Abstract
Gluconobacter oxydans is a well-known acetic acid bacterium that has long been applied in the biotechnological industry. Its extraordinary capacity to oxidize a variety of sugars, polyols, and alcohols into acids, aldehydes, and ketones is advantageous for the production of valuable compounds. Relevant G. oxydans industrial applications are in the manufacture of L-ascorbic acid (vitamin C), miglitol, gluconic acid and its derivatives, and dihydroxyacetone. Increasing efforts on improving these processes have been made in the last few years, especially by applying metabolic engineering. Thereby, a series of genes have been targeted to construct powerful recombinant strains to be used in optimized fermentation. Furthermore, low-cost feedstocks, mostly agro-industrial wastes or byproducts, have been investigated, to reduce processing costs and improve the sustainability of G. oxydans bioprocess. Nonetheless, further research is required mainly to make these raw materials feasible at the industrial scale. The current shortage of suitable genetic tools for metabolic engineering modifications in G. oxydans is another challenge to be overcome. This paper aims to give an overview of the most relevant industrial G. oxydans processes and the current strategies developed for their improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotechnology plays an important role in generating a wide range of valuable compounds, providing novel opportunities for sustainable processes. Biocatalyst based-processes have been highlighted since their use allows an increased in selectivity of chemical reactions, which lowers byproduct formation and enhances product yield compared to chemical processes (Gavrilescu and Chisti 2005). Among many microorganisms that can be applied in the biotechnological process, Gluconobacter oxydans, an obligatory aerobic and Gram-negative bacterium, has been recognized for its extraordinary capacity of incomplete regio- and stereoselective oxidation of sugars, polyols, and alcohols (De Ley et al. 1984; Gupta et al. 2001). This capacity made G. oxydans a convenient choice for synthesis of valuable compounds via a combination of biocatalysis and chemical methods.
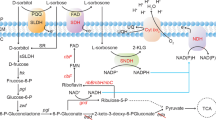
G. oxydans oxidation is catalyzed by membrane-bound dehydrogenases (mDHs), located on the outer surface of the cytoplasmic membrane and does not involve phosphorylated reactions (Fig. 1). These dehydrogenases are quinoproteins or flavoproteins that have pyrroloquinoline quinone (PQQ) and flavin adenine dinucleotide (FAD) as prosthetic groups, respectively. Furthermore, since the active site of these enzymes is oriented towards the periplasm, both substrate and oxidized products can easily cross the outer membrane. Accumulation of oxidized products occurs in the culture medium, which favors their recovery. Membrane-bound dehydrogenases are linked to the respiratory chain and the electrons are transferred to oxygen as the final electron acceptor (Matsushita et al. 1994; Deppenmeier et al. 2002).
Oxidative systems in Gluconobacter: a—Sorbitol oxidizing systems of Gluconobacter species: SLDH–PQQ dependent sorbitol dehydrogenase and SDH–FAD dependent sorbose dehydrogenase are located on the outer surface of cytoplasmic membrane; SR–NADPH dependent L-sorbose reductase; SNR–NADPH dependent L-sorbosone reductase and SNDH–NAD(P) dependent L-sorbosone dehydrogenase are present in cytoplasm; 2-KLG: 2-keto-D-gulonic acid. b—Glycerol oxidizing systems of Gluconobacter species: GLDH–PQQ-dependent glycerol dehydrogenase is located on the outer surface of cytoplasmic membrane; GK–glycerokinase and GPDH–glycerol-3-phosphate-dehydrogenase are present in cytoplasm. DHA-P, dihydroxyacetone phosphate. c—Glucose oxidizing systems of Gluconobacter species: GDH–PQQ-dependent glucose dehydrogenase, GLDH–PQQ-dependent glycerol dehydrogenase, GA2DH–FAD- dependent gluconate-2-dehydrogenase and 2KGADH–FAD-dependent 2-keto-gluconate-dehydrogenase are located on the outer surface of cytoplasmic membrane; GDH—NADP dependent glucose dehydrogenase, 2KGR–NADP dependent 2-keto-gluconate-reductase, 5KGR–NADP dependent 5-keto-gluconate-reductase, GK–glucokinase, GNK–gluconokinase and G6PD–glucose 6 phosphate dehydrogenase are present in cytoplasm. Whenever there is more than one arrow, there is more than one reaction in sequence. (Adapted from Claret et al. 1994; Matsushita et al. 1994; Deppenmeier et al. 2002; Saichana et al. 2015; Cañete-Rodríguez et al. 2016). *A FAD-dependent SLDH can also catalyzes the reaction
The direct oxidative pathways described above is related to the efficient and fast oxidation industrial reaction. There is a second oxidation pathway, in which a minor part of sugars or their oxidation products are catabolized. The substrate is taken up into the cytoplasm where the reactions occur (Fig. 1). In this case the oxidative reactions are catalyzed by NAD(P)+ dependent dehydrogenases. Phosphorylated intermediates are formed and metabolized via the pentose phosphate pathway (PPP) (Matsushita et al. 1994; Deppenmeier et al. 2002).
Gluconobater oxydans has been used in biotechnological processes, namely in the production of: vitamin C (5) (Reichstein and Grüssner 1934), miglitol (9) (Ke et al. 2018), D-gluconic acid (13) (GA) (Kulka et al. 1951; Ramachandran et al. 2006), 2-keto-D-gluconic acid (14) (2-KGA) (Wang et al. 2019), 5-keto-D-gluconic acid (16) (5-KGA) (Shinagawa et al. 1999) and 1,3 dihydroxyacetone (18) (DHA) (Claret et al. 1994). Each process requires different membrane-bound dehydrogenases to oxidize their respective substrates (Fig. 1).
The aim of this paper is overview biotechnological processes that have the versatile bacterium, Gluconobacter oxydans, as the biocatalyst. The emphasis is on current production strategies and new approaches that have been developed in the last few years to improve efficiency and profitability of these processes, focusing mostly on publications since 2014.
Recent developments rely on comprehension of metabolic features and genetic manipulation of G. oxydans. Research has been undertaken (Mientus et al. 2017; Kranz et al. 2018; Zhou et al. 2019a; Qin et al. 2021) to elucidate G. oxydans metabolism, which could pave the way for the improved performance of strains. For example, the genome of a strain with high oxidative power and low growth yield (G. oxydans ATCC 621H) was compared with that of a strain with good growth yield (G. oxydans DSM 3504) in order to understand how to boost growth and increase productivity (Kostner et al. 2015). It was found that the presence of an additional NADH dehydrogenase gene is important for increased growth yields but negatively impacts the activity of the membrane-bound polyol dehydrogenases responsible for substrate oxidation (Kostner et al. 2015).
Overexpression of genes directly related to the reaction, gene knockout and heterologous expression have been applied to G. oxydans strains to improve the synthesis of 2-keto-L-gulonic acid (2-KLG—4), 6-(2-hydroxyethyl)-amino-6-deoxy- α- L- sorbofuranose (6NSL—11), 2-keto-D-gluconic acid (2-KGA—14), 5 keto-D- gluconic acid (5-KGA -16) and dihydroxyacetone (DHA—18) (Shi et al. 2015; Chen et al. 2016; Yuan et al. 2016a; Tan et al. 2019; Liu et al. 2021). However, the scarcity of genetic tools for metabolically engineering G. oxydans limits strain development. One of the proposed approaches is the search of stronger promoters, as they are important in controlling and regulating gene expression at the transcriptional level. The choice of the promoter P2703 among 97 others resulted in a 2-KLG (4) titer three-fold higher than that obtained with other strong promoters (Chen et al. 2021). The development of the CRISPR/Cas system is also enriching the gene regulation tools in G. oxydans. An endogenous CRISPR/Cas system in G. oxydans WSH-003 allowed a multiplex gene repression and the control of central carbon metabolism flux that improved the strain growth ability and substrate conversion rate (Qin et al. 2021). Genetic modifications carried out for industrial processes by G. oxydans in the last few years are depicted in Table 1.
Regarding new strategies for G. oxydans bioprocesses, the search for cheaper feedstocks such as residual biomass or industrial byproducts, have been increased lately. Agro-industrial wastes (sugars from hydrolysis) and biodiesel byproducts (crude glycerol), have already been reported for gluconic acid (13) (Cañete-Rodríguez et al. 2015; Zhang et al. 2016a; Hou et al. 2018; Zhou and Xu 2019) and dihydroxyacetone (18) synthesis, respectively (Dikshit and Moholkar 2016a; Poljungreed and Boonyarattanakalin 2017; Stasiak-Rózańska et al. 2018). The use of residual feedstocks not only can reduce costs, but could also be a solution for waste disposal. The fermentation medium may account for 60% of processing costs, and the growing concern with sustainability make these raw materials a valuable source towards profitable and eco-friendly bioproduction (Alonso et al. 2015).
Vitamin C
L-ascorbic acid (vitamin C—5) is an essential nutrient for humans, as we are incapable of its endogenous synthesis. Prolonged vitamin C (5) deficiency causes scurvy, a deadly disease. Attempts to combat the disease led to the isolation of this vitamin in 1928 (Yang and Xu 2016). In the absence of vitamin C (5), the synthesis of collagen is impaired, leading to scurvy symptoms. Vitamin C (5) acts as coenzyme for prolyl-4-hydroxylase and lysyl hydroxylases, which are key enzymes for collagen biosynthesis. In addition, vitamin C (5) participates in several other process, such as synthesis of hormones and, carnitine, elimination of tyrosine and protection against reactive oxygen species (ROS) (Dosedel et al. 2021).
China dominates the vitamin C (5) market and supplies over 60% of demand (PR Newswire 2021). The two-step fermentation method, which is currently used for vitamin C (5) industrial production, was create under a collaboration between the Northeast Pharmaceutical Group Co., Ltda (NEPG) and the Chinese Academy of Sciences, both from China. NEPG has the largest production line in operation in China (10.000 tons) (Northeast Pharmaceutical Group—2021). Located in Jiangsu Province, DSM Jiangshan Pharmaceutical (Jiangsu) Co., Ltd, is another world leading manufacturer of vitamin C (5) (DSM Jiangshan Pharmaceutical Group - 2021). Important non-chinese producers include Abbott Laboratories and Bayer AG (CDN Newswire 2021). Global vitamin C (5) demands increased in 2020 due to the COVID-19 pandemic outbreak. Currently, the production is about 140–150 kilo tonnes annually (PR Newswire 2021). Vitamin C (5) market price is influenced not only by its demand but also by the cost of raw material that can account for 73% of total production cost. Forecasters expect the vitamin C (5) market to reach USD 1.34 billion in 2023 (PR Newswire 2021).
The industrial production of vitamin C (5) began in early of 1930 owing to its high demand. In 1933, Tadeu Reichstein, a polish chemist, developed a process for vitamin C (5) mass-production that was largely used until the late 1990s (Pappenberger and Hohmann 2013). This process involves one fermentation step and five chemical steps (Wang et al. 2018) (Fig. 2). First, glucose is reduced to D-sorbitol (2), using nickel as catalyst, followed by its oxidation to L-sorbose (3) by G. oxydans. After a series of reactions to protect the hydroxy groups against oxidation, L-sorbose (3) is oxidized to 2-keto-L-gulonic acid (4). Enolization and lactonization of 2-KLG (4) finally leads to L-ascorbic acid (5) (Reichstein and Grüssner 1934; Boudrant 1990).

