Abstract
Oleaginous microorganisms are receiving significant attention worldwide for their utility in biodiesel production and the potentiality to produce some specialty-type lipids. There is an increasing interest in isolation/adaption of robust microbe strains and design of innovative fermentation processes to make microbial lipid production a more efficient and economically feasible bio-process. Currently, the genus Rhodosporidium has been considered an important candidate, for the reason that several strains belonging to this genus have shown excellent capabilities of lipid accumulation, broad adaptabilities to various substrates, and co-production of some carotenoids. This paper reviews the current trends in the exploitation of Rhodosporidium species for microbial lipid production, including the utilization of various (single or mixed, pure or waste-derived) substrates, progress of genetic modification and metabolic engineering, innovations in fermentation mode, lipid characterizations and their potential applications. Finally, the constraints and perspectives of cultivating Rhodosporidium species for lipid production are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial lipid, also known as single cell oil, can be synthesized and accumulated intracellularly in certain oleaginous microorganisms. Microbial lipid production has several advantages over planting oil crops such as short life cycle, less need of land use, and adaptabilities to various renewable resources. Moreover, microbial lipid shows similarities in the fatty acid compositions to that of vegetable oils, and thus is considered an alternative non-food oil feedstock for biodiesel preparation. Besides, some microorganisms can produce specialty fats, which can be exploited for certain value-added applications (Papanikolaou and Aggelis 2010).
Depending on the species, microorganisms have diverse nutritional requirements, growth and lipid synthesis kinetics, lipid compositions and properties (Xu et al. 2013). In order to make microbial lipid production a more efficient and economically feasible process, there is an increasing interest in isolation/adaption of robust microbe strains. In fact, oleaginous microorganisms (those can accumulate lipid up to 20% of dry cellular weight) can be found in the genera of bacteria, yeast, fungi, and algae. Bacteria have the advantage of short doubling time, while microalgae have the attractive capability of utilizing CO2 instead of organic carbon substrate. The most widely studied candidates, however, belong to yeast or fungi, including Candida curvata, Crytococcus curvatus, Lipomyces starkeyi, Yarrowia lipolytica (Y. lipolytica), etc., largely due to their fast growth rate, high lipid content, and availability of sophisticated fermentation technologies (Xu et al. 2013). With well characterized lipid metabolism and accessible genetic tools, it was of particular interests to develop Y. lipolytica as a platform yeast, on which metabolic engineering was successfully applied to maximize lipid synthesis ability (Liang and Jiang 2013).
Recently, with recognition of the great potential shown in the wild types, genus Rhodosporidium has received an increasing interest. A number of strains have been isolated, including but not limit to Rhodosporidium toruloides (R. toruloides), Rhodosporiudm diobovatum (R. diobovatum), Rhodosporium kratochvilovae (R. kratochvilovae), Rhodosporidium fluviale (R. fluviale), which could reach a lipid content of 50%, some even up to 70% (Xu et al. 2012; Munch et al. 2015; Patel et al. 2015a). Moreover, they show native adaptability to a wide variety of carbon substrates, good tolerance to inhibitory compounds found in unrefined substrate resources, and have the potential of producing some value-added products like carotenoids. These features make Rhodosporidium an appealing research candidate for lipid and biofuel production. To date, intensive work has been done in this genus, which paved the way for developing a more economically feasible lipid production process. The aim of this article is thus to summarize and interpret the current trends in employing genus Rhodosporidium for lipid production, including the utilization of various (single or mixed, pure or waste-derived) substrates, progress in genetic modification and metabolic engineering, innovations in fermentation mode, lipid characterizations and their potential applications. Finally, the constraints and perspectives of cultivating Rhodosporidium species for lipid production are discussed.
Utilization of various substrates for lipid production
Exploitation of different carbon substrates
Since substrate cost and availability may change from time to time, the capacity of utilizing multiple feedstocks is crucial for process viability. Various carbon sources, such as glucose, glycerol, biomass-derived sugars, fatty acids, etc., can be converted into lipids via different yet integrated pathways (Fig. 1). Among these substrates, glucose is a favored carbon source. Li et al. (2007) reported the biomass and lipid titer of 106.5 and 71.9 g/L at the end of 134 h fermentation, and overall glucose to lipid conversion yield was up to 0.23 g/g in R. toruloides. In fact, various species including R. babjevae, R. fluviale, R. kratochvilovae, R. paludigenum, R. Paludigenum, R. sphaerocarpum, and R. toruloides, grow well on glucose and the intracellular lipid content can reach up to 50–60% of the cell dry weight or even higher (Garay et al. 2016). However, in order to improve the economic sustainability of lipid production, more efforts are needed to explore alternative carbon sources.
Recent progress on investigating the performance of genus Rhdosporidium for converting various carbon sources are summarized in Table 1. Among these substrates, glycerol was intensively investigated. Since biodiesel preparation generates a significant amount of glycerol, its reuse in microbial lipid production is considered beneficial for both processes. It was proved that Rhodosporidium can grow well on glycerol and reach comparable biomass and lipid titer as that of growing on glucose. The impurity composition existed in crude glycerol is dependent on the biodiesel preparation method. Within the general concentration ranges, methanol may cause inhibitive effect, while others (soap, glyceride, inorganic salt, etc.) either showed no inhibitive effect or even positive effect on lipid production. Some Rhodosporidium species could perform well in crude glycerol contained media, and even reach higher biomass and lipid titer than growing on pure glycerol (Xu et al. 2012).
Utilization of different saccharides and energy crop-derived sugars has also been investigated. Rhodosporidium species were able to utilize monosaccharide (glucose, fructose, xylose, arabinose), disaccharide (sucrose), and polysaccharide (starch, inulin) for lipid overproduction. The natural capacity of growing on different carbon substrates demonstrates their great application potential.
