Abstract
1,3-Dihydroxyacetone (DHA), a natural ketose, is widely used in the chemical, cosmetic, and pharmaceutical industries. The current method for DHA production is Gluconobacter oxydans (G. oxydans) fermentation, but the high concentration of glycerol in the fermentation broth inhibits cells growth. To overcome this obstacle, in this study, we overexpressed the glycerol transporter (GlpFp) by the use of promoters PtufB, Pgmr, Pglp1, and Pglp2 in G. oxydans 621H. The results show that the glycerol tolerances of strains overexpressing GlpF were all much better than that of the control strain. The glycerol dehydrogenase gene (Gdh) was overexpressed by the promoters PtufB and Pgdh, which increased the DHA titer by 12.7% compared with that of the control group. When GlpF and Gdh genes were co-overexpressed in G. oxydans 621H, the OD600 value of the engineered strains all increased, but the DHA titers decreased in different degrees, as compared with strains that overexpressed only Gdh. This study provides a reference for future research on DHA production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,3-Dihydroxyacetone (DHA), the simplest ketose with two hydroxyl groups and one ketone group, participates in many kinds of chemical reactions due to its active chemical properties. DHA is reported to be widely used in the cosmetics industry for its skin protection ability [1,2,3]. Also, DHA can improve human endurance [4,5,6], detoxify poisonous substances [7, 8], and fight viruses [9], so it is also widely used in the pharmaceutical [10] and chemical industries [11]. DHA can also be used as a precursor for synthesizing various chemicals such as methotrexate, lactic acid, surfactants, 1,2-propylene glycol, and pharmaceutical products [12].
Currently, microbial fermentation is the most common method used to produce DHA. G. oxydans is widely used for its high DHA production via the incomplete oxidation of glycerol. For instance, Tanamool et al. [13] isolated a G. oxydans NKC115 strain to produce DHA for which the DHA titer reached 27.50 g/L with an initial crude glycerol concentration of 100 g/L. However, the high glycerol substrate concentration inhibited the cell growth rate and the conversion efficiency of glycerol [14,15,16,17]. A high concentration of DHA affects the uptake rate of glycerol, thereby affecting the growth and metabolism of cells and indirectly reducing the DHA titer [18, 19]. For example, increasing the initial glycerol concentration from 31 to 129 g/L was reported to decrease the specific growth rate of cells by 70% and decrease the maximum specific production rate of DHA by 30% [15]. To prevent the glycerol inhibition effect, Dikshit et al. [20] used immobilized G. oxydans cells to produce DHA by batch and repeated batch fermentation and obtained a final DHA titer of 17.83 g/L, which was almost ninefold higher than that of the control groups. Researchers have also attempted to improve the metabolic pathways of DHA-producing strains by overexpressing or inhibiting-related genes [21, 22]. For instance, the G. oxydans membrane dehydrogenase gene sldAB was overexpressed using the promoters Pgdh and PtufB, respectively. When 550 mmol/L glycerol was used as the substrate, the accumulation of DHA in the fermentation broth increased by at least 25% compared with the control group [21].
The glycerol transporter and glycerol dehydrogenase are two important enzymes in DHA synthesis. The glycerol transporter encoded by GlpF is an aquaporin, which makes the uptake of glycerol more efficient [23,24,25]. The glycerol transporter is very sensitive to DHA [26]. Therefore, the inhibition of DHA on the glycerol transporter is the most important factor for inhibiting G. oxydans growth and is thus also the main inhibition effect in DHA production [27]. Overexpressing the glycerol transporter in Escherichia coli (E. coli) effectively increases the permeability of glycerol and promotes cell growth [28]. Glycerol dehydrogenase encoded by Gdh is a key enzyme for DHA synthesis in G. oxydans. This enzyme consists of two subunits and employs oxygen as the final acceptor of reduced equivalents without NADH mediation. Glycerol can be directly oxidized into DHA by the glycerol dehydrogenase present in the cell membrane. Wei et al. [29] amplified Gdh and Ndh, constructed the plasmids pET-Gdh and pET-Ndh, and transfected them into E. coli to obtain the highest DHA titer of 85 g/L. In view of the importance of the glycerol transporter and glycerol dehydrogenase in synthesizing DHA, in this study, we overexpressed GlpF and Gdh in G. oxydans via promoter engineering. The promoter PtufB from E. coli was reported to successfully enhance the production of 2-keto-L-gulonate in G. oxydans [30], and Pgmr from plasmid pBBR1MCS-5 is another frequently used promoter in G. oxydans. First, we overexpressed GlpF by changing the promoters PtufB, Pgmr, Pglp1, and Pglp2 to promote the growth of G. oxydans and improve the glycerol tolerance of G. oxydans. Next, we overexpressed Gdh using promoters PtufB and Pgdh to enhance the ability of G. oxydans in DHA production. Lastly, we co-expressed GlpF and Gdh in G. oxydans and found all the resulting strains to have a higher OD600 value than strains only expressing Gdh.
