Abstract
Klebsiella pneumoniae CGMCC 1.6366 is a bacterium isolated for 1,3-propanediol or 2,3-butanediol production previously. K. pneumoniae ΔbudA, a 2,3-butanediol synthesis pathway truncated mutant with the gene deletion of budA which encodes alpha-acetolactate decarboxylase, was found to execrate an unknown chemical at a high titer when grown in the broth using glucose as carbon source. Later this chemical was identified to be 2-ketogluconic acid, which was formed through the glucose oxidation pathway in K. pneumoniae. It was found that 2-ketogluconic can also be produced by the wild strain. The fermentation studies showed that the production of this metabolite is strictly pH dependent, when the fermenting broth was maintained at pH 6–7, the main metabolite produced by K. pneumoniae CGMCC 1.6366 was 2,3-butanediol, or some organic acids in the budA mutated strain. However, if the cells were fermented at pH 4.7, 2-ketogluconic acid was formed, and the secretion of all other organic acids or 2,3-butanediol were limited. In the 5L bioreactors, a final level of 38.2 and 30.2 g/L 2-ketogluconic acid were accumulated by the wild type and the budA mutant K. pneumoniae, respectively, in 26 and 56 h; and the conversion ratios of glucose to 2-ketogluconic acid reached 0.86 and 0.91 mol/mol for the wild and the budA mutant, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klebsiella pneumoniae is an important model species, attracting intensive investigations in the fields of medical and industrial microbiology. Some strains of K. pneumoniae are opportunistic pathogens [15], while others are classified as associative nitrogen fixers due to their capability for nitrogen fixation [4]. K. pneumonia has tremendous potential in industrial applications as a microbial cell factory because it can convert various carbon resources to many valuable chemical intermediates, such as 1,3-propanediol, 2,3-butanediol, acetoin, ethanol, acetic acid, lactic acid and succinic acid, etc. [1, 13]. It has also been reported to excrete 2-ketogluconic acid [14]. The 2-Ketogluconic acid is a valuable chemical material used for synthesis of d-isoascorbic acid, a widely used food antioxidant [2]. The 2-ketogluconic acid is usually biologically produced by Gluconobacter oxydans, Pseudogluconobacter saccharoketogenes, or Pseudomonas sorbosoxida [16].
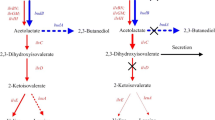
In bacteria, glucose catabolism normally starts with the phosphoenolpyruvate dependent glucose phosphotransferase system (PTS), however, some species of Enterobacteriaceae, including K. pneumoniae, have an additional PTS independent glucose oxidative pathway with which glucose is oxidized to gluconic acid and further oxidized to 2-ketogluconic acid. Gluconic acid and 2-ketogluconic acid can be re-transported inside the cells [3], and there 2-ketogluconic acid can convert back to gluconic acid, and gluconic acid is phosphorylated by adenosine triphosphate dependent kinases into 6-phosphogluconate, which is then converted to 2-dehydro-3-deoxy-gluconate-6-phosphate and enters into the Entner–Doudoroff pathway (Fig. 1).
Glucose metabolic pathway in K. pneumoniae. D1, glucose dehydrogenase; D2, gluconate dehydrogenase; D3, gluconate 2-dehydrogenase; P1, gluconate transporter; P2, 2-ketogluconate transporter; K1, gluconate kinase; PTS, phosphoenolpyruvate dependent glucose phosphotransferase system; AD, alpha-acetolactate decarboxylase. Chemicals with solid frame are various metabolic products
Klebsiella pneumoniae CGMCC 1.6366 (TUAC01) is a strain isolated for production of 1,3-propanediol or 2,3-butanediol [8]. An alpha-acetolactate decarboxylase mutation strain K. pneumoniae ΔbudA was constructed during our studies in characterization of the 2,3-butanediol metabolic pathway. However, when this mutant was cultured in a glucose containing medium, 2-ketogluconic acid was found to be accumulated in the broth at a rather higher titer. It was found that the production of 2-ketogluconic acid is pH dependent, however, not directly linked with budA deletion. This is the first report of this chemical produced by genetic engineered K. pneumoniae.