Some organic acids can be good carbon substrate as well. When cultivating R. toruloides AS 2.1389 on acetic acid, 4.35 g/L biomass with 48.2% lipid was obtained in flask culture (Huang et al. 2016). This strain could even grow on organic acid-containing wastewater (Ling et al. 2013, 2014). Since short chain organic acids, also known as volatile fatty acids, can be derived from food waste, sludge, and some acidogenic bio-processes, the potential of converting such carbon substrates into lipids offers the possibility of combining wastewater treatment with biofuel production.
In addition, a remarkable plethora of hydrophobic carbon sources have been used as substrates for ex novo lipid accumulation. The incorporated fatty acids are transformed into “new” fatty acid profiles and accumulated as storage lipids (this process is referred to as the “bio-modification”, involving enzyme-catalyzed desaturation or elongation reactions). Differed from the above described de novo lipid accumulation, that usually generate species-dependent fatty acid compositions and is mainly performed for preparation of biodiesel feedstock, ex novo lipid synthesis are principally used to improve and upgrade fatty materials for generating specialty “tailor-made” lipids of high-added value, like substitutes of cocoa butter or other exotic fats (Davies and Holdsworth 1992; Papanikolaou and Aggelis 2011a, b). For example, Gierhart (1984b) reported the utilization of pure stearic acid to produce lipid with similar total saturated fatty acid content to that of cocoa butter. It was recognized that microorganisms possessed active desaturase systems (i.e., Δ9 and Δ12 dehydrogenases). Even if the initial fatty substrates was globally of fatty materials with sugar (or alternative carbon sources) may result in significant accumulation saturated, the cellular lipids contained unsaturated fatty acids in significant quantities (Gierhart 1984b). In some investigations, utilization of mixtures of hydrophilic substrates with various fatty materials has been employed. Sugars were mainly used for growth needs while the fatty materials served to enhance the lipid accumulation. The presence of fatty materials does not completely cease the process of de novo fatty acid synthesis, thus, a proper co-utilization of lipids with similar compositions of cocoa butter (Gierhart 1984a).
Utilization of mixtures of carbon sources
In addition to efforts on exploiting appropriate single carbon substrates, utilization of mixtures of carbon sources has also drawn a lot of attention. A major motivation is for exploiting lignocellulosic biomass in biofuel production, which feedstock naturally contain a mixture of different carbon sources. Further, synergistic effects of assimilating mixed carbon sources have been observed in some cases. It is believed that exploring the substrate assimilation behavior of Rhodosporidium species can deepen the understanding of their lipid metabolism, and supports a more flexible lipid production process. The performance of Rhodosporidium species growing on substrate mixtures is summarized in Table 2. Compared to data obtained from utilizing single carbon source, these results show comparable lipid titers. However, when looking into the details of kinetics of substrate assimilation, cell growth and lipid synthesis, varying behaviors can be observed for different Rhodosporidium species growing on different mixture of carbon substrates. Some representative examples are discussed as follows.
The performance of Rhodosporidium species grown on a combination of glucose and glycerol was investigated (Bommareddy et al. 2015, 2017). Noticeably, R. toruloides DSMZ 4444 showed a catabolite repression. Diauxic growth was observed: glycerol was consumed only after glucose consumption. Interestingly, despite of the catabolite repression, the addition of glycerol enhanced the lipid yield on glucose and the specific lipid productivities. The mixed substrate culture gained dual advantages of efficient cell mass production (normally maximized with glucose) and superior lipid content (normally obtained with glycerol). Such phenomena demonstrate a benefit of using carbon substrates mixture and might be applied in other strains according to their specific metabolisms.
Co-utilization of C6 and C5 sugars was studied for biological conversion of lignocellulosic biomass and other biomass-derived feedstock. It was observed that behaviors of consuming different sugar compositions are strain dependent. For example, R. toruloides CCT 0783 could co-utilize glucose and fructose almost at the same rate (Matsakas et al. 2015). R. kratochvilovae HIMPA1 was found to consume glucose (29.45 g/L), fructose (17.98 g/L), xylose (3.87 g/L) simultaneously (Patel et al. 2015b). Many other strains, however, show sequential assimilation. When R. toruloides As. 1389 was growing on a mixture of glucose, xylose and arabinose, glucose was first consumed followed by xylose. Arabinose was not utilized (Zhao et al. 2012). To improve the efficiency of utilizing mixtures of sugars, a potential strategy is to develop engineered strain to strengthen the co-utilization characteristics, provided that molecular methodology is available. An alternative way is to modify biomass pre-treatment and saccharification process. For instance, Morikawa et al. (2014) reported the co-production of ethanol and lipid from wheat straw. Wheat straw was pretreated by dilute acid to hydrolyze most part of hemicelluloses (mainly xylan) to obtain pentose (mainly xylose), which could be utilized by R. toruloides for lipid synthesis; While the pretreated cellulosic solid was then used for ethanol production by simultaneous saccharification and fermentation. In this way, they avoided the sequential utilization effect in R. toruloides and realized whole utilization of ligocellulosic biomass.
Noticeably, another important issue coming along with utilization of biomass-derived carbon source is the tolerance of yeasts to side-products generated from biomass processing. Since the carbohydrates in lignocelluloses are usually refractory to hydrolysis, pretreatment are required to make those sugars accessible to microorganisms (Harde et al. 2016). However, existing thermochemical pretreatments lead to byproducts such as furfural, hydroxylmethyl furfural, weak acids, neutral and acidic phenolics, which can be inhibitory to microorganisms. The toxicity was found to be dependent on concentrations, and synergistic effects of these compounds could dramatically decrease the critical concentration of a single inhibitor, resulting in significant inhibitions (Parreira et al. 2015; Zhao et al. 2012). For this reason, in order to efficiently utilize carbon substrates from lignocellulosic biomass, it is imperative to apply proper detoxification and to develop tolerant strains.
