Abstract
Microbes within an infection impact neighbors’ pathogenicity. This study aimed to address in vitro virulence activity of Pseudomonas aeruginosa under the binary interaction with Acinetobacter baumannii or Enterococcus faecium, co-isolated from two chronic wound infections. The biofilm formation of Pseudomonas was enhanced 1.5- and 1.4-fold when it was simultaneously cultured with Acinetobacter and Enterococcus, respectively. Pseudomonas motility was increased by 1.9- and 1.5-fold (swimming), 3.6- and 1.9-fold (swarming), and 1.5- and 1.5-fold (twitching) in the dual cultures with Acinetobacter and Enterococcus, respectively. The synergistic hemolysis activity of Pseudomonas was observed with the heat-killed Acinetobacter and Enterococcus cells. The minimum inhibitory concentration of ciprofloxacin against Pseudomonas was increased from (μg mL−1) 25 to 400 in the individual and mixed cultures, respectively. The pyocyanin production by Pseudomonas in the single and mixed cultures with Acinetobacter and Enterococcus was (μg/mL) 1.8, 2.3, and 2.9, respectively. The expression of lasI, rhlI, and pqsR genes was up-regulated by 1.0-, 1.9-, and 16.3-fold, and 4.9-, 1.0-, and 9.3-fold when Pseudomonas was incubated with Acinetobacter and Enterococcus, respectively. Considering the entire community instead of a single pathogen may lead to a more effective therapeutic design for persistent infections caused by Pseudomonas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progress in culture-dependent studies, along with the development of culture-independent methods, demonstrates the community composition of many infections and raised the possible impact of species interaction on the progression of diseases (Peters et al 2012; Cummings et al 2020). The term poly-microbial infection is defined as pathological manifestations caused by more than one strain/species. Co-existing microorganisms can affect their neighbors through the mechanisms like direct cell–cell contact, cell–cell communication via diffusible molecules, alteration of the host environment, and the utilization of others’ metabolic by-products (Peleg et al 2010).
Chronic wounds are characterized by the impairment of the normal wound healing process in an orderly and timely manner (Frykberg and Banks 2015). They impose an enormous burden on the healthcare systems, and it is estimated that 2–3% of the healthcare budgets are related to chronic wounds. P. aeruginosa has been identified as a significant pathogen in chronic wound infections. This Gram-negative opportunistic pathogen can cause mild to life-threatening infections. P. aeruginosa often causes chronic infection due to its ability to form biofilms. The presence of microbes within the biofilms protects them from the host immune system, antibiotics, and other stressful conditions (Donlan 2002). The most studied biofilms were known to have a diverse population which means more than one species formed a cosmopolitan community. Therefore, pathogens (Like Pseudomonas) within biofilm may behave differently from single-cell planktonic life (or single sessile) due to the microbial interaction within these three-dimensional structures. Members of a poly-microbial infection can coordinate various social behaviors of neighboring populations, such as pathogenesis, by affecting their quorum sensing systems like Las and Rhl (depend on N-acyl-homo-serine lactone) and PQS (an alkylquinolone (AQ)-dependent quinolone signal system) that regulate much virulence-associated genes of Pseudomonas (Warrier et al 2021; Kostylev et al 2019).
The interactions of Pseudomonas with Staphylococcus aureus showed that poly-microbial infections are more virulent than mono-culture infections with either species (Limoli and Hoffman 2018). However, poly-microbial interactions of Pseudomonas with other bacteria have been less considered. In this study, we assessed the effect of Pseudomonas aeroginosa interaction with the two co-isolated strains from chronic wounds, i.e., Acinetobacter baumannii and E. faecium, on its in vitro virulence activities. We showed that the dual interactions could promote some virulence activity of Pseudomonas, including biofilm formation, hemolysis, motility, pigmentation, and antibiotic resistance. The up-regulations of quorum-sensing genes upon the microbial interaction were also observed.
