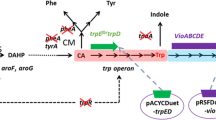
Abstract
Violacein, a therapeutic pigment synthesised naturally in specific bacterial systems, is commercially unexplored owing to low titers. The current study aimed to formulate a suitable medium and to develop a fed-batch strategy for improving the violacein productivity in a natural producer—Chromobacterium violaceum MTCC2656. The carbon and the nitrogen sources were extensively screened and their levels were optimised for maximal violacein production. The micronutrients in the medium were subjected to a two-level statistical optimisation using design of experiment approach and the final media formulation was validated. As a fed-batch approach, a combination of pulse feeding of glucose and tryptophan with optimised broth harvest of 60% (v/v) was attempted that achieved a titer of 1046 ± 16 mg/L with productivity of 26.12 ± 0.64 mg/L/h in each progressive cycle of fed-batch. The strategic sequential step of media formulation and fed-batch fermentation improved the violacein titer by ~ 5 folds. Kinetic modelling was used to understand the enhancement in fermentation performance in both the batch and fed-batch processes. The findings from the current study would enable to understand and correlate the patterns of substrate uptake and violacein formation to establish strategies for enhancing overall productivity in the fermentation processes.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Violacein is a naturally occurring indole derivative produced and secreted by certain bacteria for quorum sensing [1]. Apart from communication, violacein also manifests a range of bioactive properties such as anti-protozoan, fungicidal, anti-bacterial, anti-viral, anti-cancer, anti-tumour, immunomodulatory, nephroprotective, and anti-inflammatory properties [2, 3]. With such a broad range of activities, violacein, thus, proves to be a potential therapeutic compound.
Violacein production is mainly traced to specific species belonging to genera Alteromonas, Chromobacterium, Duganella and Janthinobacterium, of phylum Pseudomonadota [4]. Despite having immense potential as a therapeutic pigment, the commercial production of this purple-coloured compound is constrained due to lower productivity [5]. Systems biology approaches such as genetic engineering, metabolic engineering and gene overexpression have been evaluated to enhance the overall production of this water-insoluble pigment. Industrial strains such as Escherichia coli [6], Citrobacter freundii [7], Corynebacterium glutamicum [8] and Yarrowia lipolytica [9] have been modified using engineering tools for the expression of genes responsible for violacein synthesis. However, using genetically modified organisms poses a major limitation of compliance with the regulatory authorities. Plasmid instability, performance inconsistency and tedious strain development are operational difficulties associated with use of genetic engineering strategies [10]. Thus, a simpler approach towards improvement in the productivity of violacein from the natural producers is desirable for realisation of its commercial process development.
Reports on isolation, identification, characterisation and batch fermentation of common natural producers of violacein are available in literature [1]. The organism selected for this study, Chromobacterium violaceum, is commonly found in natural habitats such as soil and water. This Gram-negative bacterium is non-pathogenic and known to produce violacein, from where it draws its name. A chemically defined minimal medium formulation can help in a structured and statistical approach towards medium engineering for maximising violacein production. Glucose and glycerol are well-studied carbon sources for C. violaceum [11, 12]. However, disaccharides and polysaccharides have not been much investigated for growth of this organism. There are reports of violacein production from industrial sugar-rich wastes like bagasse and molasses [13]. Tryptophan becomes a critical component of the medium for violacein production as the biosynthetic pathway for violacein involves condensation of two tryptophan moieties under the regulation of the VioABCDE gene cluster present in the bacterium [14]. Attempts on optimisation of the fermentation conditions have been carried out previously to obtain gram-scale production of violacein [11, 15]. However, the kinetic studies and the modelling aspects of violacein production are not much explored.
Kinetic studies of the batch fermentation are instrumental in understanding and correlating, the substrate utilisation with the overall metabolism of the organism. It can also help in evaluating strategies for enhancing fermentation and predicting responses for developing fed-batch and repeated fed-batch operations [16]. Log-logistic model and Gompertz model are well-studied kinetic models for biomass accumulation in fermentation processes [17, 18]. Dose–response model is commonly used to evaluate effect of single and multiple doses of therapeutics on biological models. This model has also been used to understand the effect of inducers and enhancers on fermentation of gut bacteria [19]. This insight can be extended to study the effect of dosing specific medium components on biomass production and violacein production.
Although there are reports of fed-batch strategies for gram-scale violacein production, the organisms employed have all been genetically modified [8]. Repeated fed-batch reduces the overall efforts, money and time involved in cleaning and sterilisation of the equipment, required after every batch process thereby improving the productivity and process economics [20]. The repeated fed-batch strategy with partial biomass harvesting was, thus, attempted with natural producer of violacein which can be considered as first of a kind of the work with C. violaceum.
The difficulties associated with the genetic modifications as explained above propelled us to develop and apply an approach of combining multiple bioprocessing principles such as media engineering, fed-batch cultivation and partial broth harvest to improve the performance of fermentative production violacein. The study related to the enhanced production of this microbial secondary metabolite with insights into the industrial scale production is expected to facilitate commercial production and applications of this purple pigment.
Materials and methods
Materials
The media components used in this study were procured from Hi-Media Laboratories Private Limited, Mumbai, India and S.D. Fine Chemicals Private Limited, Mumbai, India. L-methionine and L-tryptophan were acquired from Avra Laboratories Private Limited, Hyderabad, India. The solvents used in the study were sourced from Honeywell International Inc., New Delhi, India.
Microorganism and preparation of seed inoculum
Chromobacterium violaceum MTCC2656 strain was obtained from Microbial Type Culture Collection and Gene bank, Chandigarh, India and was maintained on nutrient agar plates incubated at 30 °C. The seed culture was prepared by inoculating a loop full of culture from the maintenance plates in 100 mL of sterile nutrient broth. The seed culture was incubated at 30 °C under shaking conditions at 150 rpm for 12 h. Later, these cells were aseptically harvested, washed thrice with 0.1% (w/v) sterile saline and inoculated into the experimental flasks to achieve an initial biomass load of 7 log CFU/mL. A working volume of 50 mL was maintained in the 250 mL Erlenmeyer flasks for all the experimentations including screening studies, media engineering and optimisation studies, validation trials, kinetics and fed-batch trials.
