Abstract
Phytases are important enzymes used for eliminating the anti-nutritional properties of phytic acid in food and feed ingredients. Phytic acid is major form of organic phosphorus stored during seed setting. Monogastric animals cannot utilize this phytate-phosphorus due to lack of necessary enzymes. Therefore, phytic acid excretion is responsible for mineral deficiency and phosphorus pollution. Phytases have been reported from diverse microorganisms, however, fungal phytases are preferred due to their unique properties. Aspergillus species are the predominant producers of phytases and have been explored widely as compared to other fungi. Solid-state fermentation has been studied as an economical process for the production of phytases to utilize various agro-industrial residues. Mixed substrate fermentation has also been reported for the production of phytases. Physical and chemical parameters including pH, temperature, and concentrations of media components have significantly affected the production of phytases in solid state fermentation. Fungi produced high levels of phytases in solid state fermentation utilizing economical substrates. Optimization of culture conditions using different approaches has significantly improved the production of phytases. Fungal phytases are histidine acid phosphatases exhibiting broad substrate specificity, are relatively thermostable and protease-resistant. These phytases have been found effective in dephytinization of food and feed samples with concomitant liberation of minerals, sugars and soluble proteins. Additionally, they have improved the growth of plants by increasing the availability of phosphorus and other minerals. Furthermore, phytases from fungi have played an important roles in bread making, semi-synthesis of peroxidase, biofuel production, production of myo-inositol phosphates and management of environmental pollution. This review article describes the production of fungal phytases in solid state fermentation and their biotechnological applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a crucial element for animal and plant nutrition due to its critical role in their growth and development, but it does not have a natural replenishment cycle. All living organisms need an adequate amount of phosphorus because it is involved in the formation of cell membranes, nucleic acids, and enzyme regulation (Singh et al. 2011; Vashishth et al. 2023; Priya et al. 2023). As a result, animal diets must include sufficient inorganic phosphorus (Pi). Livestock and poultry are generally administered dicalcium phosphate as a part of their dietary intake to ensure the fulfillment of their requisite daily nutrient needs. This phosphorous and calcium supplement is incorporated into their feed to promote optimal growth and development, thereby enhancing the overall health and productivity of the animals (Jain et al. 2016; Singh et al. 2020).
Phosphorus, an essential mineral, predominantly exists in the form of phytic acid (PA), accounting for approximately 18–88% of the total phosphorus content in various plant-based sources. Phytate constitutes 1–5% of the weight in certain foodstuffs, including wheat bran, cereals, rice bran, legumes, and oilseeds (Singh and Satyanarayana 2011b, 2015; Moreira et al. 2014; Awad et al. 2014; Coban and Demirci 2014). Inability of monogastric animals to break down phytate P necessitates the addition of exogenous phosphorus to their diets, increasing the P load and resulting in large amounts of P excretion in feces in regions with high animal production. This leads to phosphorus pollution in the environment. Moreover, the defecation of unprocessed phytate along with inorganic phosphorus raises universal environmental concerns related to P eutrophication in areas with extensive cattle farming (Liu et al. 2022). Excessive phosphorus in the soil can be washed away during different weather cycles into various water bodies such as rivers and ponds, leading to rapid cyanobacterial blooms, growth of phytoplankton, algae, lack of oxygen, and the aquatic species death (Vats and Banerjee 2005; Singh et al. 2020).
Phytases have been found in mammals, plants, and microbes, with microbial phytases being predominantly researched for worldwide commercial applications (Singh et al. 2011; Singh and Satyanarayana 2011a; Jain et al. 2016; Kaur et al. 2017). Fungal phytases are potentially excellent candidates for eliminating anti-nutrients from plant-based diets due to their natural activity of breaking the phospho-monoester linkages present in the phytates (Singh et al. 2020; Kumari and Bansal 2021). Consequently, soil microorganisms have been studied for phytase production, although more sources are still required to be identified (Kalsi et al. 2016). Filamentous fungal species that can produce phytases during the fermentation process are Aspergillus oryzae, A. fumigatus, Mucor piriformis, A. niger, A. carbonarius, Rhizopus oligosporus, and Cladosporium species (Jatuwong et al. 2020). Fungi produce high levels of phytases in solid state fermentation as compared to bacteria and other sources.
Phytases break down phytic acid into Pi and myo-inositol, effectively excluding their anti-nutritional effects (Sapna and Singh 2017a, 2017b; Singh et al. 2011, 2020; Singh and Satyanarayana 2011a). Phytic acid, mainly in the form of inositol triphosphates, serves as a vital component in the regulation of signaling and cellular functions in both plant and animal cells (Goyal et al. 2022). The presence of inositol phosphates (InsP 3) in signal transduction pathways can affect the control of the cell cycle, as well as the growth and differentiation of cancerous cells (Pujol et al. 2023). Phytase reduces the ability of phytic acid to form complexes with essential minerals, enzymes, and proteins (Fig. 1). Fungi, among microorganisms, are the primary producers of phytases, and their unique attributes, such as their wide range of substrates specificity, and the ability to function effectively across a broad spectrum of pH levels and temperatures, make them potentially valuable in multiple domains. This review article narrates the classification, fungal sources, and optimization of production process, peculiar features, and advanced biotechnological applications of fungal phytases. It also describes the importance of myo inositol triphosphate, an intermediate produced during hydrolysis of phytic acid.
Classification of phytases
Based on their optimal pH for activity, phytases have been classified as acidic or alkaline. Fungal phytases and certain bacterial phytases are acidic, whereas phytases from Bacillus spp. and plants are alkaline in nature (Jain and Singh 2017; Singh et al. 2020; Pragya et al. 2021). Phytases have been categorized into 3-, 5-, and 6-phytases based on their selective attack on phosphorus linked to inositol moiety (Kumar and Sinha 2018). Only 3-phytases are documented in microorganisms, whereas 6-phytases are found in plants. Based on their catalytic processes, Phytases can be categorized into four distinct groups, namely HAP (histidine acid phosphatases), CP (cysteine phosphatases), BPP (β-propeller phytase), and PAP (purple acid phosphatases). This classification depends on reaction mechanisms, amino acid sequences, biochemical properties, and 3D conformations. HAP phytases have been reported in microbes as well as plants. Natuphos, the earliest commercial phytase, is a HAP synthesized by Aspergillus niger var ficuum (Chen et al. 2015). The enzymes exhibit a shared catalytic site structure situated at the junction of the two domains, which includes the well-preserved N-terminal active site motif RHGXRXP and the C-terminal HD. In the two-step process, the first step involves the histidine from the RHGXRXP motif initiating a nucleophilic attack on the phosphorus, resulting in the formation of a covalent phosphohistidine intermediate. Concurrently, the aspartic acid from the HD motif serves as a proton donor to the oxygen atom within the cleavable phosphomonoester bond. The necessity for the protonation of the aspartate carboxylate group to enable proton donation to the departing group explains the preference for an acidic pH for the biocatalysis (Fan et al. 2016). During substrate hydrolysis, the N- and C-terminal portions of HAPs combine to create the catalytic core (Gessler et al. 2018).
Fungal and bacterial HAPs exhibit comparable architectures, although with certain distinct features. Among these enzymes, the main detection of BPP phytases is predominantly observed in Bacillus species and similar types of bacteria. These particular phytases are occasionally referred to as alkaline phytases owing to their highest catalytic efficiency within the pH range of 7.5 to 8.0. The three-dimensional configuration of BPP phytases comprises a hexameric assembly resembling a six-bladed propeller, providing a rationale for their nomenclature (Sanangelantoni et al. 2018). The phytases under consideration exhibit distinctive catalytic characteristics, demonstrating both resistance to proteases and specificity towards phytate substrates (Jain and Singh 2017). BPPs are characterized by their remarkable thermostability, dependence on Ca2+ ions, and an optimal enzymatic activity observed within the pH range of neutrality to alkalinity (Kaur et al. 2017; Jain and Singh 2017). In contrast, phytase from B. licheniformis required low Ca2+ for their catalytic activity (Borgi et al. 2014).
Purple acid phosphatases have been identified in mammals, plants, and fungi. These enzymes, referred to as purple or pink phytases, derive their name from the presence of Fe2+, Fe3+, Mn2+, and Zn2+ in their catalytic centers. Found predominantly within plant structures, they exhibit resemblance to other plant PAPs. One specific PAP, known as GmPhy, is discovered in the developing cotyledons of sprouting soybean (Glycine max L. Merr.) and bears a structural resemblance to a kidney bean. This enzyme, described by Xiao et al. (2005), demonstrates comparable characteristics to other plant PAPs highlighted by Singh and Satyanarayana (2015). This specific phytase assembly has a limited range of catalytic activity. Notably, all PAPs in this group include five conserved blocks of metal-ligating residues, which are recognized as distinguishing features of GmPhy (Feder et al. 2020; Langeroudi et al. 2023). It is worth noting, however, that MtPHY1, a phytase produced from Medicago truncatula, lacks the entire set of all five blocks, separating it from other members of the group (Ma et al. 2012). In a recent study, a bacterium capable of producing PAP has been identified in earthworm castings (Ghorbani et al. 2018).
The CPs are phytases that belong to a new subfamily and share a catalytic mechanism with protein tyrosine phosphatases (Puhl et al. 2008; Gontia-Mishra and Tiwari 2013; Gruninger et al. 2014). They exhibit notable phytase activity in acidic environment and are selective for tyrosine phosphates. Selenomonas ruminantium, a ruminal bacterium, was the first organism to have this type of phytase identified (Mullaney and Ullah 2006). Figure 2 presents a comprehensive classification of phytases based on different criteria.