Materials and methods
Strains, plasmids and primers
Bacteria strains and plasmids used in this paper are listed in Table 1. Primers used for PCR are listed in Table 2.
Medium and culture condition
Klebsiella pneumoniae and Escherichia coli were routinely cultured in LB (Tryptone 10 g/L, yeast extract 5 g/L, NaCl 10 g/L) at 37 °C, the antibiotics were added appropriately (Ampicillin 100 μg/mL, Kanamycin 50 μg/mL, Streptomycin 50 μg/mL). The fermentation medium for K. pneumoniae contains 50 g/L glucose, 4 g/L corn steep liquor, 5 g/L (NH4)2SO4, 3 g/L Sodium acetate, 0.4 g/L KCl and 0.1 g/L MgSO4. In studies with flask culturing, 5 mL of overnight culture in LB was inoculated into 50 mL of fermentation medium (on 250 mL glass flask) and grown at 37 °C in a rotary shaker (200 rpm), 1 g CaCO3 was supplemented in every flask to adjust the pH of the broth prior to inoculation, all the flasks culturing experiments were repeated in triplicate. For bioreactor fermentation, 100 mL of seed culture in LB was inoculated to 3 L of FM in a 5 L bioreactor (BIOSTAT-A plus Sartorius) feeding with 2 L/min air at stirring rate of 350 rpm, the initial pH of the medium was set at 7.0, after the culture pH dropped to the setting point, NaOH was used for maintaining the medium pH.
Analytical methods
The biomass concentration was evaluated by determination of optical density (OD) at 640 nm with a spectrometer. The separation of the chemical compounds in the broth was quantified by a Shimadzu 20AVP high performance liquid chromatograph system (HPLC) (Shimadzu Corp., Kyoto, Japan) equipped with a RID-10A refractive index detector and a SPD-M20A photodiode array detector. The stationary and mobile phases were Aminex HPX-87H column (300 mm × 7.8 mm) (Bio-Rad, USA) and 0.005 mol/L H2SO4 solution at the velocity of 0.8 mL/min, respectively. The column temperature was set up at 65 °C.
BudA gene deletion in strain K. pneumoniae CGMCC 1.6366
The gene deletion of budA in K. pneumoniae followed the process as described previously [17]. Briefly, a DNA fragment containing budA gene was amplified with the K. pneumoniae CGMCC 1.6366 genomic DNA as template using the primers pair of budA-s and budA-a. The PCR product was linked into the clone vector pMD18-T simple® to generate pMD18-T-budA. A linear DNA with 39 and 40 nt homologous extensions flanking streptomycin resistance gene aadA was amplified using plasmid pIJ778 as template with the primers pair FRT-s1/FRT-a1. pMD18-T-ΔbudA was constructed by replacing budA with the aadA cassette in plasmid pMD18-T-budA by employing the Red/ET system in E. coli. The pMD18-T-ΔbudA was further to be used as the template for PCR preparation of a linear DNA containing the streptomycin resistance gene aadA with 500 bps of homologous regions at both sides. Finally, the linear DNA was transformed into K. pneumoniae CGMCC 1.6366 already hosting plasmid pDK6-red. Homologous recombination between the linear DNA and the chromosome that was facilitated by IPTG induced expression of Red recombinase gene in plasmid pDK6-red, led to budA deletion in K. pneumoniae CGMCC 1.6366, which was isolated on streptomycin plates. Primers pair Test778 and budA-s1 were used for PCR confirmation of the mutant.
Extraction and purification of 2-ketogluconic acid
2-ketogluconic acid was separated from a fresh culture of K. pneumoniae ΔbudA. The cell free supernatant of the culture was loaded onto a 100 mL 717 anion-exchange column, after washing the column with 200 mL of water, the components were eluted out with 20 mL of 1 M HCl; the elution was then mixed with 100 mL of anhydrous ethanol; after centrifugation of the mixture, a milky brown layer of substance was obtained and collected after vacuum dehydration.