Materials and Methods
Strains, Medium, and Culture Conditions
We used G. oxydans 621H (wild-type strain) as the original strain for DHA production and E. coli DH5a for plasmid construction. We used E. coli HB101 as the assistant bacteria for transformation. All of these strains were preserved in our laboratory. Table 1 lists all of the engineered strains used in this work.
To cultivate strains of E. coli DH5a and E. coli HB101, we used an LB medium containing 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl.
We used a sorbitol medium containing 5 g/L sorbitol, 20 g/L yeast extract, 5 g/L (NH4)2SO [1, 4], 0.5 g/L KH2PO4, and 0.5 g/L MgSO4·7H2O to cultivate G. oxydans 621H and select engineered strains by adding appropriate concentrations of antibiotics.
We used a fermentation medium containing 100 g/L glycerol, 2.5 g/L yeast extract, 2 g/L peptone, 2 g/L (NH4)2SO4, 2.62 g/L KH2PO4·3H2O, 1 g/L MgSO4·7H2O, 0.56 g/L MnSO4·H2O, 2.5 g/L CaCO3, and 1.25 g/L CaCl2 to cultivate the engineered strains for 48 h at 30 °C, 220 r/min.
DNA Manipulation
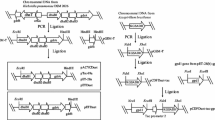
We used the promoters Pglp1 and Pglp2 as native GlpF promoters and Pgdh as the promoter of Gdh. The glycerol transporter gene GlpF, glycerol dehydrogenase gene Gdh, and DNA fragments containing GlpF1(Pglp1-GlpF), GlpF2(Pglp2-GlpF), Gdh1(Pgdh-Gdh), and promoter PtufB were amplified from the genomic DNA of G. oxydans 621H. The promoter Pgmr was amplified from the plasmid pBBR1MCS-5.
Table 2 shows a summary of all the primers used in this work.
Plasmids and Strains Construction
To obtain the plasmids pBBR-Pglp1-GlpF and pBBR-Pglp2-GlpF, we inserted DNA fragments of GlpF1(Pglp1-GlpF) and GlpF2(Pglp2-GlpF) into the plasmid pBBR1MCS-5 digested by the restriction endonucleases HindIII and EcoRI, respectively.
The DNA fragments PtufB and Pgmr were digested by the restriction endonucleases XhoI and NdeI, and GlpF was digested by the restriction endonucleases NdeI and EcoRI. We obtained the DNA fragments PtufB-GlpF and Pgmr-GlpF using T4 DNA ligase and inserted them into the plasmid pBBR1MCS-5 digested by the restriction endonucleases XhoI and EcoRI to obtain plasmids pBBR-PtufB-GlpF and pBBR-Pgmr-GlpF, respectively.
To obtain the plasmid pBBR-Pgdh-Gdh, we inserted the DNA fragment Gdh1(Pgdh-Gdh) into the plasmid pBBR1MCS-5 digested by restriction endonucleases XbaI and EcoRI.
The DNA fragment PtufB was digested by the restriction endonucleases EcoRI and BamHI, and Gdh was digested by the restriction endonucleases BamHI and XbaI. We obtained the DNA fragment PtufB-Gdh using T4 DNA ligase and then inserted it into the plasmid pBBR1MCS-5 digested by the restriction endonucleases EcoRI and XbaI to obtain the plasmid pBBR-PtufB-Gdh.
We simultaneously inserted GlpF and Gdh controlled by different promoters into the plasmid pBBR1MCS-5 to obtain different co-expression vectors.