Sources and production of fungal phytases in solid state fermentation
Phytases have been identified in mammals, plants, and microbes, although microbial phytases have been largely studied for commercial uses globally (Jain and Singh 2017; Puppala et al. 2019; Pragya et al. 2023). Therefore, soil microorganisms have been extensively screened as sources of phytases; nonetheless, it is required to uncover novel sources (Kalsi et al. 2016). Phytases have been reported from microorganisms including fungi, yeasts and bacteria (Abd-ElAziem et al. 2015; Gaind and Singh 2015; Hellström et al. 2015; Singh et al. 2015; Jain et al. 2016). Fungal phytases are favored over bacterial phytases due to their desirable characteristics, including protease resistance, high activity/yield and extracellular nature (Song et al. 2019; Priya et al. 2023). Unlike unicellular fungi, most filamentous fungi are well-suited for both submerged and solid-state fermentations because of their filamentous growth, which covers a larger surface area and allows them to penetrate solid substrates (Rizwanuddin et al. 2023a).
Over a century ago, phytase activity in microbes was first discovered in Aspergillus niger. Since then, there have been numerous scientific reports on fungal phytases, with a particular focus on those derived from the species of Penicillium, Rhizopus, Aspergillus, Thermomyces, Mucor, and Trichoderma. Both mesophilic and thermophilic fungi are known to secrete phytases. Fungi, such as Aspergillus oryzae (Sapna and Singh 2014; Pragya et al. 2023), Humicola nigrescens (Bala et al. 2014), Aspergillus flavus (Haind and Singh 2015), Sporotrichum thermophile (Singh and Satyanarayana 2011a; Kumari et al. 2016), Penicillium purpurogenum (Awad et al. 2014)d niger (Kumari and Bansal 2021) have been recorded for their ability to degrade phytate. Fungi have certain advantages in phytate degradation, including their ability to extract P proficiently from soil, easy maintenance of culture, and high enzyme yields. Additionally, fungi exhale high concentrations of organic acids that functions as a chelator and play a significant role in Pi solubilization. Microbial phytases are safe biofertilizers and do not involve the use or release any harmful substances, making them beneficial for farmers practicing organic farming (Gessler et al. 2018; Shahryari et al. 2018; Jatuwong et al. 2020; Singh et al. 2020; Rizwanuddin et al. 2023).
Mesophilic microorganisms are recognized for producing more potent phytases when operating within the temperature range of 28 to 35 °C, which aligns with the optimal conditions for their growth and development (Sapna and Singh 2014; Kumari and Bansal 2021; Rizwanuddin et al. 2023). The temperature plays a critical role in regulating factors like water activity and humidity, affecting processes such as transport across cell membranes and cellular metabolism (Suresh and Radha 2015). Similarly, many filamentous fungi including R. oligosporus (Suresh and Radha 2015), A.niger and N. sitophila (Kanti et al. 2020), A. aculeatus (Saxena et al. 2020), A. oryzae (Sapna and Singh 2014), A. flavus (Onibokun et al. 2022), A. niger (Nascimento et al. 2022), and A.oryzae (Pragya et al. 2023) also secreted phytases maximally at 30 °C.
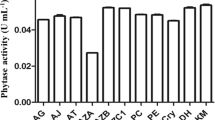
Most enzymatic activity and the transit of various components through cell membranes are influenced by the pH of the culture medium. High phytase secretion has been recorded in all filamentous fungi cultivated in SSF under acidic conditions (Singh and Satyanarayana 2008; Sapna and Singh 2014; Gupta et al. 2015; Tian and Yuan 2016; Kumari and Bansal 2021). Among all the pH tested, pH 5.0 supported high phytase production by Aspergillus sp. (Tian and Yuan 2016), A. niger (Gupta et al. 2015), A. oryzae (Sapna and Singh 2014; Pragya et al. 2023), and A. niger NT7 (Kumari and Bansal 2021). The extracellular pH influencing microbial production of phytase were pH 6.0 and 5.5 for high phytase production (Elkhateeb and Fadel 2022). In contrast, all the five fungi tested (A. niger, A. fumigatus, A. flavus, Mucor rouxii, and P. purourogenum), exhibited high phytase production at pH 7.0 (Sadaf et al. 2022). Production and properties of fungal phytases in SSF using various substrates are summarized in Table 1.
Solid state fermentation (SSF) presents the ability to use economical agro-industrial residues as substrate for the cultivation of fungi (Pragya et al. 2023). This bioprocess involves a minimal amount of free water within the interstitial space of the solid particles (Li et al. 2023). Nonetheless, the process provides sufficient moisture to sustain the growth and metabolism of microorganisms (Prado Barragán et al. 2016). The SSF approach has led to a reduction in operational costs associated with the regulation of temperature, agitation, pH, and aeration (Soccol et al. 2017; Srivastava et al. 2019; Piecha et al. 2023). Nowadays, a variety of fermentation techniques are often used in solid-state and submerged fermentation procedures to enhance the synthesis of enzymes generated by fungi (Mahendran et al. 2022; Dixit and Shukla 2023). The SSF system offers a number of advantages due to its low water, low aeration, easy fermentation medium, and low energy requirements (Kassim et al. 2022). Additionally, aeration is simple because an improved oxygen diffusion rate into wet solid substrate to assist microbial development prevents oxygen restriction from happening (Sapna and Singh 2014). Selecting the right substrate is a vital factor in ensuring the success of SSF process, as various factors such as substrate characteristics and the growth of microorganisms can have an impact on the SSF process (Kumar et al. 2021).
Fungi like Aspergillus tubingensis, A. niger, A. flavus, A. ficuum, and Rhizopus oryzae, which are filamentous in nature, are typically grown using SSF. However, SSF faces challenges such as inadequate nutrient utilization and limited biomass growth, resulting from a lack of free water content and the buildup of heat and moisture loss throughout the process. Essential substrates for fostering fungal growth and their metabolism include, wheat bran, rice bran, soybean meal, corn cobs, and citrus peels (Dahiya and Singh 2019; Rizwanuddin et al. 2023a). Triticale, containing grains like bali and barley, serves as a substrate for A. niger phytase production (Soccol et al. 2017; Rizwanuddin et al. 2023a), while corn bran and maize cob were employed for phytase production by P. purpurogenum (Awad et al. 2014). Natural substrate mixtures supply appropriate nutrients when compared to individual substrates and act as a support for microbial growth and development (Kumari et al. 2016). This is because the mixed substrate contains adequate nutrients and inducers for phytase synthesis. Higher PA concentration in wheat bran has previously been described as the primary inducer of phytase synthesis by microorganisms in SSF (Gupta et al. 2014; Tanruean et al. 2021). Lignocellulosic components (cellulose and hemicellulose) of plant residues supply carbohydrates/sugars for rapid development and metabolism of fungi (Miao et al. 2019). Similarly, the increased PA content of wheat bran and mustard oil cake allowed Rhizopus oligosporus MTCC556 to produce the most phytase in SSF (Suresh and Radha 2015). A. oryzae used mixed agri- industrial substrate (Wheat bran + rice straw) for phytase production (Pragya et al. 2023). The use of mixed substrates in phytase production by fungi offers several advantages. Firstly, by combining various substrates such as agricultural residues, oilseed cakes, and organic waste materials, a rich nutrient composition can be achieved, promoting fungal growth and enzyme production (Kumari et al. 2016). Additionally, mixed substrates can provide a cost-effective alternative compared to single substrates, as they utilize readily available and potentially inexpensive feedstocks (Kanti et al. 2020). The utilization of mixed substrates also contributes to waste management and sustainability efforts, by converting organic waste materials into valuable products (de Oliveira Ornela and Souza Guimarães 2019). This method is favored by the fermentation industry due to its reduced time consumption, simplicity, cost-effectiveness, and the ease of enzyme extraction with water (Srivastava et al. 2019).
Optimization of phytase production in solid-state fermentation
The process of optimizing conditions in experiments conventionally involves using a method called one variable at a time (OVAT) optimization, where only one factor is altered at a time while the others remain constant to optimize the process/conditions (Sagar Verma et al. 2022). The OVAT optimization, also known as single factorial optimization, is a traditional experimental approach to optimize the entire system by altering one factor at a time. This approach helps to identify critical parameters, improve manufacturing yield, and is easy to understand and apply (Sagar Verma et al. 2022).
Statistical experiment design is an efficient approach for optimization, particularly in predicting interactions between variables and identifying significant components affecting phytase production. Using factorial design and response surface methodology, a combination of factors starting at a certain optimum factor response can be determined, leading to an increase in phytase production (Kumari and Bansal 2021, Pragya et al. 2023). Statistical optimization techniques offer optimum media with minimal experiments in a short period, taking into account the interaction between selected components, which is crucial for enhancing the synthesis of phytases (Bhavsar et al. 2013; Sapna and Singh 2014; Kumari et al. 2016; Shahryari et al. 2018). The use of statistical methods in diverse bioprocessing procedures, particularly in the selection of main ingredients in the medium, has drawn considerable attention. In comparison to conventional methods, previous research has demonstrated that statistical methodology significantly enhanced phytase synthesis in SSF (Jatuwong et al. 2020; Kanti et al. 2020; Ahmed et al. 2021; Kumari and Bansal 2021) and is therefore, a preferred method for optimizing production of phytase (Ahmed et al. 2021).
Plackett-Burman design, a screening technique, efficiently identifies critical factors affecting phytase production. It allows for the screening of numerous factors in a relatively small number of experiments (Kumari and Bansal 2021). By assessing the main effects of factors, Plackett-Burman design (PBD) helps in selecting influential variables for subsequent optimization using other experimental designs (Ahmed et al. 2021). Factors such as pH, temperature, carbon and nitrogen sources, inducers, and trace elements have been identified as key variables influencing phytase production. Response surface methodology is a powerful statistical tool that combines mathematical models and experimental design to optimize process variables and their interactions (Wang et al. 2017). RSM helps to understand the complex relationships between factors and responses, enabling the identification of optimum conditions for phytase production. RSM also provides insights into the interactions between variables, enabling fine-tuning of the process for enhanced enzyme production (Shahryari et al. 2018). The combination of PBD and RSM offer a comprehensive approach for phytase production optimization (Wang et al. 2017) When compared to unoptimized conditions, phytase production has increased dramatically due to OVAT and statistical approaches (Table 2).
