Abstract
Nearly 1.4 billion people in 73 countries worldwide are threatened by lymphatic filariasis, a parasitic infection that leads to a disease commonly known as elephantiasis. Filariasis is vectored by mosquitoes, with special reference to the genus Culex. The main control tool against mosquito larvae is represented by treatments with organophosphates and insect growth regulators, with negative effects on human health and the environment. Recently, green-synthesized nanoparticles have been proposed as highly effective larvicidals against mosquito vectors. In this research, we attempted a reply to the following question: do green-synthesized nanoparticles affect predation rates of copepods against mosquito larvae? We proposed a novel method of seaweed-mediated synthesis of silver nanoparticles using the frond extract of Caulerpa scalpelliformis. The toxicity of the seaweed extract and silver nanoparticles was assessed against the filarial vector Culex quinquefasciatus. Then, we evaluated the predatory efficiency of the cyclopoid crustacean Mesocyclops longisetus against larval instars of C. quinquefasciatus in a nanoparticle-contaminated water environment. Green-synthesized silver nanoparticles were characterized by UV–vis spectrum, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and X-ray diffraction (XRD). In mosquitocidal assays, the LC50 values of the C. scalpelliformis extract against C. quinquefasciatus were 31.38 ppm (I), 46.49 ppm (II), 75.79 ppm (III), 102.26 ppm (IV), and 138.89 ppm (pupa), while LC50 of silver nanoparticles were 3.08 ppm, (I), 3.49 ppm (II), 4.64 ppm (III), 5.86 ppm (IV), and 7.33 ppm (pupa). The predatory efficiency of the copepod M. longisetus in the control treatment was 78 and 59 % against I and II instar larvae of C. quinquefasciatus. In a nanoparticle-contaminated environment, predation efficiency was 84 and 63 %, respectively. Predation was higher against first instar larvae over other instars. Overall, our study showed that seaweed-synthesized silver nanoparticles can be proposed in synergy with biological control agents against Culex larvae, since their use leads to little detrimental effects against aquatic predators, such as copepods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphatic filariasis, commonly known as elephantiasis, is a neglected tropical disease. More than 1.4 billion people in 73 countries are living in areas where lymphatic filariasis is transmitted and are at risk of being infected. Globally, an estimated 25 million men suffer with genital disease and over 15 million people are afflicted with lymphoedema (WHO 2014). Eliminating lymphatic filariasis can prevent unnecessary suffering and contribute to the reduction of poverty. Lymphatic filariasis is caused by Filariodidea nematodes, namely Wuchereria bancrofti, which is responsible for 90 % of cases, Brugia malayi, and Brugia timori. Microfilariae are transmitted to humans by different mosquitoes. Culex species, with special reference to Culex quinquefasciatus, are the most common vectors across urban and semi-urban areas of Asia (Chadee et al. 2002; WHO 2014).
Currently, the main control tool against Culex larvae is represented by treatments with organophosphates and insect growth regulators, with negative effects on human health, the environment, and non-target aquatic organisms (Brown 1986; see also Conti et al. 2014). In this scenario, botanical products constitute a valuable alternative, due to their reduced toxicity towards vertebrates and high biodegradability. Plant-borne chemicals have been used by human communities in different parts of the world as mosquitocidals, adult repellents, and oviposition deterrents, against a wide number mosquito species (e.g., Amer and Mehlhorn 2006a, b; Coelho et al. 2009; Freitas et al. 2010; Govindarajan 2010; Hafeez et al. 2011; Ravikumar et al. 2011; Benelli et al. 2013a, b, 2015a, b, c).
Nanotechnologies open new perspectives for unraveling a huge array of applications in the field of catalysis, sensors, optoelectronics, magnetic devices, drug delivery, antimicrobials, and parasitology (Shirkhanzadeh et al. 1995; Chan and Nie 1998; El-Sayed et al. 2005; Vaseashta and Dimova-Malinovska 2005; Aurel et al. 2007; Kim et al. 2007; Magana et al. 2008; Rai et al. 2009). The plant-mediated biosynthesis of nanoparticles is advantageous over chemical and physical methods, since it is cheap and eco-friendly, does not require high pressure, energy, temperature, and the use of highly toxic chemicals (Goodsell 2004). A growing number of plants and fungi have proposed for efficient and rapid extracellular synthesis of silver and gold nanoparticles (Shankar et al. 2003, 2004; Chandran et al. 2006; Song et al. 2009), which showed excellent mosquitocidal properties, also in field conditions (Soni and Prakash 2012; Dinesh et al. 2015; Suresh et al. 2015).
Concerning eco-friendly control of mosquito vectors, a further way to tackle the problem is the employment of aquatic organisms that predate Culicidae larvae in biological control programs. Good examples are odonate young instars, water bugs, tadpoles, fishes, crabs, and copepods (Bowatte et al. 2013; Kalimuthu et al. 2014 and references therein). Copepods are small aquatic crustaceans; most of them are omnivorous and can prey on immature mosquitoes, especially first-instar larvae, but rarely on later stages (Hurlbut 1938; Riviere and Thirel 1981; Marten et al. 1989; Williamson 1999). In particular, several species of copepods, such as Mesocyclops aspericornis, Mesocyclops guangxiensis, Mesocyclops longisetus, and Mesocyclops thermocyclopoides, have been reported as potential biological control agents against mosquitoes (Rawlins et al. 1997; Jekow et al. 1998; Manrique-Saide et al. 1998; Schaper 1999; Locantoni et al. 2006). Operationally, the use of M. longisetus against mosquitoes in urban and semi-urban habitats is not expensive and requires little labor for colony maintenance (Soumare and Cilek 2011; Chitra et al. 2013).