Exploitation of unconventional nitrogen source
In addition to substituting glucose with affordable carbon sources, researchers also explored various nitrogen sources for lipid production. Table 3 summarizes some representative examples of unconventional nitrogen sources, which can be derived from agricultural, industrial and bio-process residues. They usually contain organic nitrogen components in the form of amino acids, and some also contains carbohydrates and minerals, which can be assimilated by yeast cells. Although lipid overproduction in Rhodosporidium species is usually achieved under a nitrogen limit condition, a considerable amount of nitrogen is still required to support yeast growth and maintenance. Therefore, depending on the nutrient compositions, a nitrogen-sufficient feedstock can either be directly used in combination with a carbon-rich feedstock for lipid production, or used in the initial fermentation stage for quickly growing yeast cells. According to the reported data, such nitrogen sources can substitute traditional nitrogen sources such as yeast extract, peptone and ammonium sulfate, achieving comparable and even higher biomass and lipid quantity. One more issue needs to be addressed is that, similar to the problem mentioned in biomass-derived carbon substrates, for biomass-derived nitrogen substrates a proper pretreatment may also be required to release nitrogen components accessible for the microbes. It is required to take into account all the involved feedstock, pretreatment together with lipid production, and make the techno-economic evaluation.
Progress in strain engineering
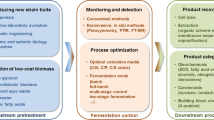
The research interest on isolating and adapting robust Rhodosporidium strains comes along with the target to improve lipid yield and to efficiently utilize those sustainable carbon/nitrogen resources, which is highly concerned for making commercialization of microbial lipid production feasible. Increasing efforts have been made to isolate superior strains, understand the molecular basis of oleaginicity, develop genetic tools and construct engineered strains. The overview of studies on strain engineering is illustrated in Fig. 2.
The adaptability of Rhodospordium species has been intensively studied, particularly for the purpose of exploiting biomass-derived carbon sources. Culture media derived from such feedstock is consisted of complex substrate combinations and may contain inhibitors, thus organisms that can utilize both C5 and C6 sugars and tolerate inhibitive components are highly desired. In addition to screening and isolating excellent strains capable of converting different carbon sources into lipids (as is summarized in Tables 1, 2), other efforts also involve strain mutagenesis and adaption. For instance, Qi et al. (2014) applied atmospheric room temperature plasma mutagenesis to obtain R. toruloides mutants, and isolated candidates that had strong tolerance to the inhibitory compounds and could grow in lignocellulosic hydrolyzate without detoxification.
Gene sequencing, RNA-sequencing assisted annotation, transcriptomic analysis and proteomic analysis provided integrated information, which helps to understand the molecular basis of Rhodosporidium for regulation of central metabolism and lipid metabolism (Zhu et al. 2012, 2015; Kumar et al. 2012; Zhang et al. 2016b; Shi et al. 2013). Functions of some genes and pathways that committed to oleaginicity, such as acetyl-CoA synthetase isoenzymes, mitochondrial NAD(+)-dependent isocitrate dehydrogenase, fatty acid desaturase, diacylglycerol acyltransferase, etc., were characterized as well (Yang et al. 2012; Cui et al. 2016; Chen et al. 2014; Liu et al. 2016). It was found that Rhodosporidium shares a number of features, for example, the nitrogen metabolism-induced lipogenesis with other well-studied yeasts, like Y. lipolytica. But different mechanisms were also noticed, for example, the oxidative pentose phosphate pathway is assumed to be the sole supplier of NADPH for lipid synthesis in Y. lipolytica (Wasylenko et al. 2015); While in R. toruloides, an alternative NADPH supply from cytosolic NADH-dependent isocitrate dehydrogenase was recognized (Zhu et al. 2012). These studies opened doors to understand the lipid metabolism in Rhodosporidium species. Furthermore, transcriptomic and proteomic analysis enriches the knowledge of molecular basis of those adapted strains. For example, Qi et al. (2017) performed mutation and adaption to obtain strains with expected characteristics. For those isolated candidates, the regulation of gene expressions could be due to mutagenesis or induced by stressful environment conditions. Through transcriptomic and proteomic analysis, genes involved in key metabolic processes can be identified and targeted for further strain engineering.
Genetic tools have been increasingly developed, including transformation and constitutive promoter set for gene expression, to establish genetically tractable system. Transformation of Rhodosporidium was rarely report in the past and was considered recalcitrant. There was a report that used PEG-mediated transformation of protoplasts in R. torulodes (Tully and Gilbert 1985), which method was limited by its low efficiency, instable chromosomal integration and auxotrophic selection (Liu et al. 2013). Based on recent research, most of the successful manipulations, however, involve the application of Agrobacterium tumefaciens-mediated transformation (ATMT). By applying ATMT, Abbott et al. (2013) transformed a vector carrying an exogenous gene (URA5) into R. kratochvilovae; Lin X et al. (2014a, b) integrated multiple genes in R. toruloides and obtained transformants with multiple antibiotic-resistant phenotype; Liu et al. (2013) employed the combination of strong promoters (RtGDP1) and the codon-optimized antibiotic resistance gene (hpt-3) to transform R. toruloides ATCC by ATMT with dominant selection. Moreover, Wang et al. (2016) constructed expression cassette and evaluated the functional evaluation of five constitutive promoters from R. toruloides. Liu et al. (2015) reported an efficient D-amino acid-inducible gene expression system with the intron 1-containing DAO1 (D-amino acid oxidase gene) promoter coupled with a DAO1 null mutant.
