Abstract
Objectives
Patients with obstructive sleep apnea (OSA) (an obstructed airway and intermittent hypoxia) negatively affect their respiratory muscles. We evaluated the effects of a 12-week threshold inspiratory muscle training (TIMT) program on OSA severity, daytime sleepiness, and pulmonary function in newly diagnosed OSA.
Methods
Sixteen patients with moderate-to-severe OSA were randomly assigned to a TIMT group and 6 to a control group. The home-based TIMT program was 30–45 min/day, 5 days/week, for 12 weeks using a TIMT training device. Their apnea-hypopnea index (AHI), Epworth sleepiness scale (ESS), and forced vital capacity (FVC) scores were evaluated pre- and post-treatment. Polysomnographic (PSG) analysis showed that 9 TIMT-group patients had positively responded (TIMT-responder group: post-treatment AHI < pre-treatment) and that 7 had not (TIMT non-responder group: post-treatment AHI > pre-treatment).
Results
Post-treatment AHI and ESS scores were significantly (both P < 0.05) lower 6% and 20.2%, respectively. A baseline AHI ≤ 29.0/h predicted TIMT-responder group patients (sensitivity 77.8%; specificity 85.7%). FVC was also significantly (P < 0.05) higher 7.2%. Baseline AHI and FEV6.0 were significant predictors of successful TIMT-responder group intervention. OSA severity and daytime sleepiness were also significantly attenuated.
Conclusions
Home-based TIMT training is simple, efficacious, and cost-effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obstructive sleep apnea (OSA), which affects 34% of men and 17% of women, has recently reached alarming levels [1]. An obstructed airway and the subsequent asphyxia will lead to increased inspiratory effort and chronic overload of inspiratory muscles [2]. In a complete upper airway occlusion (e.g., no airflow, backward-falling tongue blocks airflow) in OSA patients, the activities of the inspiratory thoracic pump muscles continue to function. The airway may be triggered to open by a protective reflex originating in the inspiratory muscles upon reaching a certain degree of contraction [3]. However, repetitive efforts against an obstructed airway and intermittent hypoxia will negatively affect the inspiratory muscles of OSA patients: they become significantly weaker and have significantly less endurance.
Two studies [2, 4] that used aerobic and aerobic plus resistance exercise for 1–1.5 h three times/week for 12 weeks with OSA patients reported that exercise had a significantly positive effect on the apnea-hypopnea index (AHI), which seems not to be dependent upon changes in body mass index (BMI). The exact mechanisms of these effects are unclear, and many different hypotheses have been proposed. A dog study [5] reported greater tone in the genioglossus (extrinsic tongue) muscle after the gastrocnemius (lower leg) muscle and sciatic nerve had been stimulated. Two later studies in humans [6, 7] raised some interest in a possible association between the strength of respiratory muscles and physical exercise in OSA patients. One viable treatment called inspiratory muscle training (IMT) is a regimen of breathing exercises aimed at increasing the strength and endurance of inspiratory muscles in people with and without chronic obstructive pulmonary disease (COPD). It tests the hypothesis that greater respiratory muscle capacity will mitigate the severity of breathlessness and augment the COPD patients’ tolerance of physical activity during their activities of daily living. Treatment duration at 30% of maximal inspiratory pressure (MIP) ranged from 8 to 10 weeks, and treatment frequency was 6 or 7 days/week for 15–30 min/session. One study [8] of OSA patients with chronic cervical cord injury reported that IMT increased inspiratory muscle strength and ameliorated the severity of AHI. However, another study [9] said that IMT treatment for OSA patients led to rapid improvement of blood pressure, plasma norepinephrine level, and reported sleep quality, it did not affect AHI.
Threshold IMT (TIMT) uses a resistive loading device to increase inspiratory muscle endurance and strength; it includes (i) a flow-independent one-way valve to ensure consistent resistance and (ii) an adjustable specific pressure setting (in cmH20) set by a healthcare professional. TIMT’s efficacy for improving maximum inspiratory pressure and the baseline dyspnea index in COPD patients has been confirmed [10]; however, how TIMT will affect OSA patients is unclear.