To the best of our knowledge, few studies investigated the non-target effects of nanoparticles against predatory copepods (Oberdorster et al. 2006; Jarvis et al. 2013; Park et al. 2014, see Fabrega et al. 2011, and Baun et al. 2008 for reviews), and no evidences are available about the toxicity of green-synthesized nanoparticles against these predaceous aquatic organisms. No efforts have been carried out to integrate classic biological control programs and nanobiotechnological tools for eco-friendly control of mosquito vectors.
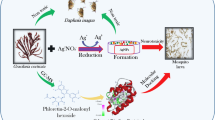
Do green-synthesized nanoparticles affect predation rates of copepods against mosquito larvae? In this research, we proposed a new method of seaweed-mediated synthesis of silver nanoparticles using the frond extract of the seaweed Caulerpa scalpelliformis (Caulerpaceae). Green-synthesized silver nanoparticles were characterized by UV–vis spectrum, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and X-ray diffraction (XRD). The toxicity of the seaweed extract and silver nanoparticles was assessed against the filarial vector C. quinquefasciatus. Then, we evaluated the predatory efficiency of the cyclopoid crustacean M. longisetus against larval instars of C. quinquefasciatus in a nanoparticle-contaminated water environment. Our research highlighted that the seaweed-synthesized silver nanoparticles can be employed in synergy with biological control agents against mosquito vectors, leading to little detrimental effects against aquatic predators, such as copepods.
Materials and methods
Seaweed study species
The marine environment is an outstanding reservoir of bioactive natural products, which have many therapeutic applications, such as antiviral, antibacterial, antifungal, antifertility, and anticancer activities (Ireland et al. 1988; Bazes et al. 2009; Ravikumar et al. 2009, 2010; Chen and Wilson 2010; Kamaraj et al. 2011; Tennyson et al. 2012). The frond extract of the seaweed C. scalpelliformis is toxic against different arthropod pests, including mosquitoes. Raniello et al. (2007) reported the phytotoxic property of caulerpenyne. Other phytochemicals include saponins, tannins, terpenoids, alkaloids, steroids, and flavonoids (Engel et al. 2006).
C. scalpelliformis collection and extraction
C. scalpelliformis was collected from coastal areas of Rameshwaram (Tamil Nadu, Southern India). C. scalpelliformis fronds were washed with tap water and shade-dried at room temperature. The dried plant material was powdered using an electrical blender. Then, 500 g of the powdered plant material were extracted using 1.5 L of ethanol for 72 h. The crude plant extract was concentrated at reduced temperature using a rotary evaporator, and stored at 22 °C. One gram of the plant residue was dissolved in 100 mL of acetone (fixative agent to separate the aqueous impurities altering the chemical composition of plant crude extract) and considered as 1 % stock solution. From this stock solution, experimental concentrations were prepared.
C. quinquefasciatus rearing
Eggs of C. quinquefasciatus were collected from local breeding habitats in Coimbatore (India) using an “O” type brush (Dinesh et al. 2015). Eggs were transferred to laboratory conditions [27 ± 2 °C, 75–85 % R.H., 14:10 (L:D) photoperiod] and placed in 18 × 13 × 4 cm plastic containers containing 500 mL of tap water, waiting for larval hatching. Larvae were reared in the plastic containers described above, and fed daily with a mixture of crushed dog biscuits and hydrolyzed yeast at 3:1 ratio (w:w). Water was renewed each 2 days. The breeding medium was checked daily and dead individuals were removed. Breeding containers were kept closed with muslin cloth to prevent contamination by foreign mosquitoes. Larvae and pupae for experiments were collected daily from culture containers and transferred to glass beakers containing 500 mL of water.
M. longisetus rearing
Copepods were collected from a pond (Muthanamkulam, Coimbatore, India) using a mesh net. All collected samples were identified as M. longisetus by Dr. Y. Ranga Reddy (Department of Zoology, Acharya Nagarjuna University, India). M. longisetus was reared following the method reported by Kosiyachinda et al. (2003). Isofemale lines were established from gravid females and maintained at Department of Zoology, Bharathiar University (Coimbatore, India). Gravid females from different isofemale lines were pooled and mass reared in dechlorinated water (pH 7) in fish tanks (15 L) at 27 ± 2 °C and natural photoperiod. Food was Paramecium spp. prepared from boiled rice straw water extract and commercial powdered fish food.
Seaweed-mediated synthesis and characterization of silver nanoparticles
The C. scalpelliformis aqueous extract was prepared adding 10 g of washed and finely cut leaves in a 300-mL Erlenmeyer flask filled with 100 mL of sterilized double distilled water and then boiling the mixture for 5 min, before finally decanting it. The extract was filtered using Whatman filter paper No. 1, stored at −15 °C and tested within 5 days. The filtrate was treated with aqueous 1 mM AgNO3 solution in an Erlenmeyer flask and incubated at room temperature (Dinesh et al. 2015). A brown-yellow solution indicated the formation of silver nanoparticles, since aqueous silver ions were reduced by the plant extract generating stable silver nanoparticles in water. Silver nitrate was purchased from the Precision Scientific Co. (Coimbatore, India).
