodies, environmental variables are highly correlated with phytoplankton parameters and act jointly as a complex forcing factor that selects seasonal groups of species sharing similar requirements (Salmaso, 2003). In high flowing rivers, factors evolving regularly on a seasonal basis became less important in the control of seasonal replacement of phytoplankton assemblages.
Conclusions
Nutrient concentrations did not appear to limit phytoplankton growth in the River Adige, although occasional regulation of algal biomass by low SRP concentrations in the upper reaches of the river cannot be excluded. The most critical forcing factors in both the northern and southern stations were physical variables, mainly water discharge and the variables directly linked to water fluxes (turbidity and, partly, dry weight). These factors acted negatively and synchronously by diluting phytoplankton cells and decreasing light availability. Even during the most favourable conditions for growth, the most abundant algae were small unicellular diatoms, with a low contribution of the other algal groups.
The low maximum algal biomass supported by River Adige is due to sustained water flow and to the Alpine flow regime, which is characterised by high flow and flooding occurring during the months when conditions for algal development are more favourable. Therefore, in this typology of rivers, flow regime is detrimental to the flourish of a rich spring and summer potamoplankton community. This is in contrast with what can be found in other European mainland rivers, where lower discharges could occur during late spring and summer. These aspects underline the need to consider also from a hydrographical perspective the ‘trophic’ status of large rivers and, more in general, their water quality.
Hydrology and flow regime are also the cause of the apparent random development of the phytoplankton community. Fast and rapid changes are also caused by the absence of a rich and well-structured community able to efficiently ‘buffer’ against sudden changes in physical factors.
References
Améziane, T., A. Dauta & R. Le Cohu, 2003. Origin and transport of phytoplankton in a large river: the Garonne, France. Archiv für Hydrobiologie 156: 385–404.
APHA, AWWA & WEF, 1995. Standard Methods for the Examination of Water and Wastewater, 19th ed. American Public Health Association, Washington.
Basu, B. K. & F. R. Pick, 1996. Factors regulating phytoplankton and zooplankton biomass in temperate rivers. Limnology and Oceanography 41: 1572–1577.
Bellin, A. & D. Zardi, 2004. Analisi climatologica di serie storiche delle precipitazioni e temperature in Trentino. Quaderni di Idronomia Montana, 23. Editoriale BIOS, Castrolibero (CS), Italy.
Bothár, A. & K. T. Kiss, 1990. Phytoplankton and zooplankton (Cladocera, Copepoda) relationship in the eutrophicated River Danube (Danubialia Hungarica, CXI). Hydrobiologia 191: 165–171.
Casper, A. F. & J. H. Thorp, 2007. Diel and lateral patterns of zooplankton distribution in the St. Lawrence River. River Research and Applications 23: 73–85.
Chételat, J., F. R. Pick & P. B. Hamilton, 2006. Potamoplankton size structure and taxonomic composition: influence of river size and nutrient concentrations. Limnology and Oceanography 51: 681–689.
Desortová, B., A. Prange & P. Punčochář, 1996. Chlorophyll-a concentrations along the River Elbe. Archiv fur Hydrobiologie Supplement 113, Large Rivers 10: 203–210.
Downing, J. A., S. B. Watson & E. McCauley, 2001. Predicting cyanobacteria dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences 58: 1905–1908.
Egiatti, G. & S. Cremonese, 2006. Considerazioni sulla scala di deflusso del Fiume Adige a Boara Pisani. ARPAV – Agenzia Regionale per la Prevenzione e Protezione Ambientale del Veneto. U.O. Rete Idrografica Regionale 01/06.
Ferrari, I., S. Viglioli, P. Viaroli & G. Rossetti, 2006. The impact of the drought event of summer 2003 on the zooplankton of the Po River (Italy). Verhandlungen - Internationale Vereinigung für Theoretische und Angewandte Limnologie 29: 2143–2149.
Garnier, J., G. Billen & M. Coste, 1995. Seasonal succession of diatoms and Chlorophyceae in the drainage network of the Seine River: observations and modeling. Limnology and Oceanography 40: 750–765.
Goslee, S. C. & D. L. Urban, 2007. The ecodist package for dissimilarity-based analysis of ecological data. Journal of Statistical Software 22(7): 1–19.
Gosselain, V., J.-P. Descy, L. Viroux, C. Joaquim-Justo, A. Hammer, A. Métens & S. Schweitzer, 1998. Grazing by large river zooplankton: a key to summer potamoplankton decline? The case of the Meuse and Moselle rivers in 1994 and 1995. Hydrobiologia 369(370): 199–216.
