Abstract
Bacterial pigments stand out as exceptional natural bioactive compounds with versatile functionalities. The pigments represent molecules from distinct chemical categories including terpenes, terpenoids, carotenoids, pyridine, pyrrole, indole, and phenazines, which are synthesized by diverse groups of bacteria. Their spectrum of physiological activities encompasses bioactive potentials that often confer fitness advantages to facilitate the survival of bacteria amid challenging environmental conditions. A large proportion of such pigments are produced by bacterial pathogens mostly as secondary metabolites. Their multifaceted properties augment potential applications in biomedical, food, pharmaceutical, textile, paint industries, bioremediation, and in biosensor development. Apart from possessing a less detrimental impact on health with environmentally beneficial attributes, tractable and scalable production strategies render bacterial pigments a sustainable option for novel biotechnological exploration for untapped discoveries. The review offers a comprehensive account of physiological role of pigments from bacterial pathogens, production strategies, and potential applications in various biomedical and biotechnological fields. Alongside, the prospect of combining bacterial pigment research with cutting-edge approaches like nanotechnology has been discussed to highlight future endeavours.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Originally identified as coloring agents, pigments isolated from natural resources including plants and microorganisms, have emerged as molecules of multitudinous applications with least toxic impact on the environment and human health (Orlandi et al. 2022). Barring their application as chromogens for foods, textiles, cosmetics, and other industries, unravelling the biological action of the pigments rendered those of immense prospect for clinical application (Agarwal et al. 2023). A number of such natural pigments are preferably extracted from microorganisms including chromogenic bacteria owing to greater stability, up-scaling, and easy-tunable down-stream processing (Barreto et al. 2023). Despite such advantages, bacterial pigments are relatively less explored compared to pigments from plant and fungal sources. Except for photosynthetic pigments like bacteriochlorophylls, bacterial pigments are diverse in terms of chemical properties and belong to groups like carotenoids, melanin, phenazines, quinones, indoles, and pyrroles (Barreto et al. 2023). Carotenoids, pyocyanin, violacein, prodigiosin, and melanin and their derivatives are the most profoundly produced bacterial pigments (Acharya et al. 2023). Producers of such pigments are ubiquitous in nature and can be isolated from various niches like marine and terrestrial environments, ambient to extreme environments, and spoilt food to industrial effluent (Chatragadda and Dufosse 2021). Well-characterized pigment producers include actinobacteria, which includes Streptomyces, unequivocally the largest genus of pigment formers. S. shaanxiensis, S. griseoviridis, and S. coelicolor are the most well-characterized pigment former species from this group (Ibrahim et al. 2023). Apart from Streptomyces, a number of pathogenic bacteria, particularly opportunistic pathogens from the genera Serratia, Pseudomonas, Staphylococcus, Chryseobacterium, and Chromobacterium have been identified as profound pigment producers (Chatragadda and Dufosse 2021). Similar to alkaloids and antibiotics bacterial pigments are secondary metabolites. Pigment anabolism requires the expression of dedicated biosynthetic gene clusters (BGC), which are regulated by various transcription factors modulated by environmental cues (Wang et al. 2021). The colossal genetic load dedicated to pigment biosynthesis and its regulation underpins the evolutionary relevance of pigment production. The majority of the pigments offer fitness advantages to the producer organism, shaping microbial communities, participating in cell-cell communication processes, and establishing infection in susceptible host (Liu et al. 2024b). For the producers, pigments provide protection against oxidative damage, genotoxicity by ultraviolet radiation and mutagens, and also impart tolerance to elevated temperature and extreme desiccation (Day et al. 2017). Biological action associated with such functions has been linked to antimicrobial, antiviral, antioxidant, antioxidant, and anticancer activities of a number of bacterial pigments. With the identification of such exotic properties, bacterial pigments are attaining mounting interest in clinical and pharmaceutical industries (Barreto et al. 2023). The estimated global market for some of the pigments has been projected in recent years. The predicted global market for natural pigments in the cosmetic industry is USD 54.5 billion in coming years (Kiki 2023). Thriving on the increasing demand for biodegradable and environment-friendly dyes, the market expansion for pigments comprising other groups are also expected to escalate. With accumulating evidence of clinical and pharmaceutical applications like antimicrobial properties, bacterial pigments are expected to offer strategies to combat antimicrobial resistance emergence (Acharya et al. 2023). A number of profound pigment-producing bacteria are opportunistic pathogens, which often hinders scaling up for production. Leveraging synthetic biology for metabolic engineering, development of semisynthetic pigment derivatives, and nanoformulations with bioactive pigments are underway to foster industrial and pharmaceutical applicability of the molecules (Muthukrishnan et al. 2019). Against this backdrop, the present review attempts to offer a comprehensive retrospect of nonphotosynthetic bacterial pigments produced by pathogenic bacteria through a thorough introspection of existing literature (Fig. 1). Some of the pigments of concern are exclusively produced by pathogenic bacteria, and for others, both pathogenic and non-pathogenic origin have been reported. The review begins with outlining the chemical identity and bioactive properties of the pigments. Subsequently, the impact of the pigments on microbial communities and their possible role in pathogenicity is accounted. A detailed discussion on various biotechnical and clinical applications is included. Alongside, an insight into the strategies for the industrial production of bacterial pigments is discussed. Finally, recent approaches for developing novel formulations exploiting the bacterial pigments are highlighted to project possible future endeavours.
Bibliographic analysis. VOSviewer generated map-view for relevant terms extracted from titles and abstracts of 632 research articles identified by DimensionsAI (https://www.dimensions.ai/). 60% of the most relevant terms are mapped on the plot with ‘occurrence’ as the weightage parameter. The map contains 329 items distributed within 7 clusters through 18,871 links
Bacterial pigments: producers and chemical properties
Bacterial pigments have been classified into various structural and functional groups. Pigments produced by non-phototrophic pathogenic bacteria have been associated with a higher spectrum of functionalities including adapting to certain environment for survival, protection against radiation and oxidative stress, and exerting antimicrobial effects to offer fitness advantage in their own habitat (Barreto et al. 2023) (Table 1). Majority of the pigments are produced as secondary metabolites, which are diverse in terms of structure (Fig. 2) and physicochemical properties (Fig. 3A and B). In this segment, a brief discussion on ten major groups of bacterial pigments produced by bacterial pathogens are discussed in terms of their chemical features linked to biological function.
Pigments produced by pathogenic bacteria. Structures of various bacterial pigments are illustrated using PubChem Sketcher V2.4 and ACD/ ChemSketch based on isomeric SIMLES retrieved from PubChem. (A) zeaxanthin (ZXT) (B) staphyloxanthin (SXT), (C) azaphenanthrene (AZP), (D) roseoflavin (RFV), (E) toxoflavin (TFV), (F) phenazine (PHZ), (G) pyocyanin (PCN), (H) pyoverdin (PVD), (I) prodigiosin (PDG), (J) violacein (VIO), (K) melanin (MEL), (L), indigoidin (IND), and (M) flexirubin (FLR)
Carotenoids
Carotenoids, are one of the most frequently observed pigment in several groups of organisms including bacteria (Devi et al. 2024), archaea, fungi, algae, plants, and even animals (Maoka 2023). More than 850 different types of carotenoids that are found in nature play important roles as photo-protection, color attractant as well as hormonal precursors of plants. In animals, carotenoids act as photo-protector, antioxidants, immunity boosters, and vitamin A precursor (Maoka 2023). The structure of all variants of carotenoids are similar. Although the common precursor for carotenoid biosynthesis is phytoene (C40), some other C30 and C50 precursors are used by a few bacterial species. All comprise of a general polyene chain with at least nine conjugated double bonds, both sides carrying an end group (Walter and Strack 2011). Phytoene gets converted to lycopene via numerous denaturation and polymerization reactions. A cyclase then converts lycopene, the red-colored pigment, to yield α-, β- and γ-carotenes (Barreto et al. 2023). Zeaxanthin (ZXT) is a yellow-pigmented carotenoid that has a linear structure β,β-carotene-3,3′-diol (Fig. 2A). ZXT can be obtained from plants and even the yolk of egg to different yellow pigmented organism. Among bacteria Flavobacterium sp., Paracoccus zeaxanthifaciens can be the source of ZXT production (Li et al. 2023a; Raman et al. 2024). Although other higher hierarchical plants, animals, and even algae produce ZXT, it is difficult to isolate the pigment from them as they also produce other carotenoids. In contrast, bacteria such as F. multivorum specifically produce 3R,3’R- ZXT (Vila et al. 2020). It also acts as an anticancer and anti-inflammatory agent due to its antioxidant properties (Raman et al. 2024) (Table 1). The pathogenic bacterium Staphylococcus aureus has been reported to synthesize a triterpenoid carotenoid, staphyloxanthin (SXT) as a possible virulence factor (Liu et al. 2005). The chemical structure of STX was determined by NMR spectroscopy, which revealed that glucose is esterified with a triterpenoid carotenoid carboxylic acid at the C1’’ position and a C15 fatty acid at C6” position (Fig. 2B). SXT provides the bacteria protection against antimicrobial attack including the immune system owing to the diaponeurosporenoic group and its ability to lower the membrane fluidity without altering conformation (Table 1), both of which are otherwise coupled events (Munera-Jaramillo et al. 2024). Astaxanthin (AXT), is an extremely important keto-carotenoid produced by nonpathogenic strains of Paracoccus and Pseudoalteromonas (Patil et al. 2022). Though AXT demonstrates immense bioactive potential, since it is produced primarily by nonpathogens, it is not further discussed here.
Azaphenanthrene
Azaphenanthrene (AZP) is a green-colored pigment that can be isolated from Bacillus cereus. Its structure is identified as 9-methyl-1,4,5,8-tetra-AZP, linked with a chromophore derivative known as 7-N, N-dibutylamino-2-AZP (Banerjee et al. 2011, 2014) (Fig. 2C). Antibacterial activity of AZP compounds was reported long ago (Gupta et al. 1970); the stability of which was demonstrated to vary depending on the arrangement of different ring structures within the pigment, including the aza, methyl, and benzyl groups (Calabrese et al. 2010). Moreover, the derivatives of AZP, identified from other resources demonstrated a range of pharmacological activities as enlisted in Table 1.
Flavins
The basic structure of the flavins, a group of yellow pigments, is composed of a tricyclic isoalloxazine ring, which is a nitrogen and oxygen-containing heterocycle. Riboflavin also known as vitamin B2 is the major microbial flavin with pigment characteristics and also the origin of all biologically active flavins. Two pentose phosphate pathway intermediates guanosine triphosphate (GTP) and ribulose-5-phosphate (Ru5P) function as the precursor for riboflavin biosynthesis contributing to the pyrimidine part and heterocyclic ring portion of the isoalloxazine ring, respectively. GTP also provides two nitrogen atoms to the ring as well as a ribityl side chain. Streptomyces spp. and Burkholderia spp. have been reported to synthesize structural analogues, roseoflavin (RFV) (Fig. 2D) and toxoflavin (TFV) (Fig. 2E) respectively, which exert antimicrobial actions (Li et al. 2019; Mora-Lugo et al. 2019) (Table 1).
Pyocyanin
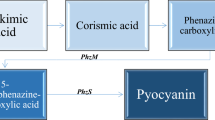
The bacterial genus Pseudomonas synthesizes a number of phenazine (PHZ) pigments (Fig. 2F) which are prominent virulence factors for the bacterium. These pigments play a crucial role in biofilm formation by P. aeruginosa by regulating gene expression (Fekete-Kertesz et al. 2024). Pyocyanin (PCN) is a nonfluorescent water-soluble blue pigment that changes color according to the oxidation status (Pierson and Pierson 2010). It is a nitrogen-containing PHZ and the heterocyclic structure is composed of two N-methyl-1-hydroxyPHZ subunits (Goncalves and Vasconcelos 2021) (Fig. 2G). The synthesis begins from the precursor chorismic acid that is converted to an intermediate PHZ-1-carboxylic acid (PCA) involving seven enzymes from two operons (phz1 and phz2). PCA is then converted to PCN either by phzM-encoded methyltransferase or phzS-encoded monooxygenase (Pierson and Pierson 2010). It stays in a zwitterionic form at neutral pH 7 hence appearing blue and also in the oxidized state under alkaline condition. On the contrary, the color turns red when in an acidic environment (Mudaliar and Bharath Prasad 2024). PCN is capable of showing antimicrobial action by modifying cellular oxidation state. Having both hydrophobic and hydrophilic moieties, PCN easily crosses the cell membrane and being a redox-active molecule kills the target cells by creating oxidative stress via the production of reactive oxygen species such as superoxide and hydrogen peroxide (Goncalves and Vasconcelos 2021). Being a redox-active molecule, PCN specifically exerts its action by oxidizing NADH and NADPH, consequently elevating cytosolic ROS levels and redox potential. This cascade of events results in diminished ATP production and a dysregulation of the reduced-to-oxidized glutathione (GSH/ GSSG) ratio (Hall et al. 2016). Thus PCN can act as an apoptosis inducer along with acting as an antibacterial, antifungal, and QS inhibitor as summarized in Table 1.
Pyoverdine
Pyoverdine (PVD) is a yellow fluorescent pigment secreted by P. aeruginosa that acts as a siderophore and helps the organism survive in iron-limiting condition by accumulating, mobilizing, and transporting iron into the cell (Dell’Anno et al. 2022). More than 100 variants of the dye are secreted by Pseudomonas spp. depicting considerable structural diversity (Ghssein and Ezzeddine 2022). The structure can be divided into three segments: a 6–12 amino acid long strain-specific peptide linked to a carboxyl group, a chromophore responsible for the fluorescence property, and a side chain that is connected to the nitrogen atom present at the C-3 position of the chromophore, which mostly are Krebs cycle intermediates or their derivatives (Schalk and Guillon 2013) (Fig. 2H). It is also a QS-regulator therefore controls its own synthesis apart from contributing to Pseudomonas pathogenesis (Dietrich et al. 2006). Non-ribosomal peptide synthetases (NRPSs) containing multiple modules are involved in the biosynthesis of PVD. Four gene products of the pvd locus, pvdL, pvdI, pvdJ, and pvdD have been identified for coding the NRPSs in PAO1 (Dell’Anno et al. 2022). Each module of the NRPS is responsible for the inclusion of individual amino acids to the peptide and bonding them with peptide linkages. The composition of the peptide varies among PVD secreted by different Pseudomonas strains and is attributed to the diverse substrate specificities of the NRPSs (Ghssein and Ezzeddine 2022). Incorporation of the chromophore moiety is also catalyzed by NRPSs which are synthesized in the cytoplasm by three enzymes PvdA, PvdF, and PvdH (Schalk and Guillon 2013). Apart from inducing biofilm formation by regulating QS pathways and exotoxin A production, PVD contributes towards overall nonresponsiveness of P. aeruginosa to antimicrobial therapies (Ullah et al. 2017). A recent screen by Vollenweider et al. (2023) with 320 natural Pseudomonas isolates against 12 human pathogens identified most potent PVD forms, which, in a concentration and iron-dependent manner, markedly dampened Acinetobacter baumannii, K. pneumonia, and S. aureus. PVD has also been reported as a potential candidate for delivering antimicrobial and/or anticancer agents to the target cells, biosensor for various molecules including pathogens and antibiotics, bioremediation, and phytostimulation (Dell’Anno et al. 2022) as summarized in Table 1.
Prodigiosin
Prodigiosine (PDG) is a member of the prodiginine family which is a red pigment and has a linear pyrrolyl dipyrromethene skeleton produced by a number of microbial groups including Serratia, Phaeocystis, Microcystis, Vibrio, Hahella, Janthinobacterium, and Streptomyces (Koksal Karayildirim et al. 2024; Mukhia et al. 2023). The structure of PDG consists of 2-methyl-3-pentyl-6-methoxyprodiginine which is a tri-pyrrole ring with red fluorescence and basic nature (Fig. 2I). Besides PDG, a few other members of the prodiginine family carry a linear chain like undecylPDG and few others are cyclic derivatives like streptorubin B, cyclononylPDG, cycloPDG, and butyl-meta-cycloheptylprodiginine (Darshan and Manonmani 2015; Williamson et al. 2006). The biosynthetic pathway of PDGs includes the condensation of a bipyrrole molecule 4-methoxy-2-2’-bipyrrole-5-carbaldehyde (MBC) with a monopyrrole. Condensation with monopyrrole 2-methyl-3-n-amyl-pyrrole (MAP) yields PDG whereas with 2-undecylpyrrole yields undecylPDG (Williamson et al. 2006). MBC biosynthesis involves successive combination of proline, malonyl- CoA and serine moieties whereas the monopyrrole portion is synthesized from different substrates and enzymes (Barreto et al. 2023). PDG can intercalate DNA and act as an inhibitor of topoisomerases I and II, thereby inducing DNA damage (Lins et al. 2015). It can compromise the integrity of the cytoplasmic membrane and bacterial outer membrane significantly (Danevcic et al. 2016) (Table 1). PDG has been reported to display an array of biological functions as antibacterial, anticancer, antiviral, antifungal, and antiparasitic agent (Islan et al. 2022). Tai et al. (2024) have reported that it can successfully down-regulate the TGF-β signalling in cancer cell lines and hence could be a potential therapeutic agent. Due to its effectiveness against UV-spectrum, PDG exhibited excellent sun protection factor, hence it is of immense interest for cosmetic industries (Lin et al. 2020).
Violacein
Violacein (VIO) is an excellent example of alkaloid pigment. This violet pigment is a bisindole (Fig. 2J) that is biosynthesized by condensation of two tryptophan molecules by forming indolocarbazole (Choi et al. 2015b). An array of enzymes coded by the vioABCDE operon catalyzes the reactions. The bacterium Chromobacterium violaceum is recognized as the most prominent producer of this pigment. Multiple Gram-negative organisms that show considerable phylogenetic distances and found in different environmental niches like members of the genera Janthinobacterium, Alteromonas, Collimonas, Duganella, Pseudoalteromonas, Massilia, and Iodobacter have been reported to synthesize the pigment (Inan Bektas et al. 2023; Kumar et al. 2022). VIO production by the organisms has been related to biofilm formation and quorum sensing system regulates the production (Batista et al. 2024). VIO is known for its diverse biological effects like antimicrobial (Inan Bektas et al. 2023; Johnson et al. 2023), antitumor (De Leon et al. 2024), and anticancer (Dahlem et al. 2022) action. The major mechanisms underpinned are extensive membrane damage and mitochondrial membrane depolarization (Aruldass et al. 2018; Duran et al. 2022). Aruldass et al. (2018) reported efficient antibacterial action of VIO against S. aureus and MRSA strains by affecting the membrane integrity (Aruldass et al. 2018). In osteosarcoma and rhabdomyosarcoma cell lines, VIO has been reported to increase apoptosis in an oxidative stress-independent manner (Milosevic et al. 2023). de Souza Oliveira et al. (2022) reported induction of apoptotic effect on colorectal cancer cells, and in a similar study by Kim et al. (2021) on hepatocellular carcinoma cells. Antioxidant (Xu et al. 2022) and anti-parasitic (Bilsland et al. 2018) properties have also been assigned to VIO (Table 1). DeoxyVIO, a VIO derivative that lacks a hydroxyl group, is extremely cytotoxic as observed against HepG2 cells. Another derivative, oxyVIO, with an additional hydroxyl group, demonstrated potent anti-Staphylococcal activity (Marinelli et al. 2015).
Melanin
Melanin (MEL) is a heterogeneous natural pigment produced by a number of bacterial genera including Proteus, Pseudomonas, Streptomyces, and several fungi (Singh et al. 2021). Streptomyces is the most extensively studied for the production of the brown/black pigment as suggested by recent reports on S. djakartensis (El-Zawawy et al. 2024) and S. nashvillensis (Restaino et al. 2024). Bacterial MEL (Fig. 2K) is a heterogeneous mix of molecules and hence the structure is quite undefined. Fungi and bacteria start the synthesis process using precursors L-tyrosine or malonyl CoA (Carletti et al. 2014). By the action of tyrosinase, L-tyrosine is initially converted to L-3,4-dihydroxyphenylalanine (L-DOPA) and subsequently transformed through intermediates into L-3,4-dihydroxyphenylalanine (L-DOPA) which is the precursor for euMELs. PyoMEL is synthesized by converting L-tyrosine into the precursor homogenistic acid via an intermediate p-hydroxyphenylpyruvate (Pralea et al. 2019). Although the pyoMEL synthesis pathway has been studied in detail in P. aeruginosa, enzyme homologs have been reported in Shewanella spp, Vibrio spp and Hypomonas spp. (Plonka and Grabacka 2006). L-tyrosine and/or L-DOPA are oxidized in the presence of L-cystein, and pheoMEL is generated which is a red-yellow colored pigment. Malonyl CoA is used as precursor to produce the fungal alloMEL (Restaino et al. 2024). Through its polymeric structure, MEL can scavenge free radicals, toxic metal ions, and drugs (El-Naggar and Saber 2022). MEL has been assigned with multiple bioactive characteristics like anti-inflammatory, antioxidant, and antimicrobial properties (Furlani et al. 2024). The pigment functions as an effective UV screen as MEL derivatives have been reported to be present in the spore coat of Bacillus thuringiensis to protect against UV-mediated damage (Zhu et al. 2022). El-Zawawy et al. (2024) have recently reported that purified MEL pigment showed antibacterial action against multidrug-resistant strains of S. aureus, Escherichia coli, Klebsiella pneumoniae, and P. aeruginosa. The biological significance of MEL is outlined in Table 1.
Indigoidine
A diverse group of bacteria produces a natural indigoidine (IND). The pigment 3′,3′-bipyridyl pigment (Fig. 2L) is formed through condensation of two molecules of L-glutamine catalyzed by a NRPS (Yu et al. 2013). Dickeya dadantii (formerly known as Erwinia chrysanthemi), a plant-pathogenic enterobacterium, is one of the prolific IND-producing bacteria (Reverchon et al. 2002). The pigment was also isolated from Streptomyces, Phaeobacter, Arthrobacter, Corynebacterium insidiosum, and other bacterial species. The bacteria can mitigate the growth of diverse microorganisms such as E. coli and S. aureus, and the fungal pathogen Candida albicans (Day et al. 2017). This pigment has wide applications in textile, food, and pharmaceutical industries (Zhao et al. 2024). Along with its antibiotic action, IND displays antioxidant properties (Xu et al. 2015) as a free radical scavenger (Table 1) that allows phytopathogens to tolerate organic peroxides and superoxide generated by plant defence response.
Flexirubin
The yellow-orange pigment is synthesized primarily by Chryseobacterium and Flexibacter with C. artocarpi, C. shigense, F. elegans, F. humi, and Cytophaga johnsonae as profound producers (Kim et al. 2019; Mogadem et al. 2022). The pigment has quite a unique chemical build-up. It has an interlocking ring structure, some with four nitrogens (pyrrole) and others with one less nitrogen (pyridine) with alternating single and double bonds (Fig. 2M), which makes flexirubins (FLR) appear yellow or orange. This molecule is composed of aryl polyenes and terminal alkyl substitution with a fatty acid tail of ω-phenyl octanoic acid chromophore with two alkylated resorcinol with ester bonds (Bukowy et al. 2008). Synthesis of FLR, therefore, involves a complex enzyme cascade (Schoner et al. 2014). The biosynthesis of the pigment initiates with the generation of a polyene moiety through a type II fatty acid synthase-like pathway as suggested by the gene composition of the BGC for the pigment, which harbors genes of several putative β-ketoacyl synthases, reductases, dehydratases, and thioesterases. Deamination of L-tyrosine to 4CA and its activation for the polyketide synthase (PKS) machinery by adenylation through the putative acyl-CoA ligase is the next step. An aryl-octane moiety synthesized utilizing 4-coumaroyl-CoA by β-ketoacyl synthases and reductases to form aryl-octane moiety. A ligase joins the aryl octane with 2,5- dialkylresorcinol (DAR). A putative polysaccharide deacetylase, a phospholipid/glycerol acyltransferase, an outer membrane lipoprotein carrier, a glycosyltransferase, and a putative exporter are predicted to be involved in the export of the pigment to outer membrane (Schoner et al. 2014). Biological activities of FLR and FLR-derived molecules include prolific free radical scavenging and anti-inflammatory activity as enlisted in Table 1 (Mogadem et al. 2021).
Role of bacterial pigment in community-level interactions
Pigments produced by bacteria often play a crucial role in determining microbial community structures. Alongside, for pigment-producing bacterial pathogens like P. aeruginosa or S. marcescens, pigments act as immunomodulators and impact host microbiomes. The impact on the bacterial community is particularly evident for secreted pigments like PHZs, where the producer bacteria gain a fitness advantage against other bacteria in polymicrobial communities. In mixed cultures with (A) baumannii or Enterococcus faecium, PCN production has been shown to get elevated compared to pure culture condition of P. aeruginosa (Laliany et al. 2022). PHZs can promote survival in anoxic condition by acting as an alternate terminal electron acceptor, particularly in biofilm communities (Saunders et al. 2020), as a cross-species signaling molecule (Dietrich et al. 2006), expediting iron acquisition (Wang et al. 2011), and eliminating competitor Gram-positive co-occupants (Wang et al. 2011). A recent report by Jean-Pierre et al. (2023) demonstrated that in an in vivo model of polymicrobial infection, mimicking cystic fibrosis (CF) with P. aeruginosa, S. aureus, Streptococcus sanguinis, and Prevotella melaninogenica, enhanced PHZ production that allows P. aeruginosa to tolerate tobramycin. PHZs like PCN can interfere with the redox status of bacterial and fungal pathogens and thereby can interfere with the metabolic activity of the pathogen in a community while it precisely controls redox balance in P. aeruginosa to reduce intracellular oxidative stress (Jacob et al. 2011; Thalhammer and Newman 2023). Dietrich et al. (2008) demonstrated that redox-active PCN shapes community structure by activating the transcription factor SoxR in Proteobacteria and Actinobacteria. The complexity of the microbial community in the CF respiratory tract is determined by PHZ content in the community. Extracellular release of PCN can impede neighbouring E. coli and induce significant transcriptional reprogramming of E. coli, related to respiration and membrane biogenesis (Yuan et al. 2021). PCN has been projected as a major determinant of antimicrobial responsiveness in communities with non-PCN-producing opportunistic pathogens, such as (B) cepacia complex, where PCN induces tolerance against fluoroquinolone antibiotics (Meirelles et al. 2021). PCN is involved in QS-mutant cheater suppression by acting as a policing toxin to selectively block the growth of cheaters. In a dual-species community with S. aureus, P. aeruginosa can determine phage-S. aureus interaction by triggering prophage induction through PCN production (Jancheva and Bottcher 2021). Contradictory results considering the impact of PHZ in determining microbial community have been observed by Ibberson et al. (2022), in wound infection model for P. aeruginosa and S. aureus dual species infection community. In rat and pig gut microbiome PCN exposure has been reported to induce dysbiosis (Peng et al. 2022).
