Abstract
Predation is known to play a prominent role in maintaining ecosystem structure and functioning. Despite metals being known to potentially affect predation in aquatic ecosystems, few studies have been conducted, so far, with the aim of evaluating this interplay. In the present study, the effects of four metal salts (copper, cadmium, mercury, and manganese) on the feeding rates and food preference of the dragonfly Tramea cophisa and of the ostracod Chlamydotheca sp. were studied by performing laboratory ecotoxicity tests. Food preference was evaluated by offering four prey species to dragonfly nymphs and three to adult large ostracods. In general, the food preference of both predator species after being exposed to metal salts was not altered, compared with controls, but the feeding rate of T. cophisa decreased in comparison with controls, after exposure to each metal salt, except manganese. Contrastingly, predation rates of Chlamydotheca sp. increased after metal salt exposure. This difference in response can be explained by differences in life-history traits of these two organisms. Both species individuals preferred soft-bodied prey (Oligochaeta, Chironomidae) over water-dwelling crustaceans that are likely to be more difficult to prey upon. Tests evaluating the effects of metals and other chemicals on predation behavior may lead to a better understanding of biotic interactions that can be restricted by chemical stress, improving our understanding of possible food web disruptions underlying chemical stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Prey capture is part of a predator foraging behavior and can influence the meta-community distribution, as well as prey behavior (Grainger et al., 2017). Predators conduct several determinant actions to assure prey capture success, such as finding, choosing, capturing, manipulating, and ingesting or rejecting prey (Kimbell & Morrell, 2015). Pollutants like metals may alter these actions, which may ultimately lead to reduced predation success rates and therefore diminished food acquisition (Walker et al., 2012).
Food intake reduction indeed appears to be a general response to contaminant exposure (McWilliam & Baird, 2002). For example, studies by Smith and Weis (1997) and Weis et al. (2003) have shown that metals like cadmium, mercury, and zinc may interfere with the ability of selection and the speed of prey capture by the fish Fundulus heteroclitus, resulting in a decrease in the amount of food ingested. Success in catching prey can be used as a sensitive behavioral biomarker to evaluate the sublethal effects of chemicals like metals on aquatic populations and indirectly on aquatic community structure (Weis et al., 2001). Crustaceans such as Gammarus pulex and Hyallela azteca have indeed been successfully used as bioindicators of metal and pesticide contamination in water and sediment through an evaluation of their feeding rates (Forrow & Maltby, 2000; Hatch & Burton, 1999).
Dragonfly and damselfly species (Odonata, Insecta) have recently been suggested as promising test species for toxicity testing (Miguel et al., 2017; Valente-Neto et al., 2016). Odonates play an important role in aquatic ecosystem dynamics (Costa et al., 2006) being relevant predators, among others, in freshwater food webs (Oliveira et al., 2013). Their diet may range from invertebrates (mainly other insects) to fish and amphibian larvae of suitable size for their ingestion (Corbet, 1999; Fulan & Dos Anjos, 2015). However, when prey-predator interaction occurs in contaminated environments, metals can be bioaccumulated in the food web due to their non-degradability (Yuan et al., 2017). In addition, because they live directly associated with the sediment in its nymph phase, they may accumulate more metals than other invertebrate taxa inhabiting the water column (Corbi et al., 2008, 2010) and may therefore be good bioindicators of metal contamination (Wayland & Crosley, 2006).
Like many Odonata, ostracods are invertebrates that are also in close contact with the sediment since most species are detritivores, feeding mainly on decomposing organic matter (Martens, 1995). However, filter-feeding and predaceous ostracod species also exist (Barnes, 1995). For example, Campbell (1995) observed that the ostracod Australocypris insularis is a predator on zooplankters, able to greatly reduce density of small copepods such as Calamoecia clitellata and C. salina besides other ostracods such as Diacypris compacta and D. dietzi. According to Duleba et al. (2005), several species of this Class are stenobiotic, i.e., very sensitive to environmental variations, and as such, good environmental indicators of changes in physicochemical water conditions. They are also known to be sensitive to pesticides, metals, and other pollutants related to oil spills (Khangarot & Das, 2009; Ruiz et al., 2013; Rocha et al. 2018).
Despite their recognized functional relevance in aquatic ecosystems, the sensitivity of Odonata and Ostracoda to chemical stress has been poorly studied worldwide, especially regarding sublethal effects like predation behavior (Sloof 1983; Meyer et al., 1986; Khangarot & Ray, 1987; Rockwood et al., 1990; Havel and Talbot 1995; Khangarot & Das, 2009; Shuhaimi-Othman et al., 2011). Considering the above, the aim of the present study was to evaluate the effect of metal salts on the predatory behavior of nymphs of the dragonfly Tramea cophisa (Libellulidae, Odonata) and adults of the ostracod Chlamydotheca sp. (Ostracoda, Cyprididae). To this end, laboratory toxicity tests were conducted with four metals (copper, cadmium, manganese, and mercury) to evaluate their effects on predation intensity and food selectivity. We hypothesized that (1) with the increase in the concentration of the contaminant (metal salts), the organisms (predators) decrease the rate of predation; (2) at lower concentrations of the contaminant, the organisms show generalist feeding characteristics; (3) under exposure to the highest concentrations of metal salts, predators present greater food selectivity, opting for more sessile prey, with greater biomass.