Yang and Xu 2016)
Pathway I: reactions IA, IB, IC, ID and IE depict stages of the Reichstein process for vitamin C synthesis. Pathway II: reactions IIA, IIB, IIC and IID depict stages of the two-step fermentation process for vitamin C synthesis. Whenever there is more than one arrow, there is more than one reaction in sequence. Adapted from (
Considered as an improvement of Reichstein’s process, a two-step fermentation is currently used for vitamin C (5) production and comprises six steps (Wang et al. 2018) (Fig. 2). Firstly, glucose is converted into D-sorbitol (2) by catalytic hydrogenation, which is efficiently transformed into L-sorbose (3) by G. oxydans through sorbitol dehydrogenase (SLDH) (Gao et al. 2014). This stage is industrially carried out in an airlift bioreactor and D-sorbitol (2) is applied as substrate (20–25%) in a batch fermentation, for 18–24 h at 32 °C and at natural pH. The oxygen transfer rate is maintained at 300–500 mmol L−1 h−1 and a productivity of 12–13 g L−1 h−1 is reached (Yang and Xu 2016). Sorbitol dehydrogenase is a major polyol dehydrogenase also known as a glycerol dehydrogenase (GDLH), which converts glycerol (17) to dihydroxyacetone (18), gluconic acid (13) to 5-keto-D-gluconic acid (16) and D-sorbitol (2) to L-sorbose (3) (Prust et al. 2005; Qin et al. 2022). This enzyme has PQQ as a prosthetic group, however a FAD dependent membrane bound SLDH also converts D-sorbitol (2) to L-sorbose (3) (Qin et al. 2022).
In the second fermentation, a mix-culture of Ketogulonicigenium vulgare and Bacillus megaterium or Bacillus cereus produces 2-KLG (4) (Fig. 2), the precursor of L-ascorbic acid (5), from L-sorbose (3). K. vulgare produces 2-KLG (4) by L-sorbose / L-sorbosone dehydrogenase (Asakura and Hoshino 1999) or by another sorbosone dehydrogenase that only catalyzes the conversion of L-sorbosone to 2-KLG (4) (Miyazaki et al. 2006). B. megaterium cannot catalyze L-sorbose (3) transformation, and act as a companion strain to enrich 2-KLG (4) production by favoring the growth of K. vulgare (Ye et al. 2014). The industrial production of 2-KLG (4) is also a batch fermentation and is carried out on an airlift bioreactor. A temperature of 29 °C and a neutral pH (adjusted by sodium hydroxide) is maintained during the process, which lasts 40–65 h. L-sorbose (3) is added as the pasteurized broth obtained from the first fermentation initially at a concentration of 10 mg ml−1and then regularly added to the fermentation medium between 10–30 h. An oxygenation of 100 mmol L−1 h−1 is kept during the process to maintain the cells growth and a satisfactory L-sorbose (3) conversion. The process reaches a final concentration of 90–110 g L−1 of 2-KLG (4) (Yang and Xu 2016). This co-cultivation of K.vulgare and Bacillus spp. is an example of a successful consortia applied industrially.
The knowledge on the type of interaction between the microorganisms is required for co-cultivation, since it affects metabolic efficiency. Due to advances in synthetic biology, metabolic and genetic engineering, researchers are working on developing efficient microbial consortia to increase productivity (Bhatia et al. 2018; Kylilis et al. 2018). Bacillus megaterium and K. vulgare form a synergistically mutualistic co-culture (Zou et al. 2013; Ghosh et al. 2016) and their interaction is crucial for increased 2-KLG (4) yield (Yang et al. 2013). Other examples of biotechnological process being devised with defined consortia of microorganism are in the production of lignocellulolytic enzymes (Hu et al. 2017a), butanol (Wu et al. 2016), lactic acid (Sun et al. 2021) and ethanol (Beri et al. 2020).
Despite effective production of vitamin C (5) by a two-step fermentation process, there are limitations such as the long incubation period and the need for additional sterilization (Wang et al. 2016a). In order to overcome these drawbacks, efforts to improve 2-KLG (4) production and develop a feasible one-step fermentation route are the major challenges of industrial vitamin C (5) production nowadays, which are discussed in greater detail by Wang and co-works (Wang et al. 2018). The design of a suitable microbial consortium and the genetic engineering of strains seem to be the paths to develop a successful one-step vitamin C (5) fermentation. Moreover, -omics technologies are important tools to understand changes occurring in microorganisms submitted for co-culture, providing insights for advancement of one-step fermentation.
Long-term adaptation of K. vulgare and B. thuringiensis strengthens their interaction due to both enhancement of amino acids metabolism and their exchange between the strains. Furthermore, the adaptation mechanism of B. thuringiensis weakened its sporulation and increased its growth rate, which positively affected K. vulgare growth and productivity towards 2-KLG (4) (Ma et al. 2014; Jia et al. 2017). In addition, when G. oxydans are in co-culture with B. endophyticus and K. vulgare, a negative impact on its carbon metabolism (PPP–pentose phosphate pathway) occurred, which affected cells growth and protein synthesis. In contrast, the presence of G. oxydans in the three microbial consortium promoted an enhancement in fatty acids and purine nucleotide synthesis (Ma et al. 2019).
Towards genetic engineering, most efforts are concentrated on construct strains able to convert D-sorbitol (2) into 2-KLG (4) in mono or co-culture. Although, Gluconobacter synthesizes the necessary enzymes to produce 2-KLG (4) from D-sorbitol (2) (Saito et al. 1997), production is very low and only a few strains are able to form 2-KLG (4) (Deppenmeier et al. 2002). Meanwhile bioconversion from D-sorbitol (2) by K. vulgare is poorly efficient compared to that using L-sorbose (3) as a substrate (Sugisawa et al. 2005). Furthermore, a high 2-KLG (4) production by K. vulgare is only achieved if a helper strain is present (Zhang et al. 2010) or by adding additional nutrients (Leduc et al. 2004). Thus, a construction of a recombinant strain of G. oxydans seems more likely.
The overexpression of K. vulgare genes in G. oxydans is also a target of research. Gao et al. (2014) expressed five genes encoding for SDH (sorbose dehydrogenase) and two genes for SNDH (sorbosone dehydrogenase) from K. vulgare WSH-01 in G. oxydans WSH-003. Ten distinct combination of SDH and SNDH genes were expressed generating ten new strains. Although all strains were able to produce 2-KLG (4) from D-sorbitol (2), the yield was low due to the accumulation of L-sorbosone. To overcome this shortcoming, linker peptides were used to fuse SDH and SNDH this led to higher yield of 2-KLG (4) (Gao et al. 2014).
Furthermore, as SLDH, SDH and SNDH are PQQ dependent, an imbalance of enzymes and their cofactor would negatively affect 2-KLG (4) production. To prevent this possible problem, the pqqA gene and pqqABCDE gene clusters were overexpressed individually in G. oxydans (G. oxydans/pGUC-k0203–GS-k0095). The overexpression of pqqABCDE gene led to the highest PQQ levels and as consequence enhanced the 2-KLG (4) yield (Gao et al. 2014). In fact, an increase in PQQ level is positively correlated to an efficient conversion of substrate into its respective products, which is promoted by PQQ—dependent dehydrogenases (Wang et al. 2016b).
Miglitol
Miglitol (9) (N-hydroxyethyl-1deoxynojirimycin) is an iminosugar developed by Bayer and has been used in the control of type 2 diabetes due to its competitive inhibition of α-glucosidase (Schedel 2000; Zhang et al. 2011a; Khobare et al. 2016). In 1996, miglitol (9) was approved by the Food and Drug Administration (FDA) as an antidiabetic drug (FDA 1996) and has been sold in many countries, such as France, USA, Japan, Spain and Australia (Sugimoto et al. 2015). Some of miglitol (9) manufacturers include Lunan Pharmaceutical Group Co., Ltd. (Linyi City, Shandong, China), Zhejiang Medicine (Shaoxing, Zhejiang, China) (Market Watch 2021) and Bayer HealthCare Pharmaceuticals Inc. (Berlin, Germany). Bayer have licensed to Pfizer the marketing rights of Glyset®, a trade name of miglitol (9) also registered by Bayer (Pfizer 2016).
Miglitol (9) can be obtained from DNJ (8), (1- Deoxynojirimycin) an imunosugar first isolated from plants in 1976 (Yagi et al. 1976). DNJ (8) can be extracted from plants or obtained by chemical synthesis or fermentation using Streptomyces and Bacillus. However, none of these methods are economically feasible on a large scale (Schedel 2000). Hence, the adopted industrial approach for miglitol (9) production is a combination of biocatalytic and chemical methods (Fig. 3). Biotransformation is applied to produce the key intermediate of miglitol (9) synthesis and then chemical steps are performed to obtain miglitol (9). Two routes with two different key intermediates (6-amino-6-deoxy-L-sorbose (7) or 6-(2-hydroxyethyl)-amino-6-deoxy- α- L- sorbofuranose (11)) are possible (Kinast and Schedel 1981; Grabner et al. 2003). Both use resting cells of G. oxydans grown in an appropriate culture medium with D-sorbitol (2) as the main carbon source (Schedel 2000).
Routes of biotechnological-chemical synthesis of miglitol. a Pathway I—occurs via biotransformation of 1-amino-1-deoxy-D-sorbitol into 6-amino-6-deoxy-L-sorbose. b Pathway II—occurs via biotransformation of N-2-hydroxyethyl glucamine (NHEG) into 6-(2-hydroxyethyl)-amino-6-deoxy- α- L- sorbofuranose (6NSL) (Kinast and Schedel 1981; Schröder and Stubbe 1989; Grabner et al. 2003)
In the first approach, D-glucose is chemically transformed through reductive amination to 1-amino-1-deoxy-D-sorbitol (6), which is regioselectively oxidated to 6-amino-6-deoxy-L-sorbose (7) by resting cells of G. oxydans. Stereoselective reductive ring closure of 6-amino-6-deoxy-L-sorbose (7) leads to DNJ (8) formation (Kinast and Schedel 1981; Schröder and Stubbe 1989; Schedel 2000). The addition of protection groups, (e.g., benzyloxycarbonyl group, and small hydrophilic groups like formyl- and dichloroacethyl-) prior the biotransformation of 1-amino-1-deoxy-D-sorbitol (6) is necessary (Kinast and Schedel 1981; Schröder and Stubbe 1989) to avoid the spontaneous intramolecular ring closure reaction. These protective groups have to be remove before the final reduction (Schedel 2000). DNJ (8) is chemically transformed into miglitol (9) (Fig. 3a).
The latest approach, also starts by a reductive amination reaction of D-glucose, which is converted into N-2-hydroxyethyl glucamine (10) (NHEG). Resting cells of G. oxydans are applied at 15 °C in sterile conditions and under aeration to the regioselective oxidation of NHEG (10) to 6-(2-hydroxyethyl)-amino-6-deoxy- α- L- sorbofuranose (11). The cell mass is removed and a catalytic hydrogenation reaction proceeds directly from the supernatant liquor to form miglitol (9) (Fig. 3b) (Grabner et al. 1999; Liu et al. 2020a). This process is more advantageous than the described above because no isolation and no protective group is needed. The conversion of NHEG (10) to 6NSL (11) requires a membrane-bound D-sorbitol dehydrogenase (SLDH) with activity and stability at the low process temperature of 15 °C. The selection or development of better strains that meet this latter requirement is until now a challenge (Ke et al. 2018; Liu et al. 2020a).