The presence of green-synthesized silver nanoparticles was confirmed by sampling the reaction mixture at regular intervals, and the absorption maxima was scanned by UV–vis spectra, at the wavelength of 200–700 nm in a UV-3600 Shimadzu spectrophotometer at 1-nm resolution. The reaction mixture was subjected to centrifugation at 15,000 rpm for 20 min, and the resulting pellet was dissolved in de-ionized water and filtered through a Millipore filter (0.45 μm). An aliquot of this filtrate containing silver nanoparticles was used for scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD) analyses, and energy dispersive X-ray (EDX) spectroscopy (Dinesh et al. 2015; Suresh et al. 2015).
FTIR spectra were recorded and analyzed using a Perkin-Elmer Spectrum 2000 FTIR spectrophotometer in the diffuse reflectance mode operating at a resolution of 4 cm−1. The structure and composition of freeze-dried purified silver particles was analyzed by using a 10 kV ultra-high-resolution scanning electron microscope with 25 μL of sample was sputter coated on copper stub and the images of nanoparticles were studied using a FEI QUANTA-200 SEM. SEM-EDX was conducted using a JEOL-MODEL 6390. The XRD pattern was phase-matched using match software version 1.10c Inc. Standard values are obtained from the International Centre for Diffraction Data ICDD. Hkl indices for the observed peaks were determined to be 111, 200, and 311 according to the Bragg’s reflection for face-centered cubic structure.
Toxicity of C. scalpelliformis extract and silver nanoparticles against C. quinquefasciatus
Following the methods reported in Suresh et al. (2015), 25 C. quinquefasciatus larvae (I, II, III or IV instar) or pupae were placed in a 500-mL glass beaker filled with 249 mL of dechlorinated water and 1 mL of the desired concentration of C. scalpelliformis extract or green-synthesized silver nanoparticles was added. Larval food (0.5 mg) was provided for each tested concentration. Each concentration was replicated five times against all instars. In control treatments, 25 larvae or pupae were transferred in 249 mL of dechlorinated water plus 1 mL of acetone (seaweed extract control), or 250 mL dechlorinated water (silver nanoparticle control). Percentage mortality was calculated as follows:
Predation of M. longisetus against C. quinquefasciatus
In this experiment, the predation efficiency of M. longisetus adults was assessed against C. quinquefasciatus larvae. For each instar, 100 mosquitoes were introduced, with 10 copepods, in a 500-mL glass beaker containing 250 mL of dechlorinated water. Mosquito larvae were replaced daily with new ones. For each mosquito instar, four replicates were conducted. Control was 250 mL of dechlorinated water without copepods. All beakers were checked after 1, 2, 3, 4, and 5 days and the number of prey consumed by copepods was recorded. Predatory efficiency was calculated using the following formula:
Predatory efficiency of M. longisetus after treatment with silver nanoparticles
In this experiment, the predation efficiency of M. longisetus adults was assessed against C. quinquefasciatus larvae, after a mosquitocidal treatment with silver nanoparticles. For each instar, 100 mosquitoes were introduced with 10 copepods in a 500-mL glass beaker filled with 249 mL of dechlorinated water and 1 mL of the desired concentration of C. scalpelliformis-synthesized silver nanoparticles (1 ppm, i.e., 1/3 of the LC50 calculated against first instar larvae of C. quinquefasciatus). Mosquito larvae were replaced daily with new ones. For each mosquito instar, four replicates were conducted. Control was 249 mL of dechlorinated water with 1 mL of acetone, without copepods. All beakers were checked after 1, 2, 3, 4, and 5 days and the number of prey consumed by copepods was recorded. Predatory efficiency was calculated using the above-mentioned formula.
Data analysis
Toxicity data were subjected to two-way ANOVA with two factors (i.e., the targeted mosquito instar and the tested dosage). Means were separated using Duncan’s multiple range tests by Alder and Rossler (1977). The average mosquito mortality data were subjected to probit analysis. LC50 and LC90, were calculated using the method by Finney (1971). Data were analyzed using the SPSS Statistical Software Package version 17.0. A probability level of P < 0.05 was used for the significance of differences between values.
Copepod predation data were analyzed by JMP 7 using a weighted general linear model with one fixed factor: y = Xß + ε where y is the vector of the observations (the number of consumed preys), X is the incidence matrix, ß is the vector of fixed effect (the targeted mosquito instar), and ε is the vector of the random residual effect. A probability level of P < 0.05 was used for the significance of differences between values.