Table 3 shows a summary of all the plasmids used in this work.
To construct engineered strains, we introduced each of the expression vectors into G. oxydans 621H by electrotransformation.
We preserved the G. oxydans strain 621H and plasmid pBBR1MCS-5 in our laboratory.
Transcriptional Gene Expression Studies by RT-PCR
We cultured the wild-type and engineered strains in sorbitol medium for 24 h at 30 °C, 220 r/min. Then, we obtained the cells by centrifugation and extracted the total RNA using a TianGen RNA kit. We used the total RNA as a template to obtain cDNA and initiated a reverse transcription polymerase chain reaction (RT-PCR) according to the methods reported by Xue et al. [36]. We used the primers listed in Table 2. As a blank control, we used the G. oxydans strain 621H containing the empty vector pBBR1MCS-5.
Shake Flask Fermentation of Strains
We cultured single colonies in a 250-mL flask containing 30 mL of sorbitol medium as the seed culture for 20 h at 30 °C, 220 r/min. Then, we inoculated a 5% (v/v) seed culture into the fermentation medium with different glycerol concentrations for 48 h at 30 °C, 220 r/min, and measured the OD600 of the fermentation broth using a UV–Vis spectrophotometer (Oppler, 752N, China). We used deionized water as a blank control.
HPLC Analysis of DHA
The fermentation broth was centrifuged at 8000 r/min for 10 min to obtain the supernatant, which was used for DHA measurement. We filtered the samples with a 0.22-μm filter membrane. To conduct high-performance liquid chromatography (HPLC), we used a Hypersil 5-NH2 (5 μm, 4.6 × 250 mm) HPLC column (Series III) from Agilent Technologies equipped with a refractive index detector and a column temperature of 30 °C. The volume of the injected sample was 20 μL. We used acetonitrile/H2O (v/v = 60: 40) as the mobile phase at a flow rate of 1 mL/min. We determined the DHA concentration using a calibration curve for standard DHA solutions.
Determination of Enzyme Activity
We cultured wild-type and engineered strains in sorbitol medium for 24 h at 30 °C, 220 r/min. The broth was then centrifuged at 10,000g for 10 min to obtain cells, after which the cells were washed twice in a 50 mmol/L phosphate buffer (pH 7.0). After washing, the cells were suspended in 20 mL of 50 mmol/L phosphate buffer (pH 7.0). Then, these suspended cells were broken by an ultrasonic wave and subsequently kept in an ice bath. The broken cells were collected by centrifugation, and the glycerol dehydrogenase activity was determined according to the method reported by Sugisawa and Hoshino [37].
Using 2,6-dichlorophenolindophenol (DCIP) as the electron acceptor, we detected changes in the absorbance at 600 nm during the reaction to determine the glycerol dehydrogenase activity in the cell membrane fraction. The basal reaction solution included 50 mmol/L of potassium phosphate buffer (pH 6.0), 0.25 mmol/L of DCIP, and 0.325 mmol/L of phenazine methosulfate (PMS). The basal reaction solution was prepared just prior to the assay.
When measuring the enzyme activity, we added 2.4 mL of basal reaction solution and 30 μL of diluted crude enzyme solution to the cuvette. After 5 min in a water bath at 30 °C, we added 600 µL of glycerol (200 mmol/L, 30 °C) to the mixture to start the reaction. We measured the results at 600 nm. The extinction coefficient of the DCIP at a pH of 6.0 was 10.8 mmol/L. In this study, we defined one enzyme unit as the amount of the enzyme that catalyzes a reduction of 1 mmol of DCIP per min when the pH is 6.0 at 30 °C. The formula we used to calculate the enzyme activity is as follows:
where \(\Delta A\) is the change in the absorption value at 600 nm in \(\Delta t\) (min); VE is the volume of the enzyme solution to be measured; ε (mmol/L) is the extinction coefficient of DCIP; and CPr (mg/mL) is the protein concentration of the enzyme solution.
