Abstract
Combination of statistical optimization and mutagenesis to isolate hypersecretory strains is studied to maximize phytase production from Aspergillus niger NCIM 563 under submerged fermentation. The overall results obtained show a remarkable 5.98-fold improvement in phytase production rates when compared to that using basal medium. Optimization of culture conditions from parent strain is studied first by the Plackett–Burman technique to evaluate the effects of 11 variables for phytase production. The results showed that glucose, MgSO4, KCl, incubation period, and MnSO4 are the most significant variables affecting enzyme production. Further optimization in these variables, using a central composite design technique, resulted in 3.74-fold increase in the yield of phytase production to 254,500 U/l when compared with the activity observed with basal media (68,000 U/l) in shake flask. Our experiments show that the phytase from A. niger NCIM 563 exhibits desirable activity in simulated gastric fluid conditions with low pH and also improved thermostability when compared to commercial phytase. The improved yield demonstrates the potential applicability of phytase enzyme as a source of phytase supplement for phosphorus nutrition and environmental protection in animal feed industry. Physical and chemical mutagenesis experiments were carried out in parallel to isolate hypersecretory mutants that could possibly further enhance the enzyme production. Using optimized media conditions of the parent strain, our results show that mutant strain A. niger NCIM 1359 increased the phytase activity by another 1.6-fold to 407,200 U/l.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus is a nonrenewable and the third most expensive nutrient in poultry production, after energy and protein. Although, plants store phosphorus in the form of phytate (inositol 6-phosphate), it is largely unavailable to mono-gastric animals due to lack of adequate levels of phytase. Therefore, inorganic phosphorous supplement of animal feeds is still the method of choice (Dvorakova 1998). Phytate acts as an antinutritional factor since it causes mineral deficiency by chelating metal ions such as Ca2+, Mg2+, Zn2+, and Fe2+ (Mitchell et al. 1997). The unutilized phytate and phosphorus supplements are excreted by animals and this creates global ecological problems (Mandviwala and Khire 2000). The use of phytase in animal feed will overcome the anti-nutritional effects of phytate, decrease environmental pollution, increase mineral bioavailability, and abolish the addition of inorganic phosphate in animal feed (Reddy et al. 1982).
The available phytase preparations used as feed additives are of fungal origin, produced by recombinant strains under submerged fermentation condition, and have shortcomings especially with regard to obtaining diluted product, sensitivity to heat, inactivation under low pH conditions present in the stomach of animals (Afinah et al. 2010), and high product recovery costs. These disadvantages need to be alleviated while at the same time producing phytase with high yield and purity. Micro-organisms produce low levels of phytase and it would be beneficial if these production rates be improved with desirable process features by employing statistical techniques and strain improvement programs.
A one variable approach at a time (OVAT) was used in our preliminary studies (Bhavsar et al. 2008) for development of production medium for phytase production at low pH. Also, we have reported high levels of phytase (optimum pH 5.0 by solid state fermentation using statistical techniques that involve a combination of Plackett–Burman (PBD) and a Box–Behnken design (Bhavsar et al. 2011). Despite the importance of phytase in poultry feed, there are very few reports on its enhanced production by statistical optimization and mutagenesis due to its low expression (Bogar et al. 2003; Chadha et al. 2004; Tanyildizi et al. 2005; Vohra and Satyanarayana 2002). The present investigation therefore aims at achieving multifold improvement in phytase production using Aspergillus niger NCIM 563 under submerged fermentation (SmF) by statistical optimization along with mutagenesis studies.
Strain improvement by mutagenesis and selection is a highly developed technique and it plays a central role in the commercial development of microbial fermentation processes (Parekh et al. 2000). Mutagenic procedures can be carried out in terms of type of mutagen and dose to obtain mutant types that may be screened for improved activity. Primary screening process studies for selection of mutant on phytase screening media showed lack of specificity and sensitivity to discriminate between phytase and acid-phosphatase activity. The time-consuming statistical optimization and mutagenesis experiments were therefore carried out in parallel because it may be reasonably expected that promising mutant strains may also be benefited from optimized media formulation obtained with the parent strain. Experiments with mutant strains were therefore carried out on the basis of this conjecture and the results obtained validate the employed approach.
