Abstract
Purpose
The aim of this study was to evaluate the effectiveness of inspiratory muscle training (IMT) on sleep and functional capacity to exercise in subjects with obstructive sleep apnea (OSA).
Methods
This is a controlled, randomized, double-blind study conducted in 16 OSA patients divided into two groups: training (IMT: n = 8) and placebo-IMT (P-IMT: n = 8). IMT was conducted during 12 weeks with a moderate load (50–60% of maximal inspiratory pressure—MIP), while P-IMT used a load < 20% of MPI. Total daily IMT time for both groups was 30 min, 7 days per week, twice a day.
Results
There was no difference comparing IMT to P-IMT group after training for lung function (p > 0.05) and respiratory muscle strength (p > 0.05). Maximal oxygen uptake (VO2Max) was not significantly different between IMT and P-IMT group (mean difference − 1.76, confidence interval (CI) − 7.93 to 4.41, p = 0.71). The same was observed for the other ventilatory and cardiometabolic variables measured (p > 0.05). A significant improvement in sleep quality was found when Pittsburgh Sleep Quality Index (PSQI) values of IMT and P-IMT group after training were compared (mean difference: 3.7, confidence interval 95% (CI95%) 0.6 to 6.9, p = 0.02) but no significant changes were seen in daytime sleepiness between both groups after the intervention (mean difference: 3.4, CI 95%: − 3.3 to 10.0; p = 0.29).
Conclusion
According to these results, 12 weeks of moderate load IMT resulted in improved sleep quality, but there were no significant repercussions on functional capacity to exercise or excessive daytime sleepiness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a breathing-related sleep disorder characterized by partial (hypopnea) or complete (apnea) occlusion of the upper airways (UA). Hypoxia/reoxygenation episodes that occur during sleep can promote or worsen cardiovascular diseases and stimulate systemic consequences such as chronic fatigue, excessive daytime sleepiness, sleep fragmentation, muscular injuries, and impaired functional capacity to exercise [1, 2]. Explanations for impaired exercise tolerance in OSA are linked to sedentarism, obesity, cardiovascular diseases, dyspnea, abnormalities of respiration, and other unknown mechanisms [3]. Moreover, an obstructed airway may lead to increased inspiratory efforts and a significantly lower functional performance in inspiratory muscles in OSA patients [4].
Many options like surgical interventions, continuous positive airway pressure (CPAP), and oral devices are considered for OSA treatment [5]. In this context, and considering comorbidities and cardiovascular consequences of OSA, exercise training is presented as a well-tolerated adjunct strategy for patients. Benefits include a reduction in the prevalence of OSA and associated comorbidities, as well as a decrease in the apnea-hypopnea index (AHI), even without changes in body weight [6]. A meta-analysis conducted in 2014 not only showed improvement in the severity of apnea, but also on sleep quality and daytime sleepiness in individuals with OSA who engaged in regular physical activity [7].
Regarding specific inspiratory muscle training (IMT), a limited number of reports have evaluated the effect of IMT in OSA [8], but numerous studies have demonstrated the effects of this type of training in several populations [9, 10]. Studies about the repercussion of IMT on functional capacity to exercise are still controversial. Asthmatic subjects using IMT showed a reduction in perception of dyspnea, improvement in inspiratory muscle fatigue, and exercise tolerance [11]. On the other hand, 6 weeks of IMT in type 2 diabetes subjects did not promote changes in pulmonary function, autonomic modulation, or functional capacity to exercise [12].
Thus, the aim of this study was to establish the effectiveness of IMT on functional capacity to exercise, sleep quality, and daytime sleepiness in OSA.
Methods
Sample
This is a double-blind and randomized clinical trial conducted with 16 patients from Pronto Socorro Cardiológico de Pernambuco (PROCAPE) and approved by the Human Research Ethics Committee of the Federal University of Pernambuco (UFPE). The research was regularly registered in clinical trials and can be accessed by the code NCT02584205. It was carried out between March 2015 and May 2016, in the Laboratory of Cardiopulmonary Physiotherapy (LACAP) at the Federal University of Pernambuco.
Randomization and blinding
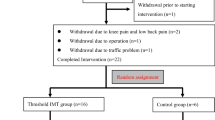
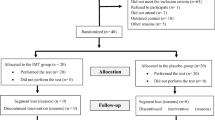
The selected patients were randomized through the randomization.com web service. A third person not involved in the research allocated the numbers of the patients in black and opaque envelopes so that patients and the first evaluator were blind. The process of capturing, filtering, randomizing, and allocating patients is described in Fig. 1.
