Abstract
The aim of the current review is to address updated research on a natural pigment called violacein, with emphasis on its production, biological activity and applications. New information about violacein’s action mechanisms as antitumor agent and about its synergistic action in drug delivery systems has brought new alternatives for anticancer therapy. Thus, violacein is introduced as reliable drug capable of overcoming at least three cancer hallmarks, namely: proliferative signaling, cell death resistance and metastasis. In addition, antimicrobial effects on several microorganisms affecting humans and other animals turn violacein into an attractive drug to combat resistant pathogens. Emphasis is given to effects of violacein combined with different agents, such as antibiotics, anticancer agents and nanoparticles. Although violacein is well-known for many decades, it remains an attractive compound. Thus, research groups have been making continuous effort to help improving its production in recent years, which can surely enable its pharmaceutical and chemical application as multi-task compound, even in the cosmetics and food industries.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
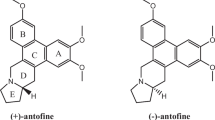
Nature has been a major source of compounds used for disease prevention and treatment purposes over the course of human history. Traditionally, marine and soil environments have provided several chemical scaffolds to help developing modern drugs, such as microbially and plant-derived metabolites (Harvey et al. 2015; Newman and Cragg 2016, 2020; Newman et al. 2000; Schreiber et al. 2002). Thus, bacterial strains are capable of synthesizing several secondary metabolites presenting biological activity (Numan et al. 2018). Among them, one finds violacein (Choi et al. 2021; Durán et al. 2012, 2016; Justo and Durán 2017), [3-(1,2-dihydro-5-(5-hydroxy-1H-indol-3-yl)-2-oxo-3H-pyrrol-3-ilydene)-1,3-dihydro-2H-indol-2-one] (Fig. 1), which is a natural purple pigment that triggers great interest due to its remarkable biological and physical properties. This pigment—which has molar mass of 343.3 amu—is insoluble in water, slightly soluble in ethanol, and soluble in methanol and dimethyl sulfoxide, as well as shows strong absorbance in the visible region of the spectrum due to resonance (Durán et al. 2007). Violacein’s high hydrophobicity (octanol–water partitioning coefficient − logPOW = 3.34) suggests that it is not secreted by bacteria into the environment (Choi et al. 2020).
Main topics covered in this review are synthetic biology violacein synthesis, using different bacteria, yeast or in situ processes. While violacein yielding species might provoke infections, violecein shows many beneficial, and synergic effects and activities against tumors, microbes, and parasites, shown in light blue
Several violacein-producing microorganisms have been identified since it was discovered more then 130 years ago (Boisbaudran 1882). Among them, one finds species Chromobacterium violaceum, which is extensively investigated as model system for violacein production, as well as species Alteromonas, Janthinobacterium, Pseudoalteromonas, Duganella, and Collimonas (Durán et al. 2016). In fact, bacterial species belonging to genus Chromobacterium, other than C. violaceum, have been used to synthesize several secondary metabolites with biocidal activity, such as the recently discovered C. subtsugae, C. sphagni and C. vaccinii (Blackburn et al. 2017; Martin and Soby 2016; Vöing et al. 2015). Several studies have recently reported new violacein sources. Two psychotropic bacterial strains capable of producing a mix of violacein and deoxyviolacein were isolated by Kuzyk et al. (2020) from Lake Winnipeg, Canada. Interestingly, Atalah et al. (2020) and Alem et al. (2020) have isolated and featured violacein produced by bacterial strains from extreme environments such as Antarctica. Recent literature has indicated increased number of studies describing violacein-producing bacteria featuring by whole genome sequencing to provide new information on violacein-producing bacteria (Bettina et al. 2018; Lamendella and Jude 2018; Xu et al. 2019). Table 1 shows a selection of violacein-producing strains, as well as the respective location bacteria were isolated from.
The first studies about violacein biosynthesis enabled observing that oxygenation in C. violaceum culture has reduced pigment production time (Tobie 1935). This finding was followed by the discovery that molecular oxygen and l-tryptophan were necessary to synthesize violacein (DeMoss and Evans 1959, 1960). From 1987 to 2000, there was great progress in the mechanistic aspects of violacein biosynthesis, such as the condensation of two l-tryptophan molecules, molecular oxygen incorporation in pyrrolidone moiety, 5-hydroxy-l-tryptophan intermediacy, independent chromopyrrolic acid production, as well as oxygenases and NAD(P)H involvement in violacein biosynthesis. Moreover, violacein biosynthesis comprises the joint action of five enzymes, called VioA-VioE (Durán et al. 2016). These first studies have increased the interest of the scientific community in the field in developing efficient routes to enable the biosynthesis of this compound by using either natural or genetically modified microbial strains, as addressed later in the current study.
Extensive studies have reported violacein’s pharmacological potential to be used as anticancer, antibacterial, antifungal, trypanocidal, antileishmanial, antinematode, antiulcerogenic, immunomodulatory and antiviral drug (Durán et al. 2016). There have been advances in understanding violacein’s action mechanisms and toxicity rates in the last years, and it contributed to improve its biological and pharmacological functionalities. In fact, there has been growing interest in investigating violacein and its activities in the last two decades, given the number of peer-reviewed articles published in the literature about it (Choi et al. 2021; Kothari et al. 2017). Although several anticancer, antiparasitic and antimicrobial properties of this purplish bisindole metabolite have been previously reviewed (Durán et al. 2016), the current study has described new features of this derivative and pointed out its value as chemotherapeutic agent. A promising application attributed to violacein lies on its potential to combat human multidrug resistant pathogens (Choi et al. 2017). In addition, violacein has been highlighted for its ability to interfere in different cancer hallmarks. It is also interesting emphasizing the symbiotic association between violacein-producing skin bacteria and amphibians, a fact that substantiates the role played by this pigment in biodiversity. Finally, attempts to introduce violacein in engineering polymers have been reported, as well as its industrial applications, mainly as high value-added product. Therefore, the current review has addressed the main biological activities performed by violacein, as well as efforts focused on improving its production and some current applications. A brief outlook of the present review is illustrated in Fig. 1.
Violacein biosynthesis
There are several ways to synthesize violacein, such as using bacteria, either wild or genetically modified strains (Park et al. 2021), yeasts (Chuang et al. 2018; Tong et al. 2021) or through synthesis performed in situ (Kanelli et al. 2018). l-Tryptophan is the substrate used to synthetize this secondary metabolite (Fig. 2), whereas the pathway counts on five enzymatic reactions and one consecutive non-enzymatic step (the sixth step) that completes the synthesis process. The secondary metabolism pathway strongly depends on oxidoreductive cofactors, such as FAD, NAD(P)H and heme-Fe(II) moieties in enzymes, as well as on oxygen supply at the final step.
Violacein is synthesized from two tryptophan molecules in a six-step pathway that involves five enzymatic steps, and one non-enzymatic step (Hoshino 2011; Tong et al. 2021). Enzymatic oxidation reactions are FAD (vioA, vioC, and vioD), NAD(P)H (vioC and vioD), and heme-Fe(II) (VioB) dependent. Also, the last step involves oxygen-guided decarboxylation. It is important to state that the enzymatic steps start with the vioA, then vioB catalyzed reactions, follows with vioE reaction as the most important step, while vioD and vioC complete the pathway
Moreover, C. violaceum is the strain mostly investigated in the violacein-production field. Durán et al. (2016) have summarized violacein-producing microorganisms, both natural and genetically engineered ones. Aye et al. (2015) have investigated marine biofilm formation bacteria and found that species Pseudoalteromonas ulvae TC14 can produce violacein, just as other strains belonging to its genus; however, it does not produce N-acylhomoserine lactones (AHLs) necessary to regulate violacein production, although it is sensitive to exogenous AHLs, at least under planktonic conditions. Violacein production was upregulated by C6-, C12-, 3-oxo-C8- and 3-oxo-C12-HSLs (homoserine lactones) and downregulated by 3-oxo-C6-HSL and 3-oxo-C8-HSL. These exogenous AHLs regulate violacein production, as well as that of other phenotypes associated with biofilm formation. This finding suggests the presence of functional AHL LuxR-type receptor in TC14 (Aye et al. 2015).
It is known that violacein found in C. violaceum presents positive regulation through the N-acylhomoserine lactone CviI/R quorum sensing system, as well as negative regulation through undistinguished putative repressor. According to Devescovi et al. (2017), violacein biosynthesis was negatively controlled by a new suppressor protein, VioS, and positively controlled by the CviI/R system. VioS does not control the CviI/R system; besides violacein, VioS and quorum sensing antagonistically control other phenotypes.
Saccharomyces cerevisiae strains were genetically engineered in the last years to produce violacein in five-enzyme biosynthetic pathway. Lee et al. (2013) have sampled 100 clones from an expression library (3% of it); they transformed the clones, featured the promoters of five heterologous genes, as well as all pathway products (violacein, deoxyviolacein, and chromoviridians). Subsequently, regression model was created to predict violacein production in the rest of the library. The aforementioned authors used this method to double violacein yield by these strains. Chuang et al. (2018) have recently described a detailed protocol used to produce violacein in S. cerevisiae by using the Versatile Genetic Assembly System (VEGAS). They have successfully inserted all five necessary transcription units in the model. Moreover, Blount et al. (2018) used S. cerevisiae strains—whose natural chromosomes are replaced by designed synthetic ones—to insert an expression plasmid for violacein production purposes and used SCRaMbLE (Synthetic Chromosome Rearrangement and Modification by LoxP-mediated Evolution) to increase violacein yield. SCRaMbLE consists in the rearrangement of non-critical sequences in the synthetic genome, when yeasts are exposed to Cre in vivo, i.e., it is a black box procedure, according to which, rapid diversification takes place and generates several phenotypes, some of them can lead to higher violacein yield (Blount et al. 2018).
On the other hand, violacein production in E. coli has also improved overtime. In fact, violacein production in Escherichia coli has gained room in the scientific field, since it is easily used, as well as presents high growth rate and protein yield. Jones et al. (2015) have created a T7 promoter library based on site-directed mutagenesis; then, they inserted all five violacein pathway genes under the control of this promoter library in E. coli specimens. The first analysis was applied to 4% of the library; results have shown 63-fold yield increase in this sample in comparison to the control. After fermentation optimization, titers were enhanced to approximately 2000 mg L−1, which corresponded to 2.6-fold improvement in titer and to 30-fold enhancement in yield in comparison to those in previous studies (Jones et al. 2015).
Kanelli et al. (2018) have optimized Janthinobacterium lividum fermentation conditions in order to maximize both violacein and biomass production. In addition, polyamide 6.6 fabrics were dyed by following three different methods, namely: simultaneous fermentation and dyeing (SFD), fabric incubation in bacterial culture after fermentation, or cell-free extract using in association with violacein. Among these methods, SFD presented maximum color change (1E) and strength (K/S), whereas dyeing has shown resistance to acid and alkaline perspiration or to water. Moreover, the fabric presented antimicrobial activity against yeasts (Candida albicans, C. parapsilosis, and C. krusei), as well as against bacteria (E. coli, Staphylococcus aureus, and methicillin-resistant S. aureus—MRSA) (Kanelli et al. 2018).
Several advances have been made in elucidating violacein-related enzyme mechanism and structure. VioD—which is the enzyme capable of converting protodeoxyviolaceinic acid into protoviolaceinic acid from Duganella sp.—was expressed in E. coli. VioD was later purified and crystalized in order to generate X-ray diffraction (Ran et al. 2015). VioA—which catalyzes the first step in violacein synthesis, i.e., l-tryptophan conversion into the corresponding α-imine—was also structurally and biochemically investigated to enable suggesting a mechanism for its activity (Füller et al. 2016). Enzyme kinetics of His163, Arg64, Lys269, Trp397, and Tyr309 mutant proteins were functionally identified as crucial residues for VioA catalysis. In addition, synthetic biology approaches were used to engineer varying violacein pathways as promising strategy for novel bisindole therapeutics’ synthesis. Accordingly, the authors proposed 7-aza-Trp, 1-methyl-Trp, 5-methyl-Trp, and 5-fluoro-Trp as interesting candidates for the synthesis of new violacein derivatives in vivo (Füller et al. 2016). Zhou et al. (2018) have also engineered E. coli to overexpress vioABCDE genes. VioE expression was reported to produce similar results to those reported by Fang et al. (2015, 2016), who overexpressed serA, trpEfbr, trpD, and vioABCDE in E. coli. Immanuel et al. (2018) used integrative approach based on the combination of constraints-based flux balance modeling to synthetic biology approach in order to optimize genetic changes to produce violacein in E. coli, in an efficient and scalable way.