Materials and methods
Chemicals
Phytic acid sodium salt was purchased from Sigma Chemical Company (St. Louis, MO, USA). All other chemicals used were of analytical grade. Rice bran was purchased from the local market.
Microorganism, culture media, and enzyme production
A. niger NCIM 563, used in the present study, was obtained from the National Collection of Industrial Microorganism (NCIM), National Chemical Laboratory, Pune India. The stock culture was maintained on Potato Dextrose Agar (PDA) slant and stored at 4 °C. Spores for inoculation were obtained by culturing the strain at 30 °C on a PDA slant for 7 days, followed by washing with 10 ml sterile saline containing 0.01% Tween 80.
The basal fermentation medium according to Bhavsar et al. 2008 contained rice bran, glucose, NaNO3, MgSO4.7H2O, KCl, and FeSO4.7H2O. The fermentation medium for optimization via statistical design of experiments included additional components, namely, MnSO4, dextrin, and Tween 80 at various concentrations as required by the experimental design. The fermentation medium pH 5.5 before sterilization (100 ml in 250 ml Erlenmeyer flask) was sterilized by autoclaving at 121 °C for 15 min. On cooling the fermentation medium was inoculated with desired spore suspension and the incubation time adjusted as per the experimental design. Flasks were incubated at 30 °C at 200 rpm and samples removed after every 24 h. Enzyme production was expressed as enzyme activity U/ml.
Analytical methods
Phytase activity was measured using 100 mM Glycine–HCl buffer, pH 2.5 at 50 °C for 30 min as described earlier (Soni and Khire 2007). One unit of phytase activity (IU) was expressed as the amount of enzyme that liberates 1 μmol phosphorus per minute under standard assay conditions. Total residual reducing sugar concentration was estimated by DNSA method (Miller 1959). Protein concentration in the culture filtrate was determined using bovine serum albumin as a standard (Lowry et al. 1951).
Partial purification of enzyme
After fermentation, the mycelium was separated by filtration followed by centrifugation at 10,000×g for 30 min and the clear supernatant was collected. Solid ammonium sulphate was added to the supernatant to 95% saturation with constant stirring. The precipitate was collected by centrifugation at 15,000×g for 20 min and dissolved in minimum volume of 100 mM Glycine–HCl buffer, pH 2.5 and the salt was removed by passing through Sephadex G-25 column. Active fractions were concentrated through YM-30 membrane (Millipore) and used for phytase activity measurement.
Release of phosphorus from soybean in simulated gastric fluid
One gram soybean meal was dissolved in 9 ml simulated gastric fluid (SGF; 250 mM Glycine–HCl containing 2.0 mg/ml NaCl and 3.2 mg/ml pepsin) and the pH was adjusted with HCl or NaOH to 1.5, 2.0, 2.5, 3.5, 5.5, or 6.5 as required. The solutions were incubated with agitation at 37 °C for 30 min, and the pH again adjusted to the corresponding values. One milliliter partially purified enzyme was then added to the solutions and incubated by agitation at 37 °C for 60 min. The amount of released phosphorus was determined as described in analytical methods.
Thermo-stabilization of phytase
Temperature stability profile (80 °C over a 5-min time course in 100 mM Glycine–HCl buffer pH 2.5) of partially purified phytase was determined in presence of 10% poly vinyl pyrrolidone (PVP). The residual phytase activity was determined by modified ammonium molybdate method as described in analytical methods.
PBD studies for screening of culture variables
The effect of 11 variables (X i ) viz, 5 basal medium variables and 6 additional variables, likely to influence phytase production were chosen for screening studies using PBD. The PBD is a two-factorial design that can be expected to identify critical chemical and physical parameters required for elevated enzyme production by screening the N variables in N + 1 experiments (Plackett and Burman 1946). Each independent variable was tested at two levels, a high (+1) level and a low (−1) level. A set of 12 experiments was thus carried out to determine phytase production as per the PBD of experiments (Table S1). The complete matrix for screening was designed using a standard Plackett–Burman orthogonal array constructed using Design Expert (DE) software Version 7.1.2, Stat-Ease, Minneapolis, MN, USA.