We therefore investigated the effects of 12 weeks of TIMT training on OSA severity, daytime sleepiness, and pulmonary function and assessed the possible predictors associated with the responsiveness of TIMT.
Material and methods
Study participants
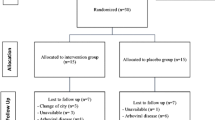
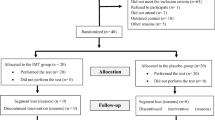
We invited 35 adult patients with newly diagnosed moderate-to-severe OSA (AHI ≥ 15/h to the Department of Otolaryngology, Tainan Hospital, Ministry of Health and Welfare, Taiwan. Exclusion criteria were inability to exercise and exercise-induced myocardial ischemia, chronic heart failure, myocardial infarction, unstable angina, respiratory or neuromuscular disease, morbid obesity, psychiatric disorder (e.g., bipolar disorder, schizophrenia), and uncontrolled chronic medical conditions (e.g., diabetes mellitus, hypertension, renal insufficiency, and hyperthyroidism). We excluded eight invitees from the study, and five withdrew for various reasons. The remaining 22 (18 men) satisfactorily completed the whole study (Fig. 1). The protocol of this study was approved by the Ethics Committee of the Human Research Committee at the National Cheng Kung University Hospital (NCKUH IRB No: A-ER-103-168) (Clinical Trials Register Number: NCT02278094). All the tests and the training program began only after the eligible participants had provided their written informed consent.
Study procedure
Before the study, the participants underwent structured interviews, sleep-related questionnaires, overnight polysomnography (PSG), and a pulmonary function test (PFT). After the baseline data had been collected, patients were randomly assigned to the TIMT group or to the control group (Fig. 1). All participants were sex-, age-, and BMI-matched (Table 1). TIMT group members were given medical treatment, routine care, and TIMT; control group members were given only medical treatment and routine care, but no TIMT (Table 2). Patients started TIMT at a threshold pressure between 11 and 21 cmH2O; the pressure increased once a week as tolerated (average weekly pressure increase was 5%; combined rating of perceived exertion (RPE) was between 13 and 15 (Table 3)). At the end of the study, assessments identical to those before baseline were done again. Patients subjectively evaluated differences between their pre- and post-TIMT OSA.
Clinical measurements
Questionnaires
A structured questionnaire was used to record demographic characteristics, health habits (drinking alcohol and smoking), medical conditions, and medication use (Table 1). Sleep quality was assessed with the Pittsburgh Sleep Quality Index (PSQI) [11]. A PSQI total score > 5 indicated poor sleep quality. Daytime sleepiness was assessed with the Epworth Sleepiness Scale (ESS) [12]. An ESS total score > 10 indicated significant daytime sleepiness (Table 2).
Overnight polysomnography
All patients underwent standard overnight hospital PSG (Somté recorder; Compumedics, Abbotsford, Victoria, Australia). AHI was calculated as the number of apnea and hypopnea events per hour of sleep. AHI during rapid eye movement (REM) sleep (AHI-REM) = the number of apnea-hypopnea events per hour of REM sleep; AHI during non-REM (NREM) sleep = AHI-NREM [13, 14]. Our PSG reader was our sleep technician (Grace Lin), who was blinded to patients’ assigned groups and to their pre-TIMT AHI levels. PSG data were interpreted by a sleep physician (C.Y. Lin) based on established criteria (Table 2).