Despite advances in recombinant DNA and protein expression technologies, classical mutation is still a strategy used to improve industrial strains. High-throughput methods are needed for efficient and rapid screening of strains with high SLDH activity among the large amount of mutants generated by this method. New strategies based on chromogenic reactions have been reported. More than 1400 G. oxydans ultraviolet irradiation mutant strains were screened for 6NSL (11) production using a chromogenic reaction between 6NSL (11) and 2,4 dinitrophenylhydrazine. The reaction products formed under alkaline conditions present color intensities proportional to the amount of 6NSL (11), which is readily recognized (Ke et al. 2018). Another approach to high-throughput screening for 6NSL (11) producer strains was developed based on the reaction of L-sorbose (3) with Fehling’s reagent, which forms a yellow to red precipitate easily identified. This approach was used for the screening of 60Co-γ irradiation and microwave mutants, resulting in more than 1000 positive strains for 6NSL (11) (Liu et al. 2020a).
Regarding metabolic engineering, the increase in 6NSL (11) production was obtained by the overexpression of genes related to the SLDH enzyme. A G. oxydans strain overexpressing the sldAB gene cluster was constructed resulting in an accelerated catalysis towards substrate, which is directly related to an increase on SLDH activity (Ke et al. 2019b). The transcriptional level of SLDH was 27.3-fold higher compared to wild strain, and relative enzymatic activity towards NHEG (10) increased 2.05 fold in the recombinant strain.
As previously discussed for vitamin C (5), the small amount of PQQ synthesized by G. oxydans (Xiong et al. 2011) may represent a bottleneck for the action of dehydrogenase enzymes dependent on this coenzyme and, consequently, for the formation of miglitol (9). To overcome this shortage, the genes responsible for PQQ synthesis (gene cluster pqqABCDE) can be overexpressed (Liu et al. 2020b) or a medium fortified with amino acids can be applied as a simpler strategy (Ke et al. 2019a, b). In fact, supplement the medium with four exogenous amino acids (Glu, Ile, Thr, Arg), which are rapidly consumed by G. oxydans for biosynthesis of PQQ precursor (PqqA), enhances PQQ levels by 48% and favors SLDH activity, which showed an increase of 19.8% (Ke et al. 2019b).
The growth of cells for biotransformation usually results in low yield and this step is responsible for 40% of the cost of producing 6NSL (11) (Hu et al. 2020). In addition, there is a loss of catalytic activity during biotransformation. Thus, cell reuse strategies help to lower the cost of the process and increase production (Hu et al. 2019, 2020). Hu et al. (2019) were able to reuse cells immobilized by adsorption four times, applying a repeated biotransformation strategy, using NH4Cl as a nitrogen source and for pH control in a bubble column bioreactor with an aeration rate of 2.5vvm. They were also able to increase the number of reuse cycles to nine by regenerating the cells in situ using fresh medium. The NHEG (10) concentration was maintained at 50 g L−1 at each repetition of the biotransformation, after verifying that above 60 g L−1 there is a drop in the reaction rate, reflecting an inhibition of enzyme activity by the substrate (Hu et al. 2020).
Gluconic acid and ketogluconic acids
Organic acids represent one of the major building blocks obtained through biotechnology processes in the global market of high-volume bulk chemicals (Anastassiadis and Morgunov 2007). The market for gluconic acid (13), also known as pentahydroxycaproic acid (C6H12O7), is expected to reach USD 1.9 billion in 2028 (Fior Markets 2021) Currently, annual production of GA (13) and its derivatives (Fig. 4) is approximately 80.000 tonnes (Karaffa and Kubicek 2021). Due to versatile chemical features, this organic acid and its derivatives have found wide applications in chemical, pharmaceutical, food, dairy, beverage and construction industries (Pal et al. 2016; Golikova et al. 2017).
Gluconic acid and ketogluconic acids from glucose oxidation by Gluconobacter oxydans; GDH glucose dehydrogenase, GL glucono-δ-lactonase, GA2DH gluconate-2-dehydrogenase, 2KGADH 2-keto-gluconate-dehydrogenase, GLDH glycerol dehydrogenase (Kataoka et al. 2015). *The reaction can be catalyzed by GL or occurs spontaneously
Even though GA (13) can be obtained through chemical or enzymatic approaches, its industrial production is based on a dehydrogenation reaction of glucose by microbial fermentation (Fig. 1c). G. oxydans and Aspergillus niger are widely employed microorganisms and starch or sucrose are used as the main feedstock (Attwood et al. 1991; Vroemen and Bévérini 1999; Sainz et al. 2016; Zhang et al. 2016b). Main manufacturers of gluconic acid (13) and/or its salts include Ruibang Laboratories (Whenzou, Zhejiang, China) (Ruibang Laboratories 2021) and Roquette Frères (France) (Roquette 2021).
The main pathway for gluconic acid (13) production is via membrane-bound D-glucose dehydrogenase that oxidizes D-glucose (1) to D-glucono-δ-lactone (12), which is subsequently converted to D-gluconate (13) spontaneously or by membrane-bound glucono-δ-lactonase (GL) (Fig. 4) (Kataoka et al. 2015). Some G. oxydans strains are able of carry out reactions transforming GA (13) into 2-keto-D-gluconate (14) or 5-keto-D-gluconate (16). Additionaly, 2-keto-D-gluconate (14) may be further oxidized into 2,5-diketo-D-gluconate (15) (2,5-KGA). GA (13) can also be formed through a second pathway, that occurs inside cells by NADP+-dependent GDH, but membrane-bound activity is 30-fold higher (Matsushita et al. 1994; Prust et al. 2005; Cañete-Rodríguez et al. 2016; Sainz et al. 2016).
Although some papers and patents report industrial production of GA (13) by Gluconobacter oxydans (Attwood et al. 1991; Vroemen and Bévérini 1999), the literature is mainly based on the process using A. niger and information on aspects of the G.oxydans process is limited. The fermentation yield in relation to glucose (1) (substrate) is higher with G. oxydans than with A. niger because the fungus uses part of the glucose (1) for its growth. The yield using G. oxydans can be close to 100%, although expensive nutrients are required for the growth of the microorganism (Vroemen and Bévérini 1999; Anastassiadis and Morgunov 2007). The process is carried out by submerged batch in aerated fermenters at controlled temperature (Anastassiadis and Morgunov 2007).
During growth on glucose (1), G. oxydans converts most of the sugar to GA (13) and ketogluconates (14, 16) (2-KGA and 5-KGA) (Fig. 4) at the expense of growth (Krajewski et al. 2010). The pH of the culture medium is the most influential factor in regulating the formation of GA (13) and its ketoacids (14, 16) (Ano et al. 2011; Cañete-Rodríguez et al. 2016). At pH between 3 and 5 the formation of 5-KGA (16) is privileged and above pH 5 this production decreases and favors the joint formation of 2-KGA (14). Gluconic acid (13) production is favored at pH below 3, but below pH 2 production is almost totally repressed (Velizarov and Beschkov 1994; Ano et al. 2011).
Glucose (1) up to a concentration of 90 g L−1 is almost completely oxidized to GA (13) (Velizarov and Beschkov 1994). Acid accumulation due glucose transformation leads to a rapid decrease of pH to about 2.5. At this pH, the enzymes of the pentose phosphate cycle are almost totally inactivated and the energy needs of cells depend on the rapid oxidation by membrane dehydrogenases. Thus, almost quantitative conversion of glucose (1) to gluconate (13) is obtained and pH control is not required for the fermentation process at this glucose concentration (Velizarov and Beschkov 1998; Zhou and Xu 2019). At elevated glucose (1) concentrations the pH of the culture medium drops to around 2, a value where gluconate (13) production is almost totally repressed (Velizarov and Beschkov 1994).
The process continues to be studied, particularly for the use of glucose (1) derived from lignocellulosic biomass as a raw material (Zhang et al. 2016a; Zhou et al. 2017). Lignocellulosic biomasses contain a large amount of sugars, in the form of cellulose and hemicelluloses, and after treatment, fermentable sugars (mainly glucose and xylose) are released and can be oxidized to GA (Fujii et al. 2009). This is especially interesting as G. oxydans, unlike A. niger, is able to oxidize hexose and pentose sugars to their corresponding acids and is tolerant to inhibitors usually present in hydrolysates (Zhang et al. 2016a; Zhou et al. 2019a). The production of GA (13) simultaneously with other products arising from the sugar content of the lignocellulosic material has been seen as a good approach to the biorefinery concept.
Glucose (1) and xylose obtained from the hydrolysis of corn stover pre-treated with diluted acid and biodetoxified were converted simultaneously into GA (13) and xylonic acid. Yields obtained were 119.10 g L−1 of GA (13) (97.12% yield) and 14.04 g L−1 of xylonic acid. The conversion of xylose to xylonic acid was not complete, as the fermentation was stopped at 32 h to avoid the possible conversion of GA (13) to keto-gluconic acid (14, 16) (Zhang et al. 2016a). This difficulty was overcome by the addition of 10 g L−1 of ZnCl2 to the reaction medium, which inhibited the further transformation of GA into the by-product 2-keto-gluconic acid. Thus, the yield of xylonic acid increased to 93.36%, keeping that of GA (13) high (93.91%) (Zhou et al. 2017). Direct improvement in the conversion efficiency of non-glucose sugar acids was also achieved by adaptive evolution of G. oxydans. The conversion of xylose to xylonic acid improved significantly and the total fermentation time was reduced to 36 h from 72 h (Jin et al. 2019).
The pH value for the fermentation of glucose (1) to GA (13) by G. oxydans is in the scope of the pH of the enzymatic hydrolysis of lignocellulosic materials, thus allowing the conduction of the process as a simultaneous saccharification and fermentation (SSF). Hou et al. (2018) proposed a cascade hydrolysis of pretreated and biodetoxified corn stover and direct fermentation of glucose hydrolysate to GA (13) and xylonic acid. The approach avoided sugar loss and energy consumption by eliminating the solid/liquid separation step. A yield of 106.0 g L−1 of GA (13) and 58.5 g L−1 of xylonic acid were obtained in a 50 L bioreactor with a 30% (w/w) solids loading (Hou et al. 2018).
Sugarcane bagasse, a residue of sugar and ethanol industries, was used as a starting material for the simultaneous production of xylooligosaccharides (XOS) and GA (13). To obtain high yield of XOS, sugarcane bagasse was pre-treated with acetic acid. The treated material was enzymatically hydrolyzed to glucose and used for the production of GA (13), leading to a good yield of 96.3%. In this process, approximately 105 g of XOS and 340 g of GA (13) were obtained from 1 kg of sugarcane bagasse (Zhou and Xu 2019).
In another report, GA (13) replaced acetic acid and proved to be effective in the treatment of bagasse for the preparation of XOS in an eco-friendly biorefinery strategy (Zhou et al. 2019b). The maximum yield of XOS was achieved with 5% gluconic acid after 60 min of treatment, without consumption of gluconic acid (13). After removing the XOS solution, the solid was enzymatically hydrolyzed to glucose and subjected to fermentation by G. oxydans, with 93.1% GA (13) yield in 14 h. It was also possible to use the newly prepared cellulose self-derived GA (13) for the production of XOS, with a maximum yield of 50.3% after 60 min at 150 °C.