The effect E (X i ) of the tested variable X i was determined by:
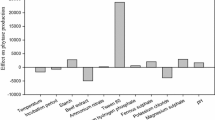
where, N denotes the number of trials with R (H) and R (L) representing a response obtained at either the high or low level, respectively, for the variable (X i ) from an experiment. Note that R (H) and R (L) are summed to obtain the average response at the high/low level, respectively, and their difference calculated to estimate the effect E (X i ). The variables with higher percent contributions were considered to influence phytase activity and chosen for further optimization studies. The percent contribution was obtained by taking the E (X i ) of each variable and dividing it by total sum of squares of E (X i ) for all the variables (Table S2).
Central composite design studies with chosen variables
Based on the results of the PBD, a central composite design (CCD) of experiments was carried out to arrive at optimized levels of the chosen variables that further maximize phytase production. According to CCD, the total number of experiments is 2k + 2 k + CP, where k is the number of independent variables and CP is the number of repetitions of the experiments at the center point. In order to ensure the design is rotatable, star points were set at ±(α) value at 1.459. This was used to develop a mathematical correlation between five variables on production of phytase. Each variable was studied at five levels, i.e., −α, −1, 0, +1, and +α as shown in Table 1. A matrix of 50 runs with 5 variables was generated using DE software to generate the response surface plot (Table S3). The optimum values of the variables and the behavior of the system were studied using the quadratic equation model. The quality for the fit of the second order equation model was expressed by the coefficient of determination R 2, and its statistical significance was determined by F test. To validate the response surface model, the maximum value was confirmed according to the optimum conditions predicted by the model. All experiments are carried out in triplicates and their mean values are presented.
Isolation of A. niger mutant
The spore suspension (107/ml) was treated with mutagens, both physical (UV) and chemical viz. ethidium bromide (0.1 mg/ml) and hydroxyl amine (0.1%) for different time intervals. The samples were suitably plated on PDA plates. The colony forming unit per milliliter (CFU/ml) was used then to calculate the 99% kill time. Mutants obtained were selected by spreading the chemically treated spore suspension on phytase screening medium (PSM) agar plates containing 0.1% calcium phytate and 0.05% NaNO3 and the selection of colony was dependent on observation of an enhanced zone of hydrolysis. Mutants were confirmed for phytase production using optimized medium under SmF and samples were removed periodically and checked for enzyme production as described earlier.
Results
Phytase production in SmF using basal medium
Our preliminary study on the effect of different process parameters on phytase production was carried using a classical OVAT approach. Among the various agriculture residues tested, rice bran showed maximal phytase activity. Optimization by OVAT showed that A. niger NCIM 563 produced best phytase activity (68,000 U/l) at low pH 2.5 on the 11th day under SmF conditions in basal medium. The basal fermentation medium (100 ml in 250 ml Erlenmeyer flask) consisted of 1 g rice bran, 5 g glucose, 0.86 g NaNO3, 0.05 g KCl, 0.05 g MgSO4, and 0.01 g FeSO4 with inoculum level 1 × E + 07 spores/ml. Other components, viz., dextrin, MnSO4, and surfactant (Tween 80), also have a positive effect on phytase production (Bhavsar et al. 2008) and were thus included in the further studies for optimization of media formulation.
Screening of important culture variables
The potential effect of 11 variables on phytase production (glucose, rice bran, NaNO3, MgSO4·7H2O, FeSO4·7H2O, KCl, incubation period, inoculum level, Tween 80, MnSO4, and dextrin) were evaluated using PBD. The PBD design matrix for experimental design of the selected variables is shown in Table S1 along with the obtained responses on phytase production. Maximum phytase production of 99,900 U/l was observed in trial number 2. The variables and their effect E (X i ), as calculated by Eq. (1) and percent contribution (Table S2) show that phytase production is significantly affected by glucose, MgSO4, KCl, incubation period, and MnSO4. These factors account for 97.8% of the total contribution while the remaining variables (NaNO3, FeSO4, inoculum level, Tween 80, and dextrin) account for only 2.2%. The variables identified by PBD to be insignificant and are therefore maintained at mean levels in further optimization studies. The best linear fit regression model obtained using the significant PBD variables gave a model F value 108.4 by ANOVA. This implies that there is only a 0.01% chance that this “Model F-Value” could occur due to noise. The coefficient of determination is R 2 = 0.98 and provides a measure of how much of the variability in the observed response values can be explained by the analysis.