Pulmonary function test
Pulmonary function was assessed using standard spirometry and included forced vital capacity (FVC), forced expiratory volume in 1.0 s (FEV1.0), FEV1.0/FVC, and FEV in 6.0 s (FEV6.0) based on the guidelines of the American Thoracic Society (ATS). For the adult population 40–80 years old, the mean FVC was 3.517 ± 0.662 L in females and 3.913 ± 0.773 L in males; the mean FEV1.0 was 2.856 ± 0.534 L in females and 4.922 ± 0.941 L in males [15]. The calculation of FEV1.0/FVC allows us to identify ventilation obstructions and restrictions. In healthy adults, the value of FEV1.0/FVC is usually approximately 70–85% (declining with age). An FEV1.0/FVC < 70% indicates a ventilation obstruction (Table 2).
TIMT
TIMT was scheduled once per week in the hospital and 4 days per week at home. Before each hospital TIMT session, PFT, inspiratory muscle function test, and RPE [16] scores were obtained (Table 3). According to the finding of inspiratory muscle function test, we set the target threshold for the TIMT device (Healthscan Products, Cedar Grove, NJ, USA). The target threshold of TIMT was adjusted every week to maintain the threshold to reach 30% of each patient’s MIP. The initial setting range of target threshold was between 11 and 21 (14 ± 6) cmH2O, which was 10 repetitions maximum (RM) [2], and the combined RPE was between 13 and 15 as the standard TIMT intensity (Table 3). PT taught patients the diaphragm breathing exercise and combined it with TIMT device training in the hospital. Patients spent 30–45 min each day 5 days/week for 12 weeks doing these exercises [17]. During training, patients were instructed to maintain diaphragmatic breathing between 15 and 20 breaths/min. Therefore, the training intensity (range: from moderate to high) was adjusted case by case for initially 60–70% of maximal workload. The final training intensity ranged from 19 to 29 (24 ± 3) cmH2O (Table 3). All TIMT assessments and training sessions were supervised by the PT, who, using weekly telephone calls, monitored patients’ progress in and adherence to the program. All patients recorded this information in diary notes that were given to the PT at the end of the program and then evaluated by the sleep technician.
Statistical analysis
The Mann-Whitney U test and the Pearson χ2 test were used to compare differences in the basic characteristics and outcome measurements of the TIMT (overall) and control groups. The Mann-Whitney U test was also used to evaluate the percentage changes in the PSG, questionnaire, and PFT scores of the participants’ pre- and post-TIMT. Pearson correlation analysis was used to determine the correlations the PSG, questionnaire, and PFT scores of the participants. The Kruskal-Wallis test was used for continuous variables, and Fisher’s exact test was used for categorical variables in the TIMT-responder group, TIMT non-responder group, and control groups. The Wilcoxon signed-rank test was used to compare pre- and post-TIMT parameters. In addition, a multivariate linear regression analysis was used to assess correlations between different variables and AHI percentage changes post-intervention in the TIMT group. The area under the receiver operating characteristic curve (AUC) was estimated for the possible predictor of responsiveness to TIMT. Youden’s index was calculated, and an optimal cutoff point was determined for the predictor. Multivariate logistic regressions were used to calculate the odds ratios (ORs) and confidence intervals (CIs) of variables after confounders had been adjusted for. STATA 11 (STATA Corp., College Station, TX, USA) was used for all analyses. Data are expressed as mean ± standard deviation (SD). Significance was set at P < 0.05.
Results
Demographics and baseline data
There were nine patients in the TIMT-responder group, seven in the TIMT non-responder group, and six in the control group (Fig. 1). Their mean age was 50.1 ± 12.3 years, and mean BMI was 26.5 ± 3.3. There were no significant differences in age, BMI, or neck circumference at baseline. The post-intervention change in BMI was not significantly different (Table 1).