Eligibility criteria
Inclusion criteria were patients between 30 and 65 years of age with body mass index (BMI) ≤ 39.9 kg/m2 with moderate or severe apnea (AHI ≥ 15 events/hour diagnosed by polygraphy, and who could perform cardiopulmonary exercise testing.
Exclusion criteria were patients using CPAP, who had history of pulmonary disease, arrhythmias, heart failure (New York Heart Association class III or IV), unstable angina, valvular heart disease, uncontrolled hypertension or diabetes mellitus, renal disease, and metabolic or endocrine disorders.
Measure of daytime sleepiness
The Epworth sleepiness scale (ESS) was used, which is a subjective scale and features commonly encountered day-to-day life situations. It promotes a self-evaluation about the possibility of dozing while performing such activities, and is scored from 0 to 3, being: 0—no chance of dozing; 1—small chance; 2—moderate chance of dozing; 3—high chance of dozing. Total score is based on a scale from 0 to 24, and scores of 11–24 represent increasing levels of excessive daytime sleepiness [13].
Measure of sleep quality
The Pittsburgh Sleep Quality Index (PSQI) is a self-rated questionnaire that evaluates the quality of sleep and disorders over the last month. It differentiates “poor” from “good” sleep by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. It scores 0–4 as good quality of sleep, 5–10 as poor quality, and above 10 as having a sleep disorder [14].
Evaluation of respiratory muscle strength
The values of maximal respiratory pressures were obtained by a digital manometer (NEPEB-LabCare/UFMG) coupled to a type of diver mouthpiece. The maneuvers were carried out with the patient seated with knees flexed to 90° and were performed using the residual volume (RV) to perform a forced inspiration. Maximal inspiratory pressure (MIP) was determined as the maximal inspiratory pressure sustainable for 1 s. The system considered three valid maneuvers (coefficient of variation below 10%). The highest value was used in the analysis, considering the reference values for the adult Brazilian population [15].
Lung function
Lung function was measured by a Minispir®light spirometer with Winspiro®light software. Patients were seated with flexed knees at 90° and held three deep breaths, inspired up to the total lung capacity (TLC) and then exhaled all the air to their residual volume (RV) to obtain the variables FEV1 (forced expiratory volume in 1 s), FVC (forced vital capacity), PEF (peak expiratory flow), and FEV1/FVC. The test was replicated at least three times until the system considered the best maneuver as more reproducible and acceptable, considering the reference values for the adult Brazilian population [16].
Cardiopulmonary exercise testing
CPET was performed using a treadmill (Centurium 300, Micromed, Brazil). The software ErgoPCElite® was associated with 12-lead electrocardiogram (Micromed, Brazil). The respiratory variables were collected in standard conditions of temperature (18–22 °C), pressure, and humidity (50–70%), with a face mask (without leaks) coupled to a gas analyzer (Cortex—Metalyzer II—Germany) during exercise. The patient was instructed not to communicate verbally during the test, informing their levels of fatigue through hand signals. The test was considered maximum when the patients reached a respiratory exchange rate (RER) ≥ 1.1, volitional exhaustion or the test was terminated by the medical monitor.
Training protocol
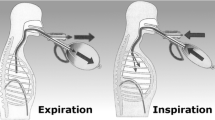
Inspiratory muscle training (IMT) was performed with the powerbreath® classic light device. The established protocol for the IMT group consisted of 12 weeks of home-based training with a moderate load (50–60% of MIP), 7 days a week, twice a day. This protocol specified that the patients should perform 90 incursions in 3 cycles (30 incursions in each cycle), with a 1-min interval between them and total daily IMT time for both groups of 30 min. Next, the training lasted approximately 15 min, twice a day, and load adjustments occurred in the quarterly visits of the patients to the lab. P-IMT group load was < 20% of MIP, which is considered insufficient to promote training of the respiratory muscles [17].
Statistical analysis
Descriptive and inferential analyses were performed using the software Statistical Package for the Social Sciences, version 20.0 (SPSS Inc., Chicago, IL, USA). Characteristics of the sample and treatment effect between groups were verified using Student’s t test for independent samples. Chi-square test corrected by Fisher’s exact test was used for the categorical variables. The results are shown as mean ± standard deviation, and as differences of means and confidence interval (95%). Statistical significance was set at p < 0.05.
Results
There were no differences between the groups regarding demographic, anthropometric, or clinical characteristics (Table 1).
Lung function and respiratory muscle strength are shown in Table 2. This sample showed no pulmonary impairment. FEV1 and FVC were greater than 80% of predicted and MIP greater than 60 cmH2O. There was no difference comparing IMT to P-IMT group after training for lung function and respiratory muscle strength.