Green, R. H., 1979. Sampling design and statistical methods for environmental biologists. Wiley, New York.
Harris, G. P., 1983. Mixed layer physics and phytoplankton populations: studies in equilibrium and non-equilibrium ecology. Progress Phycological Research 2: 1–52.
Harris, G. P., 1986. Phytoplankton ecology: structure, function and fluctuation. Chapman and Hall, London.
Havel, J. E., K. A. Medley, K. D. Dickerson, T. R. Angradi, D. W. Bolgrien, P. A. Bukaveckas & T. M. Jicha, 2009. Effect of main-stem dams on zooplankton communities of the Missouri River (USA). Hydrobiologia 628: 121–135.
Heiskary, S. & H. Markus, 2001. Establishing relationships among nutrient concentrations, phytoplankton abundance, and biochemical oxygen demand in Minnesota, USA, rivers. Lake and Reservoir Management 17: 251–267.
IPCC, 2007. Climate Change 2007: The Physical Science Basis. In Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor & H. L. Miller (eds), Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, M. F. Coveney, R. Deneke, M. T. Dokulil, B. Foy, D. Gerdeaux, S. E. Hampton, S. Hilt, K. Kangur, J. Köhler, E. H. H. R. Lammens, T. L. Lauridsen, M. Manca, M. R. Miracle, B. Moss, P. Nõges, G. Persson, G. Phillips, R. Portielje, S. Romo, C. Schelske, D. Straile, I. Tatrai, E. Willén & M. Winder, 2005. Lake responses to reduced nutrient loading–an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Kelly, M. G. & B. A. Whitton, 1998. Biological monitoring of eutrophication in rivers. Hydrobiologia 384: 55–67.
Kirk, J. T. O., 1994. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed. Cambridge University Press, Cambridge.
Komárek, J. & B. Fott, 1983. Chlorophyceae (Grünalgen). Ordnung: Chlorococcales. In: Huber-Pestalozzi - Das. Phytoplankton des Süßwassers. Systematik und Biologie 7 Teil, 1 Hälfte. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart.
Kristensen, P. & H. O. Hansen (eds), 1994. European Rivers and Lakes. Assessment of Their Environmental State. European Environment Agency, EEA Environmental Monographs 1, Copenhagen.
Legendre, P. & L. Legendre, 1998. Numerical ecology, 2nd English ed. Elsevier Science BV, Amsterdam.
Lewis, W. M. & W. A. Wurtsbaugh, 2008. Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. International Review of Hydrobiology 93: 446–465.
Lewis, W. M., S. K. Hamilton, M. A. Lasi, M. Rodríguez & J. F. Saunders III, 2000. Ecological Determinism on the Orinoco Floodplain. BioScience 50: 681–692.
Mitrovic, S. M., L. C. Bowling & R. T. Buckney, 2001. Vertical disentrainment of Anabaena circinalis in the turbid, freshwater Darling River, Australia: quantifying potential benefits from buoyancy. Journal of Plankton Research 23: 47–55.
OECD, 1982. Eutrophication of Waters. Monitoring, Assessment and Control. OECD, Paris.
Oksanen, J., R. Kindt, P. Legendre, B. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens & H. Wagner, 2008. vegan: Community Ecology Package. R package version 1.15-1 [available on internet at http://cran.r-project.org/, http://vegan.r-forge.r-project.org/].
Phillips, G., O.-P. Pietiläinen, L. Carvalho, A. Solimini, A. Lyche Solheim & A. C. Cardoso, 2008. Chlorophyll–nutrient relationships of different lake types using a large European dataset. Aquatic Ecology 42: 213–226.
R Development Core Team, 2008. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0 [available on internet at http://www.R-project.org].
Reynolds, C. S., 1988. Functional morphology and the adaptive strategies of freshwater phytoplankton. In Sandgren, C. D. (ed.), Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge University Press, Cambridge: 388–433.
Reynolds, C. S., 1999. Non-determinism to probability, or N:P in the community ecology of phytoplankton. Archiv für Hydrobiologie 146: 23–35.
Reynolds, C. S., 2006. The Ecology of Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S. & J.-P. Descy, 1996. The production, biomass and structure of phytoplankton in large rivers. Archiv für Hydrobiologie Supplement 113, Large Rivers 10: 161–187.
Richardson, W. B., E. A. Strauss, L. A. Bartsch, E. M. Monroe, J. C. Cavanaugh, L. Vingum & D. M. Soballe, 2004. Denitrification in the Upper Mississippi River: rates, controls, and contribution to nitrate flux. Canadian Journal of Fisheries and Aquatic Sciences 61: 1102–1112.