Fermentation medium for Chromobacterium violaceum
The composition of the minimal medium given by DeMoss and Happel [21] was tested with suitable modifications for cell growth and violacein production. The initial composition of the medium for this study was as follows: glucose (5 g/L), tryptone (5 g/L), NaCl (1 g/L), K2HPO4 (600 mg/L), K2SO4 (50 mg/L), MgCl2 (100 mg/L), ZnSO4·H2O (100 mg/L), FeSO4.7H2O (5 mg/L), vitamin B12 (60 µg/L), L-methionine (0.4 mM) and L-tryptophan (1.2 mM). The pH of this medium was adjusted to 7.0 ± 0.5 using ortho-phosphoric acid. All the components except amino acids and vitamin B12, were dissolved in distilled water and autoclaved at 121 °C for 15 min. Concentrated stocks of amino acids and vitamin were prepared in sterilised distilled water and sterilised by passing through a 0.2 μ pore-sized sterile syringe filter. These stocks were appropriately added to the sterilised media to achieve aforementioned concentrations. The fermentation was carried out for 72 h after inoculation at 30 °C under shaking conditions at 150 rpm.
Screening of nitrogen and carbon sources
To investigate the effect of nitrogen source, tryptone in the medium was replaced with other complex nitrogen sources such as casein–soya–meat–peptone, soybean meal, meat peptone, mycological peptone, bio-peptone, casein peptone, bacteriological peptone, veg peptone, malt extract and yeast extract, separately. Along with the complex nitrogen sources, simple nitrogen sources such as sodium nitrate, sodium nitrite, ammonia, ammonium chloride and urea were also tested. The selection criterion was maximal violacein production at the end of 72 h of incubation.
After finalizing the nitrogen source, the carbon sources were screened. Glucose in the medium was replaced with monosaccharides (fructose), disaccharides (maltose, lactose and sucrose), polysaccharides (maltodextrin and starch) and other commercial carbon sources (molasses, mannitol and glycerol) at the same concentration.
Optimisation of the levels of carbon source and nitrogen source
The concentration of the carbon source was varied keeping the concentration of the nitrogen source fixed at 5 g/L. The variations were such that the ratio of amount of carbon source to the amount of nitrogen source (C/N ratio g/g), at the initiation of the fermentation was varied as 0.25, 0.5, 1, 2, 5 and 10 (g/g). Since glucose and glycerol were selected from the screening experiment, both these carbon sources were individually assessed in this experiment for maximum violacein production. Once the C/N (g/g) ratio was finalised, the level of the nitrogen source was varied between 2.5 and 20 g/L. The levels of the carbon source were then varied to maintain the C/N (w/w) ratio.
Concentration optimisation of the remaining 9 medium components
The Plackett–Burman (PB) screening design
There were nine other components in the medium to be optimised. Hence, Plackett–Burman screening design was used to identify the components which significantly impacted violacein production. The concentrations of each of the remaining nine components were varied over two levels in the orthogonal array like arrangement of the Plackett–Burman design. Two components were kept as dummy according to the design requirements. With minimum experimentation, the main effects of each of the components were statistically compared [22]. The upper and lower limits for the medium components were selected by preliminary trials. The effects of each medium components were calculated using Eq. 1.
where E(xi) is the effect of any component, Mi+ and Mi− are the responses to higher and lower level concentration of the component, respectively, and N was the total number of trials. Accordingly, the variance amongst the dummy variables was also calculated using Eq. 2.
where Veff is the variance, Ed is effect of dummy variables and n is the number of dummy variables. Standard error was determined as the square root of the variance which was further used for determination of p-values for the model and individual factors.
The components having p-values < 0.05 and E value > 0, were considered to have significant positive effect on the violacein production and were taken ahead for further optimisation by central composite design.
Superior level optimisation using central composite design
Based on the Plackett–Burman design, four medium components (ZnSO4·H2O, MgCl2, FeSO4·7H2O and tryptophan) were selected for superior numerical optimisation. The effects of these four medium components on violacein production were examined in a face centred composite design (FCCD). Design expert version 7 was used for devising the design and analysis of the model with respect to the responses. A set of 27 experiments were performed in three replicated blocks. In one block, 16 trials were carried out at factorial points (± 1 levels) in addition to 8 trials at the axial points (± α levels) and 3 trials belonged to the centre of the domain. The experiments were carried out with both glucose and glycerol as carbon sources, separately.
A quadratic model (Eq. 3) was applied to correlate violacein production (Y) with β0, βi, βii and βij which are the regression coefficients for intercept, linearity, square and interaction, respectively; xi and xj are coded level of independent variables.
The model predicted solutions in the form of optimum concentration of the medium components for maximising violacein titers and achieving maximum desirability. The given solutions were validated experimentally. Once the medium composition was optimised, different modes of fermentation were attempted to maximize violacein production.
Evaluation of different modes of fermentation
Batch mode
In this mode, seed culture was inoculated in sterile optimised medium and sampling was conducted regularly up to 72 h to monitor growth, nutrient uptake and violacein production.
Single-nutrient pulse feed
To assess the effect of tryptophan feeding on violacein synthesis, residual tryptophan levels in the fermentation broth were analysed between the 16 h and 44 h of fermentation and replenished to 3.2 mM (initial tryptophan concentration) by adding appropriate amount of sterile tryptophan stock (64.4 mM) in the broth.
In the second set of experiments, the effect of feeding glucose pulse was assessed on the biomass accumulation in the broth. The residual glucose levels in the fermentation medium were analysed between the 6 h and 24 h of fermentation and replenished to 5 g/L (initial glucose concentration) in the medium by adding a pulse of appropriate sterile glucose stock (200 g/L) in the broth.
Fermentation in all the test flasks was terminated 72 h after initiation of fermentation. The concentration of residual nutrients at the end of fermentation was analysed. Biomass accumulation and violacein production for all the tests were compared with the batch results.
Multi-nutrient pulse feed
Based on the outcomes of experiments in single-nutrient fed-batch mode, the time for feeding glucose and concentrations of tryptophan and glucose were optimised. Accordingly, both these medium components were fed into the medium together and the effect of multi-nutrient feeding was evaluated on the biomass accumulation and violacein production.
Partial biomass harvest combined with multi-nutrient-fed-batch
To improve overall productivity, fed-batch operation with partial biomass harvest strategy was applied in combination with multi-nutrient pulse feed. Once the violacein production attained saturation after multi-nutrient pulse feeding, 40–80% of broth (v/v) was harvested, and fresh medium was added to make up the initial volume. Glucose and tryptophan contents in broth were analysed and the concentrations were adjusted to 5 g/L and 3.2 mM, respectively, using appropriate stocks. Sampling was conducted periodically to understand the growth profile, violacein production and nutrient consumption by the bacterium.
Experimental values of the kinetic parameters
Metabolic activities such as biomass accumulation, glucose consumption, violacein formation and tryptophan utilization were monitored throughout the experiments in the batch and fed-batch mode. The rates of these metabolic activities were calculated by applying Eqs. (4a–4d) to the experimental data [23]. The metabolic rates were calculated individually for the log phase, early stationary phase and late stationary phase.
where Xi, Si, Ii and Pi indicate concentration of biomass, residual glucose, residual tryptophan and violacein at the time of initiation (ti) of a particular growth phase and Xf, Sf, If and Pf indicate concentration of biomass, residual glucose, residual tryptophan and violacein concentration at the time of completion (tf) of that growth phase.