Data of the CPET are described in Table 3. Maximal oxygen uptake (VO2Max) was not significantly different between IMT and P-IMT group, with a mean difference of − 1.76, confidence interval (CI) − 7.93 to 4.41, p = 0.71). The same was observed for the other ventilatory and cardiometabolic variables measured (p > 0.05).
Significant improvement in sleep quality were found in comparing PSQI global score (mean difference: 3.7, confidence interval 95% (CI95%) 0.6 to 6.9, p = 0.02) and in sleep quality (mean difference: − 0.9, confidence interval 95% (CI95%) − 0.17 to 0.0, P = 0.049), but not in PSQI subscores for sleep duration (mean difference: 0.0, confidence interval 95% (CI95%) − 0.9 to 0.9, p = 0.04) or daytime dysfunction (mean difference: − 0.6, confidence interval 95% (CI95%) − 1.7 to 0.4, p = 0.02) for IMT and P-IMT group after training. Furthermore, no significant changes were measured in daytime sleepiness when comparing ESS values between both groups after the intervention (mean difference: 3.4, CI 95%: -3.3 to 10.0; p = 0.29) (Table 4).
Discussion
This is a double-blind, randomized controlled trial, and it is the first study to our knowledge to investigate the effectiveness of inspiratory muscle training on functional capacity to exercise in subjects with OSA. The results of the present study showed that IMT improved PSQI score in the IMT group when compared to the P-IMT group. However, IMT did not improve functional capacity to exercise, lung function, or excessive daytime sleepiness in these patients.
Respiratory muscle impairment in OSA has been reported in some studies, and seems to be related to the severity of the disease as well as the increase of respiratory drive due to the occlusion of UA occurring in these patients during sleep. Furthermore, hypoxia and hypercapnia, via the chemoreflexes, increase sympathetic activity that leads to comorbidities associated with OSA [18, 19]. In our study, the sample did not show muscle weakness or limitations in lung function under basal conditions, and there were no changes in lung function or inspiratory muscle strength after the intervention. Chien et al. [4] reported reduced respiratory muscle performance in OSA patients, but greater fatigue was only found in severe OSA subjects. It is possible that repetitive inspiratory effort against an obstructed airway may also induce deleterious effects on the inspiratory muscles in severe OSA patients. Our sample was comprised of subjects with moderate (15 ≥ AHI < 30 events/hours) to severe (AHI ≥ 30 events/hours) OSA and not only of severe OSA patients, which may explain the absence of weakness in respiratory muscles at baseline in both groups.
In our study, daytime sleepiness (one of the main symptoms of OSA) did not show differences when comparing the IMT and P-IMT group after intervention. Daytime sleepiness is more related to awakenings and micro-awakenings that occur at night than to hypoxia levels [20]. Lombardi et al. [21] analyzed apneic patients on the presence and absence of excessive sleepiness. They reported that there was lower baroreflex sensitivity in subjects with excessive sleepiness, indicating sympathetic hyperactivity. It is remarkable that the increase in sympathetic activation is involved in OSA pathophysiology, as well as with associated comorbidities.
In this study, there was not found changes on ESS score after the intervention, but it has shown significant improvement on global PSQI score and on sleep quality, sleep duration, and daytime dysfunction PSQI subscores compared to the control group, indicating good overall sleep quality. Other exercise training modalities have already been reported in the literature and put the regular practice of physical activity as an efficient method for prevention and treatment of OSA [6, 22]. Vranish and Bailey [8] carried out a study in 2016 in patients with OS, and after 6 weeks of training they observed that IMT group presented a reduction in pressure levels and serum catecholamines, and also improved the subjective quality of sleep even without changes in the AHI, thus supporting the use of IMT as a strategy for treating the disease.
Regarding the functional capacity to exercise in the studied sample according to the American Heart Association (AHA) [23] classification which considers VO2Max, age, and gender; we observed that volunteers had cardiorespiratory fitness levels ranging from regular to weak, except for one patient who showed good cardiorespiratory fitness. It has already been reported in the literature that acute and chronic hypoxia cause reduction in VO2Max. This decrease is directly proportional to the drop in hemoglobin saturation [24].
There is still no consensus on the influence of OSA on effort tolerance. Some studies report a reduction in functional capacity to exercise in the overweight and obese subjects with OSA [3, 25]. Only one study compared VO2Max values in obese and eutrophic subjects with and without OSA, and suggest that obesity alone and gender when associated with diabetes, but not OSA, influenced exercise tolerance [26]. Our study also contained overweight and obese volunteers, which may have contributed to the findings of reduced cardiorespiratory fitness, since obesity negatively interferes in the functional capacity to exercise.