Materials and methods
Bacterial strains
Pseudomonas aeroginosa/Acinetobacter baumannii and Pseudomonas aeroginosa/Enterococcus faecium were co-isolated from two skin chronic wounds. Ethics approval or waiver has been obtained with their letter number IR.UM.REC.1401.075 dated 2021. Bacterial identifications were confirmed by the conventional biochemical tests and molecular method based on the amplification and sequencing of their 16S rRNA genes.
Single and dual biofilm formation
The ability of Pseudomonas to form biofilm in the presence of Acinetobacter and Enterococcus was evaluated by the competition, displacement, and exclusion methods. All three strains were cultured overnight in Tryptic soy broth (TSB) medium at 37 °C and the standard cell concentrations (106 cells mL−1) were prepared. Regarding the competition assay, 1000 μL of each strain suspension’s (Pseudomonas and Acinetobacter/Enterococcus) was simultaneously added into the wells of 12-well polystyrene plates containing polystyrene disks with the area of 25 mm2 placed in the bottom and incubated for 24 h at 37 °C. For the displacement test, Pseudomonas cells were transferred on 12-well plates for 24 h at 37 °C, the growth medium was replaced by fresh media containing Acinetobacter/Enterococcus suspension (106 CFU/mL). Exclusion assay was achieved when Acinetobacter/Enterococcus suspensions were pre-inoculated, and after 24 h incubation at 37 °C, the medium was aspirated, and fresh medium containing Pseudomonas cells (106 CFU/mL) was added to the wells and kept at 37 °C for further 24 h. Single species biofilms were formed as control by adding Pseudomonas suspension and TSB 1:1 to wells, followed by incubation for 24 h (or 48 h for displacement) at 37 °C. The polystyrene disks were removed from wells and washed three times with PBS. The attached Pseudomonas cells were scraped, and 100 μL aliquots of the desired dilutions were seeded on plates containing TSA medium and incubated for 24 h at 37 °C. The Pseudomonas colony forming units per milliliter (CFU/mL) were determined for the single and dual biofilms.
Motility investigation
Swimming, swarming, and twitching motilities of Pseudomonas were determined in the single and dual cultures. For the swimming and swarming motilities, 5 μL of each fresh bacterial suspension (as mentioned above) was mixed and inoculated directly into the center of semisolid (0.3% W/V agar) and solid (1.0% W/V agar) TSA medium, respectively. The twitching motility was assessed by 10 μL deep inoculation of mixed bacterial suspension into the agar–Petri dish interface. The diameters of the swimming and swarming motility zones were measured after 24 h incubation at 37 °C. To visualize the twitching motility, the agar medium was carefully removed with tweezers, and the plates were stained with 0.1% (V/W) crystal violet (5 min). In the single motility experiments as the control, Pseudomonas and TSB were co-inoculated 1:1. The presence of Pseudomonas in the colonies’ edge was confirmed by the conventional Gram staining method (Cai et al 2020).
Antibiotic susceptibility test
Resistance of Pseudomonas to ciprofloxacin (Sigma-Aldrich, Germany) in the single and dual cultures was compared by the micro-dilution method. For that, TSB media contained single and dual bacterial cells (1:1 of standard fresh bacterial suspensions) were supplemented with different antibiotic concentrations of 0 to 100 ppm. The cultures were incubated at 37 °C for 24 h, and Pseudomonas growth was measured by counting colony-forming units (CFUs).