Kinetic modelling
Different models were studied to evaluate the best fitting models for biomass accumulation, substrate consumption and violacein production in the batch mode. Logistic (Eq. 5), Gompertz (Eq. 6) and Dose–response model (Eq. 7) were evaluated to assess the kinetic parameters of biomass accumulation.
where Xt is the biomass (g/L) accumulated at any time t (h), X0 is the initial biomass concentration (g/L), Xm is the maximum biomass accumulated during the cycle (g/L), μ is the specific rate of biomass accumulation (g/L/h) and tc (h) is the mid-point of the time scale.
Exponential decay model (Eq. 8) and Weibull model (Eq. 9) were applied to the residual concentration of glucose in the medium. The values of kinetic parameters and coefficient of determination were compared [24].
where St is the residual concentration (g/L) of glucose/tryptophan at any time t (h), S0 is the initial concentration of glucose/tryptophan, λ is the decay rate and n is the skewness factor such that − 1 ≤ n ≥ 1 and n ≠ 0. Since exponential decay model and Weibull model were found to be suitable for glucose consumption and consumption of tryptophan was sigmoidal in nature, Logistic decay model (Eq. 10) was applied to the kinetic data of residual tryptophan in the medium. The same model was extended in multi-nutrient fed-batch study.
where It (mM) is the residual tryptophan concentration at any time t (h), If (mM) and I0 (mM) are initial and final concentration of residual tryptophan in the batch and p (mM/h) is the decay rate of tryptophan.
The dose–response model (Eq. 11) was selected to evaluate the kinetics of violacein production in batch fermentation.
where Pt is the violacein produced (mg/L) at any time t (h), P0 is the concentration of violacein (mg/L), Pm is the maximum violacein produced during the cycle (mg/L), ρ is the specific rate of violacein production (mg/L/h) and tc (h)is the mid-point of the time scale.
The acceptable and accurate fitting of the model was evaluated on the basis of coefficient of determination (R2). The best fit model, from the all the assessed models, for each of the parameters were selected and modified to predict and assess the performance in fed-batch conditions. Based on the parameters obtained from the kinetic models, the efficiency and consistency for multiple cycles of repeated fed-batch strategy were evaluated.
Analytical techniques
Biomass accumulation was monitored by checking the optical density of an appropriately diluted culture at 764 nm on Jasco V-730 UV–Vis spectrophotometer, as suggested by Gallardo et al. [25]. Cell dry weight (CDW) was also determined to assess the biomass accumulation.
Residual glucose in the cell-free supernatant was monitored using Eco-Pak Glucose kit (Accurex) and BioTek®μQuant 96-well plate reader [26]. The cell-free supernatant was appropriately diluted with distilled water, filtered through 0.22 μ pore-sized syringe filters and analysed for residual tryptophan concentration using HPLC method described by Wang et al. [27]. Briefly, the analysis was carried out on Thermo ODS-2 HYPERSIL column, (250 mm × 4.6 mm ID, 5 µm, 120 Å) with isocratic elution using mobile phase of 90:10 (v/v) of methanol:0.03% (w/v) aqueous solution of potassium dihydrogen phosphate. The column temperature was maintained at 25 °C and flow rate was 1 mL/min throughout the run time of 25 min. The monitoring wavelength was 278 nm and 5 μL of sample was injected using the autosampler equipped in Dionex, Ultimate 3000 LC system.
For quantification of total violacein, 1 mL of broth was added to a graduated volumetric flask of 10 mL capacity. 2 mL of dimethyl sulphoxide and 5 mL of methanol were added to extract the violacein from the broth. The resultant suspension was sonicated for 60 min at 30 °C in an ultrasonic bath. Later, the volume was made up to 10 mL with methanol. After 5 min of rigorous hand mixing, the suspension was centrifuged at 6000 × g for 10 min at 30 °C. A 0.22 μ, 25 mm PTFE syringe filter was used to filter the resulting supernatant. The filtrate (5 μL) was automatically injected into the Dionex, Ultimate 3000 LC system, which was equipped with a Thermo ODS-2 HYPERSIL column (250 mm × 4.6 ID, 5 µm, 120 Å) and was maintained at 25 °C. Isocratic elution was performed at 1 mL/min with 70:30 (v/v) of methanol: water, and violacein was detected at 570 nm using a diode array detector [28]. For quantifying violacein concentration, a standard graph of pure violacein (99.89% purity) was plotted in the concentration range of 0–0.5 mg/mL.
Statistical analysis
The experimental data were analysed using one-way ANOVA and statistical analysis was performed in IBM SPSS statistics 20 programme using Tukey's honest significant difference (HSD) test at a significance level of 5%. All the data are shown as mean ± standard deviation of three independent biological replicates.
Results and discussion
Screening studies for the macronutrients
Carbon and nitrogen sources are essential components in the fermentation medium which are required for cell growth and maintenance. Amongst the complex nitrogen sources, highest violacein production was observed with tryptone (98.66 ± 0.17 mg/L) (Fig. 1a) as it is enriched with tryptophan, which is a known precursor of violacein [29]. The biomass production with all the complex nitrogen sources was comparable. However, the violacein content in the biomass did not exceed 8% (w/w) in any complex nitrogen source. Complex nitrogen sources are known to be a mixture of multiple amino acids, vitamins and growth promoters. The abundance of these growth factors might have resulted in the direction of metabolic fluxes towards overall cell growth resulting in lower violacein content. The metabolism can be directed towards violacein biosynthesis by either metabolic engineering or by limiting the content of other amino acids in the medium [30]. Thus, as an attempt of media engineering, simple nitrogen sources were also tested as a part of this minimal medium.
Studies for suitable macronutrients for the production of violacein by Chromobacterium violaceum. a Nitrogen source screening, b carbon source screening, and c optimisation of the ratio of carbon to nitrogen source in the fermentation medium (C/N, g/g). Black vertical bars represent the SD (n = 3). Small letters a, b, c and d denote significant differences in the violacein production when glucose was used as a carbon source (p < 0.05, Tukey’s test). Capital letters A, B, C and D denote significant differences in the violacein production when glycerol was used as a carbon source
Urea was the only simple nitrogen source which supported the growth of this bacterium, and it also yielded highest violacein titers of 229.51 ± 29.91 mg/L amongst all the nitrogen sources tested. A violacein content of 19.12 ± 2.49% (w/w over biomass) was obtained with urea as nitrogen source which was 3-times higher than the highest content observed amongst the complex nitrogen sources (casein peptone—7.71 ± 3.24% w/w over biomass). Unlike complex nitrogen sources, the urea containing medium lacked the excess growth factors which consequently directed the metabolic fluxes towards the assimilation of tryptophan, the only abundant amino acid in the medium. The cellular machinery thus was driven more towards violacein production. This is the first study to report on growth of C. violaceum and violacein production with urea as a nitrogen source in the medium. The phylogenetically associated organisms with this bacterium are known to have set of genes encoding for urea degradation [31]. Presence of a similar gene set in the organism under study can be anticipated with our observations. The utilization of urea will be economically beneficial for violacein production due to much lower cost than other complex nitrogen sources.