Recent article published by Tong et al. (2021) took into consideration potentially-harmful bacterial violacein-producing strains to suggest using genetically modified yeast species Yarrowia lipolytica in order to synthesize violacein. Interestingly, violacein production could increase to 70 mg L−1, and present approximately 5–10% of deoxyviolacein impurities, when the correct nitrogen and carbon supply, and medium pH were used.
Therefore, although synthetic biology solution applications have made great improvements in violacein synthesis, some issues remain unsolved, such as long production span of 100 h (or more), which requires adopting laborious purification methods.
Violacein’s antitumoral activity
Over the past two decades, several studies have reported violacein's antitumor property (Durán et al. 2016; Justo and Durán 2017); recently, a study has shown that this pigment is non-genotoxic (Alem et al. 2020). It is known that cytotoxic agents often induce cell death, partly due to reactive oxygen species (ROS) generation; thus, Leal et al. (2015) have investigated whether violacein affects cellular redox status. They observed that violacein was cytotoxic to CHO-K1 and MRC-5 (non-tumor), and to HeLa cell lines (tumor), although HeLa and MRC-5 cells were more sensitive to this pigment. In this study, no association between increased oxidative stress (ROS) and cell death induction was observed; however, enhanced mitochondrial membrane potential was observed (Fig. 3). Based on these findings, the aforementioned authors have stated that membrane hyperpolarization may be the main reason for violacein-induced cell death (Leal et al. 2015). Similar results were reported in Ehrlich ascites tumor cells, which presented fast ROS production due to violacein application (Bromberg et al. 2010). Although De Carvalho et al. (2006) identified ROS production after treating Caco-2 cells with violacein, the redox status of colorectal cancer cell line HT29 remained unchanged. Together, these findings have suggested that violacein has cell-type specific influence on redox status.
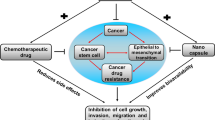
Violacein affects tumor cells in many ways by stopping proliferation and metastasis and provoking apoptosis. Hyperpolarization and permeabilization of membranes were stressed as the most important events during violacein action. Some authors use violacein with ascorbic acid loaded dendrimers. In addition, transfer of violacein biosynthetic cluster into an oncolytic bacteria strain led to an in situ production of violacein
Given the poor violacein solubility in water—which can compromise its bioavailability and, consequently, lead to low effect on biological systems—some scholars have suggested using delivery devices loaded with violacein. Accordingly, our research group has described the synthesis of violacein inclusion complexes with beta-cyclodextrin (β-CD) and their influence on HL60 cell viability (De Azevedo et al. 2000; Melo et al. 2003). Interestingly, IC50 value has dropped 15-fold when violacein was encapsulated in β-CD in comparison to values recorded for the violacein-free system (Melo et al. 2003). Fakhr et al. (2012) have also reported that violacein extracted from Janthinobacterium lividum DSM1522T presented enhanced cytotoxic activity when it was loaded on poly(amidoamine)—(PAMAM) in combination to ascorbic acid. According to the aforementioned study, human acute lymphoblastic leukemia cell line (Jurkat E6.1) was twofold more sensitive to violacein encapsulated in nanocomplex delivery system than that of the violacein-free system. Similar effect was also observed when apoptosis markers (caspases-3, -7 and -8) were analyzed; in other words, lower nanoencapsulated violacein dose was capable of activating caspases in comparison with violacein used in separate (Fakhr et al. 2012).
Another interesting aspect of violacein antitumor activity was reported by Hashimi et al. (2015), who evaluated the response of human cancer cell lines—such as A549 (lung), PC3 (prostate), HCT116 (colon), HT29 (colon), MCF-7 (breast), A431 (melanoma), HN5 (head and neck), and HeLa (cervix)—to violacein treatment under normoxic and hypoxic conditions. Almost all human tumor cells presented lower IC50 values under hypoxia, but HT29 was, by far, the most sensitive one—it recorded IC50 value 12.6-fold lower under hypoxia than the one recorded under normoxic conditions. Interestingly, the forementioned authors have found that IC50 values recorded for PC3 prostate cancer and A431 melanoma cells were not significantly affected by hypoxia (Hashimi et al. 2015).
Violacein overcomes cancer hallmarks
Durán’s group was the first to report violacein’s anticancer property in 2003 (Melo et al. 2003). Since then, molecular mechanisms supporting the strong antitumor activity of this pigment have been investigated by different groups. In fact, cancer cells treated with violacein present broad spectrum of affected key signaling transduction mediators. Therefore, this compound is currently defined as cancer hallmark modulator. Along human tumor development, which is a multistep process, cells acquire biological plasticity; it was first defined by Hanahan and Weinberg as hallmarks of cancer (Hanahan and Weinberg 2000). These scholars have initially suggested six cancer cell abilities contributing to disease complexity, namely: supporting proliferative signaling, avoiding growth repressors, holding out cell death, enabling replicative immortality, causing angiogenesis, and invasion and metastasis activations. Eleven years later, two novel emerging hallmarks were included in the previous list, namely: deregulating cellular energetics and evading immune destruction (Hanahan and Weinberg 2011). The following paragraphs pinpoint how violacein overcomes some cancer hallmarks reported, so far: supporting proliferative signaling, holding out cell death, and activating metastasis and invasion.
Violacein inhibits proliferative signaling Healthy cells tightly control the synthesis and secretion of growth factors and, consequently, they ensure cell homeostasis, whereas cancer cells acquire the ability to continuously proliferate due to mutations and overactivation of key proteins capable of promoting cell cycle progression, such as receptor tyrosine kinase protein, mitogen-activated protein (MAP)-kinase route and PI3-kinase signaling circuitry comprising their key AKT signal transducer. Reports have shown that violacein treatment led to decreased AXL—which is a specific receptor of tyrosine kinase suppressor in melanoma and CD34+/c-Kit+/P-glycoprotein+/MRP1+ TF1 leukemia progenitor cells (Liu et al. 2017; Queiroz et al. 2012). This kinase has been addressed as overexpressed in several human malignancy types, a fact that stimulates AKT, MAPK and FAK signaling pathways and evidences the role played by kinase in multiple oncogenic processes (Linger et al. 2013; Verma et al. 2011). Interestingly, AKT was inhibited by violacein in melanoma and colorectal cancer cases (Gonçalves et al. 2016; Kodach et al. 2006). According to Mojib et al. (2011), murine 2237 fibrosarcoma cells treated with violacein presented lower amounts of cyclin-dependent kinases (Cdk2, Cdk4 and Cdk6), key nuclear kinases accounting for cell cycle progression, and higher p53 and p21 (cell cycle blockers) levels. Accordingly, melanoma cell line (SKMEL-103) treated with violacein presented significantly decreased histone deacetylase-6 expression, which acts as proliferating activator in melanoma cells (Gonçalves et al. 2016). Masuelli et al. (2016) reported that violacein has inhibited head and neck cancer cell growth in vitro and in vivo.
Violacein induces cell death Although different cell death types have been described, apoptosis and autophagy are often the most explored ones. Violacein inhibits DAPK1, which contradicts both apoptosis and autophagic cell death in CD34+/c-Kit+/P-glycoprotein+/MRP1+ TF1 leukemia progenitors (Queiroz et al. 2012). Solid and hematopoietic tumors subjected to violacein treatment presented decreased number of anti-apoptotic mediators and increased number of apoptotic mediators—Bcl2 and Bax, respectively (Alshatwi et al. 2016; Kodach et al. 2006; Mojib et al. 2011). Accordingly, Kodach et al. (2006) observed that colorectal cancer cells exposed to violacein presented increased p53 protein concentration, although this protein is often deleted or downregulated in cancer cells, as strategy to limit or circumvent apoptosis. More recently, it was reported that violacein was capable of inhibiting the autophagy process in melanoma cells, as evidenced by increased amount of p62 protein (Gonçalves et al. 2016). It is well-known that melanoma sustains high basal autophagy rate as cytoprotective strategy, since it allows cells to survive even under unfavorable environmental conditions, such as nutrient deprivation and/or the presence of cytotoxic materials, through recycling (Corazzari et al. 2013). Therefore, cell death due to apoptosis is favored when violacein blocks autophagy. Findings reported by Gonçalves et al. (2016) have indicated that violacein can be useful in sensitizing cancer cells presenting high basal autophagy rate as drug-resistance strategy.
Violacein inhibits metastasis and invasion Metalloproteinases (MMPs) play essential role in metastatic processes, since metastasis requires extracellular matrix remodeling, since this matrix depends on MMPs (Conlon and Murray 2019). Platt et al. (2014) have shown that violacein was capable of decreasing MMP-2 and MMP-9 active forms. In addition, inflammatory chemokine secretion (CXCL12)—which is a positive cell migration and cancer metastasis modulator—has decreased after breast cancer cell line (MCF-7) treatment with violacein. This pigment has efficiently decreased the number of CXCR4 in the membrane through MMP-9 inhibition. Gonçalves et al. (2016) have also shown that violacein has significantly decreased the invasion capacity of a highly metastatic melanoma cell line (SKMEL-103) by using 3D culture model in matrigel.
In addition to investigate violacein’s ability to change cancer hallmark levels, the study developed by Liu et al. (2017) has traced the proteomic profile of colorectal cancer cell line (HT29) response to two violacein doses (high and low). Results have shown that violacein inhibited cell growth in a concentration- and time-dependent manner. Increased violacein concentrations have led to vacuoles in the mitochondrion and membrane blebs, as shown by Transmission Electron Microscopy (TEM). Quantitative proteomic analysis has shown differential expression in 492 and 112 proteins from cells treated with high and low violacein concentrations, repectively. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis has shown that most of these differently expressed proteins were involved in 50 signaling pathways, such as ribosome, citrate cycle and RNA degradation; 10 of them were significantly enriched (Liu et al. 2017).
We have observed that purple pellet is obtained after cells treated with violacein are subjected to centrifugation, a fact that indicates interaction between this compound and the membrane. Therefore, violacein interaction with cell membrane models was investigated by using Langmuir monolayer selected lipids in order to validate our hypothesis. Extremely low violacein amounts have affected surface pressure-area isotherms, as well as the polarization-modulation reflection–absorption infrared spectra of lipid monolayers. These results have indicated significant violacein interaction with the membrane. Brewster Angle Microscopy (morphological analysis) monitoring has indicated that violacein at air–water interface was homogenized during its incorporation to lipids. Lipids acted on floating monolayer’s viscoelastic and structural features over violacein interaction with them. These data helped better understanding violacein interaction at special membrane points (de Souza et al. 2017). Further studies by Gupta et al. (2021) have shown that violacein interaction with lipid monolayer formed at the air–water interface depends on the electrostatic nature of lipids. Studies using X-ray reflectivity on solid supported lipid monolayer on hydrophilic substrate have shown significantly increased membrane thickness in zwitterionic and positively-charged lipids. Violacein has also induced changes in lipids tilt angle and in-plane ordering. Taken together, studies by de Souza et al. (2017) and Gupta et al. (2021) provided detailed information on how violacein induces the structural reorganization of lipid molecules, which can explain the mechanisms enabling its interaction with cell membranes. Interestingly, studies conducted with Staphylococcus aureus reported that violacein was capable of inducing cell permeabilization, which was followed by the emergence of visible discontinuities in the cytoplasmic membrane (Cauz et al. 2019). If one takes into account that violacein has broad capacity of modulating different signaling pathways, such as growth factor receptors, these results can be associated, at least in part, with violacein interactions with lipid surfaces.
Antibacterial activity of violacein
Since the discovery of this purple bisindole derivative, different researchers have been carrying out studies to elucidate its antimicrobial effects on several microorganisms capable of causing severe infections to humans and to other animals. Outstanding antimicrobial activity against Gram-positive bacterial strains, such as S. aureus, S. epidermidis, Pseudomonas aeruginosa, Klebsiella pneumoniae, Vibrio cholerae, and Salmonella typhi, has been reported. Cytoplasmic membrane disruption is a major mechanism assumingly associated with this activity. According to Cauz et al. (2019), liposomes made by commercial and bacterial phospholipids were disrupted after direct interaction with violacein, leading to their increased permeability. Moreover, a two-step process was suggested as accountable for violacein-induced antibacterial action (Aruldass et al. 2018). Firstly, membrane permeabilization induces proton, ion, ATP and protein leakage from cells. Finally, intracellular content loss leads to osmotic imbalance, which may induce cell death (Aruldass et al. 2018).