Results and discussion
Toxicity of C. scalpelliformis extract against C. quinquefasciatus
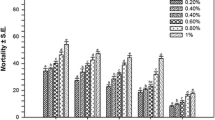
In laboratory assays, the frond ethanol extract of C. scalpelliformis was highly toxic against larval instars (I–V) and pupae of C. quinquefasciatus. Larvicidal activity was proportional to the concentration of C. scalpelliformis extract (Table 1). LC50 values were 31.38 ppm (I instar), 46.49 ppm (II), 75.79 ppm (III), 102.26 ppm (IV), and 138.89 ppm (pupa) (Table 1). To the best of our knowledge, this is the first report of mosquitocidal properties of C. scalpelliformis. However, several seaweed species have been studied for their toxic properties against mosquitoes. In agreement with our results, Kalimuthu et al. (2014) observed high mortality rates in larvae of Aedes aegypti exposed to methanol extract of the seaweed Gracilaria firma. Kumar et al. (2012) studied the larvicidal and pupicidal activity of the brown seaweed Sargassum wightii towards Anopheles sundaicus, with LC50 ranging from 0.88 mg/L (I instar) to 1.171 mg/L (pupae). Lastly, insoluble bound phenolic acids and soluble conjugated phenolic acid fractions of Chaetomorpha antennina had a larvicidal effect against A. aegypti, with LC50 of 23.4 and 44.6 μg/mL, respectively (Vimaladevi et al. 2014).
UV–vis spectrum of seaweed-synthesized silver nanoparticles
UV–vis spectrum showed maximum absorbance at 350 nm, which increased over time during the incubation of silver nitrate with the seaweed extract (Fig. 1). The fronds extract without AgNO3 did not show any change in color over time. C. scalpelliformis-mediated reduction of silver ions to silver nanoparticles was linked with changes in the UV–vis spectra. The appearance of the yellowish-brown color was an indication of formation of colloidal silver nanoparticles in the medium. The dark color may be due to the excitation of surface plasmon vibrations, typical of the silver nanoparticles (Ahmad et al. 2003; Krishnaraj et al. 2010). Our results are in agreement with previous research on color variations in fresh suspension of Vitex negundo and silver nitrate solution (Zargar et al. 2011).
Fourier transform infrared spectroscopy of seaweed-synthesized silver nanoparticles
FTIR spectra of aqueous silver nanoparticles prepared from the C. scalpelliformis frond extract exhibited prominent peaks at 3459.67, 2345.02, 1605.45, 1382.71, 1081.87, 634.466, 562.148, 519.722, 482.117, 437.762, 425.227, and 412.692 cm−1 (Fig. 2). The observed peaks were considered as major functional groups in different chemical classes such as flavonoids, triterpenoids, and polyphenols (Asmathunisha et al. 2010). A broad intense band at 3402 cm−1 in both spectra was assigned to the N–H stretching frequency arising from the peptide linkages present in the proteins of the extract (Mukherjee et al. 2008). FTIR analysis reveals that the carbonyl group from amino acid residues and proteins has the stronger ability to bind metal indicating that the proteins could possibly form a layer that cover the metal nanoparticles (i.e., capping of silver nanoparticles), preventing agglomeration and thereby stabilizing the medium. This suggests that the biological molecules could possibly perform dual functions of formation and stabilization of silver nanoparticles in the aqueous medium (Sathyavathi et al. 2010; Song et al. 2009). FTIR spectra of aqueous silver nanoparticles prepared from the C. scalpelliformis frond extract indicated that carboxyl (−C═O), hydroxyl (−OH) and amine (−NH) groups are involved in fabrication of silver nanoparticles (see also Dinesh et al. 2015; Suresh et al. 2015).
Scanning electron microscopy, energy dispersive X-ray spectroscopy, and X-ray diffraction analysis of seaweed-synthesized silver nanoparticles
Figure 3 showed a SEM micrograph of the reaction mixtures containing 50 mg of C. scalpelliformis fronds extract powder and 1.0 mM of silver nitrate incubated for 6 h magnified ×8000. Seaweed-synthesized nanoparticles were mono-dispersed with spherical and cubic structures with mean sizes of 20–35 nm. Similar morphological features of silver nanoparticles have been obtained via green-synthesis with aqueous extracts from different terrestrial plants (Chandran et al. 2006; Huang et al. 2007; Dinesh et al. 2015; Suresh et al. 2015). Recent SEM studies also showed that “capped” silver particles were stable in solution for more than 8 weeks (Suganya et al. 2013). In the spot EDX profile, strong signals from elemental silver atoms in the nanoparticles were observed (Fig. 4). Our finding is in agreement with a previous study on silver nanoparticle synthesis using the fungus Trichoderma viridae (Fayaz et al. 2010); metallic silver nanoparticles generally show typical absorption peak approximately at 3 KeV due to SPR (Magudapathy et al. 2001).
The XRD pattern of seaweed-synthesized silver nanoparticles showed a number of Bragg reflections at 2θ = 32.4, 46.4 and 28.0 (Fig. 5). They matched the face centered cubic structure of the bulk silver with the broad peaks at 32.4, 46.4, and 28.0. These corresponded to (111), (200), (311) planes, respectively. The line broadening of the peaks was primarily due to small particle size (see also Shameli et al. 2011a, b). The XRD pattern observed in this study was consistent with previous reports (Bar et al. 2009). Also, Dubey et al. (2009) reported the size of silver nanocrystals as estimated from the full width at half-maximum of (111) peak of silver using the Scherrer’s formula was 20–60 nm.