Apart from, toxic metabolites, competition for essential nutrients like iron are key in determining community structure. Pigments like PVD and pyochelin, major siderophores produced by Pseudomonas, manifest high iron-binding affinity (> 1030 M− 1) and determine species interaction in aquatic and terrestrial environments (Butaite et al. 2018). The iron-complexed PVD is imported into the cell by specific receptors. PVD shows an extraordinary structural diversity with three major classes (I, II, and III) and more than 70 described variants that differ in their peptide backbone. pH, iron content, carbon concentration, and community diversity determine PVD production. PVD is secreted extracellularly, and following extracellular iron chelation, the bacterium will uptake the complex PVD-Fe3+ to acquire iron. PVD I is stored in the periplasmic space which prevents cellular uptake of other antimicrobial metal ions (Schalk and Guillon 2013). Inhibiting PVD by novel small molecules mitigates the pathogenesis of P. aeruginosa (Schalk and Guillon 2013). PVD also plays a crucial role in enriching the soil microbial community and inter-species social dynamics comprising the siderophore-producing P. fluorescens (O’Brien et al. 2023).
The antagonistic interaction of VIO with planktonic cells of S. aureus and S. epidermidis had been documented earlier (Batista et al. 2017; Dodou et al. 2017). Albeit only very few Gram-negative bacteria are susceptible to VIO, Gram-positive bacterial strains including Staphylococcus, Bacillus, and Streptococcus are sensitive to VIO (Choi et al. 2021). At a community level, VIO production is triggered by sublethal concentrations of hygromycin B and hygromycin A from Streptomyces sp. 2AW in soil (Lozano et al. 2020). Combining VIO with predatory bacteria Bdellovibrio bacteriovorus HD100, eventuated the elimination of polymicrobial community comprising Gram-negative bacteria like Acinetobacter and Klebsiella (Im et al. 2017). VIO has been reported to affect intestinal and skin microbiome. While in the gut microbiome of Wistar rats, at low VIO dose, Bacillus and Clostridia (Firmicutes) were found as dominant, at high doses, Bacillus followed by Clostridia and Actinobacteria were identified as the abundant members (Pauer et al. 2018).
Direct contact between S. auerus and S. marcescens has been demonstrated to be a prerequisite for the anti-Staphylococcal action of S. marcescens in dual-species culture. The interaction possibly involves the TypeVI secretion system (Lim et al. 2022). PDG can modulate microbial community structure and disease outcomes in amphibian skin infection models (Madison et al. 2019). In mouse models, administration of PDG beneficially altered the structure of cecum microbiota with enrichment of Lactobacillus reuteri and depletion of Desulfovibrio (Li et al. 2021). PDG derived from chromium-resistant Serratia sp was also demonstrated to modulate gut microbiota (Nie et al. 2023) in dextran sulfate sodium-induced colitis mice. In an elaborative study, Kim et al. (2023) explored the impact of PDG on six skin microorganisms available in commercially available skin microbiome mix. The acne vulgaris Cutibacterium acnes was evidenced to be highly susceptible to PDG with an alteration of global gene expression pattern. PDG-producing S. marcescens, when grown in dual-species biofilms outcompetes A. baumannii. Moreover, in co-culture condition, S. marcescens enhanced the susceptibility of A. baumannii against ciprofloxacin (Acharya et al. 2023). In one of their recent reports, Heu et al. (2021) highlighted the possible impact of PDG on the microbiota of the insect vector Aedes aegypti.
The carotenoids in S. auerus, have been shown to influence membrane structure and physicochemical properties by increasing the order of the fatty acyl chains. Such altered membrane structure prevents the insertion of a number of antimicrobial peptides including daptomycin and magainin, and thereby prevents pore formation (Manrique-Moreno et al. 2022). STX also fosters fitness by preventing oxidative stress which renders survival in wounds and delays the healing of diabetic wounds as revealed recently by Campbell et al. (2023).
Bacterial pigment in the pathogenicity of the producer
Though a number of pigment-producing bacteria are known as opportunistic pathogens for humans, except PCN and PVD in P. aeruginosa, and MEL produced by a Vibrio cholerae mutant, no other bacterial pigments have directly been associated with virulence. Strains of S. marcescens have been reported as enteric pathogen in human (Murdoch et al. 2011). Several strains of the bacteria can infect other vertebrates in insects and insect larvae (Li et al. 2023b; Shikov et al. 2023). Though PDG elicits immunomodulatory function as observed in in vivo infection models, it does not affect virulence of the bacteria, as demonstrated in insect infection models (Zhou et al. 2016). C. violaceum is an opportunistic pathogen that causes ocular infection in humans (Venkatramanan and Nalini 2024). Another VIO-producing bacteria, J. lividum, emerged as a pathogen for aquaculture, and caused severe mortality of rainbow trout Oncorhynchus mykiss (Oh et al. 2019). Though VIO demonstrates modest cytotoxicity against some mammalian cell lines, it has not been assigned as a virulence factor for the producer (Duran et al. 2021). MEL synthesis has been associated with virulence for a variety of pathogenic fungi by mitigating the efficacy of antimicrobials and by its influence on host immune response (Nosanchuk and Casadevall 2006). For a MEL-producing V. cholerae mutant elevated production of toxin-coregulated pilli (TCP), a major virulence factor, was observed. The mutant also demonstrated improved colonization to intestinal tissue of infant mice suggesting possible involvement of MEL production with bacterial pathogenicity (Valeru et al. 2009).
PCN is a QS-regulated virulence factor for P. aeruginosa, released through into the infection loci by a type II secretion system and impacts pathophysiology of cystic fibrosis (Caldwell et al. 2009). PCN can disrupt redox homeostasis in mammalian cells with reduction in cellular ATP generation, NAD/ NADH ratio, and level of reduced glutathione (O’Malley et al. 2004). In contrast to other bacterial pigments that act as antioxidants, PCN induces the generation of ROS and perturbs mitochondrial metabolism (Hall et al. 2016). ROS induction induces MUC2 and MUC5AC expression, both encoding for mucin secretion (Jeffries et al. 2016). PCN activates MAPK signalling, particularly ERK1/2, p38, and JNK signalling (Chai et al. 2014; Hall et al. 2016). PCN can act as an immunomodulator to trigger proinflammatory response through elevated IL-2, IL-6, and prostaglandin E2 production, which eventuates T- and B- lymphocyte proliferation (Jablonska et al. 2023). It can also induce cellular senescence and therefore impair tissue regeneration in P. aeruginosa-infected wounds (Muller et al. 2009). PCN can induce dysbiosis of microbiota and damage to the gut mucosal layer (Peng et al. 2022). Moreover, with its potential to permeate the blood-brain barrier, it can influence cognitive function in the murine model (Rashid et al. 2022). Overall, PCN can result in neurotoxicity, hepatotoxicity, and cognitive impairment (Mudaliar and Bharath Prasad 2024).
The siderophore pigments produced by P. aeruginosa are also associated with virulence of the pathogen. PVD can directly kill C. elegans even in the absence of the bacteria (Kang et al. 2018; Kirienko et al. 2015). It binds with iron in 1:1 stoichiometry and due to its high affinity for Fe3+, it can outcompete host transferrin (Kang et al. 2018). The ferri-PVD complex is recognized by the receptor FpvA which triggers the alternative sigma factor PvdS. PvdS activated expression of the BGC for PVD (Cornelis et al. 2023). Such a positive loop allows PVD generation until Fe3+ requirement of the bacteria is satisfied (Cornelis et al. 2009). Accumulation of PVD in extrapharyngeal tissues of C. elegans and lung tissues of mice directly correlates with cytotoxicity. Specific PVD inhibitors like gallium, fluoropyrimidines, and LK11 can considerably ameliorate cell survival (Kang et al. 2019).
4,4’-diaponeuresporenoic acid and STX, two major carotenoids produced by S. aureus, have been detected to enhance virulence and fitness of the pathogen possibly by providing protection against host innate immune system (Xue et al. 2019). STX biosynthetic pathways have been highlighted as a prospective target for designing anti-virulent drugs (Cueno and Imai 2018). However, an extensive genetic and phenotypic profiling for pigmented and non-pigmented S. aureus by Zhang et al. (2018), suggested no significant difference in virulence between the two types of strains.
Biosynthetic gene clusters for bacterial pigments
The majority of the pigments are synthesized through defined enzymatic reaction cascades. The enzymes are encoded by genes that are part of a BGC, expression of which is precisely modulated by the regulatory circuits. In this segment composition and regulation of BGCs for seven major bacterial pigments are discussed. Alongside, use of the identified BGCs in synthetic biology for improved production of the pigments are mentioned.
BGC for carotenoids
Defined biosynthetic gene clusters (BGC) linked to carotenoid biosynthesis have been identified in a spectrum of bacteria. Overtly, the BGCs are categorized into three different gene clusters the first one is organized in crtEXYIBZ order. The second one projects an organization of crtE-idi-crtXYIBZ, and the third one contains crtE-idi-crtYIBZ (Fig. 4A). Albeit the genes and organizations are similar in different bacteria, a diverse array of carotenoids can be synthesized by the bacteria (Zhang et al. 2012). Each of the gene products participates in conversion of isopentenyl pyrophosphate (IPP) to a specific carotenoid. For efficient production of carotenoids in E. coli Bl21(DE3) metabolic engineering was performed by Yang et al. (2014). A combination of crt genes from Erwinia herbicola with geranyl diphosphate synthase2 from Abies grandis generated a high carotenoid-yielding strain of E. coli. A high-yielding ZXT mono or di glycoside synthesizing E. coli strain could be developed by expressing seven crt genes of Cronobacter sakazakii (Zhang et al. 2014). A high amount of accumulation of β-carotene was achieved by expressing crtEE, crtYB and crt I from Xanthophyllomyces dendrorhous using marker-less genome editing CRISPR/Cas9 technology (Lopez et al. 2020).
VIO Cluster
A single operon comprising five genes vioABCDE constitutes the BGC for VIO production via shikimate pathway from L-tryptophan (Xu et al. 2022) (Fig. 4B). Each of the gene products participates in independent reactions of the pathway. The operon is regulated by the CviI-CviR QS system (Fig. 5A). Expression of the genes and production of VIO, particularly from the Cv206 strain, has long been implemented in screening and identification of inhibitors of the AHL dependent QS system. Cv206, is an AHL-deficient mutant of C. violaceum. Upon AHL induction, QS modulation can be estimated quantitatively by determining VIO accumulation (Duran et al. 2016). A visualization reporter system based on Gram-negative bacterial acyl-homoserine lactone quorum-sensing (VRS-bAHL) was constructed exploiting the operon. The VRS-bAHL can be implemented in profiling gene expression in Streptomyces (Liu et al. 2022). Robust VIO-producing C. violaceum strains were developed by altering the ribosome binding site (RBS) of the VIO operon. Such altered cassettes were cloned and expressed in E. coli and Corynebacterium glutamicum to attain higher yield/ titre for industrial production (Zhang et al. 2021). Other heterologous hosts like the oleaginous yeast Yarrowia lipolytica, were used to express the genes of VIO operon through three different promoters and were assembled to a combinatorial pathway library by golden gate cloning (Nemer et al. 2023).
Quorum sensing mediated regulation of bacterial pigment production. AHL mediated QS system regulating expression of VIO gene-cluster in C. violaceum depends on CviI-CviR system (A). PCN production from P. aeruginosa is regulated by a complex network of three QS system, the AHL dependent LasI-LasR, RhlI-RhlR, and the quinone dependent PQS system
PDG gene cluster
In Serratia, the PDG gene cluster is organized in a defined order starting with pigA gene and ending with pigM gene (Fig. 4C). A certain group of the gene products including pigD, pigE, and pigB are engaged in synthesizing 2-methyl-3-n-amyl-pyrrole (MAP) and another group comprising pigA, pigF, pigG, pigH, pigI, pigJ, pigL, pigM, and pigN yields 4-methoxy-2,2’-bipyrrole-5-carbaldehyde (MBC). PigC condenses the two products at the terminal step of PDG biosynthesis (Williamson et al. 2006). Except for such conserved genes in PDG-producing Serratia strains, in some strains, an additional gene pigO is harboured in the PDG cluster (Jia et al. 2021). The orthologues of PDG biosynthetic genes in S. coelicolor are encoded by distributing 23 genes in four clusters. Two of the 23 genes in the cluster (redD and Z) are pathway-specific regulators, six are assigned to 4-methoxy-2,2′-bipyrrole-5-carboxaldehyde biosynthesis (redW, O, M, L, K, and I), eight are assigned to 2-undecylpyrrole biosynthesis (redX, R, Q, P, N, H, G, and F), and two are assigned as housekeeping genes (redU and J) (Fig. 4C). Various QS and two-component system genes are attributed to regulation of PDG gene cluster. Among the QS systems, SmaI/SmaR and SpnI/SpnR were reported to control PDG production in Serratia spp. strains. Four two-component systems including PigQ/PigW, PhoB/PhoR, RssB/RssA, and EepR/EepS can regulate the synthesis of PDG (Jia et al. 2021). OmpR and PsrA were identified as transcriptional activators for PDG genes in S. marcescens JNB5-1 through a Tn5 mutagenesis screen. A robust PDG-producing strain was constructed by cloning the transcriptional activation under a strong constitutive promoter P17 to attain a metabolically engineered strain (PG06) (Pan et al. 2022b). Robust PDG-producing S. coelicolor strain was generated by concerted metabolic engineering by (1) inactivation of a gene for repressor (ohkA), (2) knocking out the actinorhodin (ACT) and calcium-dependent antibiotic (CDA) BGCs, and (3) multi-copy chromosomal integration of the red BGC. Such a strategy resulted in a strain of ∼ 12-fold improvement for PDG production (Liu et al. 2017). Similar to PCN, heterologous expression of PDG BGC from S. marcescens ATCC274 has been accomplished in Pseudomonas putida (Cook et al. 2021).
PCN gene cluster
The conversion of chorismate to PHZ involves seven enzymes that are conserved among PHZ producers. In P. aeruginosa, two independent homologous gene clusters, phzA1B1C1D1E1F1G1 (phz1) and phzA2B2C2D2E2F2G2 (phz2) (Fig. 4D) are associated with PHZ production as revealed by Mavrodi et al. (2001). The gene products mediate the conversion of chorismate to PHZ-1-carboxylic acid (PCA) and PHZ-1,6-dicarboxylic acid (PDC). The product of phzM and phzS in combination converts PCA to PCN. In the bacteria, there are two QS systems, namely las system and rhl system. A third signalling system, integrated with the two QS systems, quinolone signalling system (pqs) characteristic also regulates PCN synthesis. While the PHZ operon is directly activated by PqsR and RhlR, the LasR and IqsR indirectly affect the activation by modulating PqsR and RhlR (Abdelaziz et al. 2023) (Fig. 5B). In order to achieve PHZ production in E. coli, da Silva et al. (2021) implemented a construct by cloning nine genes of PCN pathway in ePathBrick vectors platform. Optimal PCN production from each strain were further evaluated by altering aeration conditions in bioelectrochemical systems. Heterologous synthesis of PCN has been optimized in non-pathogenic P. putida KT2440 earlier. Here Askitosari et al. (2019) expressed one PHZ operon from PAO1 and two PHZ operon from PA14 and combined each with simultaneous expression of phzM and phzS to achieve PCN generation.
PVD gene cluster
The gene required for biosynthesis of PVD is localized in the pvd locus, which comprises larger genes like pvdL, pvdI, pvdJ, and pvdD encoding diverse groups of enzymes linked to NRPSs. Gene pvdA, pvdF, and pvdH produces chromophores and other groups (Fig. 4E). A number of substitutions in different domains of PvdJ and PvdD rendered synthesis of novel modified PVD (Puja et al. 2023).
IND gene cluster
The IND biosynthetic gene cluster was initially characterized from D. dadantii. A single module NRPS, catalyses the condensation reaction of L-glutamine to yield IND (Kong et al. 2019). The transcription PecS regulates the synthesis of indigoidine by genes indA, indB, and indC (Zhao et al. 2024). The IND synthase gene was engineered for synthetic biology purposes, for developing chemogenomic reporter system in E. coli as well as mammalian cells, including human stem cells (Muller et al. 2012; Xie et al. 2017). Exploiting a dual expression strategy Nanjaraj Urs et al. (2019), recently generated a transgenic blue rose by synthesis of IND. High-level production of IND in C. glutamicum was accomplished by heterologous expression of IND synthetase from Streptomyces lavendulae (Ghiffary et al. 2021).
FLR BGC
A hypothesized biosynthetic pathway suggests the conversion of resorcinol and aryl polyene into FLR-type pigments. Responsible genes for pigment production are darA and darB genes (Fig. 4F), which are part of a large group of gene clusters from Fjoh_1080, Fjoh_1084, Fjoh_1095, Fjoh_1097, Fjoh_1098, Fjoh_1100, Fjoh_1108 (McBride et al. 2009). Presence of such gene clusters and identification of typical orthologues were possible from data generated through genomic and metagenomic analysis in other studies including genome analysis of the bacteria C. pinensis (Keller-Costa et al. 2021; Schoner et al. 2014; Vacheron et al. 2017).
Biomedical applications of bacterial pigments
The arising problems like multidrug resistance for pathogenic infections, resistance against existing chemotherapeutics in cancer, healthcare expenses, and the irreversible impact post-treatment on the patients are major concerns warranting the quest for novel drugs. Bacterial pigments have recently been explored as a prospective alternatives to tackle some major health concerns of present era. A snapshot of therapeutic potential of the pigments is portrayed in Fig. 6A.
Networks highlighting application for bacterial pigment. (A) Nondirectional network demonstrating various therapeutic applications of bacterial pigments are demonstrated with therapeutic application as source node and the pigments as the target node. (B) Similarly a nondirectional network depicting various industrial applications of the pigments are projected. The networks were generated using Cytoscape with a network file generated from the data available in the literature
Anti-bacterial
AZP shows antibacterial activity against Pseudomonas fragi, P. putida, P. pyocyanae, V. cholerae, E. coli, S. aureus, Salmonella paratyphi, Bacillus cereus, Mycobacterium smegmetis, M. phlei (Gupta et al. 1970). A number of studies consistently showed antibacterial activity of MEL against pathogenic species of Bacillus including B. cereus and Pseudomonas including P. aeruginosa and even on E. coli, K. pneumoniae and S. aureus (Ghattavi et al. 2022; Polapally et al. 2022; Singh et al. 2021). PDG is observed to be effective even against members of the ESKCAPE group of pathogens (Acharya et al. 2023; Lapenda et al. 2015). PDG inhibits staphylococcal infection by disrupting cell membranes, leading to cell lysis and death (Koksal Karayildirim et al. 2024). Recently profound anti-adherence activity of PDG was reported (Diken-Gur 2024). An extensive transcriptomic analysis of PDG treatment (Liu et al. 2024a) indicated cell wall synthesis, cell membrane, and biofilm formation impairment as possible mechanisms. VIO is efficient in inhibiting Gram-positive bacteria like E. faecalis, S. aureus at extremely low concentrations but are ineffective even in higher concentrations against some Gram negatives like Morganella morganii, K. pneumoniae, and Proteus mirabilis (Mudaliar and Bharath Prasad 2024). VIO exerts antimycobacterial effects against M. tuberculosis (Duran et al. 2016, 2021; Inan Bektas et al. 2023). With its iron (Fe) scavenging siderophore action, PVD demonstrated concentration-dependent and iron-limited suppression of the growth of the bacteria. PVD also possesses antimicrobial activity against bacteria like Vibrio sp., and Xanthomonas oryzae (Chen et al. 2016; Zhang et al. 2016). A recent screen identified 12 effective PVD derivatives; exerting inhibitory effects on A. baumannii, K. pneumoniae, and S. aureus in a concentration- and iron-dependent manner (Vollenweider et al. 2023). PCN exhibits a robust antibacterial effect by disrupting the microbial cell membrane and thereby compromising the function of the respiratory chain (Jayaseelan et al. 2014). PCN facilitates cell lysis by increased ROS production (Abdelaziz et al. 2023). Collectively, these multifaceted antimicrobial properties position PCN as a critical factor in the persistence and proliferation of P. aeruginosa within various environments. PHZs have been shown to accept metabolic electrons and facilitate redox balancing, ATP production, and survival in P. aeruginosa (Glasser et al. 2014; Schiessl et al. 2019). Eventuating restoration of electron transport chain (ETC), like fumarate, it can potentially revert metabolic dormancy in persisters. In fact, back in 2018, halogenated derivatives of PHZ were shown to act as antipersister against M. tuberculosis and MRSA (Garrison et al. 2018). SXT pigment has been evidenced to possess antibacterial activity against pathogenic strains of E. coli (Barretto and Vootla 2018). FLR, a yellow-orange pigment from Flavobacterium sp. Ant342 (F-YOP,) was projected as a prospective compound for chemotherapy of tuberculosis (Agarwal et al. 2023).
Anti-protozoan
AZP exhibits significant anti-protozoan activity against various protozoan parasites, including Plasmodium, Trypanosoma, and Leishmania species. The mechanisms underlying their anti-protozoan effects involve disruption of essential metabolic pathways, inhibition of key enzymes vital for parasite survival, and interference with protozoan membrane integrity. Additionally, AZPs have been shown to possess low cytotoxicity towards mammalian cells, highlighting their potential as safe and effective anti-protozoan agents (Tahghighi et al. 2018). Activity of PDG against Plasmodium falciparum indicated that PDG exhibits antiparasitic activity which inhibits the growth and proliferation of malaria parasites (Castro 1967) and Leishmania sp. (Moraes et al. 2008). VIO displays anti-protozoan, anti-helminthic, and anti-parasitic activity against various pathogens such as Plasmodium spp., Leishmania spp., and Trypanosoma spp. (Duran et al. 2016). ZXT has potential activity against helminthiasis caused by nematodes, platyhelminths, and anti-malarial activity of the carotenoid was also reported (Bouyahya et al. 2021).
Anti-fungal
Limited data suggest potential antifungal activity of AZPs against clinically relevant fungi such as C. albicans and Cryptococcus neoformans (Gupta et al. 1970; Zhao et al. 2018). MEL plays a critical role in the virulence of fungal pathogens like C. neoformans. Fungi produce MEL, a dark pigment, which significantly contributes to their ability to resist the body’s immune defenses and antifungal medications. However MEL demonstrates antifungal properties against Trichophyton simii, and T. rubrum (Arun et al. 2015). VIO exhibits effectiveness as an antifungal compound, particularly against the fungi Rosellinia necatrix, Rhizopus arrhizus, and C. aurius (Duran et al. 2022). PDG demonstrated antifungal activity against plant pathogenic fungi (Islan et al. 2022). Some antifungal activity can be observed by PVD on fungi like Piricularia oryzae, Botrytis cinerea, and A. fumigatus (Liu et al. 2021). Antagonistic activity is observed against some fungi and phytopathogens. Additionally, PCN demonstrates potent antifungal activity by interfering with the electron transport chain within fungal cells. The pigment also showed antifungal actions against Aspergillus spp, Candida spp., and C. neoformans (Kaur et al. 2015; Sass et al. 2021; Shouman et al. 2023) and was also found to be effective in treating T. rubrum infection in human (El-Zawawy and Ali 2016). Antifungal activity of SXT against C. albicans has also been reported (Barretto and Vootla 2018).
Anti-viral
The mechanisms underlying MEL’s antiviral activity are multifaceted and may involve direct interaction with viral particles (El-Naggar and Saber 2022). MEL exhibits broad-spectrum antiviral activity, hindering various stages of the viral lifecycle. Studies suggest that it disrupts viral entry, replication, and maturation. Additionally, MEL possesses immunomodulatory properties, bolstering the host’s immune response against viral infections. The mechanisms underlying this antiviral activity are multifaceted and may involve interference with viral binding to host cells, and the induction of antiviral cytokines. Antiviral properties can be observed against human immunodeficiency virus (HIV), SARSCoV2, and herpes simplex virus (Abd-El-Aziz et al. 2024; Montefiori and Zhou 1991). VIO also exhibits antiviral activity against herpes simplex virus and poliovirus (Duran et al. 2016). Suryawanshi et al. (2020) reported anti-HSV activity of PDG through inhibition of prosurvival NF-κB and Akt signalling pathways and eventual death of infected cells.