Results and Discussion
Overexpression of Glycerol Transporter Gene to Improve Cell Growth
We overexpressed GlpF using the promoters PtufB, Pgmr, Pglp1 and Pglp2 to, respectively, construct the strains G1, G2, G3, and G4. Then, we cultured G. oxydans strains 621H, G1, G2, G3, and G4 in a sorbitol medium to study the effect of overexpressing the gene GlpF. Figure 1a shows the OD600 values of different strains, and we can see that the OD600 values of all engineered strains were much higher than that of the G. oxydans strain 621H. At 48 h, the OD600 values of strains G1, G2, G3, and G4 increased by 110.5%, 48.4%, 108.4%, and 72.6%, respectively, compared to that of the control group.
a OD600 values of the G. oxydans strains 621H, G1, G2, G3, and G4 in sorbitol medium. b Relative GlpF transcript levels of the G. oxydans strains 621H, G1, G2, G3, and G4 in sorbitol medium. The GlpF transcript level in G. oxydans 621H was set to 1. G. oxydans strain 621H served as the control group. The GlpF gene was overexpressed in strains G1, G2, G3, and G4 by the use of promoters PtufB, Pgmr, Pglp1, and Pglp2, respectively
To further explain this phenomenon, we analyzed the GlpF transcript levels of the G. oxydans strains 621H, G1, G2, G3, and G4 after 48 h of incubation in sorbitol medium. As shown in Fig. 1b, the GlpF transcript levels in the engineered strains were all higher than that of the control group, especially in strains G1 and G3, which was consistent with the growth levels of these strains. These results show that overexpressing GlpF can effectively improve the growth of strains, especially when using the promoters PtufB and Pglp1.
Overexpression of Glycerol Transporter Gene to Improve the Glycerol Tolerance
To investigate the tolerance of engineered strains to glycerol, we cultured the G. oxydans strains 621H, G1, G2, G3, and G4 in fermentation medium. Figure 2 shows the OD600 values of G. oxydans strains 621H, G1, G2, G3, and G4 in the fermentation medium with different glycerol concentrations, and we can see that the OD600 values of the engineered strains were all much higher than that of the G. oxydans strain 621H at the same glycerol concentrations. When the initial concentration of glycerol was 100 g/L, the OD600 values of strains G1, G2, G3, and G4 increased by 100%, 28%, 96%, and 68%, respectively, compared with that of the control group. At a glycerol concentration of 150 g/L, the OD600 values of strains G1, G2, G3, and G4 increased by 104.5%, 36.4%, 104.5%, and 81.8%, respectively, compared with that of the control group. When the initial glycerol concentration reached 200 g/L, the OD600 values of strains G1, G2, G3, and G4 increased by 211.1%, 103.7%, 196.3%, and 159.3%, respectively, compared with that of the control group. When the initial concentration of glycerol reached 250 g/L, the OD600 values of strains G1, G2, G3, and G4 increased by 1140%, 700%, 1220%, and 1004%, respectively, compared with that of the control group.
OD600 values of G. oxydans strains 621H, G1, G2, G3, and G4 in fermentation medium with different concentrations of glycerol. G. oxydans strain 621H served as the control group. The GlpF gene was overexpressed in strains G1, G2, G3, and G4 by the use of promoters PtufB, Pgmr, Pglp1, and Pglp2, respectively
The results show that for the same strain, the growth of the strain was inhibited with an increase in the glycerol concentration. However, the inhibitory degree against different strains was different. The growth of G. oxydans 621H was more severely inhibited than strains overexpressing GlpF. Strains G1 and G3, overexpressing GlpF with the promoters PtufB and Pglp1, respectively, performed better in improving glycerol tolerance. These results reveal that overexpressing GlpF can effectively improve the glycerol tolerance of strains, especially when using promoters PtufB and Pglp1.
Then, we selected the most glycerol-tolerant strain G1 for DHA production with different glycerol concentrations. Figure 3a, b shows the contents of DHA and residual glycerol, respectively.
a Concentration of DHA produced by G. oxydans strains 621H and G1 in fermentation medium. b Residual glycerol concentrations of G. oxydans strains 621H and G1 in fermentation medium. G. oxydans strain 621H served as the control group. The GlpF gene was overexpressed in G1 by the use of promoter PtufB
Figure 3a shows that GlpF overexpression did not enhance but in fact reduced the production of DHA. In Fig. 3b, we can see that the residual glycerol concentrations of G. oxydans 621H and G1 are similar. We suppose that some glycerol was converted into DHA by membrane-bound glycerol dehydrogenase in the periplasmic space of strain G1 without the need to enter the cytoplasm. In addition, GlpF overexpression improved the uptake rate of glycerol and caused more glycerol to be metabolized for cell growth after entering the cytoplasm than G. oxydans 621H. Therefore, although the production of DHA decreased, the residual glycerol concentration changed slightly and the cell growth was enhanced compared with that of G. oxydans 621H.