Polysomnography, questionnaire, and pulmonary function tests
The baseline AHI score in the control group was significantly (P = 0.003) higher than that in the TIMT group (Table 2). However, in the TIMT group, the baseline AHI score was not significantly different between responder group and non-responder group patients. After the 12-week intervention, the mean reductions in AHI scores were a nonsignificant 3.50 ± 6.2 h and 6.0 ± 21.8% in the TIMT group. However, the reduction in the responder group was significantly greater than that in the control group (P = 0.002). Similarly, the increase in ESS scores was significant in the TIMT group 20.2 ± 2.2%. The AHI and ESS improvements in the TIMT-responder group were significant (P < 0.05) 32.8% and 19.8%, respectively. PFT assessments showed significantly higher post-intervention scores for TIMT-group patients 7.2% and 9.1%, respectively (Table 2).
TIMT
The average hospital TIMT training frequency was 11.6 ± 2.0 days within 3 months, and the average home-based training was 45.0 ± 14.0 days within 3 months (Table 3). The mean of the hospital TIMT training days were 11.3 ± 6.0 days in the TIMT non-responder group within 3 months and 12.0 (SD 0.1) in the TIMT-responder group within 3 months. The mean of the home TIMT training was 13.5 ± 8.1 days within 3 months in the TIMT non-responder group and 16.1 ± 7.8 in the TIMT-responder group within 3 months.
TIMT and AHI
A multivariate linear regression analysis of the TIMT group showed that baseline AHI (ORs 3.857; 95% CIs 1.095–13.586; AUC 0.825; P = 0.012) and FEV6.0 (ORs: 1.800; 95% CIs: 1.003–3.229; P = 0.042) were significant predictors of AHI post-intervention percent change (Table 4). The baseline AHI had a high Youden’s index value that was used to predict the responsiveness of TIMT (optimal cutoff point 29.0/h; sensitivity 77.8%; specificity 85.7%).
Discussion
We found that TIMT-group patients with moderate-to-severe OSA had less daytime sleepiness and had better AHI and pulmonary function scores and that TIMT group patients with moderate OSA had significantly better AHI scores than did TIMT-group patients with severe OSA. Other studies [2, 4, 6, 18] have also reported reductions in AHI scores after 12 weeks of physical training, and one [9] reported no improvement in nighttime AHI after 6 weeks of training. We hypothesize that at least 12 weeks of TIMT is sufficient to reduce the AHI score.
One study [19] said that a 16-week respiratory muscle training program using the didgeridoo significantly reduced daytime sleepiness, but that it did not significantly mitigate sleep apnea. Another [20] claimed that 5 weeks of expiratory muscle strength training reduced the AHI scores of patients with moderate OSA more than it did for patients with mild OSA, but it did not ameliorate their ESS scores. A clinical trial [21] reported that, after 12 weeks of oropharyngeal muscle exercises, AHI, daytime sleepiness, and OSA severity was significantly lower in their patients with moderate OAS. Changes in neck circumference correlated inversely with changes in the AHI. Another study [22] reported that 10 weeks of neuromuscular stimulation (NMS)—lingual and supra-hyoid muscle strengthening—significantly reduced AHI scores in most patients with mild-to-severe OSA. Lower baseline AHI scores were associated with larger reductions in AHI. Similarly, our TIMT study found that a 12-week TIMT program significantly reduced the AHI and ESS scores of patients with moderate-to-severe OSA. Baseline AHI was significantly negatively correlated with the percentage change post-intervention. Furthermore, OSA patients with AHI scores < 29.0/h predictably responded positively to TIMT. These therapeutic effects might be attributable to the various degrees of OSA severity among our patients and to the 12-week (long) duration of the intervention. However, the reduction in AHI was only temporary. The sustained effects of IMT on OAS severity are still not known, and additional studies are warranted.