Rossetti, G., P. Viaroli & I. Ferrari, 2008. Role of abiotic and biotic factors in structuring the metazoan plankton community in a lowland river. River Research and Applications. doi:10.1002/rra.1170.
Rossi, D. & R. Veltri, 2007. Come abbiamo fronteggiato l’emergenza idrica. Adige-Etsch. Periodico trimestrale a cura dell’Autorità di Bacino del Fiume Adige. Ottobre 07: 15–19.
Rott, E., N. Salmaso & E. Hoehn, 2007. Quality control of Utermöhl based phytoplankton biovolume estimates—an easy task or a Gordian knot? Hydrobiologia 578: 141–146.
Ruse, L. P. & A. J. Hutchings, 1996. Phytoplankton composition of the River Thames in relation to certain environmental variables. Archiv für Hydrobiologie Supplement 113, Large Rivers 10: 189–201.
Salmaso, N., 2003. Life strategies, dominance patterns and mechanisms promoting species coexistence in phytoplankton communities along complex environmental gradients. Hydrobiologia 502: 13–36.
Salmaso, N. & G. Braioni, 2008. Factors controlling the seasonal development and distribution of the phytoplankton community in the lowland course of a large river in Northern Italy (River Adige). Aquatic Ecology 42: 533–545.
Salmaso, N. & J. Padisák, 2007. Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112.
Sellers, T. & P. A. Bukaveckas, 2003. Phytoplankton production in a large, regulated river: a modeling and mass balance assessment. Limnology and Oceanography 48: 1476–1487.
Sherman, B. S., I. T. Webster, G. J. Jones & R. L. Olivier, 1998. Transitions between Aulacoseira and Anabaena in a turbid river weir pool. Limnology and Oceanography 43: 1902–1915.
Skidmore, R. E., S. C. Maberly & B. A. Whitton, 1998. Patterns of spatial and temporal variation in phytoplankton chlorophyll a in the River Trent and its tributaries. The Science of the Total Environment 210(211): 357–365.
Sokal, R. R. & F. J. Rohlf, 1995. Biometry, 3rd ed. Freeman & Company, New York.
Sommer, U., Z. M. Gliwicz, W. Lampert & A. Duncan, 1986. The PEG-model of seasonal succession of planktonic events in fresh waters. Archive für Hydrobiologie 106: 433–471.
Stoyneva, M., 1994. Shallows of the lower Danube as additional sources of potamoplankton. Hydrobiologia 289: 97–108.
Thorp, J. H., A. R. Black, K. H. Haag & J. D. Wehr, 1994. Zooplankton assemblages in the Ohio River: seasonal, tributary, and navigation dam effects. Canadian Journal of Fisheries and Aquatic Sciences 51: 1634–1643.
Van Nieuwenhuyse, E. E. & J. R. Jones, 1996. Phosphorus-chlorophyll relationship in temperate streams and its variation with stream catchment area. Canadian Journal of Fisheries and Aquatic Sciences 53: 99–105.
Wehr, J. D. & J.-P. Descy, 1998. Use of phytoplankton in large river management. Journal of Phycology 34: 741–749.
Young, K., G. K. Morse, M. D. Scrimshaw, J. H. Kinniburgh, C. L. MacLeod & J. N. Lester, 1999. The relation between phosphorus and eutrophication in the Thames catchment, UK. Science of the Total Environment 228: 157–183.
Acknowledgements
This study was co-funded by the Basin Authority of River Adige. Chemical analyses were carried out at the Environmental Agency of the Trentino Province. Samplings were carried out with the logistic support of the Environmental Agencies of Bolzano and Rovigo. We wish to thank the technical staff at our Institute for chemical and biological analyses, in particular Milva Tarter and Lorena Ress. We appreciate the help of Barbara Centis in identifying diatoms and supporting the field operations. The manuscript has benefitted from the constructive comments of Dr. W. Richardson (US Geological Survey) and of an anonymous reviewer. This study was presented as a contributed article at the Bat Sheva de Rothschild seminar on Phytoplankton in the Physical Environment—The 15th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP), Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. Zohary, J. Padisák & L. Naselli-Flores / Phytoplankton in the Physical Environment: Papers from the 15th Workshop of the International Association for Phytoplankton Taxonomy and Ecology (IAP), held at the Ramot Holiday Resort on the Golan Heights, Israel, 23–30 November 2008
Rights and permissions
About this article
Cite this article
Salmaso, N., Zignin, A. At the extreme of physical gradients: phytoplankton in highly flushed, large rivers. Hydrobiologia 639, 21–36 (2010). https://doi.org/10.1007/s10750-009-0018-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-0018-0