Overexpression of Glycerol Dehydrogenase Gene to Increase the DHA Titer by Shaking Flask Fermentation
We overexpressed Gdh using promoters Pgdh and PtufB, respectively, to construct strains G5 and G6. We cultured G. oxydans strains 621H, G5, and G6 in 100-g/L glycerol fermentation medium. Figure 4a shows the OD600 values of different strains. In Fig. 4a, we can see that overexpressing Gdh did not have a significant effect on the growth of strains. The growth conditions of strains G5 and G6 were basically the same as that of G. oxydans strain 621H. At 27 h, the growth of G. oxydans strains 621H, G5, and G6 all reached the stationary phase. At 60 h, their OD600 values were 2.40, 2.30, and 2.25, respectively.
a OD600 values of G. oxydans strains 621H, G5, and G6 in 100-g/L glycerol fermentation medium. b DHA titer produced by G. oxydans strains 621H, G5, and G6 in 100-g/L glycerol fermentation medium. G. oxydans strain 621H served as the control group. We overexpressed the Gdh gene in strains G5 and G6 by the use of promoters Pgdh and PtufB, respectively
To quantify the fermentation products, we used the authentic standards curve. Figure 4b shows the DHA produced by different strains. In the figure, we can see that at the beginning of the reaction, the DHA titer increased slowly and then accelerated. During the late stages of fermentation, it increased gradually and reached the stationary phase after 48 h.
The DHA titers produced by engineered strains under the control of promoters Pgdh and PtufB were all higher than that of G. oxydans strain 621H. After fermentation, the DHA titers produced by strains G5 and G6 were about 8 g/L higher than that of G. oxydans strain 621H, which increased by almost 12.7%. This indicates that overexpressing glycerol dehydrogenase in G. oxydans 621H can increase the DHA titer. Li et al. [38] constructed an engineered strain for the industrial production of DHA by overexpressing the Gdh gene in G. oxydans M5AM, in which the gene coding for the membrane-bound alcohol dehydrogenase (Adh) is interrupted. The DHA titer in that study was 96 g/L from 100 g/L glycerol, which is higher than that obtained in our study. This may be due to the absence of the Adh gene in the G. oxydans strain M5AM.
Transcript Level and Glycerol Dehydrogenase Activity in Strains Overexpressing Glycerol Dehydrogenase Gene
Next, we cultured the G. oxydans strains 621H, G5, and G6 in a 100-g/L glycerol fermentation medium for 48 h and then analyzed the transcript levels of the Gdh gene, the results of which are shown in Fig. 5a. In the figure, we can see that all the transcript levels of the engineered strains under the control of the promoters Pgdh and PtufB were about four times higher than that of G. oxydans 621H. Glycerol dehydrogenase is the key enzyme responsible for the synthesis of DHA by the catalysis of glycerol in G. oxydans 621H. The difference in the transcript levels of the Gdh gene in the engineered and wild-type strains explains the difference in the DHA production in Fig. 4b.
a Relative transcript levels of the Gdh gene in G. oxydans strains 621H, G5, and G6 in 100-g/L glycerol fermentation medium. The transcript level of the Gdh gene in G. oxydans 621H was set to 1. b Enzyme activities of glycerol dehydrogenase in G. oxydans strains 621H, G5, and G6 in 100-g/L glycerol fermentation medium. G. oxydans strain 621H served as the control group. The Gdh gene was overexpressed in strains G5 and G6 by the use of the promoters Pgdh and PtufB, respectively
To further explain the increase in the DHA production, we measured the activity of glycerol dehydrogenase in G. oxydans strains 621H, G5, and G6, the results of which are shown in Fig. 5b. In the figure, we can see that the activity of glycerol dehydrogenase controlled by the Pgdh promoter increased from 1.74 to 2.16 U/mg, and the activity of glycerol dehydrogenase controlled by the PtufB promoter increased from 1.74 to 2.25 U/mg. The activities of glycerol dehydrogenase in strains G5 and G6 were higher than those in the G. oxydans strain 621H, which could also explain the increase in the DHA production.