Many studies have clinically important effects in inspiratory muscle strength and endurance after IMT, especially in cohorts diagnosed with chronic heart failure and COPD [9, 23, 24]. The intrathoracic pressures generated during IMT resemble pressures generated during OSA. IMT differs from OSA in that the former’s stimulus occurs when one is awake and well-oxygenated. However, the latter’s intrathoracic pressure swings, which are the typical characteristics of OSA, occur when one is asleep, and they result in hypoxemia during sleep [9]. The inspiratory muscles of OSA patients are subjected to large loads with every breath for prolonged periods during sleep and have little opportunity to rest [25]. Thus, it seems likely that the inspiratory muscles are the muscles most prone to fatigue [26]. One study reported that OSA patients were unable to increase motor unit recruitment of inspiratory muscles during incremental exercise [27]. For most OSA patients, the diaphragm appeared to be the primary inspiratory muscle during apnea. Patients with vigorous diaphragmatic and abdominal muscle responses during apnea tended to have less severe OSA [28], and one study [29] reported that inspiratory pressure threshold loading (IPTL) during TIMT was the predominant activator of the diaphragm [29]. In our study, the significant changes in FVC and FEV6.0 after 12 weeks of TIMT intervention might be because of increased diaphragm activity and more air entering the lungs. Therefore, the effects of TIMT might be more analogous to traditional forms of aerobic exercise than to TIMT [9].
Strengths and limitations
The strengths of our study included the 12-week (long) duration of the training and its effects. Moreover, our patients had a greater range of OSA severity (moderate 10 cases; severe 12 cases) than did patients in other studies we reviewed, which provided extensive estimates of the treatment effects of TIMT. However, our study had some limitations. First, we did not measure respiratory muscle strength; therefore, we could not explore the mechanisms of TIMT with respiratory muscle function. Second, overall AHI in the TIMT-group was 6.0% lower than at baseline than was AHI in the control group, which was only 4.5% lower. In both groups, AHI fell in some patients but rose in others. This is probably attributable to unpredictable nightly variability. However, the individual post-intervention changes in AHI in TIMT-responder group patients were larger than that of nightly variability [30]. Third, we identified TIMT-group members who responded to intervention with a decrease in AHI (“TIMT responders”) and then compared them with control group members after our study had ended (Fig. 2). This might lead to a selection bias in the intervention group. Confounding factors were no more equally distributed between the two groups (“TIMT responders” and controls). Nevertheless, post hoc analysis showed that a subset of patients (with a lower baseline AHI score ≤ 29.0/h) might have a positive response to TIMT intervention. Additional studies are needed to confirm this finding. Fourth, our findings cannot be generalized to dissimilar cohorts. Exclusion criteria included cardiac and pulmonary comorbidities. Moreover, the mean BMI in the intervention group was 26.2 kg/m2. This group constituted the minority of OSA patients in other countries such as the USA. Fifth, control group patients did not undergo placebo training, but their ESS scores were 9.0% lower at the end of the study. Moreover, because the ESS score was significantly better in the TIMT group, the placebo effect might partially explain this finding. Finally, our sample was small (n = 22) and the dropout rate was high. Further, subgrouping the participants resulted in smaller samples (TIMT-responder group 9; TIMT non-responder group 7; control 6) (Fig. 1). Therefore, it is not possible to analyze the influence of sex and age on the TIMT effect. Larger clinical trials with longer follow-ups are needed to confirm our preliminary findings.
Apnea-hypopnea index (AHI) level before and after receiving threshold inspiratory muscle training (TIMT), based on responders (solid line) and non-responders (dashed line) in 12-week home-based training, individually. The AHI changes of controls were also presented (dotted line). A total of 22 subjects were showed, including responders (n = 9), non-responders (n = 7), and controls (n = 6)
Conclusions
TIMT is a simple, home-based therapy and is thus cost-effective respiratory training. The severity of OSA and daytime sleepiness was significantly ameliorated after TIMT. This effect indicated the potential therapeutic benefits of TIMT for OSA. It is promising as an adjunct treatment for OSA patients who are unwilling or unable to tolerate nightly CPAP.