2-keto-D-gluconic acid
2-keto-D-gluconic acid (14) (2-KGA) is an organic acid used in cosmetics, pharmaceutical and environmental industries. Moreover, it is a key intermediate in the synthesis of several products, including erythorbic acid (isoascorbic acid), which is an important food additive, preservative and mild antioxidant approved by the FDA (Chia et al. 2008; Sun et al. 2012b). There is an annual worldwide production of 2-keto-D-gluconic acid (14) of 50.000–60.000 tons (Sun et al. 2013), and it can be obtained by a chemical or biotechnological route (enzymatic conversion and microbial fermentation). In the chemical synthesis D-glucose (1) is oxidized to 2-KGA (14) with oxygen, in the presence of a metal catalyst (e.g. platinum) (Elseviers et al. 1998), but byproducts formed lower 2-KGA (14) yield. For enzymatic conversion expensive enzymes are required (Sun et al. 2012b). Microbial fermentation presents a high selectivity and yield conversion and is the main industrial 2-KGA (14) production method (Sun et al. 2020).
Fermentation can be performed by Gluconobacter oxydans (Kulka et al. 1951; Li et al. 2016), Klebsiella pneumoniae (Wei et al. 2013; Sun et al. 2014), Pseudomonas spp. (Lockwood et al. 1941; Hou et al. 2020) and Serratia marcescens (Misenheimer et al. 1965; Zhang et al. 2011b). On the industrial scale, 2-KGA (14) has been produced mostly by Pseudomonas spp. and G. oxydans (Sun et al. 2012a). A major handicap in 2-KGA (14) fermentation is byproduct formation [GA (13), 5-KGA (16)] and inhibition caused by substrate or product, lowering its yield (Shi et al. 2015).
In an attempt to overcome the formation of by-products, H2O2 was added to the culture medium for an intensive supply of oxygen, since the bio-oxidation reaction for 2-KGA (14) is highly dependent on the supply of oxygen. When H2O2 was added to a synthetic media after 16 h of fermentation, glucose was converted to 2-KGA (14) with a rate of 63.9% compared to 43.5% conversion rate without adding H2O2. Approximately the same conversion rate was observed in a corn stover enzymatic hydrolysate medium without H2O2 addition. Further, H2O2 addition did not stimulate the production of 2-KGA (14) as it did in the synthetic medium. The researchers found that H2O2 and enzymatic hydrolysate degradation compounds up-regulated eight genes that were responsible for the production of membrane-bound enzymes, reflecting on the improved yield of 2-KGA (14) (Zhou et al. 2020).
Metabolic engineering of strains is another approach to increasing 2-KGA (14) yield. Overexpression of the ga2dh gene encoding the membrane bound gluconate-2-dehydrogenase efficiently improved the production yield of 2-KGA (14) from GA (13) (Li et al. 2016; Shi et al 2015). Research also shows the use of new and robust promoters (Shi et al. 2014) and vector modification (Shi et al 2015) to overexpress the enzyme and enhance its level of 2-KGA (14) production. Genetic modifications of other species have also been developed to increase the production of 2-KGA (14) and may contribute with a framework for the improvement of G. oxydans strains (Zeng et al. 2019; Sun et al. 2020).
5-keto-D-gluconic acid
5-keto-D-gluconic acid (16) (5-KGA) is formed by G. oxydans from GA (13) originating from the oxidation of glucose (Fig. 4). Further, this organic acid can be converted into L-tartaric acid by a chemical reaction (Matzerath et al. 1995; Merfort et al. 2006). Tartaric acid has been applied in textile and food industries, as a chiral reagent, as an acidic reducing agent and as antioxidant (Matzerath et al. 1995). Although the microbial production of 5-KGA (16) using G. oxydans is not yet industrialized, attempts are being made to increase this production, thus enable the further synthesis of tartaric acid (Yuan et al. 2016b). The main problem with the process is the accumulation of high concentrations of gluconate (13) during glucose oxidation and the production of 2-KGA (14) as a competing product. Thus, the strains used in the studies are generally genetically modified, in which the gene encoding the 2-KGA-forming enzyme has been disrupted (Merfort et al. 2006; Yuan et al. 2016a).
A recombined G.oxydans was constructed by knocking out two genes (GOX 1231 and GOX 1081) and heterologous expression of quinoprotein glucose dehydrogenase from Xanthomonas campestris. The first gene is responsible for gluconate-2-dehydrogenase (GA2DH) biosynthesis, which catalyses GA (13) to 2-KGA (14) (Fig. 4). The deletion of the gene enhanced 5-KGA (16) production by 140.33%. The second gene deleted led to the reduction of acetaldehyde and, consequently, acetic acid formation. These byproducts not only seriously affect cell growth but also may diminish 5-KGA (16) yield. In addition, X. campestris quinoprotein glucose dehydrogenase is highly active in producing 5-KGA (16) from GA (13). The recombinant strain of G. oxydans was used in fermenters with pH control at 4.5 and dissolved oxygen above 20%, resulting in a yield of 81.85% of 5-KGA (16) after 55 h of cultivation (Yuan et al. 2016a).
5-KGA (16) accumulation was also enhanced using a combinatorial metabolic engineering technique in G. oxydans strain. The genes responsible for sorbitol dehydrogenase (sldAB), PQQ (pqqABCDE and tldD) and bo3 oxidase (cyoBACD) were overexpressed with the strong promoter, P0169. After the optimization of culture conditions, the increase of 5-KGA (16) titer was tenfold higher (162 g L−1) of that produced by wild strain (G. oxydans DSM2343) in a fed-batch process to maintain the glucose at 40—50 g L−1. The dissolved oxygen was kept above 20% and the pH was controlled at 5.5 in the first stage (during glucose oxidation and cell growth) then shifted to 4.5, facilitating 5-KGA (16) formation (Yuan et al. 2016b).
Dihydroxyacetone
Dihydroxyacetone (18) (DHA) is a valuable product due to its importance in chemical, cosmetic, pharmaceutical and food industries (Rajatanavin et al. 2008; Carnali et al. 2012; Stasiak-Rózańska and Błazejak 2012; Ciriminna et al. 2018). This compound is a building block in organic synthesis of a variety of fine chemicals, including pharmaceuticals and polymers (Hekmat et al. 2003; Enders et al. 2005; Weiser et al. 2011; Lux and Siebenhofer 2015; Korley et al. 2016). DHA (18) has unique relevance in the cosmetic industry as tanning agent, and is considered one of the most suitable self-tanning product due to its satisfactory and durable results (Ciriminna et al. 2018). The tan promoted by DHA (18) on the skin surface is a consequence of its reaction (Maillard reaction) with proteins, which form brown pigments known as melanoidin (Carnali et al. 2012).
In 2019, the global market for dihydroxyacetone (18) was USD 400 million and it is expected to reach USD 500 million by 2026 (Facts and Factors 2020). Manufactures of DHA (18) include Hisun Pharmaceuticals USA Inc. (Bridgewater, NJ, USA) (Hisun Pharmaceuticals USA 2019) and Merck KGAa (Darmstadt, Germany) (Merck 2020).
The industrial production of DHA (18) is based on microbial biotransformation of glycerol (17) (Fig. 5) by high activity membrane-bound glycerol dehydrogenase present in some species of acetic acid bacteria (Stasiak-Rózańska et al. 2018). Industrial process for DHA (18) production is performed in aerated stirred tank reactors with G. oxydans as a biocatalyst and glycerol (17) as a substrate. The process is conducted in a fed-batch operation mode and the reaction time occurs up to 70 h (Hekmat et al. 2003).
Regarding oxidative biotransformation of glycerol (17), two distinct pathways can be undertaken by G. oxydans (Fig. 1b). In the periplasmic space most glycerol (17) is oxidized, which is essential for providing the energy for cell growth (Claret et al. 1994). Glycerol (17) is directly converted into DHA (18) by glycerol dehydrogenase (GLDH). In the second pathway, the substrate enters the cell and glycerol (17) is phosphorylated to glycerol-3-phosphate, which is dehydrogenated into DHA-phosphate, that further enters the pentose phosphate pathway (Claret et al. 1994; Matsushita et al. 1994).
Despite that Gluconobacter is already used industrially to oxidize glycerol (17) into DHA (18), some hindrances still need to be overcome. The viability of Gluconobacter decreases exponentially with exposure time to DHA (18), and the decrease rate is determined by DHA concentration (Ohrem and Voß 1995). Furthermore, a high initial glycerol (17) content can affect both specific growth rate of G. oxydans and specific rate of DHA (18) production. However, DHA (18) shows more influence in causing an inhibitory effect on microbial growth than glycerol (17) (Claret et al. 1992; de la Morena et al. 2019a). Despite cell damage caused by DHA (18) accumulation in the medium, the formation of this product continues for a period, due to robustness of glycerol dehydrogenase activity (Bauer et al. 2005).
The efforts to enhance industrial DHA (18) production mostly involve the search for new methods to prevent or decrease inhibitory effects of the substrate/product (Hu and Zheng 2011; Dikshit and Moholkar 2016a), strain improvement in bioconversion (Lin et al. 2016; Tan et al. 2019) and optimization of process conditions (Hu et al. 2017b; Sudarshan and Sanjay 2018; de la Morena et al. 2019a). Regarding reduction of costs, the use of biodiesel-derived crude glycerol (17) has been tested as a substrate for DHA (18) fermentation due to its low price on the market (Jun et al. 2010; Stasiak-Rózańska et al. 2018; Dikshit and Moholkar 2019; Jittjang et al. 2020). The environmental commitment for the use of a by-product can also be highlighted. In the last few years, different approaches have been proposed to produce DHA (18) from crude glycerol (17), without prior purification, as shown in Table 2.
Another way to reduce costs is to obtain a purer product already in the biocatalysis phase, in order to reduce the costs of the downstream process since large amounts of impurities and high DHA (18) solubility hinder its purification and crystallization (Xie 2018). Hu et al. (2017b) successfully obtained a near colorless DHA (18) solution with few impurities by applying inorganic nitrogen sources or amino acids in the fermentation medium, instead of a yeast extract that hinders DHA (18) recovery.
In order to optimize DHA (18) production the control of the factors that most influence the process is important. De la Morena et al. (2020) successfully established four kinetic model equations to correlate the oxygen transfer rate (OTR) and DHA (18) production, which represents a guide towards the influence of OTR on glycerol (17) to DHA (18) transformation by resting cells of G. oxydans. Other recent studies that analyzed oxygen interference on DHA (18) production have been published (Zheng et al. 2016; Zhou et al. 2016; Poljungreed and Boonyarattanakalin 2017; de la Morena et al. 2019b).
Regarding the use of genetic engineering, efficient strains can be constructed by overexpressing or suppressing the genes related to metabolic pathways of DHA (18) production. A 2014 patent (Xu et al. 2014b) claimed an invention that provides a method for constructing a DHA (18) high-producing strain. The inventors constructed a new strain excessively expressing the membrane system of glycerol dehydrogenase, which positively affect DHA (18) production. Tan et al.(2019) overexpressed the gene encoded glycerol transporter (GlpF) in G. oxydans ATCC 621H aiming to overcome the inhibition caused by high glycerol (17) concentration and to improve cell growth. The authors have been able to efficiently construct strains tolerant to high glycerol (17) concentrations.