Peculiar features of fungal phytases
Phytases from distinct sources display different characteristics. Their qualities include glycoprotein nature, thermal stability, substrate specificity, enzyme levels, and protease-resistance (Singh and Satyanarayana 2015; Singh et al. 2020). Fungal phytases are mostly monomeric proteins with molecular weights in the range of 38 to 500 kDa and are secreted in larger quantities as compared to bacterial and other sources. For usage in the feed and food sectors, phytases that can tolerate protease action and acidic conditions are mostly preferable. Fungal and bacterial phytases respond differently to pepsin and trypsin (Singh et al. 2020). Fungal phytases are acidic phytases and have been studied in more depth than alkaline phytases due to their multifarious applications. Fungal phytases are highly useful in improving soil fertility and in aquaculture, where lower temperature conditions favour their activity and suitability under these conditions (Pragya et al. 2023; Priya et al. 2023).
Acidic phytases
Acidic phytases have been studied in more depth than alkaline phytases due to their roles in enhancing nutritional quality of food and feed ingredients. Fungal phytases are acidic phytases as compared to neutral and alkaline phytases from bacteria. The optimum pH of fungal phytases plays a critical role in determining their efficacy in catalyzing the breakdown of phytic acid. The search of an ideal phytase involves evaluating its efficacy within the stomachs of both humans and animals during the digestive process, where the pH levels typically range from 1.5 to 3.5. Phytases that remain active under acidic conditions can significantly enhance their applicability in the food industry. According to Saxena et al. (2020), the partially purified phytase obtained from Aspergillus aculeatus APF1 exhibited its highest level of activity at an acidic pH of 3.0. Furthermore, the purified phytase from Aspergillus niger BIONCL8 demonstrated a wide range of pH stability, reaching its peak activity at pH 2.1 (Bhandari et al. 2023). While a general pH range of 3.0 to 5.0 is commonly observed among many fungal phytases, variability exists among different fungal species (Filippovich et al. 2023). Phytase of P. oxalicum PJ3 (Lee et al. 2014), A. niger CFR335 (Shivanna and Venkateswaran 2014), A.oryzae SBS50 (Pragya et al. 2023) and A. niger (Neira-Vielma et al. 2018) were optimally active between pH 4.0–5.3. The maximum activity of phytase was obtained from T. purpureogenus NSA20 (Ahmed et al. 2021)d polonicum MF82 (Kalkan et al. 2020) at pH 5.5. Phytase of Aspergillus flavus showed optimal activity at pH 6.0 (Onibokun et al. 2022). The crude phytase from A. niger NT7 displayed activity under acidic conditions (Kumari and Bansal 2021). Recent advances in biotechnology and enzyme engineering have allowed for the manipulation of pH activity profile of fungal phytases (Zhou et al. 2022).
Thermostable phytases
Temperature is a significant parameter that influence the activity of fungal phytases (Priya et al. 2023; Pragya et al. 2023). The optimum temperature of fungal phytases can vary depending on the fungal species. However, the optimal temperature for phytase activity typically ranges from 40 to 60 °C, depending on the sources of the enzymes (Goyal et al. 2022). The purified phytases from A. fumigatus (Sanni et al. 2019) and A. niger S2 (Sandhya et al. 2019) had temperature optima at 40 °C (Sanni et al. 2019). The ideal temperature for phytase derived from Yersinia intermedia (Lahiji et al. 2021) and A. oryzae (Pragya et al. 2023) was determined as 50 °C. Phytase from P. polonicum MF82 had optimal activity at 60 °C (Kalkan et al. 2020).
The pH and thermo-stability of phytases are critical factors that significantly influence their catalytic efficiency, functionality, and applicability in various industries (Pragya et al. 2021). In the feed and food processing industry, the feed is treated at a high temperature and this is the phase where phytases are typically added to the feed before pelletization. Therefore, the thermal stability of phytase is critical and required for this treatment (Coutinho et al. 2020). Phytase from A. niger displayed high thermostability, maintaining 70% of its activity at 80 °C (Neira-Vielma et al. 2018). Phytases derived from A. fumigatus and A. niger experienced denaturation at 50 and 70 °C, respectively (Wyss et al. 1998), while Thermomyces lanuginosus TL-7 phytase demonstrated high tolerance at 70 °C by maintaining 70% of its activity (Gulati et al. 2007). Phytase from Aspergillus aculeatus APF1 showed high activity at 55 °C (Saxena et al. 2020) and from A. niger NT7 at 60 °C (Kumari and Bansal 2021). Phytase derived from A. niger remained stable even at 80 °C and retained 30% of its activity (Bhandari et al. 2023). Partially purified phytase from A. oryzae was thermostable upto 60 °C (Pragya et al. 2023).
Protease-insensitive phytases
Proteases are naturally present in the digestive systems of all living organisms. Therefore, the ability to withstand protease activity is highly esteemed among feed enzymes and is essential for their effectiveness as additives in animal feed (Gordeeva et al. 2023). Proteolysis, the enzymatic degradation of proteins, is a common challenge that enzymes face, especially during industrial applications and gastrointestinal transit. Fungal phytases are no exception, as they are exposed to proteolytic enzymes in animal digestive systems, food processing conditions, and various biotechnological processes (Jatuwong et al. 2020). The susceptibility of phytases to proteolytic degradation can reduce their efficacy and limit their applications. Fungal phytases exhibit significant diversity unlike bacterial ones in their ability to withstand the actions of pepsin and trypsin (Yu et al. 2015). A. niger phytase showed noticeable protease resistance (Ushasree et al. 2014). The protease susceptibility test revealed that the enzyme maintained 80% of its activity following exposure to pepsin. The enzyme retained 60% of its activity after treatment with higher levels of pepsin (Ushasree et al. 2014). Recombinant phytase of S. thermophile also showed resistance to proteases, as it retained 95% of its initial activity against pepsin and trypsin (Ranjan et al. 2015). The phytase from P. polonicum MF82 maintained its full activity after exposure to trypsin (Kalkan et al. 2020). Phytases from A. oryzae SBS50 (Sapna and Singh 2017a), A. aculeatus APF1 (Saxena et al. 2020)d tubingensis TEM 37 (Çalışkan-Özdemir et al. 2021) also observed high resistance to proteases. When subjected to trypsin, phytases from A. niger, R. mucilaginosa, and A. oryzae retained 10%, 75%, and 84% of their respective activities (Yu et al. 2015). Phytase from P. polonicum MF82 maintained 100% activity after exposure to trypsin (Kalkan et al. 2020).
Broad substrate specificity
Substrate specificity refers to the ability of an enzyme to recognize and bind specific substrates, initiating the enzymatic reaction. Fungal phytases possessing a wide range of substrate compatibility can efficiently break down phytate into myo-inositol monophosphate without significant buildup of intermediate compounds. Conversely, bacterial phytases with a limited substrate range lead to the accumulation of myoinositol tris- and bisphosphate as intermediates during the degradation of phytate (Singh et al. 2018; Kaur et al. 2021). Phytases from fungi showed broad-substrate specificity in contrast to bacterial phytases that are phytate-specific (Singh et al. 2011; Singh and Satyanarayana 2015; Jain et al. 2016; Jatuwong et al. 2020). The purified phytase from A.niger dephosphorylated various substrates such as, 1- naphthyl phosphate, phenyl phosphate and 2-naphthyl phosphate in addition to sodium phytate (Neira-Vielma et al. 2018). The purified recombinant phytase from Myceliophthora thermophila (syn. Sporotrichum thermophile) effectively hydrolyzed various organic phosphates besides phytic acid (Ranjan and Satyanarayana 2016). Moreover, the process of molecular docking of phytase with different substrates revealed distinct binding patterns (Singh et al. 2018). The docking simulations demonstrated a high binding affinity with phytic acid and ATP, while phosphoenol pyruvate and AMP exhibited the lowest binding affinity (Singh et al. 2018). The phytase from Sporotrichum thermophile possesses a larger catalytic pocket, contributing to its wide substrate specificity. This larger pocket of fungal phytases enables them to hydrolyze a diverse range of organic phosphates as compared to bacterial phytases (Kumari et al. 2016; Singh et al. 2018).
Products of phytate degradation
Phytases work by cleaving the phosphate groups from the inositol ring of phytate, leading to the formation of lower inositol phosphates, including inositol pentakisphosphate (InsP5), inositol tetrakisphosphate (InsP4), and ultimately inositol trisphosphate (InsP3) or even inositol bisphosphate (InsP2) (Gupta et al. 2014). Phytate degradation products using fungal phytases are mentioned in Table 3. The breakdown of phytate into these lower-phosphate forms not only improves the nutritional value of food and feed but also has positive environmental implications. The combination of A. fumigatus phytase and A. niger acid phosphatase successfully released all six phosphate groups results in production of myo-inositol 1-monophosphate (Wyss et al. 1999). Studies have also demonstrated that fungal phytases can effectively hydrolyze phytate and liberate phosphorus in the form of inorganic phosphate, reducing sugars and soluble proteins (Sapna Singh 2017). Aspergillus oryzae SBS50 phytase supplementation enhanced the release of inorganic phosphate, reducing sugars and soluble proteins from the flours (Pragya et al. 2023). The products of phytate degradation play a vital role in enhancing phosphorus availability for monogastric animals, such as poultry and swine, in their diets (Priya et al. 2023).
Biotechnological applications of fungal phytases
Phytases are widely used in many sectors, including the food industry. Their function in the production of animal feed, human food, the production of bread, and the processing of various cereal grains has been investigated (Nadeem et al. 2023). Numerous studies have shown that the uses for microbial phytases in food and feed are the most promising. In addition, recent advancements in biotechnological research have led to the creation of microbial phytases that may aid in the promotion of plant growth and the reduction of environmental phosphorus pollution (Singh and Satyanarayana 2011a, 2015; Bhavsar et al. 2013). Fungal phytases have been tested for multifarious applications (Fig. 3). Phytases are predominantly sourced from genetically modified strains due to the limited protein production by wild-type strains in comparison to the commercial demand. The leading product in the market is Natuphos, which contains a phytase derived from A. niger var. ficuum. In 2016, BASF introduced an improved version of Natuphos known as Natuphos E (Correa et al. 2020). This upgraded version exhibits enhanced resistance to pepsin, tolerance to adverse pH conditions, sustained activity under high processing temperatures, and an extended shelf life. The possible microbial strains that have the ability to produce phytase, along with their potential functions, are outlined in Table 4.