The emergence of multidrug resistant bacteria is a health issue worldwide; S. aureus is one of the most opportunistic pathogens associated with the development of multidrug resistance and hospital-associated infections (Cardozo et al. 2013). It is well-known that the number of MRSA infection cases has increased in the last few decades, and it was followed by increased resistance to current antibiotic therapies (Nathwani et al. 2014). Thus, new drugs capable of overcoming resistance issues are extremely desirable. Interestingly, violacein synthesized by C. violaceum UTM5 was also effective against MRSA ATCC 43,300. It recorded minimum inhibitory concentration (MIC) value of 15.6 µg mL−1, which was twice the MIC value recorded for S. aureus ATCC 29,213 (Aruldass et al. 2015). Nevertheless, violacein has shown bacteriostatic activity against both S. aureus strains (ATCC 43,300 and ATCC 29,213); it recorded MIC value of 3.9 μg mL−1. Furthermore, fluorescent dyes used for membrane integrity evaluation and TEM analysis have confirmed membrane rupture as violacein’s major antibacterial action mechanism (Aruldass et al. 2018).
Interestingly, violacein has also shown strong antimicrobial effect on S. aureus isolated from bovine mastitis, which is a chronic dairy farm-associated disease that typically presents multidrug resistance and leads to considerable economic losses (Cazotto et al. 2011). It is also important mentioning that, in addition to its antibacterial activity, violacein has shown synergistic effect, together with other antimicrobial compounds, on mastitis; this outcome suggested violacein use in combined treatments (Cazotto et al. 2011). Thus, it is reasonable prospecting violacein as antibiotic in vivo used to treat both human and animal diseases.
Several reports have shown synergistic effect of violacein associated with commercial antibiotics. Dodou et al. (2017) have shown the antimicrobial activity of violacein combined to other antibiotics against S. epidermidis. According to another study, violacein interacts with commercial antibiotics in a synergistic manner, and it enhances drug effectiveness (Priya et al. 2018). MIC recorded for violacein used in association with other commercial antibiotics against uropathogenic E. coli is summarized in Table 2. It was possible observing that violacein acts synergistically (> 55%) with commercial antibiotics and that it could be certainly used as antibiotic in association with other antimicrobial agents (Priya et al. 2018).
Subramaniam et al. (2014) have tested the activity of violacein-macrolide and violacein-aminoglycoside class combinations against major pathogens, such as S. aureus, K. pneumoniae, P. aeruginosa and V. cholera. These combinations have shown Fractional Inhibitory Concentration Indices (FICI) lower than 0.5, and it has evidenced synergistic effect (Subramaniam et al. 2014). According to this very same study, violacein-azithromycin and violacein-kanamycin association resulted in high synergistic effect (FICI = 0.3) on Salmonella enterica serovar Typhi strain. Therefore, violacein can be used as potential synergistic antibiotic in combination to other antimicrobial agents.
Microbial growth in biofilm also plays important role in providing different defense mechanisms against microorganisms during infection processes. Besides preventing the access of immune cells to the infection site, biofilm matrix increases microorganisms’ resistance to antimicrobial agents and contributes to infectious processes (Savage et al. 2013). Violacein has shown high antimicrobial activity in suppressing and killing S. aureus in biofilm formation processes and in planktonic cultures. Minimum bactericidal concentration (MBC) of 5 µg mL−1 violacein has killed S. aureus after 3–4 h exposure, whereas MIC of 1.25 µg mL−1 has suppressed bacterial growth within the first 8 h of interaction. Biofilm formation was also strongly inhibited at this very same violacein concentration. On the other hand, higher S. aureus resistance (40 µg mL−1) was observed in mature biofilm (Batista et al. 2017). These results suggest that increased bacterial metabolic activity (exponential growth phase) may favor violacein action, which appears to involve macromolecule synthesis. However, since violacein has also shown antimicrobial activity against S. aureus at all growth stages, although at different intensities, different mechanisms may have operated to explain violacein’s action (Batista et al. 2017). Dodou et al. (2019) have also investigated the antimicrobial potential of violacein deriving from C. violaceum ATCC 12,472 against S. epidermidis biofilm. Violacein, at concentrations of 20 µg mL−1 and 160 µg mL−1, was capable of inhibiting biofilm formation and eradicating mature biofilm, respectively. Moreover, these effects took place within less than 3 h of incubation.
Another interesting approach adopted to overcome multidrug resistance was reported by Mitchell’s group. They used a combination of violacein—which is effective against Gram-positive bacteria—and Bdellovibrio bacteriovorus HD100—which predates on Gram-negative strains—as strategy against resistant mixed species communities (Im et al. 2017). These agents were used alone, in mixed bacterial cultures comprising Gram-positive and Gram-negative strains, but they were only effective against their respective strains. On the other hand, the combined treament reduced by 84,500 times the total numbers of pathogens. Decrease by 99.98% in cell viability was observed after treatment application to a mixed culture of Acinetobacter baumannii, S. aureus, K. pneumoniae and Bacillus cereus based on the association between violacein and B. bacteriovorus. On the other hand, reductions by only 19% and 68% were observed when violacein and B. bacteriovorus were used alone, respectively (Im et al. 2017).
Nanoparticles associated with drugs tend to increase their effectiveness, delivery and safety profiles. Accordingly, violacein deriving from C. violaceum and silver nanoparticles (AgNPs) deriving from Fusarium oxysporum have shown synergistic effect on several resistant microorganisms (Sarmiento et al. 2016). Arif et al. (2017) took advantage of AgNPs’ therapeutic properties to synthesize violacein capped silver nanoparticles (vAgNPs), and compared their antimicrobial and antialgal properties to that of starch capped silver NPs (cAgNPs). MICs of vAgNPs were far lower than those recorded for cAgNPs, which showed 3–10 times higher therapeutic action against multidrug resistant bacteria (P. aeruginosa, E. coli, and S. aureus), and algae (Dictyosphaerium sp. strain DHM 1, Pectinodesmus sp. strain PHM 3 and Dictyosphaerium sp. strain DHM 2). Importantly, violacein capping seems to induce AgNPs’ selectivity to Gram-positive bacteria. Thus, the aforementioned report has indicated a promising strategy to enhance not only AgNPs' stability and bioavailability, but also to modulate their therapeutic mechanism (Arif et al. 2017).
Violacein effects on parasites and on their vectors
In 2000, WHO has estimated 12–15 million leishmaniosis cases worldwide, with approximately 70,000 deaths per year, as well as that 350 million individuals would be at risk of acquiring the infection in 89 countries (Torres-Guerrero et al. 2017). With respect to trypanosomiasis, approximately 8 million individuals are infected with Trypanosoma cruzi in America (i.e. Chagas disease) and more than 10,000 patients die of this infection on a yearly basis; whereas approximately 60 million people are at risk of getting infected with Human African trypanosomiasis (i.e. sleeping sickness). In addition, 212 million new malaria cases and approximately half a million death cases were estimated worldwide, among other parasitic diseases (Martin and Soby 2016; World Health Organization 2017). These data do not reflect small advances in therapies used to treat human parasites in the last decades, although most antiparasitic drugs were developed 25 to 40 years ago. Moreover, increased pathogen resistance to commercial drugs, restricted biocide activity, and undesirable secondary effects on humans during extended treatments (Islan et al. 2017) effectively contribute to increase the number of infected invidivuals.
The main interest in using violacein as antiparasitic molecule is based on its reported leishmanicidal, trypanocidal, antioxidant and antiprotozoal activities, among others (Leon et al. 2001; Matz et al. 2004). Since Chromobacterium spp. produces multiple biocide compounds, the role played by violacein as antiplasmodial and trypanocidal agent was investigated by cloning vioABCDE or vioABCE operons for violacein and deoxyviolacein, respectively, in several plasmids. This procedure was followed by E. coli BL21 transformation and by the expression of both molecules (Bilsland et al. 2018). Violacein IC50 was 1.51 ± 0.4 µM, which corresponded to half benzinidazole’s IC50—benzinidazole is the drug most often used to treat trypanosomiasis; its IC50 in T. cruzi (Tulahuen strain) is 3.07 ± 0.6 µM. The same experiment was performed with Plasmodium falciparum, wild-type (3D7 strain, sensitive to chloroquine) and chloroquine-resistant (W2 strain) strains; results have shown violacein IC50 values of 0.4 µM and 0.5 µM, respectively, whereas deoxyviolacein IC50 values were approximately 11 µM and 14 µM, respectively. Comparative violacein and dexoyviolacein toxicity analysis applied to P. falciparum and red blood cells has shown approximately 5 and 20 times more sensitivity to the parasite than the sentisivity recorded for mammalian cells, respectively (Bilsland et al. 2018). Similar results were previously reported for violacein extracts used against P. falciparum and P. chabaudi (Lopes et al. 2009). In addition, Canuto et al. (2019) reported ROS induction and changes in mitochondrial transmembrane using as potential action mechanisms.
Another study focused on investigating violacein’s effect on Acanthamoeba castellanii—which is a protozoan species capable of causing severe diseases, such as amebic keratitis and granulomatous amebic encephalitis—has shown that 1 µM of violacein was capable of producing 100% amoeba lysis, which was triggered by apoptotic mechanisms determined through TUNEL and caspase-3 assays (Matz et al. 2008).
Several pathologies affecting humans are caused by nematodes, such as angiostrongyliasis, ascariasis, enterobiasis, filariasis, hookworm and trichinosis, among others. The biocide effect of violacein on nematode species Caenorhabditis elegans was evaluated (Ballestriero et al. 2014). Besides C. elegans resistance to violacein, LC50 > 30 µM (although lower than that of common drugs), its accumulation in nematodes’ intestine was considered the main cause for their increased mortality rate. The authors of the aforementioned study have speculated that violacein has compromised nematodes’ immune system, which led to reduced ability to control bacterial concentrations in the gut and, consequently, to death (Ballestriero et al. 2014). Interestingly, they further showed that the investigated worms presented learned aversion to bacterial metabolites (Ballestriero et al. 2016). Yoon et al. (2020) have recently reported that violacein is not only toxic to adults, but it also inhibits C. elegans larval development. In addition, matriphagy—i.e., mothers hold their eggs within their bodies until young larvae hatch inside them—happens to avoid some of the toxic effects of violacein. Moreover, the aforementioned study also helped elucidating that consumption of unsaturated fatty acids, such as oleate—which is produced by nematode mothers—has mitigated violacein toxicity (Yoon et al. 2020). If one takes into consideration mechanisms elicited by oleate in C. elegans, the study by Yoon et al. (2020) have also explained violacein activity against other pathologies. In another interesting study, violacein-5’-O-glucoside was produced by E. coli harboring the violacein biosynthetic gene cluster vioABCDE and expressing a Bacillus glycosyltransferase (YjiC) enzyme (Lee et al. 2019). This derivative increased violacein’s solubility in water and presented effective anti-nematodal activity against the causative agent of pine wilt disease, namely Bursaphelenchus xylophilus (Lee et al. 2019).
Similarly, changes in midgut susceptibility to viruses and parasites in mosquitoes fed on violacein or violacein extracts were observed (Bahia et al. 2014; Ramirez et al. 2014). Chromobacterium spp. strains, such as C. subtsugae, C. sphagni and C. vaccinii, have shown insecticidal activity capable of killing Aedes aegypti and Anopheles spp. larvae, as well as vectors of Plasmodium spp., which is the etiological agent of malaria (Blackburn et al. 2017; Farrar et al. 2018; Vöing et al. 2015). For example, MWU205 strain produces approximately 7.9 × 10–6 ng/cell and 1.3 × 10–6 ng/cell extra- and intra-cellular of violacein, respectively. This amount of violacein is capable of killing diamondback moth (Plutella xylostella) larvae, although it only kills 40% and 50% of A. aegypti larvae 16 h and 72 h after insect hatching. However, C. vaccinii strains MWU300 and MWU328 are capable of killing A. aegypti larvae 16 h after hatching; this outcome is comparable to the mosquitocidal activity of B. thuringiensis IBL10003 toxin, which is often used as reference strain (Martin and Soby 2016).
Moreover, bacterial extracts of novel isolated C. sphagni strains IIBBL 14B-1 (NRRL B-67130) and 37-2 (NRRL B67131) were toxic to some lepidopteran insects (Blackburn et al. 2018). Gypsy moth (Lymantria dispar), diamondback moth (Plutella xylostella), tobacco hornworm (Manduca sexta), and cabbage looper (Tricoplusia ni) larvae fed on C. sphagni strain extracts presented decreased pupation, or larvae weight, or survival by approximately 30% to 40% (Blackburn et al. 2018). Moreover, Blackburn et al. (2018) and Martin et al. (2007) reported larvae inhibition in corn earworm (Helicoverpa zea), Colorado potato beetle (Leptinotarsa decemlineata), beet armyworm (Spodoptera exigua), fall armyworm (Spodoptera frugiperda), European corn borer (Ostrinia nubilalis), and tobacco budworm (Heliothis virescens) speciemens fed on C. sphagni IIBBL 14B-1 (NRRL B-67130) and 37-2 (NRRL B67131) cultures.