Structure determination
The mass spectrum were recorded by using an Agilent 1100 LC/MSD mass spectrometer, performed both in positive and negative ion mode. The 1H and 13C nuclear magnetic resonance (NMR) spectra data were obtained in a D2O solution using a Bruker AV-400 (400 MHz) spectrometer. The chemical shift values were reported in ppm (δ) and the coupling constants were given in Hz (J).
Results and discussion
Construction of K. pneumoniae CGMCC 1.6366 budA deletion strain
As seen in the metabolic pathway from glucose to 2,3-butanediol (Fig. 1), two molecules of pyruvic acid forms acetolactate, which is decarboxylated to acetoin, and acetoin converts to 2,3-butanediol by reduction. Alpha-acetolactate decarboxylase, encoded by budA, catalyzes the decarboxylation reaction of acetolactate to acetoin. Supposedly the deletion of budA would interrupt the 2,3-butanediol synthesis pathway, and our initial motive to build a budA mutant was to study how that will affect the formation of 2,3-butanediol in K. pneumoniae. After the mutant was isolated, the replacement of 433 bp budA internal fragment by streptomycin resistance gene aadA was confirmed by PCR identification.
2-ketogluconic acid extraction and purification from fermentation broth
When K. pneumoniae ΔbudA was cultured in flasks without pH adjustment, an unknown compound was found accumulated at a very high level in the broth. The retention time of this chemical is 6.3 min when checked by HPLC, and has the UV absorbance detected by the photodiode array detector. However, this compound was not found by HPLC analysis after the sample flew through a Cl− anion-exchange column. The charging property of this compound indicated this compound is probably an organic acid. By using a 717 anion-exchange column, this chemical was purified.
Structure identification of 2-ketogluconic acid
The mass spectrum of the sample showed a distinguished peak m/z 193.1 in the negative mode, this peak corresponds to [M-H]−. The majority peaks in positive mode are m/z 217.1, 239.0 and 261.2, corresponding to [M + Na]+, [M + 2Na-H]+ and [M + 3Na-2H]+ ,respectively. Therefore, the molecular weight of this compound was deduced as to be 194.1.
1H and 13C NMR spectra acquired in D2O solution are shown in Fig. 2, and the results of the chemical shift of 13C resonance are listed in Table 3. The multiple peaks seen in 1H NMR spectrum indicated the supposedly purified sample tested was instead a mixture of different compounds, displaying the discrepancy with the high purity of the sample shown in the test by HPLC.
The 13C NMR spectrum evidently showed the chemical shifts of carbon signals of the unknown compound agreed well with that of the known compound 2-ketogluconic acid which Crawford had previously described (Table 3) (Crawford call it d-arabino-2-hexulosonate) [6]; and, according to that reference, in aqueous solution, 2-ketogluconic acid would have four different sugar-ring forms (Fig. 3); the 13C NMR spectrum demonstrated that α-pyranose and β-furanose are the major tautomer types in the solution, although a small amount of α-furanose isomer may coexist as well. The molar ratio of α-pyranose tautomer, β-pyranose tautomer, α-furanose tautomer, β-furanose tautomer was approximately 21: trace: 1: 5, which coincides very well to the reported ratio of 80: trace: 3: 17 by Crawford [6].
The 13C NMR spectrum solved the discrepancy between the analysis result of 1H NMR spectrum and HPLC, and, it proved that the sample was the mixture of three forms of tautomers of the same chemical. Taking into account all these proofs, the unknown sample was determined to be 2-ketogluconic acid.
2-Ketogluconic acid is an important chemical intermediate used for synthesis of d-isoascorbic acid. There were only a few references decades ago about its biological production using Klebsiella. Spp., therefore it would be interesting to further characterize and optimize the fermentation process in order to characterize the 2-ketogluconic acid produce by K. pneumoniae CGMCC 1.6366.
