Abstract
Objective
The study aimed to evaluate whether the inflammatory marker “high-sensitivity C-reactive protein (hsCRP)” level was associated with impaired heart rate recovery at 1 min after exercise termination (HRR-1) in middle-aged patients with severe obstructive sleep apnea (OSA).
Methods
Thirty middle-aged male patients (40–64 years old) with severe OSA (apnea–hypopnea index [AHI] ≥ 30 h−1) and 30 subjects without OSA (AHI < 5 h−1), matched with age and body mass index (BMI), were recruited. All subjects underwent an overnight polysomnography and completed a symptom-limited maximal exercise test. Cardiopulmonary parameters included peak oxygen consumption (VO2peak) and heart rate response during and immediately after exercise. Fasting blood samples were drawn for hsCRP analysis.
Result
Patients with severe OSA had significantly higher hsCRP levels (0.18 vs. 0.07 mg/dl, P < 0.01), lower reduced HRR-1, peak heart rate, and VO2peak values than those in the controls. The hsCRP levels significantly correlated with HRR-1 in the OSA group (r = −0.69, P < 0.01) after adjustment for VO2peak (r = −0.66, P < 0.01). Furthermore, stepwise multiple regression analysis showed that HRR-1 and AHI were significant predictors of hsCRP levels in all participants (adjusted R 2 = 0.53, P < 0.01).
Conclusions
Blunted HRR was shown in middle-aged men with severe OSA, and it was associated with high hsCRP levels significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Culminated evidence indicates that obstructive sleep apnea (OSA) is associated with metabolic and cardiovascular abnormalities [1]. The underlying mechanisms linking OSA and cardiovascular disease have not been clearly elucidated. However, unique features of OSA, including intermittent hypoxia and sleep fragmentation from sleep, have been proposed to initiate many pathophysiologic processes that promote cardiovascular disease, such as increased sympathetic activation [2], altered cardiovascular variability [3], and low-grade inflammation [4]. The pathophysiological hallmark of OSA, chronic sympathetic hyperactivity, has also been reported to play an important role in the pathogenesis of left ventricular dysfunction [5].
Slow heart rate recovery (HRR) has been reported to be important in predicting mortality in healthy individuals [6] and heart failure [7]. HRR after exercise is mediated by a combination of sympathetic withdrawal and parasympathetic reactivation, primarily by vagal reactivation [8]. HRR at 1 min after exercise termination (HRR-1) was thus used as a simple measure indicative of decreased autonomic nervous system activity [9]. There is emerging evidence that autonomic nervous system function is related to inflammation [10–12]. Some studies have shown that decreased HRR-1 was associated with high high-sensitivity C-reactive protein (hsCRP) levels in elderly individuals [13] and in unselected patients [9]. The concept of “inflammatory reflex” has been proposed to elucidate the association between impaired HRR-1 and CRP levels, which refers to vagus nerve stimulation that can modulate inflammatory cytokines through the cholinergic anti-inflammatory pathway [14]. In other words, the nervous system can reflexively regulate the inflammatory response, just as it controls heart rate and other vital functions [14].
OSA has been reported to be associated with increased circulating levels of hsCRP [15]. Several studies have demonstrated an association between HRR-1 and the severity of OSA for young men [16, 17]. In addition, continuous positive airway pressure (CPAP) treatment has been shown to result in an improvement in HRR in OSA [18]. However, whether the elevated hsCRP level is associated with the impaired HRR-1 in middle-aged men with OSA who did not have cardiovascular diseases, diabetes, or very overweight or obesity is still not clear. Studies in this age group have provided divergent results whether or not HRR is impaired in OSA [19, 20].
Therefore, the aim of this study was to test our hypothesis that heart rate response to exercise and recovery may be blunted in middle-aged patients with severe OSA and elevated hsCRP level may associate with impaired HRR.