Amongst the different carbon sources tested, glucose and glycerol are well reported to support the growth of C. violaceum. These two carbon sources yielded maximal violacein titers amongst all the carbon sources tested. Fructose just supported the growth of the organism (Fig. 1b). The difficulty to assimilate carbohydrates other than the monosaccharides can be attributed to the limited ability of the bacterium to produce glucosidases enzyme [32]. Thus, glucose and glycerol were identified as the most suitable carbon sources to support the growth and violacein production. Both these carbon sources were individually investigated for further optimisations.
Levels of macronutrients in the fermentation medium
Initially, the ratio of carbon and nitrogen sources (C/N ratio, g/g) in the medium was optimised by keeping the concentration of urea fixed at 5 g/L. In case of both glucose and glycerol, it was observed that a ratio of C/N ratio of 0.5 (w/w) produced a significantly higher violacein concentration (Fig. 1c). To the best of our knowledge, this is the first study of its kind to investigate such variations for Chromobacterium violaceum and report the optimum values for maximal violacein production.
Once the C/N ratio was fixed at 0.5 (w/w), the concentration of urea and glucose/glycerol were varied keeping this ratio constant. It was found that urea at 10 g/L supported maximal violacein production. Both glucose and glycerol, when tested individually, yielded maximal titers at 5 g/L (Table 1). Thus, these two concentrations were fixed for further studies. As a result of this set of experiments with carbon and nitrogen source, a substantial increase of ~ twofold in the violacein titer from 98.66 ± 0.17 to 199.73 ± 0.73 mg/L was evident. The violacein content over biomass also increased up to 16.9% (w/w) which was ~ 3.5-times of the initial content of 5.03 ± 0.25 (w/w).
Optimisation of the concentration of the remaining medium components
The remaining 9 components in the basal medium, other than the carbon and nitrogen sources, comprised of metabolic enhancers, co-factors, growth essentials and buffering agents. A two-step statistical and design of experiment-based optimisations were carried out for these 9 medium components. Media formulations with glucose and glycerol were discretely assessed.
Screening of medium components with significant effect on violacein titers
The screening for medium components with the most significant effect on violacein production was carried out with 12 runs of the Plackett–Burman (PB) design. The variations were undertaken at the higher level (+ 1) and lower level (− 1). The responses obtained were statistically analysed using ANOVA. The design parameters and the details of ANOVA are provided in the supplementary data (Tables S1–S2). The percentage contribution of the dummy variables accounted for 1.96 ± 0.04%, indicating the contribution of error. Plackett–Burman screening showed that ZnSO4·H2O, MgCl2, FeSO4·7H2O and tryptophan had a significant and positive effect on violacein production. These four medium components had a higher positive value of the impact coefficients. The p-values for these components were below 0.05, which indicated a “real” or “significant” effect of these medium components over all the others under study. The concentration of these four medium components was further optimised using face centred composite design (FCCD) as a superior optimisation strategy—response surface methodology (RSM). As aforementioned, tryptophan is a known precursor of violacein. Thus, a positive impact coefficient was anticipated and was very well observed in the study. Ions and electrolytes in the form of ZnSO4·H2O, MgCl2 and FeSO4·7H2O are required as modulators and co-factors for various cellular operations such as inter-membrane transport and enzymatic reactions [33, 34]. K2HPO4 was one of the components which exhibited a slight negative effect on violacein production when its quantity was increased. This can be attributed to the increase in the pH (up to 8.7 ± 0.2) at (+ 1) higher level, when compared to the pH 7 of the basal medium. The increase in pH, due to increase in the K2HPO4 levels had a deteriorating effect on biomass accumulation and violacein production. This can be correlated to the existing literature, where the pH of medium was controlled to 7 [13]. L-Methionine and vitamin B12 are crucial for the growth and maintenance of C. violaceum and their presence in the medium promotes cell proliferation and biomass [21, 35]. Accordingly, the PB design matrix showed that increasing methionine beyond basal levels in the medium directed the metabolic flux more towards the growth and compromised with violacein production. The latter behaviour was hence considered as a negative effect. This was the reason of keeping L-methionine at the basal level in the medium for the sole purpose of biomass maintenance. All the other components, which did not have a significant positive effect on violacein production, were fixed at levels as those in the basal medium.
Face-centred composite design (FCCD) for optimisation
The effect of the components selected from the Plackett–Burman design was further assessed using a FCCD matrix. These responses were analysed in the software and an ANOVA for the quadratic regression model was fitted. The design parameters and the details of ANOVA are provided in the supplementary data (Tables S3–S4). The p-values for the model with glucose was < 0.0001 and with glycerol was 0.0002, indicating a suitable fit. The high F values (11.89 for glucose and 9.36 for glycerol) validated the statistical significance of the quadratic models applied in both the cases. The p-values for ZnSO4·H2O, FeSO4.7H2O and L-tryptophan were < 0.05, indicating a profound impact on the violacein titers. With both glucose and glycerol, the p-values for MgCl2 were > 0.05. The significant impact of tryptophan on the violacein titers can be related to the biosynthesis pathway [29]. The presence of zinc ions in the fermentation medium is reported to improve the violacein production. These ions are known to be co-factors for specific enzymatic reactions in the VioABCDE operon which is responsible for violacein production in the bacterium. The effect of these ions on violacein production is thus well anticipated [11]. Similarly, the redox potential of ferrous ions make them versatile co-factors for various enzymatic reactions. C. violaceum is known to have specialised cellular machinery for intake of this crucial ion [33]. This study conclusively establishes a correlation of the metabolic requirements of the organisms with the production of this secondary metabolite.
Equations 12 and 13 show the correlations of these medium components with the violacein titers in the glucose-based and glycerol-based mediums, respectively.
where A, B, C and D are coded concentrations of ZnSO4·H2O (mg/L), MgCl2 (mg/L), FeSO4·7H2O (mg/L) and L-tryptophan (mM), respectively.