Violacein deriving from C. violaceum, and its combination to silver and gold nanoparticles (phytosynthesized), were reported for P. falciparum and T. brucei gambiense growth inhibition in vitro. Violacein association (IC50 = 51.8 µg mL−1) with silver nanoparticles has shown reduction by ~ 1.2-fold in IC50 values recorded for both parasites, and it slightly reduced the cytotoxicity in mammalian cells (peripheral blood mononuclear cells and cancer cell lines, such as HeLa and MCF7) (Rahul et al. 2015). However, previous reports have shown values close to 2 µg mL−1 for P. chabaudi chabaudi and 0.68 µg mL−1 for P. falciparum (Lopes et al. 2009). This difference could be associated with violacein purity level.
Antifungal activity of violacein
Most studies focused on investigating violacein’s antifungal activity were based on diseases affecting amphibians, such as chytridiomycosis (Scheele et al. 2019), or with fungi capable of damaging soybean crops.
Barreto et al. (2008) carried out a study with C. violaceum, which was used as model representative of the Amazonian biodiversity, against seven fungal pathogen strains affecting soybeans crops in Brazil. They used the paring method to analyze whether C. violaceum cells and metabolites, such as violacein, found in the supernatant of the investigated cultures were capable of inhibiting the selected fungal species growth. Seven-day inhibition results have shown that cells and supernatants of a whole variety of strains presented different antifungal activities against Fusarium sp., Phomopsis sp., Corynespora sp., Aspergillus sp., Colletotric kikuchi and Cercospora kikuchi strains (Barreto et al. 2008). The aforementioned study has emphasized the paramount importance of Brazilian biodiversity, since different C. violaceum strains were capable of acting against six different pathogenic fungal types that harm soybean tillage and cause significant economic losses in Brazil.
Another important environmental issue caused by fungi lies on the decrease and even extinction of different amphibian species worldwide, mainly due to chytridiomycosis, for which fungal pathogen Batrachochytrium dendrobatidis is the key agent (Brucker et al. 2008a, b; Harris et al. 2009; Sasidharan et al. 2015; Woodhams et al. 2018). Brucker et al. (2008b) have used J. lividum culture to investigate a mutualism mechanism observed in nature to inhibit B. dendrobatidis growth on amphibian skins. The study was based on the comparison between skin samples from salamander species P. cinereus to other organisms investigated in the literature. In addition, two main metabolites were isolated from J. lividum, namely: indole-3-carboxaldehyde and violacein; they presented antifungal activity against B. dendrobatidis, even at relatively low concentrations (68.9 and 1.82 μM, respectively), over a seven-day period-of-time (Brucker et al. 2008b).
Harris et al. (2009) conducted a studty with another amphibian species and also found decreased mortality and morbidity rates associated with chytridiomycosis, due to the presence of J. lividum. Amphibians were divided into three major groups, namely: the ones only subjected to B. dendrobatidis zoospores, the ones only subjected to J. lividum, and the ones subjected to both. Swab collections were performed at different times to estimate violacein and B. dendrobatidis concentrations. Violacein was only found in frogs subjected to J. lividum, and it suggested that the additional presence of this bacterium can activate the production of secondary metabolites capable of protecting these amphibians (Harris et al. 2009).
Woodhams et al. (2018) have also investigated the important role played by symbiosis as defense mechanism in amphibian species. They used 20 amphibian species to investigate the presence of two metabolites—prodigiosin and violacein—and they tested their antifungal activity against B. dendrobatidis (Bd) and B. salamandrivorans (Bsal). Results have shown violacein in six J. lividum isolates, as well as prodigiosin in five Serratia plymuthica and S. marcescens isolates. With respect to the antifungal activity of commercial metabolites, prodigiosin has significantly inhibited fungal growth—it recorded MIC values of 10 and 50 μM for Serratia plymuthica and S. marcescens isolates, respectively,—whereas violacein MIC value recorded for Bsal and Bd was approximately 15 μM. According to the aforementioned authors, even if the relative abundance of bacteria generating these protective secondary metabolites is low, lower than 1%, their prevalence is high (Woodhams et al. 2018). Thus, the presence of one, or more, bacteria in amphibians’ skin can considerably decrease their chytridiomycosis-associated mortality rates.
Sasidharan et al. (2015) have isolated a new Chromobacterium sp. strain, NIIST (MTCC 5522), from clay mine acidic sediment, which was capable of producing large violacein amounts. The aims were to develop a high-yield violacein purification process and to test its antifungal activity. Fungi accountable for causing diseases in plants and humans, such as Aspergillus flavus MTCC 183, Cryptococcus gastricus MTCC 1715, and Trichophyton rubrum MTCC 296; as well as agriculturally significant fungi, such as Fusarium oxysporum MTCC 284, Rhizoctonia solani MTCC 4634, and Penicillium expansum MTCC 2006, were selected based on disc diffusion method. Violacein’s antifungal potential was compared to that of commercial antifungals, such as bavistin and amphotericin B; violacein was more efficient against 4 of the 8 tested fungi. The aforementioned authors believe that violacein associated with other compounds is a product with great potential to be used against fungal infections (Sasidharan et al. 2015).
Arif et al. (2017) have coupled silver nanoparticles to violacein (vAgNPs) and evaluated the pharmacological profile of this new molecule against different fungal species, such as Aspergillus tamari, A. tubingensis and F. proliferatum, as an attempt to solve the violacein solubility issue. Results have shown increased efficiency in vAgNPs' antifungal activity (10 mg and 1 mg) in comparison to that of starch capped AgNPs (cAgNPs). The overall susceptibility pattern of fungal species against vAgNPs presented the following order: A. tamari > A. tubingensis > F. proliferatum (Arif et al. 2017). Thus, although still modest, nanotechnology is an important tool used to help improving the antifungal activity of new or already used compounds in therapies.
Inflammation
Despite the accumulating evidence suggesting that violacein has several pharmacologic effects, its anti-inflammatory and anti-oxidant activities have only recently started to be investigated in acute and chronic diseases (Durán et al. 2016; Justo and Durán 2017). Verinaud et al. (2015) were the first to report violacein’s ability to protect mice from acute and chronic autoimmune inflammation. Violacein was capable of reducing the production of inflammatory cytokines such as IL-6 and CXCL1 when it was intraperitoneally administered to mice (3.5 mg kg−1) at the same time they received 1 μg LPS. Violacein has also reduced neutrophil infiltration in the peritoneal cavity; thus, no changes in T- and dendritic cell (DC) populations, or in the frequency of B cells, were observed. Curiously, serum IL-10 levels have increased after violacein treatment, and it suggested the modulatory effect of this bisindole on cytokine and chemokine. This outcome is consistent with violacein’s ability to downregulate the interaction between inflammatory CXCL12 and CXCR4, which play important role in breast cancer cell adhesion and metastasis processes (Platt et al. 2014). Experimental autoimmune encephalomyelitis (EAE) was induced in mice to evaluate violacein’s ability to counteract chronic inflammation progression. Treatment with 3.5 mg kg−1 of violacein for 3 days has significantly reduced the clinical course of the disease, central nervous system (CNS) inflammation and cellular infiltration in mice. In addition, regulatory T cells (Tregs) play important role in controlling autoimmune inflammation processes (Sakaguchi et al. 2008; Thomé et al. 2013, 2014; Thornton and Shevach 1998). Increased Tregs frequency was also observed in mice treated with violacein. Most importantly, CNS gene expression profile has evidenced that violacein significantly induced increase in IL-10 and indoleamine 2,3-dioxygenase (IDO) expression levels, which appears to modulate DC and Tregs. Furthermore, adoptive transfer of violacein-elicited Tregs (CD4+CD25+) has significantly reduced EAE, and it confirmed violacein’s ability to suppress the disease.
As previously reported (Bromberg et al. 2010), Verinaud et al. (2015) have also found violacein toxicity at higher dose (7 mg kg−1). However, antimalarials also present toxicity; similar to chloroquine, violacein-induced DC immature phenotype may be an interesting approach to induce anti-inflammatory profile (Thomé et al. 2014). Interestingly, recent study has calculated violacein properties such as Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) (Verma and Pandey 2017). LD50 of 500 mg kg−1 was observed, and it suggested that it may be safe for humans at doses lower than 300 mg kg−1. Furthermore, the aforementioned study has also shown that this compound follows the Lipinski’s rule, a fact that suggests its potential to be used as drug.
Venegas et al. (2019) have recently attempted to clarify the role played by TNF-α in violacein activity, since violacein’s ability to induce or suppress this cytokine remains unclear (Alshatwi et al. 2016; Antonisamy et al. 2014; Ferreira et al. 2004; Platt et al. 2014; Verinaud et al. 2015). These authors have used different immune cell lines and peripheral blood mononuclear cells (PMBCs) to show that violacein has immunostimulatory effect on different cell lines, as well as on PBMCs. Furthermore, TLR-transfected HEK-293 cells and molecular docking studies have clearly indicated that this effect could be associated with signaling through TLR8, thus stimulating pro-inflammatory response, which is consistent with previous reports (Alshatwi et al. 2016; Ferreira et al. 2004). Gene expression analysis has further suggested negative TLR signaling feedback mechanism, which could explain the anti-inflammatory effect observed in animal models (Antonisamy et al. 2014; Verinaud et al. 2015).
Several reports (reviewed in Durán et al. 2016; Justo and Durán 2017) have indicated that molecular mechanisms subjacent to violacein targets are diverse and suggested associations of multiple signaling pathways. Based on these previous studies, Verma and Pandey (2017) performed molecular docking analysis in violacein, MMP-2 and MMP-9 in order to investigate the inhibitory potential of this indole derivative. Matrix metalloproteinases play important role in cerebral ischemia, as well as in many other diseases, such as cancer, cardiovascular diseases and osteoarthritis (Dong et al. 2009; Lakhan et al. 2013; Romanic et al. 1998). The study by Verma and Pandey (2017) has clearly provided support for direct MMP-2 and MMP-9 inhibition by violacein—which recorded Ki values of 2.12 and 56.14 nM, respectively—and high binding energies. Most importantly, these Ki values are approximately 100 (MMP-2) and 6 times (MMP-9) lower than those recorded for resveratrol and quercetin in a study based on this very same approach, respectively (Pandey et al. 2012, 2015). In fact, several natural products have been suggested as MMP inhibitors to treat ischemic conditions (Lee et al. 2010; Sarkar et al. 2016). Thus, ADMET analysis, together with violacein’s MMP inhibitory activity, supports its use to treat ischemic pathologies, as well as other potential inflammatory diseases.
Violacein applications
Violacein is a purple pigment with potential to be used in a wide range of biological applications, such as photoprotective, antioxidant, anticancer, antiprotozoal and antibacterial agent (Berti et al. 2019; Choi et al. 2015b, 2017; Durán et al. 2016, 2020). However, one of the most interesting violacein applications lies on a natural process. Species Duganella spp. lives in the soil and in the rhizosphere of cultivated and wild olive trees in Southern Spain; it protects them from pathogens (Aranda et al. 2011). In addition, the combined antibiotic effect of violacein and B. bacteriovorus HD100 on Gram-positive MRSA was interesting, since they did not antagonize each other (Im et al. 2017). There was expressive reduction in bacterial viability when these agents were used in combination to one another, a fact that reinforced their prospective application against mixed species populations, as discussed above (Im et al. 2017). Nakazato et al. (2019) have recently suggested the combined use of violacein and biologically prepared silver nanoparticles (bioAgNPs). Violacein-bioAgNPs have shown increased synergistic antimicrobial activity against S. aureus and E. coli. According to Choi et al. (2020), C. violaceum produced extracellular membrane vesicles that can be used to solubilize and transport violacein to other microorganisms, such as S. aureus, against which violacein has shown antibacterial activity; 90% of microorganisms died after 6 h of treatment with 3.1 mg L−1 of violacein. Further studies conducted by Batista et al. (2020) have also shown that outer membrane vesicles produced by C. violaceum delivered violacein to mediate its toxicity at long distance. Furthermore, this membrane vesicle release was achieved by the concerted expression of two quorum sensing-regulated pathways, namely: violacein biosynthesis and VacJ/Yrb system. Thus, violacein can make its own secretion easier when membrane vesicle production decreases due to high cell density.