Abbreviations
- AE:
-
Aerobic exercise
- AHI:
-
Apnea-hypopnea index
- AHI-NREM:
-
Apnea-hypopnea index during non-rapid eye movement (NREM) sleep
- AHI-REM:
-
Apnea-hypopnea index during rapid eye movement (REM) sleep
- ATS:
-
American Thoracic Society
- BMI:
-
Body mass index
- EEG:
-
Electroencephalography
- ESS:
-
Epworth sleepiness scale
- FEV1.0 :
-
Forced expiratory volume in 1 s
- FEV6.0 :
-
Forced expiratory volume in 6 s
- FVC:
-
Forced vital capacity
- IMT:
-
Inspiratory muscle training
- NREM:
-
Non-rapid eye movement
- OSA:
-
Obstructive sleep apnea
- PFT:
-
Pulmonary function test
- PSG:
-
Polysomnography
- PSQI:
-
Pittsburgh sleep quality index
- PT:
-
Physical therapist
- REM:
-
Rapid eye movement
- RM:
-
Repetition maximum
- ROC:
-
Receiver operating characteristic
- RPE:
-
Rating of perceived exertion
- TIMT:
-
Threshold inspiratory muscle training program
References
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177(9):1006–1014. https://doi.org/10.1093/aje/kws342
Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN (2011) The effect of exercise training on obstructive sleep apnea and sleep quality:a randomized controlled trial. SLEEP 34(12):1631–1640. https://doi.org/10.5665/sleep.1422
Vincken W, Guilleminault C, Silvestri L, Cosio M, Grassino A (1987) Inspiratory muscle activity as a trigger causing the airways to open in obstructive sleep apnea. Am Rev Respir Dis 135(2):372–377. https://doi.org/10.1164/arrd.1987.135.2.372
Sengul YS, Ozalevli S, Oztura I, Iti lO, Baklan B (2011) The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep Breath 15(1):49–56. https://doi.org/10.1007/s11325-009-0311-1
Haxhiu MA, Lunteren EV, Mitra J, Cherniack NS, Strohl KP (1984) Comparison of the responses of the diaphragm and upper airway muscles to central stimulation of the sciatic nerve. Respir Physiol 58:65–76. https://doi.org/10.1016/0034-5687(84)90045-8
Norman JF, Von Essen SG, Fuchs RH, McElligott M (2000) Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online 3(3):121–129
Chen N-H, Li H-Y, Gliklich RE, Chu C-C, Liang S-C, Wang P-C (2002) Validation assessment of the Chinese version of the snore outcomes survey. Qual Life Res 11(6):601–607. https://doi.org/10.1023/A:1016337008763
Boswell-Ruys C, Lewis C, Gandevia S, Butler J (2015) Respiratory muscle training may improve respiratory function and obstructive sleep apnoea in people with cervical spinal cord injury. Spinal Cord Ser Cases 1:15010. https://doi.org/10.1038/scsandc.2015.10
Vranish J, Bailey E (2016) Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep 39(6):1179–1185. https://doi.org/10.5665/sleep.5826
Chuang HY, Chang HY, Fang YY, Guo SE (2017) The effects of threshold inspiratory muscle training in patients with chronic obstructive pulmonary disease: a randomised experimental study. J Clin Nurs 26(23–24):4830–4838. https://doi.org/10.1111/jocn.13841
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14(6):540–545. https://doi.org/10.1093/sleep/14.6.540
Haba-Rubio J, Janssens J-P, Rochat T, Sforza E (2005) Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest 128(5):3350–3357. https://doi.org/10.1378/chest.128.5.3350
Yamauchi M, Fujita Y, Kumamoto M, Yoshikawa M, Ohnishi Y, Nakano H, Strohl KP, Kimura H (2015) Nonrapid eye movement-predominant obstructive sleep apnea: detection and mechanism. J Clin Sleep Med 11(09):987–993. https://doi.org/10.5664/jcsm.5010
Ostrowski S, Grzywa-Celinska A, Mieczkowska J, Rychlik M, Lachowska-Kotowska P, Lopatynski J (2005) Pulmonary function between 40 and 80 years of age. J Physiol Pharmacol 56:127
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14(5):377–381. https://doi.org/10.5271/sjweh.1815