Copper, manganese, cadmium, and mercury were selected as test compounds because they are common contaminants in aquatic environments (Campagna et al., 2008; Dornfeld et al., 2018; Gomes et al., 2020; Vogt et al., 2010; Watts & Pascoe, 2000). These compounds enter the aquatic environment both through natural sources and through human activity, e.g., mining activities agricultural runoff and industrial effluents (Hudspith et al., 2017; Lesch & Bouwman, 2018). Although copper and manganese are essential elements, in high concentrations, they are known to have adverse effects on aquatic organisms (Lima et al., 2019; Majumdar & Gupta, 2012). Cadmium is a highly toxic element with a high potential for bioaccumulation (Moiseenko & Gashkina, 2018), and mercury also has the potential to cause serious effects on growth, survival, and reproduction of invertebrates (Buch et al., 2017).
2 Material and Methods
2.1 Test Organisms and Culture Conditions
Tramea cophisa dragonfly nymphs were collected in the Mayaca reservoir, located in the municipality of São Carlos, SP, Brazil (21° 01′ S, 47° 53′ W). This small shallow reservoir is located in a permanent preservation area and has a surface area of 0.17 ha and a maximum depth of 1.20 m (Albuquerque 1990). The ostracod Chlamydotheca sp. was collected from 1,000-L plastic tanks at the Fish Farming Station of the Federal University of São Carlos (UFSCar). Care was taken in selecting nymphs of similar size to run the predation experiments, which required large sampling effort for obtaining both predator and prey. The need to not compromise T. cophisa population and the large amount of prey used, the number of replicas had to be limited.
In the laboratory, organisms were kept in plastic trays of 29 cm (width) × 34 cm (length) × 6.7 cm (height) and gradually acclimated to reconstituted water (ABNT, 2016) and test conditions (see below) for 7 days before starting tests. A preliminary study was made to determine best conditions to maintain healthy individuals in the laboratory (Lima et al., 2019). T. cophisa nymphs were fed with Chironomus inquinatus larvae (Insecta, Diptera) and small juveniles of Artemia franciscana (Crustacea, Anostraca), whereas Chlamydotheca sp. was fed with dense suspension of Chlorella sorokiniana (≈ 108 cells L−1). Both species were fed daily ad libitum.
2.2 Test Concentrations and Chemical Analysis of Metal Salts
Compounds were evaluated and their respective stock solutions were copper sulfate (10 mg L−1, CuSO4, CAS no: 7758–98-7), manganese sulfate (100 mg L−1, MnSO4, CAS no: 7785–87-7), cadmium chloride (10 mg L−1, CdCl2, CAS no: 10108–64-2, Carlo Erba), and mercury chloride (10 mg L−1, HgCl2, CAS no: 7487–94-7, ACS Merck). The nominal test concentrations of each chemical were obtained by diluting their respective stock solutions in reconstituted water. The stock solutions and test concentrations were prepared immediately before testing. The concentrations of cadmium, copper, and manganese were determined in stock solutions using a Perkin Elmer PinAAcle 900 T flame and longitudinal Zeeman atomic absorption spectrometer which was calibrated with Cd, Cu, and Mn standards (Trace Metals Basis, Sigma-Aldrich) and for stock solutions of mercury chloride using an AAS, AAnalyst 400, PerkinElmer.
2.3 Experimental Design
To evaluate the effects of metal salts on predation and food selectivity of T. cophisa nymphs (mean length: 8.54 mm ± 2.88 mm) and Chlamydotheca sp. adult individuals (mean length: 4.32 mm ± 0.59 mm) were exposed for 24 h to four sublethal concentrations of copper sulfate (CuSO4), cadmium chloride (CdCl2), mercury chloride (HgCl2), and manganese sulfate (MnSO4) (Table 1), besides the control treatment (reconstituted water only). The selected test concentrations corresponded for LC1, LC5, LC10, and LC20 values (LCx = lethal concentration to x % of the test population) were derived from acute toxicity tests conducted in our laboratory with each metal salt, for both species (Lima et al., 2019).