Methods
Participants
Male patients with the age range of 40–65 years who were referred to the Center of Sleep Disorder of National Taiwan University Hospital for evaluation of sleep apnea were prospectively recruited in this study since September 2007. The consecutive participants were newly diagnosed severe OSA with whole-night polysomnography (PSG) (apnea–hypopnea index (AHI) of ≥30 h−1) were recruited. The control group were age- (±3 years), weight- (±3 kg), and height-matched (±5 cm) subjects without OSA (AHI < 5 h−1). Control subjects were recruited from patients who were referred to a sleep laboratory of snoring, sleep disturbance, or excessive daytime sleepiness and confirmed without OSA. The exclusion criteria were as follows: subjects treated with negative chronotropic drugs (i.e., β-blockers, amiodarone, verapamil, or diltiazem), glucose-lowering drugs (i.e., metformin), lipid-lowering drugs (i.e. statin), and those with a history of coronary artery disease or other manifestations of atherosclerosis, heart failure, renal failure, diabetes mellitus, or any conditions that may limit exercise capacity (e.g., osteoarthritis). In addition, they were excluded if they had any missing value of our primary measures. All participants have never been treated for OSA. We determined that a target sample size of 30 participants per group was needed to provide a power of 0.80 at an alpha level of 0.05 according to data from a previous study [17]. The study was approved by the Institutional Ethics Committee of the National Taiwan University Hospital (NCT00813852), and all subjects provided their written informed consent prior to enrolment.
Diagnosis of OSA
Full-night PSG (Embla N7000, Medicare Flaga, Reykjavik, Iceland) was performed in the sleep laboratory following the protocol as previously described [21]. The sleep stage and respiratory event was scored according to the American Academy of Sleep Medicine standard [22]. Briefly, apnea was defined as the absence of airflow ≥10 s and hypopnea was a ≥50% decrease in airflow ≥10 s associated with reduced arterial oxygen saturation in ≥3% or an arousal. The oxygen desaturation index (ODI) was defined as the amount of reduction in arterial oxygen saturation (SaO2) in 4%/h. All of the sleep studies were analyzed by the same investigator to maximize interscorer and intrascorer reliability.
The level of daytime sleepiness was assessed using the Epworth sleepiness scale in the morning after nocturnal PSG. Normal values ranged from 2 to 10, with scores >10 indicating daytime sleepiness [23].
High-sensitivity C-reactive protein
A venous blood sample was collected from the antecubital vein the next morning of PSG while the patient was in a supine position. Measurement of hsCRP levels were carried out using the CRP-Latex (II) immunoturbidimetric assay kit (Denka Seiken, Tokyo, Japan) on a Hitachi 911 immunoanalyzer (Roche Diagnostics, Indianapolis, IN, USA). This assay has a minimal detectable concentration of 0.03 mg/L and a total imprecision of 5.1% and 2.5% at concentrations of 0.2 and 1.9 mg/L, respectively [24]. All testing was performed by personnel blinded to clinical outcomes.
Vital signs and body composition measurements
Upon arrival at the laboratory, subjects rested in a chair for 5 min at least before heart rate and blood pressure were taken. The hypertensive patients were defined as those whose resting blood pressures are ≥140/90 mmHg or who reported the use of antihypertensive medication, such as diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor antagonist.
Weight was measured to the nearest 0.5 kg on a calibrated scale, and height was determined using a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared.
Body composition was then measured by a bioelectrical impedance analyzer (BIA) (Maltron BioScan 920, Esgender, UK) using an 800-μA current at a frequency of 50 kHz. The subjects were asked to lie in the supine position on a nonconducting surface for 5 min with their arms abducted away from their trunk and their legs slightly separated. Four surface electrodes and cables were attached to subject’s right hand and ankle, as shown in the user’s manual. When the measurements had stabilized, the analyzer calculated the percentage of body fat directly from the equation and displayed the value. Previous studies have demonstrated excellent test–retest reliability for BIA-obtained measurements, with correlation coefficients ranging from 0.96 to 0.99 for resistance measurements [25]. Hydrostatic weighing and BIA-predicted correlation coefficients range from 0.71 to 0.93, with standard errors of estimate ranging from 2.7% to 4.7% body fat [25, 26]. In addition, a good level of agreement between BIA and dual X-ray absorptiometry in estimating the percentage of body fat among Singapore Chinese adults was reported [27].