Shake flask experiments of K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudA
Both the K. pneumoniae CGMCC 1.6366 and the ΔbudA were initially cultured in flasks using CaCO3 to maintain pH. Figure 4 shows the results of those experiments. It was found that the final pH in the wild type cells’ culture remained stable at 6.0; 2-ketogluconic acid from wild type cells was accumulated only at an earlier phase, its level reached its culmination at 2.0 g/L, and dropped gradually to be almost undetectable; 2,3-butanediol, however, reached 17.3 g/L and was the main product in the broth.
Batch culture of K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudA in flasks a Glucose consumption and 2-ketogluconic acid formation by K. pneumoniae CGMCC 1.6366; b organic acid and 2,3-butanediol formation by K. pneumoniae CGMCC 1.6366; c Glucose consumption and 2-ketogluconic acid formation by K. pneumoniae ΔbudA; d organic acid formation by K. pneumoniae ΔbudA
A comparison of the results from the ΔbudA strain clearly presented the effect of deleting budA on the metabolic network in K. pneumoniae. As expected, the production of 2,3-butanediol was almost abolished, instead the level of 2-ketogluconic acid peaked at the end of growth and reached 41.2 g/L, and its level increased gradually during the whole process, accompanied by a low level of production of some other organic acids, such as 0.3 g/L succinic acid and 1.5 g/L acetic acid. The other decided difference in budA deletion strain shown in the experiment is that the final pH of the culture was only 4.7, which can probably be explained by the fact that more organic acids were produced by the mutant cells.
As seen in Fig. 1, glucose is normally transported into cytoplasm through the phosphoenolpyruvate dependent glucose phosphotransferase system and oxidized through glycolysis pathway to provide reducing cofactors and a carbon source for synthesis of other metabolites such as 2,3-butanediol, succinic acid, lactic acid, acetic acid, acetoin and ethanol. However, it can also go through the glucose oxidation pathway, there it will be oxidized by pyroloquinoline quinone (PQQ) dependent glucose dehydrogenase and gluconate dehydrogenase located at the outer membrane of the cells [3]. This explains why 2-ketogluconic acid can only be detected at an earlier fermentation phase and then disappeared, because the oxidized product, 2-ketogluconic acid, though accumulated in the medium, can be transferred into the cytoplasm, where it converted back to gluconic acid and phosphorylated to 6-phospho-gluconate which entered back into the metabolic network for production of 2,3-butanediol. As expected, the elimination of budA almost abolished the production of 2,3-butanediol, though a lower level of 2,3-butanediol was still detected, which suggests there may exist an alternative synthesis pathway or isoenzymes for converting alpha-acetolactate to acetoin. Nevertheless, most carbon flux was successfully channeled into the glucose oxidation pathway upon deletion of budA, and the mutant reduced the excretion of 2,3-butanediol greatly.
Production of 2-ketogluconic acid by K. pneumoniae CGMCC 1.6366 and ΔbudA in bioreactors is pH dependent
It was found that the ending pH in cultures of the wild type of K. pneumoniae and ΔbudA was different; this brought up an interesting question that, whether the increased production of 2-ketogluconic acid was favored by the low pH or the low pH was just caused by the 2-ketogluconic acid formed by mutant cells. The experiments set to precisely maintaining the broth at different pH during fermentation were performed using both wild type K. pneumoniae and ΔbudA. In 5L bioreactors, the medium pH was maintained differently at pH 4.7, pH 6.0 or pH 7.0. The growth experiment results summarizing the production of the main metabolites at different pHs were demonstrated in Figs. 5, 6 and 7, respectively.