Hemolysin assay
Hemolysin activity of Pseudomonas singly and in the presence of Acinetobacter/Enterococcus live, dead, and cell-free supernatants (CFS) was investigated as described elsewhere (Sperandio et al 2010). The released hemoglobin in the supernatant was measured at 540 nm using an ELISA reader, and the percentage (%) of total cell lysis was calculated as follows:
The negative and positive controls were prepared by mixing RBC suspension with RPMI and SDS (1% W/V), respectively. The fractional inhibitory concentration (FIC) index defined additive, antagonist, synergism, and indifferent hemolysis activity. The sum of the FICs (ΣFIC) was calculated for all experiments with the below equation:
where FICA was the hemolysis percent of Pseudomonas in combination/ hemolysis percent of Pseudomonas alone, and FICB was the Acinetobacter/Enterococcus live, dead, and CFS hemolysis in combination/ Acinetobacter/Enterococcus live, dead and CFS hemolysis activity alone. FICI was interpreted as synergism, indifferent, additive and antagonism when ≤ 0.5, > 0.5 and ≤ 1, > 1.0 and ≤ 4.0, and > 4.0, respectively (Fadwa et al 2021).
Pigmentation
P. aeruginosa strain singly and mixed with Acinetobacter/Enterococcus was cultured in nutrient broth medium supplemented by glycerol and statically incubated at 37 °C for four days. The pigment was extracted from filtrated supernatant by chloroform following the methods described by DeBritto et al. (2020). The UV–visible spectrum of the pigment was recorded between 200 and 800 nm, and the intensity of pyocyanin concentration (µg ml−1) was calculated according to the following equation:
Electron microscope image
The cell pellets were fixed with 2.5% glutaraldehyde for two hours, serially dehydrated with ethanol, and then coated with gold. The samples were observed under LEO 1450 VP-Zeiss scanning electron microscope (SEM) (Jena, Germany) at the resolution of 2.5 nm and an accelerating voltage of 20 kV.
Gene expression analysis
Quantitative reverse transcriptase PCR (qRT-PCR) was performed against Rhl, LasI, and PQS genes using The ABI 7500 Real-Time PCR system (Applied Biosystems Co, California, USA). The transcripts were normalized to GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) levels (Shi et al 2019).
Statistical analysis
The standard error mean was calculated and shown as error bars on the figures. One-way analysis of variance and t test were performed by SPSS ver. 16 software (IBM Co) to determine the significance of results (p value less than 0.05).
Results
Bacterial strains were identified as P. aeruginosa (GenBank/EMBL/DDBJ accession number ON514172), A. baumannii (ON514174), and E. faecium (ON514173) and confirmed by biochemical tests (data not shown).
Single and dual biofilms of Pseudomonas with either Acinetobacter or Enterococcus
Pseudomonas biofilm was enhanced 1.5- and 1.4-fold when simultaneously cultured with Acinetobacter and Enterococcus, respectively (competition). Pseudomonas biofilm was decreased to 0.51- and 0.37-fold when the pre-formed biofilms were treated by Acinetobacter and Enterococcus strains, respectively (displacement). When Pseudomonas was added to the substratum with the attached Acinetobacter and Enterococcus cells in the exclusion methods, its biofilm formation was not changed (Acinetobacter) (p > 0.05) or reduced by half in comparison to the single biofilm (Enterococcus) (Fig. 1). However, Pseudomonas growth was not affected in the planktonic co-cultures with Acinetobacter or Enterococcus (Fig. 1. inset).
Biofilm formation of Pseudomonas aeruginosa in the mono-culture (solid) and co-cultures with Acinetobacter (sphere) and Enterococcus (diamond) by competition, displacement, and exclusion. Error bars indicate standard deviation for three replicates, and different letters indicate significant differences between strains (p < 0.05). Inlet: Pseudomonas growth curve; alone (solid line), and in the presence of Acinetobacter (dash-dot line) and Enterococcus (dot line)
Pseudomonas motility in the single and dual cultures
The swimming motility of Pseudomonas was enhanced 1.9- and 1.5-fold in the presence of Acinetobacter and Enterococcus, respectively. The swarming motility was much affected in the co-culture with Acinetobacter and Enterococcus and was enhanced 3.7- and 1.9-fold compared to the axenic Pseudomonas culture, respectively. Regarding the twitching motility, no significant difference was observed between Acinetobacter and Enterococcus, and both strains increased 1.5-fold Pseudomonas motility in the dual cultures (Fig. 2).