Conclusion
This review highlights the industrial versatility of Gluconobacter oxydans, an outstanding bacterium that has been widely used in biotechnology processes. All this versatility is linked to the extraordinary capacity of incomplete regio- and stereoselective oxidation of sugars, polyols, and alcohols. All G. oxydans products reported here have diverse and important applications and comprise a high value market. The vitamin C production process is one of the oldest industrial applications of G. oxydans; the microbial production of 5-keto-D-gluconic acid is still potential, but of great industrial interest. Strategies for process improvement mainly include optimization of fermentation medium and culture conditions, use of residues and by-products as raw materials, cell immobilization, microbial consortia and genetically improved strains.
The application of agro-industrial wastes or by-products as substrate have been explored to lower process costs and make processes more environmentally friendly. Lignocellulosic biomass and biodiesel-derived glycerol have shown potential to be applied to biotechnological processes and a future advance towards this area can be glimpsed. Regardless of the bioprocesses reviewed, all displayed efforts to genetically improve G. oxydans strains, despite the current shortage of suitable genetic tools. The construction of robust strains successfully enhanced bioproduction yields, whether by deleting genes that generate byproducts, expressing heterologous genes or using more potent promoters and vectors. Through -omics technologies, a better understanding of metabolic networks of microbial consortia has been reached. The great versatility already demonstrated by G. oxydans points to a potential yet to be explored in new future applications.
Abbreviations
- 2-KGA:
-
2-Keto-D-gluconic acid
- 2KGADH:
-
2-Keto-D-gluconate dehydrogenase
- 2KGR:
-
2-Keto-gluconate-reductase
- 2-KLG:
-
2-Keto-L-gulonic acid
- 2,5-KGA:
-
2,5-Diketogluconic acid
- 5-KGA:
-
5-Keto-D-gluconic acid
- 5KGR:
-
5-Keto-gluconate-reductase
- 6NSL:
-
6-(N-hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose
- DHA:
-
1,3 Dihydroxyacetone
- DHA-P:
-
Dihydroxyacetone phosphate
- DNJ:
-
1- Deoxynojirimycin
- FAD:
-
Flavin adenine dinucleotide
- FDA:
-
Food and Drug Administration
- GA:
-
D-gluconic acid
- GA2DH:
-
D-gluconate-2-dehydrogenase
- GDH:
-
D-glucose dehydrogenase
- GK:
-
Glucokinase
- GK:
-
Glycerokinase or glycerol kinase
- GL:
-
Glucono-δ-lactonase
- GLDH:
-
Glycerol dehydrogenase
- GNK:
-
Gluconokinase
- GPDH:
-
Glycerol-3-phosphate-dehydrogenase
- mDHs:
-
Membrane- bound dehydrogenases
- NHEG:
-
N-2-hydroxyethyl glucamine
- PPP:
-
Pentose phosphate pathway
- PQQ:
-
Pyrroloquinoline quinone
- SDH:
-
Sorbose dehydrogenase
- SLDH:
-
D-sorbitol dehydrogenase
- SNDH:
-
Sorbosone dehydrogenase
- SSDH:
-
Sorbose/sorbosone dehydrogenase
References
Alonso S, Rendueles M, Díaz M (2015) Microbial production of specialty organic acids from renewable and waste materials. Crit Rev Biotechnol 35:497–513. https://doi.org/10.3109/07388551.2014.904269
Anastassiadis S, Morgunov I (2007) Gluconic acid production. Recent Pat Biotechnol 1:1–13. https://doi.org/10.2174/187220807780809472
Ano Y, Shinagawa E, Adachi O et al (2011) Selective, high conversion of D-glucose to 5-keto-D-gluoconate by Gluconobacter suboxydans. Biosci Biotechnol Biochem 75:586–589. https://doi.org/10.1271/bbb.100701
Asakura A, Hoshino T (1999) Isolation and characterization of a new quinoprotein dehydrogenase, L-sorbose/L-sorbosone dehydrogenase. Biosci Biotechnol Biochem 63:46–53. https://doi.org/10.1271/bbb.63.46
Attwood MM, van Dijken JP, Pronk JT (1991) Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus. J Ferment Bioeng 72:101–105. https://doi.org/10.1016/0922-338X(91)90317-A
Bauer R, Katsikis N, Varga S, Hekmat D (2005) Study of the inhibitory effect of the product dihydroxyacetone on Gluconobacter oxydans in a semi-continuous two-stage repeated-fed-batch process. Bioprocess Biosyst Eng 28:37–43. https://doi.org/10.1007/s00449-005-0009-0
Beri D, York WS, Lynd LR et al (2020) Development of a thermophilic coculture for corn fiber conversion to ethanol. Nat Commun. https://doi.org/10.1038/s41467-020-15704-z
Bhatia SK, Bhatia RK, Choi YK et al (2018) Biotechnological potential of microbial consortia and future perspectives. Crit Rev Biotechnol 38:1209–1229. https://doi.org/10.1080/07388551.2018.1471445
Boudrant J (1990) Microbial processes for ascorbic acid biosynthesis: a review. Enzyme Microb Technol 12:322–329. https://doi.org/10.1016/0141-0229(90)90159-N
Cañete-Rodríguez AM, Santos-Dueñas IM, Torija-Martínez MJ et al (2015) Preparation of a pure inoculum of acetic acid bacteria for the selective conversion of glucose in strawberry purée into gluconic acid. Food Bioprod Process 96:35–42. https://doi.org/10.1016/j.fbp.2015.06.005
Cañete-Rodríguez AM, Santos-Dueñas IM, Jiménez-Hornero JE et al (2016) Gluconic acid: properties, production methods and applications—An excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem 51:1891–1903. https://doi.org/10.1016/j.procbio.2016.08.028
Carnali JO, Madison SA, Shah P, Qiu Q (2012) Structure/property relationship for ethylenediamine derivatives as aids in sunless tanning. Ind Eng Chem Res 51:15573–15581. https://doi.org/10.1021/ie301929w
CDN Newswire (2021) Global vitamin C market is projected to grow at a CAGR of 4.6% during the forecast period 2020–2027. https://www.einnews.com/pr_news/550934484/global-vitamin-c-market-is-projected-to-grow-at-a-cagr-of-4-6-during-the-forecast-period-2020-2027. Accessed 19 Dec 2021
Chen S, Jia N, Ding MZ, Yuan YJ (2016) Comparative analysis of l - sorbose dehydrogenase by docking strategy for 2 - keto - l - gulonic acid production in Ketogulonicigenium vulgare and Bacillus endophyticus consortium. J Ind Microbiol Biotechnol 43:1507–1516. https://doi.org/10.1007/s10295-016-1829-4
Chen Y, Liu L, Yu S et al (2021) Identification of gradient promoters of Gluconobacter oxydans and their applications in the biosynthesis of 2-keto-L-gulonic acid. Front Bioeng Biotechnol 9:1–9. https://doi.org/10.3389/fbioe.2021.673844
Chia M, Van Nguyen TB, Choi WJ (2008) DO-stat fed-batch production of 2-keto-d-gluconic acid from cassava using immobilized Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78:759–765. https://doi.org/10.1007/s00253-008-1374-9
Ciriminna R, Fidalgo A, Ilharco LM, Pagliaro M (2018) Dihydroxyacetone: an updated insight into an important bioproduct. ChemistryOpen 7:233–236. https://doi.org/10.1002/open.201700201
Claret C, Bories A, Soucaille P (1992) Glycerol inhibition of growth and dihydroxyacetone production by Gluconobacter oxydans. Curr Microbiol 25:149–155. https://doi.org/10.1007/BF01571023
Claret C, Salmon JM, Romieu C, Bories A (1994) Physiology of Gluconobacter oxydans during dihydroxyacetone production from glycerol. Appl Microbiol Biotechnol 41:359–365. https://doi.org/10.1007/BF00221232
de la Morena S, Acedos MG, Santos VE, García-Ochoa F (2019a) Dihydroxyacetone production from glycerol using Gluconobacter oxydans: study of medium composition and operational conditions in shaken flasks. Biotechnol Prog 35:1–9. https://doi.org/10.1002/btpr.2803
de la Morena S, Santos VE, García-Ochoa F (2019b) Influence of oxygen transfer and uptake rates on dihydroxyacetone production from glycerol by Gluconobacter oxydans in resting cells operation. Biochem Eng J 147:20–28. https://doi.org/10.1016/j.bej.2019.03.021
de la Morena S, Wojtusik M, Santos VE, Garcia-Ochoa F (2020) Kinetic modeling of dihydroxyacetone production from glycerol by Gluconobacter oxydans ATCC 621 resting cells: effect of fluid dynamics conditions. Catalysts. https://doi.org/10.3390/catal10010101
De Ley J, Swings J, Gossele F (1984) The genus Gluconobacter. In: Krieg NR, Holt JG (eds) Bergey’s manual of systematic bacteriology. Williams & Wilkins, Baltimore, pp 267–278
Deppenmeier U, Hoffmeister M, Prust C (2002) Biochemistry and biotechnological applications of Gluconobacter strains. Appl Microbiol Biotechnol 60:233–242. https://doi.org/10.1007/s00253-002-1114-5
Dikshit PK, Moholkar VS (2016a) Kinetic analysis of dihydroxyacetone production from crude glycerol by immobilized cells of Gluconobacter oxydans MTCC 904. Bioresour Technol 216:948–957. https://doi.org/10.1016/j.biortech.2016.06.042
Dikshit PK, Moholkar VS (2016b) Optimization of 1,3-dihydroxyacetone production from crude glycerol by immobilized Gluconobacter oxydans MTCC 904. Bioresour Technol 216:1058–1065. https://doi.org/10.1016/j.biortech.2016.01.100
Dikshit PK, Moholkar VS (2019) Batch and repeated-batch fermentation for 1,3-dihydroxyacetone production from waste glycerol using free, immobilized and resting Gluconobacter oxydans cells. Waste and Biomass Valorization 10:2455–2465. https://doi.org/10.1007/s12649-018-0307-9
Dikshit PK, Padhi SK, Moholkar VS (2017) Process optimization and analysis of product inhibition kinetics of crude glycerol fermentation for 1,3-dihydroxyacetone production. Bioresour Technol 244:362–370. https://doi.org/10.1016/j.biortech.2017.07.136
Dikshit PK, Kharmawlong GJ, Moholkar VS (2018) Investigations in sonication-induced intensification of crude glycerol fermentation to dihydroxyacetone by free and immobilized Gluconobacter oxydans. Bioresour Technol 256:302–311. https://doi.org/10.1016/j.biortech.2018.02.024
Dosedel M, Jirkovský E, Macáková K et al (2021) Vitamin C—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 13:1–34
DSM Jiangshan Pharmaceutical Group - 2021 DSM Jiangshan Pharmaceutical Group Co., Ltd. https://www.dsm.com/jiangshan/en_us/home.html. Accessed 12 Dec 2021
Elseviers M, Lemmens HOJ, Coomans SMJ, Röper HWW (1998) Process for the production of 2-keto-D-gluconic acid. Eur Pat Appl Patent Number EP 0867446:A1