Four replicates were used for each treatment, with each replicate consisting of a circular non-toxic plastic vessel (height 8 cm; diameter 11 cm) filled with 200 mL test solution and one individual of either T. cophisa or Chlamydotheca sp. Only one predator individual was tested per replica due to the observation, from preliminary tests, of cannibalism occurrence for both predator species, thus making impossible to use more than one predator individual per test recipient. During the 24 h exposure period, no food was offered to test organisms. After this period, organisms were rinsed with non-contaminated reconstituted water and transferred to test vessels containing 200 mL fresh prepared non-contaminated reconstituted water. Five specimens of each of the following prey species were offered to T. cophisa making prey density per replicate, n = 20: Ilyocryptus spinifer (Crustacea, Ilyocryptidae), Artemia franciscana (Crustacea, Artemiidae), Chironomus inquinatus (Diptera, Chironomidae), and Dero furcatus (Annelida, Randiellidae). For the ostracod predator Chlamydotheca sp., three specimens of each prey C. inquinatus, A. fransciscana, and I. spinifer were added (total prey per replicate = 12). Test organisms were held with their prey for 24 h, after which the number of each prey species consumed was verified.
2.4 Data Analysis
After exposure to metal salts, predation rate and food selectivity of predator species were obtained by Ivlev Index of Electivity (Ivlev, 1961) calculated by the equation: E = (ri − Pi) / (ri + Pi), where E is the electivity index, ri is the relative abundance of each item in the stomach contents, and Pi is the relative abundance of each item in the environment after consumption. Stomach content was not analyzed. The relative abundance of stomach contents (ri) was calculated by subtracting the number of individuals of each prey remaining in each test vessel after 24 h of exposure, from the respective amount at the beginning of the test. Index values vary from − 1 to + 1 with the value zero indicating no selectivity; values lower than 0 indicate rejection of the food item and values higher than 0 denote positive selection (Harrison et al., 2005). Prior to statistical data analysis, all prey abundance values were logarithm transformed (log x + 1) to down-weight high abundance values aiming to approximate data normality. An array using the results of the Bray–Curtis similarity test was constructed and a permutational multivariate analysis of variance (PERMANOVA) was performed to compare results. Comparison between concentrations tested and controls was performed using the statistical program BioEstat 5.0 for analysis of variance (ANOVA).
3 Results and Discussion
3.1 Chemical Analyses
For all experiments, nominal concentrations of metal salts were measured for stock solutions. Concentrations of copper sulfate (10 mg L−1), manganese sulfate (100 mg L−1), cadmium chloride (10 mg L−1), and mercury chloride (10 mg L−1) were quantified. Accuracy (%) values obtained were 10.3 mg L−1 (100.3 ± 0.9%); 107 mg L−1 (107.3 ± 0.2%); 9.1 mg L−1 (91.1 ± 0.9%); and 9.3 mg L−1 (93 ± 1.8%), respectively. Nominal concentrations did not vary more than 10% from measured concentrations. Therefore, nominal initial concentrations were used to represent treatment concentrations.
3.2 Predation Rates
A voracious feeding behavior by unaffected Tramea cophisa nymphs was observed in the first hour after being transferred from treatment test solution to clean culture medium. This was very likely due to the food restriction that nymphs experienced during the 24 h exposure period. In line with this, Tomazelli et al. (2011) observed the same behavior for Neuraeschna sp. (Odonata, Aeshnidae) nymphs when offering the common carp Cyprinus carpio larvae (Cypriniformes, Cyprinidae) after a 27 h exposure period without food.
Prey consumption rates of T. cophisa indicate that most metal salt concentrations tested led to a significant decrease in predation rates as compared with controls, supporting our first hypothesis. However, for cadmium chloride, there was greater variability in prey consumption among replicates as observed at one of the highest concentrations tested (240 µg L−1) resulting no significant difference from control. This might be explained by the low number of replicates used, in face of restrict availability of nymphs of similar size (Table 2). And only for nymphs exposed to manganese sulfate, there were no statistically significant differences in predation rates at any of the concentrations tested. In line with this, Lima et al. (2019) noted that this species is relatively non-sensitive to short-term exposure to manganese. However, this metal is an essential micronutrient being more or less toxic depending on the species, the stage of life and physical, and chemical characteristics of the water (Vieira et al., 2012).
So far, few studies have been conducted to evaluate metal effect on dragonfly feeding behavior, hampering comparisons between our results with studies also evaluating the metals tested in the present study. Nevertheless, previous studies have demonstrated a decrease in feeding rates of dragonflies following exposure to other chemicals. For example, a decrease in predation rate of Pantala sp. nymphs (Odonata, Libellulidae) when feeding on Ictalurus punctatus larvae (Siluriformes, Ictaluridae) after exposure in tanks chemically treated with larvicides (McGrinty, 1980). Tomazelli et al. (2011) also reported a reduction for predation rates of dragonfly nymphs (Neuraeschna sp.) on C. carpio fingerlings when nymphs were exposed to Melia azedarach (Meliaceae) extract. These authors also noted slowness in movements of dragonfly nymphs exposed to plant extract, which was concluded to be the cause of reduced predation rate.