These available genetic tools enable functional integration and expression of exogenous genes, or gene knockout, in Rhodosporidium strains. For example, Lee et al. (2016) used ATMT to transport the gene encoding for the membrane transporter, pleiotropic drug resistance (Pdr10) from Saccharomyces cerevisiae into R. toruloides. When this modified strain was cultured in a two-phase system, it facilitated the export of carotenoids in situ without the need for any extraction steps. Targeted gene depletion was also reported in Rhodosporidium species, although such reports were comparatively rare than that of gene expression. For instance, Koh et al. (2014) reported the targeted gene deletion in R. toruloides ATCC via ATMT and the generation of KU70 null mutant. The KU70-deficient mutant was further found to be effective in improving gene deletion frequency. Liu et al. (2016) characterized two genes encoding two acetyl-CoA synthetase isoenzymes in R. diobovatum. Noticeably, they used CRISPR-Cas9 system to delete two gene fragments (ACS1 and ACS2) in haploid R. diobovatum, and applied plasmid transformation with a Bio-Rad Gene Pulser, which showed application of different genetic tools comparing to most other reported literatures.
Furthermore, with the increased knowledge in Rhodosporidium’s oleaginicity and the developed genetic tools, metabolic engineering can be applied to these naturally productive strains. The successful examples are summarized in Fig. 2. Zhang et al. (2016b) reported one of the first examples of metabolic engineering in R. toruloides. They overexpressed the native acetyl-CoA carboxylase (ACC1) and diacylaglycerol acyltransferase (DGA1) genes using ATMT. These two genes encoded enzymes that catalyze the first and last steps in triacylglycerol biosynthesis. This strategy had been applied in diverse microorganisms, most notably in Y. lipolytica, and was commented as the “push and pull” strategy (Tai and Stephanopoulos 2013). By applying it in R. toruloides, they successfully increased lipid titer and yield significantly due to more carbon being directed toward lipid synthesis. They further explored a number of different metabolic engineering strategies (Zhang et al. 2016a), and found that overexpression of malic enzyme and stearoyl-CoA desaturase could significantly increase lipid production. Another successful strategy was to overexpress stearoyl-CoA desaturase in the previously engineered strain with overexpressed ACC1 and DGA1. In summary, strategies for strain engineering mainly include increasing carbon flux toward lipid anabolism, strengthening the supply of NADPH, and optimizing the function of desaturases in the fatty acid synthesis pathways. With deeper understating of features of genus Rhodosporidium in the central metabolism and lipid metabolism, it will be possible to identify more key steps involved in lipid synthesis, apply metabolic engineering and develop global regulations. It can be expected that, with advanced genetic tools, R. toruloides might be developed as the next platform microorganism for producing lipid and lipid-based chemicals.
Progress in fermentation technologies
Industrial implementation of microbial lipid production is strongly dependent on the raw material cost, unitary cost, as well as fermenter and process design. For the stage of fermentation, improvements on substrate utilization efficiency, lipid productivities and lipid titer, as well as energy-saving techniques are highly expected (Koutinas et al. 2014). Research efforts on fermentation technologies are largely driven by the demands of utilizing various feedstocks (as summarized in Tables 1, 2, 3), and obtaining high lipid productivity. Researchers are under the way to solve problems coming along with utilization of various feedstocks, such as substrate-associated growth inhibition, inhibitive compositions, low strength substrates, etc. For instance, Fei et al. (2016) reported an automated online sugar control feeding mode using an automated cell-free sampling system coupled with an online sugar analyzer, which was designed for feeding glucose and xylose-containing corn stover hydrolysates. This strategy reduced the substrate-associated growth inhibition by maintaining a desired glucose at 10 g/L, and resulted in a promoted lipid yield (0.29 g/g) and productivity (0.4 g/L/h). Xu et al. (2017) proposed a glycerol-based two-stage process to separately meet the nutritional demand of both the cell proliferation phase and lipid accumulation phase, consequently achieved a promoted lipid yield and productivity. Signori et al. (2016) developed a feeding strategy for crude glycerol utilization, which was designed to prevent the inhibitory effect in the lag phase, and supported a progressive cells adaptation to increasing inhibitors concentrations, leading to an improved bioconversion of glycerol into lipids. Huang et al. (2016) proposed a repeated batch culture mode for lipid production from acetic acid, which strategy could avoid substrate inhibition and make utilization of low-content acetic acid feasible. These researches provide some hints on way to utilize different kinds of unrefined or waste-derived resources.
On the other hand, nowadays researchers have been increasingly paying attention to the microbial ecosystem and their potential application in biotechnologies. Some co-culture systems were investigated to take advantage of metabolic abilities of a different microbe to support Rhodosporidium for lipid production. For example, Ling et al. (2014) reported the co-culture of R. toruloides with microalgae, Chlorella pyreoidosa. Microalgae are effective in nitrogen and phosphorus removal, uptake of CO2, and O2 production, while yeasts are effective in organic matter removal and CO2 production. Based on this symbiotic relationship, mixture culture was conducted in distillery and municipal wastewater, and was able to remove COD, total nitrogen and total phosphorus while producing lipids. Another example was to combine the starch hydrolysis with lipid production in a co-culture system dominated by R. toruloides and an immobilized amylases-producing yeast, Saccharomycopsis fibuligera. Saccharomycopsis fibuligera synthesized and secreted amylases into the medium, which could hydrolyze cassava starch into glucose. Glucose was then converted into lipid by R. toruloides. In a 168 h culture, R. toruloides biomass and lipid content reached 20 g/L and 65%, respectively (Gen et al. 2014). Although very few studies using co-culture have been done for lipid production, and such research to date haven’t been optimized and did not show a significantly superior lipid productivity compared to traditional culture systems, they potentially provide some new possibilities for waste-derived resource utilization in combination with lipid production.
Besides, means of energy cost reduction deserve to be studied as well. Main factors involved in a fermentation system include aeration, agitation, temperature control, sterilization, etc. Major research efforts undertaken involve fermenter design and process optimization. In the case of lipid production by genus Rhodosporidium, an interesting fermentation mode was recently reported to save energy from sterilization. Inspired by the fact that Rhodoporidium sp. strains have been isolated from hyper-saline habitats and therefore could potentially grow on media presenting relatively high salinity. Tchakouteu et al. (2017) investigated the performance of R. toruloides DSM 4444 on pasteurized media supplemented with NaCl. In addition to confirming that supplementing 4.0% w/v NaCl could stimulate lipid accumulation, the combination of NaCl and high glucose concentrations was found to suppress bacterial contamination. The accomplishment of microbial lipid production under nonaseptic conditions can potentially save a significant amount of unitary cost for industrialized lipid production.