Anti-cancer
AZPs exhibit activity against multiple signaling cascades implicated in cancer progression and can target the leukemic cells K562 (Lucio et al. 2011). MEL pigment shows anticancer properties against skin cancer cell lines and anti-tumor properties by controlling tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6) (El-Obeid et al. 2006), and vascular endothelial growth factor (VEGF) synthesis by monocytes (El-Naggar and El-Ewasy 2017). MEL is a potential singlet oxygen scavenger and hence shows antioxidant properties (Ju et al. 2011). MEL has a unique skin wound healing and regeneration capacity, and coated nano-hydroxyapatite formulation is used for healing (Furlani et al. 2024). It also poses an anti-hemolytic effect by neutralizing free radicals from erythrocytes membrane and cell lysis. Across species, MEL acts as a protector against radiation-induced and free radical stress (Kordjazi et al. 2024) and has also been reported to have anticancer effects (El-Zawawy et al. 2024). PDG has low cytotoxicity and can show anticancer and antitumor activity by programmed cell death system for cancer cell line and inhibition of cell cycle (Anwar et al. 2022). VIO possesses antitumoral and anti-cancer properties, and can function as an immunomodulator. VIO prompts myeloid leukemia cells and TF1 leukemia cells to program cell death. In breast cancer cells VIO showed a non-canonical mechanism of cell death. VIO also acts on glioblastoma and lung cancer cell lines and reduces metastasis and glioblastoma migration (Duran et al. 2016; Mehta et al. 2015; Queiroz et al. 2012). PCN demonstrated significant cytotoxic effects on human pancreatic cancer cell line PANC-1 cells, triggering both apoptotic and necrotic pathways. Subsequent in vivo studies employing animal models are imperative to assess its efficacy as a potential anti-tumor therapy (Moayedi et al. 2018). PCN suppresses the cell proliferation of human melanoma cells SK-MEL-30 and human colon cancer cells HT-29. PCN shows anti-cancer properties against human breast cancer cell line MCF-7, human hepatoma cell HepG2, and colorectal carcinoma HCT-116 (Zhao et al. 2014). FLR produced by C. artocarpi CECT8497 demonstrated a proapoptotic effect against human breast cancer cell line MCF7. The pigment also demonstrated anti-cancer activity for 7,12-dimethylbenz(a)anthracene (DMBA) induced breast cancer in the Sprague Dawley rat model (Venil et al. 2016, 2021). STX isolated from S. gallinarum against Dalton’s lymphoma ascites, Ehrlich ascites carcinoma, adenocarcinomic human alveolar basal epithelial cells, and Mus musculus skin melanoma (B16F10) (Barretto and Vootla 2018).
Antioxidant
The antioxidant potential of AZPs has been evaluated using established assays, such as DPPH radical scavenging and ferric-reducing antioxidant power (FRAP) assays. These compounds also inhibit lipid peroxidation. This dual mechanism mitigates cellular damage caused by oxidative stress. Furthermore, AZPs appear to up-regulate the activity of endogenous antioxidant enzymes, including superoxide dismutase (SOD) and catalase (CAT), resulting in enhanced overall antioxidant capacity (Girgis et al. 2018). MEL showed oxygen-scavenging properties and metal-chelating activity, hence having high antioxidant properties (Manivasagan et al. 2013). IND has high antibiotic activity with antioxidant properties (Cude et al. 2012; Xu et al. 2015). The structure of the pigment suggests hydroxyl-radical-scavenging properties (Ali et al. 2013). ZXT protects from reactive oxygen intoxication and hence is an effective antioxidant and plays a major role in condensing central fovea of retina to protect it from light-initiated oxidative damage and macular degeneration (Landrum and Bone 2001; Widomska et al. 2020). Antioxidant property is evaluated to be high in an FLR-type pigment from C. artocarpi CECT 8497 by a number of standard antioxidant assay. The pigment demonstrated scavenging of superoxide, hydroxyl free radicals, and H2O2, along with mitigation of lipid peroxidation. FLR can bind directly with SOD and modulate its activity (Amorim et al. 2022a; Mogadem et al. 2021).
Anti-inflammatory
MEL has some biological applications such as anti-tumoral, reduced ROS production oxidative liver damage, and DNA damage (Tong et al. 2023). Other utilities of MEL are that it can be used in implantable devices like biosensors, and fluorescent probes and as hydrogel in photothermal therapy (Kim et al. 2020; Vahidzadeh et al. 2018). MEL has been suggested to act as a free radical scavenger, thereby mitigating oxidative stress often associated with inflammatory processes. Different proinflammatory cytokine levels decrease with VIO administration and different growth factors like endothelial, hepatocyte, epidermal, and hepatocyte increase, leading to major activity in mucin secretion and ulcer healing (Antonisamy et al. 2014). PCN exerts its cytotoxic effects through the generation of ROS. While ROS are endogenously produced during cellular respiration, their excessive accumulation triggers oxidative stress. This disrupts cellular homeostasis, compromising metabolic processes and ultimately leading to cell death. PCN specifically mediates its toxicity by oxidizing NADH and NADPH, consequently elevating cytosolic ROS levels and redox potential. This cascade of events results in diminished ATP production and a dysregulation of the reduced-to-oxidized glutathione ratio. Notably, PCN has been implicated in the pathogenesis of various physiological systems, including the urological, nervous, hepatic, and vascular systems (Hall et al. 2016). The impact of PCN is demonstrated to be dose-dependent. At lower concentrations, PCN exhibits immunomodulatory properties, stimulating the proliferation of T and B lymphocytes, enhancing IL-2 production, and promoting B cell differentiation (Ulmer et al. 1990). Conversely, in vivo studies demonstrated that PCN accelerates neutrophil apoptosis, thereby mitigating local inflammation and potentially favouring P. aeruginosa persistence during infection (Allen et al. 2005). ZXT acts as an anticancer and anti-inflammatory due to its antioxidant properties (Raman et al. 2024). SXT is a carotenoid pigment that reduces the activity of ROS, and thereby increases neutrophil resistance and virulence in the host for the bacteria (Xue et al. 2019). FLR demonstrated hepatoprotective effects to ameliorate oxidative stress, steatosis, ballooning degeneration, leukocytic infiltration, and necrosis (Mogadem et al. 2022).
Neuroprotection
The role of MEL in the central nervous system (CNS) has gained interest, especially in the context of neurodegenerative diseases. The potential neuroprotective effects stem from its proposed functions. One key function is its ability to act as a free radical scavenger. By neutralizing free radicals, MEL helps reduce oxidative stress, a cellular condition implicated in neuronal damage and neurodegeneration. Furthermore, MEL might play a role in regulating neurotransmitter levels and synaptic function, both crucial for maintaining healthy and functional neurons (Petrosyan 2015; Petrosyan et al. 2012; Tang et al. 2022). The tripyrrole, PDG shows promise as a neuroprotectant. Studies suggest it improves chronic unpredictable mild stress (CUMS)-induced depression-like behaviour in rats (Albrakati et al. 2021). Beyond its antioxidant effects, PDG also combats inflammation in the brain, potentially aiding neurodegenerative diseases. PDG further protects neurons by interfering with cell death pathways, making it a strong candidate for the treatment of neurodegenerative diseases. Its multifaceted approach includes boosting natural antioxidants and lowering harmful molecules (ROS) in neurons, further protecting them from damage (Salem et al. 2022). Furthermore, PDG modulates neuroinflammatory responses by inhibiting the NF-κB signalling pathway and down-regulating the expression of pro-inflammatory cytokines, thereby creating a neuroprotective milieu. Because of its well-known bioactive qualities, PDG from S. marcescens has been proposed as a potential medication for the treatment of neurodegenerative along with cancerous disorders as summarized by Tunca Koyun et al. (2022). Acetylcholine esterase (AChE) enzyme activity responsible for neurodegenerative diseases can be inhibited using PCN (Mudaliar and Bharath Prasad 2024). Even neural injury caused by AChE induced by H2O2 can be protected by PCN (Ibberson et al. 2022). ZXT reduces Alzheimer’s disease and neural disorders related to visualization and auditory signals (Wong et al. 2017). Even regular ZXT in a diet reduces pro-inflammatory hormones, anxiety, depression, and diabetics (Stringham et al. 2019).
Bacterial pigment and antibiotic interaction
MEL can bind to certain antibiotics, potentially affecting their distribution and bioavailability within the body. This interaction may consequently influence the pharmacokinetics and pharmacodynamics of the antibiotics. MEL can bind to fluoroquinolones like ciprofloxacin and moxifloxacin, potentially affecting their distribution and bioavailability (Alyami et al. 2022; Beberok et al. 2011). The pigment also interacts with β-lactam antibiotics, including penicillins and cephalosporins, influencing the pharmacokinetics of β-lactams and impacting their effectiveness against bacteria (Barza et al. 1976). MEL binding to tetracyclines such as doxycycline and minocycline has been observed. This may affect their distribution and efficacy within the body (Rok et al. 2021). Some potential interactions between MEL and macrolide antibiotics like erythromycin and azithromycin, potentially influence their pharmacokinetics and bioavailability (Barza et al. 1976). VIO can be used to treat bovine mastitis either in single or in combinatorial treatment with antibiotics. It is observed that combinatorial usage of VIO along with antibiotics like azithromycin, cefadroxil, gentamycin, and kanamycin accentuates antibacterial activity against multidrug-resistant pathogenic bacteria (Duran et al. 2016). During combinatorial treatment along with antibiotics, VIO-gentamicin and VIO-cefadroxil treatments, effective results can be observed in S. epidermidis, Salmonella typhi, V. cholerae, P. aeruginosa, K. pneumoniae, and S. aureus (Dodou et al. 2017; Subramaniam et al. 2014). PDG exhibits synergistic or additive effects against many bacteria like E. coli, S. aureus, Bacillus cereus, C. violaceum, M. smegmatis, and P. aeruginosa when used in a combinatorial antimicrobial system (Gohil et al. 2020). When PCN is used to treat along with novobiocin and nalidixic acid then it shows potentiation of antibacterial activity against S. aureus. In combination with ciprofloxacin and nalidixic acid it shows such activity against E. coli. With meropenem synergy was observed against S. marcescens and Proteus mirabilis (Abdelaziz et al. 2023). Subinhibitory concentrations of ciprofloxacin, tobramycin, and meropenem can modulate PCN production by various strains of P. aeruginosa (Mojsoska et al. 2021).
Industrial application of bacterial pigment
Though a number of bioactive pigments are profoundly produced by opportunistic pathogenic bacteria, considering their potential of as natural dye, the molecules have immense prospect in food, agriculture, textile, and cosmetic industries. Alongside, the pigments are gaining novel implication in bioremediation, biofuel cell designing, and biosensor development. An association network map for the diverse industrial use of the pigments is provided in Fig. 6B to offer a snippet of their industrial application.
Food
In order to make their food appealing, the food industries began to use synthetic colorants. Since the synthetic colorants were made out of petroleum by-products they pose health risks to the consumers, which insist industries to switch to natural colorants. In the food industry, among the bacterial pigments, riboflavin, β-carotene, PDG, PCN, MEL, VIO, and lycopene have been identified as safe and edible colorants (Sen et al. 2019). In contrast, yellow-colored water-soluble pigment riboflavin has been reported to have applications as a dietary supplement and additive in dairy products, baby foods, and energy drinks for their ability to break down polymeric components like carbohydrates, proteins, and fat to release energy. It is also extensively used as a component of the vitamin B complex to treat specific deficiency (Peechakara et al. 2024). Red-orange colored bacterial pigment β-carotene is an excellent source (provitamin) of vitamin A that helps boosting the immune system and is necessary to prevent night blindness in human (Eroglu et al. 2012). Some other members of the carotenoid family have also been reported to have applications as food additives for animal and fish feed for aquaculture, and pharmaceutical fields (Stafsnes et al. 2010). PDG, the red pigment produced by a number of bacteria has been recognized as a multipurpose pigment that can be used extensively in commercial preparations of milk, yoghurt, and carbonated drinks (Namazkar 2013). Another blue-colored pigment, PCN, can be used in sweets, ice creams, and in proteinaceous dietary supplements. It can also purposed as a protective supplement due to its anti-bacterial, anti-fungal, and neuroprotective properties (Jayaseelan et al. 2014). MEL has also been reported to have application as food additive (Sen et al. 2019). VIO is in demand for use in cosmetics, medicine, textile as well as food industries (Sutherland et al. 2011) owing to its diverse bioactivities including antiulcerogenic, anticancer, antimicrobial, enzyme modulation, and anti-parasitic activities (Soliev et al. 2011). Furthermore, a number of pigments are undergoing laboratory analysis and may soon be used in the food industry as non-toxic, therapeutic food colorants. Examples of these pigments include undecylprodigiosin (isolated from S. marcescenes), and STX (derived from S. aureus) (Agarwal et al. 2023). A new class of immune-fortified foods may soon become widespread as a result of increased studies into the quest for new bacterial pigments. These foods would not only be aesthetically pleasing to eat, but they would also provide therapeutic immunity to the consumer—a valuable benefit in the current period where infectious diseases and lifestyle problems are more prevalent than ever.
Textile
The application of microbial pigments as industrial fabric dyes is not yet common and further exploration is warranted. In recent times, textile industries are venturing into bacterial pigments rather than synthetic ones because they are non-carcinogenic, eco-friendly, and also have antimicrobial activity. A number of bacterial pigments have found application in the field of textile dyeing owing to their ability to bind to the textile fiber and the most commonly used ones are PDG, MEL, and VIO. PDG from S. marcescens SB08 is used frequently for dyeing fibers like nylon, acrylics, cotton, and silk, and is quite stable when tested at variable conditions of washing, perspiration, and rubbing. PDG from Vibrio sp. is used for dying nylon, acrylics, silk, and wool (Barreto et al. 2023). Similarly, VIO extracted from C. violaceum has been used for dyeing pure silk, cotton, rayon, and polyester. The coloring could be obtained by either dipping the fabric into the dye solution or by boiling the fabric along with the bacteria and the intensity varies with the time and the temperature of exposure of the fabric to the dye. The process is divided into three stages – preparation of dyeing solution with pigment fixing additives, hot dyeing at 60 °C – 80 °C, washing and drying of dyed fabric. Dissolution of pigment depends on the nature of the pigment requiring solvents like ethanol, acetone, and methanol (Kramar et al. 2021). Dye baths produced using acetone, water, or ethanol are also considered eco-friendly. Appropriate pH optimization is also necessary while preparing dye baths depending on the type of textile material. Protein-based fibres like wool and silk require an acidic dye-bath whereas plant-based materials like cellulose require higher pH as acidic conditions may cause the cellulose to degrade. Again pigments are also sensitive towards pH change. Pigments from Serratia sakuensis change color at different pH: pH 4 (pink), pH 5 (red), pH 7 (orange), and pH 9 (yellow) (Ren et al. 2018). PDG from the strains Streptomyces sp. NP2 and NP4 showed brownish-to-red colour at low pH 3.5 and 4.5 and grey-to-blue at a pH of 8, thus dying at different pH induce different colour in multifibre fabric (Kramar et al. 2014). Pigment isolated from P. aeruginosa under alkaline conditions is blue but when dying polyester at 130 °C, the polyester dyed yellow. This is because of the pyrolysis of the PCN pigment; hence it is important to determine the sensitivity of both the pigment and the fabric towards pH and temperature. Frequently salts are used as additives in dyebaths for improving the fixation of natural dyes to fibres. These salts are called mordents, which form a complex with the pigment and are also able to attach to the fibre. Some conventional mordents are iron, copper, aluminium. Mordants have an effect on the resulting color of the fabric. Mordents like Al and Ti have significant positive impact on washing fastness, perspiration, and dry-cleaning of the dyed silk using PDG extracted from Zooshikella rubidus (Kim and Choi 2015). The synergistic antimicrobial activity of the pigment and silver nanoparticle (AgNP) exhibited remarkable effect against bacteria and C. albicans (Kim and Choi 2015). Synergy between the antimicrobial property of the pigment VIO extracted from J. lividum, and silver and titanium dioxide nanoparticles was observed when a viscose fabric was coated with the nanoparticle. After dying with the pigment, it showed greater antimicrobial activity against E. coli than the regular-dyed fabric (Khaksar et al. 2021). The works highlighted obstacles that must be surmounted in order to make microbial pigments widely used for commercial dyeing, including the high costs, yield, and color stability, as well as advancements in the extraction methods. But in spite of all of this, the market is growing, full of creative and innovative opportunities, and offering more environment-friendly products, opening up new avenues for biotechnological solutions.
Cosmetics
Cosmetic industries are also switching to the safer alternative i.e. the microbial pigments especially the carotenoids as they also have excellent capacity to reduce ROS production and are the major active ingredients in anti-aging creams. External environmental factors such as the UV exposure, smoke pollution, and intrinsic factors like genetics and lifestyle resulting in damage and degradation of the dermis and epidermis are the major factors in skin aging (Guillerme et al. 2017). A number of bacterial pigments have found their application in the cosmetics industry owing to their antioxidant properties. Carotenoids like lycopene, β-carotene, and canthaxanthin belong to this category (Wan et al. 2014). The red pigment PDG has also been found to be incorporated in a number of dermatological formulations to enhance their UV protection ability as measured by sunscreen protection factor by 25–65%. The combination of PDG with aloe Vera and Cucumis sativus fruit extract was also found to enhance the order of protection (Suryawanshi et al. 2015). VIO, isolated from the genus Pseudoalteromonas has been extensively tested in various cosmetic preparations due to the nonpathogenic character of the bacterium (Duran et al. 2016). It has been examined as an ingredient of products that come in direct and prolonged contact with the airways, mucous membrane, and skin, like antiperspirants, lipsticks, and eye makeup.
Biosensor
Gu and Cheung (2001) projected IND production from Vogesella indigofera up on exposure to Cr6+ as a biosensor for hexavalent Cr. Subsequently, IND production has been used in various metal ion detection with sensitivity between 200 and 300 µg/ml (Bereza-Malcolm et al. 2015). In an intensive effort, Gustavsson et al. (2016), engineered E. coli, for outer membrane expression of tyrosinase for complete oxidation and polymerization of tyrosine to melanin. Thus an efficient system in removal of pharmaceutical contaminants from polluted waters was generated with a rapid regeneration of the melanin matrix by simple pH cycling. A number of whole-cell biosensors based on pigment biosynthesis have been engineered in the recent past. For detecting low concentrations of QS signal, a N-butyryl homoserine lactone sensing biosensor was developed by genetically modifying P. aeruginosa CGMCC 1.860 RhlR (Yong and Zhong 2009). A strategy for developing copper biosensors was configured by Chen et al. (2017), using the production of the plant pigment β-xanthene. Using a similar strategy, bacterial pigment biosynthetic genes have been implemented in developing biosensors, particularly for metal and metalloid ions. Hui et al. (2020) described development of a whole-cell biosensor for lead with E. coli cells by integrating a circuit for expression of vio-genes under the control of PbrR, a Pb(II) dependent transcriptional regulator. Direct visualization for Pb contamination was achieved with a linear detection for VIO accumulation (OD490 nm) within a range of 0.19–1.5 µM. Further improvement of the sensor was accomplished with the incorporation of triggers for vio ABE-catalysed production of green prodeoxyVIO, vio ABDE-catalysed production of blue proVIO, vio ABCE-catalysed production of purple deoxyVIO, and vioABCDE-catalysed production of navyVIO to detect varied concentrations of Pb(II) (Hui et al. 2022b). A whole-cell biosensor for detecting cadmium was subsequently developed using the cd (II) sensory element fused with IND BGC and expression of CadR. Naked-eye detection for induction of blue coloration was achieved along with a colorimetric detection system with a limit of detection as low as 0.024 µM. The system also demonstrated modest nonspecificity as it could weakly detect other metal ions like Zn(II), Pb(II), and Hg(II) (Hui et al. 2022a). Through incorporation of vio-genes under mercury resistance (mer) promoter and mercury resistance regulator (MerR), a Hg(II) detection biosensor was developed with a colorimetric detection range of 0.78–12.5 µM (Guo et al. 2021).
Implementing two Zn-responsive transcription factors and regulatory element system, a tricolor sensor system for Zn present in serum was developed by Watstein and Styczynski (2018). The gene for conversion of lycopene to β-carotene (crtY) was placed in the promoter PzntA. Under PznuC, with additional “decoy” binding sites to sequester zinc-bound zinc uptake regulator (Zur), VIO-producing genes were placed. Thus two systems responding to two different concentrations of Zn, rendered development of a three-colored biosensor for Zn. Recently, in an effort to develop a bacterial pigment-based biosensor for detecting bacterial pathogens in water samples constructed on the basis of the QscR quorum sensing signal generated by the pathogenic bacteria. The system was primarily optimized with eGFP as reporter and subsequently the red pigment lycopene synthesizing module ctrE, ctrB, and ctrI genes were introduced in the system with ctrI under the regulation of QscR. The configured strain enabled point-of-care detection of water contamination by P. aeruginosa and Burkholderia pseudomallei (Wu et al. 2021). In the recent most effort to develop bacterial pigment-based biosensor, Hui et al. (2023) designed a high throughput system through profiling nine stress-responsive promoters. A set of such promoters was fused with purple deoxyVIO synthetic enzyme cluster and another set was fused with the blue IND gene cluster. Through this system, sensitive and efficient detection of genotoxic compounds like mitomycin C and nalidixic acid was accomplished.
Agriculture
Safe agricultural practices have included the use of biological agents mostly due to safety and specificity in their actions. Bacterial pigments have also been explored with the same goal. VIO was found to be effective as a component of an insecticide in preventing fungal infection in plants like grass sclerotinia stem rot, bean sprout seedling blight, pythium blight, as well as parasitic infections like Meloidogyne spp. diseases in watermelon (Orlandi et al. 2022). Similarly, PDG was found to be effective in inhibiting Drosophila larvae, Aedes aegypti, and Anopheles stephensi (Suryawanshi et al. 2015). PDG has also been found to be effective in restricting viral infections involved in sericulture. This dye has also been effective in restricting the environmental pollution caused by bloom of the cyanobacteria Microcystis aeruginosa (Wei et al. 2020). Scientists have reported a melanin-synthesizing mutant strain of B. thuringiensis to be resistant against UV-mediated damage (Zhu et al. 2022). This finding has opened the pathway for industrial production of light-stable eco-friendly insecticide. PHZ has been reported to have beneficial effect on plant roots as it helps to overcome ROS-mediated stress. Some PHZ-derivatives display inhibitory effects on the plant pathogen Rhizoctonia solanii (Xiang et al. 2018). Antifungal activity has also been reported for PVD against Aspergillus (Sass et al. 2021).
Bioremediation
A major cause of low crop yield is iron deficiency, even though Fe being the fourth most abundant metal on Earth. Fe in soil reacts with the insoluble bicarbonates causing the micronutrient deficiency in plants. This is mostly noted in soils with pH of 7.4–8.5 as in calcareous and alkaline soil. To counter this reduced availability, chelators such as ethylendiamine-N-N’bis(o-hydroxyphenylacetic) acid (o, o-EDDHA) are employed to efficiently chelate Fe but are harmful to the environment and are expensive. Siderophores produced and released by bacteria have high affinity for Fe due to groups like catecholate, hydroxamate, carboxylate, and can act as chelators (Cordero et al. 2017). PVD-type siderophores are produced by P. fluorescens, in uranium mines. These are able to enhance mobility and reduce heavy metal toxicity (Edberg et al. 2010). The binding capability of siderophores to Fe is stronger than to toxic heavy metals (Baysse et al. 2000; Braud et al. 2009), still, siderophores bind to toxic heavy metals, like Cr3+, Cu3+, Pb2+, Cu2+, V4+, and Al3+ (Braud et al. 2009), thus the detoxifying and binding capability of siderophore plays a remarkable role in plant growth on heavy metal-polluted lands.
E. coli can be genetically engineered to melanize its surface and remove pharmaceutical pollutants from wastewater with high efficiency (Cordero et al. 2017). Melanotic microorganisms are particularly attractive given their remarkable ability to grow in highly radioactive sites. The capacity of melanin pigments to readily adsorb radionuclides such as uranium and cobalt is advantageous. For instance, MEL has a significantly greater capacity to adsorb uranium (∼ 10-fold) than activated carbon (Saini and Melo 2013).
Fuel cell
PHZ, is a class of pigments that has electron transfer abilities and thus is used as an electron shuttle by a number of bacteria including the producer. This particular characteristic of the dye has made it a potent candidate for the production of biofuel cells (Simoska et al. 2023). Respiratory electrons generated by a group of microorganisms are diverted toward an electrode for the production of electrical energy. Constant oxidation of the dye additionally ensures overproduction aided by increasing cell density.
The use of bacterial pigments is currently supported and regulated by laws, and this trend is expected to persist in the coming years (Sen et al. 2019). Commercial processes for this are either already operational or in the developmental stages. According to Dufossé (2018), the industrial production of bacterial pigments such as PDG has already commenced for use as anticancer drugs and immunosuppressants. On the other hand, bacterial pigments such as rubrolone, heptylPDG, and ZXT are still in their developmental stages (Dufossé 2018). The present colorant production figure consists 75% of petroleum derivatives and 25% of plant extraction, both of which are challenged in terms of sustainability and environmental impact. Keeping aside the technical aspects in translating bacterial pigment to market ready product, the business perspective, in terms of embracing newer sustainable technologies within a reasonable financial limit, remains a major challenge in expanding market for natural pigments. With Europe widening the avenue for natural colorants including pigments originating from bacteria with raising their use in various industries including food and textile, an escalation of market entry for bacterial pigment-based product across the globe is anticipated (Venil et al. 2020b). The market share for carotenoids, particularly in food and nutraceutical industry, is till date overwhelmingly dominated by synthetic carotenoids with 68.24% market share for synthetic AXT, beta-carotene, and canthaxanthin (Market Research Report, 2022). Though ZXT can be produced from Chryseobacterium proteolyticum and Flavobacterium granuli with a yield of > 90%, and more than 99% yield could be achieved for canxanthin when produced by Dietzia schimae (Lopez et al. 2023), high production cost remains a prime factor. Deinove, a cleantech company, earlier pointed out that high cost of natural carotenoids against synthetic carotenoids ($350–7,500/ kg vs. $250–2,000/ kg) is restricting its market. Deinove recently identified deinoxanthin from Deinococcus sp. along with the identification of terminal enzymes for synthesizing various carotenoids and optimizing the yield and market finish (https://www.labiotech.eu/trends-news/synbio-carotenoids-market-deinove-greentech-industry-milestones/). In a major leap towards application of bacterial pigment in textile industry, Colorifix, a UK-based biotech company have engaged in implementing synthetic biology in genetically modifying bacteria to produce a range of natural pigments. Colorfix implements an on-site live whole dying process for fabrics in fermenter, which significantly reduces use of water and other chemicals. The industry trial for Colorfix in Portugal demonstrated 30-80% lesser impact on 10 critical environmental impact assessment parameters (https://colorifix.com/app/uploads/2022/12/colorifix-environmental-impact.pdf and https://colorifix.com/colorifix-proves-lower-environmental-impact-at-every-stage-of-its-biological-dyeing-process/). Pili Bio, a France based biotech farm, has developed bacterial biofactories integrated with pigment-producing enzyme cascades to produce pigments emphatically with least pollution (https://www.pili.bio/9/technology).