The predation rate of the ostracod Chlamydotheca sp. increased compared to control in metal salts-affected treatments (Table 3). This increased predation rate could be caused by increased energy demand due to greater stress, which can be offset with greater predation. Alternatively, the increased predation rate could also be partly related with a greater energy demand of the predator for possible defense mechanisms against the toxic stress. In real aquatic ecosystems, indirect effects of chemical stress on predation rates may also play a prominent role. Pearson et al. (1981), for example, observed that the predator crab Cancer magister consumed a larger number of the snail Protothaca staminea in oil-contaminated sand when compared to uncontaminated sand. These authors associated this with a slower penetration of snails in the contaminated sand, making them easier to be preyed upon by the crabs.
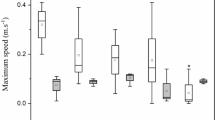
Ostracoda Chlamydotheca sp. showed increased rates of predation in relation to the control (Fig. 1). Ostracods, for example, can ingest large quantities of food, in few minutes in contaminated systems and survive for several weeks without feeding, thus increasing their rate of predation at times (Vannier et al. 1998). On the other hand, T. cophisa showed lower rates of predation compared to control (Fig. 1).
3.3 Food Preferences: Ivlev Electivity Index
Figure 2 shows the consumption rates of T. cophisa dragonfly nymphs on each of four prey taxa. From this figure, it appears that C. inquinatus and D. furcatus were the most preferred prey species. The Ivlev Electivity Index (IEI) confirms this overall preference of T. cophisa for the D. furcatus (IEI = 0.66 ± 0.23; mean ± SD for all treatments) and Chironomus inquinatus (IEI = 0.46 ± 0.26; mean ± SD for all treatments), with not a single negative value, meaning no rejection for these preys at any tested metal salt concentrations (Table 4). These results confirm the food preference of T. cophisa for organisms with higher biomass when exposed to higher concentrations of metal salts. Although these preys are not sessile, they have slower locomotion than I. spinifer and A. franciscana, corroborating our third hypothesis. These results also agree with those obtained by Alzmann et al. (1999) who found that the dragonfly Gomphus pulchellus preferred oligochaete and chironomid larvae over amphipods and ephemeropteran larvae. The relatively lower preference for I. spinifer (IEI = 0.14 ± 0.30) and, to a lesser extent, Artemia franciscana (IEI = 0.24 ± 0.30) may have several underlying reasons, including (i) their overall smaller size; (ii) their movement through the water column rather than being sessile; and, for I. spinifer specifically, (iii) the presence of thorns around the carapace (Kotov & Williams, 2000). However, in the lowest concentrations of the tested metal salts, T. cophisa showed a generalist feeding behavior, as indicated in our second hypothesis.
Prey consumption rates of Tramea cophisa in relation to the number initially offered of each prey, in treatments without exposure (control) and in treatments with exposure to copper sulfate (CuSO4), cadmium chloride (CdCl2), mercury chloride (HgCl2), and manganese sulfate (MnSO4) for 24 h. Asterisks indicate significant differences
For Chlamydotheca sp., C. inquinatus chironomid larvae were the most consumed prey in the control in all tests performed, followed by I. spinifer and A. franciscana (Fig. 3; Table 4). C. inquinatus was also the most consumed prey species in the metal salt exposure treatments, for all tests performed, except in the treatment of 60 µg L−1 of CuSO4, followed by I. spinifer that was less consumed only at concentrations of 60 µg. L−1 (CuSO4) and in controls of CuSO4 and MnSO4. A. franciscana was most consumed only at the concentration of 60 µg. L−1 of CuSO4. Thus, with these results, for Chlamydotheca sp., our second hypothesis that in low concentrations, the test organisms would be generalists, is not fully supported. Studies on the predation behavior of ostracods are scarce, but they are known to prey on a wide range of organisms including Daphnia magna (Ottonello and Romano 2011) and other cladocerans, as well as copepods, ostracods, oligochaetes, and insect larvae (Ganning, 1971; Meisch, 2000; Wilkinson et al., 2007). The preference for the chironomid prey over the two crustacean preys may have been due to the same reasons as discussed above for T. cophisa.
Consumption rate of Chlamydotheca sp. (expressed as %) in relation to the number initially offered of each prey, for treatments without exposure (control) and in treatments with previous exposure to copper sulfate (CuSO4), cadmium chloride (CdCl2), mercury chloride (HgCl2), and manganese sulfate (MnSO4), during 24 h. Asterisks indicate significant differences
4 Conclusions
In this study, we observed that the tested metal salts did not influence food selectivity, but the predation behavior of nymphs of the dragonfly T. cophisa and adults of ostracod Chlamydotheca sp. was altered in relation to the control tests. In addition, this study evidenced that these effects are species specific. Such complex species-specific predator–prey interactions may have pronounced impacts on ecosystem structure and functioning following chemical stress. Future studies are needed to shed more light on predator species-specific prey preferences and how these are affected by metal and other chemical contamination.