Lipid characterizations and potential applications
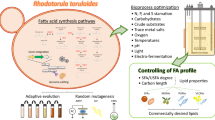
A major driven force for producing microbial lipid is its utilization as a biodiesel feedstock. Intensive research has been done to evaluate the fatty acid compositions in microbial lipids, because characteristics like carbon chain length and degree of unsaturation, can influence the biodiesel property such as cold flow feature, oxidative stability, etc. Fatty acid compositions of lipids obtained from Rhodosporidium species are summarized in Table 4. It can be seen that fatty acid profiles are primarily strain specific. In general, for most strains the major compositions are palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1) and linoleic acid (C18:2). Some strains of R. toruloides, R. diobovatum and R. fluviale can produce small amount of linolenic acid (C18:3) and fatty acids with carbon chain length between C20 and C24, the total content of which is usually below 6%. R. kratochvilovae HIMPA1 was reported to produce about 30% of long chain fatty acid (C20-C27), whose lipid composition is quite unusual comparing to other Rhodosporidium species. Added to strain specific fatty acid synthesis as the major determinant, a variety of factors, such as carbon/nitrogen substrates, media composition, and environmental conditions, can affect fatty acids compositions to different degrees. For example, Patel et al. (2015a) cultivated R. toruloides HIMPA1 on different carbon sources, and found mono-unsaturated fatty acids content was high when grown on glucose and fructose, while on sucrose poly-unsaturated fatty acids content was comparatively high. Nitrogen source could influence fatty acids compositions as well. It was found that lipids produced in the rapeseed meal hydrolysate-containing media included higher linoleic (C18:2) but lower palmitic acid (C16:0) compared to those obtained using yeast extract (Kiran et al. 2013). Polburee et al. (2016) found that cultivation at a low temperature of 25 °C yielded a higher percentage of unsaturated fatty acids, especially linoleic (C18:2) and linolenic acid (C18:3), than the two-stage cultivation with a temperature shift from 30 to 25 °C. Besides, Bommareddy et al. (2015) revealed the influential effects of oxygen supply on lipid compositions.
The second research interest is to generate specialty “tailor-made” lipids, like substitutes of cocoa butter or other exotic fats, which has been considered a process potentially economically viable, specifically if various low- or negative cost raw materials are utilized as substrates (Papanikolaou and Aggelis 2011b). As shown in Table 4, de novo lipid synthesis by genus Rhodosporidium generates lipids with a major percentage of unsaturated fatty acids, whereas cocoa butter is principally composed of saturated fatty acids. An average fatty acid profile is: C16:0 23–30%, C18:1 30–37%, C18:0 32–37%, C18:2 2–4% (Papanikolaou and Aggelis 2011b). Various strategies have been investigated in order to increase the saturated fatty acid content in microbial lipids, including separation of the synthesized cellular lipid (Davies and Holdsworth 1992), utilization of natural or synthetic desaturation inhibitors such as sterculic acid and malvalic acid (Moreton 1985; Moreton and Clode 1988), as well as performing fermentation condition control such as increasing sulfate limitation (Wu et al. 2011) and cultivating yeasts on mixtures of sugars or polyols with fatty materials (Gierhart 1984a).
In addition to the major interest of obtaining neutral lipids, mainly in the form of triacylglycerols for preparation of biodiesel or cocoa butter substitutes, some research is focused on obtaining other fatty acid-derived products. Since oleaginous yeasts hold effective fatty acid synthesis pathways and have the capacity for lipid overproduction, they are considered attractive candidates for fatty acid-derived chemicals (Xu et al. 2016). Although such research was rarely reported in Rhodosporidium species, now with the developed genetic tools, some promising results has been obtained. Fillet et al. (2015) reported the engineering of R. toruloides to produce fatty alcohols by expressing a fatty acyl-CoA reductase from Marinobacter aquaeolei VT8. Cultivation of this strain in a 75 h fed-batch mode resulted in over 8 g/L C16-C18 fatty alcohols. Since Rhodosporidium species are productive in converting a variety of carbon substrates into lipids, they might be exploited as the next platform for fatty acids-derived chemicals, those can be further used to produce valuable oleochemicals.
Besides, compared to other oleaginous microorganisms, Rhodosporidium species hold a unique capability, that is, to produce carotenoids. A handful of species, such as R. toruloides and R. diobovatum, are carotenoid producer, and thus are known as red yeasts (Mannazzu et al. 2015). The yield and composition of carotenoids vary according to strain, medium components and environmental conditions. Lee et al. (2014) reported the metabolomics profiling of three strains of R. toruloides grown on glycerol. These species could produce torularhodin, torulene and β-carotene, whose composition were species dependent. It was recognized that carotenoid production may share the same trigger mechanism with lipid synthesis, that is, lower production of the TCA cycle and amino acid metabolites promoted energy and metabolic flux toward the carotenoid synthesis pathways. This metabolism suggests that carotenoid synthesis can be co-produced with neutral lipids under proper culture conditions. Since carotenoids can be widely used in chemical, pharmaceutical, cosmetic, food and feed industries, they are considered as high-value product. With further understanding of carotenoid synthesis, fermentation and separation technologies, it may potentially become a value-added side-product of lipid production by those red yeasts.
Prospects of employing genus Rhodosporidium for lipid production
Rhodosporidium species are considered as promising candidates for lipid production due to their flexible substrate adaptability, superior lipid content, and potential of production fatty acid-derived chemicals and carotenoids. However, currently microbial lipid production is still not viable when biofuels are considered as final products, mainly due to the high raw material cost and unitary cost involving aeration, agitation, sterilization, etc. (Davies 1988; Koutinas et al. 2014) According to a R. toruloides-based techno-economic evaluation, lipid production is strongly dependent on the feedstock used, fermenter and process design, and value of the final products (Koutinas et al. 2014). In order to realize an economically viable lipid production process, more efforts are required to utilize renewable and affordable raw materials, achieve higher lipid titer and productivities, and decrease the unitary costs.