Industrial production of bacterial pigments
The pigments discussed here are produced profoundly by pathogenic bacteria, and are often associated with infection establishment. However pathogenic strains of bacteria are not considered as an ideal cell factories considering the risk as public and occupational health hazard. To our advantage, most of the pigments are not exclusively produced by pathogenic strains and a number of non-pathogenic producer strains, generally considered as safe (GRAS), have been identified. For pigments like VIO, PDG, and IND a number of such non-pathogenic producers have been isolated from soil or other environmental sources (Pailliè-Jiménez et al. 2020). Apart from native pigment producers, pigment production by expression of the BCGs in amicable hosts by implementing synthetic biology strategy have been achieved (Banerjee et al. 2020). Even cell-free multienzyme systems have been optimized for pigment production at industrial scale (Hooe et al. 2024).
In the context of industrial production of pigments, traditional methods are limited by their dependence on natural sources and the typically low yield of target molecules. To address these challenges and scale up industrial production, significant efforts have been made to enhance pigment yields from natural producers (Lyu et al. 2022). The quality and yield of bacterial pigments synthesized in different species depend on specific growth conditions and growth medium-supplementation for optimal synthesis of specific pigment at a desired growth phase (Galasso et al. 2017). Hence intense observation of production kinetics and yield at laboratory scale is required prior to scaling up the production (Agarwal et al. 2023). Physical stress associated with the scaling up includes the hydrostatic pressure gradient, which affects the membrane integrity, cell viability, and metabolic flux (Wehrs et al. 2019b). Major chemical stresses linked to scaling up pigment production are the raw material, microbial contaminants, and toxic by-products (Dasgupta Mandal and Majumdar 2023). Agro-industrial wastes like sugarcane juice, sugar beet, and molasses are utilized for pigment production at minimal production cost. Optimization of fermentation media through advanced statistical methods like response surface methodology (RSM), artificial neural networks (ANNs), and genetic algorithms (GAs) has enhanced production efficiency, although challenges remain in optimizing complex factor-response interactions (Padhan et al. 2021; Singh et al. 2015). Scale-up optimization with intense monitoring hence becomes a prerequisite to minimize the physical and chemical stress for industrial pigment production. Like other metabolites, strain improvement is exigent for the industrial production of pigments. Certain strategies have been adopted to develop high-yield strains that enhance pigment production along with cost rationalization for stringent fermentation conditions aiming an increase in pigment production per unit mass. Innovative bioreactor designs, such as bubble columns, contribute to higher pigment production rates by maintaining optimal oxygen transfer and reducing energy consumption, thereby improving overall process economics (Lyu et al. 2022). Following production, the downstream method used in the purification of pigment from the fermentation media defines the pigment quality, making a systematic purification scheme essential for pigment purification. Bacterial pigments can either be extracellular where they permeate from the biosynthesizing cell, or they can be intracellular where it is contained and stored within the producing cell (Agarwal et al. 2023). The extracellular pigment can be isolated from the culture matrix through chromatography. For intracellular or insoluble pigments, isolation first requires the cells to be disrupted to release the pigment into the surrounding medium (Agarwal et al. 2023). Most of the bacterial pigments are hydrophobic and are soluble selectively in organic solvents. After obtaining the pigment in desired organic solvent the solvent is allowed to evaporate and the powdered residue is analysed further (Agarwal et al. 2023). An enormous amount of effort has been put into optimizing the production of all the industrially relevant bacterial pigments. Industrial production of MEL, and PHZs including PCN has recently been elegantly elaborated by Orlandi et al. (2022) and Jabłońska et al. (2023). Here we discuss recent progress made in industrial production of the industrially relevant bacterial pigments- VIO, IND, PDG, and FLR with emphasis on strain isolation and improvement by genetic engineering, combinatorial strategy integrating synthetic biology, metabolic engineering. Alongside, development of production media and scale up, and extraction and down-stream processing are delineated.
Rettori et al. (1998) reported successful isolation of VIO from C. violaceum cultivated on cotton and extracted by ethanol. Subsequently, various agricultural waste materials (sugarcane bagasse, solid pineapple waste, molasses, and brown sugar) were examined for optimal VIO production with tryptophan supplementation (Ahmad et al. 2012). Kanelli et al. (2018) reported an optimal fedbatch culture condition for VIO with production as high as 1.8 g/ L from J. lividum. Addition of a sub-inhibitory concentration of ampicillin and 1%V/V glycerol for fed-batch fermentation substantially improved VIO-producing biomass. Recently Cheng et al. (2022) reported that augmenting QS with formic acid enhanced the production of VIO from C. violaceum. The group successfully scaled up the formulation up to bioreactor level to achieve an elevated VIO level. Optimization of VIO production from a number of native-producing species has been attempted for quite a long time. Gohil et al. (2022) recently formulated a soybean meal-based cost-effective growth medium for achieving a crude VIO yield of 1.5 g/L from C. violaceum. Using C. violaceum MTCC2626 strain Gharat and Singhal (2024), formulated a medium and fed-batch condition with pulse feeding of glucose and tryptophan to attain markedly enhanced yield of VIO. Apart from C. violaceum, a number of VIO-producing strains have been identified through a consistent effort by several groups. From the Pacific coast of Japan, VIO-producing Pseudoalteromonas strains were identified by Yada et al. (2008). For VIO production, an extremely high yielding strain of Duganella violaceinigra str. NI28 was identified (Choi et al. 2015a). Sequencing and functional characterization of VIO BGC was performed by August et al. (2000), which widened the avenue for metabolic engineering-based strain improvement. Wu et al. (2017) published the draft genome sequence of J. lividum which further unveiled the regulation of VIO BGC expression. A VIO-producing recombinant Citrobacter freundii strain was developed by cloning and expression of VIO gene cluster in a plasmid. The fed-batch fermentation condition was optimized with controlled dissolved oxygen and pH with continuous feeding of glycerol, ammonium chloride, and tryptophan to attain a final production for crude VIO of 4.13 g/ L (Yang et al. 2011). E. coli was engineered for VIO production utilizing glucose through combinatorial knockout of three genes linked to regulation of tryptophan metabolism (trpR, tnaA, and pheA) and overexpression of two rate-limiting genes of tryptophan metabolism (trpE and trpD). Subsequently, VIO BCG was introduced for downstream expression. At a bioreactor level scale-up, the strain could produce a VIO titre of 1.75 g/ L (Fang et al. 2015). Strong heterologous expression of vioB, vioC, and vioD in the yeast Y. lipolytica enabled increased yield of VIO production. With optimization of the medium and culture condition, a production of 70.04 mg/L could be achieved in shake-flask scale. In the same study, strong correlation of weak expression of vioD and deoxy-VIO production was established (Tong et al. 2021). Nemer et al. (2023), recently optimized a protocol of VIO extraction from engineered Y. lipolytica through extraction by ethyl acetate/cyclohexane mixture and subsequent column chromatography. Kholany et al. (2020) earlier described a two-step downstream process based on solid-liquid extraction and a second step addressing the separation of VIO from contaminant proteins using aqueous bi-phasic separation with Tween 20 and cholinium-based ionic liquids. A synthetic VIO BGC expressing construct was generated for expression in C. glutamicum ATCC 21,850 with a host-specific RBS positioned upstream of VIO BGC. When optimized for fermentation condition and medium for bio-reactor scale a VIO titre of 5436 mg/ L was attained (Sun et al. 2016). Aiming for further optimization of the bioprocess of VIO production a number of synthetic biology-based designing of its synthesis in heterologous system has been attempted. In one of the earliest of such efforts, Lee et al. (2013) screened a library generated and analysed by TaqMan Rapid Analysis of Combinatorial assemblies (TRAC) of a set of constitutive promoters with the genes for the five-enzyme VIO biosynthetic cascade. Satisfactory production of VIO, deoxyVIO, proVIO, and prodeoxyVIO was observed in downstream analysis. Using a cell-free multi-enzyme system, Hooe et al. (2024), explored substrate analogues for enzymes of VIO biosynthetic cascade. A number of homo-substituted compounds like 6,6′-difluoroVIO could be synthesized without interrupting tryptophan metabolism. A CRISPRi-mediated metabolic flux engineering method was optimized recently by designing a single-guide RNA (sgRNA) library to function with dCas9. An extensive metabolic rewiring was possible to tune metabolic flux through VIO biosynthetic cascade employing the approach (Byun et al. 2023). With further optimization of such processes implementing robust prediction models, striking advancement for industrial production of VIO in the near future is anticipated.
The natural blue pigment, IND, is produced by several bacteria formed through the condensation of two molecules of L-glutamine to 3′,3′-bipyridyl pigment by a single NRPS (Takahashi et al. 2007). Being a monoenzymatically synthesized pigment, in recent years production of the pigment has been immensely improved by employing various genetic engineering strategies. Brachmann et al. (2012), successfully activated the silent IND BGC in Photorhabdus luminescens by promoter exchange. In the same work, the group reported heterologous expression of IND genes in E. coli. Cloning and expression of IND-synthase and its activator in E. coli resulted in IND synthesis. The fermentation was further optimized by supplementation with L-glutamine or by in situ L-glutamine synthesis by over-expression of glutamine synthetase. The media optimization with various nitrogen sources enabled the production of 7 g/ L IND (Xu et al. 2015). S. lavendulae NRPS (blue-pigment indigoidine synthetase, BpsA) was heterologously expressed in S. cerevisiae. With media optimization for carbon sources and process optimization at the bioreactor-scale 980 mg/l production was attainable for IND (Wehrs et al. 2018). BpsA and sfp were expressed in the basidiomycete Rhodosporidium toruloides to produce IND in a low-cost renewable carbon and nitrogen sources enriched medium. With sorghum lignocellulosic hydrolysate in a batch process ∼ 3 g/ L IND could be produced with the strain while in a glucose fed-batch process, ∼ 86 g/ L production could be attained (Wehrs et al. 2019a). Heterologous expression of bpsA from S. lavendulae in C. glutamicum was performed for IND production. IND production from this strain was further improved by tuning the influx of precursors L-glutamate and L-glutamine, bolstering glucose uptake, and minimizing by product formation. An optimized fed-batch fermentation protocol yielded 49.30 g/L IND from the engineered strain (Kim et al. 2020). Recently Aspergillus oryzae has been utilized as a platform cell factory for expression of IND synthetase gene from Streptomyces chromofuscu s. Media optimization and addition of Tween 20 for fostering pigment release resulted in a production of ∼ 1.4 g/ L (Panchanawaporn et al. 2022). Cell-free systems have been adopted for synthesizing secondary metabolites, particularly to avoid the complexity of cellular metabolic cross-talks that impair product yield. For IND, the minimal complex PURE system was exploited to express the NRPS bpsA from S. lavendulae. The enzyme produced from cell-free system can be evaluated for efficacy of IND production (Siebels et al. 2020). In an intense effort to optimize genome-scale rewiring for metabolite production Banerjee et al. (2020), aimed IND production by P. putida. A minimum cut set model was implemented to set up strong growth-coupled IND production leveraging the genome-scale metabolic model (GSMM) for P. putida KT2440. A multiplex CRISPRi-based knockdown approach was exploited to edit P. putida and rewire the host metabolically through detailed introspection of the multi-gene engineered production strain. At a bioreactor-scale production of 12.5–25 g/ L was achieved through the approach. Recently, the same group rewired P. putida KT2440 for the production of IND from para-coumarate. Following optimization of para-coumarate minimal medium, 7.3 g/L IND production could be obtained from the final growth-coupled strain (Eng et al. 2023). Thus rationalizing genome engineering combined with omics data is expediting higher production of the pigment for industrial use.
Though increasing reports on diverse bioactivity of PDG is warranting extensive therapeutic and industrial application of the pigment, industrial production of the pigments remains a major hindrance to the broader applicability of the pigment. Though PDG was initially identified from S. marcescens, in subsequent years a number of species of Serratia and Pseudoalteromonas rubra, Janthinobacterium, Hahella chejuensis, and S. coelicolor were reported to produce and accumulate PDG. These isolated strains demonstrated variation in terms of PDG generation. S. marcescens is considered as the most prolific PDG producer with the highest recorded production of 49.5 g/L from UCP 1549 strain, which was isolated from a semiarid soil by de Araújo et al. (2010). A number of indigenous PDG-producing strains have been isolated from diverse ecological niches including soil, sea, freshwater lakes, polar region, and glaciers. A comprehensive profile of such isolated strains and their PDG-producing potential has been aptly summarized by Han et al. (2021). Though S. marcescens isolates from soil have been projected as profound PDG producers for high-level industrial production of PDG, strain improvement is exigent. Following the identification of the biosynthetic pathway for PDG (Williamson et al. 2005), the understanding of the regulation of PDG biosynthetic cassette has gradually accumulated. PigP, a positive regulator of pig operon, is negatively regulated by LysR-type regulator MetR (Pan et al. 2020). OmpR family transcriptional regulator, CpxR was identified as a temperature-sensitive regulator of pig operon (Sun et al. 2020). A transcriptional regulator RcsB represses pig operon by regulating FhlDC, which is an activator of pig operon (Pan et al. 2021). The MarR-family transcriptional repressor OhrR was identified as a negative regulator of PDG synthesis (Sun et al. 2022). Disruption of barA or/and uvrY results in elevated level PDG production in S. marcescens (Liu et al. 2023). A recent genome-wide Tn5 random loss of function mutagenesis screen by Jia et al. (2021) identified essential genetic elements for PDG biosynthesis. Exploiting such knowledge recombinant PDG producers have been designed by engineering on Serratia or by heterologous expression of PDG-biosynthetic network in P. putida. CpxR-deleted S. marcescens strains demonstrated considerable improvement in PDG production with a production of ∼ 6 g/ L (Sun et al. 2020). P. putida has been exploited as a heterologous host to express pig genes by chromosomal integration. With promoter shuffling and media optimization, a production of 1.1 g/ L was attained in non-baffled shake-flask level (Cook et al. 2021). Pan et al. (2022a), recently reported a constitutive promoter screening linked to the application of expression of pig genes in S. marcescens to enhance PDG production. However, such directed engineered strain development involves huge effort and also there are finite chances of genetic compensation against secondary metabolite production. In this regard, random mutagenesis might provide culture condition-specific selective advantage to the hyperproducers. However, barring some intermittent trials with ethyl methane sulfonate (EMS) or γ-ray (Elkenawy et al. 2017), intensive effort to exploit random mutagenesis for PDG production is still awaited.
Scaling up of PDG production using diverse strategies and conditions has been attempted by different groups (Abdul Manas et al. 2020; de Araujo et al. 2010; Dos Santos et al. 2021; Elkenawy et al. 2017; Nguyen et al. 2020), albeit satisfactory yield and purity remain unattained through any kind of economical production strategy. Hence, mass-scale production of PDG by S. marcescens remains highly expensive as the culture medium should contain glucose, sucrose, and fructose as pure carbon sources, at least 1.5% casein hydrolysate as nitrogen source, and plant seed oils for satisfactory growth. So, the development of an economical culture medium is exclusively needed to make the process economically favorable. Consistent efforts through the last decade to optimize media composition for PDG production underscored the nutritional prerequisites for its production. All such efforts engaged robust statistical methodology. PDG production of 2.6 g/ L was attained from H. chejuensis M3349 by Kim et al. (2008), with a media containing sucrose 10.0 g/L, peptone 8.0 g/L, and yeast extract 2.0 g/L. Chen et al. (2013), identified starch 6/peptone as C and N- source for SmC3 strain. The composition elevated PDG production up to ∼ 7 g/ L. Su et al. (2011) subsequently attained PDG production of ∼ 2.5 g/ L with 0.454% peptone, 0.5% sucrose as C and N-source respectively. Miglani et al. (2023) optimised a medium with xylose derived from rice straw and peanut de-oiled cake as an economical carbon source. An extremely high production of PDG (∼ 6 g/ L) was attained through the fermentation condition. A number of media supplementation improved PDG production. Elkenawy et al. (2017) demonstrated that crude glycerol induced PDG production by several folds in S. marcescens strains. A number of oil supplements including olive oil and plum oil have been tested for PDG production by S. marcescens by Abdul Manas et al. (2020). A number of amino acid supplementations have been profiled with diverse impacts on PDG production by S. marcescens (Han et al. 2021). A medium was developed for PDG production by Serratia from Cassava waste water with 2% mannitol supplementation to attain 45 g/ L PDG production (de Araujo et al. 2010). A low-cost scaling-up approach was adopted using demineralized crab shell powder by Nguyen et al. (2020), for pilot scaling up of PDG production to ∼ 5.1 g/ L of PDG. Dos Santos et al. (2021) optimized a solid substrate fermentation on agro-waste to attain PDG production of 119.8 g/kg dry substrate with a medium containing wheat bran and soybean oil. Using bagasse as an inert matrix excelled PDG yield up to 40.86 g/kg dry solid was attained by Xia et al. (2016). A detailed list of such media optimization and supplementation is reviewed by Han et al. (2021). Downstream processing and isolation of PDG after fermentation is also challenging as PDG is intracellular and insoluble in water. Following culture, PDG-containing cells are harvested by centrifugation. Acidified ethanol (95%, pH3.0) is added following harvest and lysis of cells. The PDG is trapped in the organic phase and can be separated from ethanol by evaporation. Subsequently, the dried mass is resuspended in ethanol and PDG is purified by liquid chromatography or column chromatography. PDG is purified from the columns using organic elution by hexane-acetone (Paul et al. 2022). The extracted PDG fraction can be further purified by TLC. Since the use of organic solvent for extraction is expensive and toxicogenic to a certain extent, optimization of environment-friendly low-cost methods like the use of chitin or resin for separation of PDG is underway. Often incorporation of additives in culture broth facilitates isolation or secretion of pigment from the cells. Addition of SDS at suboptimal concentration was observed to promote release of PDG possibly by forming an effective negative macromolecular charge cloud around cells. Hydrophobic polyurethane foam cubes allow absorption of PDG from the lysates, which can be subsequently washed off from the foam cubes by organic solvents (Han et al. 2021).
FLRs are unique type of bacterial pigments produced by the bacteria from the genera Flavobacterium and Chryseobacterium. For industrial production of the pigment, C. artocarpi CECT 8497, has been exploited for optimizing maximum yield by Venil et al. (2015). Optimizing a medium with lactose and tryptophan, and culture condition variables a maximum production of 521.64 mg/L could be achieved in a 50 L bioreactor scale. The pigment is soluble in acetone, alkaline aqueous, and DMSO while insoluble in water and most organic solvents (Venil et al. 2014) which makes the extraction arduous.
Bacterial pigments implication in nanotechnology
Bacteria-derived pigments are looked in to as a possible beneficial replacement for synthetic pigments in the field of natural dyes because of their many benefits, which include color stability, improved environmental friendliness, economics, and ease of production. However, in the presence of extreme heat, radiation, pH, or oxygen, bacterial pigments frequently show noticeable instability, finding it difficult to retain their properties under certain natural conditions (Chiba et al. 2006; Devi et al. 2024; Narsing Rao et al. 2017; Pagano et al. 2018). To address this challenge, microencapsulation emerges as a promising technique, which is capable of enhancing the solubility, stability, and photo-oxidation of materials by encapsulating active ingredients within micro/nanoparticles (Martinez-Alvarez et al. 2020). Encapsulated pigments within polymers enhance their stability and solubility under ambient conditions, consequently extending the shelf life of the final product (Soukoulis and Bohn 2018; Zabot et al. 2022). Microencapsulation significantly improved the stability of FLR isolated from C. artocarpi CECT8497 compared to their unencapsulated counterparts, offering increased protection. Additionally, the improved characteristics and potent antioxidant activity of the microcapsules, the pigment derived from C. artocarpi CECT8497 may find application as a natural colorant in the food sector (Mogadem et al. 2021). Using the human breast cancer cell line MCF-7, Venil et al. (2016) examined the anticancer effects of FLR-mediated AgNP and discovered that they suppressed 99% of the cells. Their method is unique because it produces stable AgNPs that have strong anticancer effects in an environmentally friendly manner. The findings indicate that AgNP mediated by FLR have potential as sophisticated chemotherapeutic interventions.
A recent study shows light, pH changes, and variations in temperature cause poor stability for PDG. Encapsulated PDG, on the other hand, improves stability and solubility, and presents a viable alternative to the synthetic colorants that are presently marketed (Desai 2012). In a study, the water-in-oil emulsion approach was implemented to create 40 to 60 μm chitosan microspheres loaded with PDG, and glutaraldehyde as the cross-linker. Breast cancer cells (MDA-MB-231 cells), when utilized to assess drug release, exhibited significantly reduced viability following 24 h of PDG therapy (Dozie-Nwachukwu et al. 2017). An alternative approach for administering cancer chemotherapy entails crafting microparticles composed of biodegradable poly (lactide-co-glycolide) (PLGA) encapsulating PDG by the evaporation of a single emulsion solvent, wherein poly-vinyl alcohol served as the emulsifying agent. In addition, as a control, paclitaxel (PTX)-loaded particles were created. Compared to the PLGA microspheres loaded with PTX, these PDG-loaded microspheres showed comparatively high and comparable drug loading and encapsulation efficiency, with particle diameters ranging from 5 to 50 μm. This renders them appropriate for controlled and targeted drug delivery system in cancer therapy. Upon testing their cytotoxicity on MDA-MB-231 cells, the results were comparable to those of PTX, causing apoptosis by inhibiting the growth of the cells during a mitotic phase of the cell cycle (Obayemi et al. 2016). A rapid single-step method was employed to produce PDG-conjugated AgNPs, harnessing the amphoteric properties of silver oxide in an alkaline solution. With an average diameter of 10 nm, these very stable and spherical nanoparticles showed an IC50 value of 29.85 µg/ mL against the human liver cancer cell line HepG2, while free PDG showed an IC50 of 44.83 µg/ mL (El-Batal et al. 2017). In a similar targeted delivery approach, dendrigraft poly-L-lysines (DGL) underwent modification with a synthetic peptide capable of binding to placental chondroitin sulfate (CSA), known as plCSA-BP, derived from the malarial protein VAR2CSA. This produced PDG-loaded nanoparticles, which had a negatively charged surface and an average diameter of 396 nm. These nanoparticles demonstrated an encapsulating efficacy of 90% and a loading capacity of around 41%. Studies conducted on the in vitro release revealed a rapid initial release within 12 h at pH 5.3 and 7.4, indicating its prospect as a delivery system. Assessment of their cytotoxic impact on choriocarcinoma cells (JEG3 cells) demonstrated significantly enhanced anticancer efficacy both in vitro and in the JEG3 tumor model in vivo, as compared to free PDG (Zhao et al. 2019). Utilizing PDG nano-micelles, a cotton dyeing technique was developed to exploit the antimicrobial properties of PDG. The hydrophobic pigment was encapsulated into micelles by means of microbial fermentation with constant agitation in presence of a nonionic surfactant, Tween 80. The dyed cotton demonstrated strong bacteriostatic effects against E. coli and S. aureus, with efficacy rates of 85% and 99%, respectively (Gong et al. 2017).
The restricted water solubility, low bioavailability, oxidation propensity, photo- and heat instability, and poor solubility all contribute to their limited use. In order to preserve ZEA from degradation and enhance their intestinal stability and permeability, the work reported by Radic et al. (2023) aimed to create nano-structured lipid carriers (NLCs) loaded with these substances. For the first time, they have demonstrated a considerable increase in intestinal absorption of ZEA-NLCs, which may help their applications in the food and pharmaceutical industries.
Due to their capability to function as either electron donors or acceptors in enzyme reactions, PHZs exhibit significant promise as support materials for enzyme immobilization and biosensing purposes. This feature improves the efficiency of the catalyst as well as long-term stability (da Silva et al. 2020). Moreover, when combined with Fe2O3 nanoparticles to form a nanocomposite, the complementary electrochemical catalytic characteristics of polyPHZ films and Fe2O3 nanoparticles suggest improved performance in both conductivity and sensing applications. Additionally, these novel nanostructured materials create an ideal environment for biomolecule immobilization, thereby further enhancing biosensing capabilities (da Silva et al. 2020).
MEL nanoparticles (MNPs) have been shown in several studies to have potential applications in a variety of fields; their semiconductor qualities have prompted the development of electronic films (Vahidzadeh et al. 2018). They have been used as adjuvants in cancer radiation therapy (Cuzzubbo and Carpentier 2021; Yue and Zhao 2021; Zhou et al. 2019) and sun protection against UV radiation (Mavridi-Printezi et al. 2020) because of their strong radiation-absorption and antioxidant abilities.