Batch culture of K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudA in bioreactor, the culture pH was stable at 4.7. a Biomass, glucose consumption and 2-ketogluconic acid formation by K. pneumoniae CGMCC 1.6366; b organic acid and 2,3-butanediol formation by K. pneumoniae CGMCC 1.6366; c Biomass, glucose consumption and 2-ketogluconic acid formation by K. pneumoniae ΔbudA; d organic acid formation by K. pneumoniae ΔbudA
Batch culture of K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudA in the bioreactor, the culture pH was stable at 6; a Biomass, glucose consumption and 2-ketogluconic acid formation by K. pneumoniae CGMCC 1.6366; b organic acid and 2,3-butanediol formation by K. pneumoniae CGMCC 1.6366; c Biomass, glucose consumption and 2-ketogluconic acid formation by K. pneumoniae ΔbudA; d organic acid formation by K. pneumoniae ΔbudA
Batch culture of K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudA in the bioreactor, the culture pH was stable at 7; a Biomass, glucose consumption and 2-ketogluconic acid formation by K. pneumoniae CGMCC 1.6366; b organic acid and 2,3-butanediol formation by K. pneumoniae CGMCC 1.6366; c Biomass, glucose consumption and 2-ketogluconic acid formation by K. pneumoniae ΔbudA; d organic acid formation by K. pneumoniae ΔbudA
It is shown that, at pH 4.7, both the wild strain and the mutant K. pneumoniae grew very slowly, the final cell density of the wild strain was only 2.2, and for budA deletion mutant, it was only 0.6. Nevertheless, a surprisingly high titer of 2-ketogluconic acid was produced from wild type cells, and the conversion ratio of glucose to 2-ketogluconic acid was as high as that of 0.86 mol/mol. The experiments also showed that the glucose was exhausted at 26 h, and only 1.3 g/L 2,3-butanediol was synthesized, and succinic acid was never detected throughout the fermentation. For acetic acid, its level slightly decreased during fermentation, and the concentration of lactic acid in the medium kept decreasing and finally this chemical disappeared from the broth at 11 h. For K. pneumoniae ΔbudA, 30.2 g/L 2-ketogluconic acid was produced after consuming 30.7 g/L glucose at 56 h culture, and the conversion ratio of glucose to 2-ketogluconic acid was calculated to be 0.91 mol/mol. The concentration of succinic acid and acetic acid were only 0.7 and 0.3 g/L, respectively, though slightly higher than observed in the culture of the wild strain. Similarly, the lactic acid also disappeared after 11 h of fermentation.
When the medium pH was controlled at 6.0, both the cells of the wild type and the mutant seemed to be much healthier and grew much faster than in the medium at pH 4.7. Glucose was exhausted at 11 and 17 h with the wild type and mutant strain, respectively. The final OD600 of the wild type cells reached 8.9 and produced no 2-ketogluconic acid at the end, although 1.9 g/L of this chemical was accumulated at 5 h. In contrast, 2,3-butanediol’s accumulative titer reached 14.7 g/L, besides, succinic acid was gradually produced, its final concentration reached 1.6 g/L. At pH 6.0, K. pneumoniae ΔbudA execrated a very low level of 2-ketogluconic acid (0.2 g/L) although it’s OD600 reached 12.6. The accumulative titer of succinic acid, lactic acid and acetic acid were 0.7, 6.0 and 2.7 g/L, respectively.
When culturing in the medium at pH 7.0, K. pneumoniae CGMCC 1.6366 and K. pneumoniae ΔbudA grew even better than at pH 6.0. The carbon source of glucose was consumed at 9 and 15 h, respectively, for the wild strain and the mutant, their final cell density of OD600 reached 9.3 and 14.2. Almost no 2-ketogluconic acid was produced for both strains. The 2,3-Butanediol was still the main fermenting product from the wild type cells, its final level was 12.0 g/L. In contrast, the concentration of succinic acid, lactic acid and acetic acid were gradually increased, their accumulative levels were 2.8, 3.8 and 5.9 g/L, respectively. At pH 7.0, K. pneumoniae ΔbudA still produced some organic acids, the ending concentrations of succinic acid, lactic acid and acetic acid, were 3.1, 6.2 and 9.2 g/L, respectively.