Enders D, Voith M, Lenzen A (2005) The dihydroxyacetone unit—A versatile C3 building block in organic synthesis. Angew Chemie Int Ed 44:1304–1325. https://doi.org/10.1002/anie.200400659
Facts and Factors (2020) Global 1,3-dihydroxyacetone market is projected to reach USD 500 million by 2026. https://www.fnfresearch.com/news/global-13-dihydroxyacetone-market-is-projected-to-reach. Accessed 10 Dec 2021
FDA (1996) Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020682. Accessed 12 Dec 2021
Fujii T, Fang X, Inoue H et al (2009) Enzymatic hydrolyzing performance of Acremonium cellulolyticus and Trichoderma reesei against three lignocellulosic materials. Biotechnol Biofuels 2:1–8. https://doi.org/10.1186/1754-6834-2-24
Gao L, Hu Y, Liu J et al (2014) Stepwise metabolic engineering of Gluconobacter oxydans WSH-003 for the direct production of 2-keto-L-gulonic acid from D-sorbitol. Metab Eng 24:30–37. https://doi.org/10.1016/j.ymben.2014.04.003
Gavrilescu M, Chisti Y (2005) Biotechnology—A sustainable alternative for chemical industry. Biotechnol Adv 23:471–499. https://doi.org/10.1016/j.biotechadv.2005.03.004
Ghosh S, Chowdhury R, Bhattacharya P (2016) Mixed consortia in bioprocesses: role of microbial interactions. Appl Microbiol Biotechnol 100:4283–4295. https://doi.org/10.1007/s00253-016-7448-1
Golikova EP, Lakina NV, Grebennikova OV et al (2017) The study of biocatalysts on the base of the glucose oxidase. Faraday Discuss 202:303–314. https://doi.org/10.1039/C7FD00042A
Grabner RW, Landis BH, Wang P tu, et al (2003) Salt forms of 6-N-butylamino-6-deoxy-alpha-L-sorbofuranose. Patent Number US 6552176 B2
Grabner RW, Landis BH, Wang PT, et al (1999) Process for microbially oxidizing N-substituted glucamines. Patent numer US5916784A
Gupta A, Singh VK, Qazi GN, Kumar A (2001) Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456
Hekmat D, Bauer R, Fricke J (2003) Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess Biosyst Eng 26:109–116. https://doi.org/10.1007/s00449-003-0338-9
Hisun Pharmaceuticals USA (2019) API Products. http://www.hisunusa.com/products/api-products/. Accessed 10 Dec 2021
Hou W, Zhang M, Bao J (2018) Cascade hydrolysis and fermentation of corn stover for production of high titer gluconic and xylonic acids. Bioresour Technol 264:395–399. https://doi.org/10.1016/j.biortech.2018.06.025
Hou Z, Sun L, Wang D et al (2020) Production of 2-keto-gluconic acid from glucose by immobilized Pseudomonas plecoglossicida resting cells. 3 Biotech 10:1–9. https://doi.org/10.1007/s13205-020-02243-z
Hu ZC, Zheng YG (2011) Enhancement of 1,3-dihydroxyacetone production by a uv-induced mutant of Gluconobacter oxydans with do control strategy. Appl Biochem Biotechnol 165:1152–1160. https://doi.org/10.1007/s12010-011-9332-x
Hu Y, Wan H, Li J, Zhou J (2015) Enhanced production of L-sorbose in an industrial Gluconobacter oxydans strain by identification of a strong promoter based on proteomics analysis. J Ind Microbiol Biotechnol 42:1039–1047. https://doi.org/10.1007/s10295-015-1624-7
Hu J, Xue Y, Guo H et al (2017a) Design and composition of synthetic fungal-bacterial microbial consortia that improve lignocellulolytic enzyme activity. Bioresour Technol 227:247–255. https://doi.org/10.1016/j.biortech.2016.12.058
Hu ZC, Tian SY, Ruan LJ, Zheng YG (2017b) Repeated biotransformation of glycerol to 1,3-dihydroxyacetone by immobilized cells of Gluconobacter oxydans with glycerol- and urea-feeding strategy in a bubble column bioreactor. Bioresour Technol 233:144–149. https://doi.org/10.1016/j.biortech.2017.02.096
Hu ZC, Bu JL, Wang RY et al (2019) Enhanced production of 6-(N-Hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose by immobilized Gluconobacter oxydans on corn stover with a pH control strategy in a bubble column bioreactor. Appl Biochem Biotechnol 188:297–309. https://doi.org/10.1007/s12010-018-2924-y
Hu ZC, Zhao ZY, Ke X, Zheng YG (2020) Repeated production of 6-(N-hydroxyethyl)-amino-6-deoxy-α-l-sorbofuranose by immobilized Gluconobacter oxydans cells with a strategy of in situ exhaustive cell regeneration. Bioprocess Biosyst Eng 43:1781–1789. https://doi.org/10.1007/s00449-020-02368-8
Jackson E, Ripoll M, Betancor L (2019) Efficient glycerol transformation by resting Gluconobacter cells. Microbiologyopen. https://doi.org/10.1002/mbo3.926
Jia N, Ding MZ, Zou Y et al (2017) Comparative genomics and metabolomics analyses of the adaptation mechanism in Ketogulonicigenium vulgare-Bacillus thuringiensis consortium. Sci Rep. https://doi.org/10.1038/srep46759
Jin C, Hou W, Yao R et al (2019) Adaptive evolution of Gluconobacter oxydans accelerates the conversion rate of non-glucose sugars derived from lignocellulose biomass. Bioresour Technol 289:121623. https://doi.org/10.1016/j.biortech.2019.121623
Jittjang S, Jiratthiticheep I, Kajonpradabkul P et al (2020) Effect of NaCl removal from biodiesel-derived crude glycerol by ion exchange to enhance dihydroxyacetone production by Gluconobacter thailandicus in minimal medium. J Chem Technol Biotechnol 95:281–288. https://doi.org/10.1002/jctb.6234
Jun S, Moon C, Kang C (2010) Microbial Fed-batch Production of 1, 3-propanediol using raw glycerol with suspended and immobilized Klebsiella pneumoniae. Appl Biochem Biotechnol 161:491–501. https://doi.org/10.1007/s12010-009-8839-x
Karaffa L, Kubicek CP (2021) Production of organic acids by fungi. Encycl Mycol 2:406–419. https://doi.org/10.1016/b978-0-12-809633-8.21066-2
Kataoka N, Matsutani M, Yakushi T, Matsushita K (2015) Efficient production of 2,5-diketo-d-gluconate via heterologous expression of 2-ketogluconate dehydrogenase in Gluconobacter japonicus. Appl Environ Microbiol 81:3552–3560. https://doi.org/10.1128/AEM.04176-14
Ke X, Wang NN, Yu PH et al (2018) Biosynthesis of miglitol intermediate 6-(N-hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose by an improved D-sorbitol dehydrogenase from Gluconobacter oxydans. 3 Biotech 8:231. https://doi.org/10.1007/s13205-018-1251-x
Ke X, Lu YH, Yu PH et al (2019a) Glutamate addition improves the activity of membrane-bound sorbitol dehydrogenase in a pyrroloquinoline quinone-dependent manner: a feasible strategy for the cost-effective fermentation of Gluconobacter oxydans. Process Biochem 84:1–8. https://doi.org/10.1016/j.procbio.2019.06.009
Ke X, Pan-Hong Y, Hu ZC et al (2019b) Synergistic improvement of PQQ-dependent D-sorbitol dehydrogenase activity from Gluconobacter oxydans for the biosynthesis of miglitol precursor 6-(N-hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose. J Biotechnol 300:55–62. https://doi.org/10.1016/j.jbiotec.2019.05.007
Khobare SR, Gajare V, Reddy EV et al (2016) One pot oxidative dehydration - oxidation of polyhydroxyhexanal oxime to polyhydroxy oxohexanenitrile: a versatile methodology for the facile access of azasugar alkaloids. Carbohydr Res 435:1–6. https://doi.org/10.1016/j.carres.2016.09.003
Kim T, Gao H, Li J et al (2019) Overcoming NADPH product inhibition improves D-sorbitol conversion to L-sorbose. Sci Rep 9:815. https://doi.org/10.1038/s41598-018-37401-0
Kinast G, Schedel M (1981) Production of N-substituted derivatives of 1-desoxynojirimycin. Patent number CN4266025
Korley JN, Yazdi S, McHugh K et al (2016) One-step synthesis, biodegradation and biocompatibility of polyesters based on the metabolic synthon, dihydroxyacetone. Biomaterials 98:41–52. https://doi.org/10.1016/j.biomaterials.2016.04.042
Kostner D, Luchterhand B, Junker A et al (2015) The consequence of an additional NADH dehydrogenase paralog on the growth of Gluconobacter oxydans DSM3504. Appl Microbiol Biotechnol 99:375–386. https://doi.org/10.1007/s00253-014-6069-9
Krajewski V, Simić P, Mouncey NJ et al (2010) Metabolic engineering of Gluconobacter oxydans for improved growth rate and growth yield on glucose by elimination of gluconate formation. Appl Environ Microbiol 76:4369–4376. https://doi.org/10.1128/AEM.03022-09
Kranz A, Steinmann A, Degner U et al (2018) Global mRNA decay and 23S rRNA fragmentation in Gluconobacter oxydans 621H. BMC Genomics 19:1–17
Kulka A, Hall AN, Walker TK (1951) Formation of 2-keto-D-gluconic acid, 5-keto-D-gluconic acid, and tartronic acid by Acetobacter species. Nature 167:905–906. https://doi.org/10.1038/167905b0
Kylilis N, Tuza ZA, Stan GB, Polizzi KM (2018) Tools for engineering coordinated system behaviour in synthetic microbial consortia. Nat Commun. https://doi.org/10.1038/s41467-018-05046-2
Ruibang Laboratories (2021) Over 60 years of experience with the pharmaceutical field. https://www.china-ruibang.com/company/history. Accessed 8 Dec 2021
Leduc S, De Troostembergh JC, Lebeault JM (2004) Folate requirements of the 2-keto-L-gulonic acid-producing strain Ketogulonigenium vulgare LMP P-20356 in L-sorbose/CSL medium. Appl Microbiol Biotechnol 65:163–167. https://doi.org/10.1007/s00253-004-1562-1
Li K, Mao X, Liu L et al (2016) Overexpression of membrane-bound gluconate-2-dehydrogenase to enhance the production of 2-keto-D-gluconic acid by Gluconobacter oxydans. Microb Cell Fact 15:1–10. https://doi.org/10.1186/s12934-016-0521-8
Liebminger S, Hofbauer R, Siebenhofer M et al (2014) Microbial conversion of crude glycerol to dihydroxyacetone. Waste Biomass Valor 5:781–787. https://doi.org/10.1007/s12649-013-9288-x
Lin X, Liu S, Xie G et al (2016) Enhancement of 1,3-dihydroxyacetone production from Gluconobacter oxydans by combined mutagenesis. J Microbiol Biotechnol 26:1908–1917. https://doi.org/10.4014/jmb.1604.04019
Liu D, Hu ZC, Ke X, Zheng YG (2020a) Breeding of Gluconobacter oxydans with high PQQ-dependent D-sorbitol dehydrogenase for improvement of 6-(N-hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose production. Biochem Eng J 161:107642. https://doi.org/10.1016/j.bej.2020.107642