Optimization of the screened variables
The five medium components (Table 1) identified by PBD, as significant factors, for phytase production were further optimized by CCD. The CCD design matrix and levels of the variables comprising and the obtained response values are shown in Table S3. It may be seen that trial number 39 showed the highest phytase activity (259,800 U/l) while trial number 12 showed the lowest phytase activity (2,100 U/l). The wide range in activity shows the sensitivity of the process to experimental conditions and the need for process optimization. The obtained results were fitted to polynomial equation to three significant digits:
where ŷ is the predicted response, 91700 is the intercept with variables A, B, and D are corresponding to glucose, MgSO4, and KCl. It may be seen that an interaction term AD is present in the above model. Non-significant variable and interaction terms were excluded by systematically carrying out ANOVA analysis to obtain the above reduced but improved model description Eq. (2). The Fisher F test (Table 2) for the model gave a very low probability value (P model > F = 0.0001) and this favorably suggests a very high significance of the regression model. The coefficient of determination (R 2) is 0·937 for phytase production, suggesting that 93·7% of the variability is explained in the model. The adjusted R 2 (0.93) is also very high and corroborates the significance of the model. The value of correlation coefficient (predicted R 2) for phytase production is 0·87 and suggests a strong agreement between the observed and model predicted values of phytase production. Adequate precision measures signal to noise ratio and a ratio greater than four is desirable. The obtained value of adequate precision (= 33.5) for phytase production indicates that the model Eq. (2) can be used to navigate the design space. The model F value of 106.3 implies there is only a 0.01% chance that this model F value occurs due to noise. Values of p > F (<0.05) indicate that the model terms A, D, AD, B 2, and D 2 in Eq. (2) are significant. The values of p > F did not satisfy this criteria for the other terms.
Validation of model
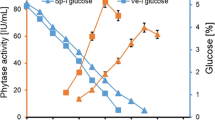
To validate the model, a time course for phytase production was carried out under conditions predicted by RSM as shown in Fig 1. The experimental response for phytase production was 254,500 U/l which is very close to the predicted response. A sharp decrease in phytase activity after 13th day is observed due to depletion of glucose in fermentation medium. The 3D response surface plot (Fig S1) shows the positive effect of the interaction between glucose and KCl for phytase production. An increase in phytase production is seen as the concentration of these variables is increased towards the +1 level. Overall, optimum values of the tested variables were obtained as 8% glucose, 0.1% MgSO4, 0.1% KCl, and 0.005% MnSO4 with 13 days of incubation period.
Release of phosphorus from soybean in simulated gastric fluid
Partially purified phytase exhibited high efficacy in phytate hydrolysis at different pH releasing 400, 1340, 1342, 800.7, 240, and 882 mg inorganic phosphorus/kg soybean meal at pH 1.5, 2.0, 2.5, 3.5, 5.5, and 6.5, respectively, under simulated gastric conditions.
Thermo-stabilization of phytase
The phytase thermostability was improved at 80 °C in presence of PVP which imparts 100% protection to enzyme up to 1 min (Fig S2). This thermostability profile is superior when compared to Allzyme and Natuphos (commercial phytase) that show only 50% and 75% residual activity (Casey and Walsh 2003).
Isolation of phytase producing A. niger mutant
Mutants were selected on the basis of small compact colony with large zone of hydrolysis on calcium phytate plate as compared to parent strain. The mutants A. niger NCIM 1359 and 1360 also exhibited 407,200 and 389,700 U/l phytase activity on 13th and 10th day, respectively using RSM optimized media. A remarkable difference among these two mutants strains was the delay in the sporulation time of the mutant strains (7th day) as compared to parent (4th day). The morphological patterns of mutant strains such as mycelial morphology, sporangium shape, and sporangium size were evaluated by using microscopy (Fig. 2).
Discussion
Although a broad range of microorganisms can produce phytase, their expression levels of phytase are too low for economic considerations. Productivity of any fungal fermentation is affected by process parameters and media composition and therefore the present investigation was performed to statistically optimize the medium components for the production of the phytase from A. niger using PBD and CCD methodologies. All significant variables involved in phytase production were evaluated by PBD because it can test a large number of variables while avoiding the loss of any essential information in subsequent optimization studies. Based on analysis of PBD, five key variables, viz., glucose, MgSO4, KCl, incubation period, and MnSO4, were found to affect the phytase production by A. niger. This was followed by use of a multifactorial response surface approach employing CCD, an effective design strategy, for studying the effects of key variables and their mutual interactions. This study resulted in an overall 3.74-fold enhancement in phytase activity of parent strain which is 274% higher than that observed with the basal medium.