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
ABNT - Associação Brasileira de Normas Técnicas (2016). Aquatic ecotoxicology - acute toxicity - Test with Daphnia spp. (Cladocera, Crustacea). NBR 12713. ABNT, Rio de Janeiro.
Alzmann, N., Köhler, B., & Maier, G. (1999). Spatial distribution, food and activity of Gomphus pulchellus SELYS 1840 (Insecta; Odonata; Gomphidae) from a still water habitat. International Review of Hydrobiology, 84, 299–313.
Anholt, B. (1992). Sex and habitat differences in feeding by an adult damselfly. Oikos, 65, 428–432.
Barnes, R.D. (1995). Zoologia de Invertebrados. 4ed. Ed. Roca, 1178 pp.
Buch, A. C., Brown, G. G., Correia, M. E. F., Lourençato, L. F., & Silva-Filho, E. V. (2017). Ecotoxicology of mercury in tropical forest soils: Impact on earthworms. Science of the Total Environment, 589, 222–231.
Campagna, A. F., Fracácio, R., Rodrigues, B. K., Eler, M. N., Verani, N. F., & Espíndola, E. L. G. (2008). Analyses of the sediment toxicity of Monjolinho River, São Carlos, São Paulo State, Brazil, using survey, growth and gill morphology of two fish species. Brazilian Archives of Biology and Technology, 51, 193–201.
Campbell, C. E. (1995). The influence of a predatory ostracod, Australocypris insularis, on zooplankton abundance and species composition in a saline lake. Hydrobiologia, 302, 229–239.
Clark, R. B. (1992). Marine pollution (p. 172). Clarendon Press.
Corbi, J. J., Strixino, S. T., Santos, A. D., & Del Grande, M. (2006). Diagnóstico ambiental de metais e organoclorados em córregos adjacentes a áreas de cultivo de cana-de-açúcar (Estado de São Paulo, Brasil). Química Nova, 29, 61–65.
Corbi, J. J., Strixino, S. T., & Santos, A. D. (2008). Environmental evaluation of metals in sediments and dragonflies due to sugar cane cultivation in Neotropical streams. Water Air and Soil Pollution, 195, 325–333.
Corbi, J. J., Froehlich, C. G., Trivinho-Strixino, S., & Dos Santos, A. (2010). Bioaccumulation of metals in aquatic insects of streams located in areas with sugar cane cultivation. Química Nova, 33, 644–648.
Corbet, P. S. (1999). Dragonflies: Behavior and ecology. University Press.
Correa, M., Coler, R. A., & Yin, C. M. (1985). Changes in oxygen consumption and nitrogen metabolism in the dragonfly Somatochlora cingulata exposed to aluminum in acid waters. Hydrobiologia, 121, 151–156.
Costa, C., Ide, S., & Simonka, C. E. (2006). Insetos imaturos: metamorfose e identificação. Ribeirão Preto: Holos. 249 pp.
Deschiens, R. (1954). Mécanisme de l’actionléthale de Cypridopsis hartwigi surles mollusques vecteurs des bilharzioses. Bulletin De La Société De Patholoie Exotique, 47, 399–401.
Deschiens, R., Lamy, L., & Lamy, H. (1953). Sur un ostracode prédateur de Bullinset de Planorbes. Bulletin De La Société De Pathologie Exotique, 46, 956–958.
Dornfeld, C. B., Rodgher, S., Negri, R. G., Espíndola, E. L. G., & Daam, M. A. (2018). Chironomus sancticaroli (Diptera, Chironomidae) as a sensitive tropical test species in laboratory bioassays evaluating metals (copper and cadmium) and field testing. 2018. Archives of Environmental Contamination and Toxicology, 76, 42–50.
Duleba, W., Coimbra, J. C. S., Petri, S., & Barbosa, C. F. (2005). Foraminíferos tecamebas e ostracodes recentes utilizados como bioindicadores em estudos ambientais brasileiros. In: C. R. G. Sousa, K. Suguio, M. Santos, & P. E. Oliveira (Eds.), Quaternário do Brasil. ABEQUA. Editora Holos, Ribeirão Preto, 176–201 pp.
Elmoor-Loureiro, L. M. A. (1997). Manual de identificação de cladóceros límnicos do Brasil. Brasília. Ed. Universitária. 156 pp.
Forrow, D. M., & Maltby, L. (2000). Toward a mechanistic understanding of contaminant-induced changes in detritus processing in streams: Direct and indirect effects on detritivore feeding. Environmental Toxicology and Chemistry, 19, 2100–2106.
Fulan, J. A., & Dos Anjos, M. R. D. (2015). Predation by Erythemis nymphs (Odonata) on Chironomidae (Diptera) and Elmidae (Coleoptera) in different conditions of habitat complexity. Acta Limnologica Brasiliensia, 27, 454–458.