Despite the low levels of cardiorespiratory fitness found in the present study, there was no improvement in the parameters related to exercise tolerance provided by the cardiopulmonary exercise test. Not even variables such as VO2Max and VO2 at the first threshold were affected by IMT. The increase in VO2Max with training is proportional to the muscle mass used in exercise [24]. The fact that IMT is restricted to the respiratory muscles may not generate enough physiological overload on the cardiovascular system in order to provide significant gains in maximum oxygen consumption [27].
In agreement with our findings in evaluating healthy subjects submitted to IMT for 4 weeks, Edwards (2013) also found no alterations in the ventilatory variables of the cardiopulmonary exercise test. However, he found an increase in time to volitional exhaustion and the speed at which the individual reached peakVO2. It is possible that changes related to functional capacity to exercise resulting from IMT are initially related to the attenuated perception of fatigue by reducing the afferent input on the respiratory center, thus improving effort tolerance [28]. The group of neuronal afferents III and IV is directly involved with cardiovascular and respiratory control during exercise [29]. These groups of neurons after plasticity inhibit the afferent feedback to the respiratory center, and delay fatigue onset in peripheral and respiratory musculature [30].
Our study has some limitations. First, a small number of subjects involved in the research make a group stratification based on OSA severity impossible. In addition, other variables related to exercise tolerance and associated with cardiopulmonary exercise test such as volitional exhaustion were not measured, and could also reflect an improvement in the performance of these individuals.
Conclusion
According to the results of the present study, 12 weeks of moderate load IMT resulted in improved sleep quality, but did not seem to cause significant repercussions on functional capacity to exercise, inspiratory muscle strength, lung function, or excessive daytime sleepiness.
From this perspective, IMT could emerge as an adjunct strategy for treating OSA. Despite it not showing an increase in the functional capacity to exercise, IMT can be considered as an alternative for OSA management, since an important improvement in the quality of sleep of these subjects was found. We can consider not only the isolated practice of IMT, but also its association with other types of training, optimizing the gains and intensifying the results.
Thus, we recommend further studies with a larger sample size, in different groups of OSA severity following the same methodological rigor in comparing different training intensities and establishing the physiological mechanisms through which IMT would bring greater benefits to OSA subjects.
References
Jackson MJ, O'Farrell S (1993) Free radicals and muscle damage. Br Med Bull 49(3):630–641
Bradley TD, Floras J (2009) Obstructive sleep apnea and its cardiovascular consequences. Lancet 373(9657):82–93. https://doi.org/10.1016/S0140-6736(08)61622-0
Rizzi CF, Cintra F, Risso T, Pulz C, Tufik S, de Paola A, Poyares D (2010) Exercise capacity and obstructive sleep apnea in lean subjects. Chest 137(1):109–114. https://doi.org/10.1378/chest.09-1201
Chien MY, YT W, Lee PL, Chang YJ, Yang PC (2010) Inspiratory muscle dysfunction in patients with severe obstructive sleep apnoea. Eur Respir J 35(2):373–380. https://doi.org/10.1183/09031936.00190208
Deacon N, Jen R, Li Y, Malhotra A (2016) Treatment of obstructive sleep apnea. Prospects for personalized combined modality therapy. Ann Am Thorac Soc 13(1):101–108. https://doi.org/10.1513/AnnalsATS.201508-537FR
Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine J, Davis JM, Youngstedt SD (2011) The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep 34(12):1631–1640. https://doi.org/10.5665/sleep.1422
Iftikhar IH, Kline CE, Youngstedt SD (2014) Effects of exercise training on sleep apnea: a meta-analysis. Lung 192(1):175–184
Vranish JR, Bailey EF (2016) Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep 39(6):1179–1185
Hajghanbari B, Yamabayashi C, Buna TR, Coelho JD, Freedman KD, Morton TA, Palmer SA, Toy MA, Walsh C, Sheel AW, Reid WD (2013) Effects of respiratory muscle training on performance in athletes: a systematic review with meta-analyses. J Strength Cond Res 27(6):1643–1663
Bavarsad MB, Shariati A, Eidani E, Latifi M (2015) The effect of home-based inspiratory muscle training on exercise capacity, exertional dyspnea and pulmonary function in COPD patients. Iran J Nurs Midwifery Res 20(5):613–618. https://doi.org/10.4103/1735-9066.164588