Phytase from A. niger NCIM 563 is more resistant to pepsin and therefore releases more inorganic phosphorus from soybean meal under emulated gastric conditions over a much broader pH range (1.5–6.5) as compared to Yersinia rhodei and commercial phytase from A. niger (Huoqing et al. 2008). This brings out a significant advantage of A. niger over other phytases as it functions well under gastric conditions.
Liquid and dry enzyme formulations are used on a commercial scale by the feed industry. We have earlier reported the liquid formulation specifically used in mash feed, which can be added to the feed after pelleting (Shah et al. 2009). This is done to avoid heat inactivation of the phytase that may occur due to temperature of pelleting process (60–95 °C). However, this technique has certain disadvantages such as difficulties in mixing (due to small enzyme amounts) leading to the heterogeneous distribution of enzyme in feed. In addition, specialized equipment is needed to add liquids to the feed after pelleting and this is not currently available at most feed mills due to the extra cost involved. Thermostability is considered an important and useful criterion for industrial application of phytase. As shown, enzyme from A. niger NCIM 563 is more heat stable and hence should have great potential use in pelleted feed than available commercial preparations.
In parallel with media optimization studies, mutagenesis studies were conducted, using hydroxyl amine, for obtaining a hypersecretory strain. The selection of mutants was based on enhanced hydrolysis zone on PSM plates. However, the method is unable to differentiate between phytase activity and acid production. Hence, all the positive mutants were quantified and confirmed for phytase production using the statistically optimized media for the parent strain in shake flask condition. Among the hypersecretory mutants, the mutant A. niger NCIM 1359 exhibited the highest phytase production (407,200 U/l) and improved the yield 1.6 times as compared to production by parent strain. Exploitation of this observation could have tremendous value addition from the phytate feed-conversion and environmental point-of-view. Furthermore, the results of the present study provide an excellent basal medium formulation for studying phytase production with the mutant strain and assessing its properties.
Strain improvement is associated with disadvantages, such as low mutation frequency in the desired gene. Even if mutants with the desired phenotype are isolated, there is no guarantee that the mutation has occurred in the gene of interest. These shortcomings need to be alleviated and with the recent development of gene-cloning and sequencing techniques we can now locate whether mutation has occurred in gene by a single base change of DNA (an insertion or a deletion). From a simpler viewpoint, however, we can reasonably assume that genetic variability amongst parent and mutant strains exist if morphological differences are seen through mutagenesis studies. In the present study, we exploit this observation.
Low yield, high production costs, and lack of desirable characteristics in the currently available commercial phytases have limited its widespread use (Krishna and Nokes 2001). Thus there is a need for identifying novel phytases with high yield and improved desired enzymatic characteristics. The combined results of statistical optimization and mutagenesis show a remarkable 6-fold improvement in phytase production rates to 407,200 U/l and suggest its potential for industrial application. Also, A. niger outperforms the phytase production rates by an exceptional 32-fold increase in comparison to other organisms, viz., Sporotrichum thermophile with 10,100 (Singh and Satyanarayana 2006) and 12,500 U/l (Singh and Satyanarayana 2008) for two different media formulations and recombinant Escherichia coli with 2,250 U/l (Sunitha et al. 1999). Currently A. niger is “Generally Recognized as Safe (GRAS)” in food and feed applications (Bhavsar et al. 2011). Along with the high yield, pH tolerance, and temperature stability characteristics, it appears to be a viable option when compared to other available commercial phytase supplements.