Co-overexpression of Glycerol Transporter and Glycerol Dehydrogenase Genes to Produce DHA by Shaking Flask Fermentation
Overexpressing GlpF can effectively improve the glycerol tolerance of strains, and overexpressing Gdh in G. oxydans can increase the DHA titer. As such, we attempted to overexpress GlpF and Gdh in G. oxydans simultaneously. We linked GlpF with promoters PtufB, Pgmr, Pglp1, and Pglp2, respectively, and linked Gdh with the promoters PtufB and Pgdh, respectively. Then, they were combined to construct strains G7, G8, G9, G10, G11, G12, G13, and G14. All these strains were cultured in 100-g/L glycerol fermentation medium. After 48 h, we identified and recorded the fermentation products by HPLC. Figure 6a shows the DHA titers produced by different strains, and Fig. 6b shows the OD600 values of these strains.
a DHA titers produced by G. oxydans strains 621H, G5, G6, G7, G8, G9, G10, G11, G12, G13, and G14 in 100-g/L glycerol fermentation medium. b OD600 values of G. oxydans strains 621H, G5, G6, G7, G8, G9, G10, G11, G12, G13, and G14 in 100-g/L glycerol fermentation medium. The term 621H indicates the G. oxydans strain 621H, which served as the control group. The Gdh gene was overexpressed in strains G5 and G6 by the use of promoters Pgdh and PtufB, respectively. The GlpF and Gdh genes were co-overexpressed in strains G7, G8, G9, G10, G11, G12, G13, and G14
As shown in Fig. 6, the DHA titers of strains G7, G8, G9, G10, G11, G12, G13, and G14 are less than those in G. oxydans strains 621H, G5, and G6. However, the OD600 values of strains G7, G8, G9, G10, G11, G12, G13, and G14 were all higher than those of G. oxydans strains 621H, G5, and G6. Although the growth of strains co-overexpressing GlpF and Gdh was successfully enhanced, the DHA titers were reduced, which indicates that co-overexpressing GlpF and Gdh does not improve DHA production.
Transcript Level and Activity of Glycerol Dehydrogenase in Strains Co-overexpressing Glycerol Transporter Gene and Glycerol Dehydrogenase Gene
We measured the transcript levels of the Gdh gene in engineered strains, the results of which are shown in Fig. 7a. In the figure, we can see that the Gdh gene transcript levels in strains G7, G8, G9, G10, G11, G12, G13, and G14 and strains G5 and G6 are similar. Both are about five times the transcript level of G. oxydans strain 621H, which indicates that the overexpression of the glycerol transporter did not affect the transcript level of glycerol dehydrogenase.
a Relative transcript levels of Gdh in G. oxydans strains 621H, G5, G6, G7, G8, G9, G10, G11, G12, G13 and G14 in 100-g/L glycerol fermentation medium. The Gdh transcript level in G. oxydans 621H was set to 1. b Enzyme activities of G. oxydans strains 621H, G5, G6, G7, G8, G9, G10, G11, G12, G13, and G14 in 100-g/L glycerol fermentation medium. The term 621H indicates G. oxydans strain 621H, which served as the control group. The Gdh gene was overexpressed in strains G5 and G6 by the use of promoters Pgdh and PtufB, respectively. GlpF and Gdh were co-overexpressed in strains G7, G8, G9, G10, G11, G12, G13, and G14
Next, we measured the activity of glycerol dehydrogenase in the engineered strains, the results of which are shown in Fig. 7b. In the figure, we can see that the overexpression of GlpF had very little effect on the enzyme activity of glycerol dehydrogenase.
The effect of co-overexpressing GlpF and Gdh in G. oxydans was not as good as we had expected. High Gdh transcript levels and high enzyme activities of glycerol dehydrogenase in strains co-overexpressing GlpF and Gdh did not lead to high DHA titers. This indicates that although overexpressing GlpF can enhance cell growth and improve the glycerol tolerance of G. oxydans, it had negative effects on DHA production. We assume that GlpF overexpression improved the uptake rate of glycerol and caused more glycerol to be metabolized for cell growth after entering the cytoplasm than strains not overexpressing GlpF. The more glycerol that enters the cytoplasm for cell growth, the less glycerol is converted into DHA by the membrane-bound glycerol dehydrogenase in the periplasmic space. Therefore, the production of DHA decreased.