Improving monogastric nutrition
Most of the monogastric animals (poultry, piggery, fish) and humans use the plant-based foods to meet their nutritional requirements. However, many of nutritious components are not available for absorption during digestion owing to their interactions with the phytates (Kebreab et al. 2012; Priya et al. 2023). Therefore, phytase treatment is necessary in order to increase the bioavailability of minerals and nutrients with simultaneous reduction of anti-nutritional factor. Therefore, phytases used in food and feed industries must retain their activities in digestive tracts of monogastric animals (Bhandari et al. 2023). This is a requisite to ensure the successful biodegradation of phytate present in plant-based diets. Intestinal simulation studies have been conducted to study the efficacy of fungal phytases (Rodriguez et al. 2018; Lopes et al. 2021). Coutinho et al. (2020) observed similar action of immobilized and free phytase under simulated conditions. However, immobilized phytase exhibited high action at lower pH values than the free-phytase. In fishes, empty stomach pH varies from 5 to 7.0 that become highly acidic during digestion (Rodriguez et al. 2018). Therefore, highly acidic and acid-stable phytases are highly suitable for application in aquaculture (Moriarty 1973; Lopes et al. 2021; Priya et al. 2023).
The addition of phytase with citric acid to granulated feed has the potential to benefit the environment in carp farming, as it can reduce the phosphorus excretion from fish, thereby mitigating its environmental impact (Maly et al. 2023). The research findings indicated that using phytase as a feed additive for Tilapia sp. offers numerous advantages with no adverse effects. Among various fungal species tested, Aspergillus tubingensis demonstrated the highest yield of phytase (Mahendran et al. 2022). Mahmood et al. (2023) reported that supplementation of A. niger phytase to poultry diets improves the growth rate of broiler chickens, leading to increased body weight gain. Aspergillus niger BIONCL8 strain demonstrated a substantial reduction in phytate content in six poultry feed ingredients, making it a potential supplement for improving poultry feed (Bhandari et al. 2023). Because phytase has a specific target application, it cannot be considered universally ideal for both in vivo and in vitro use in all situations. For instance, in poultry, neutral phytases perform better, while acidic phytases are more effective in piggery. Additionally, the temperature optima for swine or poultry diets differ from those in aquaculture, leading to the use of distinct microbial phytases for various applications (Rizwanuddin et al. 2023a).
Bread making
The enzyme phytase is employed more prevalently in the food industry as compared to the feed industry, a preference mainly attributed to its unique property of complexing with crucial minerals like iron, zinc, and calcium in the human body (Longin et al. 2023). This distinctive characteristic of phytase facilitates its extensive utilization in the food sector, emphasizing its role in enhancing the bioavailability of these essential minerals, thereby contributing significantly to human health. This binding reduces mineral deficiencies and increases the bioavailability of essential minerals, ultimately improving the health of individuals who lack sufficient minerals (Handa et al. 2020; Rizwanuddin et al. 2023a). Addition of fungal phytase to whole wheat breads resulted in improved bread making (Goyal et al. 2022). Phytase of P. anomala effectively dephytinized whole wheat unleavened flat Indian breads like naan and tandoori (Joshi and Satyanarayana 2015). Phytase of S. thermophile has effectively reduced phytic acid in breads with concomitant amelioration of nutrition (Singh et al. 2011). Recombinant phytase of S. thermophile resulted in dephytinization of roti, naan, tandoori and bread with improved nutritional properties (Ranjan and Satyanarayana 2016).
Synthesis of peroxidases
Vanadium is an inhibitor of acid phosphatases due to similarity with phosphate. Vanadate-treated acid phosphatase demonstrated the activity of a peroxidase (Tanaka et al. 2005; Sharma et al. 2020). Histidine acid phosphatases (HAP-phytases) showed similarities with vanadium haloperoxidases by substitution of phosphate with vanadate (Renirie et al. 2003). These haloperoxidases have great potential as catalysts in oxidative reactions/processes. Fungal phytases are HAP-phytases, therefore, can easily be converted into peroxidases due to incorporation of vanadate in their active sites (Velde et al. 2000). Phytase was synthesized as a CLEA and vanadium-haloperoxidase activity due to incorporation of vanadate into active site of HAP-phytase was employed in thioanisolesulfoxidation in the presence of hydrogen peroxide. This enzyme showed selectivity, and recyclability with high conversion rate (Correia et al. 2008). Vanadate ion incorporated into the P. anomala and S. thermophile phytases converted into haloperoxidases (Joshi and Satyanarayana 2015; Singh et al. 2018). Molecular docking studies also supported the sharing of binding site by vanadate with phytic acid (Joshi and Satyanarayana 2015; Singh et al. 2018). A notable increase in peroxidase activity was detected in the case of A.oryzae phytase when it was exposed to ammonium metavanadate as compared to sodium metavanadate. Additionally, there was increase in haloperoxidase activity with concomitant decline in phytase activity (Pragya et al. 2023).
Plant growth promotion
In many regions of the globe, phosphorus is a crucial macronutrient for agricultural crops that restricts plant development and crop yield (Singh et al. 2020). In the soil, phosphorus and other chemicals combine to create insoluble complexes. Soil P exists in two forms viz. organic and inorganic. The organic form predominantly comprises phytates, which account for approximately 50–80% of the total soil phosphorus pool (Singh et al. 2020). The specific proportion depends on the particular soil type and this form of phosphorus is often derived from plant residues and compost materials. Conversely, the inorganic form, often denoted as Pi, primarily consists of apatite that is complexed with other elements such as calcium, iron, and aluminum phosphate. Additionally, phosphorus can also be adsorbed onto clay particles in the soil matrix, enhancing its retention in the soil environment. Recent empirical investigations suggest potential strategies for optimizing the acquisition of phosphorus from soil phytate by plants (Rizwanuddin et al. 2023b). One such approach entails the inoculation of soil with a specific microbial strain known to produce the enzyme phytase. An alternate strategy involves the direct addition of phytase to the soil. Both methods aim to enhance the bioavailability of phosphorus from phytates, thereby potentially improving nutrient uptake and plant productivity (Ige et al. 2011). Phialocephala fortinii DSE2 demonstrated the capacity to colonize the roots of Vaccinium macrocarpon, a different plant species. This colonization resulted in an increase in the plant’s phosphorus content and overall biomass. Additionally, the fungus exhibited the ability to hydrolyze phytates and accumulate polyphosphates (Mikheev et al. 2022). Out of the fungi examined, Chaetomium globosum displayed the most effective extracellular phytase, facilitating the mobilization of soil organic phosphorus for plant nutrition (Dhariwal et al. 2023).
Biofuel production
Phytic acid a prevalent compound in grain-derived raw materials has an inherent propensity to form complexes with multivalent cations including zinc, iron, calcium, and magnesium as well as proteins and starch (Mikulski et al. 2015). This complexation hampers its availability to yeast during the alcohol fermentation sequence. The chelation with polysaccharides confers a degree of resistance to enzymatic degradation, consequently reducing the quantity of sugars eligible for fermentation. The interaction with starch could occur directly via hydrogen bonds or indirectly through affiliated proteins. A feasible mitigation strategy to this issue 35 involves the hydrolysis of phytic acid utilizing phytase. The liberation of inositol from phytic acid has the potential to augment the ethanol endurance of yeast, thereby facilitating enhanced ethanol generation (Khullar et al. 2011; Mrudula Vasudevan et al. 2019). Moreover, the utilization of phytase serves to enhance the accessibility of liberated phosphorus, minerals, and vitamins to fermenting yeast. This, in turn, increases the production of ethanol and prevents the interference of phytic acid with minerals like Ca2+, Mg2+, Zn2+, and Fe2+. These minerals have a destabilizing effect on amylases, particularly those sourced from Bacillus sp. and A. niger, which are commonly employed in ethanol manufacturing (He et al. 2017). In another study, a heat and acid-resistant phytase obtained from the thermophilic mold Thermomyces lanuginosus SSBP was utilized to enhance the production of bioethanol from Colocasia esculenta. By reducing the phytate content in the starch of Colocasia esculenta from 1.43 mg/g to 0.05 mg/g, this enzyme increased the number of fermentable sugars available and decreased the viscosity, resulting in a significant 1.59-fold increase in ethanol yield (Makolomakwa et al. 2017). A cell-bound phytase from Williopsis saturnus NCIM 3298 was utilized in the saccharification of corn. It was observed that the saccharification of phytase-treated corn resulted in enhanced production of reducing sugars. Furthermore, the bioethanol production was also increased 18 % using phytase-treated corn hydrolysate (Pable et al. 2019). Further studies need to be carried out on the role of fungal phytases in biofuel production from lignocellulosic substrates.
Environmental pollution management
Phosphorus is a crucial element for both plant and animal nutrition, as it plays a significant role in growth and development processes (Kumar et al. 2016; Mir et al. 2022). However, the indiscriminate and persistent use of P can lead to various environmental issues. By increasing the bioavailability of phosphorus in animal feed, the addition of fungal phytases helps in reducing aquatic P pollution and hence, representing an essential strategy to address environmental pollution (Zhou et al. 2022). Phosphorus is vital for metabolic and regulatory functions in living organisms, including animals and humans, and is required for proper growth and development. PA, a primary source of phosphorus in food, can bind with metal ions and form an undigested, insoluble complex called phytate. This complex lower phosphorus bioavailability due to the limited activity of phytases in monogastric animals and humans, highlighting the importance of phytases in these organisms. Fungal phytases exhibit significant potential for sustainable phosphorus management through efficient utilization of soil phytate (Vashishth et al. 2023).