Liu D, Ke X, Hu ZC, Zheng YG (2020b) Combinational expression of D-sorbitol dehydrogenase and pyrroloquinoline quinone increases 6-(N-hydroxyethyl)-amino-6-deoxy-α-L-sorbofuranose production by Gluconobacter oxydans through cofactor manipulation. Enzyme Microb Technol 141:109670. https://doi.org/10.1016/j.enzmictec.2020.109670
Liu D, Ke X, Hu ZC, Zheng YG (2021) Improvement of pyrroloquinoline quinone-dependent D-sorbitol dehydrogenase activity from Gluconobacter oxydans via expression of Vitreoscilla hemoglobin and regulation of dissolved oxygen tension for the biosynthesis of 6-(N-hydroxyethyl)-amino-6-deoxy-α-. J Biosci Bioeng 131:518–524. https://doi.org/10.1016/j.jbiosc.2020.12.013
Lockwood LB, Tabenkin B, Ward GE (1941) The production of gluconic acid and 2-keto-gluconic acid from glucose by species of Pseudomonas and Phytomonas. J Bacteriol 42:51–61
Lux S, Siebenhofer M (2015) Catalytic conversion of dihydroxyacetone to lactic acid with Brønsted acids and multivalent metal ions. Chem Biochem Eng Q 29:575–585. https://doi.org/10.15255/CABEQ.2014.2110
Ma Q, Zou Y, Lv Y et al (2014) Comparative proteomic analysis of experimental evolution of the Bacillus cereus-Ketogulonicigenium vulgare co-culture. PLoS ONE. https://doi.org/10.1371/journal.pone.0091789
Ma Q, Bi YH, Wang EX et al (2019) Integrated proteomic and metabolomic analysis of a reconstructed three-species microbial consortium for one-step fermentation of 2-keto-L-gulonic acid, the precursor of vitamin C. J Ind Microbiol Biotechnol 46:21–31. https://doi.org/10.1007/s10295-018-2096-3
Fior Markets (2021) Global gluconic acid market is expected to reach USD 1.9 billion by 2028: Fior Markets. In: GlobeNewswire. https://www.globenewswire.com/news-release/2021/05/04/2221889/0/en/Global-Gluconic-Acid-Market-Is-Expected-to-Reach-USD-1-9-billion-by-2028-Fior-Markets.html. Accessed 8 Dec 2021
Matsushita K, Toyama H, Adachi O (1994) Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microb Physiol 36:247–301. https://doi.org/10.1016/s0065-2911(08)60181-2
Matzerath I, Kläui W, Klasen R, Sahm H (1995) Vanadate catalysed oxidation of 5-keto-d-gluconic acid to tartaric acid: the unexpected effect of phosphate and carbonate on rate and selectivity. Inorganica Chim Acta 237:203–205. https://doi.org/10.1016/0020-1693(95)04665-V
Merck (2020) 110150 Dihydroxyacetone extra pure for cosmetic purposes. https://www.merckgroup.com/en/products/pm/110150.html. Accessed 13 Dec 2021
Merfort M, Herrmann U, Ha SW et al (2006) Modification of the membrane-bound glucose oxidation system in Gluconobacter oxydans significantly increases gluconate and 5-keto-D-gluconic acid accumulation. Biotechnol J 1:556–563. https://doi.org/10.1002/biot.200600032
Mientus M, Kostner D, Peters B et al (2017) Characterization of membrane-bound dehydrogenases of Gluconobacter oxydans 621H using a new system for their functional expression. Appl Microbiol Biotechnol 101:3189–3200. https://doi.org/10.1007/s00253-016-8069-4
Misenheimer TJ, Anderson RF, Lagoda AA, Tyler DD (1965) Production of 2-ketogluconic acid by Serratia marcescens. Appl Microbiol 13:393–396
Miyazaki T, Sugisawa T, Hoshino T (2006) Pyrroloquinoline quinone-dependent dehydrogenases from Ketogulonicigenium vulgare catalyze the direct conversion of L-sorbosone to L-ascorbic acid. Appl Environ Microbiol 72:1487–1495. https://doi.org/10.1128/AEM.72.2.1487-1495.2006
Northeast Pharmaceutical Group - 2021 Northeast Pharmaceutical Group Co., Ltda. http://www.nepharm.com/intro/1.html. Accessed 8 Dec 2021
Ohrem HL, Voß H (1995) Inhibitory effects of dihydroxyacetone on Gluconobacter cultures. Biotechnol Lett 17:981–984. https://doi.org/10.1007/BF00127438
Pal P, Kumar R, Banerjee S (2016) Manufacture of gluconic acid: a review towards process intensification for green production. Chem Eng Process Process Intensif 104:160–171. https://doi.org/10.1016/j.cep.2016.03.009
Pappenberger G, Hohmann H-P (2013) Industrial Production of L-ascorbic acid (Vitamin C) and D-isoascorbic acid. Biotechnol Food Feed Addit 123:143–188. https://doi.org/10.1007/10_2013_243
Pfizer (2016) GLYSET. https://www.pfizermedicalinformation.com/en-us/glyset/other. Accessed 14 Jan 2022
Poljungreed I, Boonyarattanakalin S (2017) Dihydroxyacetone production by Gluconobacter frateurii in a minimum medium using fed-batch fermentation. J Chem Technol Biotechnol 92:2635–2641. https://doi.org/10.1002/jctb.5281
PR Newswire (2021) Vitamin C market size to be valued at $ 1.34 billion by 2023, says Beroe Inc. https://www.prnewswire.com/news-releases/vitamin-c-market-size-to-be-valued-at-1-34-billion-by-2023--says-beroe-inc-301276570.html. Accessed 8 Dec 2021
Prust C, Hoffmeister M, Liesegang H et al (2005) Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechnol 23:195–200. https://doi.org/10.1038/nbt1062
Qin Z, Yang Y, Yu S et al (2021) Repurposing the endogenous type I-E CRISPR/Cas system for gene repression in Gluconobacter oxydans WSH-003. ACS Synth Biol 10:84–93. https://doi.org/10.1021/acssynbio.0c00456
Qin Z, Yu S, Chen J, Zhou J (2022) Dehydrogenases of acetic acid bacteria. Biotechnol Adv 54:107863. https://doi.org/10.1016/j.biotechadv.2021.107863
Rajatanavin N, Suwanachote S, Kulkollakarn S (2008) Dihydroxyacetone: a safe camouflaging option in vitiligo. Int J Dermatol 47:402–406. https://doi.org/10.1111/j.1365-4632.2008.03356.x
Ramachandran S, Fontanille P, Pandey A, Larroche C (2006) Gluconic acid: properties, applications and microbial production. Food Technol Biotechnol 44:185–195
Reichstein T, Grüssner A (1934) Eine ergiebige synthese der l-ascorbinsäure (C-Vitamin). Helvetica 17:311–328. https://doi.org/10.1002/hlca.19340170136
Ripoll M, Jackson E, Trelles JA, Betancor L (2021) Dihydroxyacetone production via heterogeneous biotransformations of crude glycerol. J Biotechnol 340:102–109. https://doi.org/10.1016/j.jbiotec.2021.08.011
Roquette (2021) Gluconic acid. https://www.roquette.com/animal-nutrition-gluconic-acid. Accessed 10 Dec 2021
Saichana N, Matsushita K, Adachi O et al (2015) Acetic acid bacteria: a group of bacteria with versatile biotechnological applications. Biotechnol Adv. 33:1260–1271. https://doi.org/10.1016/j.biotechadv.2014.12.001
Sainz F, Navarro D, Mateo E et al (2016) Comparison of D-gluconic acid production in selected strains of acetic acid bacteria. Int J Food Microbiol 222:40–47. https://doi.org/10.1016/j.ijfoodmicro.2016.01.015
Saito Y, Ishii Y, Hayashi H et al (1997) Cloning of genes coding for L-sorbose and L-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-L-gulonate, a precursor of L-ascorbic acid, in a recombinant G. oxydans strain. Appl Environ Microbiol 63:454–460. https://doi.org/10.1128/aem.63.2.454-460.1997
Schedel M (2000) Regioselective oxidation of aminosorbitol with Gluconobacter oxydans, key reaction in the industrial 1-deoxynojirimycin synthesis. In: Rehm HJ, Reed G (eds) Biotechnology Second, Second. Wiley, Wuppertal, pp 296–311
Schröder T, Stubbe M (1989) Process for preparing 1-deoxynojirimycin and N-derivatives thereof. Patent number US4806650
Shi L, Li K, Zhang H et al (2014) Identification of a novel promoter gHp0169 for gene expression in Gluconobacter oxydans. J Biotechnol 175:69–74. https://doi.org/10.1016/j.jbiotec.2014.01.035
Shi YY, Li KF, Lin JP et al (2015) Engineered expression vectors significantly enhanced the production of 2-keto-D-gluconic acid by Gluconobacter oxidans. J Agric Food Chem 63:5492–5498. https://doi.org/10.1021/acs.jafc.5b01652
Shinagawa E, Matsushita K, Toyama H, Adachi O (1999) Production of 5-keto-D-gluconate by acetic acid bacteria is catalyzed by pyrroloquinoline quinone (PQQ)-dependent membrane-bound D-gluconate dehydrogenase. J Mol Catal B Enzym 6:341–350. https://doi.org/10.1016/S1381-1177(98)00112-X
Stasiak-Różańska L, Błażejak S, Gientka I et al (2017) Utilization of a waste glycerol fraction using and reusing immobilized Gluconobacter oxydans ATCC 621 cell extract. Electron J Biotechnol 27:44–48. https://doi.org/10.1016/j.ejbt.2017.03.003
Stasiak-Rózańska L, Błazejak S (2012) Production of dihydroxyacetone from an aqueous solution of glycerol in the reaction catalyzed by an immobilized cell preparation of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur Food Res Technol 235:1125–1132. https://doi.org/10.1007/s00217-012-1846-0
Stasiak-Rózańska L, Berthold-Pluta A, Dikshit PK (2018) Valorization of waste glycerol to dihydroxyacetone with biocatalysts obtained from Gluconobacter oxydans. Appl Sci. https://doi.org/10.3390/app8122517
Sudarshan BL, Sanjay KR (2018) Optimization of fermentation conditions for production of 1, 3-dihydroxyacetone from glycerol obtained as a byproduct during biodiesel production. Microbiol Res J Int 25:1–9. https://doi.org/10.9734/mrji/2018/17137
Sugimoto S, Nakajima H, Kosaka K, Hosoi H (2015) Review: Miglitol has potential as a therapeutic drug against obesity. Nutr Metab. https://doi.org/10.1186/s12986-015-0048-8
Sugisawa T, Miyazaki T, Hoshino T (2005) Microbial production of L-ascorbic acid from D-sorbitol, L-sorbose, L-gulose, and L-sorbosone by Ketogulonicigenium vulgare DSM 4025. Biosci Biotechnol Biochem 69:659–662. https://doi.org/10.1271/bbb.69.659
Sun WJ, Liu CF, Yu L et al (2012a) A novel bacteriophage KSL-1 of 2-keto-gluconic acid producer Pseudomonas fluorescens K1005: Isolation, characterization and its remedial action. BMC Microbiol 12:1–8. https://doi.org/10.1186/1471-2180-12-127
Sun WJ, Zhou YZ, Zhou Q et al (2012b) Semi-continuous production of 2-keto-gluconic acid by Pseudomonas fluorescens AR4 from rice starch hydrolysate. Bioresour Technol 110:546–551. https://doi.org/10.1016/j.biortech.2012.01.040
Sun WJ, Yun QQ, Zhou YZ et al (2013) Continuous 2-keto-gluconic acid (2KGA) production from corn starch hydrolysate by Pseudomonas fluorescens AR4. Biochem Eng J 77:97–102. https://doi.org/10.1016/j.bej.2013.05.010