Swelling pectin-gelatin and TWEEN® 20 matrices loaded with violacein were developed by Berti et al. (2019) and tested against HCT116 colorectal cancer cells. Pectin-gelatin matrix had protective effect on the digestive tract, until the colon was reached, a fact that showed reduced cytotoxicity to cells. Polysorbate 20 microemulsions prepared with violacein avoided pigment precipitation inside the human body and effectively reduced colon cancer cell viability in a dose-dependent manner (Berti et al. 2019). An interesting strategy was recently developed to overcome violacein’s poor solubility in aqueous solutions and to reduce its toxicity to non-tumorigenic cells. Based on the interesting physicochemical properties of ionic liquids (ILs) and on the likelihood of tailoring biological aspects, such as cytotoxicity and biodegradability, these ILs have shown important applications in several fields, such as biomedicine. Surface-active ionic liquids (SAILs) are an important class of ILs, which has surfactant properties and can be used to help developing drug delivery devices for hydrophobic molecules. According to Berti et al. (2020), SAILs based on cation 1-alkylimidazolium were used to dissolve violacein in micellar aqueous media [Viol-([C16Him]-S)]; then, this complex was used to develop an efficient hybrid solid lipid nanoparticle (SLN) carrier tailored with folate in order to target cancer cells. The uptake of these SLN was tested in cancer (HCT116 and HeLa) and non-tumorigenic (A549) cell lines; remarkably higher incorporation was observed in the former cells—this outcome is consistent with their higher expression of folate receptors. The study of Berti et al. (2020) has provided a new effective formulation for violacein delivery to cancer cells with potential application in therapy.
In addition, violacein and its derivative have shown interesting properties for cosmetic applications, mainly if one takes into consideration its antimicrobial protection property in decorative cosmetics and pharmaceutical products (Kallmayer et al. 2005). German pharmaceutical industry Beiersdorf has protected several cosmetic formulations, such as lipstick, gloss lip, paste, lipstick against herpes, mascaras, foundation, and nail polish, as well as healthcare emulsions and formulations for antimicrobial skin protection, deodorant, antiperspirant, and anti-dandruff shampoo, which were produced based on the addition of violacein, deoxyviolacein and/or violacein, and of their derivatives tri-acetyl-violacein and diacetyl-(di)-methyl-violacein and/or its furan analogs (Kallmayer et al. 2005). Violacein is also acknowledged as oxidase tyrosinase inhibitor, which has catalytic activity in melanin generation in skin pigmentation processes, such as freckles and spots (Hidachi et al. 2017). Satoshi and Takatoshi (1998) focused on producing a natural antimicrobial antioxidant to be applied on the skin. They proposed a process of constant large scale violacein production from Chromobacterium sp. or Janthinobacterium sp., and natural antioxidant and antimicrobial application as cosmetic products, such as gel, oil, surfactant, polyhydric alcohol, lower alcohol, perfume, pigment, dye antioxidant, ultraviolet absorber, ultraviolet scattering agent, purified water, moisturizing agent and cosmetic ingredients, among others (Satoshi and Takatoshi 1998). Another interesting violacein application was introduced in a Taiwan patent as environmentally friendly nail polish product and cuticle cream. The cosmetic and pharmaceutical industry has been making great effort in the last 20 to 30 years to reduce the use of synthetic dyes capable of causing skin rashes and allergies (Rongzhen and Yuwen 2017). A World patent from 2012 has described the use of violacein isolated from Corbetia marina as dye additive in aquaculture to help increasing fish immunity (Tapia et al. 2012).
Kato et al. (1998) have acknowledged the dyeing or coloring additive properties of violacein and of its derivatives. Purple pigments dyed cotton fibers and silk fabric by dispersing the textile in a mix of cell body suspension comprising microorganisms deriving from C. violaceum, A. luteoviolacea or J. lividum S9601 strains. Accordingly, a violacein-AgNPs combination was recently used to dye silk composite with violacein presenting antimicrobial activity (Gao et al. 2019). Excellent antimicrobial activity was observed, since the investigated combination was capable of reducing by more than 99% the incidence of microorganisms, such as S. aureus, E. coli and C. albicans.
Gomez-Gomez et al. (2019) have shown that violacein production can be also controlled at quorum signaling molecule level. They used selenium nanoparticles (SeNPs) as quorum sensing-mediated violacein synthesis inhibitors in C. violaceum. Schaeffer et al. (2019) adopted a sustainable approach based on thermo-responsive system that combined sodium dodecyl sulfate surfactant to tetrabutylammonium chloride salt to enable solid–liquid violacein extraction from bio-engineered Yarrowia lipolytica yeast. This procedure was followed by 1:1 menthol:thymol hydrophobic eutectic solvent (HES) for violacein back-extraction, which also resulted in contaminant protein precipitation at the solvent interface. This thermo-responsive aqueous surfactant-based system is a reasonable alternative for the extraction of highly-hydrophobic value-added products.
Venil et al. (2015) have proposed the technique of new commercial violacein powder for application as pigment in jelly and yogurt. Products added with C. violaceum violet pigment remained stable for one storage month; most importantly, they achieved stability for 30 days at pH 7, and temperature ranging from 25 to 60 °C.
Conclusions
Violacein is a multi-task compound used in food and cosmetics; however, given its potent anticancer, antiprotozoal and antimicrobial effects, it has huge potential to be deeply explored as potent drug, mainly if its side effects are mitigated and different methods focused on large-scale production are developed. In addition, further investigation is necessary to identify new violacein applications. For instance, Pauer et al. (2018) have recently reported violacein impact on gut microbiota. Treatment with low and high violacein doses has differentially shifted the composition of bacterial communities, a fact that may benefit the hosts, such as the ones affected by syndromes associated with inflammatory diseases. Better understanding violacein activities and its effects on healthy microbioma will certainly provide the basis to the development of new therapeutic strategies. Another important research line focuses on improving violacein production. There is a vast literature on violacein production by using bacteria, recombinant DNA technology, yeast or even on its production in situ, which may be extremely useful to help controlling violacein load, as well as to direct its effects towards target cells. Other hot topics associated with violacein research comprise investigations on guided drug delivery, due to differential uptake by cancer cells, as well as on potentiating violacein effects through synergistic actions with other drugs, such as antibiotics. This biopigment is one of the promising agents used to combat multidrug-resistant pathogens, and it opens significant room to treat emerging diseases capable of overcoming traditional therapy. It is also worth mentioning the great advances in the knowledge about violacein mechanisms of action, mainly about its interaction with membranes to induce cell death. Furthermore, according to Choi et al. (2020), violacein is secreted within membrane vesicles of bacteria capable of producing it; these violacein-carrying vesicles show effective bactericidal activity. Finally, secondary metabolites may also play complex roles in microbial communities. Lozano et al. (2020) have recently reported violacein production by C. violaceum in response to sublethal hygromycin A concentrations deriving from Streptomyces sp., in a mechanism dependent on translational polypeptide elongation inhibition and on a previously unknown two-component regulatory system. Studies in this field not only bring insights into the dynamics of soil communities, but they also shed light on undesired effects of clinical antibiotic administration. However, there is yet much to be discovered for such great applications; thus, it is necessary performing extensive research about violacein effects to avoid cumulative and undesired reactions in humans and in the environment.
Data availability
Not applicable.
Code availability
Not applicable.
References
Agematu H, Suzuki K, Tsuya H (2011) Massilia sp. BS-1, a novel violacein-producing bacterium isolated from soil. Biosci Biotechnol Biochem 75(10):2008–2010. https://doi.org/10.1271/bbb.100729
Ahmad WA, Yusof NZ, Nordin N, Zakaria ZA, Rezali MF (2012) Production and characterization of violacein by locally isolated Chromobacterium violaceum grown in agricultural wastes. Appl Biochem Biotechnol 167(5):1220–1234. https://doi.org/10.1007/s12010-012-9553-7
Alem D, Marizcurrena JJ, Saravia V, Davyt D, Martinez-Lopez W, Castro-Sowinski S (2020) Production and antiproliferative effect of violacein, a purple pigment produced by an Antarctic bacterial isolate. World J Microbiol Biotechnol 36:120. https://doi.org/10.1007/s11274-020-02893-4
Alshatwi AA, Subash-Babu P, Antonisamy P (2016) Violacein induces apoptosis in human breast cancer cells through up regulation of BAX, p53 and down regulation of MDM2. Exp Toxicol Pathol 68:89–97. https://doi.org/10.1016/j.etp.2015.10.002
Antonisamy P, Kannan P, Aravinthan A, Duraipandiyan V, Arasu MV, Ignacimuthu S, Al-Dhabi NA, Kim JH (2014) Gastroprotective activity of violacein isolated from Chromobacterium violaceum on indomethacin-induced gastric lesions in rats: investigation of potential mechanisms of action. Sci World J. https://doi.org/10.1155/2014/616432
Aranda S, Montes-Borrego M, Landa BB (2011) Purple-pigmented violacein-producing Duganella spp. inhabits the rhizosphere of wild and cultivated olives in Southern Spain. Microb Ecol 62:446–459. https://doi.org/10.1007/s00248-011-9840-9
Arif S, Batool A, Khalid N, Ahmed I, Janjua HA (2017) Comparative analysis of stability and biological activities of violacein and starch capped silver nanoparticles. RSC Adv 7:4468–4478. https://doi.org/10.1039/C6RA25806A
Aruldass CA, Rubiyatno VCK, Ahmad WA (2015) Violet pigment production from liquid pineapple waste by Chromobacterium violaceum UTM5 and evaluation of its bioactivity. RSC Adv 5:51524–51536. https://doi.org/10.1039/C5RA05765E
Aruldass CA, Masalamany SRL, Venil CK, Ahmad WA (2018) Antibacterial mode of action of violacein from Chromobacterium violaceum UTM5 against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus (MRSA). Environ Sci Pollut Res 25:5164–5180. https://doi.org/10.1007/s11356-017-8855-2
Atalah J, Blamey L, Munoz-Ibacache S, Gutierrez F, Urzua M, Encinas MV, Páez M, Sun J, Blamey JM (2020) Isolation and characterization of violacein from an Antarctic Iodobacter: a non-pathogenic psychrotolerant microorganism. Extremophiles 24:43–52. https://doi.org/10.1007/s00792-019-01111-w
Avguštin JA, Bertok DZ, Kostanjšek R, Avguštin G (2013) Isolation and characterization of a novel violacein-like pigment producing psychrotrophic bacterial species Janthinobacterium svalbardensis sp. nov”, Antonie van Leeuwenhoek. Int J Gen Mol Microbiol 103:763–769. https://doi.org/10.1007/s10482-012-9858-0
Aye AM, Bonnin-Jusserand M, Brian-Jaisson F, Ortalo-Magne A, Culioli G, Nevry RK, Rabah N, Blache Y, Molmeret M (2015) Modulation of violacein production and phenotypes associated with biofilm by exogenous quorum sensing N-acylhomoserine lactones in the marine bacterium Pseudoalteromonas ulvae TC14. Microbiology 161:2039–2052. https://doi.org/10.1099/mic.0.000147