Toxicity of seaweed-synthesized silver nanoparticles against C. quinquefasciatus
C. scalpelliformis-synthesized silver nanoparticles were highly toxic against larvae and pupae of the filarial vector C. quinquefasciatus (Table 2). LC50 values were 3.08 ppm (I instar), 3.49 ppm (II), 4.64 ppm (III), 5.86 ppm (IV), and 7.33 ppm (pupa). Results of larvicidal and pupicidal assays indicated that the percentage of mortality was directly proportional to concentration of the silver nanoparticles. No mortality was observed in control treatment. To the best of our knowledge, few studies have been conducted to evaluate the toxic activity of seaweed-synthesized nanoparticles against insects and bacteria. Recently, Vinayaga Moorthi et al. (2015) reported the insecticidal activity of silver nanoparticles synthesized using Sargassum muticum against the common castor butterfly Ariadne merione, outlining changes in the protein profile of hemolymph, morphology of hemocytes, and deteriorated midgut inclusions. Furthermore, Sargassum longifolium-synthesized silver nanoparticles have been found toxic against the pathogenic fungi Aspergillus fumigatus, Candida albicans, and Fusarium sp., even at low dosages (Rajeshkumar et al. 2014).
Predation of M. longisetus against C. quinquefasciatus before and after treatment with seaweed-synthesized silver nanoparticles
M. longisetus adults actively predate C. quinquefasciatus young larval instars. The predatory efficiency per copepod per day was 7.8, 5.9, 1.4, and 0.7 larvae (I, II, III, and IV, respectively) (Table 3). Our results are in agreement with previous evidences on other species. Indeed, adult copepods have been found very effective to control young larval instars of different mosquitoes (e.g., Aedes albopictus and A. aegypti), while little predation rates have been observed against late-instar larvae (Kay et al. 1992; Schreiber et al. 1993; Marten et al. 1994; Murugan et al. 2011, 2013).
Post-treatment with seaweed-synthesized silver nanoparticles, the predatory efficiency of a single M. longisetus per day was 8.0, 6.3, 0.8, and 0.2 larvae (I, II, III, and IV, respectively) (Table 4). Also in this experiment, copepods were effective predators of first and second instars of mosquitoes, while they are not active control agents against late larval instars. Our results highlighted that a combined approach using green-synthesized silver nanoparticles and predaceous aquatic organisms is effective against the filarial vector C. quinquefasciatus. The higher predation rates of M. longisetus against C. quinquefasciatus young larvae may be due to the impact of nanoparticles treatment on the prey organism, since they can affect the physiological and metabolic activities, thus motility. This has been hypothesized also by Murugan et al. (2011), reporting higher predation rates of the copepod M. aspericornis against A. aegypti, after the treatment with neem seed kernel extract. Similarly, the predatory efficiency of a single adult copepod of M. thermocyclopoides was 6.5, 4.6, 0.76, and 0.14 C. quinquefasciatus larvae per day (I, II, III, and IV instar, respectively), while it was 8.7, 5.9, 1.2, and 0.36 larvae day (I, II, III, and IV instar, respectively), after treatment with Solanum xanthocarpum fruit extract (Mahesh Kumar et al. 2012).
Conclusions
This research highlighted that seaweed-borne compounds are highly effective against larval populations of the filarial vector C. quinquefasciatus, and can be used as effective reducing agent for the synthesis of mosquitocidal silver nanoparticles. The novel method we proposed is simple and cheap. Furthermore, we consider the predatory copepod M. longisetus as a reliable biological control agents against young larvae of C. quinquefasciatus. Interestingly, in a nanoparticle-contaminated environment, the predation efficiency of this crustacean is still high. Overall, our study firstly showed that seaweed-synthesized silver nanoparticles can be proposed in synergy with biological control agents against Culex larvae, since their use leads to little detrimental effects on predation efficacy of aquatic predators, such as copepods. However, further research is needed to shed light on long-term toxicity and sub-lethal effects of nanoparticles against copepods (Baun et al. 2008; Fabrega et al. 2011).