Pseudomonas swimming, swarming, and twitching motility in the mono-culture (solid) and co-cultures with Acinetobacter (sphere) and Enterococcus (diamond). Error bars indicate the standard deviation for three replicates, and different letters indicate significant differences between strains (p < 0.05)
The ciprofloxacin susceptibility and pigment production
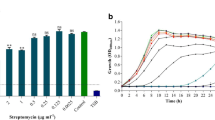
The concentration of ciprofloxacin inhibited the single cell Pseudomonas biofilm was equal to 25 μg mL−1. However, the Pseudomonas cells could tolerate up to 400 μg ciprofloxacin in the mixed biofilms with Acinetobacter/Enterococcus (Fig. 3). The UV–Vis spectrum of pigment showed a similar pattern in the single and dual Pseudomonas cultures except for the absorbance peaks seen in the Pseudomonas–Acinetobacter at 290 nm (Fig. 1. inlet). The crude pyocyanin production titer in the single Pseudomonas culture was 1.8 μg/ml, whereas it increased up to 2.3, and 2.9 μg/ml in the co-culture with Acinetobacter and Enterococcus, respectively.
Hemolysis activity
The synergistic hemolysis activity of Pseudomonas was observed with dead Acinetobacter and Enterococcus cells. Simultaneous culture of Pseudomonas and live Acinetobacter/Enterococcus reduced its hemolysis activity (antagonist). Acinetobacter CFS showed an additive effect on Pseudomonas hemolysis, while the Enterococcus cell-free supernatant did not increase nor decrease the Pseudomonas hemolysis (indifferent) (Table 1).
Las, Rhl, and PQS genes expression in single and dual Pseudomonas cultures
Rhl was the most affected Pseudomonas quorum-sensing system where its expression increased 16.3- and 9.3-fold in the mixed culture with Acinetobacter and Enterococcus, respectively. The expression of PQS and LasI was not significantly varied in Pseudomonas co-cultured with Acinetobacter/Enterococcus, respectively (P > 0.05), but increased 4.9- and 1.9-fold when Pseudomonas were co-cultured with Enterococcus and Acinetobacter, respectively (Fig. 4).
Relative expression of lasI, rhlI, and pqsR genes in dual Pseudomonas- Acinetobacter/Enterococcus cultures. Sphere: Acinetobacter, and diamond: Enterococcus. Error bars indicate the standard deviation for three replicates. *Not significant difference with control (P > 0.05), **Significant differences with control (p < 0.05). Inlet: scanning electron micrographs of Pseudomonas in co-culture with Acinetobacter (a) and Enterococcus (b)
Scanning electron microscope image
SEM analysis was conducted to represent the morphology of the Pseudomonas cells in the presence of Acinetobacter and Enterococcus. The micrographs represent that Pseudomonas morphology was not affected by the co-cultivation, and the intact rod-shaped cells could be observed in the mixed culture by Acinetobacter and Enterococcus (Fig. 4 inlet).
Discussion
It is estimated that more than 60% of the chronic wounds are caused by microbial biofilms (Høiby et al 2015). Dual biofilm formation of Pseudomonas with S. aureus, E. coli, and A. baumannii was previously reported (Solis-Velazquez et al 2021). We showed here that Acinetobacter/Enterococcus enhanced biofilm formation of Pseudomonas in dual biofilms. The correlation between cell auto-aggregation and biofilm formation and the dependence of Pseudomonas auto-aggregation on the fimbrial adhesins were previously determined (D'Argenio et al 2002; Sorroche et al 2012). The ability of P. aeruginosa cells to produce adhesins and attachment to epithelial cells was reported to be dependent on the Rhl quorum-sensing system (Glessner 1999). Accordingly, because the Pseudomonas Rhl system was over-expressed in the dual cultures with Acinetobacter/Enterococcus, we hypothesize that Acinetobacter/Enterococcus enhanced Pseudomonas biofilm formation by enhancing its auto-aggregation due to the overproduction of some aggregative adhesins. Interestingly, adding Acinetobacter/Enterococcus to the pre-formed Pseudomonas biofilm reduced its attachment. While the surfactant production was reported for both Acinetobacter and Enterococcus strains, it is not surprising that they could disrupt the pre-formed Pseudomonas biofilms (Gupta et al 2020; Chaurasia et al 2022). Cells could also leave the biofilm by dispersion, the active mechanisms that cause cells to separate from the biofilm. The involvement of PQS and Rhl quorum sensing systems in Pseudomonas dispersal seems evident from our data that these genes were much expressed in the presence of Acinetobacter and Enterococcus (Kim and Lee 2016).