Cardiopulmonary exercise test
Each subject performed a symptom-limited maximal exercise test with continuous electrocardiographic monitoring following an initial practice session on a cycle ergometer on the same day. All participants were encouraged to exercise until exhaustion. The criteria for termination were adopted from the recommendations by the American College of Sports Medicine [28], including the subject’s desire to stop or evidence of ischemia or hypertensive response. In addition, various criteria have also been suggested to confirm that a maximal effort has been elicited during graded exercise, such as failure of heart rate to increase with further increase in exercise intensity, a plateau in oxygen uptake with increased workload, or a respiratory exchange ratio >1.1 [28]. The graded exercise protocol consisted of 3-min stages, starting at 25 W with a 25-W increment at each successive stage, while maintaining a pedaling rate of 50–60 rpm. After attaining maximal exercise, a cool-down exercise protocol consisting of 3 min at 25 W was performed. Heart rate was measured 1 min after exercise cessation while the subjects sat upright to calculate HRR-1. The value for HRR-1 was defined as the reduction in heart rate from the rate at peak exercise to the rate 1 min after the cessation of exercise (HRR-1) [6]. The intraclass correlation coefficients of HRR after 1, 2, 3, and 5 min of exercise cessation were 0.58–0.61 [29].
Modified Borg effort scales (out of 10) for evaluating the intensity of both dyspnea and leg discomfort were recorded at peak exercise. Respiratory gas exchange measurements, including peak ventilation (V Epeak) and oxygen consumption (VO2peak), were obtained during exercise using a computer-controlled, breath-by-breath metabolic measurement system (Vmax29 Metabolic Measurement System; SensorMedics, Anaheim, CA, USA). The tester was blinded to the results of biochemical analysis.
Exercise capacity was expressed as peak VO2 indexed to body weight (per milliliter per kilogram/minute) and peak VO2 indexed to fat-free mass (per milliliter per kilogram/minute). The respiratory exchange ratio, defined as VCO2 divided by VO2, was determined at peak exercise. The anaerobic threshold was determined by the V-slope method. The breathing reserve was defined as [1 − (V E/MVV)] × 100.
For assessment of the chronotropic response, peak heart rate (HRpeak) was expressed as the percentage of HRpredicted, where age-predicted heart rate was defined as 220 minus the subject’s age (in years). Chronotropic incompetence was defined as failure to achieve at least 85% of HRpredicted or failure to use 85% of heart rate reserve during exercise [30]. Abnormal HRR was defined as heart rate declines of ≤13 bpm in the first minute after exercise for cycle exercise protocols that employed a cool-down period [31].
Data analysis and statistics
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) v.13 for Windows (SPSS Inc., Chicago, IL, USA). Normal distribution of data was assured by the use of the Shapiro–Wilk test. The skewed data were log transformed and analyzed by parametric statistics if normality test was passed. Otherwise, nonparametric statistics were used. Continuous variables were expressed as means ± standard deviations, and categorical variables as numbers and percentages. Between-group comparisons at baseline were performed using independent Student’s t test, Mann–Whitney U test, or chi-squared test. The differences of exercise test parameters between the two groups were examined using a general linear model while controlling for age and BMI. Pearson’s correlation was first used to examine the associations among the variables and partial correlation analysis was performed, adjusted by peak oxygen consumption for each group. The stepwise multiple regression analysis was used to determine the significant predictors of log-transformed hsCRP in all participants and in the severe OSA group by including those variables in Pearson’s correlation analysis. The α value was set at 0.05.
Results
There were 67 men who participated in this study, and 7 subjects (3 in the OSA group and 4 in the control group) were excluded for lack of hsCRP results. Therefore, the results of 60 men were included in the analysis. Table 1 shows the basic demographic characteristics, sleep examination results, and hsCRP levels of the patients with severe OSA and controls. The two groups were matched for age and anthropometric characteristics. Significantly higher values of ESS, AHI, ODI, arousal index, and blood pressure were observed in the severe OSA group than in the control group (P < 0.05). However, both systolic and diastolic blood pressures were considered “normal” based on the most recent American Heart Association recommendations [32]. In addition, the numbers of participants with hypertension were also not significantly different between the two groups (P > 0.05). The average and lowest SaO2 in the severe OSA group were significantly lower than those in the control group (P < 0.001). The hsCRP levels were significantly higher in the severe OSA group (0.18 mg/dl; ranges, 0.05–0.42 mg/dl) than in the control group (0.07 mg/dl; ranges, 0.02–0.25 mg/dl) (P < 0.05).