Validation of the optimised levels of medium components
The overall experiments for optimisation of the levels of the components in chemically defined medium for C. violaceum MTCC2656 yielded following composition: glucose/glycerol (5 g/L), urea (10 g/L), NaCl (1 g/L), K2HPO4 (600 mg/L), K2SO4 (50 mg/L), MgCl2 (367.47 mg/L), ZnSO4·H2O (219.83 mg/L), FeSO4.7H2O (2.2 mg/L), vitamin B12 (60 µg/L), L-methionine (0.4 mM) and L-tryptophan (3.2 mM). With glucose and glycerol as the carbon source, the violacein production predicted in the final step of optimisation was 529.96 mg/L and 413.43 mg/L, respectively. The models and the predicted values were experimentally validated and found to be 501.65 ± 36.0 mg/L and 421.12 ± 22.20 mg/L for glucose-based and glycerol-based medium, respectively. Since higher titer was achieved with glucose, it was selected as a carbon source for further studies. The results demonstrated a successful media engineering approach, which improved the titer of violacein in the chemically defined medium from 98.66 ± 0.17 to 501.65 ± 36.0 mg/L. The violacein content in biomass also improved from 5.03 ± 0.25% (w/w over biomass) to 31.31 ± 0.54% (w/w over biomass), indicating a six-fold increase. The kinetics of batch fermentation in the optimised media formulation was carried out and the strategies for fed-batch fermentation were evaluated.
Batch fermentation
The biomass accumulation of C. violaceum and violacein production along with utilization of the two substrates, glucose and tryptophan is shown in Fig. 2a. Figure 2b shows the trend of the rates of metabolic activities calculated in this study for the batch fermentation. It was observed that the log phase of biomass accumulation corresponded with the exponential glucose consumption phase, which lasted up to 16 h. The rates of biomass production and glucose consumption were significantly higher in log phase of growth (interval 0–16 h). Tryptophan consumption was significantly slower in the log phase than the early stationary growth phase. Post 16 h, biomass growth profile entered the stationary phase due to exhaustion of glucose in the medium. Bapat et al. [36] have reported that the uptake of amino acids in bacteria is energy dependant which is the reason for such preferential and sequential consumption of carbon source and amino acids in fermentation medium.
Batch fermentation kinetics of Chromobacterium violaceum. a Kinetic profile of biomass accumulation, glucose consumption, tryptophan utilization and violacein production. b Experimental values of kinetic parameters of the metabolic activities in different growth phases. Black vertical bars represent the SD (n = 3). Small letters a, b and c denote significant differences in the rates of metabolic activities in different growth phases (p < 0.05), Tukey’s test
In the early stationary phase, tryptophan uptake by the bacterium improved and maximum tryptophan uptake rate was observed in this phase. The production of violacein in the broth corresponded with active tryptophan uptake and significant violacein production took place during the early stationary phase (16–48 h). The rate of violacein production was highest in this phase which later decreased in late stationary phase as the tryptophan levels reached the minimum concentration levels in the broth. Thus, feeding tryptophan was pivotal to improve the violacein production which is also reported in literature [15]. It was seen that the residual levels of glucose and tryptophan had saturated to minimal levels at the end of the batch indicating maximum consumption of these medium components (Fig. 2a). The terminal concentration of residual glucose was 0.22 ± 0.01 g/L, while that of residual tryptophan was 0.13 mM. Based on this, the yield of violacein over glucose was calculated as 0.11 mg violacein/g of glucose, while that over tryptophan was 0.89 ± 0.04 mg violacein/mg tryptophan.
Single-nutrient pulse feed strategy
The batch fermentation kinetics showed that tryptophan was consumed exponentially in the early stationary phase (16–48 h). Hence, tryptophan pulse feeding was carried out during this phase of growth. A slight increase in the violacein titer up to 530.38 ± 0.71 mg/L was observed when tryptophan pulse was fed between 16 and 20 h. Tryptophan feeding at 0.42 ± 0.12 mM after 16 h showed a violacein content of 31.44 ± 3.96% (w/w) over biomass. Addition of tryptophan beyond 16 h did not improve the violacein content in biomass which is indication of saturation in tryptophan uptake by the bacterium. Hence, pulse feeding of glucose in the broth was attempted in an effort to further increase the violacein titer. Glucose supplementation is a commonly attempted strategy to improve the biomass accumulation in fed-batch fermentation [37, 38]. Experiments on glucose feeding carried out during the log phase (6–24 h) did increase biomass production up to 3.98 ± 0.68 g/L, when a pulse equivalent to 5 g/L of glucose was fed at 16th hour of fermentation.
Improvement in the violacein production by multi-nutrient pulse feeding
From the studies with individual feeding of glucose and tryptophan, the time and concentration for pulse feeding of these two medium components were optimised for maximum biomass, and thereby maximum violacein production. Combined feeding of 5 g/L glucose and 0.42 mM tryptophan was carried out at the 16th hour. This combined feeding yielded 2.8 ± 0.12 g/L of biomass and 820.97 ± 15.97 mg/L violacein which gave ~ 1.5-fold enhancement over the batch mode. The feeding strategy was found to be economical as the yields of biomass and violacein over glucose and tryptophan, respectively, remained consistent with respect to the batch mode. Gohil et al. [15] did report on strategic supplementation of tryptophan to enhance the violacein production up to gram scale using soya meal as an organic nitrogen source. However, in the present study, similar titer was obtained with urea which is a much cheaper and simple nitrogen source. The time of addition of tryptophan had a crucial role in enhancement of the violacein content which was in line with that reported by Cheng et al. [39].
Partial harvest of biomass for increasing overall productivity of the system
Partial harvest of broth is a well-studied strategy for achieving operation in continual fed-batch mode [20, 40]. The harvest percentage of broth had a significant impact on growth of the bacterium in the subsequent cycles of repeated fed-batch. Figure 3 shows the growth profile of the bacteria in the medium with different harvest percentage in repeated fed-batch. In case of 40% and 60% (v/v) broth harvest, a 34–36% enhancement in biomass accumulation was observed in the second cycle. In case of 40% (v/v) harvest, as the fermentation progressed in the third cycle, a significant reduction in the biomass accumulation was observed. The main reason for this inconsistent and lowered performance in the third cycle could be the accumulation and dominance of stationary phase cells in the broth. In the case of 80% (v/v) broth harvest, an extended log phase was observed in the second cycle which can be attributed to a very low starting cell density (0.90 ± 0.07 g/L) after broth harvest.