References
Afinah S, Yazid AM, Anis Shobirin MH, Shuhaimi M (2010) Phytase: application in food industry. Inter Food Research J 17:13–21
Bhavsar KP, Shah PC, Soni SK, Khire JM (2008) Influence of pre-treatment of agriculture residues on phytase production by Aspergillus niger NCIM 563, under submerged fermentation conditions. Afr J Biotechnol 7:1101–1106
Bhavsar KP, Ravi Kumar V, Khire JM (2011) High level phytase production by A. niger NCIM 563 in solid state culture: response surface optimization, up scaling and its partial characterization. J Ind Microbiol Biotech 38:1407–1417
Bogar B, Szakacs G, Pandey A, Abdulhameed S, Linden JC, Tengerdy RP (2003) Production of phytase by Mucor racemosus in solid-state fermentation. Biotechnol Prog 19:312–319
Casey A, Walsh G (2003) Purification and characterization of extracellular phytase from Aspergillus niger ATCC 9142. Bioresour Technol 86:183–188
Chadha BS, Gulati H, Minhas M, Saini HS, Singh N (2004) Phytase production by the thermophilic fungus Rhizomucor pusillus. World J Microbiol Biotechnol 20:105–109
Dvorakova J (1998) Phytase source, preparation and exploitation. Folia Microbiol 43:323–338
Huoqing H, Huiying L, Wang Y, Fu D, Shao N, Wang G, Yang P, Yao B (2008) A novel phytase from Yersinia rohdei with high phytate hydrolysis activity under low pH and strong pepsin conditions. Appl Microbiol Biotechnol 80:417–426
Krishna C, Nokes SE (2001) Predicting vegetative inoculum performance to maximize phytase production in solid-state fermentation using response surface methodology. J Ind Microbiol Biotechnol 26:161–170
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mandviwala TN, Khire JM (2000) Production of high activity thermostable phytase from thermotolerant Aspergillus niger in solid-state fermentation. J Ind Microbiol Biotechnol 24:237–243
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:427–431
Mitchell DB, Vogel K, Weimann BJ, Pasamontes L, van Loon APGM (1997) The phytase subfamily of histidine acid phosphatase: isolation of genes for two novel phytases from fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 143:245–252
Parekh S, Vinci VA, Strobel RJ (2000) Improvement of microbial strains and fermentation processes. Appl Microbiol Biotechnol 54:287–301
Plackett RL, Burman JP (1946) The design of optimum multifactor experiments. Biometrika 33:305–325
Reddy NR, Sathe SK, Salunkhe DK (1982) Phytases in legumes and cereals. Adv Food Res 82:1–92
Shah P, Bhavsar K, Soni SK, Khire JM (2009) Strain improvement and up scaling of phytase production by Aspergillus niger NCIM 563 under submerged fermentation conditions. J Ind Microbiol Biotechnol 36:373–380
Singh B, Satyanarayana T (2006) A marked enhancement in phytase production by a thermophilic mould Sporotrichum thermophile using statistical designs in a cost-effective cane molasses medium. J Appl Microbiol 101:344–352
Singh B, Satyanarayana T (2008) Improved phytase production by a thermophilic mould Sporotrichum thermophile in submerged fermentation due to statistical optimization. Bioresour Technol 99:824–830
Soni SK, Khire JM (2007) Production and partial characterization of two types of phytase from Aspergillus niger NCIM 563 under submerged fermentation conditions. World J Microbiol Biotechnol 23:1585–1593
Sunitha K, Lee JK, Oh TK (1999) Optimization of medium components for phytase production by E. coli using response surface methodology. Bioproc Eng 21:477–481
Tanyildizi MS, Ozer D, Elibol M (2005) Optimization of α-amylase production by Bacillus sp. using response surface methodology. Proc Biochem 40:2291–2296
Vohra A, Satyanarayana T (2002) Statistical optimization of the medium components by response surface methodology to enhance phytase production by Pichia anomala. Proc Biochem 37:999–1004
Acknowledgments
The authors, Ms. Kavita Bhavsar and Ms. Pradnya Gujar, thank Council of Scientific and Industrial Research, Government of India for the financial assistance. The authors also gratefully acknowledge the support and facilities provided by the Center of Excellence in Scientific Computing, National Chemical Laboratory, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 265 kb)
Rights and permissions
About this article
Cite this article
Bhavsar, K., Gujar, P., Shah, P. et al. Combinatorial approach of statistical optimization and mutagenesis for improved production of acidic phytase by Aspergillus niger NCIM 563 under submerged fermentation condition. Appl Microbiol Biotechnol 97, 673–679 (2013). https://doi.org/10.1007/s00253-012-3965-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3965-8