Bahia AC, Dong Y, Blumberg BJ, Mlambo G, Tripathi A, Ben Marzouk-Hidalgo OJ, Chandra R, Dimopoulos G (2014) Exploring anopheles gut bacteria for plasmodium blocking activity. Environ Microbiol 16:2980–2994. https://doi.org/10.1111/1462-2920.12381
Ballestriero F, Daim M, Penesyan A, Nappi J, Schleheck D, Bazzicalupo P, Di Schiavi E, Egan S (2014) Antinematode activity of violacein and the role of the insulin/IGF-1 pathway in controlling violacein sensitivity in Caenorhabditis elegans. PLoS ONE 8:e109201. https://doi.org/10.1371/journal.pone.0109201
Ballestriero F, Nappi J, Zampi G, Bazzicalupo P, Di Schiavi E, Egan S (2016) Caenorhabditis elegans employs innate and learned aversion in response to bacterial toxic metabolites tambjamine and violacein. Sci Rep 6:29284. https://doi.org/10.1038/srep29284
Barreto ES, Torres AR, Barreto MR, Vasconcelos ATR, Astolfi-Filho S, Hungria M (2008) Diversity in antifungal activity of strains of Chromobacterium violaceum from the Brazilian Amazon. J Ind Microbiol Biotechnol 35:783–790. https://doi.org/10.1007/s10295-008-0331-z
Batista AHM, Moreira ACD, de Carvalho RM, Sales GWP, Nogueira PCN, Grangeiro TB, Medeiros SC, Silveira ER, Nogueira NAP (2017) Antimicrobial effects of violacein against planktonic cells and biofilms of Staphylococcus aureus. Molecules 22:1534. https://doi.org/10.3390/molecules22101534
Batista JH, Leal FC, Fukuda TTH, Alcoforado Diniz J, Almeida F, Pupo MT, da Silva Neto JF (2020) Interplay between two quorum sensing-regulated pathways, violacein biosynthesis and VacJ/Yrb, dictates outer membrane vesicle biogenesis in Chromobacterium violaceum. Environ Microbiol 22(6):2432–2442. https://doi.org/10.1111/1462-2920.15033
Berti IG, Rodenak-Kladniew B, Perez AA, Santiago L, Durán N, Castro RG (2019) Development of biocarrier for violacein controlled release in the treatment of cancer. React Funct Polym 136:122–130. https://doi.org/10.1016/j.reactfunctpolym.2019.01.001
Berti IR, Rodenak-Kladniew BR, Onaindia C, Adam CG, Islan GA, Durán N, Castro GR (2020) Assessment of in vitro cytotoxicity of imidazole ionic liquids and inclusion in targeted drug carriers containing violacein. RSC Adv 10:29336–29346. https://doi.org/10.1039/d0ra05101b
Bettina AM, Doing G, O’Brien K, Perron GG, Jude BA (2018) Draft genome sequences of phenotypically distinct Janthinobacterium sp. isolates cultured from the Hudson Valley Watershed. Genome Announc 6:e01426-e1517. https://doi.org/10.1128/genomeA.01426-17
Bilsland E, Tavella TA, Krogh R, Stokes JE, Roberts A, Ajioka J, David R, Spring DR, Andricopulo AD, Costa FTM, Oliver SG (2018) Antiplasmodial and trypanocidal activity of violacein and deoxyviolacein produced from synthetic operons. BMC Biotechnol 18:22. https://doi.org/10.1186/s12896-018-0428-z
Blackburn MB, Farrar RR Jr, Sparks ME, Kuhar D, Mitchell A, Gundersen-Rindal DE (2017) Chromobacterium sphagni sp. nov., an insecticidal bacterium isolated from Sphagnum bogs. Int J Syst Evol Microbiol 67:3417–3422. https://doi.org/10.1099/ijsem.0.002127
Blackburn MB, Gundersen-Rindal DE, Farrar RR, Kuhar DJ, Mitchell AD (2018) Chromobacterium species with insecticidal activity. US Patent 0103646 A1
Blount BA, Gowers G-OF, Ho JCH, Ledesma-Amaro R, Jovicevic D, McKiernan RM, Xie ZX, Li BZ, Yuan YJ, Ellis T (2018) Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome. Nat Commun 9:1932. https://doi.org/10.1038/s41467-018-03143-w
Boisbaudran LD (1882) Matiere colarante se formant dans la colle de farine. C R Acad Biol 94:562–563
Brazilian National Genome Project Consortium (2003) The complete genome sequence of Chromobacterium violaceum reveals remarkable and exploitable bacterial adaptability. Proc Natl Acad Sci USA 100:11660–11665. https://doi.org/10.1073/pnas.1832124100
Bromberg N, Dreyfuss JL, Regatieri CV, Palladino MV, Durán N, Nader HB, Haun M, Justo GZ (2010) Growth inhibition and pro-apoptotic activity of violacein in Ehrlich ascites tumor. Chem Biol Interact 186:43–52. https://doi.org/10.1016/j.cbi.2010.04.016
Brucker RM, Baylor CM, Walters RL, Lauer A, Harris RN, Minbiole KPC (2008a) The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J Chem Ecol 34:39–43. https://doi.org/10.1007/s10886-007-9352-8
Brucker RM, Harris RN, Schwantes CR, Gallaher TN, Flaherty DC, Lam BA, Minbiole KPC (2008b) Amphibian chemical defense: antifungal metabolites of the micro-symbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J Chem Ecol 34:1422–1429. https://doi.org/10.1007/s10886-008-9555-7
Canuto JA, Lima DB, de Menezes R, Batista AHM, Nogueira P, Silveira ER, Grangeiro TB, Nogueira NAP, Martins AMC (2019) Antichagasic effect of violacein from Chromobacterium violaceum. J Appl Microbiol 127:1373–1380. https://doi.org/10.1111/jam.14391
Cardozo VF, Oliveira AG, Nishio EK, Perugini MRE, Andrade CGTJ, Silveira WD, Durán N, Andrade G, Kobayashi RKT, Nakazato G (2013) Antibacterial activity of extracellular compounds produced by a Pseudomonas strain against methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann Clin Microbiol Antimicrob 12:12. https://doi.org/10.1186/1476-0711-12-12
Cauz ACG, Carretero GPB, Saraiva GKV, Park P, Mortara L, Cuccovia IM, Brocchi M, Gueiros-Filho FJ (2019) Violacein targets the cytoplasmic membrane of bacteria. ACS Infect Dis 5(4):539–549. https://doi.org/10.1021/acsinfecdis.8b00245
Cazotto LL, Martins D, Ribeiro MG, Durán N, Nakazato G (2011) Antibacterial activity of violacein against Staphylococcus aureus from bovine mastitis. J Antibiotics 64:395–397. https://doi.org/10.1038/ja.2011.13
Choi SY, Kim S, Lyuck S, Kim SB, Mitchell RJ (2015a) High-level production of violacein by the newly isolated Duganella violaceinigra str. NI28 and its impact on Staphylococcus aureus. Sci Rep 5:15598. https://doi.org/10.1038/srep15598
Choi SY, Yoon K-H, Lee JI, Mitchell RJ (2015b) Violacein: properties and production of a versatile bacterial pigment. BioMed Res Int 105:465056. https://doi.org/10.1155/2015/465056
Choi SY, Im H, Mitchell RJ (2017) Violacein and bacterial predation: promising alternatives for priority multidrug resistant human pathogens. Future Microbiol 12:835–838. https://doi.org/10.2217/fmb-2017-0090
Choi SY, Lim S, Cho G, Kwon J, Mun W, Im H, Mitchell RJ (2020) Chromobacterium violaceum delivers violacein, a hydrophobic antibiotic, to other microbes in membrane vesicles. Environ Microbiol 20:705–713. https://doi.org/10.1111/1462-2920.14888
Choi SY, Lim S, Yoon KH, Lee JI, Mitchell RJ (2021) Biotechnological activities and applications of bacterial pigments violacein and prodigiosin. J Biol Eng 15(1):10. https://doi.org/10.1186/s13036-021-00262-9
Chuang J, Boeke JD, Mitchell LA (2018) Coupling yeast golden gate and VEGAS for efficient assembly of the violacein pathway in Saccharomyces cerevisiae. In: Jensen MK, Keasling JD (eds) Synthetic metabolic pathways: methods and protocols, methods in molecular biology. Springer, Berlin, pp 211–295
Corazzari M, Fimia GM, Lovat P, Piacentini M (2013) Why is autophagy important for melanoma? Molecular mechanisms and therapeutic implications. Semin Cancer Biol 23:337–343. https://doi.org/10.1016/j.semcancer.2013.07.001
Conlon GA, Murray GI (2019) Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol 24:629–640. https://doi.org/10.1002/path.5225
Cress BF, Erkert KA, Barquera B, Koffas MA (2013) Draft genome sequence of Pseudoalteromonas luteoviolacea strain B (ATCC 29581). Genome Announc 1:e0004813. https://doi.org/10.1128/genomeA.00048-13
Dang HT, Yotsumoto K, Enomoto K (2014) Draft genome sequence of violacein-producing marine bacterium Pseudoalteromonas sp. 520P1. Genome Announc 2:e01346-e1414. https://doi.org/10.1128/genomeA.01346-14
de Azevedo MBM, Alderete J, Rodriguez JA, Souza AO, Rettori D, Torsoni MA, Faljoni-Alário A, Haun M, Durán N (2000) Biological activities of violacein: a new antitumoral indole derivative in an inclusion complex with β-cyclodextrin. J Incl Phenom Macrocycl Chem 37:93–101. https://doi.org/10.1023/A:1008138807481
de Carvalho DD, Costa FT, Durán N, Haun M (2006) Cytotoxic activity of violacein in human colon cancer cells. Toxicol in Vitro 20:1514–1521. https://doi.org/10.1016/j.tiv.2006.06.007
de Souza KD, Perez KR, Durán N, Justo GZ, Caseli L (2017) Interaction of violacein in models for cellular membranes: regulation of the interaction by the lipid composition at the air-water interface. Colloids Surf b: Biointerf 160:247–253. https://doi.org/10.1016/j.colsurfb.2017.09.027
DeMoss RD, Evans NR (1959) Physiological aspects of violacein biosynthesis in nonproliferating cells. J Bacteriol 78(4):583–588
DeMoss RD, Evans NR (1960) Incorporation of C14-labeled substrates into violacein. J Bacteriol 79(5):729–733
Devescovi G, Kojic M, Covaceuszach S, Cámara M, Williams P, Bertani I, Subramoni S, Venturi V (2017) Negative regulation of violacein biosynthesis in Chromobacterium violaceum. Front Microbiol 8:349. https://doi.org/10.3389/fmicb.2017.00349
Dodou HV, de Morais Batista AH, Sales GWP, de Medeiros SC, Rodrigues ML, Nogueira PCN, Silveira ER, Nogueira NAP (2017) Violacein antimicrobial activity on Staphylococcus epidermidis and synergistic effect on commercially available antibiotics. J Appl Microbiol 123:853–860. https://doi.org/10.1111/jam.13547
Dodou HV, Batista AHM, Medeiros SC, Sales GWP, Rodrigues ML, Pereira PIO, Nogueira PCN, Silveira ER, Grangeiro TB, Nogueira NAP (2019) Violacein antimicrobial activity on Staphylococcus epidermidis biofilm. Nat Prod Res 13:1–4. https://doi.org/10.1080/14786419.2019.1569654
Doing G, Perron GG, Jude BA (2018) Draft genome sequence of a violacein-producing Iodobacter sp. from the Hudson Valley Watershed. Genome Announc 6:e01428-e1517. https://doi.org/10.1128/genomeA.01428-17
Dong X, Song YN, Liu WG, Guo XL (2009) MMP-9, a potential target for cerebral ischemic treatment. Curr Neuropharmacol 7:269–275. https://doi.org/10.2174/157015909790031157
Durán N, Justo GZ, Ferreira CV, Melo PS, Cordi L, Martins D (2007) Violacein: properties and biological activities. Biotechnol Appl Biochem 48:127–133. https://doi.org/10.1042/BA20070115
Durán M, Ponezi AN, Faljoni-Alario A, Teixeira MFS, Justo GZ, Durán N (2012) Potential applications of violacein: a microbial pigment. Med Chem Res 21:1524–1532. https://doi.org/10.1007/s00044-011-9654-9
Durán N, Justo GZ, Durán M, Brocchi M, Cordi L, Tasic L, Castro GR, Nakazato G (2016) Advances in Chromobacterium violaceum and properties of violacein, its main secondary metabolite: a review. Biotechnol Adv 34:1030–1045. https://doi.org/10.1016/j.biotechadv.2016.06.003
Durán N, Fávaro WJ, Brocchi M, Justo GZ, Castro GR, Durán M, Nakazato G (2020) Patents on violacein: a compound with great diversity of biological activities and industrial potential. Recent Pat Biotechnol. https://doi.org/10.2174/2213476X07666201221111655
Fakhr FA, Khanafari A, Baserisalehi M, Yaghoobi RF, Shahghasempour S (2012) An investigation of antileukemia activity of violacein loaded dendrimer in Jurkat cell lines. Afr J Microbiol Res 6:6235–6242. https://doi.org/10.5897/AJMR11.741
Fang M-Y, Zhang C, Yang S, Cui J-Y, Jiang P-X, Lou K, Wachi M, Xing X-H (2015) High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microb Cell Fact 14:8. https://doi.org/10.1186/s12934-015-0192-x
Fang M, Wang T, Zhang C, Bai J, Zheng X, Zhao X, Lou K, Xing X-H (2016) Intermediate-sensor assisted push-pull strategy and its application in heterologous deoxyviolacein production in Escherichia coli. Metab Eng 33:41–51. https://doi.org/10.1016/j.ymben.2015.10.006
Farrar RR, Gundersen-Rindal DE, Kuhar D, Blackburn MB (2018) Insecticidal activity of Chromobacterium vaccinii. J Entomol Sci 53:339–346. https://doi.org/10.18474/JES17-108.1