Conclusions
Our results indicate that overexpressing GlpF alone effectively promotes the growth of cells and improves the glycerol tolerance of G. oxydans, but it reduces the synthesis of DHA. Overexpressing Gdh alone increases the DHA titer by 12.7% from that of its parental strain. However, co-overexpressing GlpF and Gdh does not achieve a good balance between enhancing cell growth and increasing DHA production. Although it enhances cell growth, it fails to increase the DHA titer.
Future research should focus on analyzing the role of the glycerol transporter in DHA synthesis using transcriptomics and metabolomics methods and searching for new targets with which to construct recombinant strains to produce DHA at high glycerol concentrations.
References
Meybeck A (1977) A spectroseopic study of reaction products of dihydroxyacetone with aminoacids. J Soc Cosmet Chem 28:25–35
Bobin MF, Martii MC, Cotte J (1984) Effect of color adjuvants on the tanning effect of dihydroxyacetone. J Soc Cosmet Chem 35:265–272
Fesq H, Brockow K, Strom K et al (2001) Dihydroxyacetone in a new formulation: a powerful therapeutic option in vitiligo. Dermatology 203(3):241–243. https://doi.org/10.1159/000051757
Stanko RT, Robertson RJ, Spina RJ et al (1990) Enhancement of arm exercise endurance capacity with dihydroxyacetone and pyruvate. J Appl Physiol 68(1):119
Ivy JL (1998) Effect of pyruvate and dihydroxyacetone on metabolism and aerobic endurance capacity. Med Sci Sports Exerc 30(6):837–843
Schlifke AC (1999) Can pyruvate and dihydroxyacetone(DHA) improve athletic performance. Nutr Bytes 5:1–5
Niknahad H, Ghelichkhani E (2002) Antagonism of cyanide poisoning by dihydroxyacetone. Toxicol Lett (Shannon) 132(2):95–100
Cummings TF (2004) The treatment of cyanide poisoning. Occup Med 54(2):82–85
Oh CH, Hong JH (2007) Short synthesis and antiviral evaluation of c-fluoro-branched cyclopropyl nucleosides. Nucleosides, Nucleotides Nucleic Acids 26(4):403–411
Henderson PW, Kadouch DJ, Singh SP et al (2010) A rapidly resorbable hemostatic biomaterial based on dihydroxyacetone. J Biomed Mater Res Part A 93(2):776–782
Hekmat D, Bauer R, Fricke J (2003) Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess Biosyst Eng 26(2):109–116
Green SR, Whalen EA, Molokie E (1961) Dihydroxyacetone: production and uses. Biotechnol Bioeng 3(4):351–355
Tanamool V, Hongsachart P, Soemphol W (2018) Bioconversion of biodiesel-derived crude glycerol to 1, 3-dihydroxyacetone by a potential acetic acid bacteria. Sains Malays 47(3):481–488
Dikshit PK, Moholkar VS (2016) Kinetic analysis of dihydroxyacetone production from crude glycerol by immobilized cells of Gluconobacter oxydans MTCC 904. Bioresour Technol 216:948–957
Claret C, Bories A, Soucaille P (1992) Glycerol inhibition of growth and dihydroxyacetone production by Gluconobacter oxydans. Curr Microbiol 25(3):149–155
Dikshit PK, Kharmawlong GJ, Moholkar VS (2018) Investigations in sonication–induced intensification of crude glycerol fermentation to dihydroxyacetone by free and immobilized Gluconobacter oxydans. Biores Technol 256:302–311
Stasiak-Różańska L, Berthold-Pluta A, Dikshit PK (2018) Valorization of waste glycerol to dihydroxyacetone with biocatalysts obtained from Gluconobacter oxydans. Appl Sci 8(12):2517
Bauer R, Katsikis N, Varga S et al (2005) Study of the inhibitory effect of the product dihydroxyacetone on Gluconobacter oxydans in a semi-continuous two-stage repeated-fed-batch process. Bioprocess Biosyst Eng 28(1):37–43
Claret C, Bories A, Soucaille P (1993) Inhibitory effect of dihydroxyacetone on Gluconobacter oxydans: kinetic aspects and expression by mathematical equations. J Ind Microbiol 11(2):105–112