The main hindrance to the delivery and bioavailability of VIO is its hydrophobic character (Arif et al. 2017). Compared to starch-capped silver NPs (cAgNPs), VIO-capped silver NPs (vAgNPs) are more stable and have therapeutic efficacy against multidrug-resistant bacteria and fungi that are three to ten times higher. The attributes of VIO-capped nanoparticles (VNPs) include antibacterial, anticancer, and anticancer effects (Konzen et al. 2006). There have been prior investigations on the antibacterial and anticancer properties of VNPs (Arif et al. 2017). Comparing low doses of VNPs to high doses of free VIO, the former demonstrated stronger antioxidant properties. The scavenging ability of peroxides and superoxides by VIO was the plausible reason for such observation. The characteristics and advantages of various nano combinations with bacterial pigments are summarized Table 2. Also, the benefits offered by the nanoformulations to enhance sustainability and bioavailability of the pigments are highlighted.
Concluding remarks
Despite their striking bioactive potential, bacterial pigments have been overlooked to a certain extent with limited effort to systemically explore, evaluate, and invent strategies for application. A plethora of reports emerging throughout the last decade or so in their impact on pathogenicity, function in community and host interface, have precisely illuminated the significance of various groups of bacterial pigments. Attempts for optimization of industrial production of bacterial pigments and developing formulations to maximally exploit properties like antioxidant, antimicrobial, anti-inflammatory, and anticancer are underway for pharmacological application along with the obvious application as dye by replacing chemical dyes in various industries.
Bacterial pigments represent a part of the vast spectrum of organic pigments which are biodegradable and sustainable. The global organic pigment market is set to reach around 4.89 billion USD by the present year (https://menafn.com/1097992026/Global-Organic-Pigments-Market-Worth-Reach-USD-489-Billion-By-2024). Though till now pigments extracted from microalgae and fungi are predominating over the bacterial pigments in terms of use in industry, diverse groups of bacterial pigments are gaining attention, particularly for their diverse bioactive potential and advent of effective encapsulation strategies to increase sustainability. In contrast to the industrially important pigment-producing bacterial strains, a number of the pigment-producing fungi synthesize mycotoxins along with the pigments as enlisted by Poorniammal et al. (2021). Coproduction of such toxic metabolites impairs safety of the pigments and thereby restricts application in food, pharmaceutical, and cosmetic industry (Lin and Xu 2022). A second impediment for fungal pigment production is the product yield. As a cell factory fungal cells are less tuneable compared to bacteria and the array of chemical entities present in the pigment-containing biomass limits its yield through purification (Chadni et al. 2017). Apart from purification, media and process optimization for maximizing pigment production involves substantial cost, which limits scalability for fungal pigments (Kalra et al. 2020). Owing to such pluses in comparion to fungal pigments, and immense prospect as natural pigment for biotechnological and other industrial application considering the detrimental impact of synthetic pigments, the market for bacterial pigment is anticipated to surge gradually. With large industrial markets are inclining toward natural pigment, the 2021 market value for natural pigments attained a value of USD 1.21 billion. As summarized by Barreto et al. (2023), for natural pigments, the market carotenoid segment alone is projected to reach USD 2.7 billion. Among the bacterial pigments, AXT have already been approved by FDA for direct human consumption. Though plants have been a traditional source of natural colorant, their production and scalability are dependent of seasonal cultivation and weather conditions (Di Salvo et al. 2023). Compared to plant carotenoids, bacterial carotenoids are conveniently isolated and their production has escalated in recent years (Venil et al. 2020b).
A major group of bioactive pigment producers are opportunistic pathogens which remains a possible bottleneck in terms of industrial production of pigment like PDG or PCN. However the advancement of strategies for strain development, genetic engineering, and synthetic biology integrated to AI-ML, allowing to maneuver over the strong foundation of the colossal omics data available for tractable systems such hindrances are expected to get resolved in near future. For industrial production, the other bottleneck of cost optimization is being addressed by formulating medium with agro and dairy industrial wastes (Grewal et al. 2022). Process optimization with cutting-edge simulations and tuning the downstream processing for maximal yield are expected to overcome existing impediments in near future. A major area of research delving into the sustainability of bacterial pigments in various applications is nanotechnological exploration for developing novel formulations with bacterial pigments (Venil et al. 2020a). Along with discovering novel pigments and pigment producers, intensive effort is still warranted to exploit the complete potential of bacterial pigments.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AXT:
-
Astaxanthin
- BGC:
-
Biosynthetic gene cluster
- FLR:
-
Flexirubin
- IND:
-
Indigoidin
- AZP:
-
Azaphenanthrene
- MEL:
-
Melanin
- MRSA:
-
Methicillin resistant Staphylococcus aureus
- PHZ:
-
Phenazine
- PCN:
-
Pyocyanin
- PVD:
-
Pyoverdin
- PDG:
-
Prodigiosin
- QS:
-
Quorum Sensing
- RFV:
-
Roseoflavin
- ROS:
-
Reactive oxygen species
- SXT:
-
Staphyloxanthin
- TFV:
-
Toxoflavin
- VIO:
-
Violacein
- ZXT:
-
Zeaxanthin
References
Abd-El-Aziz AS, Abed NN, Mahfouz AY, Fathy RM (2024) Production and characterization of melanin pigment from black fungus curvularia soli AS21 ON076460 assisted gamma rays for promising medical uses. Microb Cell Fact 23(1):68. https://doi.org/10.1186/s12934-024-02335-y
Abdelaziz AA, Kamer AMA, Al-Monofy KB, Al-Madboly LA (2022) A purified and lyophilized Pseudomonas aeruginosa derived pyocyanin induces promising apoptotic and necrotic activities against MCF-7 human breast adenocarcinoma. Microb Cell Fact 21(1):262. https://doi.org/10.1186/s12934-022-01988-x
Abdelaziz AA, Kamer AMA, Al-Monofy KB, Al-Madboly LA (2023) Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: its production and biological activities. Microb Cell Fact 22(1):110. https://doi.org/10.1186/s12934-023-02122-1
Abdul Manas NH, Chong LY, Tesfamariam YM, Zulkharnain A, Mahmud H, Abang Mahmod DS, Mohamad Fuzi SFZ, Wan Azelee NI (2020) Effects of oil substrate supplementation on production of prodigiosin by Serratia nematodiphila for dye-sensitized solar cell. J Biotechnol 317:16–26. https://doi.org/10.1016/j.jbiotec.2020.04.011
Acharya K, Borborah S, Chatterjee A, Ghosh M, Bhattacharya A (2023) A comprehensive profiling of quorum quenching by bacterial pigments identifies quorum sensing inhibition and antibiofilm action of prodigiosin against Acinetobacter baumannii. Arch Microbiol 205(12):364. https://doi.org/10.1007/s00203-023-03710-w
Agarwal H, Bajpai S, Mishra A, Kohli I, Varma A, Fouillaud M, Dufosse L, Joshi NC (2023) Bacterial pigments and their multifaceted roles in contemporary biotechnology and pharmacological applications. Microorganisms 11(3). https://doi.org/10.3390/microorganisms11030614
Ahmad WA, Yusof NZ, Nordin N, Zakaria ZA, Rezali MF (2012) Production and characterization of violacein by locally isolated Chromobacterium violaceum grown in agricultural wastes. Appl Biochem Biotechnol 167(5):1220–1234. https://doi.org/10.1007/s12010-012-9553-7
Albrakati A, Alsharif KF, Al Omairi NE, Alsanie WF, Almalki ASA, Abd Elmageed ZY, Elshopakey GE, Lokman MS, Bauomy AA, Abdel Moneim AE, Kassab RB (2021) Neuroprotective Efficiency of Prodigiosins Conjugated with selenium nanoparticles in rats exposed to chronic unpredictable mild stress is mediated through Antioxidative, Anti-Inflammatory, Anti-apoptotic, and neuromodulatory activities. Int J Nanomed 16:8447–8464. https://doi.org/10.2147/IJN.S323436
Ali HM, Abo-Shady A, Sharaf Eldeen HA, Soror HA, Shousha WG, Abdel-Barry OA, Saleh AM (2013) Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem Cent J 7(1):53. https://doi.org/10.1186/1752-153X-7-53
Allen L, Dockrell DH, Pattery T, Lee DG, Cornelis P, Hellewell PG, Whyte MK (2005) Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J Immunol 174(6):3643–3649. https://doi.org/10.4049/jimmunol.174.6.3643
Alyami BA, Alqarni AO, Alqahtani YS, Mahnashi MH, Javed Q, Hassan M, Tahir T, Ali A, Kotwica-Mojzych K, Mojzych M (2022) Fluoroquinolones as Tyrosinase Inhibitors; Enzyme Kinetics and Molecular Docking Studies to Explore Their Mechanism of Action Applied Sciences. vol 12
Amorim LFA, Fangueiro R, Gouveia IC (2022a) Characterization of Bioactive Colored materials produced from bacterial cellulose and bacterial pigments. Mater (Basel) 15(6). https://doi.org/10.3390/ma15062069
Amorim LFA, Fangueiro R, Gouveia IC (2022b) Novel functional material incorporating flexirubin-type pigment in polyvinyl alcohol_kefiran/polycaprolactone nanofibers. J Appl Polym Sci 139(48). https://doi.org/10.1002/app.53208
Antonisamy P, Kannan P, Aravinthan A, Duraipandiyan V, Arasu MV, Ignacimuthu S, Al-Dhabi NA, Kim JH (2014) Gastroprotective activity of violacein isolated from Chromobacterium violaceum on indomethacin-induced gastric lesions in rats: investigation of potential mechanisms of action. ScientificWorldJournal 2014:616432. https://doi.org/10.1155/2014/616432
Anwar MM, Albanese C, Hamdy NM, Sultan AS (2022) Rise of the natural red pigment ‘prodigiosin’ as an immunomodulator in cancer. Cancer Cell Int 22(1):419. https://doi.org/10.1186/s12935-022-02815-4
Arif S, Batool A, Khalid N, Ahmed I, Janjua HA (2017) Comparative analysis of stability and biological activities of violacein and starch capped silver nanoparticles. RSC Adv 7(8):4468–4478. https://doi.org/10.1039/C6RA25806A
Aruldass CA, Masalamany SRL, Venil CK, Ahmad WA (2018) Antibacterial mode of action of violacein from Chromobacterium violaceum UTM5 against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). Environ Sci Pollut Res Int 25(6):5164–5180. https://doi.org/10.1007/s11356-017-8855-2
Arun G, Eyini M, Gunasekaran P (2015) Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (fries). Indian J Exp Biol 53(6):380–387
Askitosari TD, Boto ST, Blank LM, Rosenbaum MA (2019) Boosting Heterologous Phenazine production in Pseudomonas putida KT2440 through the exploration of the natural sequence space. Front Microbiol 10:1990. https://doi.org/10.3389/fmicb.2019.01990
August PR, Grossman TH, Minor C, Draper MP, MacNeil IA, Pemberton JM, Call KM, Holt D, Osburne MS (2000) Sequence analysis and functional characterization of the violacein biosynthetic pathway from Chromobacterium violaceum. J Mol Microbiol Biotechnol 2(4):513–519
Banerjee D, Chatterjee S, Banerjee UC, Guha AK, Ray L (2011) Green pigment from Bacillus cereus M(1)(16) (MTCC 5521): production parameters and antibacterial activity. Appl Biochem Biotechnol 164(6):767–779. https://doi.org/10.1007/s12010-011-9172-8
Banerjee D, Mondal A, Gupta M, Guha AK, Ray L (2014) Optimization of fermentation conditions for green pigment production from Bacillus cereus M(1) 16 (MTCC 5521) and its pharmacological application. Lett Appl Microbiol 58(1):25–30. https://doi.org/10.1111/lam.12151
Banerjee D, Eng T, Lau AK, Sasaki Y, Wang B, Chen Y, Prahl JP, Singan VR, Herbert RA, Liu Y, Tanjore D, Petzold CJ, Keasling JD, Mukhopadhyay A (2020) Genome-scale metabolic rewiring improves titers rates and yields of the non-native product indigoidine at scale. Nat Commun 11(1):5385. https://doi.org/10.1038/s41467-020-19171-4
Barreto JVO, Casanova LM, Junior AN, Reis-Mansur M, Vermelho AB (2023) Microbial pigments: Major groups and Industrial Applications. Microorganisms 11(12). https://doi.org/10.3390/microorganisms11122920
Barretto DA, Vootla SK (2018) In Vitro Anticancer activity of Staphyloxanthin Pigment extracted from Staphylococcus gallinarum KX912244, a gut microbe of Bombyx mori. Indian J Microbiol 58(2):146–158. https://doi.org/10.1007/s12088-018-0718-0
Barza M, Baum J, Kane A (1976) Inhibition of antibiotic activity in vitro by synthetic melanin. Antimicrob Agents Chemother 10(3):569–570. https://doi.org/10.1128/AAC.10.3.569
Batista AHM, Moreira ACD, de Carvalho RM, Sales GWP, Nogueira PCN, Grangeiro TB, Medeiros SC, Silveira ER, Nogueira NAP (2017) Antimicrobial effects of Violacein against Planktonic cells and biofilms of Staphylococcus aureus. Molecules 22(10). https://doi.org/10.3390/molecules22101534
Batista BB, de Lima VM, Picinato BA, Koide T, da Silva Neto JF (2024) A quorum-sensing regulatory cascade for siderophore-mediated iron homeostasis in Chromobacterium violaceum. mSystems e013972310.1128/msystems.01397-23
Baysse C, De Vos D, Naudet Y, Vandermonde A, Ochsner U, Meyer JM, Budzikiewicz H, Schafer M, Fuchs R, Cornelis P (2000) Vanadium interferes with siderophore-mediated iron uptake in Pseudomonas aeruginosa. Microbiol (Reading) Pt 10146. https://doi.org/10.1099/00221287-146-10-2425
Beberok A, Buszman E, Wrzesniok D, Otreba M, Trzcionka J (2011) Interaction between ciprofloxacin and melanin: the effect on proliferation and melanization in melanocytes. Eur J Pharmacol 669(1–3):32–37. https://doi.org/10.1016/j.ejphar.2011.08.003
Bereza-Malcolm LT, Mann G, Franks AE (2015) Environmental sensing of heavy metals through whole cell microbial biosensors: a synthetic biology approach. ACS Synth Biol 4(5):535–546. https://doi.org/10.1021/sb500286r
Bilsland E, Tavella TA, Krogh R, Stokes JE, Roberts A, Ajioka J, Spring DR, Andricopulo AD, Costa FTM, Oliver SG (2018) Antiplasmodial and trypanocidal activity of violacein and deoxyviolacein produced from synthetic operons. BMC Biotechnol 18(1):22. https://doi.org/10.1186/s12896-018-0428-z
Bouyahya A, El Omari N, Hakkur M, El Hachlafi N, Charfi S, Balahbib A, Guaouguaou F-E, Rebezov M, Maksimiuk N, Shariati MA, Zengin G, El Menyiy N, Chamkhi I, Bakrim S (2021) Sources, health benefits, and biological properties of zeaxanthin. Trends Food Sci Technol 118:519–538. https://doi.org/10.1016/j.tifs.2021.10.017
Brachmann AO, Kirchner F, Kegler C, Kinski SC, Schmitt I, Bode HB (2012) Triggering the production of the cryptic blue pigment indigoidine from Photorhabdus luminescens. J Biotechnol 157(1):96–99. https://doi.org/10.1016/j.jbiotec.2011.10.002
Braud A, Jezequel K, Bazot S, Lebeau T (2009) Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 74(2):280–286. https://doi.org/10.1016/j.chemosphere.2008.09.013
Bukowy Z, Harrigan JA, Ramsden DA, Tudek B, Bohr VA, Stevnsner T (2008) WRN exonuclease activity is blocked by specific oxidatively induced base lesions positioned in either DNA strand. Nucleic Acids Res 36(15):4975–4987. https://doi.org/10.1093/nar/gkn468
Butaite E, Kramer J, Wyder S, Kummerli R (2018) Environmental determinants of pyoverdine production, exploitation and competition in natural Pseudomonas communities. Environ Microbiol 20(10):3629–3642. https://doi.org/10.1111/1462-2920.14355
Byun G, Yang J, Seo SW (2023) CRISPRi-mediated tunable control of gene expression level with engineered single-guide RNA in Escherichia coli. Nucleic Acids Res 51(9):4650–4659. https://doi.org/10.1093/nar/gkad234
Calabrese V, Quici S, Rossi E, Cariati E, Dragonetti C, Roberto D, Tordin E, De Angelis F, Fantacci S (2010) Highly stable 7-N,N-dibutylamino-2-azaphenanthrene and 8-N,N-dibutylamino-2-azachrysene as a new class of second order NLO-active chromophores. Chem Commun (Camb) 46(44):8374–8376. https://doi.org/10.1039/c0cc02781b
Caldwell CC, Chen Y, Goetzmann HS, Hao Y, Borchers MT, Hassett DJ, Young LR, Mavrodi D, Thomashow L, Lau GW (2009) Pseudomonas aeruginosa Exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol 175(6):2473–2488. https://doi.org/10.2353/ajpath.2009.090166
Campbell AE, McCready-Vangi AR, Uberoi A, Murga-Garrido SM, Lovins VM, White EK, Pan JT, Knight SAB, Morgenstern AR, Bianco C, Planet PJ, Gardner SE, Grice EA (2023) Variable staphyloxanthin production by Staphylococcus aureus drives strain-dependent effects on diabetic wound-healing outcomes. Cell Rep 42(10):113281. https://doi.org/10.1016/j.celrep.2023.113281
Carletti G, Nervo G, Cattivelli L (2014) Flavonoids and melanins: a common strategy across two kingdoms. Int J Biol Sci 10(10):1159–1170. https://doi.org/10.7150/ijbs.9672
Castro AJ (1967) Antimalarial activity of Prodigiosin. Nature 213(5079):903–904. https://doi.org/10.1038/213903a0
Chadni Z, Rahaman MH, Jerin I, Hoque KMF, Reza MA (2017) Extraction and optimisation of red pigment production as secondary metabolites from Talaromyces Verruculosus and its potential use in textile industries. Mycology 8(1):48–57. https://doi.org/10.1080/21501203.2017.1302013
Chai W, Zhang J, Duan Y, Pan D, Liu W, Li Y, Yan X, Chen B (2014) Pseudomonas pyocyanin stimulates IL-8 expression through MAPK and NF-kappaB pathways in differentiated U937 cells. BMC Microbiol 14:26. https://doi.org/10.1186/1471-2180-14-26
Chatragadda R, Dufosse L (2021) Ecological and biotechnological aspects of pigmented microbes: a Way Forward in Development of Food and Pharmaceutical Grade pigments. Microorganisms 9(3). https://doi.org/10.3390/microorganisms9030637
Chen W-C, Yu W-J, Chang C-C, Chang J-S, Huang S-H, Chang C-H, Chen S-Y, Chien C-C, Yao C-L, Chen W-M, Wei Y-H (2013) Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem Eng J 78:93–100. https://doi.org/10.1016/j.bej.2013.02.001
Chen WJ, Kuo TY, Hsieh FC, Chen PY, Wang CS, Shih YL, Lai YM, Liu JR, Yang YL, Shih MC (2016) Involvement of type VI secretion system in secretion of iron chelator pyoverdine in Pseudomonas taiwanensis. Sci Rep 6:32950. https://doi.org/10.1038/srep32950
Chen P-H, Lin C, Guo K-H, Yeh Y-C (2017) Development of a pigment-based whole-cell biosensor for the analysis of environmental copper. RSC Adv 7(47):29302–29305. https://doi.org/10.1039/C7RA03778C
Cheng KC, Hsiao HC, Hou YC, Hsieh CW, Hsu HY, Chen HY, Lin SP (2022) Improvement in Violacein production by utilizing formic acid to Induce Quorum sensing in Chromobacterium violaceum. Antioxid (Basel) 11(5). https://doi.org/10.3390/antiox11050849
Chiba S, Tsuyoshi N, Fudou R, Ojika M, Murakami Y, Ogoma Y, Oguchi M, Yamanaka S (2006) Magenta pigment produced by fungus. J Gen Appl Microbiol 52(4):201–207. https://doi.org/10.2323/jgam.52.201
Choi SY, Kim S, Lyuck S, Kim SB, Mitchell RJ (2015a) High-level production of violacein by the newly isolated Duganella violaceinigra str. NI28 and its impact on Staphylococcus aureus. Sci Rep 5:15598. https://doi.org/10.1038/srep15598
Choi SY, Yoon KH, Lee JI, Mitchell RJ (2015b) Violacein: Properties and Production of a Versatile Bacterial Pigment. Biomed Res Int 2015:465056 https://doi.org/10.1155/2015/465056
Choi SY, Lim S, Yoon KH, Lee JI, Mitchell RJ (2021) Biotechnological activities and applications of bacterial pigments Violacein and Prodigiosin. J Biol Eng 15(1):10. https://doi.org/10.1186/s13036-021-00262-9
Cook TB, Jacobson TB, Venkataraman MV, Hofstetter H, Amador-Noguez D, Thomas MG, Pfleger BF (2021) Stepwise genetic engineering of Pseudomonas putida enables robust heterologous production of prodigiosin and glidobactin A. Metab Eng 67:112–124. https://doi.org/10.1016/j.ymben.2021.06.004
Cordero RJB, Vij R, Casadevall A (2017) Microbial melanins for radioprotection and bioremediation. Microb Biotechnol 10(5):1186–1190. https://doi.org/10.1111/1751-7915.12807
Cornelis P, Matthijs S, Van Oeffelen L (2009) Iron uptake regulation in Pseudomonas aeruginosa. Biometals 22(1):15–22. https://doi.org/10.1007/s10534-008-9193-0
Cornelis P, Tahrioui A, Lesouhaitier O, Bouffartigues E, Feuilloley M, Baysse C, Chevalier S (2023) High affinity iron uptake by pyoverdine in Pseudomonas aeruginosa involves multiple regulators besides Fur, PvdS, and FpvI. Biometals 36(2):255–261. https://doi.org/10.1007/s10534-022-00369-6
Cude WN, Mooney J, Tavanaei AA, Hadden MK, Frank AM, Gulvik CA, May AL, Buchan A (2012) Production of the antimicrobial secondary metabolite indigoidine contributes to competitive surface colonization by the marine roseobacter Phaeobacter sp strain Y4I. Appl Environ Microbiol 78(14):4771–4780. https://doi.org/10.1128/AEM.00297-12
Cueno ME, Imai K (2018) Network analytics approach towards identifying potential antivirulence drug targets within the Staphylococcus aureus staphyloxanthin biosynthetic network. Arch Biochem Biophys 645:81–86. https://doi.org/10.1016/j.abb.2018.03.010
Cuzzubbo S, Carpentier AF (2021) Applications of melanin and melanin-like nanoparticles in Cancer Therapy: a review of recent advances. Cancers (Basel) 13(6). https://doi.org/10.3390/cancers13061463
da Silva W, Queiroz AC, Brett CMA (2020) Nanostructured Poly(Phenazine)/Fe2O3 nanoparticle film modified electrodes formed by electropolymerization in ethaline - deep eutectic solvent. Microscopic and electrochemical characterization. Electrochim Acta 347:136284. https://doi.org/10.1016/j.electacta.2020.136284
da Silva AJ, Cunha JS, Hreha T, Micocci KC, Selistre-de-Araujo HS, Barquera B, Koffas MAG (2021) Metabolic engineering of E. Coli for pyocyanin production. Metab Eng 64:15–25. https://doi.org/10.1016/j.ymben.2021.01.002
Dahlem C, Chanda S, Hemmer J, Schymik HS, Kohlstedt M, Wittmann C, Kiemer AK (2022) Characterization of anti-cancer activities of Violacein: actions on Tumor cells and the Tumor Microenvironment. Front Oncol 12:872223. https://doi.org/10.3389/fonc.2022.872223
Danevcic T, Boric Vezjak M, Zorec M, Stopar D (2016) Prodigiosin - A Multifaceted Escherichia coli Antimicrobial Agent. PLoS ONE 11(9):e0162412. https://doi.org/10.1371/journal.pone.0162412
Darshan N, Manonmani HK (2015) Prodigiosin and its potential applications. J Food Sci Technol 52(9):5393–5407. https://doi.org/10.1007/s13197-015-1740-4