All subjects completed the exercise tests to exhaustion without complications. Exercise capacity was significantly reduced in the severe OSA group, with lower VO2peak, VO2peak index to fat-free mass, and peak work rate achieved (P < 0.05). HRpeak, HRpeak in percentage of HRpredicted, and HRR-1 were also lower in the OSA group (P < 0.05) (Table 2). However, O2 pulse and ventilatory variables (V Epeak and breathing reserve) were not significantly different between the two groups (P > 0.05). In addition, subjective reasons for exercise termination assessed by the modified Borg scale for evaluation of both dyspnea and leg effort were not different between the two groups (P > 0.05).
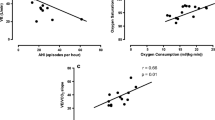
The Pearson’s correlation coefficients of log-transformed hsCRP levels and various parameters in all participants and each group are provided in Table 3. Log-transformed hsCRP levels were positively correlated with AHI (r = 0.53, P < 0.01), ODI, arousal index, smoking status, and mean arterial blood pressure (MAP), and it was negatively correlated with HRR-1 (r = −0.69, P < 0.01), VO2peak, SaO2, and sleep efficiency in all participants. The log-transformed hsCRP levels was significantly associated with HRR-1 only in the OSA group (r = −0.69, P < 0.01) (Table 3, Fig 1a), but associated with smoking status (r = 0.36, P < 0.05), HRR-1 (r = −0.52, P < 0.01), and VO2peak (r = −0.47, P < 0.01) in the control group (Table 3, Fig 1b). Partial correlation analysis was performed to find out the real relationship between log-transformed hsCRP and HRR-1 by controlling VO2peak. The significant correlation still existed in the OSA group (r = −0.66, P < 0.01), but no longer in the control group (r = −0.33, P = 0.08) (Fig 2).
In the stepwise multiple regression analysis which included all participants, predictors of log-transformed hsCRP levels included HRR-1 (β = −0.54, P < 0.001) and AHI (β = 0.30, P = 0.005, adjusted R 2 = 0.531) (Table 4). In the stepwise multiple regression analysis of the solely severe OSA group, the predictors of log-transformed hsCRP levels would only be HRR-1 (β = −0.69, P < 0.001, adjusted R 2 = 0.462) (Table 4).
Discussion
In this study, we found that patients with severe OSA exhibited higher hsCRP levels, slower HRR-1, and a lower cardiopulmonary capacity (VO2peak) than those in the control group. Moreover, there was a significant correlation between hsCRP and HRR-1 in the OSA group. The present study consisted of a well-matched non-OSA control group, which strengthened our point, impaired HRR-1 in middle-aged men with severe OSA.
Inflammation has been reported to play an important role in the development and progression of atherosclerosis. Previous studies have reported that patients with hsCRP levels in the highest quartile (0.38 to 1.5 mg/dl) had higher risks of stroke, peripheral vascular diseases, or myocardial infarction [33, 34]. Many previous studies have reported an independent relationship between OSA and elevated CRP levels [15, 35]. First, Shamsuzzaman and coworkers reported significantly elevated CRP levels in 22 OSA patients (0.09 to 2.73 mg/dl) compared with 20 healthy control subjects (0.02 to 0.90 mg/dl) matched for age and BMI [15]. Recently, Lui and coworkers demonstrated that CRP levels were associated with OSA independently of visceral obesity [35]. In Lui’s study, the hsCRP levels of patients with severe OSA was 0.056 to 0.188 mg/dl. Our result was in agreement with these reports, which showed that patients with severe OSA had higher CRP levels even when their inflammatory status was relatively low. However, the issue of an association between CRP levels and OSA is still highly controversial, and there have been some studies finding no such association [36, 37]. The negative findings might be due to these studies not taking into consideration the analytical confounders, such as central obesity, smoking status, or hypertension.
One of the merits of our study was using VO2peak to adjust HRR-1 in the correlation analysis. The present results showed significantly lower VO2peak in patients with OSA than in controls, which supported the results of Lin and colleagues [38]. However, some studies have reported no differences in VO2peak between patients with OSA and matched controls [19, 39]. The discrepancy might stem from the differences between studies with respect to sample size, severity of OSA, comorbid conditions, or habitual levels of physical activity. In addition, our severe OSA group had significantly lower HRpeak values but not lower V Epeak and breathing reserve than those in the control group. This suggested that the exercise limitation in the OSA group was due to circulatory rather than pulmonary factors.