References
Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B: Biointerfaces 28:313–318
Alder HL, Rossler EB (1977) Introduction to probability and statistics, 6th edn. Freeman, San Francisco
Amer A, Mehlhorn H (2006a) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006b) Repellency effect of forty-one essential oils against Aedes, Anopheles and Culex mosquitoes. Parasitol Res 99:478–490
Asmathunisha N, Kathiresan K, Anburaj, Nabeel MA (2010) Synthesis of antimicrobial silver nanoparticles by callus leaf extracts from salt marsh plant Sesuvium portulacastrum L. Colloids Surf B: Biointerfaces 79:488–493
Bar H, Bhui DK, Sahoo GP, Sarkar P, Pyne S, Misra A (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 348:212–216
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17:387–395
Bazes A, Silkina A, Douzenel P, Fay F, Kervarec N, Morin D et al (2009) Investigation of the antifouling constituents from the brown alga Sargassum sp (Yendo) Fensholt. J Appl Phycol 21:395–403
Benelli G, Flamini G, Fiore G, Cioni PL, Conti B (2013a) Larvicidal and repellent activity of the essential oil of Coriandrum sativum L. (Apiaceae) fruits against the filariasis vector Aedes albopictus Skuse (Diptera: Culicidae). Parasitol Res 112:1155–1161
Benelli G, Canale A, Conti B (2013b) Eco-friendly control strategies against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae): repellency and toxic activity of plant essential oils and extracts. Pharmacologyonline 47:44–51
Benelli G, Bedini S, Cosci F, Toniolo C, Conti B, Nicoletti M (2015a) Larvicidal and ovideterrent properties of neem oil and fractions against the filariasis vector Aedes albopictus (Diptera: Culicidae): a bioactivity survey across production sites. Parasitol Res 114:227–236
Benelli G, Bedini S, Flamini G, Cosci F, Cioni PL, Amira S, Benchikh F, Laouer H, Di Giuseppe G, Conti B (2015b) Mediterranean essential oils as effective weapons against the West Nile vector Culex pipiens and the Echinostoma intermediate host Physella acuta: what happens around? An acute toxicity survey on non-target mayflies. Parasitol Res. doi:10.1007/s00436-014-4267-0
Benelli G, Murugan K, Panneerselvam C, Madhiyazhagan P, Conti B, Nicoletti M (2015c) Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol Res 114:391–397
Bowatte G, Perera P, Senevirathne G, Meegaskumbura S, Meegaskumbura M (2013) Tadpoles as dengue mosquito (Aedes aegypti) egg predators. Biol Control 67:469–47
Brown AWA (1986) Insecticide resistance in mosquitoes: a pragmatic review. J Am Mosq Control Assoc 2:123–140
Chadee DD, Williams SA, Ottesen EA (2002) Xenomonitoring of Culex quinquefasciatus mosquitoes as a guide for detecting the presence or absence of lymphatic filariasis: a preliminary protocol for mosquito sampling. Ann Trop Med Parasitol 96:47–53
Chan WCS, Nie S (1998) Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016–2018
Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M (2006) Synthesis of gold nanotriangles and silver nanoparticles using aloe vera plant extract. Biotechnol Prog 22:577–583
Chen LH, Wilson ME (2010) Dengue and chikungunya infections in travelers. Curr Opin Infect Dis 23:438–444
Chitra T, Murugan K, Naresh Kumar A, Madhiyazhagan P, Nataraj, Indumathi D, Hwang JS (2013) Laboratory and field efficacy of Pedalium murex and predatory copepod Mesocyclops longisetus on rural malaria vector Anopheles culicifacies. Asia Pac J Trop Dis 3:111–118
Coelho JS, Santos NDL, Napoleao TH, Gomes FS, Ferreira RS, Zingali RB (2009) Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti larvae. Chemosphere 77:934–938
Conti B, Flamini G, Cioni PL, Ceccarini L, Macchia M, Benelli G (2014) Mosquitocidal essential oils: are they safe against non-target aquatic organisms? Parasitol Res 113:251–259
Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, Benelli G, Chandramohan B, Suresh U (2015) Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi? Parasitol Res. doi:10.1007/s00436-015-4336-z
Dubey M, Bhadauria S, Kushwah BS (2009) Green synthesis of nanosilver particles from extract of Eucalyptus Hybrida (Safeda) leaf. Dig J Nanomater Biostruct 4(3):537–543
El-Sayed IH, Huang XH, El-Sayed MA (2005) Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett 5:829–834
Engel S, Puglisi MP, Jensen PR, Fenical W (2006) Antimicrobial activities of extracts from tropical Atlantic marine plants against marine pathogens and saprophytes. Mar Biol 149:991–1002
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R (2010) Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol Biol Med 6:103–109
Finney DJ (1971) Probit analysis. Cambridge University, London, pp 68–78
Freitas FP, Freitasb SP, Lemosb GCS, Vieirac IJC, Gravinad GA, Lemosa FJA (2010) Comparative larvicidal activity of essential oils from three medicinal plants against Aedes aegypti L. Chem Biodivers 7:2801–2807
Goodsell DS (2004) Bionanotechnology: lessons from nature. Wiley, Hoboken
Govindarajan M (2010) Larvicidal efficacy of Ficus benghalensis L plant leaf extracts against Culex quinquefasciatus Say Aedes aegypti L and Anopheles stephensi L (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 14:107–111