Dasgupta Mandal D, Majumdar S (2023) Bacteria as biofactory of pigments: evolution beyond therapeutics and biotechnological advancements. J Biosci Bioeng 135(5):349–358. https://doi.org/10.1016/j.jbiosc.2023.01.008
Day PA, Villalba MS, Herrero OM, Arancibia LA, Alvarez HM (2017) Formation of indigoidine derived-pigments contributes to the adaptation of Vogesella sp strain EB to cold aquatic iron-oxidizing environments. Antonie Van Leeuwenhoek 110(3):415–428. https://doi.org/10.1007/s10482-016-0814-2
de Araujo HW, Fukushima K, Takaki GM (2010) Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules 15(10):6931–6940. https://doi.org/10.3390/molecules15106931
De Leon ME, Wilson HS, Jospin G, Eisen JA (2024) Corrigendum: ‘Genome sequencing and multifaceted taxonomic analysis of novel strains of violacein-producing bacteria and non-violacein-producing close relatives’. Microb Genom 10(2). https://doi.org/10.1099/mgen.0.001204
de Souza Oliveira PF, Faria AVS, Clerici SP, Akagi EM, Carvalho HF, Justo GZ, Duran N, Ferreira-Halder CV (2022) Violacein negatively modulates the colorectal cancer survival and epithelial-mesenchymal transition. J Cell Biochem 123(7):1247–1258. https://doi.org/10.1002/jcb.30295
Dell’Anno F, Vitale GA, Buonocore C, Vitale L, Palma Esposito F, Coppola D, Della Sala G, Tedesco P, de Pascale D (2022) Novel insights on pyoverdine: from biosynthesis to Biotechnological Application. Int J Mol Sci 23(19). https://doi.org/10.3390/ijms231911507
Desai N (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J 14(2):282–295. https://doi.org/10.1208/s12248-012-9339-4
Devi M, Ramakrishnan E, Deka S, Parasar DP (2024) Bacteria as a source of biopigments and their potential applications. J Microbiol Methods 219:106907. https://doi.org/10.1016/j.mimet.2024.106907
Di Salvo E, Lo Vecchio G, De Pasquale R, De Maria L, Tardugno R, Vadala R, Cicero N (2023) Natural pigments production and their application in Food, Health and Other industries. Nutrients 15(8). https://doi.org/10.3390/nu15081923
Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK (2006) The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61(5):1308–1321. https://doi.org/10.1111/j.1365-2958.2006.05306.x
Dietrich LE, Teal TK, Price-Whelan A, Newman DK (2008) Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science 321(5893):1203–1206. https://doi.org/10.1126/science.1160619
Diken-Gur S (2024) Investigation of anti-adherence and antimicrobial properties of prodigiosin-functionalized bacterial cellulose membrane for biomedical applications. J Biotechnol 385:58–64. https://doi.org/10.1016/j.jbiotec.2024.03.002
Dodou HV, de Morais Batista AH, Sales GWP, de Medeiros SC, Rodrigues ML, Nogueira PCN, Silveira ER, Nogueira NAP (2017) Violacein antimicrobial activity on Staphylococcus epidermidis and synergistic effect on commercially available antibiotics. J Appl Microbiol 123(4):853–860. https://doi.org/10.1111/jam.13547
Dos Santos RA, Rodriguez DM, da Silva LAR, de Almeida SM, de Campos-Takaki GM, de Lima MAB (2021) Enhanced production of prodigiosin by Serratia marcescens UCP 1549 using agrosubstrates in solid-state fermentation. Arch Microbiol 203(7):4091–4100. https://doi.org/10.1007/s00203-021-02399-z
Dozie-Nwachukwu SO, Danyuo Y, Obayemi JD, Odusanya OS, Malatesta K, Soboyejo WO (2017) Extraction and encapsulation of prodigiosin in chitosan microspheres for targeted drug delivery. Mater Sci Eng C Mater Biol Appl 71:268–278. https://doi.org/10.1016/j.msec.2016.09.078
Dufossé L (2018) Microbial Pigments From Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries Natural and Artificial Flavoring Agents and Food Dyes. pp 113–132
Duran N, Justo GZ, Duran M, Brocchi M, Cordi L, Tasic L, Castro GR, Nakazato G (2016) Advances in Chromobacterium violaceum and properties of violacein-its main secondary metabolite: a review. Biotechnol Adv 34(5):1030–1045. https://doi.org/10.1016/j.biotechadv.2016.06.003
Duran N, Nakazato G, Duran M, Berti IR, Castro GR, Stanisic D, Brocchi M, Favaro WJ, Ferreira-Halder CV, Justo GZ, Tasic L (2021) Multi-target drug with potential applications: violacein in the spotlight. World J Microbiol Biotechnol 37(9):151. https://doi.org/10.1007/s11274-021-03120-4
Duran N, Castro GR, Portela RWD, Favaro WJ, Duran M, Tasic L, Nakazato G (2022) Violacein and its antifungal activity: comments and potentialities. Lett Appl Microbiol 75(4):796–803. https://doi.org/10.1111/lam.13760
Edberg F, Kalinowski BE, Holmstrom SJ, Holm K (2010) Mobilization of metals from uranium mine waste: the role of pyoverdines produced by Pseudomonas fluorescens. Geobiology 8(4):278–292. https://doi.org/10.1111/j.1472-4669.2010.00241.x
El-Batal AI, El-Hendawy HH, Faraag AHI (2017) In silico and In vitro cytotoxic effect of prodigiosin-conjugated silver nanoparticles on liver cancer cells (HepG2). BioTechnologia 98(3):225–243 https://doi.org/10.5114/bta.2017.70801
El-Naggar NE, El-Ewasy SM (2017) Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci Rep 7:42129. https://doi.org/10.1038/srep42129
El-Naggar NE, Saber WIA (2022) Natural melanin: current trends, and future approaches, with Especial Reference to Microbial source. Polym (Basel) 14(7). https://doi.org/10.3390/polym14071339
El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A (2006) Herbal melanin modulates tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine 13(5):324–333. https://doi.org/10.1016/j.phymed.2005.03.007
El-Zawawy NA, Ali SS (2016) Pyocyanin as anti-tyrosinase and anti tinea corporis: a novel treatment study. Microb Pathog 100:213–220. https://doi.org/10.1016/j.micpath.2016.09.013
El-Zawawy NA, Kenawy ER, Ahmed S, El-Sapagh S (2024) Bioproduction and optimization of newly characterized melanin pigment from Streptomyces djakartensis NSS-3 with its anticancer, antimicrobial, and radioprotective properties. Microb Cell Fact 23(1):23. https://doi.org/10.1186/s12934-023-02276-y
Elkenawy NM, Yassin AS, Elhifnawy HN, Amin MA (2017) Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol Rep (Amst) 14:47–53. https://doi.org/10.1016/j.btre.2017.04.001
Eng T, Banerjee D, Menasalvas J, Chen Y, Gin J, Choudhary H, Baidoo E, Chen JH, Ekman A, Kakumanu R, Diercks YL, Codik A, Larabell C, Gladden J, Simmons BA, Keasling JD, Petzold CJ, Mukhopadhyay A (2023) Maximizing microbial bioproduction from sustainable carbon sources using iterative systems engineering. Cell Rep 42(9):113087. https://doi.org/10.1016/j.celrep.2023.113087
Eroglu A, Hruszkewycz DP, dela Sena C, Narayanasamy S, Riedl KM, Kopec RE, Schwartz SJ, Curley RW Jr., Harrison EH (2012) Naturally occurring eccentric cleavage products of provitamin a beta-carotene function as antagonists of retinoic acid receptors. J Biol Chem 287(19):15886–15895. https://doi.org/10.1074/jbc.M111.325142
Fang MY, Zhang C, Yang S, Cui JY, Jiang PX, Lou K, Wachi M, Xing XH (2015) High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microb Cell Fact 14:8. https://doi.org/10.1186/s12934-015-0192-x
Fekete-Kertesz I, Berkl Z, Buda K, Fenyvesi E, Szente L, Molnar M (2024) Quorum quenching effect of cyclodextrins on the pyocyanin and pyoverdine production of Pseudomonas aeruginosa. Appl Microbiol Biotechnol 108(1):271. https://doi.org/10.1007/s00253-024-13104-7
Furlani F, Pota G, Rossi A, Luciani G, Campodoni E, Mocerino F, D’Errico G, Pezzella A, Panseri S, Vitiello G, Sandri M (2024) Designing bioinspired multifunctional nanoplatforms to support wound healing and skin regeneration: Mg-hydroxyapatite meets melanins. Colloids Surf B Biointerfaces 235:113756. https://doi.org/10.1016/j.colsurfb.2024.113756
Galasso C, Corinaldesi C, Sansone C (2017) Carotenoids from Marine organisms: Biological functions and Industrial Applications. Antioxid (Basel) 6(4). https://doi.org/10.3390/antiox6040096
Garrison AT, Abouelhassan Y, Kallifidas D, Tan H, Kim YS, Jin S, Luesch H, Huigens RW 3rd (2018) An efficient Buchwald-Hartwig/Reductive cyclization for the Scaffold diversification of Halogenated Phenazines: potent antibacterial targeting, Biofilm Eradication, and Prodrug Exploration. J Med Chem 61(9):3962–3983. https://doi.org/10.1021/acs.jmedchem.7b01903
Gharat K, Singhal RS (2024) A strategic media engineering and fed-batch approach enhances violacein production by Chromobacterium violaceum MTCC2656. Syst Microbiol Biomanufacturing. https://doi.org/10.1007/s43393-023-00230-y
Ghattavi K, Homaei A, Kamrani E, Kim S-K (2022) Melanin pigment derived from marine organisms and its industrial applications. Dyes Pigm 201:110214. https://doi.org/10.1016/j.dyepig.2022.110214
Ghiffary MR, Prabowo CPS, Sharma K, Yan Y, Lee SY, Kim HU (2021) High-level production of the Natural Blue Pigment Indigoidine from metabolically Engineered Corynebacterium glutamicum for sustainable fabric dyes. ACS Sustain Chem Eng 9(19):6613–6622. https://doi.org/10.1021/acssuschemeng.0c09341
Ghssein G, Ezzeddine Z (2022) A review of P seudomonas aeruginosa Metallophores: pyoverdine, Pyochelin and pseudopaline. Biology (Basel) 11(12). https://doi.org/10.3390/biology11121711
Girgis A, Aziz M, Shalaby E, Asaad F, Ahmed Farag I (2018) Synthesis and X-ray studies of Novel azaphenanthrenes. J Chem Res 42:90–95. https://doi.org/10.3184/174751918X15183538282993
Glasser NR, Kern SE, Newman DK (2014) Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol Microbiol 92(2):399–412. https://doi.org/10.1111/mmi.12566
Gohil N, Bhattacharjee G, Singh V (2020) Synergistic bactericidal profiling of prodigiosin extracted from Serratia marcescens in combination with antibiotics against pathogenic bacteria. Microb Pathog 149:104508. https://doi.org/10.1016/j.micpath.2020.104508
Gohil N, Bhattacharjee G, Gayke M, Narode H, Alzahrani KJ, Singh V (2022) Enhanced production of violacein by Chromobacterium violaceum using agro-industrial waste soybean meal. J Appl Microbiol 132(2):1121–1133. https://doi.org/10.1111/jam.15277
Goncalves T, Vasconcelos U (2021) Colour Me Blue: the history and the Biotechnological potential of Pyocyanin. Molecules 26(4). https://doi.org/10.3390/molecules26040927
Gong J, Ren Y, Fu R, Li Z, Zhang J (2017) pH-Mediated antibacterial dyeing of cotton with Prodigiosins Nanomicelles produced by Microbial Fermentation. Polym (Basel) 9(10). https://doi.org/10.3390/polym9100468
Grewal J, Wola Cewicz M, Pyter W, Joshi N, Drewniak L, Pranaw K (2022) Colorful treasure from Agro-industrial Wastes: a sustainable chassis for Microbial Pigment production. Front Microbiol 13:832918. https://doi.org/10.3389/fmicb.2022.832918
Gu J-D, Cheung KH (2001) Phenotypic expression of Vogesella indigofera upon exposure to hexavalent chromium, Cr6+. World J Microbiol Biotechnol 17(5):475–480. https://doi.org/10.1023/A:1011917409139
Guillerme J-B, Couteau C, Coiffard L (2017) Applications for Marine Resources in Cosmetics Cosmetics. vol 4
Guo Y, Hui CY, Liu L, Chen MP, Huang HY (2021) Development of a bioavailable hg(II) sensing system based on MerR-regulated visual pigment biosynthesis. Sci Rep 11(1):13516. https://doi.org/10.1038/s41598-021-92878-6
Gupta KG, Kessar SV, Singh B (1970) Antibacterial effect of some azaphenanthrene compounds. Appl Microbiol 19(6):1017–1018. https://doi.org/10.1128/am.19.6.1017-1018.1970
Gustavsson M, Hornstrom D, Lundh S, Belotserkovsky J, Larsson G (2016) Biocatalysis on the surface of Escherichia coli: melanin pigmentation of the cell exterior. Sci Rep 6:36117. https://doi.org/10.1038/srep36117
Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV, Davey AK, Chess-Williams R, Kiefel MJ, Arora D, Grant GD (2016) Cellular effects of Pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins (Basel) 8(8). https://doi.org/10.3390/toxins8080236
Han R, Xiang R, Li J, Wang F, Wang C (2021) High-level production of microbial prodigiosin: a review. J Basic Microbiol 61(6):506–523. https://doi.org/10.1002/jobm.202100101
Heu K, Romoli O, Schonbeck JC, Ajenoe R, Epelboin Y, Kircher V, Houel E, Estevez Y, Gendrin M (2021) The Effect of secondary metabolites produced by Serratia marcescens on Aedes aegypti and its Microbiota. Front Microbiol 12(645701). https://doi.org/10.3389/fmicb.2021.645701
Hooe SL, Thakur M, Lasarte-Aragones G, Breger JC, Walper SA, Medintz IL, Ellis GA (2024) Exploration of the in vitro violacein synthetic pathway with substrate analogues. ACS Omega 9(3):3894–3904. https://doi.org/10.1021/acsomega.3c08233
Hui CY, Guo Y, Liu L, Zhang NX, Gao CX, Yang XQ, Yi J (2020) Genetic control of violacein biosynthesis to enable a pigment-based whole-cell lead biosensor. RSC Adv 10(47):28106–28113. https://doi.org/10.1039/d0ra04815a
Hui C-y, Guo Y, Gao C-x, Li H, Lin Y-r, Yun J-p, Chen Y-t, Yi J (2022a) A tailored indigoidine-based whole-cell biosensor for detecting toxic cadmium in environmental water samples. Environ Technol Innov 27:102511. https://doi.org/10.1016/j.eti.2022.102511
Hui CY, Guo Y, Zhu DL, Li LM, Yi J, Zhang NX (2022b) Metabolic engineering of the violacein biosynthetic pathway toward a low-cost, minimal-equipment lead biosensor. Biosens Bioelectron 214:114531. https://doi.org/10.1016/j.bios.2022.114531
Hui CY, Hu SY, Yang XQ, Guo Y (2023) A panel of visual bacterial biosensors for the rapid detection of genotoxic and oxidative damage: a proof of concept study. Mutat Res Genet Toxicol Environ Mutagen 888:503639. https://doi.org/10.1016/j.mrgentox.2023.503639
Ibberson CB, Barraza JP, Holmes AL, Cao P, Whiteley M (2022) Precise spatial structure impacts antimicrobial susceptibility of S. Aureus in polymicrobial wound infections. Proc Natl Acad Sci U S A 119(51):e2212340119. https://doi.org/10.1073/pnas.2212340119
Ibrahim WM, Olama ZA, Abou-Elela GM, Ramadan HS, Hegazy GE, El Badan DES (2023) Exploring the antimicrobial, antiviral, antioxidant, and antitumor potentials of marine Streptomyces tunisiensis W4MT573222 pigment isolated from Abu-Qir sediments, Egypt. Microb Cell Fact 22(1):94. https://doi.org/10.1186/s12934-023-02106-1
Im H, Choi SY, Son S, Mitchell RJ (2017) Combined application of bacterial predation and Violacein to kill Polymicrobial Pathogenic communities. Sci Rep 7(1):14415. https://doi.org/10.1038/s41598-017-14567-7
Inan Bektas K, Nalcaoglu A, Kati H, Ceylan E, Nalcacioglu R, Belduz AO, Canakci S (2023) Janthinobacterium kumbetense sp nov., a violacein-producing bacterium isolated from spring water in Turkey, and investigation of antimicrobial activity of violacein. FEMS Microbiol Lett 370. https://doi.org/10.1093/femsle/fnac119
Islan GA, Rodenak-Kladniew B, Noacco N, Duran N, Castro GR (2022) Prodigiosin: a promising biomolecule with many potential biomedical applications. Bioengineered 13(6):14227–14258. https://doi.org/10.1080/21655979.2022.2084498
Jablonska J, Augustyniak A, Dubrowska K, Rakoczy R (2023) The two faces of pyocyanin - why and how to steer its production? World J Microbiol Biotechnol 39(4):103. https://doi.org/10.1007/s11274-023-03548-w
Jacob C, Jamier V, Ba LA (2011) Redox active secondary metabolites. Curr Opin Chem Biol 15(1):149–155. https://doi.org/10.1016/j.cbpa.2010.10.015
Jancheva M, Bottcher T (2021) A metabolite of Pseudomonas triggers Prophage-Selective Lysogenic to Lytic Conversion in Staphylococcus aureus. J Am Chem Soc 143(22):8344–8351. https://doi.org/10.1021/jacs.1c01275
Jayaseelan S, Ramaswamy D, Dharmaraj S (2014) Pyocyanin: production, applications, challenges and new insights. World J Microbiol Biotechnol 30(4):1159–1168. https://doi.org/10.1007/s11274-013-1552-5
Jean-Pierre F, Hampton TH, Schultz D, Hogan DA, Groleau MC, Deziel E, O’Toole GA (2023) Community composition shapes microbial-specific phenotypes in a cystic fibrosis polymicrobial model system. Elife 12. https://doi.org/10.7554/eLife.81604
Jeffries JL, Jia J, Choi W, Choe S, Miao J, Xu Y, Powell R, Lin J, Kuang Z, Gaskins HR, Lau GW (2016) Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewis(x) to increase binding to airway epithelial cells. Mucosal Immunol 9(4):1039–1050. https://doi.org/10.1038/mi.2015.119
Jia X, Liu F, Zhao K, Lin J, Fang Y, Cai S, Lin C, Zhang H, Chen L, Chen J (2021) Identification of essential genes Associated with Prodigiosin Production in Serratia marcescens FZSF02. Front Microbiol 12:705853. https://doi.org/10.3389/fmicb.2021.705853
Johnson ET, Bowman MJ, Gomes RP, Carneiro LC, Dunlap CA (2023) Identification of 2,4-diacetylphloroglucinol production in the genus Chromobacterium. Sci Rep 13(1):14292. https://doi.org/10.1038/s41598-023-41277-0
Ju KY, Lee Y, Lee S, Park SB, Lee JK (2011) Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 12(3):625–632. https://doi.org/10.1021/bm101281b
Kalra R, Conlan XA, Goel M (2020) Fungi as a potential source of pigments: Harnessing Filamentous Fungi. Front Chem 8:369. https://doi.org/10.3389/fchem.2020.00369
Kamer AMA, Abdelaziz AA, Al-Monofy KB, Al-Madboly LA (2023) Antibacterial, antibiofilm, and anti-quorum sensing activities of pyocyanin against methicillin-resistant Staphylococcus aureus: in vitro and in vivo study. BMC Microbiol 23(1):116. https://doi.org/10.1186/s12866-023-02861-6
Kanelli M, Mandic M, Kalakona M, Vasilakos S, Kekos D, Nikodinovic-Runic J, Topakas E (2018) Microbial production of Violacein and process optimization for Dyeing Polyamide Fabrics with Acquired Antimicrobial properties. Front Microbiol 9:1495. https://doi.org/10.3389/fmicb.2018.01495
Kang D, Kirienko DR, Webster P, Fisher AL, Kirienko NV (2018) Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. Elegans, removes iron, and activates a distinct host response. Virulence 9(1):804–817. https://doi.org/10.1080/21505594.2018.1449508
Kang D, Revtovich AV, Chen Q, Shah KN, Cannon CL, Kirienko NV (2019) Pyoverdine-dependent virulence of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Front Microbiol 10:2048. https://doi.org/10.3389/fmicb.2019.02048
Kaur J, Pethani BP, Kumar S, Kim M, Sunna A, Kautto L, Penesyan A, Paulsen IT, Nevalainen H (2015) Pseudomonas aeruginosa inhibits the growth of Scedosporium Aurantiacum, an opportunistic fungal pathogen isolated from the lungs of cystic fibrosis patients. Front Microbiol 6:866. https://doi.org/10.3389/fmicb.2015.00866
Keller-Costa T, Lago-Leston A, Saraiva JP, Toscan R, Silva SG, Goncalves J, Cox CJ, Kyrpides N, Nunes da Rocha U, Costa R (2021) Metagenomic insights into the taxonomy, function, and dysbiosis of prokaryotic communities in octocorals. Microbiome 9(1):72 https://doi.org/10.1186/s40168-021-01031-y
Khaksar F, Rigi G, Mirdamadian SH (2021) Creation of a violacein pigment hybrid with silver and titanium dioxide nanoparticles to produce multifunctional textiles with antimicrobial properties. Nanomed Res J 6(1):60–72. https://doi.org/10.22034/nmrj.2021.01.007
Kholany M, Trébulle P, Martins M, Ventura SPM, Nicaud J-M, Coutinho JAP (2020) Extraction and purification of violacein from Yarrowia lipolytica cells using aqueous solutions of surfactants. J Chem Technol Biotechnol 95(4):1126–1134. https://doi.org/10.1002/jctb.6297
Kiki MJ (2023) Biopigments of Microbial Origin and Their Application in the Cosmetic Industry Cosmetics. vol 10
Kim Y, Choi J (2015) Dyeing properties of microbial prodiginine from Zooshikella rubidus for silk fabrics. Fibers Polym 16(9):1981–1987. https://doi.org/10.1007/s12221-015-5211-3
Kim SJ, Lee HK, Lee YK, Yim JH (2008) Mutant selection of Hahella chejuensis KCTC 2396 and statistical optimization of medium components for prodigiosin yield-up. J Microbiol 46(2):183–188. https://doi.org/10.1007/s12275-008-0037-y
Kim I, Kim J, Chhetri G, Seo T (2019) Flavobacterium Humi Sp nov., a flexirubin-type pigment producing bacterium, isolated from soil. J Microbiol 57(12):1079–1085. https://doi.org/10.1007/s12275-019-9350-x
Kim MA, Yoon SD, Lee JS, Lee C-M (2020) Melanin-PEG nanoparticles as a photothermal agent for tumor therapy. Mater Today Commun 25:101575. https://doi.org/10.1016/j.mtcomm.2020.101575
Kim YJ, Yuk N, Shin HJ, Jung HJ (2021) The natural pigment Violacein potentially suppresses the proliferation and stemness of Hepatocellular Carcinoma cells in Vitro. Int J Mol Sci 22(19). https://doi.org/10.3390/ijms221910731
Kim HJ, Lee MS, Jeong SK, Lee SJ (2023) Transcriptomic analysis of the antimicrobial activity of prodigiosin against Cutibacterium acnes. Sci Rep 13(1):17412. https://doi.org/10.1038/s41598-023-44612-7
Kirienko NV, Ausubel FM, Ruvkun G (2015) Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 112(6):1821–1826. https://doi.org/10.1073/pnas.1424954112
Koksal Karayildirim C, Sahiner A, Caliskan S, Soylu FE, Gokhan A, Eroglu E, Uyanikgil Y, Karayildirim T (2024) Isolation, identification, and Antimicrobial evaluation of secondary metabolite from Serratia marcescens via an. Vivo Epicutaneous Infect Model ACS Omega 9(7):8397–8404. https://doi.org/10.1021/acsomega.3c09522
Kong L, Xu G, Liu X, Wang J, Tang Z, Cai YS, Shen K, Tao W, Zheng Y, Deng Z, Price NPJ, Chen W (2019) Divergent biosynthesis of C-Nucleoside minimycin and Indigoidine in Bacteria. iScience 22:430–440. https://doi.org/10.1016/j.isci.2019.11.037
Konzen M, De Marco D, Cordova CA, Vieira TO, Antonio RV, Creczynski-Pasa TB (2006) Antioxidant properties of violacein: possible relation on its biological function. Bioorg Med Chem 14(24):8307–8313. https://doi.org/10.1016/j.bmc.2006.09.013
Kordjazi T, Mariniello L, Giosafatto CVL, Porta R, Restaino OF (2024) Streptomycetes as microbial cell factories for the Biotechnological Production of Melanin. Int J Mol Sci 25(5). https://doi.org/10.3390/ijms25053013
Kramar A, Ilic-Tomic T, Petkovic M, Radulović N, Kostic M, Jocic D, Nikodinovic-Runic J (2014) Crude bacterial extracts of two new Streptomyces sp isolates as bio-colorants for textile dyeing. World J Microbiol Biotechnol 30(8):2231–2240. https://doi.org/10.1007/s11274-014-1644-x
Kramar AD, Ilic-Tomic TR, Lađarević JM, Nikodinovic-Runic JB, Kostic MM (2021) Halochromic cellulose textile obtained via dyeing with biocolorant isolated from Streptomyces sp strain NP4. Cellulose 28(13):8771–8784. https://doi.org/10.1007/s10570-021-04071-7
Kumar V, Kashyap P, Kumar S, Thakur V, Kumar S, Singh D (2022) Multiple adaptive strategies of Himalayan Iodobacter sp. PCH194 to high-altitude stresses. Front Microbiol 13:881873. https://doi.org/10.3389/fmicb.2022.881873