Organophosphate pesticides are commonly used in agriculture for inhibiting the growth of insects and pests. These pesticides remain in soil for prolonged duration due to poor degradation and hence, result in biomagnification in food chain. These pesticides are highly toxic to animals and humans due to adverse effects on nervous system. Fungal phytases have been shown effective in degradation of these organic phosphorus pesticides (Shah et al. 2017). Phytase from Aspergillus niger NCIM 563 degraded 72% of chlorpyrifos at pH 7.0 and 35 °C. Phytase also degraded monocrotophos and methyl parathion up to 53 and 77%, respectively. Chlorpyrifos was degraded up to 91% at 50 °C (Shah et al. 2017).
Role of phytase in nano-drug delivery
Recently, nanoparticles-loaded protein drug carriers are considered as promising materials for cancer and other therapies. The current development of a nanoscale drug delivery system harnesses the enzymatic properties of phytase, coupled with a platinum coating, presenting a novel therapeutic approach for the treatment of various cancer cell lines, specifically THP-1, Hep-G2, and MCF-7 (Sodhi et al. 2022). Materials from biological sources hold a distinct advantage for developing novel materials with potential applications (Wang et al. 2008). Unique structural characteristics of proteins make them naturally compatible with biological systems. These attributes enable proteins to effectively encapsulate diverse substances, including drugs, food components, and nutrients, in aqueous solutions, positioning them as robust delivery carriers (Hermenson et al. 2007). Soni et al. (2015) devised a method to create self-assembled nanospheres of the phytase, which significantly enhanced their effectiveness by incorporating platinum nanoparticles and the anticancer drug curcumin. The process of self-assembly involving the phytase within the ionic liquid,1-butyl-3-methylimidazolium tetrafluoroborate, leads to the creation of functionally active phytase nanospheres. A remarkable increase in anticancer effect was observed with phytase nanosphere (25%), platinum-phytase nanosphere (37%), phytase curcumin (78%) and platinum-phytase-curcumin nanosphere (90%). This innovative methodology potentially introduces a new paradigm in targeted cancer therapy to bridge the gap between nanotechnology and oncology (Sodhi et al. 2022).
Synthesis of myo-inositol phosphates
Fungal phytases catalyze the hydrolysis of phytic acid and generate myo-inositol phosphates intermediates. Lower myo-inositol phosphate derivatives have an important role in cell signaling pathways and mobilization of calcium ions from intracellular spaces (Jain et al. 2016). Plant-based materials are rich in inositol polyphosphates, primarily in the form of phytic acid or its salt (Gonzalez-Uarquin et al. 2020). Super-dosing effects of phytases have shown improvements in weight gain and overall performance as compared to the standard phytase dosage (Cowieson et al. 2011). This high dose of phytase facilitates almost complete degradation of phytate and increases the levels of inositol and intermediates (Walk et al. 2014). It is important to note that lower inositol polyphosphate esters exhibit greater solubility with lesser anti-nutritional effect (Schlemmer et al. 2001). Fungal phytases play a role in the gradual release of phosphate groups from phytate, generating intermediate products such as penta- (IP5), tetra- (IP4), tri- (IP3), di- (IP2), and mono- (IP1) phosphate esters of inositol (Table 3). When exogenous phytase is add to the animal diet, it initiates the hydrolysis of phytate in the acidic conditions of the stomach or gizzard, thereby releasing these lower esters into the intestinal tract (Lee et al. 2018). The animal’s own alkaline phosphatase then completes the process by hydrolyzing IP1, resulting in the release of free inositol (Pirgozliev et al. 2017).
Release of Ins (1,4,5)P3 triggers the release of Ca2+ from internal stores (Irvine et al. 1984). Inositol plays a fundamental role in signal transduction in various tissues, including the brain, kidneys, reproductive organs, and others, responding to neurotransmitters, hormones, and growth factors. Multiple genes are involved in inositol metabolism and related pathways (Kiani et al. 2021). Partial degradation of dietary InsP is carried out by phosphatases, phytases, microbial phytases, and pancreatic phospholipases in the digestive tract (Walk et al. 2018). In humans, nearly all (99.8%) of the myo-inositol is absorbed by the gastrointestinal tract (Kiani et al. 2021). Cowieson et al. (2015) demonstrated that plasma inositol levels increased in broiler chickens when they were fed with phytase-supplemented diets. These findings imply that dietary phytases leads to increased dephosphorylation of phytate, resulting in enhanced release of inositol phosphates, which play important role in cell signaling pathways (Lee et al. 2018).
Conclusions
This article discusses the production of phytases by fungi in solid state fermentation and their industrial applications. Fungal phytases are secreted in large amounts using economical substrates in SSF. Fungal phytases have features of ideal phytases, which are suitable for applications in food and feed industries. Fungal phytases are acidic, thermostable and protease-resistant. These properties make them suitable for improving nutrition of monogastric animals including humans, pigs, poultry and fishes. Fungal phytases have garnered significant interest in food production and feed industries, aiming to enhance nutrition quality and reduce phosphorus pollution. Investigating various biological properties of fungal phytases is essential to enhance their activity and stability for both nutritional and industrial purposes. However, only a limited number of fungal strains have been studied for phytase production, necessitating the identification of novel fungal species with advanced phytase characteristics and stability levels. Among fungi, Aspergillus niger and Aspergillus oryzae are classified as ‘Generally Recognized as Safe’ (GRAS) status by the FDA. Plants-based food and feed materials have been employed in poultry, piggery and aquaculture for economical production at large scale. The price of this commodity has increased due to growing demand for fishmeal in aquaculture, the livestock and poultry industries, and piggery production. It has been proven both in-vitro and in-vivo that addition of phytase to diets has improved the bioavailability of nutrients including phosphorus, sugars, minerals and proteins for absorption by the body. Phytase supplementation has reduced the excretion of phytic acid significantly and hence, resulting in mitigation of environmental phosphorus pollution. Thermophilic fungi have been explored as a potential source for phytases that are highly stable as compared to mesophilic fungi. Therefore, there is need to explore more natural resources for the isolation of thermophilic fungi for phytase production. Furthermore, cloning and protein engineering of potential phytase-producing fungi can provide valuable advantages. The growing demand for phytases offers opportunities for discovering catalysts with improved properties suitable for industrial implementation.
Data availability
All the data are included in the manuscript.
References
Abd-ElAziem F, Abdulelah NTA et al (2015) Inducible secretion of phytate-degrading enzymes from bacteria associated with the medical plant Rosa damascena cv. Taifi using rice bran. Afr J Biotechnol 14:425–433
Ahmed NE, Salem SS, Hashem AH (2021) Statistical optimization, partial purification, and characterization of phytase produced from Talaromyces purpureogenus NSA20 using potato peel waste and its. Biointerface Res 12:4417–4431
Awad GEA, Helal MMI, Danial EN et al (2014) Optimization of phytase production by Penicillium purpurogenum GE1 under solid state fermentation by using box–behnken design. Saudi J Biol Sci 21:81–88 (4)
Bala A, Sapna, Jain J et al (2014) Production of an extracellular phytase from a thermophilic mould Humicola nigrescens in solid state fermentation and its application in dephytinization. Biocatal Agricult Biotechnol 3:259–264
Berikten D, Kivanc M (2014) Optimization of solid-state fermentation for phytase production by Thermomyces lanuginosus using response surface methodology. Prep Biochem 44:834–848
Bhandari Y, Sonwane B, Vamkudoth KR (2023) Isolation and biochemical characterization of acid phytase from Aspergillus niger and its applications in dephytinization of phytic acid in poultry feed ingredients. Microbiol 92:221–229
Bhavsar K, Ravi Kumar V, Khire JM (2011) High level phytase production by Aspergillus niger NCIM 563 in solid state culture: response surface optimization, up-scaling, and its partial characterization. J Ind Microbiol Biotechnol 38:1407–1417
Bhavsar K, Gujar P, Shah P et al (2013) Combinatorial approach of statistical optimization and mutagenesis for improved production of acidic phytase by Aspergillus niger NCIM 563 under submerged fermentation condition. Appl Microbiol Biotechnol 97:673–679
Borgi MA, Boudebbouze S, Aghajari N, Szukala F, Pons N, Maguin E, Rhimi M (2014) The attractive recombinant phytase from Bacillus licheniformis: biochemical and molecular characterization. Appl Microbiol Biotechnol 98:5937–5947
Buddhiwant P, Bhavsar K, Ravi Kumar V et al (2016) Phytase production by solid-state fermentation of groundnut oil cake by Aspergillus niger: a bioprocess optimization study for animal feedstock applications. Prep Biochem Biotechnol 46:531–538