Ferreira CV, Bos CL, Versteeg HH, Justo GZ, Durán N, Peppelenbosch MP (2004) Molecular mechanism of violacein-mediated human leukemia cell death. Blood 104:1459–1464. https://doi.org/10.1182/blood-2004-02-0594
Füller JJ, Röpke R, Krausze J, Rennhack KE, Daniel N, Blankenfeldt W, Schulz S, Jahn D, Moser J (2016) Biosynthesis of violacein: structure and function of L-tryptophan oxidase VioA from Chromobacterium violaceum. J Biol Chem 291:20068–20084. https://doi.org/10.1074/jbc.M116.741561
Gao A, Chen H, Hou A, Xie K (2019) Efficient antimicrobial silk composites using synergistic effects of violacein and silver nanoparticles. Mater Sci Eng C Mater Biol Appl 103:109821. https://doi.org/10.1016/j.msec.2019.109821
Gomez-Gomez B, Arregui L, Serrano S, Santos A, Perez-Corona T, Madrid Y (2019) Selenium and tellurium-based nanoparticles as interfering factors in quorum sensing-regulated processes: violacein production and bacterial biofilm formation. Metallomics 11:1104–1114. https://doi.org/10.1039/C9MT00044E
Gonçalves PR, Rocha-Brito KJP, Fernandes MRN, Abrantes JL, Durán N, Ferreira-Halder CV (2016) Violacein induces death of RAS-mutated metastatic melanoma by impairing autophagy process. Tumor Biol 37:14049–14058. https://doi.org/10.1007/s13277-016-5265-x
Gupta R, Mitra S, Chowdhury S, Das G, Priyadarshini R, Mukhopadhyay MK, Ghosh SK (2021) Discerning perturbed assembly of lipids in a model membrane in presence of violacein. Biochim Biophys Acta Biomembr 1863:183647. https://doi.org/10.1016/j.bbamem.2021.183647
Hakvåg S, Fjaervik E, Klinkenberg G, Borgos SE, Josefsen KD, Ellingsen TE, Zotchev SB (2009) Violacein-producing Collimonas sp. from the sea surface microlayer of costal waters in Trøndelag. Norway Mar Drugs 7:576–588. https://doi.org/10.3390/md7040576
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT, Minbiole KPC (2009) Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3:818–824. https://doi.org/10.1038/ismej.2009.27
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129. https://doi.org/10.1038/nrd4510
Hashimi SM, Xu T, Wei MQ (2015) Violacein anticancer activity is enhanced under hypoxia. Oncol Rep 33:1731–1736. https://doi.org/10.3892/or.2015.3781
Hidachi K, Mitsuishi S, Ueno M, Toda K, Yuki H, Takiguchi Y, Sasaki O, Tomiyama M, Kawakami R, Sakai A, Sakurai T, Yasukawa T, Abe J, Ogata H (2017) Tyrosinase activity inhibitor. Japan Patent 2017210451
Hoshino T (2011) Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: biosynthetic mechanism and pathway for construction of violacein core. Appl Microbiol Biotechnol 91:1463–1475. https://doi.org/10.1007/s00253-011-3468-z
Im H, Choi SY, Son S, Mitchell RJ (2017) Combined application of bacterial predation and violacein to kill polymicrobial pathogenic communities. Sci Rep 7:14415. https://doi.org/10.1038/s41598-017-14567-7
Immanuel SRC, Banerjee D, Rajankar MP, Raghunathan A (2018) Integrated constraints based analysis of an engineered violacein pathway in Escherichia coli. Biosystems 171:10–19. https://doi.org/10.1186/s12918-017-0427-z
Islan GA, Durán M, Cacicedo ML, Nakazato G, Kobayashi RKT, Martinez DST, Castro GR, Durán N (2017) Nanopharmaceuticals as a solution to neglected diseases: is it possible? Acta Trop 170:16–42. https://doi.org/10.1016/j.actatropica.2017.02.019
Jones JA, Vernacchio VR, Lachance DM, Lebovich M, Fu L, Shirke AN, Schultz VL, Cress B, Linhardt RJ, Koffas MAG (2015) ePathOptimize: a combinatorial approach for transcriptional balancing of metabolic pathways. Sci Rep 5:11301. https://doi.org/10.1038/srep11301
Jude BA (2019) Draft genome sequence of a Chitinimonas species from Hudson Valley waterways that expresses violacein pigment. Microbiol Resour Announc 8:e00683-e719. https://doi.org/10.1128/MRA.00683-19
Justo GZ, Durán N (2017) Action and function of Chromobacterium violaceum in health and disease: violacein as a promising metabolite to counteract gastroenterological disease. Best Pract Res Clin Gastroenterol 31:649–656. https://doi.org/10.1016/j.bpg.2017.10.002
Kallmayer V, Lanzendoerfer G, Meiring U, Mocigemba N, Reidel H, Schaefer J, Viala S (2005) Cosmetic preparation, useful e.g. for the protection of skin and (semi)mucous membrane against bacteria and/or virus, comprises violacein dye in combination with lipophilic and/or hydrophilic substances. Germany Patent DE102005051869 A1
Kanelli M, Mandic M, Kalakona M, Vasilakos S, Kekos D, Nikodinovic-Runic J, Topakas E (2018) Microbial production of violacein and process optimization for dyeing polyamide fabrics with acquired antimicrobial properties. Front Microbiol 9:1495. https://doi.org/10.3389/fmicb.2018.01495
Kato K, Yasui T, Akira S, Tsukamoto T, Qin H, Atsushi K (1998) Bluish purple pigment produced by bacterium and its use as dye or coloring additive. Japan Patent 10113169
Kodach LL, Bos CL, Durán N, Peppelenbosch MP, Ferreira CV, Hardwick JCH (2006) Violacein synergistically increases 5-fluorouracil cytotoxicity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogenesis 27:508–516. https://doi.org/10.1093/carcin/bgi307
Kothari V, Sharma S, Padia D (2017) Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Med 10:744–752. https://doi.org/10.1016/j.apjtm.2017.07.022
Kuzyk SB, Pritchard AO, Plouffe J, Sorensen JL, Yurkov V (2020) Psychrotrophic violacein-producing bacteria isolated from Lake Winnipeg, Canada. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2020.04.008
Lakhan SE, Kirchgessner A, Tepper D, Leonard A (2013) Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 4:32. https://doi.org/10.3389/fneur.2013.00032
Lamendella R, Jude BA (2018) Draft genome sequences of violacein-producing Duganella sp. isolates from a waterway in eastern Pennsylvania. Microbiol Resour Announc 7:e01196-e1218. https://doi.org/10.1128/MRA.01196-18
Leal AMS, de Queiroz JDF, de Medeiros SRB, Lima TKS, Agnez-Lima LF (2015) Violacein induces cell death by triggering mitochondrial membrane hyperpolarization in vitro. BMC Microbiol 15:115. https://doi.org/10.1186/s12866-015-0452-2
Lee JK, Kwak HJ, Piao MS, Jang JW, Kim SH, Kim HS (2010) Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir (wien) 153:1321–1329. https://doi.org/10.1007/s00701-010-0889-x
Lee ME, Aswani A, Han AS, Tomlin CJ, Dueber JE (2013) Expression-level optimization of a multi-enzyme pathway in the absence of a high-throughput assay. Nucleic Acids Res 41:10668–10678. https://doi.org/10.1093/nar/gkt809
Lee YJ, Bashyal P, Pandey RP, Sohng JK (2019) Enzymatic and microbial biosynthesis of novel violacein glycosides with enhanced water solubility and improved anti-nematode activity. Biotechnol Bioproc Eng 24(2):366–374. https://doi.org/10.1007/s12257-018-0466-3
Leon LL, Miranda CC, De Souza AO, Durán N (2001) Antileishmanial activity of the violacein extracted from Chromobacterium violaceum. J Antimicrob Chemother 48:449–450. https://doi.org/10.1093/jac/48.3.449
Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, Lu X, Barón AE, Franklin WA, Merrick DT, Jedlicka P, DeRyckere D, Heasley LE, Graham DK (2013) Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene 32:3420–3431. https://doi.org/10.1038/onc.2012.355
Liu L, Lu J, Wang Y, Pang XY, Xu M, Zhang SW, Lu JP (2017) Antitumor effect of violacein against HT29 by comparative proteomics. Sci Agric Sinica 50:1604–1704. https://doi.org/10.3864/j.issn.0578-1752.2017.09.015
Lopes SCP, Blanco YC, Justo GZ, Nogueira PA, Rodrigues FLS, Goelnitz U, Wunderlich G, Facchini G, Brocchi M, Durán N, Costa FTM (2009) Violacein extracted from Chromobacterium violaceum inhibits plasmodium growth in vitro and in vivo. Antimicrob Agents Chemother 53:2149–2152. https://doi.org/10.1128/AAC.00693-08
Lozano GL, Guan C, Cao Y, Borlee BR, Broderick NA, Stabb EV, Handelsman J (2020) A chemical counterpunch: Chromobacterium violaceum ATCC 31532 produces violacein in response to translation-inhibiting antibiotics. Mbio 11(3):e00948-e01020. https://doi.org/10.1128/mBio.00948-20
Martin PA, Soby S (2016) Insecticidal strains of Chromobacterium vaccinii sp. nov. for control of insects. US Patent 9,339,039 B1
Martin PA, Gundersen-Rindal D, Blackburn M, Buyer J (2007) Chromobacterium subtsugae sp. nov., a betaproteobacterium toxic to Colorado potato beetle and other insect pests. Int J Syst Evol Microbiol 57:993–999. https://doi.org/10.1099/ijs.0.64611-0
Masuelli L, Pantanella F, La Regina G, Benvenuto M, Fantini M, Mattera R, Di Stefano E, Mattei M, Silvestri R, Schippa S, Manzari V, Modesti A, Bei R (2016) Violacein, an indole-derived purple-colored natural pigment produced by Janthinobacterium lividum, inhibits the growth of head and neck carcinoma cell lines both in vitro and in vivo. Tumour Biol 37(3):3705–3717. https://doi.org/10.1007/s13277-015-4207-3
Matz C, Deines P, Boeings J, Arndt H, Eberl L, Kjelleberg S, Jürgens K (2004) Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl Environ Microbiol 70:1593–1599. https://doi.org/10.1128/aem.70.3.1593-1599.2004
Matz C, Webb JS, Schupp PJ, Phang SY, Penesyan A, Egan S, Peter Steinberg P, Kjelleberg S (2008) Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS ONE 3:e2744. https://doi.org/10.1371/journal.pone.0002744
Melo PS, Justo GZ, de Azevedo MB, Durán N, Haun M (2003) Violacein and its beta-cyclodextrin complexes induce apoptosis and differentiation in HL60 cells. Toxicology 186:217–225. https://doi.org/10.1016/s0300-483x(02)00751-5
Mojib N, Nasti TH, Andersen DT, Attigada VR, Hoover RB, Yusuf N, Bej AK (2011) The antiproliferative function of violacein-like purple violet pigment (PVP) from an Antarctic Janthinobacterium sp. Ant5-2 in UV-induced 2237 fibrosarcoma. Int J Dermatol 5:1223–1233. https://doi.org/10.1111/j.1365-4632.2010.04825.x
Myeong NR, Seong HJ, Kim HJ, Sul WJ (2016) Complete genome sequence of antibiotic and anticancer agent violacein producing Massilia sp. strain NR 4–1. J Biotechnol 223:36–37. https://doi.org/10.1016/j.jbiotec.2016.02.027
Nakazato G, Gonçalves MC, das Neves MS, Kobayashi RKT, Brocchi M, Durán N (2019) Violacein@Biogenic Ag system: synergistic antibacterial activity against Staphylococcus aureus. Biotechnol Lett 41:1433–1437. https://doi.org/10.1007/s10529-019-02745-8
Nathwani D, Eckmann C, Lawson W, Solem CT, Corman S, Stephens JM, Macahilig C, Simoneau D, Chambers R, Li JZ, Haider S (2014) Influence of real-world characteristics on outcomes for patients with methicillin-resistant Staphylococcal skin and soft tissue infections: a multi-country medical chart review in Europe. BMC Infect Dis 14:476. https://doi.org/10.1186/1471-2334-14-476
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661. https://doi.org/10.1021/acs.jnatprod.5b01055
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Newman DJ, Cragg GM, Snader KM (2000) The influence of natural products upon drug discovery. Nat Prod Rep 17:215–234. https://doi.org/10.1039/a902202c
Numan M, Bashir S, Mumtaz R, Tayyab S, Rehman NU, Khan AL, Shinwari ZK, Al-Harrasi A (2018) Therapeutic applications of bacterial pigments: a review of current status and future opportunities. 3 Biotech 8:207. https://doi.org/10.1007/s13205-018-1227-x
Pandey AK, Verma S, Bhattacharya P, Paul S, Mishra A, Patnaik R (2012) An in-silico strategy to explore neuroprotection by quercetin in cerebral ischemia: a novel hypothesis based on inhibition of matrix metalloproteinase (MMPs) and acid sensing ion channel 1a (ASIC1a). Med Hypotheses 79:76–81. https://doi.org/10.1016/j.mehy.2012.04.005
Pandey AK, Bhattacharya P, Shukla SC, Paul S, Patnaik R (2015) Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection. Neural Regen Res 10:568–575. https://doi.org/10.4103/1673-5374.155429