Ganning, B. (1971). On the ecology of Heterocypris salinus, H. incongruens and Cypridopsis aculeata (Crustacea, Ostracoda) from Baltic brackish-water rockpools. Marine Biology, 8, 271–279.
Gerhardt, A., De Bisthoven, L. J., & Soares, A. M. V. M. (2004). Macroinvertebrate response to acid mine drainage: Community metrics and on-line behavioral toxicity bioassay. Environmental Pollution, 130, 263–274.
Gomes, D. F., Moreira, R. A., Sanches, N. A. O., Vale, C. A., Daam, M. A., Gorni, G. R., & Bastos, W. R. (2020). Dynamics of (total and methyl) mercury in sediment, fish, and crocodiles in an Amazonian Lake and risk assessment of fish consumption to the local population. Environmental Monitoring and Assessment, 192, 1–01.
Grainger, P., Christie, M., Thomas, G., Dole, S., Heck, D., Marshman, M., & Carey, M. (2017). Improving the quality of assessment by using a community of practice to explore the optimal construction of assessment rubrics. Reflective Practice, 18, 410–422.
Harrison, S. S. C., Bradley, D. C., & Harris, I. T. (2005). Uncoupling strong predator-prey interactions in streams: The role of marginal macrophytes. Oikos, 108, 443–448.
Hatch, A. C., & Burton, G. A. (1999). Phototoxicity of fluoranthene to two freshwater crustaceans, Hyalella azteca and Daphnia magna: Measures of feeding inhibition as a toxicological endpoint. Hydrobiologia, 400, 243–248.
Havel, J. E., & Talbott, B. L. (1995). Life history characteristics of the freshwater ostracod Cyprinotus incongruens and their application to toxicity testing. Ecotoxicology, 4, 206–218.
Hudspith, M., Reichelt-Brushett, A., & Harrison, P. L. (2017). Factors affecting the toxicity of trace metals to fertilization success in broadcast spawning marine invertebrates: A review. Aquatic Toxicology, 184, 1–13.
Irving, E. C., Baird, D. J., & Culp, J. M. (2003). Ecotoxicological responses of the mayfly Baetis tricaudatus to dietary and waterborne cadmium: Implications for toxicity testing. Environmental Toxicology and Chemistry, 22, 1058–1064.
Ivlev, V. S. (1961). Experimental ecology of the feeding of fishes (p. 302). Yale University Press.
Khangarot, B. S., & Das, S. (2009). Acute toxicity of metals and reference toxicants to a freshwater ostracod, Cypris subglobosa Sowerby, 1840 and correlation to EC50 values of other test models. Journal of Hazardous Materials, 172, 641–649.
Khangarot, B. S., & Ray, P. K. (1987). Response of a freshwater ostracod (Cypris subglobosa Sowerby) exposed to copper at different pH levels. Acta Hydrochimica Et Hydrobiologica, 15, 553–558.
Kimbell, H. S., & Morrell, L. J. (2015). Turbidity influences individual and group level response to predation in guppies, Poecilia reticulata. Animal Behaviour, 103, 179–185.
Kotov, A. A., & Williams, J. L. (2000). Ilyocryptus spinifer Herrick 1882 (Anomopoda, Branchiopoda): A redescription based on North American material and designation of a neotype from Minnesota. Hydrobiologia, 428, 67–84.
Lailson-Brito, J., Jr., Dorneles, P. R., Da Silva, V. M., Martin, A. R., Bastos, W. R., Azevedo-Silva, C. E., & Malm, O. (2009). Dolphins as indicators of micropollutant trophic flow in Amazon Basin. Oecologia Brasiliensis, 12, 531–541.
Lesch, V., & Bouwman, H. (2018). Adult dragonflies are indicators of environmental metallic elements. Chemosphere, 209, 654–665.
Lima, J. C. S., Neto, A. J. G., Andrade, D. P., Freitas, E. C., Moreira, R. A., Miguel, M., Daam, M. A., & Rocha, O. (2019). Acute toxicity of four metals to three tropical aquatic invertebrates: The dragonfly Tramea cophysa and the ostracods Chlamydotheca sp. and Strandesia trispinosa. Ecotoxicology and Environmental Safety, 180, 535–541.
Majumdar, T. N., & Gupta, A. (2012). Acute and chronic toxicity of copper on aquatic insect Chironomus ramosus from Assam, India. Journal of Environmental Biology, 33, 139–142.
Martens, K. (1995). Recent non-marine Ostracoda. Workshop on Neotropical Aquatic Invertebrates University of São Paulo, Brazil, 31, 1–18.
McGrinty, A. S. (1980). Survival, growth and variation growth of channel catfish fry and fingerlings. Alabama, 1980. (Thesis Doctoral) Auburn University.