Laliany G, Jamehdar SA, Makhdoumi A (2022) In vitro virulence activity of Pseudomonas aeruginosa, enhanced by either Acinetobacter baumannii or Enterococcus faecium through the polymicrobial interactions. Arch Microbiol 204(12):709. https://doi.org/10.1007/s00203-022-03308-8
Landrum JT, Bone RA (2001) Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys 385(1):28–40. https://doi.org/10.1006/abbi.2000.2171
Lapenda JC, Silva PA, Vicalvi MC, Sena KX, Nascimento SC (2015) Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J Microbiol Biotechnol 31(2):399–406. https://doi.org/10.1007/s11274-014-1793-y
Lee ME, Aswani A, Han AS, Tomlin CJ, Dueber JE (2013) Expression-level optimization of a multi-enzyme pathway in the absence of a high-throughput assay. Nucleic Acids Res 41(22):10668–10678. https://doi.org/10.1093/nar/gkt809
Li X, Li Y, Wang R, Wang Q, Lu L (2019) Toxoflavin produced by Burkholderia gladioli from Lycoris aurea is a New Broad-Spectrum Fungicide. Appl Environ Microbiol 85(9). https://doi.org/10.1128/AEM.00106-19
Li X, Tan X, Chen Q, Zhu X, Zhang J, Zhang J, Jia B (2021) Prodigiosin of Serratia marcescens ZPG19 alters the gut microbiota composition of Kunming Mice. Molecules 26(8). https://doi.org/10.3390/molecules26082156
Li S, Feng D, Li E, Gilbert RG (2023a) Formation, structural characterization, and Functional Properties of Corn Starch/Zeaxanthin Composites. Foods 12(10). https://doi.org/10.3390/foods12102076
Li Y, Wang S, Zhang K, Yin Y, Zhang X, Zhang Q, Kong X, Tang L, Zhang R, Zhang Z (2023b) Serratia marcescens in the intestine of housefly larvae inhibits host growth by interfering with gut microbiota. Parasit Vectors 16(1):196. https://doi.org/10.1186/s13071-023-05781-6
Lim S, Bhak J, Jeon S, Mun W, Bhak J, Choi SY, Mitchell RJ (2022) The kiss of death: Serratia marcescens antibacterial activities against Staphylococcus aureus requires both de novo Prodigiosin Synthesis and Direct Contact. Microbiol Spectr 10(3):e0060722. https://doi.org/10.1128/spectrum.00607-22
Lin L, Xu J (2022) Production of fungal pigments: molecular processes and their applications. J Fungi (Basel) 9(1). https://doi.org/10.3390/jof9010044
Lin SR, Chen YH, Tseng FJ, Weng CF (2020) The production and bioactivity of prodigiosin: quo vadis? Drug Discov Today 25(5):828–836. https://doi.org/10.1016/j.drudis.2020.03.017
Lins JC, ME DEM, SC DON, Adam ML (2015) Differential genomic damage in different tumor lines induced by prodigiosin. Anticancer Res 35(6):3325–3332
Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202(2):209–215. https://doi.org/10.1084/jem.20050846
Liu P, Zhu H, Zheng G, Jiang W, Lu Y (2017) Metabolic engineering of Streptomyces coelicolor for enhanced prodigiosins (RED) production. Sci China Life Sci 60(9):948–957. https://doi.org/10.1007/s11427-017-9117-x
Liu Y, Dai C, Zhou Y, Qiao J, Tang B, Yu W, Zhang R, Liu Y, Lu SE (2021) Pyoverdines are essential for the antibacterial activity of Pseudomonas chlororaphis YL-1 under low-Iron conditions. Appl Environ Microbiol 87(7). https://doi.org/10.1128/AEM.02840-20
Liu X, Li J, Li Y, Li J, Sun H, Zheng J, Zhang J, Tan H (2022) A visualization reporter system for characterizing antibiotic biosynthetic gene clusters expression with high-sensitivity. Commun Biol 5(1):901. https://doi.org/10.1038/s42003-022-03832-9
Liu X, Xu D, Wu D, Xu M, Wang Y, Wang W, Ran T (2023) BarA/UvrY differentially regulates prodigiosin biosynthesis and swarming motility in Serratia marcescens FS14. Res Microbiol 174(3):104010. https://doi.org/10.1016/j.resmic.2022.104010
Liu X, Wang Z, You Z, Wang W, Wang Y, Wu W, Peng Y, Zhang S, Yun Y, Zhang J (2024a) Transcriptomic analysis of cell envelope inhibition by prodigiosin in methicillin-resistant Staphylococcus aureus. Front Microbiol 15:1333526. https://doi.org/10.3389/fmicb.2024.1333526
Liu Y, McQuillen EA, Rana P, Gloag ES, Parsek MR, Wozniak DJ (2024b) A bacterial pigment provides cross-species protection from H(2)O(2)- and neutrophil-mediated killing. Proc Natl Acad Sci U S A 121(2):e2312334121. https://doi.org/10.1073/pnas.2312334121
Lopez J, Bustos D, Camilo C, Arenas N, Saa PA, Agosin E (2020) Engineering Saccharomyces cerevisiae for the overproduction of beta-ionone and its Precursor beta-carotene. Front Bioeng Biotechnol 8:578793. https://doi.org/10.3389/fbioe.2020.578793
Lopez GD, Alvarez-Rivera G, Carazzone C, Ibanez E, Leidy C, Cifuentes A (2023) Bacterial carotenoids: extraction, characterization, and applications. Crit Rev Anal Chem 53(6):1239–1262. https://doi.org/10.1080/10408347.2021.2016366
Lozano GL, Guan C, Cao Y, Borlee BR, Broderick NA, Stabb EV, Handelsman J (2020) A Chemical Counterpunch: Chromobacterium violaceum ATCC 31532 produces Violacein in response to translation-inhibiting antibiotics. mBio 11(3). https://doi.org/10.1128/mBio.00948-20
Lucio AS, Almeida JR, Barbosa-Filho JM, Pita JC, Branco MV, Diniz Mde F, Agra Mde F, da-Cunha EV, da Silva MS, Tavares JF (2011) Azaphenanthrene alkaloids with antitumoral activity from Anaxagorea Dolichocarpa Sprague & Sandwith (Annonaceae). Molecules 16(8):7125–7131. https://doi.org/10.3390/molecules16087125
Lyu X, Lyu Y, Yu H, Chen W, Ye L, Yang R (2022) Biotechnological advances for improving natural pigment production: a state-of-the-art review. Bioresour Bioprocess 9(1):8. https://doi.org/10.1186/s40643-022-00497-4
Madison JD, Ouellette SP, Schmidt EL, Kerby JL (2019) Serratia marcescens shapes cutaneous bacterial communities and influences survival of an amphibian host. Proc Biol Sci 286(1914):20191833 https://doi.org/10.1098/rspb.2019.1833
Manivasagan P, Venkatesan J, Senthilkumar K, Sivakumar K, Kim SK (2013) Isolation and characterization of biologically active melanin from Actinoalloteichus sp. MA-32. Int J Biol Macromol 58:263–274. https://doi.org/10.1016/j.ijbiomac.2013.04.041
Manrique-Moreno M, Jemiola-Rzeminska M, Munera-Jaramillo J, Lopez GD, Suesca E, Leidy C, Strzalka K (2022) Staphylococcus aureus carotenoids modulate the Thermotropic Phase Behavior of Model systems that mimic its membrane composition. Membr (Basel) 12(10). https://doi.org/10.3390/membranes12100945
Maoka T (2023) Carotenoids: distribution, function in Nature, and analysis using LC-Photodiode array detector (DAD)-MS and MS/MS system. Mass Spectrom (Tokyo) 12(1):A0133. https://doi.org/10.5702/massspectrometry.A0133
Marinelli F, Genilloud O, Fedorenko V, Ron EZ (2015) Specialized Bioactive Microbial metabolites: from gene to product. Biomed Res Int 2015(276964). https://doi.org/10.1155/2015/276964
Martinez-Alvarez O, Calvo MM, Gomez-Estaca J (2020) Recent Advances in Astaxanthin Micro/Nanoencapsulation to improve its Stability and Functionality as a food ingredient. Mar Drugs 18(8). https://doi.org/10.3390/md18080406
Mavridi-Printezi A, Guernelli M, Menichetti A, Montalti M (2020) Bio-applications of multifunctional melanin nanoparticles: from nanomedicine to Nanocosmetics. Nanomaterials (Basel) 10(11). https://doi.org/10.3390/nano10112276
Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS (2001) Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183(21):6454–6465. https://doi.org/10.1128/JB.183.21.6454-6465.2001
McBride MJ, Xie G, Martens EC, Lapidus A, Henrissat B, Rhodes RG, Goltsman E, Wang W, Xu J, Hunnicutt DW, Staroscik AM, Hoover TR, Cheng YQ, Stein JL (2009) Novel features of the polysaccharide-digesting gliding bacterium Flavobacterium johnsoniae as revealed by genome sequence analysis. Appl Environ Microbiol 75(21):6864–6875. https://doi.org/10.1128/AEM.01495-09
Mehta T, Vercruysse K, Johnson T, Ejiofor AO, Myles E, Quick QA (2015) Violacein induces p44/42 mitogen-activated protein kinase-mediated solid tumor cell death and inhibits tumor cell migration. Mol Med Rep 12(1):1443–1448. https://doi.org/10.3892/mmr.2015.3525
Meirelles LA, Perry EK, Bergkessel M, Newman DK (2021) Bacterial defenses against a natural antibiotic promote collateral resilience to clinical antibiotics. PLoS Biol 19(3):e3001093. https://doi.org/10.1371/journal.pbio.3001093
Miglani K, Singh S, Singh DP, Krishania M (2023) Sustainable production of prodigiosin from rice straw derived xylose by using isolated Serratia marcescens (CMS 2): statistical optimization, characterization, encapsulation & cost analysis. Sustainable Food Technol 1(6):837–849. https://doi.org/10.1039/D3FB00100H
Milosevic E, Stanisavljevic N, Boskovic S, Stamenkovic N, Novkovic M, Bavelloni A, Cenni V, Kojic S, Jasnic J (2023) Antitumor activity of natural pigment violacein against osteosarcoma and rhabdomyosarcoma cell lines. J Cancer Res Clin Oncol 149(13):10975–10987. https://doi.org/10.1007/s00432-023-04930-9
Moayedi A, Nowroozi J, Akhavan Sepahy A (2018) Cytotoxic effect of pyocyanin on human pancreatic cancer cell line (Panc-1). Iran J Basic Med Sci 21(8):794–799. https://doi.org/10.22038/IJBMS.2018.27865.6799
Mogadem A, Almamary MA, Mahat NA, Jemon K, Ahmad WA, Ali I (2021) Antioxidant activity evaluation of FlexirubinType Pigment from Chryseobacterium Artocarpi CECT 8497 and related docking study. Molecules 26(4). https://doi.org/10.3390/molecules26040979
Mogadem A, Naqvi A, Almamary MA, Ahmad WA, Jemon K, El-Alfy SH (2022) Hepatoprotective effects of flexirubin, a novel pigment from Chryseobacterium Artocarpi, against carbon tetrachloride-induced liver injury: an in vivo study and molecular modeling. Toxicol Appl Pharmacol 444:116022. https://doi.org/10.1016/j.taap.2022.116022
Mojsoska B, Ghoul M, Perron GG, Jenssen H, Alatraktchi FA (2021) Changes in toxin production of environmental Pseudomonas aeruginosa isolates exposed to sub-inhibitory concentrations of three common antibiotics. PLoS ONE 16(3):e0248014. https://doi.org/10.1371/journal.pone.0248014
Montefiori DC, Zhou JY (1991) Selective antiviral activity of synthetic soluble L-tyrosine and L-dopa melanins against human immunodeficiency virus in vitro. Antiviral Res 15(1):11–25. https://doi.org/10.1016/0166-3542(91)90037-r
Mora-Lugo R, Stegmuller J, Mack M (2019) Metabolic engineering of roseoflavin-overproducing microorganisms. Microb Cell Fact 18(1):146. https://doi.org/10.1186/s12934-019-1181-2
Moraes CS, Seabra SH, Castro DP, Brazil RP, de Souza W, Garcia ES, Azambuja P (2008) Leishmania (Leishmania) chagasi interactions with Serratia marcescens: ultrastructural studies, lysis and carbohydrate effects. Exp Parasitol 118(4):561–568. https://doi.org/10.1016/j.exppara.2007.11.015
Mudaliar SB, Bharath Prasad AS (2024) A biomedical perspective of pyocyanin from Pseudomonas aeruginosa: its applications and challenges. World J Microbiol Biotechnol 40(3):90. https://doi.org/10.1007/s11274-024-03889-0
Mukhia S, Kumar A, Kumar R (2023) Antioxidant prodigiosin-producing cold-adapted Janthinobacterium Sp ERMR3:09 from a glacier moraine: genomic elucidation of cold adaptation and pigment biosynthesis. Gene 857:147178. https://doi.org/10.1016/j.gene.2023.147178
Muller M, Li Z, Maitz PK (2009) Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: role for p38 mitogen-activated protein kinase. Burns 35(4):500–508. https://doi.org/10.1016/j.burns.2008.11.010
Muller M, Auslander S, Auslander D, Kemmer C, Fussenegger M (2012) A novel reporter system for bacterial and mammalian cells based on the non-ribosomal peptide indigoidine. Metab Eng 14(4):325–335. https://doi.org/10.1016/j.ymben.2012.04.002
Munera-Jaramillo J, Lopez GD, Suesca E, Carazzone C, Leidy C, Manrique-Moreno M (2024) The role of staphyloxanthin in the regulation of membrane biophysical properties in Staphylococcus aureus. Biochim Biophys Acta Biomembr 1866(3):184288. https://doi.org/10.1016/j.bbamem.2024.184288
Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, Coulthurst SJ (2011) The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol 193(21):6057–6069. https://doi.org/10.1128/JB.05671-11
Muthukrishnan L, Chellappa M, Nanda A, Thukkaram S, Selvaraj G, Muthiah B, Sagadevan S, Lett JA (2019) Bio-fabrication of pigment-capped silver nanoparticles encountering antibiotic-resistant strains and their cytotoxic effect towards human epidermoid larynx carcinoma (HEp-2) cells. RSC Adv 9(28):15874–15886. https://doi.org/10.1039/c9ra01072f
Namazkar S (2013) Spray-dried Prodigiosin from Serratia marcescens as a colorant. Biosci Biotechnol Res Asia 10:69–76. https://doi.org/10.13005/bbra/1094
Nanjaraj Urs AN, Hu Y, Li P, Yuchi Z, Chen Y, Zhang Y (2019) Cloning and expression of a nonribosomal peptide synthetase to Generate Blue Rose. ACS Synth Biol 8(8):1698–1704. https://doi.org/10.1021/acssynbio.8b00187
Narsing Rao MP, Xiao M, Li WJ (2017) Fungal and bacterial pigments: secondary metabolites with wide applications. Front Microbiol 8:1113. https://doi.org/10.3389/fmicb.2017.01113
Nemer G, Louka N, Rabiller Blandin P, Maroun RG, Vorobiev E, Rossignol T, Nicaud JM, Guenin E, Koubaa M (2023) Purification of natural pigments Violacein and Deoxyviolacein produced by Fermentation using Yarrowia Lipolytica. Molecules 28(11). https://doi.org/10.3390/molecules28114292
Nguyen VB, Nguyen DN, Nguyen AD, Ngo VA, Ton TQ, Doan CT, Pham TP, Tran TPH, Wang SL (2020) Utilization of crab Waste for cost-effective bioproduction of Prodigiosin. Mar Drugs 18(11). https://doi.org/10.3390/md18110523
Nie H, Li Y, Lu XL, Yan J, Liu XR, Yin Q (2023) Prodigiosin derived from chromium-resistant Serratia sp prevents inflammation and modulates gut microbiota homeostasis in DSS-induced colitis mice. Int Immunopharmacol 116:109800. https://doi.org/10.1016/j.intimp.2023.109800
Nielsen H, Nielsen GL (1989) Preprandial blood glucose values: influence on glycemic response studies. Am J Clin Nutr 49(6):1243–1246. https://doi.org/10.1093/ajcn/49.6.1243
Nosanchuk JD, Casadevall A (2006) Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50(11):3519–3528. https://doi.org/10.1128/AAC.00545-06
O’Brien S, Culbert CT, Barraclough TG (2023) Community composition drives siderophore dynamics in multispecies bacterial communities. BMC Ecol Evol 23(1):45. https://doi.org/10.1186/s12862-023-02152-8
O’Malley YQ, Reszka KJ, Spitz DR, Denning GM, Britigan BE (2004) Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287(1):L94–103. https://doi.org/10.1152/ajplung.00025.2004
Obayemi JD, Danyuo Y, Dozie-Nwachukwu S, Odusanya OS, Anuku N, Malatesta K, Yu W, Uhrich KE, Soboyejo WO (2016) PLGA-based microparticles loaded with bacterial-synthesized prodigiosin for anticancer drug release: effects of particle size on drug release kinetics and cell viability. Mater Sci Eng C Mater Biol Appl 66:51–65. https://doi.org/10.1016/j.msec.2016.04.071
Oh WT, Giri SS, Yun S, Kim HJ, Kim SG, Kim SW, Kang JW, Han SJ, Kwon J, Jun JW, Park SC (2019) Janthinobacterium lividum as an emerging pathogenic bacterium affecting Rainbow Trout (Oncorhynchus mykiss) fisheries in Korea. Pathogens 8(3). https://doi.org/10.3390/pathogens8030146
Orlandi VT, Martegani E, Giaroni C, Baj A, Bolognese F (2022) Bacterial pigments: a colorful palette reservoir for biotechnological applications. Biotechnol Appl Biochem 69(3):981–1001. https://doi.org/10.1002/bab.2170
Padhan B, Poddar K, Sarkar D, Sarkar A (2021) Production, purification, and process optimization of intracellular pigment from novel psychrotolerant Paenibacillus sp BPW19. Biotechnol Rep (Amst) 29:e00592. https://doi.org/10.1016/j.btre.2021.e00592
Pagano APE, Khalid N, Kobayashi I, Nakajima M, Neves MA, Bastos EL (2018) Microencapsulation of betanin in monodisperse W/O/W emulsions. Food Res Int 109:489–496. https://doi.org/10.1016/j.foodres.2018.04.053
Pailliè-Jiménez ME, Stincone P, Brandelli A (2020) Natural pigments of Microbial Origin. Front Sustainable Food Syst 4. https://doi.org/10.3389/fsufs.2020.590439
Pan X, Sun C, Tang M, You J, Osire T, Zhao Y, Xu M, Zhang X, Shao M, Yang S, Yang T, Rao Z (2020) LysR-Type Transcriptional Regulator MetR controls Prodigiosin Production, Methionine Biosynthesis, Cell Motility, H(2)O(2) Tolerance, Heat Tolerance, and Exopolysaccharide Synthesis in Serratia marcescens. Appl Environ Microbiol 86(4). https://doi.org/10.1128/AEM.02241-19
Pan X, Tang M, You J, Liu F, Sun C, Osire T, Fu W, Yi G, Yang T, Yang ST, Rao Z (2021) Regulator RcsB Controls Prodigiosin synthesis and various Cellular processes in Serratia marcescens JNB5-1. Appl Environ Microbiol 87(2). https://doi.org/10.1128/AEM.02052-20
Pan X, Tang M, You J, Hao Y, Zhang X, Yang T, Rao Z (2022a) A novel method to screen strong constitutive promoters in Escherichia coli and Serratia marcescens for Industrial Applications. Biology (Basel) 12(1). https://doi.org/10.3390/biology12010071
Pan X, You J, Tang M, Zhang X, Xu M, Yang T, Rao Z (2022b) Improving prodigiosin production by transcription factor engineering and promoter engineering in Serratia marcescens. Front Microbiol 13:977337. https://doi.org/10.3389/fmicb.2022.977337
Panchanawaporn S, Chutrakul C, Jeennor S, Anantayanon J, Rattanaphan N, Laoteng K (2022) Potential of aspergillus oryzae as a biosynthetic platform for indigoidine, a non-ribosomal peptide pigment with antioxidant activity. PLoS ONE 17(6):e0270359. https://doi.org/10.1371/journal.pone.0270359
Patil AD, Kasabe PJ, Dandge PB (2022) Pharmaceutical and nutraceutical potential of natural bioactive pigment: astaxanthin. Nat Prod Bioprospect 12(1):25. https://doi.org/10.1007/s13659-022-00347-y
Pauer H, Hardoim CCP, Teixeira FL, Miranda KR, Barbirato DDS, de Carvalho DP, Antunes LCM, Leitao A, Lobo LA, Domingues R (2018) Impact of violacein from Chromobacterium violaceum on the mammalian gut microbiome. PLoS ONE 13(9):e0203748. https://doi.org/10.1371/journal.pone.0203748
Paul T, Bandyopadhyay TK, Mondal A, Tiwari ON, Muthuraj M, Bhunia B (2022) A comprehensive review on recent trends in production, purification, and applications of prodigiosin. Biomass Convers Biorefinery 12(4):1409–1431. https://doi.org/10.1007/s13399-020-00928-2
Peechakara BV, Sina RE, Gupta M (2024) Vitamin B2 (Riboflavin) StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Reddog Sina declares no relevant financial relationships with ineligible companies. Mohit Gupta declares no relevant financial relationships with ineligible companies, Disclosure
Peng W, Li H, Zhao X, Shao B, Zhu K (2022) Pyocyanin modulates gastrointestinal Transformation and Microbiota. J Agric Food Chem 70(8):2722–2732. https://doi.org/10.1021/acs.jafc.1c07726
Petrosyan T (2015) Bacterial melanin in rat models of Parkinson’s disease: a potential neuroprotective strategy. Neural Regen Res 10(2):211–212. https://doi.org/10.4103/1673-5374.152372
Petrosyan TR, Gevorkyan OV, Meliksetyan IB, Hovsepyan AS, Manvelyan LR (2012) Neuroprotective action of bacterial melanin in rats after corticospinal tract lesions. Pathophysiology 19(2):71–80. https://doi.org/10.1016/j.pathophys.2011.12.003
Pierson LS 3rd, Pierson EA (2010) Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl Microbiol Biotechnol 86(6):1659–1670. https://doi.org/10.1007/s00253-010-2509-3
Plonka PM, Grabacka M (2006) Melanin synthesis in microorganisms–biotechnological and medical aspects. Acta Biochim Pol 53(3):429–443
Polapally R, Mansani M, Rajkumar K, Burgula S, Hameeda B, Alhazmi A, Bantun F, Almalki AH, Haque S, El Enshasy HA, Sayyed RZ (2022) Melanin pigment of Streptomyces puniceus RHPR9 exhibits antibacterial, antioxidant and anticancer activities. PLoS ONE 17(4):e0266676. https://doi.org/10.1371/journal.pone.0266676
Poorniammal R, Prabhu S, Dufosse L, Kannan J (2021) Safety evaluation of fungal pigments for Food Applications. J Fungi (Basel) 7(9). https://doi.org/10.3390/jof7090692
Pralea IE, Moldovan RC, Petrache AM, Ilies M, Heghes SC, Ielciu I, Nicoara R, Moldovan M, Ene M, Radu M, Uifalean A, Iuga CA (2019) From extraction to Advanced Analytical methods: the challenges of melanin analysis. Int J Mol Sci 20(16). https://doi.org/10.3390/ijms20163943
Puja H, Mislin GLA, Rigouin C (2023) Engineering Siderophore Biosynthesis and Regulation pathways to increase diversity and availability. Biomolecules 13(6). https://doi.org/10.3390/biom13060959
Queiroz KC, Milani R, Ruela-de-Sousa RR, Fuhler GM, Justo GZ, Zambuzzi WF, Duran N, Diks SH, Spek CA, Ferreira CV, Peppelenbosch MP (2012) Violacein induces death of resistant leukaemia cells via kinome reprogramming, endoplasmic reticulum stress and Golgi apparatus collapse. PLoS ONE 7(10):e45362. https://doi.org/10.1371/journal.pone.0045362
Radic K, Barbosa AI, Reis S, Marijan M, Lima SAC, Cepo DV (2023) Preparation of astaxanthin/zeaxanthin-loaded nanostructured lipid carriers for enhanced bioavailability: Characterization-, stability-and permeability study. Acta Pharm 73(4):581–599. https://doi.org/10.2478/acph-2023-0038
Raman J, Ko YJ, Kim JS, Kim DH, Kim SJ (2024) Overproduction of Xanthophyll Pigment in Flavobacterium Sp JSWR-1 under Optimized Culture conditions. J Microbiol Biotechnol 34(3):710–724. https://doi.org/10.4014/jmb.2310.10034
Rashid MI, Rashid H, Andleeb S, Ali A (2022) Evaluation of Blood-Brain-Barrier Permeability, Neurotoxicity, and Potential Cognitive Impairment by Pseudomonas aeruginosa’s Virulence Factor Pyocyanin. Oxid Med Cell Longev 2022:3060579 https://doi.org/10.1155/2022/3060579
Ren Y, Gong J, Fu R, Zhang J, Fang K, Liu X (2018) Antibacterial dyeing of silk with prodigiosins suspention produced by liquid fermentation. J Clean Prod 201:648–656. https://doi.org/10.1016/j.jclepro.2018.08.098
Restaino OF, Manini P, Kordjazi T, Alfieri ML, Rippa M, Mariniello L, Porta R (2024) Biotechnological Production and characterization of Extracellular melanin by Streptomyces nashvillensis. Microorganisms 12(2). https://doi.org/10.3390/microorganisms12020297
Rettori D, Durán N (1998) Production, extraction and purificationof violacein: an antibiotic pigment producedby Chromobacterium violaceum. World J Microbiol Biotechnol 14(5):685–688. https://doi.org/10.1023/A:1008809504504
Reverchon S, Rouanet C, Expert D, Nasser W (2002) Characterization of indigoidine biosynthetic genes in Erwinia chrysanthemi and role of this blue pigment in pathogenicity. J Bacteriol 184(3):654–665. https://doi.org/10.1128/JB.184.3.654-665.2002
Rok J, Rzepka Z, Kowalska J, Banach K, Beberok A, Wrzesniok D (2021) Molecular and biochemical basis of Minocycline-Induced hyperpigmentation-the study on normal human melanocytes exposed to UVA and UVB Radiation. Int J Mol Sci 22(7). https://doi.org/10.3390/ijms22073755