Those researches conducted for exploitation of various renewable and waste-derived feedstocks are meaningful, which in turn, demonstrate the great potential of Rhodosporidium species in their broad substrate adaptabilities. However, it is known that those raw materials usually require certain unit operations to release carbon or nitrogen substrates that are accessible for microorganisms. In consequence, utilization of mixture of substrates and overcoming inhibitive effects of complex compositions, can constitute two major bottlenecks for efficient utilization of those renewable feedstocks. In addition to the research efforts on developing advanced feedstock processing technologies, other solutions may be sought from investigation of metabolic behaviors for utilizing various substrates, as well as strain isolation, adaption and engineering for strengthening the ability of substrate assimilation and inhibitor tolerance. On the other hand, with increasing understanding in lipid metabolism and more genetic tools available, metabolic engineering of Rhodosporidium species will become possible. In the past, intensive research has been conducted in the model oleaginous yeast, Y. lipolytica, and made it a platform for producing neutral lipids and fatty-acid derived products. In the near future, Rhodospordium may become the next platform. Wild-type strains in this genus already show great potential in utilizing waste-derived raw materials, and hold some specialty in their metabolites, such as co-producing carotenoids as value-added products. The capabilities of Rhdosporidium species for lipid production should be further exploited in the future.
In conclusion, in order to fully exploit the potential of Rhodorsporidium species and make it an appealing platform for lipid production, comprehensive research efforts are required, including process design for efficient utilization of low-cost raw materials, understanding and optimization of substrate assimilations, and metabolic engineering for maximizing lipid synthesis efficiency, and exploitation of value-added lipid products.
References
Abbott E, Ianiri G, Castoria R, Idnurm A (2013) Overcoming recalcitrant transformation and gene manipulation in Pucciniomycotina yeasts. Appl Microbiol Biotechnol 97:283–295
Bommareddy R, Sabra W, Maheshwari G, Zeng A (2015) Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb Cell Fact 14:36
Bommareddy R, Sabra W, Zeng A (2017) Glucose-mediated regulation of glycerol uptake in Rhodosporidium toruloides: Insights through transcriptomic analysis on dual substrate fermentation. Eng life Sci. doi:10.1002/elsc.201600010
Chen Z, Liu P, Liu Y, Tang H, Chen Y, Zhang L (2014) Identification and characterization of a type-2 diacylglycerol acyltransferase (DGAT2) from Rhodosporidium diobovatum. Antonie Van Leeuwenhoek 106:1127–1137
Cui J, He S, Ji X, Lin L, Wei Y, Zhang Q (2016) Identification and characterization of a novel bifunctional Delta(12) Delta(15)-fatty acid desaturase gene from Rhodosporidium kratochvilovae. Biotechnol Lett 38:1155–1164
Davies R (1988) Yeast oil from cheese whey: process development. In: Moreton R (ed) Single cell oil. Longman Scientific & Technical, Harlow, pp 99–145
Davies R, Holdsworth J (1992) Synthesis of lipids in yeasts: biochemistry, physiology and production. Adv Appl Lipid Res 1: 119–159
Fei Q, O’Brien M, Nelson R, Chen X, Lowell A, Dowe N (2016) Enhanced lipid production by Rhodosporidium toruloides using different fed-batch feeding strategies with lignocellulosic hydrolysate as the sole carbon. Biotechnol Biofuels 9:130
Fillet S, Gibert J, Suarez B, Lara A, Ronchel C, Adrio J (2015) Fatty alcohols production by oleaginous yeast. J Ind Microbiol Biotechnol 42:1463–1472
Garay L, Sitepu I, Cajka T, Chandra I, Shi S, Lin T, German J, Fiehn O, Boundy-Mills K (2016) Eighteen new oleaginous yeast species. J Ind Microbiol Biotechnol 43:887–900
Gen Q, Wang Q, Chi Z (2014) Direct conversion of cassava starch into single cell oil by co-cultures of the oleaginous yeast. Renew Energy 62:522–526
Gierhart D (1984a) Multistage process for the preparation of fats and oils. US patent 4485172
Gierhart D (1984b) Preparation of fats and oils. US patent 4485173
Harde S, Wang Z, Horne M, Zhu J, Pan X (2016) Microbial lipid production from SPORL-pretreated Douglas fir by Mortierella isabellina. Fuel 175:64–74
Huang X, Liu J, Lu L, Peng K, Yang G, Liu J (2016) Culture strategies for lipid production using acetic acid as sole carbon source by Rhodosporidium toruloides. Bioresour Technol 206:141–149
Kiran E, Salakkam A, Trzcinski A, Bakir U, Webb C (2012) Enhancing the value of nitrogen from rapeseed meal for microbial oil production. Enzym Microb Technol 50:337–342
Kiran E, Trzcinski A, Webb C (2013) Microbial oil produced from biodiesel by-products could enhance overall production. Bioresour Technol 129:650–654
Koh C, Liu Y, Moehninsi, Du M, Ji L (2014) Molecular characterization of KU70 and KU80 homologues and exploitation of a KU70-deficient mutant for improving gene deletion frequency in Rhodosporidium toruloides. BMC Microbiol 14:50
Koutinas A, Chatzifragkou A, Kopsahelis N, Papanikolaou S, Kookos I (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 116:566–577