Motility plays a significant role in the bacterial pathogenesis (at least in the initial phases of the infection), where non-motile bacteria are severely reduced in their virulence (Josenhans and Suerbaum 2002). Acinetobacter and Enterococcus induced all motility types of P. aeruginosa in the interspecies interactions. Overhage et al. present the association between swarming and up-regulation of rhlR (Overhage et al 2008). While both Acinetobacter and Enterococcus strains induced rhlI expression, it is not surprising that they enhanced Pseudomonas flagella-mediated motility. A proteomic study of P. aeruginosa and Staphylococcus aureus dual-species biofilm also represents the abundance of Pseudomonas flagellar and pilus proteins which result in the increase of the swimming/swarming and twitching motilities (Reigada et al 2021). Pigments like pyocyanin and pyoverdine play an essential role in the pathogenesis of P. aeruginosa by suppressing the host immune response and their role in iron acquisition. Pigmentation was significantly reduced in the Pseudomonas–Staphylococcus co-culture (Hall et al 2016). The sufficient iron concentration in this mixed biofilm can decrease the need for production of the iron acquisition system (pyocyanin) and pigmentation (Reigada et al 2021). However, we observed that Pseudomonas produces more pyocyanin in the presence of Acinetobacter and Enterococcus. While the iron acquisition systems were well determined in Acinetobacter and Enterococcus, competition between these strains and Pseudomonas to uptake iron may cause more pigmentation in co-cultures in comparison to monocultures.
In many poly-microbial biofilms, interspecies interactions significantly decrease bacterial susceptibility to antimicrobials compared to single species biofilm (Bottery et al 2022). Staphylococcus increased Pseudomonas susceptibility to ciprofloxacin, gentamicin, and amikacin but decreased to polymyxin B (Trizna et al 2020; Reigada et al 2021). The role of Rhl quorum sensing system in Pseudomonas antibiotic resistance was determined through the induction of the resistance genes like drug efflux pumps and is consistent with the results of the gene expression study in our research (Rezaie et al 2018).
The LasR is the central regulator of Pseudomonas QS and triggers the expression of most virulence factor-encoding genes. The third Pseudomonas QS signal is a quinolone-based QS system (PQS), where its null mutation may result in reduced biofilm formation and decreased production of some virulence factors (Lee and Zhang 2015). In this study, we observed that the Rhl is the central QS system and is affected in the dual cultures with Acinetobacter/ Enterococcus, with the more effects for Acinetobacter. This QS system plays a crucial role in the regulation of Pseudomonas virulence and biofilm formation, which is promoted by interspecies interactions. The result may relate to unknown metabolites produced by Acinetobacter/Enterococcus to induce the Pseudomonas QS system; however, the exact active molecule(s) remains to be identified.