Most importantly, we found that severe OSA was associated with impaired chronotropic responsiveness to exercise (inability to use most of the heart rate reserve, i.e., chronotropic incompetence) and abnormal HRR-1 (inability to promptly slow the heart rate immediately after exercise). According to the definitions [30, 31], 50% and 27% of the patients with severe OSA in the present study showed chronotropic incompetence and impaired HRR-1, respectively. The present study was in agreement with previous studies that indicated an impaired heart rate response in patients with OSA [17, 38].
The mechanisms underlying the impaired heart rate response to exercise in OSA are not entirely clear. It has been proposed that repetitive blood pressure surges during sleep and increased sympathetic activity during wakefulness may result in the downregulation of cardiac β-adrenergic receptors, which would cause an inability to quickly elevate heart rate sufficiently to meet the physiologic demands of exercise [40]. In addition, the changes in sympathetic activity in OSA could alter the baroreflex set point to higher levels of pressure [41]. The baroreceptors play a major role in the reflex regulation of both blood pressure and heart rate response. Previous study had reported that baroreflex control of heart rate during sleep depressed in OSA and improved after treatment with CPAP [42]. This might contribute to the impaired heart rate response to exercise in OSA.
Recently, Lombardi and coworkers have demonstrated an association of excessive daytime sleepiness with baroreflex sensitivity and heart rate variability in patients with sleep-related breathing disorders [43]. The link between these phenomena of the OSA population might be through complex interaction between dysfunctions in cerebral regions responsible for sleep regulation, daytime vigilance, and autonomic cardiovascular control. Further analysis of the correlation between ESS and HRR-1 of our participants revealed a significant correlation coefficient between the two parameters (r = −0.38, P < 0.05). However, this issue deserves to be investigated in future studies.
One of the important findings of the present study was a significant inverse correlation between hsCRP levels and HRR-1 that persisted in the OSA group with or without adjustment for VO2peak,but this relationship no longer existed after adjustment for VO2peak in the control group (r = −0.33, P > 0.05). Previous study has shown individuals with higher cardiopulmonary capacity increased in parasympathetic nervous system tone and decreased in sympathetic tone [44]. Recently, Jae and coworkers reported that autonomic nervous system function affects the relationship between cardiopulmonary fitness and CRP levels significantly [45]. Therefore, it speculated a complex relationship among HRR-1, CRP levels, and VO2peak. The gross relationship between HRR-1 and CRP levels was similar in both groups; however, it would be masked by good VO2peak in the control group. Thus, our results revealed that blunted HRR was associated with significantly high hsCRP levels in middle-aged men with severe OSA, but additional studies are needed to confirm this finding.
Our results are consistent with previous observational studies showing that decreased parasympathetic activity was associated with an elevation of inflammatory markers [9, 13]. However, the linking mechanism for HRR-1 and CRP levels is not clear. Recent researches have suggested a cholinergic anti-inflammatory reflex pathway that monitors and regulates the inflammatory response reflexively [14, 46]. In this reflex pathway, vagal efferents release acetylcholine, which interacts with macrophages of the reticuloendothelial system to inhibit proinflammatory cytokine release [46]. However, the relationship between inflammation and autonomic system function is still debated. Other studies have shown that the inflammation affected the autonomic nervous system [47]. IL-6 may affect autonomic balance by disturbing the hypothalamic–pituitary–adrenal axis at the level of the pituitary and adrenal glands [48]. It is still unclear whether higher inflammation levels are associated with decreasing HRR over time or whether slow HRR is associated with increasing inflammation. Future studies are needed to explore this issue.
This study has some limitations. First, given the cross-sectional nature of this study, any causal relationship between these constructs remains to be determined. Future longitudinal studies are needed in order to verify the relationships found in this study. Second, 50% of patients with severe OSA in the present study showed chronotropic incompetence, which would limit their ability to achieve the same level of peak exercise as the controls. Therefore, the unequal exercise challenge might complicate comparisons of HRR-1 between the two groups. Third, the control subjects in this study might not be true “controls” because they were referred for sleep examination due to snoring, sleep disturbance, or excessive daytime sleepiness. However, our control group was generally healthy, and the ESS and parameters of sleep examination were significantly better than those in the severe OSA group. Fourth, our participants were limited to middle-aged men. Thus, the results could not be extrapolated to women or other ethnics. In addition, we did not adjust diet pattern and take into consideration other behavioral and environmental factors that might influence hsCRP levels. Nevertheless, the numbers of current smokers and patients with hypertension were similar in both groups.