Kumar S, Kushwaha H, Bachhawat A, Raghava G, Ganesan K (2012) Genome Sequence of the Oleaginous Red Yeast Rhodosporidium toruloides MTCC 457. Eukaryot Cell 11:1083–1084
Lee J, Chen L, Shi J, Trzcinski A, Chen W (2014) Metabolomic profiling of Rhodosporidium toruloides grown on glycerol for carotenoid production during different growth phases. J Agric Food Chem 62:10203–10209
Lee J, Chen L, Cao B, Chen W (2016) Engineering Rhodosporidium toruloides with a membrane transporter facilitates production and separation of carotenoids and lipids in a bi-phasic culture. Appl Microbiol Biotechnol 100:869–877
Leiva-Candia D, Tsakona S, Kopsahelis N, Garcia I, Papanikolaou S, Dorado M, Koutinas A (2015) Biorefining of by-product streams from sunflower-based biodiesel production plants for integrated synthesis of microbial oil and value-added co-products. Bioresouc Technol 190: 57–65
Li Y, Zhao Z, Bai F (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzym Microb Technol 41:312–317
Liang M, Jiang J (2013) Advancing oleaginous microorganisms to produce lipid via metabolic engineering technology. Prog Lipid Res 52:395–408
Lin X, Wang Y, Zhang S, Zhu Z, Zhou Y, Sun W, Wang X, Zhao Z (2014a) Functional integration of multiple genes into the genome of the oleaginous yeast Rhodosporidium toruloides. FEMS Yeast Res 14:547–555
Lin J, Li S, Sun M, Zhang C, Yang W, Zhang Z, Li X, Li S (2014b) Microbial lipid production by oleaginous yeast in D-xylose solution using a two-stage culture mode. RSC Adv 4:34944–34949
Ling J, Nip S, Shim H (2013) Enhancement of lipid productivity of Rhodosporidium toruloides in distillery wastewater by increasing cell density. Bioresour Technol 146:301–309
Ling J, Nip S, Cheok W, de Toledo R, Shim H (2014) Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol 173:132–139
Liu Y, Koh C, Sun L, Hlaing M, Du M, Peng N, Ji L (2013) Characterization of glyceraldehyde-3-phosphate dehydrogenase gene RtGPD1 and development of genetic transformation method by dominant selection in oleaginous yeast Rhodosporidium toruloides. Appl Microbiol Biotechnol 97:719–729
Liu Y, Koh C, Ngoh S, Ji L (2015) Engineering an efficient and tight d-amino acid-inducible gene expression system in Rhodosporidium Rhodotorula species. Microb Cell Fact 14:170
Liu Y, Zhang M, Wang T, Shi X, Li J, Jia L, Tang H, Zhang L (2016) Two acetyl-CoA synthetase isoenzymes are encoded by distinct genes in marine yeast Rhodosporidium diobovatum. Biotechnol Lett 38:417–423
Luque L, Orr V, Chen S, Westerhof R, Oudenhoven S, Rossum G, Kersten S, Berruti F, Rehmann L (2016) Lipid accumulation from pinewood pyrolysates by Rhodosporidium diobovatum and Chlorella vulgaris for biodiesel production. Bioresour Technol 214:660–669
Mannazzu I, Landolfo S, Silva T, Buzzini P (2015) Red yeasts and carotenoid production: outlining a future for non-conventional yeasts of biotechnological interest. World J Microbiol Biotechnol 31:1665–1673
Matsakas L, Bonturi N, Miranda E, Rova U, Christakopoulos P (2015) High concentrations of dried sorghum stalks as a biomass feedstock for single cell oil production by Rhodosporidium toruloides. Biotechnol Biofuels 8:6
Moreton R (1985) Modification of fatty acid composition of lipid accumulating yeasts with cyclopropene fatty acid desaturase inhibitors. Appl Microbiol Biotechnol 22:41–45
Moreton R, Clode D (1988) Microbial desaturase enzyme inhibitors and their use in a method of producing lipids. US patent 4778630
Morikawa Y, Zhao X, Liu D (2014) Biological co-production of ethanol and biodiesel from wheat straw- a case of dilute acid pretreatment. RSC Adv 4:37878–37888
Munch G, Sestric R, Sparling R, Levin D, Cicek N (2015) Lipid production in the under-characterized oleaginous yeasts Rhodosporidium babjevae and Rhodosporidium diobovatum from biodiesel-derived waste glycerol. Bioresour Technol 185:49–55
Papanikolaou S, Aggelis G (2010) Yarrowia lipolytica: A model microorganism used for the production of tailor-made lipids. Eur J Lipid Sci Technol 112:639–654
Papanikolaou S, Aggelis G (2011a) Lipids of oleaginous yeasts. Part Ι: Biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Papanikolaou S, Aggelis G (2011b) Lipids of oleaginous yeasts. Part ΙΙ: technology and potential applications. Eur J Lipid Sci Technol 113:1052–1073
Parreira T, Freitas C, Reis A, Roseiro J, Silva T (2015) Carbon concentration and oxygen availability affect lipid and carotenoid production by carob pulp syrup. Eng. Life Sci 15:815–823
Patel A, Pravez M, Deeba F, Pruthi V, Singh R, Pruthi P (2014) Boosting accumulation of neutral lipids in Rhodosporidium kratochvilovae HIMPA1 grown on hemp seed. Bioresour Technol 165:214–222
Patel A, Pruthi V, Singh R, Pruthi P (2015a) Synergistic effect of fermentable and non-fermentable carbon sources enhances TAG accumulation in oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour Technol 188:136–144
Patel A, Sindhu D, Arora N, Singh R, Pruthi V, Pruthi P (2015b) Biodiesel production from non-edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour Technol 197:91–98
Polburee P, Yongmanitchai W, Honda K, Ohashi T, Yoshida T, Fujiyama K, Limtong S (2016) Lipid production from biodiesel-derived crude glycerol by Rhodosporidium fluviale DMKU-RK253 using temperature shift with high cell density. Biochem Eng J 112:208–218