Fermentation studies clearly showed that the culturing pH played a major role in directing the glucose oxidation pathway. For the wild strain, at lower pH the main fermentative product was switched from 2,3-butanediol to 2-ketogluconic acid. As for the ΔbudA strain, since the 2,3-butanediol synthesis pathway was impaired, when growing in a medium of higher pH, its fermentative products were some organic acids, such as succinic acid, lactic acid and acetic acid, but when cultured at pH 4.7, it synthesized almost nothing else other than 2-ketogluconic acid. These results reproduced the fermentation results done in flasks and lead to the speculation that the glucose oxidative pathway is at a certain level affected by medium pH. The difference in metabolic pattern caused by budA deletion probably can be explained by the fact that the mutant strain produced more organic acids which lowered the medium pH to a threshold point where the cells switch the intermediates of normal organic acids to 2-ketogluconic acid.
Therefore, as shown in Fig. 5, at pH 4.7, the biological synthesis pathway of 2,3-butanediol, succinic acid, lactic acid and acetic acid were all inhibited. And, as shown in Figs. 6 and 7, the effect of pH on 2,3-butanediol synthesis and succinic acid, lactic acid and acetic acid synthesis was different, pH 6.0 favored the production of 2,3-butanediol; increasing the medium pH incurred the increased production of succinic acid, lactic acid and acetic acid. The K. pneumoniae CGMCC 1.6366 was also tested to be growing in the medium of pH 8.0, and it was found that only 0.3 g/L 2,3-butanediol was produced, the carbon resource was mostly converted to succinic acid, lactic acid or acetic acid. All these data combined indicated that the metabolic flux between glucose oxidation pathway and glycolysis pathway was pH dependent.
Unlike the Embden–Meyerhof pathway, the enzymes in the glucose strictly oxidative pathway are located on the outer surface cytoplasmic membrane. Glucose dehydrogenase is a PQQ dependent dehydrogenase, and gluconate dehydrogenase uses riboflavin as the cofactor. The activity of the glucose dehydrogenase in K. pneumoniae was tightly related to both the level of the glucose dehydrogenase apo-enzyme synthesis and the cofactor PQQ [10]. As Hommes has pointed out, the medium pH had a profound influence on the activity and synthesis of the glucose dehydrogenase and the gluconate dehydrogenase in K. pneumoniae [11]. The optimal pH for glucose dehydrogenase and gluconate dehydrogenase were all between pH 5 and pH 6.
In a natural environment, the production of 2-ketogluconic acid is correlative to the solubilized phosphate in soil and this phenomenon is important for supporting plant growth [12]. The production of 2-ketogluconic acid by K. pneumoniae NCTC 418 was stimulated in a phosphate and potassium limited environment, and the synthesis of glucose dehydrogenase is possibly regulated by factors that may impose a nutritional stress upon the cells [9]. When K. pneumoniae NCTC 418 was cultured at pH ranging from pH 5.5 to pH 6.0, more than 80 % of the glucose consumed was converted into gluconic acid and 2-ketogluconic acid under potassium- or phosphate-limited condition [11, 14].
It has been reported that the industrial strain pseudomonas fluorescens K1005 can yield 178.45 g/L 2-ketogluconic acid in 72 h [16], another strain, Pseudomonas aeruginosa IFO 3448 can convert 110 g of dried cassava to 72 g of 2-ketogluconic acid in a repeated fed batch at a maximum production rate of 0.55 g/L/h [5]. On the studies of production of 2-ketogluconate by K. pneumoniae, K. pneumoniae NCTC 418 is the only strain to be investigated and reported, and this strain also produces gluconic acid besides 2-ketogluconic acid. However, K. pneumoniae CGMCC 1.6366 and its budA deletion mutant produce only 2-ketogluconic acid, no gluconate was detected in the broth. Our recent studies showed that a final cumulative titer of 150 g/L 2-ketogluconic acid can be achieved in 24 h, and the conversion ratio from glucose to 2-ketogluconic acid was as high as 0.9 mol/mol. Therefore, K. pneumoniae CGMCC 1.6366 could be a novel cell factory for the production of 2-ketogluconic acid in the future.