In conclusion, dual-species cultures of P. aeruginosa with two co-isolated species, viz. Acinetobacter and Enterococcus from chronic wounds were characterized. Multiple Pseudomonas virulence factors, including biofilm formation, motility, hemolysis, antibiotic resistance, and pigmentation, were promoted in the dual cultures. The Rhl quorum sensing of Pseudomonas was sharply over-expressed in the dual cultures and may activate various virulence factors. Since the synergistic effects of Acinetobacter/Enterococcus on Pseudomonas pathogenesis are probably due to its QS system, the detection of quorum quenching molecules can be used to overcome the Pseudomonas poly-microbial chronic infection.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bottery MJ, Matthews JL, Wood AJ, Johansen HK, Pitchford JW, Friman VP (2022) Inter-species interactions alter antibiotic efficacy in bacterial communities. ISME 16:812–821. https://doi.org/10.1038/s41396-021-01130-6
Cai YM, Hutchin A, Craddock J, Walsh MA, Webb JS, Tews I (2020) Differential impact on motility and biofilm dispersal of closely related phosphodiesterases in Pseudomonas aeruginosa. Sci Rep 10:6232. https://doi.org/10.1038/s41598-020-63008-5
Chaurasia LK, Tirwa RK, Tamang B (2022) Potential of Enterococcus faecium LM5. 2 for lipopeptide biosurfactant production and its effect on the growth of maize (Zea mays L.). Arch Microbiol 204:223. https://doi.org/10.1007/s00203-022-02834-9
Cummings LA, Hoogestraat DR, Rassoulian-Barrett SL, Rosenthal CA, Salipante SJ, Cookson BT et al (2020) Comprehensive evaluation of complex polymicrobial specimens using next generation sequencing and standard microbiological culture. Sci Rep 10:5446. https://doi.org/10.1038/s41598-020-62424-x
D’Argenio DA, Calfee MW, Rainey PB, Pesci EC (2002) Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J Bacteriol 184:6481–6489. https://doi.org/10.1128/JB.184.23.6481-6489.2002
DeBritto S, Gajbar TD, Satapute P, Sundaram L, Lakshmikantha RY, Jogaiah S et al (2020) Isolation and characterization of nutrient dependent pyocyanin from Pseudomonas aeruginosa and its dye and agrochemical properties. Sci Rep 10:1542. https://doi.org/10.1038/s41598-020-58335-6
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. https://doi.org/10.3201/eid0809.020063
Fadwa AO, Alkoblan DK, Mateen A, Albarag AM (2021) Synergistic effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J Biol Sci 28:928–935. https://doi.org/10.1016/j.sjbs.2020.09.064
Frykberg RG, Banks J (2015) Challenges in the treatment of chronic wounds. Adv Wound Care 4:560–582. https://doi.org/10.1089/wound.2015.0635
Glessner A, Smith RS, Iglewski BH, Robinson JB (1999) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J Bacteriol 181:1623–1629. https://doi.org/10.1128/JB.181.5.1623-1629.1999
Gupta B, Puri S, Thakur IS, Kaur J (2020) Enhanced pyrene degradation by a biosurfactant producing Acinetobacter baumannii BJ5: growth kinetics, toxicity and substrate inhibition studies. Environ Technol Innov 19:100804. https://doi.org/10.1016/j.eti.2020.100804
Hall S, McDermott C, Anoopkumar-Dukie S, McFarland AJ, Forbes A, Perkins AV et al (2016) Cellular effects of Pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins (basel) 8:236. https://doi.org/10.3390/toxins8080236
Høiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T, Donelli G et al (2015) ESCMID∗ guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. https://doi.org/10.1016/j.cmi.2014.10.024
Josenhans C, Suerbaum S (2002) The role of motility as a virulence factor in bacteria. Int J Med Microbiol 291:605–614. https://doi.org/10.1078/1438-4221-00173
Kim SK, Lee JH (2016) Biofilm dispersion in Pseudomonas aeruginosa. J Microbiol 54:71–85. https://doi.org/10.1007/s12275-016-5528-7