Qi F, Kitahara Y, Wang Z, Zhao X, Du W, Liu D (2014) Novel mutant strains of Rhodosporidium toruloides by plasma mutagenesis approach and their tolerance for inhibitors in lignocellulosic hydrolysate. J Chem Technol Biotechnol 89:735–742
Qi F, Zhao X, Kitahara Y, Li T, Ou X, Du W, Liu D, Huang J (2017) Integrative transcriptomic and proteomic analysis of the mutant lignocellulosic hydrolysate-tolerant Rhodosporidium toruloides. Eng. Life Sci. doi:10.1002/elsc.201500143
Shi J, Feng H, Lee J, Chen W (2013) Comparative proteomics profile of lipid-cumulating oleaginous yeast: An iTRAQ-coupled 2-D LC-MS MS analysis. PloS ONE 8:e85532
Signori L, Ami D, Posteri R, Giuzzi A, Mereghetti P, Porro D, Branduardi P (2016) Assessing an effective feeding strategy to optimize crude glycerol utilization as sustainable carbon source for lipid accumulation in oleaginous yeasts. Microb Cell Fact 15:75
Tai M, Stephanopoulos G (2013) Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab Eng 15:1–9
Tchakouteu S, Kalantzi O, Gardeli C, Koutinas A, Aggelis G, Papanikolaou S (2015) Lipid production by yeasts growing on biodiesel-derived crude glycerol strain selection and impact of substrate concentration on the fermentation efficiency. J Appl Microbiol 118:911–927
Tchakouteu S, Kopsahelis N, Chatzifragkou A, Kalantzi O, Stoforos N, Koutinas A, Aggelis G, Papanikolaou S (2017) Rhodosporidium toruloides cultivated in NaCl-enriched glucose-based media: Adaptation dynamics and lipid production. Eng. Life Sci. doi:10.1002/elsc.201500125
Tully M, Gilbert H (1985) Transformation of Rhodosporidium toruloides. Gene 36:235–240
Vieira J, Ienczak J, Rossell C, Pradella J, Franco T (2014) Microbial lipid production- screening with yeasts grown on Brazilian molasses. Biotechnol Lett 36:2433–2442
Wang Z, Fu W, Xu H, Chi Z (2014) Direct conversion of inulin into cell lipid by an inulinase-producing yeast Rhodosporidium toruloides 2F5. Bioresour Technol 161:131–136
Wang Y, Lin X, Zhang S, Sun W, Ma S, Zhao Z (2016) Cloning and evaluation of different constitutive promoters in the oleaginous yeast Rhodosporidium toruloides. Yeast 33:99–106
Wasylenko T, Ahn W, Stephanopoulos G (2015) The oxidative pentose phosphate pathway is the primary source of NADPH for lipid overproduction from glucose in Yarrowia lipolytica. Metab Eng 30:27–39
Wiebe M, Koivuranta K, Renttila M, Ruohonen L (2012) Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol 12:26
Wu S, Zhao X, Shen H, Wang Q, Zhao Z (2011) Microbial lipid production by Rhodosporidium toruloides under sulfate-limited conditions. Bioresour Technol 102:1803–1807
Xu J, Zhao X, Wang W, Du W, Liu D (2012) Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem Eng J 65:30–36
Xu J, Du W, Zhao X, Zhang G, Liu D (2013) Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuels Bioprod Biorefin 7:65–77
Xu P, Qiao K, Ahn W, Stephanopoulos G (2016) Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc Natl Acad Sci 113:10848–10853
Xu J, Zhao X, Du W, Liu D (2017) Bioconversion of glycerol into lipids by Rhodosporidium toruloides in a two-stage process and characterization of lipid properties. Eng. Life Sci. doi:10.1002/elsc.201600062
Yang F, Zhang S, Zhou Y, Zhu Z, Lin X, Zhao Z (2012) Characterization of the mitochondrial NAD(+)-dependent isocitrate dehydrogenase of the oleaginous yeast Rhodosporidium toruloides. Appl Microbiol Biotechnol 94:1095–1105
Yang X, Jin G, Gong Z, Shen H, Bai F, Zhao Z (2015) Recycling microbial lipid production wastes to cultivate oleaginous yeasts. Bioresour Technol 175:91–96
Zhang S, Ito M, Skerker J, Arkin A, Rao C (2016a) Metabolic engineering of the oleaginous yeast Rhodosporidium toruloides IFO0880 for lipid overproduction during high-density fermentation. Appl Microbiol Biotechnol 100:9393–9405
Zhang S, Skerker J, Rutter C, Maurer M, Arkin A, Rao C (2016b) Engineering Rhodosporidium toruloides for increased lipid production. Biotechnol Bioeng 113:1056–1066
Zhang X, Shen H, Yang X, Wang Q, Yu X, Zhao Z (2016c) Microbial lipid production by oleaginous yeasts on Laminaria residue hydrolysates. RSC Adv 6:26752–26756
Zhao X, Peng F, Du W, Liu C, Liu D (2012) Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides. Bioprocess Biosyst Eng 35:993–1004
Zhou W, Wang W, Li Y, Zhang Y (2013) Lipid production by Rhodosporidium toruloides Y2 in bioethanol wastewater and evaluation of biomass energetic yield. Bioresour Technol 127:435–440
Zhu Z, Zhang S, Liu H, Shen H, Lin X, Yang F, Zhou Y, Jin G, Ye M, Zou H, Zhao Z (2012) A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides. Nat Commun 3:1112
Zhu Z, Ding Y, Gong Z, Yang L, Zhang S, Zhang C, Lin X, Shen H, Zou H, Xie Z, Yang F, Zhao X, Liu P, Zhao Z (2015) Dynamics of the Lipid Droplet Proteome of the Oleaginous Yeast Rhodosporidium toruloides. Eukaryot Cell 14:252–264
Acknowledgements
The authors express their gratitude to the support from China Scholarship Council and Zhejiang Provincial Education Department Support Program (Y201533267).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Liu, D. Exploitation of genus Rhodosporidium for microbial lipid production. World J Microbiol Biotechnol 33, 54 (2017). https://doi.org/10.1007/s11274-017-2225-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2225-6