Park HA, Park SA, Yang Y-H, Choi K-Y (2021) Microbial synthesis of violacein pigment and its potential applications. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2021.1892579
Pauer H, Hardoim CCP, Teixeira FL, Miranda KR, Barbirato DS, Pires de Carvalho DP, Antunes LCM, Leitão AAC, Lobo LA, Domingues RMCP (2018) Impact of violacein from Chromobacterium violaceum on the mammalian gut microbiome. PLoS ONE 13:e0203748. https://doi.org/10.1371/journal.pone.0203748
Platt D, Amara S, Mehta T, Vercuyssee K, Myles EL, Johnson T, Tiriveedhi V (2014) Violacein inhibits matrix metalloproteinase mediated CXCR4 expression: potential anti-tumor effect in cancer invasion and metastasis. Biochem Biophys Res Commun 455:107–112. https://doi.org/10.1016/j.bbrc.2014.10.124
Priya D, Kannan SRS, Thanga MK (2018) Production of violacein pigment from Chromobacterium violaceum and its antibacterial activity and synergism on E. coli isolated from UTI samples. Int J Recent Sci Res 9:24479–24484. https://doi.org/10.24327/ijrsr.2018.0902.1669
Puranik S, Talkal R, Qureshi A, Khardenavis A, Kapley A, Purohit HJ (2013) Sequence of the pigment-producing bacterium Pseudogulbenkiania ferrooxidans, isolated from Loktak Lake. Genome Announc 1:e01115-e1213. https://doi.org/10.1128/genomeA.01115-13
Queiroz KC, Milani R, Ruela-de-Sousa RR, Fuhler GM, Justo GZ, Zambuzzi WF, Duran N, Diks SH, Spek CA, Ferreira CV, Peppelenbosch MP (2012) Violacein induces death of resistant leukaemia cells via kinome reprogramming, endoplasmic reticulum stress and Golgi apparatus collapse. PLoS ONE 7:e45362. https://doi.org/10.1371/journal.pone.0045362
Rahul S, Chandrashekhar P, Hemant B, Bipinchandra S, Mouray E, Grellier P, Patil S (2015) In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol Int 64:353–356. https://doi.org/10.1016/j.parint.2015.05.004
Ramirez JL, Short SM, Bahia AC, Saraiva RG, Dong Y, Kang S, Tripathi A, Mlambo G, Dimopoulos G (2014) Chromobacterium Csp_P reduces malaria and dengue infection in vector mosquitoes and has entomopathogenic and in vitro anti-pathogen activities. PLoS Patholog 10:e1004398. https://doi.org/10.1371/journal.ppat.1004398
Ran T, Gao M, Wei Q, He J, Tang L, Wang W, Xu D (2015) Expression, crystallization and preliminary crystallographic data analysis of VioD, a hydroxylase in the violacein-biosynthesis pathway. Acta Crystal Sect F Struct Biol Commun 71:149–152. https://doi.org/10.1107/s2053230x14027617
Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC (1998) Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29:1020–1030. https://doi.org/10.1161/01.str.29.5.1020
Rongzhen X, Yuwen W (2017) Use of natural pigment as nail polish pigment enabling to develop a healthy and environmentally friendly nail polish pigment. Taiwan Patent TW I570191
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787. https://doi.org/10.1016/j.cell.2008.05.009
Santos AB, Costa PS, do Carmo AO, da Rocha Fernandes G, Scholte LLS, Ruiz J, Kalapothakis E, Chartone-Souza E, Nascimento AMA (2018) Insights into the genome sequence of Chromobacterium amazonense isolated from a tropical freshwater lake. Int J Genomics. https://doi.org/10.1155/2018/1062716
Sarkar J, Nandy SK, Chowdhury A, Chakrabor T, Chakrabor S (2016) Inhibition of MMP-9 by green tea catechins and prediction of their interaction by molecular docking analysis. Biomed Pharmacother 84:340–347. https://doi.org/10.1016/j.biopha.2016.09.049
Sarmiento JJP, Cardozo VF, Durán N, Brocchi M, Kobayashi RKT, Nakazato G (2016) Composição contendo nanopartículas de prata biológica e um pigmento produzido por Chromobacterium violaceum com atividade antibacteriana. Brazilian Patent PIBR-10 003373 0
Sasidharan A, Sasidharan NK, Amma DBNS, Vasu RK, Nataraja AV, Bhaskaran K (2015) Antifungal activity of violacein purified from a novel strain of Chromobacterium sp. NIIST (MTCC 5522). J Microbiol 53:694–701. https://doi.org/10.1007/s12275-015-5173-6
Satoshi A, Takatoshi N (1998) Production of natural antimicrobial antioxidant and its cosmetic formulation. Japan Patent 10139612
Savage VJ, Chopra I, O’Neill AJ (2013) Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother 57:1968–1970. https://doi.org/10.1128/AAC.02008-12
Schaeffer N, Kholany M, Veloso TLM, Pereira JL, Ventura SPM, Nicaud JM, Coutinho JAP (2019) Temperature-responsive extraction of violacein using a tuneable anionic surfactant-based system. Chem Commun (camb) 55(59):8643–8646. https://doi.org/10.1039/c9cc03831k
Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, Acevedo AA, Burrowes PA, Carvalho T, Catenazzi A, De la Riva I, Fisher MC, Flechas SV, Foster CN, Frías-Álvarez P, Garner TWJ, Gratwicke B, Guayasamin JM, Hirschfeld M, Kolby JE, Kosch TA, La Marca E, Lindenmayer DB, Lips KR, Longo AV, Maneyro R, McDonald CA, Mendelson J 3rd, Palacios-Rodriguez P, Parra-Olea G, Richards-Zawacki CL, Rödel MO, Rovito SM, Soto-Azat C, Toledo LF, Voyles J, Weldon C, Whitfield SM, Wilkinson M, Zamudio KR, Canessa S (2019) Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 363:1459–1463. https://doi.org/10.1126/science.aav0379
Schreiber SL, Nicolaou KC, Davies K (2002) Diversity-oriented organic synthesis and proteomics. New frontiers for chemistry & biology. Chem Biol 9:1–2. https://doi.org/10.1016/s1074-5521(02)00088-1
Smith HJ, Foreman CM, Akiyama T, Franklin MJ, Devitt NP, Ramaraj T (2016) Genome sequence of Janthinobacterium sp. CG23_2, a violacein-producing isolate from an Antarctic supraglacial stream. Genome Announc 4:e01468-e1515. https://doi.org/10.1128/genomeA.01468-15
Subramaniam S, Ravi V, Sivasubramanian A (2014) Synergistic antimicrobial profiling of violacein with commercial antibiotics against pathogenic micro-organisms. Pharm Biol 52:86–90. https://doi.org/10.3109/13880209.2013.815634
Tapia MAD, Herrera JRO, Quiroga CJI (2012) Strain of Cobetia marina and biosurfactant extract obtained from same. European Patent EP2716749
Thøgersen MS, Delpin MW, Melchiorsen J, Kilstrup M, Månsson M, Bunk B, Sproer C, Overmann J, Nielsen KF, Gram L (2016) Production of the Bioactive compounds violacein and indolmycin is conditional in a maeA mutant of Pseudoalteromonas luteoviolacea S4054 lacking the malic enzyme. Front Microbiol 7:1461. https://doi.org/10.3389/fmicb.2016.01461
Thomé R, Moraes AS, Bombeiro AL, Farias A, Francelin C, da Costa TA, Di Gangi R, Santos LMB, de Oliveira ALR, Verinaud L (2013) Chloroquine treatment enhances regulatory T cells and reduces the severity of experimental autoimmune encephalomyelitis. PLoS ONE 8:e65913. https://doi.org/10.1371/journal.pone.0065913
Thomé R, Issayama LK, DiGangi R, Bombeiro AL, da Costa TA, Ferreira IT, de Oliveira ALR, Machado DRS, Verinaud L (2014) Dendritic cells treated with chloroquine modulate experimental autoimmune encephalomyelitis. Immunol Cell Biol 92:124–132. https://doi.org/10.1038/icb.2013.73
Thornton AM, Shevach EM (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188:287–296. https://doi.org/10.1084/jem.188.2.287
Tobie WC (1935) The pigment of Bacillus violaceus. I. The production, extraction, and purification of violacein. J Bacteriol 29(3):223–227
Tong Y, Zhou J, Zhang L, Xu P (2021) A golden-gate based cloning toolkit to build violacein pathway libraries in Yarrowia lipolytica. ACS Synth Biol 10(1):115–124. https://doi.org/10.1021/acssynbio.0c00469
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R (2017) Leishmaniasis: a review. F1000Res 6:750. https://doi.org/10.12688/f1000research.11120.1
Valdes N, Soto P, Cottet L, Alarcon P, Gonzalez A, Castillo A, Corsini G, Tello M (2015) Draft genome sequence of Janthinobacterium lividum strain MTR reveals its mechanism of capnophilic behavior. Stand Genomic Sci 10:110. https://doi.org/10.1186/s40793-015-0104-z
Venegas FA, Kollisch G, Mark K, Diederich WE, Kaufmann A, Bauer S, Max Chavarría M, Juan J, Araya JJ, Alfonso J, García-Piñeres AJ (2019) The bacterial product violacein exerts an immunostimulatory effect via TLR8. Sci Rep 9:13661. https://doi.org/10.1038/s41598-019-50038-x
Venil CK, Aruldass CA, Halim MHA, Khasim AR, Zakaria ZA, Ahmad WA (2015) Spray drying of violet pigment from Chromobacterium violaceum UTM 5 and its application in food model systems. Int Biodeterior Biodegrad 102:324–329. https://doi.org/10.1016/j.ibiod.2015.02.006
Verma S, Pandey AK (2017) An in-silico approach to explore the possible multifunctional neuroprotective efficacy of violacein against ischemic stroke. J Pharmacol 3:17. https://doi.org/10.21767/2469-6692.100017
Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S (2011) Targeting Axl and Mer kinases in cancer. Mol Cancer Ther 10:1763–1773. https://doi.org/10.1158/1535-7163.MCT-11-0116
Verinaud L, Lopes SCP, Prado ICN, Zanucoli F, da Costa TA, Di Gangi R, Issayama LK, Carvalho AC, Bonfanti AP, Niederauer GF, Durán N, Costa FTM, de Oliveira ALR, Thomé R (2015) Violacein treatment modulates acute and chronic inflammation through the suppression of cytokine production and induction of regulatory T cells. PLoS ONE 10:e0125409. https://doi.org/10.1371/journal.pone.0125409
Vöing K, Harrison A, Soby SD (2015) Draft genome sequence of Chromobacterium vaccinii, a potential biocontrol agent against mosquito (Aedes aegypti) larvae. Genome Announc 3:e00477–e00515. https://doi.org/10.1128/genomeA.00477-15
Vöing K, Harrison A, Soby SD (2017) Draft genome sequence of Chromobacterium subtsugae MWU12-2387 isolated from a Wild Cranberry Bog in Truro, Massachusetts. Genome Announc 5:e01633-e1716. https://doi.org/10.1128/genomeA.01633-16
Woodhams DC, LaBumbard BC, Barnhart KL, Becker MH, Bletz MC, Escobar LA, Flechas SV, Forman ME, Iannetta AA, Joyce MD, Rabemananjara F, Gratwicke B, Vences M, Minbiole KPC (2018) Prodigiosin, violacein, and volatile organic compounds Produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb Ecol 75:1049–1062. https://doi.org/10.1007/s00248-017-1095-7
World Health Organization (2017) World malaria report 2017. pp 197. https://www.who.int/malaria/publications/world-malaria-report-2017/en/. Accessed 28 Sept 2020
Wu YH, Cheng H, Xu L, Jin XB, Wang CS, Xu XW (2017) Physiological and genomic features of a novel violacein-producing bacterium isolated from surface seawater. PLoS ONE 12(6):e0179997. https://doi.org/10.1371/journal.pone.0179997
Xu X, Tian L, Zhang S, Jiang L, Zhang Z, Huang H (2019) Complete genome sequence of Janthinobacterium sp. B9–8, a violacein-producing bacterium isolated from low-temperature sewage. Microb Pathog 128:178–183. https://doi.org/10.1016/j.micpath.2019.01.003
Yoon KH, Lee TY, Moon JH, Choi SY, Choi YJ, Mitchell RJ, Il Lee J (2020) Consumption of oleic acid during matriphagy in free-living nematodes alleviates the toxic effects of the bacterial metabolite violacein. Sci Rep 10(1):8087. https://doi.org/10.1038/s41598-020-64953-x
Zhou Y, Fang MY, Li G, Zhang C, Xing XH (2018) Enhanced production of crude violacein from glucose in Escherichia coli by overexpression of rate-limiting key enzyme(s) involved in violacein biosynthesis. Appl Biochem Biotechnol 186:909–916. https://doi.org/10.1007/s12010-018-2787-2
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarships and sponsorship of projects that enabled our research and literature access.
Author information
Authors and Affiliations
Contributions
All the authors cooperated in the formal analysis, writing, reviewing and editing the original draft. GZJ and ND cooperated in the conceptualization and final revision of the manuscript. GZJ edited the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durán, N., Nakazato, G., Durán, M. et al. Multi-target drug with potential applications: violacein in the spotlight. World J Microbiol Biotechnol 37, 151 (2021). https://doi.org/10.1007/s11274-021-03120-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03120-4