Leith DE, Bradley M (1976) Ventilatory muscle strength and endurance training. J Appl Physiol 41(4):508–516. https://doi.org/10.1152/jappl.1976.41.4.508
Ueno LM, Drager LF, Rodrigues AT, Rondon MPB, WM J (2009) Effects of exercise training in patients with chronic heart failure and sleep apnea. SLEEP 32(5):637–647. https://doi.org/10.1093/sleep/32.5.637
Puhan MA, Suarez A, Cascio CL, Zahn A, Heitz M, Braendli O (2006) Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ 332:266–270. https://doi.org/10.1136/bmj.38705.470590.55
Kuo Y-C, Song T-T, Bernard JR, Liao Y-H (2017) Short-term expiratory muscle strength training attenuates sleep apnea and improves sleep quality in patients with obstructive sleep apnea. Respir Physiol Neurobiol 243:86–91. https://doi.org/10.1016/j.resp.2017.05.007
Guimaraes KC, Drage LF, Gent PR, Marcondes BF, Lorenzi-Filho G (2009) Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med 179(10):962–966. https://doi.org/10.1164/rccm.200806-981OC
Lequeux T, Chantrain G, Bonnand M, Chelle A, Thill MP (2005) Physiotherapy in obstructive sleep apnea syndrome: preliminary results. Eur Arch Oto-Rhino-Laryngology Head Neck 262(6):501–503. https://doi.org/10.1007/s00405-004-0806-0
Dall’Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP (2006) Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 47(4):757–763. https://doi.org/10.1016/j.jacc.2005.09.052
Beckerman M, Magadle R, Weiner M, Weiner P (2005) The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest 128(5):3177–3182. https://doi.org/10.1378/chest.128.5.3177
Force AASMT (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 22(5):667–689. https://doi.org/10.1093/sleep/22.5.667
Mezzanotte WS, Tangel DJ, White DP (1992) Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Investig 89(5):1571–1579. https://doi.org/10.1172/JCI115751
Chien MY, Chang YJ, Lee P, Yang PC, Wu YT (2013) Electrophysiologic changes with incremental exercise in obstructive sleep apnea. Muscle Nerve 48(2):212–218. https://doi.org/10.1002/mus.23745
Wilcox PG, Paré PD, Road JD, Fleetham JA (1990) Respiratory muscle function during obstructive sleep apnea. Am Rev Respir Dis 142(3):533–539. https://doi.org/10.1164/ajrccm/142.3.533
Walterspacher S, Pietsch F, Walker DJ, Rocker K, Kabitz HJ (2018) Activation of respiratory muscles during respiratory muscle training. Respir Physiol Neurobiol 247:126–132. https://doi.org/10.1016/j.resp.2017.10.004
Mjid M, Ouahchi Y, Toujani S, Snen H, Ben NS, Ben AH, Louzir B, Mhiri N, Cherif J, Beji M (2016) Night-to-night variability of the obstructive sleep apnoea-hypopnoea syndrome. Rev Mal Respir 33(9):775–780. https://doi.org/10.1016/j.rmr.2016.04.023
Acknowledgments
We thank Professor Yu-Wen Chen, Grace Chen, and the sleep medicine center staff members at National Cheng Kung University Hospital and Tainan Hospital who participated in the study.
Funding
This study was funded by Shu-Zen Junior College of Medicine and Management, under contract SZPT10403008, and by the Gary & Amy Foundation, and by National Cheng-Kung University Hospital (grant number NCKUH-10802018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were approved by the Institutional Review Board of National Cheng Kung University (IRB No. A-ER-103-168).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, HC., Chiang, LL., Ong, JH. et al. The effects of threshold inspiratory muscle training in patients with obstructive sleep apnea: a randomized experimental study. Sleep Breath 24, 201–209 (2020). https://doi.org/10.1007/s11325-019-01862-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-019-01862-y