It can be seen from Table 2, that the trend of variation in violacein production after harvests was similar to that with biomass accumulation. A 33–38% enhancement in violacein production was observed in cycle 2 and 3 of 60% (v/v) broth harvest. A similar enhancement of about 29% was observed in the second cycle of 40% (v/v) harvest which later decreased to 20% in cycle 3. Biomass accumulation was also adversely affected in this cycle. A significantly lower violacein titer was obtained in the second cycle of 80% (v/v) broth harvest. Thus, 60% (v/v) harvest for broth was selected for repeated fed-batch as it provided significant enhancement in violacein production and also displayed consistency in performance over three cycles of repeated fed-batch. The optimised multi-nutrient pulse feed followed by repeated fed-batch strategy, thus, provided a violacein titer of 1045.65 ± 15.72 mg/L and a productivity 26.12 ± 0.65 mg/L/h in each cycle, considering the 60% (v/v) harvest after every 40 h. The yield of biomass obtained over glucose was 0.37 ± 0.001 (g/g) and the yield of violacein over tryptophan was 1.56 ± 0.03 (mg/mg).
Gram-scale production of this microbial therapeutic compound by a natural producer is reported by Gohil et al. [15]. However the fermentation time reported in that study is significantly long (114 h) which brings down the overall productivity of violacein to 13.26 mg/L/h [15]. Cheng et al. [39] have reported higher violacein productivity up to 23.33 mg/L/h using formic acid as quorum sensing inducer in addition to feeding tryptophan. In the present study, a comparable gram-scale titer with respect to the available literature was achieved. However the combined feeding of tryptophan and glucose, and partial harvesting at optimum time improved the violacein productivity to 26.13 ± 0.65 mg/L/h, which to the best of our knowledge, is the highest amongst the studies reported for C. violaceum.
Kinetic modelling of the batch and fed-batch mode of fermentation
Kinetics of biomass accumulation
The biomass accumulation in batch fermentation was plotted and non-linear fitting was attempted using log-logistic model, Gompertz model and dose–response model (Fig. 4a) Log-logistic and Gompertz did not show a good fit for the prediction of the biomass production rate with experimental values. A very high COD of 0.991 was achieved with the curve fitting with the dose–response model, and which could predict the kinetic parameters of biomass accumulation suitably. The proposed pulse feed strategy was studied by modifying this model to the bi-dose–response model as given in Eq. 14.
where Xt is the biomass (g/L) accumulated at any time t (h), X0 is the initial biomass concentration (g/L), Xm is the maximum biomass accumulated during the cycle (g/L), and μ1 and μ2 are the specific rate of biomass accumulation (g/L/h) after first dosing at time t0 (h) and second dosing at time tc (h), respectively.
Kinetic models applied to the fermentation, a Biomass accumulation in batch mode (Log-logistic model, Gompertz model and dose response model), b Bi-dose (B-D) response model applied to biomass accumulation in three cycles of repeated fed-batch with multi-nutrient pulse feed and 60% (v/v) broth harvest, c reduction in residual glucose levels in batch mode (Exponential decay model and Weibull model). d Logistic decay model for the reduction in residual tryptophan in batch mode, e Dose response model for the production of violacein in batch mode, f Bi-dose (B-D) response model applied to violacein production in three cycles of repeated fed-batch with multi-nutrient pulse feed fermentation and 60% (v/v) broth harvest
Figure 4b shows the bi-dose (B-D) response fit plots for the three cycles of fed-batch fermentation with multi-nutrient pulse feed and 60% (v/v) broth harvest. It can be seen that consistent higher values of biomass accumulation were achieved in Cycle 2 and Cycle 3 and this improvement can be related to higher initial biomass concentration at the initiation of these cycle.
Kinetics of substrate utilization
Exponential decay model and Weibull model were applied to the kinetic data of residual glucose concentration in the batch mode of fermentation. Bedade et al. [24] had previously applied these models to study the uptake of carbon sources and specific inducers in fermentation of metabolites. Accordingly, the model fitting was attempted in this study. Figure 4c shows the curve fitting for both Exponential decay model and Weibull model. Exponential decay model fit had a R2 value of 0.807, which was a comparatively better fit than Weibull’s model. The decay rate evaluated in both the models was a measure of the velocity of glucose consumed. The decay rate predicted by the Weibull model (0.53 ± 0.01 g/L/h) was significantly overestimated while that predicted with exponential decay model (0.42 ± 0.01 g/L–h) was of lower magnitude, closer to the experimental value (0.31 ± 0.01 g/L/h). The exponential decay model was thus applied to individual decay curves of glucose pulses in the three cycles of 60% (v/v) harvest associated muti-nutrient fed-batch fermentation. The values of COD for each decay curve were > 0.8. The values of COD for each decay curve were > 0.8. Detailed data are provided in supplementary files.
The consumption of tryptophan was slower in the log phase of growth which later improved in early stationary phase and further declined in late stationary phase. Thus, the consumption of residual tryptophan displayed a sigmoidal profile unlike the exponential profile of residual glucose in broth (Fig. 4d). Hence, when curve fitting was attempted with exponential decay model, the fit parameters were not optimum (R2 < 0.8). The logistic model was, thus, suitably fitted (R2 = 0.990) and the decay rate of tryptophan was predicted as 0.095 ± 0.006 mM/h. The values for coefficient of determination were > 0.85 for each fit and the predicted values were coherent with experimental ones. The detailed data of this model fitting in fed-batch mode are given in supplementary file.
Kinetics of violacein production
Based on the outcomes of “Kinetics of biomass accumulation” section, the dose–response model was found to be optimum to study the effect of pulse feeds on biomass accumulation. A similar approach was undertaken to study the violacein production in batch mode as well as in fed-batch mode. Figure 4e shows the kinetic profile of violacein production in batch mode of fermentation. It can be seen that the profile was sigmoidal which indicated no significant production of violacein in the log phase but a significant production of violacein in the stationary phase of growth. The maximum violacein production rate was 9.92 ± 0.02 mg/L, and was observed in the early stationary phase. The predicted value of the overall violacein production rate from dose–response model (R2 = 0.996) was 8.64 ± 0.01 mg/L. The experimental and predicted values of maximum violacein produced in the batch were 508.23 ± 32.3 mg/L and 517.71 ± 7.17 mg/L indicating suitable prediction by the model. A modification of dose response model to bi-dose response model (Eq. 15) was developed to study the impact of dosing tryptophan on violacein production in repeated fed-batch model.
where Pt is the violacein produced (mg/L) at any time t (h), P0 is the concentration of violacein (mg/L), Pm is the maximum violacein produced during the cycle (mg/L), ρ1 and ρ2 are the specific rate of biomass accumulation (g/L/h) after first dosing at time t0 (h) second dosing at time tc (h), respectively (Table 3).