Çalışkan-Özdemir S, Önal S, Uzel A (2021) Partial purification and characterization of a thermostable phytase produced by thermotolerant aspergillus tubingensis TEM 37 isolated from hot spring soil in gediz geothermal field, Turkey. Geomicrobiol J 38:895–904
Chen R, Zhang C, Yao B et al (2013) Corn seeds as bioreactors for the production of phytase in the feed industry. J Biotechnol 165:120–126
Chen CC, Cheng KJ, Ko TP, Guo RT (2015) Current progresses in phytase research: three-dimensional structure and protein engineering. ChemBioEng Rev 2:76–86
Coban HB, Demirci A (2014) Screening of phytase producers and optimization of culture conditions for submerged fermentation. Bioprocess Biosyst Eng 37:609–616
Correia I, Aksu S, Adão P et al (2008) Vanadate substituted phytase: immobilization, structural characterization and performance for sulfoxidations. J Inorg Biochem 102:318–329
Corrêa TLR, de Araújo EF (2020) Fungal phytases: from genes to applications. Brazilian J Microbiol 51:1009–1020
Coutinho TC, Tardioli PW, Farinas CS (2020) Phytase immobilization on hydroxyapatite nanoparticles improves its properties for use in animal feed. Appl Biochem Biotechnol 190:270–292
Cowieson AJ, Wilcock P, Bedford MR (2011) Super-dosing effects of phytase in poultry and other monogastrics. World Poul Sci J 67(2):225–236
Cowieson AJ, Ruckebusch JP, Knap I, Guggenbuhl P, Fru-Nji F (2015) Phytate-free nutrition: a new paradigm in monogastric animal production. Anim Feed Sci Technol 222:180–189
Dahiya S, Singh B (2019) Enhanced endoxylanase production by Myceliophthora thermophila with applicability in saccharification of agricultural substrates. 3 Biotech 9:1–10
de Oliveira Ornela PH, Souza Guimarães LH (2019) Purification and characterization of an alkalistable phytase produced by Rhizopus microsporus var. microsporus in submerged fermentation. Process Biochem 81:70–76
Dhariwal AG, Tarafdar JC, Dhariwal AG et al (2023) A comparison of phytase efficiency originated from plant and fungal sources. GSC Biol Pharm Sci 23:117–126
Din M, Nelofer R, Salman M et al (2019) Production of nitrogen fixing Azotobacter (SR-4) and phosphorus solubilizing Aspergillus niger and their evaluation on Lagenaria siceraria and Abelmoschus esculentus. Biotechnol Rep 22:e00323
Dixit M, Shukla P (2023) Multi-efficient endoglucanase from Aspergillus niger MPS25 and its potential applications in saccharification of wheat straw and waste paper deinking. Chemosphere 313:137298
Duliński R, Zdaniewicz M, Pater A, Poniewska D, Żyła K (2020) The impact of phytases on the release of bioactive inositols, the profile of inositol phosphates, and the release of selected minerals in the technology of buckwheat beer production. Biomolecules 10(2):166
Elkhateeb YAM, Fadel M (2022) Bioinformatic studies, experimental validation of phytase production and optimization of fermentation conditions for enhancing phytase enzyme production by different microorganisms under solid-state fermentation. Open Microbiol J. https://doi.org/10.2174/18742858-v16-e2202160
Evstatieva Y, Ilieva A, Valcheva V et al (2020) Production of plant growth regulatory metabolites of Rhizopus arrhizus KB-2. Bulg J Agric Sci 26:551–557
Fan CM, Wang YH, Zheng CY et al (2016) Fingerprint motifs of phytases. Afr J Biotechnol 12:1138–1147
Feder D, McGeary RP, Mitić N, Lonhienne T, Furtado A, Schulz BL, Henry RJ, Schmidt S, Guddat LW, Schenk G (2020) Structural elements that modulate the substrate specificity of plant purple acid phosphatases: avenues for improved phosphorus acquisition in crops. Plant Sci 294:110445
Filippovich SY, Isakova EP, Gessler NN et al (2023) Advances in immobilization of phytases and their application. Bioresour Technol 379:129030
Gaind S, Singh S (2015) Production, purification and characterization of neutral phytase from thermotolerant Aspergillus flavus ITCC 6720. Int Biodeter Biodegrad 99:15–22
Gessler NN, Serdyuk EG, Isakova EP, Deryabina YI (2018) Phytases and the prospects for their application (review). Appl Biochem Microbiol 54:352–360
Ghorbani Nasrabadi R, Greiner R, Yamchi A et al (2018) A novel purple acid phytase from an earthworm cast bacterium. J Sci Food Agric 98:3667–3674
Gontia-Mishra I, Tiwari S (2013) Molecular characterization and comparative phylogenetic analysis of phytases from fungi with their prospective applications. Food Technol Biotechnol 51:313–326
Gonzalez-Uarquin F, Kenéz Á, Rodehutscord M, Huber K (2020) Dietary phytase and myo-inositol supplementation are associated with distinct plasma metabolome profile in broiler chickens. Animal 14(3):549–559
Goyal S, Nagar S, Mallesh G et al (2022) A review on role of phytic acid and phytase in food and feed. Chem Sci Rev Lett 2022:510–518
Gruninger RJ, Thibault J, Capeness MJ et al (2014) Structural and biochemical analysis of a unique phosphatase from Bdellovibrio bacteriovorus reveals its structural and functional relationship with the protein tyrosine phosphatase class of phytase. PLoS ONE 9:e94403
Gulati H, Chadha B, Saini H (2007) Production, purification and characterization of thermostable phytase from thermophilic fungus Thermomyces lanuginosus TL-7 Acta. Microbiol Immunol Hung 54(2):121–138
Gupta RK, Gangoliya SS, Singh NK (2014) Isolation of thermotolerant phytase producing fungi and optimisation of phytase production by Aspergillus niger NRF9 in solid state fermentation using response surface methodology. Biotechnol Bioprocess Eng 19:996–1004
Gupta RK, Gangoliya SS, Singh NK (2015) Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol 52:676–684
Handa V, Sharma D, Kaur A et al (2020) Biotechnological applications of microbial phytase and phytic acid in food and feed industries. Biocatal Agricult Biotechnol 25:101600
He Q, Reis CER, Wang F, Hu B (2017) Phytate extraction from coproducts of the dry-grind corn ethanol process. RSC Adv 7(9):5466–5472
Hellström A, Qvirist L, Svanberg U et al (2015) Secretion of non-cell‐bound phytase by the yeast Pichia kudriavzevii TY13. J Appl Microbiol 118:1126–1136
Hermanson KD, Huemmerich D, Scheibel T, Bausch AR (2007) Engineered microcapsules fabricated from reconstituted spider silk. Adv Mater 19(14):1810–1815
Ige DV, Abioye OS, Akinremi OO et al (2011) Phosphorus solubility in Manitoba soils treated with pig manure from phytase supplemented diets. Can J Soil Sci 91:947–955
Irvine RF, Brown KD, Berridge MJ (1984) Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J 222(1):269–272
Jafari-Tapeh H, Hamidi-Esfahani Z, Azizi MH (2012) Culture condition improvement for phytase production in solid state fermentation by Aspergillus ficuum using statistical method. Int Sch Res Netw ISRN Chem Eng 2012:5
Jain J, Singh B (2017) Phytase production and development of an ideal dephytinization process for amelioration of food nutrition using microbial phytases. Appl Biochem Biotechnol 181:1485–1495
Jain J, Sapna, Singh B (2016) Characteristics and biotechnological applications of bacterial phytases. Process Biochem 51:159–169
Jatuwong K, Kumla J, Suwannarach N et al (2020) Bioprocessing of agricultural residues as substrates and optimal conditions for phytase production of chestnut mushroom, pholiota adiposa. Solid State Ferment 6:384
Joshi S, Satyanarayana T (2015) Characteristics and applicability of phytase of the yeast Pichia anomala in synthesizing haloperoxidase. Appl Biochem Biotechnol 176:1351–1369
Kalkan SO, Bozcal E, Hames Tuna EE et al (2020) Characterisation of a thermostable and proteolysis resistant phytase from Penicillium polonicum MF82 associated with the marine sponge Phorbas sp. Biocatal Biotransform 38:469–479
Kalsi HK, Singh R, Dhaliwal HS et al (2016) Phytases from Enterobacter and Serratia species with desirable characteristics for food and feed applications. 3 Biotech 6:1–13
Kanti A, Idris I, Sudiana IM (2020) Aspergillus niger Str 3 and Neurospora sitophila for phytase production on coconut oil cake supplemented with rice brand in solid-state fermentation. IOP Conf Ser Earth Environ Sci 439:12020
Kassim MA, Meng TK, Kamaludin R et al (2022) Bioprocessing of sustainable renewable biomass for bioethanol production. In: Yusup S, Rashidi NA (eds) Value-chain biofuels fundam technol stand. Elsevier, Amsterdam, pp 195–234
Kaur R, Saxena A, Sangwan P et al (2017) Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nusant Biosci 9:68–76
Kaur P, Vohra A, Satyanarayana T (2021) Developments in fungal phytase research: characteristics and multifarious applications. Springer, Singapore
Kebreab E, Hansen AV, Strathe AB (2012) Animal production for efficient phosphate utilization: from optimized feed to high efficiency livestock. Curr Opin Biotechnol 23:872–877
Kiani AK, Paolacci S, Calogero AE, Cannarella R, Di Renzo GC, Gerli S, Della Morte C, Busetto GM, De Berardinis E, Del Giudice F, Stuppia L (2021) From Myo-inositol to D-chiro-inositol molecular pathways. Eur Rev Med Pharmacol Sci 25(5):2390–2402
Kour D, Lata Rana K, Yadav N et al (2019) Agriculturally and industrially important fungi: current developments and potential biotechnological applications. In: Yadav AN, Singh S, Mishra S, Gupta A (eds) Recent advancement in white biotechnology through fungi. Springer, Cahm, pp 1–64
Kumar V, Sinha AK (2018) General aspects of phytases. In: Nunes CS, Kumar V (eds) Enzymes in human and animal nutrition. Elsevier, Amsterdam, pp 53–72
Kumar A, Chanderman A, Makolomakwa M et al (2016) Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit Rev Environ Sci Technol 46:556–591
Kumar A, Singh B, Raigond P, Sahu C, Mishra UN, Sharma S, Lal MK (2021) Phytic acid: Blessing in disguise, a prime compound required for both plant and human nutrition. Food Res Int 142:110193