Sun Y, Wei D, Shi J et al (2014) Two-stage fermentation for 2-ketogluconic acid production by Klebsiella pneumoniae. J Microbiol Biotechnol 24:781–787. https://doi.org/10.4014/jmb.1401.01038
Sun L, Wang DM, Sun WJ et al (2020) Two-stage semi-continuous 2-keto-gluconic acid (2KGA) Production by Pseudomonas plecoglossicida JUIM01 from rice starch hydrolyzate. Front Bioeng Biotechnol 8:1–10. https://doi.org/10.3389/fbioe.2020.00120
Sun Y, Li X, Wei C et al (2021) An aptly industrialized bioprocess for lactic acid production from corn stover using thermotolerant microbial consortia. Bioprocess Biosyst Eng 44:2445–2454. https://doi.org/10.1007/s00449-021-02616-5
Tan J, Yang X, Lu W (2019) Research of 1,3-dihydroxyacetone production by overexpressing glycerol transporter and glycerol dehydrogenase. Trans Tianjin Univ 25:549–558. https://doi.org/10.1007/s12209-019-00207-w
Tanamool V, Hongsachart P, Soemphol W (2018) Bioconversion of biodiesel-derived crude glycerol to 1,3-dihydroxyacetone by a potential acetic acid bacteria. Sains Malays 47:481–488. https://doi.org/10.17576/jsm-2018-4703-07
Velizarov S, Beschkov V (1994) Production of free gluconic acid by cells of Gluconobacter oxydans. Biotechnol Lett 16:715–720. https://doi.org/10.1007/BF00136477
Velizarov S, Beschkov V (1998) Biotransformation of glucose to free gluconic acid by Gluconobacter oxydans: Substrate and product inhibition situations. Process Biochem 33:527–534. https://doi.org/10.1016/S0032-9592(98)00000-4
Vroemen AJ, Bévérini M (1999) Enzymatic production of gluconic acid or its salts. Patent Number US5897995
Wang EX, Ding MZ, Ma Q et al (2016a) Reorganization of a synthetic microbial consortium for one - step vitamin C fermentation. Microb Cell Fact 15:1–11. https://doi.org/10.1186/s12934-016-0418-6
Wang P, Xia Y, Li J et al (2016b) Overexpression of pyrroloquinoline quinone biosynthetic genes affects L-sorbose production in Gluconobacter oxydans WSH-003. Biochem Eng J 112:70–77. https://doi.org/10.1016/j.bej.2016.04.011
Wang P, Zeng W, Xu S et al (2018) Current challenges facing one-step production of L-ascorbic acid. Biotechnol Adv 36:1882–1899. https://doi.org/10.1016/j.biotechadv.2018.07.006
Wang DM, Sun L, Sun WJ et al (2019) A membrane-bound gluconate dehydrogenase from 2-keto-D-gluconic acid industrial producing strain Pseudomonas plecoglossicida JUIM01: purification, characterization, and gene identification. Appl Biochem Biotechnol 188:897–913. https://doi.org/10.1007/s12010-019-02951-0
Market Watch (2021) Miglitol (CAS 72432–03–2) market 2021. https://www.marketwatch.com/press-release/miglitol-cas-72432-03-2-market-2021-industry-size-overview-segmentation-and-geographical-forecast-till-2026-with-dominant-countries-data-2021-11-18. Accessed 8 Dec 2021
Wei D, Xu J, Sun J et al (2013) 2-Ketogluconic acid production by Klebsiella pneumoniae CGMCC 1.6366. J Ind Microbiol Biotechnol 40:561–570. https://doi.org/10.1007/s10295-013-1261-y
Weiser JR, Zawaneh PN, Putnam D (2011) Poly(carbonate-ester)s of dihydroxyacetone and lactic acid as potential biomaterials. Biomacromolecules 12:977–986. https://doi.org/10.1021/bm101342p
Wu P, Wang G, Wang G et al (2016) Butanol production under microaerobic conditions with a symbiotic system of Clostridium acetobutylicum and Bacillus cereus. Microb Cell Fact 15:1–11. https://doi.org/10.1186/s12934-016-0412-z
Xie G (2018) Method for preparing DHA dry powder through microbial fermentative liquid. Patent number CN108315362
Xiong XH, Zhao Y, Ge X et al (2011) Production and radioprotective effects of pyrroloquinoline quinone. Int J Mol Sci 12:8913–8923. https://doi.org/10.3390/ijms12128913
Xu S, Wang X, Du G et al (2014a) Enhanced production of L-sorbose from D-sorbitol by improving the mRNA abundance of sorbitol dehydrogenase in Gluconobacter oxydans WSH-003. Microb Cell Fact. https://doi.org/10.1186/s12934-014-0146-8
Xu X, He Y, Xia B, Zhao Q (2014b) 1,3-dihydroxyacetone high-yielding strain and construction method thereof. Patent number CN103789250
Yagi M, Kouno T, Aoyagi Y, Murai H (1976) The structure of moranoline, a piperidine alkaloid from morus species. Nippon Nogeikagaku Kaishi 50:571–572
Yang W, Xu H (2016) Industrial Fermentation of Vitamin C. In: Vandamme EJ, Revuelta JL (eds) Industrial biotechnology of vitamins, biopigments, and antioxidants, 1st edn. Wiley-VCH Verlag GmbH & Co KGaA, Weinheim, pp 161–192
Yang W, Han L, Mandlaa M et al (2013) Spaceflight-induced enhancement of 2-keto-L-gulonic acid production by a mixed culture of Ketogulonigenium vulgare and Bacillus thuringiensis. Lett Appl Microbiol 57:54–62
Ye C, Zou W, Xu N, Liu L (2014) Metabolic model reconstruction and analysis of an artificial microbial ecosystem for vitamin C production. J Biotechnol 182–183:61–67. https://doi.org/10.1016/j.jbiotec.2014.04.027
Yuan J, Wu M, Lin J, Yang L (2016a) Enhancement of 5-keto-D-gluconate production by a recombinant Gluconobacter oxydans using a dissolved oxygen control strategy. J Biosci Bioeng 122:10–16. https://doi.org/10.1016/j.jbiosc.2015.12.006
Yuan J, Wu M, Lin J, Yang L (2016b) Combinatorial metabolic engineering of industrial Gluconobacter oxydans DSM2343 for boosting 5-keto-D-gluconic acid accumulation. BMC Biotechnol 16:1–15. https://doi.org/10.1186/s12896-016-0272-y
Zeng W, Cai W, Liu L et al (2019) Efficient biosynthesis of 2-keto-D-gluconic acid by fed-batch culture of metabolically engineered Gluconobacter japonicus. Synth Syst Biotechnol 4:134–141. https://doi.org/10.1016/j.synbio.2019.07.001
Zeng W, Wang P, Li N et al (2020) Production of 2-keto-L-gulonic acid by metabolically engineered Escherichia coli. Bioresour Technol 318:1–8. https://doi.org/10.1016/j.biortech.2020.124069
Zhang J, Liu J, Shi Z et al (2010) Manipulation of B. megaterium growth for efficient 2-KLG production by K. vulgare. Process Biochem 45:602–606. https://doi.org/10.1016/j.procbio.2009.11.016
Zhang JB, Zhang XL, Wang DH et al (2011a) Biocatalytic regioselective oxidation of N-hydroxyethyl glucamine for synthesis of miglitol. Adv Mater Res 197–198:51–55. https://doi.org/10.4028/www.scientific.net/AMR.197-198.51
Zhang W, Xie Z, Luo W, Zhang J (2011b) Fermentation process optimization and kinetics studies of 2-keto-D-gluconic acid production by Serratia sp. BK-98. J Chem Ind Eng 62:1371–1376 ((in Chinese))
Zhang H, Liu G, Zhang J, Bao J (2016a) Fermentative production of high titer gluconic and xylonic acids from corn stover feedstock by Gluconobacter oxydans and techno-economic analysis. Bioresour Technol 219:123–131. https://doi.org/10.1016/j.biortech.2016.07.068
Zhang H, Zhang J, Bao J (2016b) High titer gluconic acid fermentation by Aspergillus niger from dry dilute acid pretreated corn stover without detoxification. Bioresour Technol 203:211–219. https://doi.org/10.1016/j.biortech.2015.12.042
Zheng XJ, Jin KQ, Zhang L et al (2016) Effects of oxygen transfer coefficient on dihydroxyacetone production from crude glycerol. Brazilian J Microbiol 47:129–135. https://doi.org/10.1016/j.bjm.2015.11.020
Zhou X, Xu Y (2019) Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour Technol 282:81–87. https://doi.org/10.1016/j.biortech.2019.02.129
Zhou X, Zhou X, Xu Y, Yu S (2016) Improving the production yield and productivity of 1, 3- dihydroxyacetone from glycerol fermentation using Gluconobacter oxydans NL71 in a compressed oxygen supply-sealed and stirred tank reactor ( COS-SSTR ). Bioprocess Biosyst Eng 39:1315–1318. https://doi.org/10.1007/s00449-016-1595-8
Zhou X, Zhou X, Huang L et al (2017) Efficient coproduction of gluconic acid and xylonic acid from lignocellulosic hydrolysate by Zn(II)-selective inhibition on whole-cell catalysis by Gluconobacter oxydans. Bioresour Technol 243:855–859. https://doi.org/10.1016/j.biortech.2017.07.023
Zhou P, Yao R, Zhang H, Bao J (2019a) Unique glucose oxidation catalysis of Gluconobacter oxydans constitutes an efficient cellulosic gluconic acid fermentation free of inhibitory compounds disturbance. Biotechnol Bioeng 116:2191–2199. https://doi.org/10.1002/bit.27020
Zhou X, Zhao J, Zhang X, Xu Y (2019b) An eco-friendly biorefinery strategy for xylooligosaccharides production from sugarcane bagasse using cellulosic derived gluconic acid as efficient catalyst. Bioresour Technol 289:121755. https://doi.org/10.1016/j.biortech.2019.121755
Zhou X, Shen Y, Xu Y, Balan V (2020) Directing cell catalysis of glucose to 2-keto-D-gluconic acid using Gluconobacter oxydans NL71. Process Biochem 94:365–369. https://doi.org/10.1016/j.procbio.2020.04.038
Zou Y, Hu M, Lv Y et al (2013) Enhancement of 2-keto-gulonic acid yield by serial subcultivation of co-cultures of Bacillus cereus and Ketogulonigenium vulgare. Bioresour Technol 132:370–373. https://doi.org/10.1016/j.biortech.2012.10.151
Acknowledgements
The authors thank the brazilian agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Finance Code 001 for fellowship and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). We are grateful to Norman Ratcliffe for editing and suggestions for improvements of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES)- Finance Code 001 and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Author information
Authors and Affiliations
Contributions
GARdS: designed study, performed research, analyzed data, wrote the paper; SSSO: designed study, performed research. SFL: designed study, performed research. RPdN: revised the work. ARBdS: revised the work. SBF: revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, G.A.R., Oliveira, S.S.d., Lima, S.F. et al. The industrial versatility of Gluconobacter oxydans: current applications and future perspectives. World J Microbiol Biotechnol 38, 134 (2022). https://doi.org/10.1007/s11274-022-03310-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-022-03310-8