The violacein production in cycle 2 and cycle 3 of the repeated fed-batch process was higher than the first cycle (Fig. 4f) Similar to the batch fermentation, the violacein production in the first cycle of fed-batch was clearly non-growth associated. The violacein production rate improved after the second dosing of tryptophan. From Table 4 it can be seen that the predicted and the experimental data of violacein production rate validated this finding. However, in the second and the third cycles, the violacein production rate was higher during the initial phase of the cycles and clearly decreased after the second dosing of tryptophan. Thus, it was observed that the production of violacein was synchronous with biomass accumulation in Cycles 2 and 3 of the repeated fed-batch. The resulting titers of violacein were higher and productivity improved accordingly. Yang et al. [41] had claimed significantly higher titer of violacein when its production was growth associated. However, the organism used in that study was genetically modified. Our study is exclusively on the natural producer of violacein and we could achieve similar patterns of violacein production with the optimised media formulation and fed-batch strategy.
Conclusion
Media engineering was attempted for maximal violacein production from Chromobacterium violaceum MTCC2656. A two-level design of experiment approach helped in screening and optimising the levels of media components. Initially, for fed-batch studies, pulse feeding of glucose and tryptophan were combined, and later an optimised partial broth harvest strategy was applied for achieving maximum violacein productivity. The overall kinetic studies indicated that the performance of fermentation improved after the first harvest and was consistent in the progressive cycles for the proposed fed-batch operation.
Data availability
All the data sets generated and anlaysed during this study are provided in the manuscript and supplementary data. If any further information is needed, it can be provided on a reasonable request to the corresponding author.
References
Ahmed A, Ahmad A, Li R, AL-Ansi W, Fatima M, Mushtaq BS, Basharat S, Li Y, Bai Z. Recent advances in synthetic, industrial and biological applications of violacein and its heterologous production. J Microbiol Biotechnol. 2021;31:1465–80. https://doi.org/10.4014/jmb.2107.07045.
Pang L, Antonisamy P, Ali G, Fahad A, Al-dhabi NA, Valan M, Ponmurugan K, Ock Y, Kim H, Kim H. Nephroprotective effect of pigmented violacein isolated from Chromobacterium violaceum in wistar rats, Saudi. J Biol Sci. 2020;27:3307–12. https://doi.org/10.1016/j.sjbs.2020.10.004.
Dahlem C, Chanda S, Hemmer J, Schymik HS, Kohlstedt M, Wittmann C, Kiemer AK, Corbet C. Characterization of anti-cancer activities of violacein: actions on tumor cells and the tumor microenvironment. Front Oncol. 2022;12:1–11. https://doi.org/10.3389/fonc.2022.872223.
Alem D, Marizcurrena JJ, Saravia V, Davyt D, Martinez-Lopez W, Castro-Sowinski S. Production and antiproliferative effect of violacein, a purple pigment produced by an Antarctic bacterial isolate. World J Microbiol Biotechnol. 2020;36:1–11. https://doi.org/10.1007/s11274-020-02893-4.
Choi SY, Lim S, Yoon K, Lee JI, Mitchell RJ. Biotechnological activities and applications of bacterial pigments violacein and prodigiosin. J Biol Eng. 2021;9(2021):1–16. https://doi.org/10.1186/s13036-021-00262-9.
Fang MY, Zhang C, Yang S, Cui JY, Jiang PX, Lou K, Wachi M, Xing XH. High crude violacein production from glucose by Escherichia coli engineered with interactive control of tryptophan pathway and violacein biosynthetic pathway. Microb Cell Fact. 2015;14:1–13. https://doi.org/10.1186/s12934-015-0192-x.
Yang C, Jiang P, Xiao S, Zhang C, Lou K, Xing XH. Fed-batch fermentation of recombinant Citrobacter freundii with expression of a violacein-synthesizing gene cluster for efficient violacein production from glycerol. Biochem Eng J. 2011;57:55–62. https://doi.org/10.1016/j.bej.2011.08.008.
Sun H, Zhao D, Xiong B, Zhang C, Bi C. Engineering Corynebacterium glutamicum for violacein hyper production. Microb Cell Fact. 2016;15:1–9. https://doi.org/10.1186/s12934-016-0545-0.
Kholany M, Trébulle P, Martins M, Ventura SPM, Nicaud JM, Coutinho JAP. Extraction and purification of violacein from Yarrowia lipolytica cells using aqueous solutions of surfactants. J Chem Technol Biotechnol. 2020;95:1126–34. https://doi.org/10.1002/jctb.6297.
Liu Y, Feng J, Pan H, Zhang X, Zhang Y. Genetically engineered bacterium: principles, practices, and prospects. Front Microbiol. 2022;10:1–15. https://doi.org/10.3389/fmicb.2022.997587.
Mendes AS, De Carvalho JE, Duarte MCT, Durán N, Bruns RE. Factorial design and response surface optimization of crude violacein for Chromobacterium violaceum production. Biotechnol Lett. 2001;23:1963–9. https://doi.org/10.1023/A:1013734315525.
Rettori D, Dura N. Production, extraction and purification of violacein: an antibiotic pigment produced by Chromobacterium violaceum. World J Microbiol Biotechnol. 1998;14:685–8. https://doi.org/10.1023/A:1008809504504.
Ahmad WA, Yusof NZ, Nordin N, Zakaria ZA, Rezali MF. Production and characterization of violacein by locally isolated Chromobacterium violaceum grown in agricultural wastes. Appl Biochem Biotechnol. 2012;167:1220–34. https://doi.org/10.1007/s12010-012-9553-7.
Park HA, Park SA, Yang YH, Choi KY. Microbial synthesis of violacein pigment and its potential applications. Crit Rev Biotechnol. 2021;41:879–901. https://doi.org/10.1080/07388551.2021.1892579.
Gohil N, Bhattacharjee G, Gayke M, Narode H, Alzahrani KJ, Singh V. Enhanced production of violacein by Chromobacterium violaceum using agro-industrial waste soybean meal. J Appl Microbiol. 2021. https://doi.org/10.1111/jam.15277.
Ghimire A, Sah AK. Kinetics and modeling of growth and lactic acid production in Gundruk, a Himalayan fermented vegetable dish. Food Sci Nutr. 2020. https://doi.org/10.1002/fsn3.1854.
Cante RC, Gallo M, Nigro F, Passannanti F, Budelli A, Nigro R. Mathematical modeling of Lactobacillus paracasei cba l74 growth during rice flour fermentation performed with and without pH control. Appl Sci. 2021;11:1–11.
Ramakrishnan V, Goveas LC. Kinetic modeling, production and characterization of an acidic lipase produced by Enterococcus durans NCIM5427 from fish waste. J Food Sci Technol. 2015;52:1328–38. https://doi.org/10.1007/s13197-013-1141-5.
Schilde M, Von Soosten D, Hüther L, Kersten S, Meyer U, Zeyner A, Dänicke S. Dose–response effects of 3-nitrooxypropanol combined with low- and high-concentrate feed proportions in the dairy cow ration on rermentation parameters in a rumen simulation technique. Animals. 2021;11:1–18.