Kumari N, Bansal S (2021) Statistical modeling and optimization of microbial phytase production towards utilization as a feed supplement. Biomass Convers Biorefinery 1:1–11
Kumari A, Satyanarayana T, Singh B (2016) Mixed substrate fermentation for enhanced phytase production by thermophilic mould Sporotrichum thermophile and its application in beneficiation of poultry feed. Appl Biochem Biotechnol 178:197–210
Khullar E, Shetty JK, Rausch KD, Tumbleson ME, Singh V (2011) Use of phytases in ethanol production from E-Mill corn processing. Cereal Chem 88(3):223–227
Lahiji S, Hemmati R, Homaei A et al (2021) Improved thermal stability of phytase from Yersinia intermedia by physical adsorption immobilization on amino-multiwalled carbon nanotubes. Bioprocess Biosyst Eng 44:2217–2228
Langeroudi JA, Sabet MS, Jalali-Javaran M, Zamani K, Lohrasebi T, Malboobi MA (2023) Functional assessment of AtPAP17; encoding a purple acid phosphatase involved in phosphate metabolism in Arabidopsis thaliana. Biotechnol Lett 45(5):719–739
Lee SH, Cho J, Bok J et al (2014) Characterization, gene cloning, and sequencing of a fungal phytase, PhyA, from Penicillium oxalicum PJ3. Prep Biochem Biotechnol 45:336–347
Lee SA, Dunne J, Febery E, Brearley CA, Mottram T, Bedford MR (2018) Exogenous phytase and xylanase exhibit opposing effects on real-time gizzard pH in broiler chickens. Br Poul Sci 59(5):568–578
Liu X, Han R, Cao Y, Turner BL, Ma LQ (2022) Enhancing phytate availability in soils and phytate-P acquisition by plants: a review. Environ Sci Technol 56(13):9196–9219
Longin CFH, Afzal M, Pfannstiel J et al (2023) Mineral and phytic acid content as well as phytase activity in flours and breads made from different wheat species. Int J Mol Sci 24:2770
Lopes MM, Coutinho TC, Malafatti JOD et al (2021) Immobilization of phytase on zeolite modified with iron(II) for use in the animal feed and food industry sectors. Process Biochem 100:260–271
Ma XF, Tudor S, Butler T, Ge Y, Xi Y, Bouton J, Harrison M, Wang ZY (2012) Transgenic expression of phytase and acid phosphatase genes in alfalfa (Medicago sativa) leads to improved phosphate uptake in natural soils. Mol Breed 30:377–391
Mahendran S, Sankaralingam S, Maheswari P et al (2022) Production, characterization, and feed supplement applications of phytase enzyme from Aspergillus tubingensis isolated from western Ghats soil. Biomass Convers Biorefinery 1:1–11
Mahmood S, Shahid MG, Nadeem M et al (2023) Stimulatory effect of medium components on phytase production by Aspergillus niger and biotechnological application as a poultry feed additive. Kuwait J Sci. https://doi.org/10.48129/kjs.17947
Makolomakwa M, Puri AK, Permaul K, Singh S (2017) Thermo-acid-stable phytase-mediated enhancement of bioethanol production using Colocasia esculenta. Bioresour Technol 235:396–404
Malý O, Zugárková I, Radojičić M et al (2023) Increasing phosphorus digestibility in common carp (Cyprinus carpio L.) farming using phytase and citric acid. Aquac Res 2023:1–10
Miao J, Wang M, Ma L, Li T, Huang Q, Liu D, Shen Q (2019) Effects of amino acids on the lignocellulose degradation by Aspergillus fumigatus Z5: Insights into performance, transcriptional, and proteomic profiles. Biotechnol Biofuel 12:1–19
Mikheev VS, Struchkova IV, Ageyeva MN et al (2022) The role of Phialocephala fortinii in improving plants’ phosphorus nutrition: new puzzle pieces. J Fungi 8:1225
Mikulski D, Kłosowski G, Rolbiecka A (2015) Influence of phytase and supportive enzymes applied during high gravity mash preparation on the improvement of technological indicators of the alcoholic fermentation process. Biomass Bioenergy 80:191–202
Mir S, Dervash MA, Shikari AB et al (2022) Microbial consortium: a biotechnological tool for enhanced bioremediation in pollution-affected environments. Environ Biotechnol. https://doi.org/10.1201/9781003277279-5
Moreira KA, Herculano PN, De M et al (2014) Optimization of phytase production by Aspergillus japonicus Saito URM 5633 using cassava bast as substrate in solid state fermentation. Afr J Microbiol Res 8:929–938
Moriarty DJW (1973) The physiology of digestion of blue-green algae in the cichlid fish, Tilapia nilotica. J Zool 171:25–39
Mrudula Vasudevan U, Jaiswal AK, Krishna S, Pandey A (2019) Thermostable phytase in feed and fuel industries. Bioresour Technol 278:407
Mullaney EJ, Ullah AHJ (2006) Phytases: attributes, catalytic mechanisms and applications. In: Turner BL, Richardson AE, Mullaney EJ (eds) Inositol phosphates: linking agriculture and the environment. CABI, Wallingford
Nadeem H, Shah SZH, Fatima M et al (2023) Prospects of microbial phytases in the food and feed industry. In: Kumar A, Bilal M et al (eds) Microbial biomolecules: emerging approach in agriculture, pharmaceuticals and environment management. Elsevier, Amsterdam, pp 325–351
Nascimento JCS, Ribeiro AG, Pessoa RAS, Rabello CBV, Venâncio A, Porto TS, Teixeira JAC, Porto ALF (2022) Effect of pH and temperature on phytase and biomass production by submerged fermentation with Aspergillus niger var phoenicis URM 4924. Res Soc Dev 11(6):e41311628994
Neira-Vielma AA, Aguilar CN, Ilyina A et al (2018) Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnol Rep 17:49–54
Onibokun EA, Eni AO, Oranusi SU (2022) Purification and characterization of phytase from a local poultry isolate of Aspergillus flavus MT899184. In: Ayeni AO, Sanni SE, Oranusi SU (eds) Bioenergy and biochemical processing technologies. Springer, Cham, pp 99–112
Pable AA, Shah S, Ravi Kumar V et al (2019) Use of Plackett-Burman design for enhanced phytase production by Williopsis saturnus NCIM 3298 for applications in animal feed and ethanol production. 3 Biotech. https://doi.org/10.1007/s13205-019-1764-y. (3 Biotech 9)
Piecha CR, Alves TC, de Zanini MLO et al (2023) Application of the solid-state fermentation process and its variations in PHA production: a review. Arch Microbiol 205:1–16
Pires EBE, de Freitas AJ, Souza FFE et al (2019) Production of fungal phytases from agroindustrial byproducts for pig diets. Sci Rep 9(1):9256
Pirgozliev V, Bedford MR, Rose SP, Whiting IM, Oluwatosin OO, Oso AO, Oke FO, Ivanova SG, Staykova GP (2017) Phosphorus utilisation and growth performance of broiler chicken fed diets containing graded levels of supplementary myo-inositol with and without exogenous phytase. J World Poul Res 7(1):1–10
Prado Barragán LA, Figueroa JJB, Rodríguez Durán LV et al (2016) Fermentative production methods. In: Poltronieri P, D’Urso OF (eds) Biotransformation of agricultural waste and by-products in the 4F economy: the food, feed, fiber, fuel (4F) economy. Elsevier, Amsterdam, pp 189–217
Priya, Pragya, Virmani I et al (2023) Role of microbial phytases in improving fish health. Rev Aquac. https://doi.org/10.1111/raq.12790
Pragya, Sharma KK, Kumar A et al (2021) Immobilized phytases: an overview of different strategies, support material, and their applications in improving food and feed nutrition. Crit Rev Food Sci Nutr 2021:1–23
Pragya, Sharma KK, Kumar S et al (2023) Enhanced production and immobilization of phytase from Aspergillus oryzae: a safe and ideal food supplement for improving nutrition. Lett Appl Microbiol 76(2):ovac077
Pragya Sharma KK, Singh B (2023) Phytase from Aspergillus oryzae SBS50: Biocatalytic reduction of antinutritional factor and exhibiting vanadium-dependent haloperoxidase activity. Biocatal Agric Biotechnol 52:102840
Puhl AA, Greiner R, Selinger LB (2008) A protein tyrosine phosphatase-like inositol polyphosphatase from Selenomonas ruminantium subsp. lactilytica has specificity for the 5-phosphate of myo-inositol hexakisphosphate. Int J Biochem Cell Biol 40:2053–2064
Pujol A, Sanchis P, Grases F, Masmiquel L (2023) Phytate intake, health and disease:“let thy food be thy medicine and medicine be thy food.” Antioxidants 12(1):146
Puppala KR, Ravi Kumar V, Khire J et al (2019) Dephytinizing and probiotic potentials of Saccharomyces cerevisiae (NCIM 3662) strain for amelioration of nutritional quality of functional foods. Probiotics Antimicrob Proteins 11:604–617
Ranjan B, Satyanarayana T (2016) Recombinant HAP phytase of the thermophilic mold Sporotrichum thermophile: expression of the codon-optimized phytase gene in Pichia pastoris and applications. Mol Biotechnol 58:137–147
Ranjan B, Singh B, Satyanarayana T (2015) Characteristics of recombinant phytase (rSt-Phy) of the thermophilic mold Sporotrichum thermophile and its applicability in dephytinizing foods. Appl Biochem Biotechnol 177:1753–1766
Renirie R, Pierlot C, Aubry JM et al (2003) Vanadium chloroperoxidase as a catalyst for hydrogen peroxide disproportionation to singlet oxygen in mildly acidic aqueous environment. Adv Synth Catal 345:849–858
Rizwanuddin S, Kumar V, Naik B et al (2023a) Microbial phytase: their sources, production, and role in the enhancement of nutritional aspects of food and feed additives. J Agric Food Res 12:100559
Rizwanuddin S, Kumar V, Singh P et al (2023b) Insight into phytase-producing microorganisms for phytate solubilization and soil sustainability. Front Microbiol 14:1127249
Rodriguez YE, Laitano MV, Pereira NA et al (2018) Exogenous enzymes in aquaculture: Alginate and alginate-bentonite microcapsules for the intestinal delivery of shrimp proteases to Nile tilapia. Aquaculture 490:35–43
Sadaf N, Haider MZ, Iqbal N, Abualreesh MH, Alatawi A (2022) Harnessing the phytase production potential of soilborne fungi from wastewater irrigated fields based on eco-cultural optimization under shake flask method. Agriculture 12(1):103
Sagar Verma V, Kumar Jain H, Kumar Ramchandra Badwaik H et al (2022) Statistical optimization of oxidative derivatization of polyethylene glycol to polyethylene carboxylate using custom design approach. Int J Health Sci (Qassim) 6:7086–7097