In conclusion, the middle-aged men with severe OSA had higher levels of inflammatory markers, slower HRR, and impaired cardiopulmonary capacity than their age- and BMI-matched controls. In addition, elevated hsCRP level was associated with blunted HRR in patients with OSA.
References
Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE (2004) Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 160:521–530
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–1904
Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK (1998) Altered cardiovascular variability in obstructive sleep apnea. Circulation 98:1071–1077
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112:2660–2667
Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K (1989) Reduction in sympathetic activity after long-term CPAP in sleep apnea: cardiovascular implications. Eur Respir J 8:222–229
Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS (1999) Heart rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 341:1351–1357
Arena R, Guazzi M, Myers J, Peberdy MA (2006) Prognostic value of heart rate recovery in patients with heart failure. Am Heart J 151:e7–e13
Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, Takeda H, Inoue M, Kamada T (1994) Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 24:1529–1535
Jae SY, Ahn ES, Heffernan KS, Woods JA, Lee MK, Park WH, Fernhall B (2007) Relation of heart rate recovery after exercise to C-reactive protein and white blood cell count. Am J Cardiol 99:707–710
Lanza GA, Sgueglia GA, Cianflone D, Rebuzzi AG, Angeloni G, Sestito A, Infusino F, Crea F, Maseri A, SPAI (Stratificazione Prognostica dell'Angina Instabile) Investigators (2006) Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am J Cardiol 97:1702–1706
Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T (2007) RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med 13:178–184
Lampert R, Bremner JD, Su S, Miller A, Lee F, Cheema F, Goldberg J, Vaccarino V (2008) Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J 156:759.e1–759.e7
Vieira VJ, Valentine RJ, McAuley E, Evans E, Woods JA (2007) Independent relationship between heart rate recovery and C-reactive protein in older adults. J Am Geriatr Soc 55:747–751
Tracey KJ (2002) The inflammatory reflex. Nature 420:1622–1673
Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK (2002) Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation 105:2462–2464
Hargens TA, Guill SG, Zedalis D, Gregg JM, Nickols-Richardson SM, Herbert WG (2008) Heart rate recovery following exercise testing in overweight young men with untreated obstructive sleep apnea. Sleep 31:104–110
Maeder MT, Münzer T, Rickli H, Schoch OD, Korte W, Hürny C, Ammann P (2008) Association between heart rate recovery and severity of obstructive sleep apnea syndrome. Sleep Med 9:753–761
Maeder MT, Ammann P, Münzer T, Schoch OD, Korte W, Hürny C, Myers J, Rickli H (2009) Continuous positive airway pressure improves exercise capacity and heart rate recovery in obstructive sleep apnea. Int J Cardiol 132:75–83
Kaleth AS, Chittenden TW, Hawkins BJ, Hargens TA, Guill SG, Zedalis D, Gregg JM, Herbert WG (2007) Unique cardiopulmonary exercise test responses in overweight middle-aged adults with obstructive sleep apnea. Sleep Med 8:160–168
Vanhecke TE, Franklin BA, Zalesin KC, Sangal RB, deJong AT, Agrawal V, McCullough PA (2008) Cardiorespiratory fitness and obstructive sleep apnea syndrome in morbidly obese patients. Chest 134:539–545
Lee P, Su YN, Yu CJ, Yang PC, Wu HD (2009) PHOX2B mutation-confirmed congenital central hypoventilation syndrome in a Chinese family: presentation from newborn to adulthood. Chest 135:537–544
American Academy of Sleep Medicine (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689
John MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545
Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Nader R (2001) Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem 47:418–425
Pollock ML, Graves JE, Mahar MT (1988) Reliability and validity of bioelectrical impedance in determining body composition. J Appl Physiol 64:529–534
Lukaski HC, Bolonchuk WW, Hall CB, Siders WA (1986) Validation of tetrapolar bioelectrical impedance method to assess human body composition. J Appl Physiol 60:1327–1332
Gupta N, Balasekaran G, Govindaswamy VV, Hwa CY, Shun LM (2011) Comparison of body composition with bioelectric impedance (BIA) and dual energy X-ray absorptiometry (DEXA) among Singapore Chinese. J Sci Med Sports 14:33–35