Turner LA, Mickleborough TD, Mcconnell AK, Stager JM, Tecklenburg-Lund S, Lindley MR (2011) Effect of inspiratory muscle training on exercise tolerance in asthmatic individuals. Med Sci Sports Exerc 43(11):2031–2038. https://doi.org/10.1249/MSS.0b013e31821f4090
Corrêa AP, Ribeiro JP, Balzan FM, Mundstock L, Ferlin EL, Moraes RS (2011) Inspiratory muscle training in type 2 diabetes with inspiratory muscle weakness. Med Sci Sports Exerc 43(7):1135–1141. https://doi.org/10.1249/MSS.0b013e31820a7c12
Bertolazi AN, Fagondes SC, Hoff LS, Pedro VD, Barreto SSM, Johns MW (2009) Validação da escala de sonolência de Epworth em português para uso no Brasil. J Bras Pneumol 35(9):877–883
Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, de Barba ME, Barreto SS (2011) Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med 12(1):70–75. https://doi.org/10.1016/j.sleep.2010.04.020
Pessoa IM, Houri Neto M, Montemezzo D, Silva LAM, Andrade AD, Parreira VF (2014) Predictive equations for respiratory muscle strength according to international and Brazilian guidelines. Braz J Phys Ther 18(5):410–418
Pereira CA, Duarte AA, Gimenez A, Soares MR (2014) Comparison between reference values for FVC, FEV1, and FEV1/FVC ratio in White adults in Brazil and those suggested by the Global Lung Function Initiative 2012. J Bras Pneumol 40(4):397–402
Hill K, Cecins NM, Eastwood PR, Jenkins SC (2010) Inspiratory muscle training for patients with cronic obstructive pulmonar disease: a pratical guide for clinicians. Arch Phys Med Rehabil 91(9):1466–1470. https://doi.org/10.1016/j.apmr.2010.06.010
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA 283(14):1829–1836
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342(19):1378–1384
Roehrs T, Zorick F, Wittig R, Conway W, Roth T (1989) Predictors of objective level of daytime sleepiness in patients with sleep-related breathing disorders. Chest 95(6):1202–1206
Lombardi C, Parati G, Cortelli P, Provini F, Vetrugno R, Plazzi G, Vignatelli L, Di Rienzo M, Lugaresi E, Mancia G, Montagna P, Castiglioni P (2008) Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res 17(3):263–270. https://doi.org/10.1111/j.1365-2869.2008.00659.x
Mendelson M, Lyons OD, Yadollahi A, Inami T, Oh P, Bradley TD (2016) Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J 48(1):142–150. https://doi.org/10.1183/13993003.01897-2015
Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD Jr, Winters WL, Yanowitz FG, Ritchie JL, Gibbons RJ, Cheitlin MD, Eagle KA, Gardner TJ, Garson A Jr, Lewis RP, O'Rourke RA, Ryan TJ (1997) ACC/AHA guidelines for exercise testing: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on exercise testing). J Am Coll Cardiol 30(1):260–231
Ferretti G (2014) Maximal oxygen consumption in healthy humans: theories and facts. Eur J Appl Physiol 114(10):2007–2036. https://doi.org/10.1007/s00421-014-2911-0
Chien MY, Lee P, Tsai YF, Yang PC, YT W (2012) C-reactive protein and heart rate recovery in middle-aged men with severe obstructive sleep apnea. Sleep Breath 16(3):629–637. https://doi.org/10.1007/s11325-011-0549-2
Rizzi CF, Cintra F, Mello-Fujita L, Rios LF, Mendonca ET, Feres MC, Tufik S, Poyares D (2013) Does obstructive sleep apnea impair the cardiopulmonary response to exercise? Sleep 36(4):547–553. https://doi.org/10.5665/sleep.2542
Edwards AM, Cooke CB (2004) Oxygen uptake kinetics and maximal aerobic power are unaffected by inspiratory muscle training in healthy subjects where time to exhaustion is extended. Eur J Appl Physiol 93(1–2):139–144
Edwards AM (2013) Respiratory muscle training extends exercise tolerance without concomitant change to peak oxygen uptake: physiological, performance and perceptual responses derived from the same incremental exercise test. Respirology 18(6):1022–1027. https://doi.org/10.1111/resp.12100
Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2010) Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol (1985) 109(4):966–976. https://doi.org/10.1152/japplphysiol.00462.2010
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81(4):1725–1789
Funding
The Coordination for the Improvement of Higher Education Personnel (CAPES) provided financial support in the form of data collection funding. The sponsor had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Souza, A.K.F., Dornelas de Andrade, A., de Medeiros, A.I.C. et al. Effectiveness of inspiratory muscle training on sleep and functional capacity to exercise in obstructive sleep apnea: a randomized controlled trial. Sleep Breath 22, 631–639 (2018). https://doi.org/10.1007/s11325-017-1591-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-017-1591-5