Hafeez F, Akram W, Abdul E, Shaalan S (2011) Mosquito larvicidal activity of citrus limonoids against Aedes albopictus. Parasitol Res 109:221–229
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18:1–11
Hurlbut HS (1938) Copepod observed preying on first instar larva of Anopheles quadrimaculatus. J Parasitol 24:281
Ireland CM, Roll DM, Molinski TF, Mckee TC, Zarbriske TM, Swersey JC (1988) Uniqueness of the marine environment: categories of marine natural products from invertebrates In: Biomedical importance of marine organisms. Calif Acad Sci, California, United States of America, pp. 41–47
Jarvis TA, Miller RJ, Lenihan HS, Bielmyer GK (2013) Toxicity of ZnO nanoparticles to the copepod Acartia tonsa, exposed through a phytoplankton diet. Environ Toxicol Chem 32:1264–1269
Jekow PS, Schaper D, Gunther P, Tavares HW (1998) Crystallization and preliminary X-ray crystallographic studies of the 13-fold symmetric portal protein of bacteriophage SPP1. Acta Crystallogr Sect D: Biol Crystallogr 54:1008–1011
Kalimuthu K, Lin SM, Tseng LC, Murugan K, Hwang J-S (2014) Bio-efficacy potential of seaweed Gracilaria firma with copepod, Megacyclops formosanus for the control larvae of dengue vector Aedes aegypti. Hydrobiologia 741:113–123
Kamaraj C, Bagavan A, Elango G, Zahir AA, Rajakumar G, Marimuthu S et al (2011) Larvicidal activity of medicinal plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Indian J Med Res 134:101–106
Kay BH, Cabral CP, Sleigh AC, Brown MD, Ribeiro ZM, Vasconcelos AW (1992) Laboratory evaluations of Mesocyclops (Cyclopoida: Copepoda) for mosquito control. J Med Entomol 29:599–602
Kim JS, Kuk E, Yu KN, Kim JS, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med 3:95–101
Kosiyachinda P, Bhumiratana A, Kittayapong P (2003) Enhancement of the efficacy of a combination of Mesocyclops aspericornis and Bacillus thuringiensis var. israelensis by community-based products in controlling Aedes aegypti larvae in Thailand. Am J Trop Med Hyg 69:206–212
Krishnaraj C, Jagan EG, Rajasekar S, Selvakumar P, Kalaichelvan PT, Mohan N (2010) Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B: Biointerfaces 76:50–56
Kumar KP, Murugan K, Kovendan K, Kumar AN, Hwang JS, Barnard DR (2012) Combined effect of seaweed (Sargassum wightii) and Bacillus thuringiensis var. israelensis on the coastal mosquito, Anopheles sundaicus, in Tamil Nadu, India. Sci Asia 38:141–146
Locantoni L, Giusti F, Cristofaro M, Pasqualini L, Esposito F, Lupetti P, Habluetzel A (2006) Effect of neem extract on blood feeding oviposition and oocyte ultra structure in Anopheles stephensi Liston (Diptera: Culicidae). Tissue Cell 38:361–371
Magana SM, Quintana P, Aguilar DH, Toledo JA, Chavez CA, Cortes MA, Leon L, Pelegrin YF, Lopez T, Sanchez RMT (2008) Antibacterial activity of montmorillonites modified with silver. J Mol Catal A 281:192–199
Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S (2001) Electrical transport studies of Ag nanoclusters embedded in glass matrix. Physica 299:142–146
Mahesh Kumar P, Murugan K, Kovendan K, Panneerselvam C, Prasanna Kumar K, Amerasan D, Subramaniam J, Kalimuthu K, Nataraj T (2012) Mosquitocidal activity of Solanum xanthocarpum fruit extract and copepod Mesocyclops thermocyclopoides for the control of dengue vector Aedes aegypti. Parasitol Res 111:609–618
Manrique-Saide PS, Ibanez-Bernal, Delfin-Gonzalez H, Parra Tabla V (1998) Mesocyclops longisetus effects on survivorship of Aedes aegypti immature stages in car tyres. Med Vet Entomol 12:386–390
Marten GG, Astaiza R, Suarez MF, Monje C, Reid JW (1989) Natural control of larval Anopheles albuminus (Diptera: Culicidae) by the predator Mesocyclops (Copepoda: Cyclopoida). J Med Entomol 26:624–627
Marten GG, Borjas G, Cush M, Fernandez E, Reid JW (1994) Control of larval Aedes aegypti in peridomestic breeding containers. J Med Entomol 31:36–44
Mukherjee P, Roy M, Mandal BP, Dey GK, Mukherjee PK, Ghatak J, Tyagia K, Kale SP (2008) Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 19:075103. doi:10.1088/0957-4484/19/7/075103
Murugan K, Hwang SJ, Kovendan K, Kumar KP, Vasugi C, Kumar AN (2011) Use of plant products and copepods for control of the dengue vector, Aedes aegypti. Hydrobiologia 666:331–338
Murugan K, Kalimuthu K, Mahesh Kumar P, Hwang JS, Nicoletti M (2013) Larval and pupal toxicity effects of Plectranthus amboinicus, Bacillus sphaericus and predatory copepods for the control of the dengue vector, Aedes aegypti. Phytoparasitica 41:307–316
Oberdorster E, Zhu S, Michelle Blickley T, McClellan-Green P, Haasch ML (2006) Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C60) on aquatic organisms. Carbon 44:1112–1120
Park J, Kim S, Yoo J, Lee JS, Park JW, Jung J (2014) Effect of salinity on acute copper and zinc toxicity to Tigriopus japonicus: the difference between metal ions and nanoparticles. Mar Pollut Bull 85:526–531
Rai MA, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Rajeshkumar S, Malarkodi C, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G (2014) Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int J Metals Article 692643:1–8
Raniello R, Mollo E, Lorenti M, Gavagnin M, Cristina BM (2007) Phytotoxic activity of caulerpenyne from the Mediterranean invasive variety of Caulerpa racemosa: a potential allelochemical. Biol Invasions 9:361–368