McWilliam, R. A., & Baird, D. J. (2002). Application of post exposure feeding depression bioassays with Daphnia magna for assessment of toxic effluents in rivers. Environmental Toxicology and Chemistry, 21, 1462–1468.
Meisch, C. (2000). Freshwater Ostracoda of Western and Central Europe (p. 522). Spektrum Akademischer Verlag.
Meyer, W., Harisch, G., & Sagredos, A. N. (1986). Biochemical and histochemical aspects of lead exposure in dragonfly larvae (Odonata: Anisoptera). Ecotoxicology and Environmental Safety, 11, 308–319.
Miguel, T. B., Oliveira-Junior, J. M. B., Ligeiro, R., & Juen, L. (2017). Odonata (Insecta) as a tool for the biomonitoring of environmental quality. Ecological Indicators, 81, 555–566.
Moiseenko, T. I., & Gashkina, N. A. (2018). Biogeochemistry of cadmium: Anthropogenic dispersion, bioaccumulation, and ecotoxicity. Geochemistry International, 5, 798–811.
Murugan, K., Benelli, G., Ayyappan, S., Dinesh, D., Panneerselvam, C., Nicoletti, M., & Suresh, U. (2015). Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasitology Research, 114, 2243–2253.
Oliveira, E., Takeuchi, S. S., & Cerutti, V. E. (2013). Assembleia de Larvas de Odonata (Insecta) em ambientes límnicos do Parque Estadual de Vila Velha, Paraná, Brasil. Estudos De Biologia, 35, 163–176.
Ottonello, D., & Romano, A. (2011). Ostracoda and Amphibia in temporary ponds: Who is the prey? Unexpected trophic relation in a Mediterranean freshwater habitat. Aquatic Ecology, 45, 55–62.
Pearson, W. H., Woodruff, D. L., Sugarman, P. C., & Olla, B. L. (1981). Effects of oiled sediment on predation on the littleneck clam, Protothaca staminea, by the Dungeness crab, Cancer magister. Estuarine, Coastal and Shelf Science, 13, 445–454.
Pinder, L. C. V. (1995). The habitats of chironomid larvae. In: P. D. Armitage, P. S. Cranston, & L. C. V. Pinder. Biology and ecology of non-biting midges. Chapman and Hall, London, U.K. 107–135 pp.
Pritchard, G. (1965). Prey capture by dragonfly larvae (Odonata; Anisoptera). Canadian Journal of Zoology, 43, 271–289.
Rockwood, J. P., Jones, D. S., & Coler, R. A. (1990). The effect of aluminum in soft water at low pH on oxygen consumption by the dragonfly Libellula julia Uhler. Hydrobiologia, 190, 55–59.
Ruiz, F., Abad, M., Bodergat, A. M., Carbonel, P., Rodríguez-Lázaro, J., González Regalado, M. L., Toscano, A., García, E. X., & Prenda, J. (2013). Freshwater ostracods as environmental tracers. International Journal of Environmental and Science Technology, 10, 1115–1128.
Shuhaimi-Othman, M., Yakub, N., Ramle, N. A., & Abas, A. (2011). Toxicity of metals to a freshwater ostracod: Stenocypris major. Journal of Toxicology, 2011, 1–8.
Sloff, W. (1983). Benthic macroinvertebrates and water quality assessment: Some toxicological considerations. Aquatic Toxicology, 4, 73–82.
Smith, G. M., & Weis, J. S. (1997). Predator-prey relationships in mummichogs (Fundulus heteroclitus (L.): Effects of living in a polluted environment. Journal of Experimental Marine Biology and Ecology, 209, 75–87.
Soares, C. M., Hayashi, C., & Faria, A. C. E. A. (2001). Influência da disponibilidade de presas, do contraste visual e do tamanho das larvas de Pantala sp. (Odonata, Insecta) sobre a predação de Simocephalus cerrulatus (Cladocera, Crustacea). Acta Scientarum. Biological Sciences, 23, 357–362.
Soares, C. M., Hayashi, C., & Reidel, A. (2003). Predação de pós-larvas de curimba (Prochilodus lineatus, Valenciennes, 1836) por larvas de Odonata (Pantala sp., Fabricius, 1798) em diferentes tamanhos. Acta Scientarum. Biological Sciences, 25, 95–100.
Sohn, I. G., & Kornicker, L. S. (1972). Predation of schistosomiasis vector snails by Ostracoda (Crustacea). Science, 175, 1258–1259.
Suhling, F., Martens, A., & Suhling, I. (2017). Long-distance dispersal in Odonata: Examples from arid Namibia. Austral Ecology, 42, 544–552.
Tave, D., Rezk, M., & Smitherman, R. O. (1990). Effect of body color of Oreochromis mossambicus (Peters) on predation by dragonfly nymphs. Aquaculture Research, 21, 157–161.
Thetmeyer, H., & Kils, U. (1995). To see and not be seen: The visibility of predator and prey with respect to feeding behaviour. Marine Ecology, 126, 1–8.