Conclusions
The 2-ketogluconic acid, a valuable chemical, rarely produced by a common microorganism, was identified in the broth of K. pneumoniae CGMCC 1.6366 and its ΔbudA mutant. The production of 2-ketogluconic acid in K. pneumtoniae CGMCC 1.6366 was found to be pH dependent. When cultured at pH 4.7, almost all the glucose in the medium was converted to 2-ketogluconic acid, the synthesis of 2,3-butanediol and other organic acid were all limited. Owing to the advantages of high productivity, a higher conversion ratio and the ability of using a low cost carbon source, K. pneumoniae CGMCC 1.6366 and its derivatives represent a group of novel workhorses for microbial production of 2-ketogluconic acid.
References
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1, 3-propanediol. Appl Microbiol Biotechnol 52(3):289–297
Bisht SS, Mishra R (2011) Antioxidants and their characterization. J Pharm Res 4(8):2744–2746
Bouvet OMM, Lenormand P, Grimont PAD (1989) Taxonomic diversity of the d-glucose oxidation pathway in the Enterobacteriaceae. Int J Syst Bacteriol 39(1):61–67
Brisse S, Grimont F, Grimont P (2006) The genus Klebsiella. Prokaryotes 6:159–196
Chia M, Van Nguyen TB, Choi WJ (2008) DO-stat fed-batch production of 2-keto-d-gluconic acid from cassava using immobilized Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78(5):759–765
Crawford TC, Andrews GC, Faubl H, Chmurny GN (1980) The structure of biologically important carbohydrates. A carbon-13 nuclear magnetic resonance study of tautomeric equilibriums in several hexulosonic acids and related compounds. J Am Chem Soc 102(7):2220–2225
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci USA 100(4):1541
Hao J, Lin R, Zheng Z, Liu H, Liu D (2008) Isolation and characterization of microorganisms able to produce 1, 3-propanediol under aerobic conditions. World J Microbiol Biotechnol 24(9):1731–1740
Hommes R, Hell B, Postma P, Neijssel O, Tempest D (1985) The functional significance of glucose dehydrogenase in Klebsiella aerogenes. Arch Microbiol 143(2):163–168
Hommes R, Herman P, Postma P, Tempest D, Neijssel O (1989) The separate roles of PQQ and apo-enzyme syntheses in the regulation of glucose dehydrogenase activity in Klebsiella pneumoniae NCTC 418. Arch Microbiol 151(3):257–260
Hommes R, Postma P, Tempest D, Neijssel O (1989) The influence of the culture pH value on the direct glucose oxidative pathway in Klebsiella pneumoniae NCTC 418. Arch Microbiol 151(3):261–267
Hwangbo H, Park RD, Kim YW, Rim YS, Park KH, Kim TH, Suh JS, Kim KY (2003) 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol 47(2):87–92
Ma C, Wang A, Qin J, Li L, Ai X, Jiang T, Tang H, Xu P (2009) Enhanced 2, 3-butanediol production by Klebsiella pneumoniae SDM. Appl Microbiol Biotechnol 82(1):49–57
Neijssel O, Tempest D (1975) Production of gluconic acid and 2-ketogluconic acid by Klebsiella aerogenes NCTC 418. Arch Microbiol 105(1):183–185
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11(4):589–603
Sun WJ, Liu CF, Yu L, Cui F, Zhou Q, Yu S, Sun L (2012) A novel bacteriophage KSL-1 of 2-keto-gluconic acid producer pseudomonas fluorescens K1005: isolation, characterization and its remedial action. BMC Microbiol 12(1):127
Wei D, Wang M, Shi J, Hao J (2012) Red recombinase assisted gene replacement in Klebsiella pneumoniae. J Ind Microbiol Biotechnol 39:1219–1226
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20906076 and 31270122); the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry; Serbian—Chinese Science & Technology Cooperation (1-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, D., Xu, J., Sun, J. et al. 2-Ketogluconic acid production by Klebsiella pneumoniae CGMCC 1.6366. J Ind Microbiol Biotechnol 40, 561–570 (2013). https://doi.org/10.1007/s10295-013-1261-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1261-y