Kostylev M, Kim DY, Smalley NE, Salukhe I, Greenberg EP, Dandekar AA (2019) Evolution of the Pseudomonas aeruginosa quorum-sensing hierarchy. Proc Natl Acad Sci 116:7027–7032. https://doi.org/10.1073/pnas.1819796116
Lee J, Zhang L (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6:26–41. https://doi.org/10.1007/s13238-014-0100-x
Limoli DH, Hoffman LR (2018) Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax 7:4684–4692. https://doi.org/10.1136/thoraxjnl-2018-212616
Overhage J, Bains M, Brazas MD, Hancock RE (2008) Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J Bacteriol 190:2671–2679. https://doi.org/10.1128/JB.01659-07
Peleg AY, Hogan DA, Mylonakis E (2010) Medically important bacterial-fungal interactions. Nat Rev Microbiol 8:340–349. https://doi.org/10.1038/nrmicro2313
Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME (2012) Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 25:193–213. https://doi.org/10.1128/CMR.00013-11
Reigada I, San-Martin-Galindo P, Gilbert-Girard S, Chiaro J, Cerullo V, Savijoki K et al (2021) Surfaceome and exoproteome dynamics in dual-species Pseudomonas aeruginosa and Staphylococcus aureus Biofilms. Front Microbiol 12:672975. https://doi.org/10.3389/fmicb.2021.672975
Rezaie P, Pourhajibagher M, Chiniforush N, Hosseini N, Bahador A (2018) The effect of quorum-sensing and efflux pumps interactions in Pseudomonas aeruginosa against photo oxidative stress. J Lasers Med Sci 9:161–167. https://doi.org/10.15171/jlms.2018.30
Shi N, Gao Y, Yin D, Song Y, Kang J, Li X et al (2019) The effect of the sub-minimal inhibitory concentration and the concentrations within resistant mutation window of ciprofloxacin on MIC, swimming motility and biofilm formation of Pseudomonas aeruginosa. Microb Pathog 137:103765. https://doi.org/10.1016/j.micpath.2019.103765
Solis-Velazquez OA, Gutiérrez-Lomelí M, Guerreo-Medina PJ, Rosas-García ML, Iñiguez-Moreno M, Avila-Novoa MG (2021) Nosocomial pathogen biofilms on biomaterials: Different growth medium conditions and components of biofilms produced in vitro. J Microbiol Immunol Infect 54:1038–1047. https://doi.org/10.1016/j.jmii.2020.07.002
Sorroche FG, Spesia MB, Zorreguieta A, Giordano W (2012) A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl Environ Microbiol 78:4092–4101. https://doi.org/10.1128/AEM.07826-11
Sperandio D, Rossignol G, Guerillon J, Connil N, Orange N, Feuilloley MG et al (2010) Cell-associated hemolysis activity in the clinical strain of Pseudomonas fluorescens MFN1032. BMC Microbiol. https://doi.org/10.1186/1471-2180-10-124
Trizna EY, Yarullina MN, Baidamshina DR, Mironova AV, Akhatova FS, Rozhina EV et al (2020) Bidirectional alterations in antibiotics susceptibility in Staphylococcus aureus-Pseudomonas aeruginosa dual-species biofilm. Sci Rep 10:14849. https://doi.org/10.1038/s41598-020-71834-w
Warrier A, Satyamoorthy K, Murali TS (2021) Quorum-sensing regulation of virulence factors in bacterial biofilm. Future Microbiol 16:1003–1021. https://doi.org/10.2217/fmb-2020-0301
Acknowledgements
This work was supported by a grant from Ferdowsi University of Mashhad (50614/3).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
A.M and S.A contributed to the study conception and design. G.H and A.M contributed to Material preparation, data collection and analysis. The first draft of the manuscript was written by A.M and G.H. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Muhammad Bilal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Laliany, G., Jamehdar, S.A. & Makhdoumi, A. In vitro virulence activity of Pseudomonas aeruginosa, enhanced by either Acinetobacter baumannii or Enterococcus faecium through the polymicrobial interactions. Arch Microbiol 204, 709 (2022). https://doi.org/10.1007/s00203-022-03308-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03308-8