American College of Sports Medicine (2006) ACSM's guidelines for exercise testing and prescription, 7th edn. Lippincott Williams & Wilkins, Philadelphia
Bosquet L, Gamelin FX, Berthoin S (2008) Reliability of postexercise heart rate recovery. Int J Sports Med 29:238–243
Lauer MS, Francis GS, Okin PM, Pashkow FJ, Snader CE, Marwick TH (1999) Impaired chronotropic response to exercise stress testing as a predictor of mortality. JAMA 281:524–529
Maeder MT, Duerring C, Engel RP, Boesch C, Pfisterer ME, Myers J, Müller-Brand J, Zellweger MJ (2010) Predictors of impaired heart rate recovery: a myocardial perfusion SPECT study. Eur J Cardivasc Prev Rehabil 17:303–308
Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ (2005) Recommendations for blood pressure measurement in humans and experimental animals. Part I: blood pressure measurement in humans: statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111:697–716
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH (1998) Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 97:425–428
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently health men. N Engl J Med 336:973–979
Lui MM, Lam JC, Mak HK, Xu A, Ooi C, Lam DC, Mak JC, Khong PL, Ip MS (2009) C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest 135:950–956
Sharma SK, Mishra HK, Sharma H, Goel A, Sreenivas V, Gulati V, Tahir M (2008) Obesity, and not obstructive sleep apnea, is responsible for increased serum hs-CRP levels in patients with sleep-disordered breathing in Delhi. Sleep Med 9:149–156
Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E (2007) Correlates of serum C-reactive protein (CRP)—no association with sleep duration or sleep disordered breathing. Sleep 30:991–996
Lin CC, Hsieh WY, Chou CS, Liaw SF (2006) Cardiopulmonary exercise testing in obstructive sleep apnea syndrome. Respir Physiol Neurobiol 150:27–34
Alonso-Fernandez A, Garcia-Rio F, Arias MA, Mediano O, Pino JM, Martinez I (2006) Obstructive sleep apnoea–hypoapnoea syndrome reversibly depresses cardiac response to exercise. Eur Heart J 27:207–215
Grote L, Kraiczi H, Hender J (2000) Reduced alpha- and beta-adrenergic vascular response in patients with obstructive sleep apnea. Am J Crit Care Med 162:1480–1487
Carlson JT, Hender JA, Sellgren J, Elam M, Wallin BC (1996) Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 154:1490–1496
Bonsignore MR, Parati G, Insalaco G, Castiglioni P, Marrone O, Romano S, Salvaggio A, Mancia G, Bonsignore G, Di Rienzo M (2006) Baroreflex control of heart rate during sleep in severe obstructive sleep apnoea: effects of acute CPAP. Eur Respir J 27:128–135
Lombardi C, Parati G, Cortelli P, Provini F, Vetrugno R, Plazzi G, Vignatelli L, Di Rienzo M, Lugaresi E, Mancia G, Montagna P, Castiglioni P (2008) Daytime sleepiness and neural cardiac modulation in sleep-related breathing disorders. J Sleep Res 17:263–270
Rennie KL, Hemingway H, Kumari M, Brunner E, Malik M, Marmot M (2003) Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol 157:135–143
Jae SY, Heffernan KS, Yoon ES, Lee MK, Fernhall B, Park WH (2009) The inverse association between cardiorespiratory fitness and C-reactive protein is mediated by autonomic function: a possible role of the cholinergic anti-inflammatory pathway. Mol Med 15:291–296
Czura CJ, Tracey KJ (2005) Autonomic neural regulation of immunity. J Intern Med 257:156–166
Jüttler E, Tarabin V, Schwaninger M (2002) Interleukin-6 (IL-6): a possible neuromodulator induced by neuronal activity. Neuroscientist 8:268–275
Ruzek MC, Miller AH, Opal SM, Pearce BD, Biron CA (1997) Characterization of early cytokine responses and an interleukin (IL)-6-depenednet pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med 185:1185–1192
Acknowledgement
The authors thank the National Science Council (Taiwan) for the financial support (NSC 96-2314-B-002-022-MY3).
Conflict of interest statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical trial registration
ClinicalTrials.gov identifier: NCT00813852
Rights and permissions
About this article
Cite this article
Chien, MY., Lee, P., Tsai, YF. et al. C-reactive protein and heart rate recovery in middle-aged men with severe obstructive sleep apnea. Sleep Breath 16, 629–637 (2012). https://doi.org/10.1007/s11325-011-0549-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-011-0549-2