Dikshit PK, Moholkar VS (2018) Batch and repeated-batch fermentation for 1,3-dihydroxyacetone production from waste glycerol using free, immobilized and resting Gluconobacter oxydans cells. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-018-0307-9
Gätgens C, Degner U, Bringer-Meyer S et al (2007) Biotransformation of glycerol to dihydroxyacetone by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 76(3):553–559
Habe H, Fukuoka T, Morita T et al (2010) Disruption of the membrane-bound alcohol dehydrogenase-encoding gene improved glycerol use and dihydroxyacetone productivity in Gluconobacter oxydans. Biosci Biotechnol Biochem 74(7):1391–1395
Calmes R, Deal SJ (1972) Glycerol transport by Nocardia asteroides. Can J Microbiol 18(11):1703–1708
Richey DP, Lin EC (1972) Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol 112(2):784–790
Saheb SA (1972) Perméation du glycérol et sporulation chez Bacillus subtilis. Can J Microbiol 18(8):1307–1313
Stasiak-Różańska L, Błażejak S, Gientka I (2014) Effect of glycerol and dihydroxyacetone concentrations in the culture medium on the growth of acetic acid bacteria Gluconobacter oxydans ATCC 621. Eur Food Res Technol 239(3):453–461
Claret C, Salmon JM, Romieu C et al (1994) Physiology of Gluconobacter oxydans during dihydroxyacetone production from glycerol. Appl Microbiol Biotechnol 41(3):359–365
Hedfalk K, Bill RM, Hohmann S et al (2000) Overexpression and purification of the glycerol transport facilitators, fps1p and GlpF, in Saccharomyces Cerevisiae and Escherichia coli. Mol Biol Physiol Water Solut Transp. https://doi.org/10.1007/978-1-4615-1203-5_4
Wei DZ, Ma XY, Zheng Y et al (2007) Gene engineering bacteria for producing dihydroxy acetone, constructing method, and application: CN, 101092604A. 2007-12-26 (in Chinese)
Saito Y, Ishii Y, Hayashi H et al (1997) Cloning of genes coding for L-sorbose and L-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-L-gulonate, a precursor of L-ascorbic acid, in a recombinant G. oxydans strain. Appl Environ Microbiol 63(2):454–460
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166(4):557–580
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41(3):459–472
Yang XP, Wei LJ, Lin JP et al (2008) Membrane-bound pyrroloquinoline quinone-dependent dehydrogenase in Gluconobacter oxydans M5, responsible for production of 6-(2-hydroxyethyl) amino-6-deoxy-L-sorbose. Appl Environ Microbiol 74(16):5250–5253
Kovach ME, Elzer PH, Steven Hill D et al (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166(1):175–176
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci 76(4):1648–1652
Xue CY, Zhang XM, Yu ZR et al (2013) Up-regulated spinosad pathway coupling with the increased concentration of acetyl-CoA and malonyl-CoA contributed to the increase of spinosad in the presence of exogenous fatty acid. Biochem Eng J 81:47–53
Sugisawa T, Hoshino T (2002) Purification and properties of membrane-bound D-sorbitol dehydrogenase from Gluconobacter suboxydans IFO 3255. Biosci Biotechnol Biochem 66(1):57–64
Li MH, Wu J, Liu X et al (2010) Enhanced production of dihydroxyacetone from glycerol by overexpression of glycerol dehydrogenase in an alcohol dehydrogenase-deficient mutant of Gluconobacter oxydans. Bioresour Technol 101(21):8294–8299
Acknowledgements
This work was supported by the Major Research Plan of Tianjin (16YFXTSF00460).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, J., Yang, X. & Lu, W. Research of 1,3-Dihydroxyacetone Production by Overexpressing Glycerol Transporter and Glycerol Dehydrogenase. Trans. Tianjin Univ. 25, 549–558 (2019). https://doi.org/10.1007/s12209-019-00207-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-019-00207-w