Sanangelantoni AM, Malatrasi M, Trivelloni E et al (2018) A novel β-propeller phytase from the dioxin-degrading bacterium Sphingomonas wittichii RW-1. Appl Microbiol Biotechnol 102:8351–8358
Sandhya A, Sridevi A, Suvarnalathadevi P (2019) Biochemical characterization of phytase purified from Aspergillus niger S2. EurAsian J Biosci 13:99–103
Sanni DM, Lawal OT, Enujiugha VN (2019) Purification and characterization of phytase from Aspergillus fumigatus isolated from African giant snail (Achatina fulica). Biocatal Agric Biotechnol 17:225–232
Sapna, Singh B (2014) Phytase production by Aspergillus oryzae in solid-state fermentation and its applicability in dephytinization of wheat bran. Appl Biochem Biotechnol 173:1885–1895
Sapna, Singh B (2015) Biocatalytic potential of protease-resistant phytase of Aspergillus oryzae SBS50 in ameliorating food nutrition. Biocatal Biotransform 33:167–174
Sapna, Singh B (2017a) Purification and characterization of a protease-resistant phytase of Aspergillus oryzae SBS50 whose properties make it exceptionally useful as a feed supplement. Int J Biol Macromol 103:458–466
Sapna, Singh B (2017b) Free and immobilized Aspergillus oryzae SBS50 producing protease-resistant and thermostable phytase. 3 Biotech 7:1–8
Sapna Singh B (2017) Free and immobilized Aspergillus oryzae SBS50 producing protease-resistant and thermostable phytase. 3 Biotech 7(3):213
Saxena A, Verma M, Singh B et al (2020) Characteristics of an acidic phytase from Aspergillus aculeatus APF1 for dephytinization of biofortified wheat genotypes. Appl Biochem Biotechnol 191:679–694
Saxena A, Verma M, Singh B, Sangwan P, Yadav AN, Dhaliwal HS, Kumar V (2020) Characteristics of an acidic phytase from Aspergillus aculeatus APF1 for dephytinization of biofortified wheat genotypes. Appl Biochem Biotechnol 191:679–694
Shah PC, Kumar VR, Dastager SG et al (2017) Phytase production by Aspergillus niger NCIM 563 for a novel application to degrade organophosphorus pesticides. AMB Express 7(1):1–11
Shahryari Z, Fazaelipoor MH, Setoodeh P et al (2018) Utilization of wheat straw for fungal phytase production. Int J Recycl Org Waste Agric 7:345–355
Sharma A, Ahluwalia O, Tripathi AD et al (2020) Phytases and their pharmaceutical applications: mini-review. Biocatal Agric Biotechnol 23:101439
Shivanna GB, Venkateswaran G (2014) Phytase production by Aspergillus niger CFR 335 and Aspergillus ficuum SGA 01 through submerged and solid-state fermentation. Sci World J. https://doi.org/10.1155/2014/392615
Singh B, Satyanarayana T (2008) Phytase production by a thermophilic mould Sporotrichum thermophile in solid state fermentation and its potential applications. Bioresour Technol 99:2824–2830
Singh B, Satyanarayana T (2011a) Phytases from thermophilic molds: their production, characteristics and multifarious applications. Process Biochem 46:1391–1398
Singh B, Satyanarayana T (2011b) Microbial phytases in phosphorus acquisition and plant growth promotion. Physiol Mol Biol Plants 17:93–103
Singh B, Kunze G, Satyanarayana T (2011) Developments in biochemical aspects and biotechnological applications of microbial phytases. Biotechnol Mol Biol Rev 6:69–87
Singh B, Satyanarayana T (2015) Fungal phytases: characteristics and amelioration of nutritional quality and growth of non-ruminants. J Anim Physiol Anim Nutr (Berl) 99:646–660
Singh N, Kumari A, Gakhar SK, Singh B (2015) Enhanced cost-effective phytase production by Aspergillus niger and its applicability in dephytinization of food ingredients. Microbiology (Russian Fed) 84:219–226
Singh B, Sharma KK, Kumari A et al (2018) Molecular modeling and docking of recombinant HAP-phytase of a thermophilic mould Sporotrichum thermophile reveals insights into molecular catalysis and biochemical properties. Int J Biol Macromol 115:501–508
Singh B, Boukhris I, Pragya et al (2020) Contribution of microbial phytases to the improvement of plant growth and nutrition: a review. Pedosphere 30:295–313
Soccol CR, da Costa ESF, Letti LAJ et al (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Innov 1:52–71
Sodhi AS, Sharma N, Bhatia S et al (2022) Insights on sustainable approaches for production and applications of value added products. Chemosphere 286:131623
Song HY, El Sheikha AF, Hu DM (2019) The positive impacts of microbial phytase on its nutritional applications. Trends Food Sci Technol 86:553–562
Soni SK, Sarkar S, Selvakannana PR et al (2015) Intrinsic therapeutic and biocatalytic roles of ionic liquid mediated self-assembled platinum–phytase nanospheres. RSC Adv 5:62871–62881
Srivastava N, Srivastava M, Ramteke PW et al (2019) Solid-state fermentation strategy for microbial metabolites production: an overview. In: Gupta VK, Pandey A (eds) New and future developments in microbial biotechnology and bioengineering: microbial secondary metabolites biochemistry and applications. Elsevier, Amsterdam, pp 345–354
Suresh S, Radha KV (2015) Effect of a mixed substrate on phytase production by Rhizopus oligosporus MTCC 556 using solid state fermentation and determination of dephytinization activities in food grains. Food Sci Biotechnol 24:551–559
Tanaka T, Izawa S, Inoue Y (2005) GPX2, encoding a phospholipid hydroperoxide glutathione peroxidase homologue, codes for an atypical 2-Cys peroxiredoxin in Saccharomyces cerevisiae. J Biol Chem 280:42078–42087
Tanruean K, Penkhrue W, Kumla J et al (2021) Valorization of lignocellulosic wastes to produce phytase and cellulolytic enzymes from a thermophilic fungus, Thermoascus aurantiacus SL16W, under semi-solid state fermentation. J Fungi 7:286
Tian M, Yuan Q (2016) Optimization of phytase production from potato waste using Aspergillus ficuum. 3 Biotech 6(2):256
Ushasree MV, Vidya J, Pandey A (2014) Gene cloning and soluble expression of Aspergillus niger phytase in E. coli cytosol via chaperone co-expression. Biotechnol Lett 36:85–91
Vashishth A, Tehri N, Tehri P et al (2023) Unraveling the potential of bacterial phytases for sustainable management of phosphorous. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.2466
Vats P, Banerjee UC (2005) Biochemical characterisation of extracellular phytase (myo-inositol hexakisphosphate phosphohydrolase) from a hyper-producing strain of Aspergillus niger van Teighem. J Ind Microbiol Biotechnol 32:141–147
van de Velde F, Lourenço N, Bakker M et al (2000) Improved operational stability of peroxidases by coimmobilization with glucose oxidase. Biotechnol Bioeng 69:286–291
Venkataraman S, Vaidyanathan VK (2023) Dephytinization of wheat and rice bran by cross-linked enzyme aggregates of Mucor indicus phytase: a viable prospect for food and feed industries. J Sci Food Agric 103:1935–1945
Walk CL, Santos TT, Bedford MR (2014) Influence of superdoses of a novel microbial phytase on growth performance, tibia ash, and gizzard phytate and inositol in young broilers. Poul Sci 93(5):1172–1177
Walk CL, Bedford MR, Olukosi OA (2018) Effect of phytase on growth performance, phytate degradation and gene expression of myo-inositol transporters in the small intestine, liver and kidney of 21 day old broilers. Poul Sci 97(4):1155–1162
Wang ZL (2008) Splendid one-dimensional nanostructures of zinc oxide: a new nanomaterial family for nanotechnology. ACS Nano 2(10):1987–1992
Wang ZH, Dong XF, Zhang GQ et al (2017) Waste vinegar residue as substrate for phytase production. Waste Manag Res J Sustain Circ Econ 29:1262–1270
Wyss M, Brugger R, Kronenberger A, Rémy R, Fimbel R, Oesterhelt G, Lehmann M, Van Loon AP (1998) Biochemical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): catalytic properties. Appl Environ Microbiol 65(2):367–373
Wyss M, Pasamontes L, Friedlein A, Rémy R, Tessier M, Kronenberger A, Middendorf A, Lehmann M, Schnoebelen L, Röthlisberger U, Kusznir E, (1999) Biophysical characterization of fungal phytases (myo-inositol hexakisphosphate phosphohydrolases): molecular size, glycosylation pattern, and engineering of proteolytic resistance. Appl Environ Microbiol 65(2):359–366
Xiao K, Harrison MJ, Wang ZY (2005) Transgenic expression of a novel M. truncatula phytase gene results in improved acquisition of organic phosphorus by Arabidopsis. Planta 222:27–36
Yu P, Wang XT, Liu JW (2015) Purification and characterization of a novel cold-adapted phytase from Rhodotorula mucilaginosa strain JMUY14 isolated. Antarct J Basic Microbiol 55(8):1029–1039
Zeller E, Schollenberger M, Witzig M, Shastak Y, Kühn I, Hoelzle LE, Rodehutscord M (2015) Interactions between supplemented mineral phosphorus and phytase on phytate hydrolysis and inositol phosphates in the small intestine of broilers. Poul Sci 94(5):1018–1029
Zhou Y, Anoopkumar AN, Tarafdar A et al (2022) Microbial engineering for the production and application of phytases to the treatment of the toxic pollutants: a review. Environ Pollut 308:119703
Zsheng L, Wang J, Cai RJ et al (2023) Heat dissipation performance improvement of a solid-state fan using copper foams as collecting electrode. Int J Heat Mass Transf 202:123730
Author information
Authors and Affiliations
Contributions
BS and Pragya wrote the main text of the manuscript. Pragya and BS prepared all the tables and figures, BS, SKT, DS, SK and VM improved the text and designed all the figures. All authors edited the original manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, B., Pragya, Tiwari, S.K. et al. Production of fungal phytases in solid state fermentation and potential biotechnological applications. World J Microbiol Biotechnol 40, 22 (2024). https://doi.org/10.1007/s11274-023-03783-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-023-03783-1