Liu L, Wang F, Pei G, Cui J, Diao J, Lv M. Repeated fed-batch strategy and metabolomic analysis to achieve high docosahexaenoic acid productivity in Crypthecodinium cohnii. Microb Cell Fact. 2020;19(91):1–14. https://doi.org/10.1186/s12934-020-01349-6.
DeMoss RD, Happel ME. Nutritional requirements of Chromobacterium violaceum. J Bacteriol. 1963;77:137–41. https://doi.org/10.1128/jb.77.2.137-141.1959.
Plackett RL, Burman JP. Design of optimum multifactorial experiments. Biometrika. 1946;33(1946):305–25. https://doi.org/10.2307/2332195.
Stanbury PF, Whitaker A, Hall SJ. Chapter 2-Microbial growth kinetics. In: Stanbury PF, Whitaker A, Hall SJ, editors, Princ. Ferment. Teschnology, 3rd ed., Butterworth-Heinemann; 2017. p. 21–74. https://doi.org/10.1016/B978-0-08-099953-1.00002-8.
Bedade DK, Dev MJ, Singhal RS. Bioreactor studies on acrylamidase produced from Cupriavidus oxalaticus ICTDB921: production, kinetic modeling, and puri fi cation. Biochem Eng J. 2019;149:107–17. https://doi.org/10.1016/j.bej.2019.107245.
Gallardo MJ, Staforelli JP, Meza P, Bordeu I, Torres S. Characterization of Chromobacterium violaceum pigment through a hyperspectral imaging system. AMB Express. 2014;4:1–9. https://doi.org/10.1186/2191-0855-4-4.
Agarwal A, Patil S, Gharat K, Pandit RA, Lali AM. Modulation in light utilization by a microalga Asteracys sp. under mixotrophic growth regimes. Photosynth Res. 2018;139:553–67. https://doi.org/10.1007/s11120-018-0526-8.
Wang J, Huang J, Shi J, Xu Q. Fermentation characterization of an L-tryptophan producing Escherichia coli strain with inactivated phosphotransacetylase. Ann Microbiol. 2013;63:1219–24. https://doi.org/10.1007/s13213-012-0579-4.
Wang H, Wang F, Zhu X, Yan Y, Yu X, Jiang P, Xing X. Biosynthesis and characterization of violacein, deoxyviolacein and oxyviolacein in heterologous host, and their antimicrobial activities. Biochem Eng J. 2012;67:148–55. https://doi.org/10.1016/j.bej.2012.06.005.
Füller JJ, Röpke R, Krausze J, Rennhack KE, Daniel NP, Blankenfeldt W, Schulz S, Jahn D, Moser J. Biosynthesis of violacein, structure and function of L-tryptophan oxidase VioA from Chromobacterium violaceum. J Biol Chem. 2016;291:20068–84. https://doi.org/10.1074/jbc.M116.741561.
Zhou Y, Fang M, Li G, Zhang C, Xing XH. Enhanced production of crude violacein from glucose in Escherichia coli by overexpression of rate-limiting key enzyme (s) involved in violacein biosynthesis. Appl Biochem Biotechnol. 2018;186:909–16. https://doi.org/10.1007/s12010-018-2787-2.
Chernogor L, Bakhvalova K, Belikova A, Belikov S. Isolation and properties of the bacterial strain Janthinobacterium sp. SLB01. Microorganisms. 2022;10:1–13. https://doi.org/10.3390/microorganisms10051071.
Zala DB, Khan V, Sanghai AA, Vohra M, Das VK. A case of Chromobacterium violaceum. J Microbiol Infect Dis. 2018;8:76–9. https://doi.org/10.5799/jmid.434632.
Batista BB, De Souza Santos RER, Ricci-Azevedo R, Da Silva Neto JF. Production and uptake of distinct endogenous catecholate-type siderophores are required for iron acquisition and virulence in Chromobacterium violaceum. Infect Immun. 2019;87:1–15. https://doi.org/10.1128/IAI.00577-19.
Batista JH, Leal FC, Fukuda TTH, Alcoforado Diniz J, Almeida F, Pupo MT, da Silva Neto JF. Interplay between two quorum sensing-regulated pathways, violacein biosynthesis and VacJ/Yrb, dictates outer membrane vesicle biogenesis in Chromobacterium violaceum. Environ Microbiol. 2020;22:2432–42. https://doi.org/10.1111/1462-2920.15033.
DeMoss RD, Evans NR. Physiological aspects of violacein biosynthesis in non-proliferating cells. J Bacteriol. 1959;78:583–8. https://doi.org/10.1128/jb.78.4.583-588.1959.
Bapat PM, Das D, Sohoni SV, Wangikar PP. Hierarchical amino acid utilization and its influence on fermentation dynamics: Rifamycin B fermentation using Amycolatopsis mediterranei S699, a case study. Microb Cell Fact. 2006;5:1–14. https://doi.org/10.1186/1475-2859-5-32.
Bafrncov P, Šmogroviˇ D. Improvement of very high gravity ethanol fermentation by media supplementation using Saccharomyces cerevisiae. Biotechnol Lett. 1999;21:337–41.
Pan X, Cai J. Improved fermentation yield of doramectin from Streptomyces avermitilis N72 by strain selection and glucose supplementation strategies. Fermentation. 2023;9:21–34. https://doi.org/10.3390/fermentation9020121.
Cheng K, Hsiao H, Hou Y, Hsieh C, Hsu H, Chen H, Lin S. Improvement in violacein production by utilizing formic acid to induce quorum sensing in Chromobacterium violaceum. Antioxidants. 2022;11:1–12. https://doi.org/10.3390/antiox11050849.
Giridhar R, Srivastava AK. Fed-batch sorbose fermentation using pulse and multiple feeding strategies for productivity improvement. Biotechnol Bioprocess Eng. 2000;5:340–4. https://doi.org/10.1007/BF02942209.
Yang D, Park SY, Lee SY. Production of rainbow colorants by metabolically engineered Escherichia coli. Adv Sci. 2021;8:1–13. https://doi.org/10.1002/advs.202100743.
Funding
This study is funded by the All-India Council of Technical Education, New Delhi, India.
Author information
Authors and Affiliations
Contributions
KG: Conceptualization, Methodology, Validation, Analysis, Investigation, Writing original draft, Visualization, Software RSS: Conceptualization, Supervision, Project administration, Resources, Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
Both authors agree to submit this article for publication in Systems Microbiology and Biomanufacturing.
Ethics approval and consent to participate
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gharat, K., Singhal, R.S. A strategic media engineering and fed-batch approach enhances violacein production by Chromobacterium violaceum MTCC2656. Syst Microbiol and Biomanuf 4, 1106–1120 (2024). https://doi.org/10.1007/s43393-023-00230-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00230-y