Ravikumar S, Ramanathan G, Subhakaran M, Inbaneson SJ (2009) Antimicrobial compounds from marine halophytes for silkworm disease treatment. Int J Med Sci 1:184–191
Ravikumar S, Abideen S, Ali MS, Selvam MB (2010) In vitro human sperm immobilizing activity of marine halophytes. J Pharm Res 4:1291–1293
Ravikumar S, Ali MS, Beula JM (2011) Mosquito larvicidal efficacy of seaweed extracts against dengue vector of Aedes aegypti. Asia Pac J Trop Biomed 1:S143–S146
Rawlins SC, Martinez R, Wiltshire S, Clarke D, Prabhakar P, Spinks M (1997) Evaluation of Caribbean strains of Macrocyclops and Mesocyclops (Cyclopoida: Cyclopidae) as biological control tools for the dengue vector Aedes aegypti. J Am Mosq Control Assoc 13:18–23
Riviere F, Thirel R (1981) La predation du copepods Mesocyclops leuckarti pilosa sur les larves de Aedes (Stegomyia) aegypti et Ae. St. polynesiensis essais preliminaries d’utilization comme de lutte biologique. Entomophaga 26:427–439
Sathyavathi R, Balamurali Krishna M, Venugopal Rao S, Saritha R, Narayana Rao (2010) Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett 3:138–143
Schaper S (1999) Evaluation of Costa Rican copepods (Crustacea: Eudecapoda) for larval Aedes aegypti control with special reference to Mesocyclops thermocyclopoides. J Am Mosq Control Assoc 15:510–519
Schreiber ET, Thrner WL, Lopez AM, Hallmon CE, Marten GG (1993) Evaluation of two cyclopoid copepods for Aedes albopictus control in tires in the panhandle of Florida at low introduction rates. J Fla Mosq Control Assoc 64:7317
Shameli K, Ahmad MB, Yunus WMZW, Ibrahim NA, Zargar M (2011) Int J Nanomed (6) 581–590
Shameli K, Ahmad MS, Zargar M, Yunus WMZW, Rustaiyan A, Ibrahim NA (2011b) Synthesis of silver nanoparticles in montmorillonite and their antibacterial behavior. Int J Nanomedicine 6:581–590
Shankar SS, Ahmad A, Sastry M (2003) Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog 19:1627–1631
Shankar SS, Rai A, Ahmad A, Sastry M (2004) Biosynthesis of silver and gold nanoparticles from extracts of different parts of the geranium plant. Appl Nano Sci 1:69–77
Shirkhanzadeh M, Azadegan M, Liu GQ (1995) Bioactive delivery systems for the slow release of antibiotics: incorporation of Ag ions into micro-porous hydroxyapatite coatings. Mater Lett 24:7–12
Song YJ, Jang HK, Kim SB (2009) Biological synthesis of gold nanopaticles using Magnolia kobus and Diopyros kaki leaf extract. Process Biochem 44:1133–1138
Soni N, Prakash S (2012) Efficacy of fungus mediated silver and gold nanoparticles against Aedes aegypti larvae. Parasitol Res 110:175–184
Soumare MKF, Cilek JE (2011) The effectiveness of Mesocyclops longisetus (Copepoda) for the control of container-inhabiting mosquitoes in residential environments. J Am Mosq Control Assoc 27:376–383
Suganya A, Murugan K, Kovendan K, Mahesh Kumar P, Hwang JS (2013) Green synthesis of silver nanoparticles using Murraya koenigii leaf extract against Anopheles stephensi and Aedes aegypti. Parasitol Res 112:1385–1397
Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, Mahesh Kumar P, Subramaniam J, Dinesh D, Chandramohan B (2015) Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res. doi:10.1007/s00436-015-4339-9
Tennyson S, Ravindran KJ, Arivoli S (2012) Screening of twenty five plant extracts for larvicidal activity against Culex quinquefasciatus Say (Diptera:Culicidae). Asia Pac J Trop Biomed 2:S1130–S1134
Vaseashta A, Dimova-Malinovska D (2005) Nanostructured and nanoscale devices sensors and detectors. Sci Technol Adv Mater 6:312–318
Vimaladevi S, Mahesh A, Dhayanithi BN, Karthikeyan N (2014) Mosquito larvicidal efficacy of phenolic acids of seaweed Chaetomorpha antennina (Bory) Kuetz. against Aedes aegypti. Biologia 67:212–216
Vinayaga Moorthi P, Balasubramanian C, Mohan S (2015) An improved insecticidal activity of silver nanoparticle synthesized by using Sargassum muticum. Appl Biochem Biotechnol 175:135–140
WHO (2014) Lymphatic filariasis. Fact sheet N°102
Williamson CE (1999) Ecology and classification of North American freshwater invertebrates. Academic, San Diego, pp 787–822
Zargar M, Hamid AA, Bakar FA, Shamsudin MN, Shameli K, Jahanshiri F, Farahani F (2011) Green synthesis and antibacterial effect of silver nanoparticles using Vitex negundo L. Molecules 16:6667–6676
Acknowledgments
Kadarkarai Murugan is grateful to the Department of Science and Technology (New Delhi, India), Project No. DST/SB/EMEQ-335/2013, for providing financial support. Giovanni Benelli is supported by a Mis. 124 MODOLIVI Grant. Funds were also provided by the Italian Ministry of Education, University and Research (MIUR). Funds were also provided by the Italian Ministry of Education, University and Research (MIUR). Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Compliance with ethical standards
The authors declare no conflicts of interest. All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murugan, K., Benelli, G., Ayyappan, S. et al. Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus . Parasitol Res 114, 2243–2253 (2015). https://doi.org/10.1007/s00436-015-4417-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4417-z