Thornton, I., Ramsey, M., & Atkinson, N. (1995). Metals in the global environment: Facts and misconceptions (p. 103). International Council on Metals and Environment (ICME).
Tomazelli, O., Jr., Franco, G. M. S., Casaca, J. M., Munarini, A. C., & Dal Magro, J. (2011). Effect of the Melia azedarach L. on the predation of common carp fingerlings (Cyprinus carpio) by larvae of Neuraeschna (Odonata: Aeshnidae). Braz Journal of Aquatic Science & Technology, 15, 19–25.
Valente-Neto, F., De Oliveira Roque, F., Rodrigues, M. E., Juen, L., & Swan, C. M. (2016). Toward a practical use of Neotropical odonates as bioindicators: Testing congruence across taxonomic resolution and life stages. Ecological Indicators, 61, 952–959.
Van Stappen, G. (1996). Introduction, biology and ecology of Artemia. In: P. Lavens, & P. Sorgeloos (Eds). Manual on the Production and Use of Live Food for Aquaculture. FAO Fisheries Technical Paper, 295 pp.
Vannier, J., Abe, K., & Ikuta, K. (1998). Feeding in myodocopid ostracods: Functional morphology and laboratory observations from videos. Marine Biology, 132, 391–408.
Vieira, M. C., Torronteras, R., Córdoba, F., & Canalejo, A. (2012). Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicology and Environmental Safety, 78, 212–217.
Vogt, C., Hess, M., Nowak, C., Diogo, J. B., Oehlmann, J., & Oetken, M. (2010). Effects of cadmium on life-cycle parameters in a multi-generation study with Chironomus riparius following a pre-exposure of populations to two different tributyltin concentrations for several generations. Ecotoxicology, 19, 1174–1182.
Walker, C. H., Hopkin, S. P., Sibly, R. M., & Peakall, D. B. (2012). Principles of ecotoxicology (4th ed., p. 360). Taylor and Francis.
Watts, M. M., & Pascoe, D. A. (2000). Comparative study of Chironomus riparius Meigen and Chironomus tentans Fabricius (Diptera: Chironomidae) in aquatic toxicity tests. Archives of Environmental Contamination and Toxicology, 39, 299–306.
Wayland, M., & Crosley, R. (2006). Selenium and other trace elements in aquatic insects in coalmine-affected streams in the Rocky Mountains of Alberta, Canada. Archives of Environmental Contamination and Toxicology, 50, 511–522.
Weis, J. S., Samson, J., Zhou, T., Skurnick, J., & Weis, P. (2001). Prey capture ability of mummichogs (Fundulus heteroclitus) as a behavioral biomarker for contaminants in estuarine systems. Canadian Journal of Fisheries and Aquatic Science, 58, 1442–1452.
Weis, J. S., Samson, J., Zhou, T., Skurnick, J., & Weis, P. (2003). Evaluating prey capture by larval mummichogs (Fundulus heteroclitus) as a potential biomarker for contaminants. Marine Environmental Research, 55, 27–38.
Williams, M., Perrier, V., Bennett, C., & Hearing, T. (2015). Ostracods: The ultimate survivors. Geology Today, 31, 193–200.
Wilkinson, I. P., Wilby, P. R., Williams, M., Siveter, D. J., & Vannier, J. (2007). Ostracod carnivory through time. In: Elewa ATM (Ed.). Predation in organisms: a distinct phenomenon. Springer Berlin, Heidelberg., 39–57 pp.
Yuan, D., Huang, L., Zeng, L., & Liu, S. (2017). Acute toxicity of mercury chloride (HgCl2) and cadmium chloride (CdCl2) on the behavior of freshwater fish, Percocypris pingi. International Journal of Aquaculture Fisheries and Science, 7, 66–70.
Acknowledgements
The authors are indebted to the Brazilian National Research Council (CNPq, Process: 140400/2015-0) and CAPES for scholarships to the first author in different periods, and to Dr. Norma Luiza Würdig from the Federal University of Rio Grande do Sul, RS, Brazil, for the taxonomic identification of the Ostracoda species. R.A.M. has a postdoctoral fellowship from FAPESP (grant no. 2017/24126-4). This work was also supported by the Portuguese government (FCT) through a postdoc grant for M. Daam (SFRH/BPD/109199/ 2015) and the research unit UID/AMB/04085/2013 (CENSE). The work was also partially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Consent to Participate
All authors inform consent to participate in this manuscript.
Consent for Publication
Authors inform consent for the publication of any associated data and accompanying images.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos Lima, J., Moreira, R., Neto, A. et al. Metal Toxicity Can Affect Dragonfly Nymphs and Ostracods Predation Rates and Food Selectivity: Ecological Implications on Food Webs. Water Air Soil Pollut 232, 288 (2021). https://doi.org/10.1007/s11270-021-05248-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-021-05248-3