Saini AS, Melo JS (2013) Biosorption of uranium by melanin: kinetic, equilibrium and thermodynamic studies. Bioresour Technol 149:155–162. https://doi.org/10.1016/j.biortech.2013.09.034
Salem FE, Yehia HM, Korany SM, Alarjani KM, Al-Masoud AH, Elkhadragy MF (2022) Neurotherapeutic effects of prodigiosin conjugated with silver-nanoparticles in rats exposed to cadmium chloride-induced neurotoxicity. Food Sci Technol 42
Sass G, Nazik H, Chatterjee P, Stevens DA (2021) Under nonlimiting iron conditions pyocyanin is a major antifungal molecule, and differences between prototypic Pseudomonas aeruginosa strains. Med Mycol 59(5):453–464. https://doi.org/10.1093/mmy/myaa066
Saunders SH, Tse ECM, Yates MD, Otero FJ, Trammell SA, Stemp EDA, Barton JK, Tender LM, Newman DK (2020) Extracellular DNA promotes efficient Extracellular Electron transfer by Pyocyanin in Pseudomonas aeruginosa Biofilms. Cell 182(4):919–932e19. https://doi.org/10.1016/j.cell.2020.07.006
Schalk IJ, Guillon L (2013) Pyoverdine biosynthesis and secretion in Pseudomonas aeruginosa: implications for metal homeostasis. Environ Microbiol 15(6):1661–1673. https://doi.org/10.1111/1462-2920.12013
Schiessl KT, Hu F, Jo J, Nazia SZ, Wang B, Price-Whelan A, Min W, Dietrich LEP (2019) Phenazine production promotes antibiotic tolerance and metabolic heterogeneity in Pseudomonas aeruginosa biofilms. Nat Commun 10(1):762. https://doi.org/10.1038/s41467-019-08733-w
Schoner TA, Fuchs SW, Schonau C, Bode HB (2014) Initiation of the flexirubin biosynthesis in Chitinophaga pinensis. Microb Biotechnol 7(3):232–241. https://doi.org/10.1111/1751-7915.12110
Sen T, Barrow CJ, Deshmukh SK (2019) Microbial pigments in the Food Industry-Challenges and the Way Forward. Front Nutr 6:7. https://doi.org/10.3389/fnut.2019.00007
Shikov AE, Merkushova AV, Savina IA, Nizhnikov AA, Antonets KS (2023) The man, the plant, and the insect: shooting host specificity determinants in Serratia marcescens pangenome. Front Microbiol 14:1211999. https://doi.org/10.3389/fmicb.2023.1211999
Shouman H, Said HS, Kenawy HI, Hassan R (2023) Molecular and biological characterization of pyocyanin from clinical and environmental Pseudomonas aeruginosa. Microb Cell Fact 22(1):166. https://doi.org/10.1186/s12934-023-02169-0
Siebels I, Nowak S, Heil CS, Tufar P, Cortina NS, Bode HB, Grininger M (2020) Cell-free synthesis of Natural compounds from genomic DNA of Biosynthetic Gene clusters. ACS Synth Biol 9(9):2418–2426. https://doi.org/10.1021/acssynbio.0c00186
Simoska O, Cummings DA, Gaffney EM, Langue C, Primo TG, Weber CJ, Witt CE, Minteer SD (2023) Enhancing the performance of Microbial fuel cells via Metabolic Engineering of Escherichia coli for Phenazine Production. ACS Sustain Chem Eng 11(32):11855–11866. https://doi.org/10.1021/acssuschemeng.3c01593
Singh N, Goel G, Singh N, Kumar Pathak B, Kaushik D (2015) Modeling the red pigment production by Monascus Purpureus MTCC 369 by Artificial Neural Network using rice water based medium. Food Bioscience 11:17–22. https://doi.org/10.1016/j.fbio.2015.04.001
Singh S, Nimse SB, Mathew DE, Dhimmar A, Sahastrabudhe H, Gajjar A, Ghadge VA, Kumar P, Shinde PB (2021) Microbial melanin: recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol Adv 53:107773. https://doi.org/10.1016/j.biotechadv.2021.107773
Soliev AB, Hosokawa K, Enomoto K (2011) Bioactive pigments from marine bacteria: applications and physiological roles. Evid Based Complement Alternat Med 2011:670349. https://doi.org/10.1155/2011/670349
Soukoulis C, Bohn T (2018) A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit Rev Food Sci Nutr 58(1):1–36. https://doi.org/10.1080/10408398.2014.971353
Stafsnes MH, Josefsen KD, Kildahl-Andersen G, Valla S, Ellingsen TE, Bruheim P (2010) Isolation and characterization of marine pigmented bacteria from Norwegian coastal waters and screening for carotenoids with UVA-blue light absorbing properties. J Microbiol 48(1):16–23. https://doi.org/10.1007/s12275-009-0118-6
Stringham JM, Johnson EJ, Hammond BR (2019) Lutein across the Lifespan: from Childhood Cognitive performance to the Aging Eye and Brain. Curr Dev Nutr 3(7):nzz066. https://doi.org/10.1093/cdn/nzz066
Su W-T, Tsou T-Y, Liu H-L (2011) Response surface optimization of microbial prodigiosin production from Serratia marcescens. J Taiwan Inst Chem Eng 42(2):217–222. https://doi.org/10.1016/j.jtice.2010.05.009
Subramaniam S, Ravi V, Sivasubramanian A (2014) Synergistic antimicrobial profiling of violacein with commercial antibiotics against pathogenic micro-organisms. Pharm Biol 52(1):86–90. https://doi.org/10.3109/13880209.2013.815634
Sun H, Zhao D, Xiong B, Zhang C, Bi C (2016) Engineering Corynebacterium glutamicum for violacein hyper production. Microb Cell Fact 15(1):148. https://doi.org/10.1186/s12934-016-0545-0
Sun Y, Wang L, Pan X, Osire T, Fang H, Zhang H, Yang ST, Yang T, Rao Z (2020) Improved Prodigiosin production by relieving CpxR temperature-sensitive inhibition. Front Bioeng Biotechnol 8:344. https://doi.org/10.3389/fbioe.2020.00344
Sun D, Liu W, Zhou X, Ru Y, Liu J, Zhu J, Liu C (2022) Organic Hydroperoxide induces Prodigiosin Biosynthesis in Serratia Sp ATCC 39006 in an OhrR-Dependent manner. Appl Environ Microbiol 88(5):e0204121. https://doi.org/10.1128/AEM.02041-21
Suryawanshi RK, Patil CD, Borase HP, Narkhede CP, Salunke BK, Patil SV (2015) Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic Biochem Physiol 123:49–55. https://doi.org/10.1016/j.pestbp.2015.01.018
Suryawanshi RK, Koujah L, Patil CD, Ames JM, Agelidis A, Yadavalli T, Patil SV, Shukla D (2020) Bacterial pigment Prodigiosin demonstrates a unique Antiherpesvirus activity that is mediated through inhibition of Prosurvival Signal Transducers. J Virol 94(13). https://doi.org/10.1128/JVI.00251-20
Sutherland I, Ignatova S, Hewitson P, Janaway L, Wood P, Edwards N, Harris G, Guzlek H, Keay D, Freebairn K, Johns D, Douillet N, Thickitt C, Vilminot E, Mathews B (2011) Scalable technology for the extraction of Pharmaceutics (STEP): the transition from academic knowhow to industrial reality. J Chromatogr A 1218(36):6114–6121. https://doi.org/10.1016/j.chroma.2011.01.016
Tahghighi A, Karimi S, Parhizgar AR, Zakeri S (2018) Synthesis and antiplasmodial activity of novel phenanthroline derivatives: an in vivo study. Iran J Basic Med Sci 21(2):202–211
Tai SB, Huang CY, Chung CL, Sung PJ, Wen ZH, Chen CL (2024) Prodigiosin inhibits transforming growth factor beta signaling by Interfering Receptor Recycling and subcellular translocation in epithelial cells. Mol Pharmacol 105(4):286–300. https://doi.org/10.1124/molpharm.123.000776
Takahashi H, Kumagai T, Kitani K, Mori M, Matoba Y, Sugiyama M (2007) Cloning and characterization of a Streptomyces single module type non-ribosomal peptide synthetase catalyzing a blue pigment synthesis. J Biol Chem 282(12):9073–9081. https://doi.org/10.1074/jbc.M611319200
Tang C, Luo J, Yan X, Huang Q, Huang Z, Luo Q, Lan Y, Chen D, Zhang B, Chen M, Kong D (2022) Melanin nanoparticles enhance the neuroprotection of mesenchymal stem cells against hypoxic-ischemic injury by inhibiting apoptosis and upregulating antioxidant defense. Cell Biol Int 46(6):933–946. https://doi.org/10.1002/cbin.11781
Thalhammer KO, Newman DK (2023) A phenazine-inspired framework for identifying biological functions of microbial redox-active metabolites. Curr Opin Chem Biol 75:102320. https://doi.org/10.1016/j.cbpa.2023.102320
Tong Y, Zhou J, Zhang L, Xu P (2021) A Golden-gate based Cloning Toolkit to build violacein pathway libraries in Yarrowia Lipolytica. ACS Synth Biol 10(1):115–124. https://doi.org/10.1021/acssynbio.0c00469
Tong C, Luo J, Xie C, Wei J, Pan G, Zhou Z, Li C (2023) Characterization and biological activities of melanin from the Medicinal Fungi Ophiocordyceps Sinensis. Int J Mol Sci 24(12). https://doi.org/10.3390/ijms241210282
Tunca Koyun M, Sirin S, Aslim B, Taner G, Nigdelioglu Dolanbay S (2022) Characterization of prodigiosin pigment by Serratia marcescens and the evaluation of its bioactivities. Toxicol Vitro 82:105368. https://doi.org/10.1016/j.tiv.2022.105368
Ullah W, Qasim M, Rahman H, Jie Y, Muhammad N (2017) Beta-lactamase-producing Pseudomonas aeruginosa: phenotypic characteristics and molecular identification of virulence genes. J Chin Med Assoc 80(3):173–177. https://doi.org/10.1016/j.jcma.2016.08.011
Ulmer AJ, Pryjma J, Tarnok Z, Ernst M, Flad HD (1990) Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect Immun 58(3):808–815. https://doi.org/10.1128/iai.58.3.808-815.1990
Vacheron J, Dubost A, Chapulliot D, Prigent-Combaret C, Muller D (2017) Draft Genome Sequence of Chryseobacterium sp JV274 Isolated from Maize Rhizosphere. Genome Announc 5(15). https://doi.org/10.1128/genomeA.00122-17
Vahidzadeh E, Kalra AP, Shankar K (2018) Melanin-based electronics: from proton conductors to photovoltaics and beyond. Biosens Bioelectron 122:127–139. https://doi.org/10.1016/j.bios.2018.09.026
Valeru SP, Rompikuntal PK, Ishikawa T, Vaitkevicius K, Sjoling A, Dolganov N, Zhu J, Schoolnik G, Wai SN (2009) Role of melanin pigment in expression of Vibrio cholerae virulence factors. Infect Immun 77(3):935–942. https://doi.org/10.1128/IAI.00929-08
Venil CK, Zakaria ZA, Usha R, Ahmad WA (2014) Isolation and characterization of flexirubin type pigment from Chryseobacterium sp UTM-3T. Biocatal Agric Biotechnol 3(4):103–107. https://doi.org/10.1016/j.bcab.2014.02.006
Venil CK, Zakaria ZA, Ahmad WA (2015) Optimization of culture conditions for flexirubin production by Chryseobacterium Artocarpi CECT 8497 using response surface methodology. Acta Biochim Pol 62(2):185–190. https://doi.org/10.18388/abp.2014_870
Venil CK, Sathishkumar P, Malathi M, Usha R, Jayakumar R, Yusoff ARM, Ahmad WA (2016) Synthesis of flexirubin-mediated silver nanoparticles using Chryseobacterium Artocarpi CECT 8497 and investigation of its anticancer activity. Mater Sci Eng C Mater Biol Appl 59:228–234. https://doi.org/10.1016/j.msec.2015.10.019
Venil C, Devi P, Dufossé L (2020a) Synthesis of Pigment-Mediated Nanoparticles and Its Pharmacological Applications. pp 331–346
Venil CK, Dufossé L, Renuka Devi P (2020b) Bacterial pigments: sustainable compounds with market potential for Pharma and Food Industry. Front Sustainable Food Syst 4. https://doi.org/10.3389/fsufs.2020.00100
Venil CK, Malathi M, Devi PR, Ahmad WA (2021) Chemo-preventive potential of flexirubin against human breast cancer: An in vitro, in vivo and in silico approach. Materials Today: Proceedings 47:2138–2147 https://doi.org/10.1016/j.matpr.2021.05.153
Venkatramanan M, Nalini E (2024) Regulation of virulence in Chromobacterium violaceum and strategies to combat it. Front Microbiol 15:1303595. https://doi.org/10.3389/fmicb.2024.1303595
Vila E, Hornero-Mendez D, Lareo C, Saravia V (2020) Biotechnological production of zeaxanthin by an Antarctic Flavobacterium: evaluation of culture conditions. J Biotechnol 319:54–60. https://doi.org/10.1016/j.jbiotec.2020.05.014
Vollenweider V, Rehm K, Chepkirui C, Pérez-Berlanga M, Polymenidou M, Piel J, Bigler L, Kümmerli R (2023) Antimicrobial activity of iron-depriving pyoverdines against human opportunistic pathogens. Cold Spring Harbor Laboratory
Walter MH, Strack D (2011) Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep 28(4):663–692. https://doi.org/10.1039/c0np00036a
Wan M, Hou D, Li Y, Fan J, Huang J, Liang S, Wang W, Pan R, Wang J, Li S (2014) The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresour Technol 163:26–32. https://doi.org/10.1016/j.biortech.2014.04.017
Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK (2011) Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol 193(14):3606–3617. https://doi.org/10.1128/JB.00396-11
Wang X, Isbrandt T, Christensen EO, Melchiorsen J, Larsen TO, Zhang SD, Gram L (2021) Identification and Verification of the Prodigiosin Biosynthetic Gene Cluster (BGC) in Pseudoalteromonas rubra S4059. Microbiol Spectr 9(2):e0117121. https://doi.org/10.1128/Spectrum.01171-21
Watstein DM, Styczynski MP (2018) Development of a pigment-based whole-cell zinc Biosensor for Human serum. ACS Synth Biol 7(1):267–275. https://doi.org/10.1021/acssynbio.7b00292
Wehrs M, Prahl JP, Moon J, Li Y, Tanjore D, Keasling JD, Pray T, Mukhopadhyay A (2018) Production efficiency of the bacterial non-ribosomal peptide indigoidine relies on the respiratory metabolic state in S. Cerevisiae. Microb Cell Fact 17(1):193. https://doi.org/10.1186/s12934-018-1045-1
Wehrs M, Gladden JM, Liu Y, Platz L, Prahl J-P, Moon J, Papa G, Sundstrom E, Geiselman GM, Tanjore D, Keasling JD, Pray TR, Simmons BA, Mukhopadhyay A (2019a) Sustainable bioproduction of the blue pigment indigoidine: expanding the range of heterologous products in R. toruloides to include non-ribosomal peptides. Green Chem 21(12):3394–3406. https://doi.org/10.1039/C9GC00920E
Wehrs M, Tanjore D, Eng T, Lievense J, Pray TR, Mukhopadhyay A (2019b) Engineering Robust Production microbes for large-scale cultivation. Trends Microbiol 27(6):524–537. https://doi.org/10.1016/j.tim.2019.01.006
Wei J, Xie X, Huang F, Xiang L, Wang Y, Han T, Massey IY, Liang G, Pu Y, Yang F (2020) Simultaneous Microcystis algicidal and microcystin synthesis inhibition by a red pigment prodigiosin. Environ Pollut 256:113444. https://doi.org/10.1016/j.envpol.2019.113444
Widomska J, SanGiovanni JP, Subczynski WK (2020) Why is Zeaxanthin the most concentrated Xanthophyll in the Central. Fovea? Nutrients 12(5). https://doi.org/10.3390/nu12051333
Williamson NR, Simonsen HT, Ahmed RA, Goldet G, Slater H, Woodley L, Leeper FJ, Salmond GP (2005) Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2-methyl-3-n-amyl-pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol 56(4):971 – 89 https://doi.org/10.1111/j.1365-2958.2005.04602.x
Williamson NR, Fineran PC, Leeper FJ, Salmond GP (2006) The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol 4(12):887–899. https://doi.org/10.1038/nrmicro1531
Wong JC, Kaplan HS, Hammond BR (2017) Lutein and zeaxanthin status and auditory thresholds in a sample of young healthy adults. Nutr Neurosci 20(1):1–7. https://doi.org/10.1179/1476830514Y.0000000138
Wu X, Deutschbauer AM, Kazakov AE, Wetmore KM, Cwick BA, Walker RM, Novichkov PS, Arkin AP, Chakraborty R (2017) Draft genome sequences of two J anthinobacterium lividum strains, isolated from Pristine Groundwater collected from the Oak Ridge Field Research Center. Genome Announc 5(26). https://doi.org/10.1128/genomeA.00582-17
Wu Y, Wang CW, Wang D, Wei N (2021) A whole-cell Biosensor for Point-of-care detection of Waterborne bacterial pathogens. ACS Synth Biol 10(2):333–344. https://doi.org/10.1021/acssynbio.0c00491
Xia Y, Wang G, Lin X, Song X, Ai L (2016) Solid-state fermentation with Serratia marcescens Xd-1 enhanced production of prodigiosin by using bagasse as an inertia matrix. Ann Microbiol 66(3):1239–1247. https://doi.org/10.1007/s13213-016-1208-4
Xiang Y, Zhang Y, Wang C, Liu S, Liao X (2018) Effects and inhibition mechanism of phenazine-1-carboxamide on the mycelial morphology and ultrastructure of Rhizoctonia solani. Pestic Biochem Physiol 147:32–39. https://doi.org/10.1016/j.pestbp.2017.10.006
Xie Z, Zhang Z, Cao Z, Chen M, Li P, Liu W, Qin H, Zhao X, Tao Y, Chen Y (2017) An external substrate-free blue/white screening system in Escherichia coli. Appl Microbiol Biotechnol 101(9):3811–3820. https://doi.org/10.1007/s00253-017-8252-2
Xu F, Gage D, Zhan J (2015) Efficient production of indigoidine in Escherichia coli. J Ind Microbiol Biotechnol 42(8):1149–1155. https://doi.org/10.1007/s10295-015-1642-5
Xu X, Chu X, Du B, Huang C, Xie C, Zhang Z, Jiang L (2022) Functional characterization of a novel violacein biosynthesis operon from Janthinobacterium sp. B9-8. Appl Microbiol Biotechnol 106(8):2903–2916. https://doi.org/10.1007/s00253-022-11929-8
Xue L, Chen YY, Yan Z, Lu W, Wan D, Zhu H (2019) Staphyloxanthin: a potential target for antivirulence therapy. Infect Drug Resist 12:2151–2160. https://doi.org/10.2147/IDR.S193649
Yada S, Wang Y, Zou Y, Nagasaki K, Hosokawa K, Osaka I, Arakawa R, Enomoto K (2008) Isolation and characterization of two groups of novel marine bacteria producing violacein. Mar Biotechnol (NY) 10(2):128–132. https://doi.org/10.1007/s10126-007-9046-9
Yang C, Jiang P, Xiao S, Zhang C, Lou K, Xing X-H (2011) Fed-batch fermentation of recombinant Citrobacter freundii with expression of a violacein-synthesizing gene cluster for efficient violacein production from glycerol. Biochem Eng J 57:55–62. https://doi.org/10.1016/j.bej.2011.08.008
Yong YC, Zhong JJ (2009) A genetically engineered whole-cell pigment-based bacterial biosensing system for quantification of N-butyryl homoserine lactone quorum sensing signal. Biosens Bioelectron 25(1):41–47. https://doi.org/10.1016/j.bios.2009.06.010
Yu D, Xu F, Valiente J, Wang S, Zhan J (2013) An indigoidine biosynthetic gene cluster from Streptomyces chromofuscus ATCC 49982 contains an unusual IndB homologue. J Ind Microbiol Biotechnol 40(1):159–168. https://doi.org/10.1007/s10295-012-1207-9
Yuan Y, Li J, Lin J, Pan W, Chu Y, Prithiviraj B, Guo Y, Wang X, Zhao K (2021) Extracellular products-mediated interspecific interaction between Pseudomonas aeruginosa and Escherichia coli. J Microbiol 59(1):29–40. https://doi.org/10.1007/s12275-021-0478-0
Yue Y, Zhao X (2021) Melanin-like Nanomedicine in Photothermal Therapy Applications. Int J Mol Sci 22(1). https://doi.org/10.3390/ijms22010399
Zabot GL, Schaefer Rodrigues F, Polano Ody L, Vinicius Tres M, Herrera E, Palacin H, Cordova-Ramos JS, Best I, Olivera-Montenegro L (2022) Encapsulation of Bioactive compounds for Food and Agricultural Applications. Polym (Basel) 14(19). https://doi.org/10.3390/polym14194194
Zhang J, Lu L, Yin L, Xie S, Xiao M (2012) Carotenogenesis gene cluster and phytoene desaturase catalyzing both three- and four-step desaturations from Rhodobacter azotoformans. FEMS Microbiol Lett 333(2):138–145. https://doi.org/10.1111/j.1574-6968.2012.02604.x
Zhang W, Hu X, Wang L, Wang X (2014) Reconstruction of the carotenoid biosynthetic pathway of Cronobacter sakazakii BAA894 in Escherichia coli. PLoS ONE 9(1):e86739. https://doi.org/10.1371/journal.pone.0086739
Zhang W, Liang W, Li C (2016) Inhibition of marine Vibrio sp by pyoverdine from Pseudomonas aeruginosa PA1. J Hazard Mater 302:217–224. https://doi.org/10.1016/j.jhazmat.2015.10.003
Zhang J, Suo Y, Zhang D, Jin F, Zhao H, Shi C (2018) Genetic and virulent difference between pigmented and non-pigmented Staphylococcus aureus. Front Microbiol 9:598. https://doi.org/10.3389/fmicb.2018.00598
Zhang Y, Chen H, Zhang Y, Yin H, Zhou C, Wang Y (2021) Direct RBS Engineering of the biosynthetic gene cluster for efficient productivity of violaceins in E. Coli. Microb Cell Fact 20(1):38. https://doi.org/10.1186/s12934-021-01518-1
Zhao J, Wu Y, Alfred AT, Wei P, Yang S (2014) Anticancer effects of pyocyanin on HepG2 human hepatoma cells. Lett Appl Microbiol 58(6):541–548. https://doi.org/10.1111/lam.12224
Zhao W, Xu LL, Zhang X, Gong XW, Zhu DL, Xu XH, Wang F, Yang XL (2018) Three new phenanthrenes with antimicrobial activities from the aerial parts of Juncus effusus. Fitoterapia 130:247–250. https://doi.org/10.1016/j.fitote.2018.09.007
Zhao K, Li D, Cheng G, Zhang B, Han J, Chen J, Wang B, Li M, Xiao T, Zhang J, Zhou D, Jin Z, Fan X (2019) Targeted delivery prodigiosin to Choriocarcinoma by peptide-guided dendrigraft Poly-l-lysines nanoparticles. Int J Mol Sci 20(21). https://doi.org/10.3390/ijms20215458
Zhao M, Zhang XS, Xiong LB, Liu K, Li XF, Liu Y, Wang FQ (2024) Establishment of an efficient expression and regulation system in Streptomyces for economical and high-level production of the Natural Blue Pigment Indigoidine. J Agric Food Chem 72(1):483–492. https://doi.org/10.1021/acs.jafc.3c05696
Zhou W, Li J, Chen J, Liu X, Xiang T, Zhang L, Wan Y (2016) The red pigment prodigiosin is not an essential virulence factor in entomopathogenic Serratia marcescens. J Invertebr Pathol 136:92–94. https://doi.org/10.1016/j.jip.2016.03.011
Zhou Z, Yan Y, Wang L, Zhang Q, Cheng Y (2019) Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 203:63–72. https://doi.org/10.1016/j.biomaterials.2019.02.023
Zhu L, Chu Y, Zhang B, Yuan X, Wang K, Liu Z, Sun M (2022) Creation of an industrial Bacillus thuringiensis strain with high melanin production and UV tolerance by Gene Editing. Front Microbiol 13:913715. https://doi.org/10.3389/fmicb.2022.913715
https://colorifix.com/app/uploads/2022/12/colorifix-environmental-impact.pdf. Accessed 15th June 2024
https://colorifix.com/colorifix-proves-lower-environmental-impact-at-every-stage-of-its-biological-dyeing-process/. Accessed 15th June 2024
https://menafn.com/1097992026/Global-Organic-Pigments-Market-Worth-Reach-USD-489-Billion-By-2024. Accessed 6th July 2024
https://www.pili.bio/9/technology. Accessed 15th June 2024
https://www.labiotech.eu/trends-news/synbio-carotenoids-market-deinove-greentech-industry-milestones/. Accessed 15th June 2024
Acknowledgements
The authors acknowledge all the open-source software and server providers. AB is funded by Startup Research Grant- SRG/2020/000702 (SERB, Govt. of India), Adhoc Grant- Project ID: AMR/Adhoc/284/2022-ECD-II (ICMR, Govt. of India), and SEED grant, Adamas University.
Funding
AB is funded by ICMR Adhoc research grant- Project ID: AMR/Adhoc/284/2022-ECD-II (ICMR, Govt. of India) and Start-up research Grant project ID: SRG/2020/000702 (Science and Engineering Research Board, Govt. of India).
Author information
Authors and Affiliations
Contributions
KA, Swarna Shaw, SPB, SBh, and AB drafted the manuscript. SBi was involved in preparing figures, editing, and correcting the manuscript. AB conceptualized the work and prepared the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The study does not involve any human and/or animal subjects or clinical isolates. No personally identifiable patient/ human subject information was disclosed to the researchers.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Acharya, K., Shaw, S., Bhattacharya, S.P. et al. Pigments from pathogenic bacteria: a comprehensive update on recent advances. World J Microbiol Biotechnol 40, 270 (2024). https://doi.org/10.1007/s11274-024-04076-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-024-04076-x