Abstract
Despite that chironomids are the most widely used benthic insect test species worldwide, little research has been conducted so far with tropical chironomid representatives. This study was designed to evaluate the indigenous midge Chironomus sancticaroli as a candidate test species for use in tropical toxicity assessments. To this end, laboratory water-only toxicity tests were conducted evaluating copper and cadmium. Obtained lethal concentration values were overall comparable or lower than those reported for other chironomids, including those most commonly used in temperate regions (C. riparius and C. dilutus). In addition, C. sancticaroli was deployed in situ in the Monjolinho River (São Paulo State, Brazil), and toxicity of sediment from this river was evaluated in the laboratory. Several field water and sediment quality parameters also were measured to enable correlating these with the effects observed in these toxicity tests. Field sediment toxicity to C. sancticaroli appeared to be related with sediment endosulfan concentrations, whereas effects noted in the in situ test were likely due to low pH values measured in river water. Chironomus sancticaroli appears to be a suitable candidate for inclusion as a test species in tropical toxicity evaluations in both the laboratory and the field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Human activities (industrial, agricultural, and urban) have resulted in an increase in the release of various pollutants that eventually may find their way into natural waters and hence into those sediments (Ma et al. 2011). Sediments are important parts of aquatic ecosystems because of the niche provided for a variety of aquatic organisms, including economically important species as well as species that play a key role in the food web (Burton et al. 2003; Martínez-Jerónimo et al. 2008). Protecting sediment quality has been viewed as a needed extension of water-quality protection (US-EPA 2000; EFSA 2015).
Aquatic test species may be used in laboratory bioassays to derive toxicity data to set regulatory thresholds for both the water and the sediment compartments (e.g., EFSA 2013, 2015). They also may serve as indicators in biomonitoring programs by testing their sensitivity in situ or by evaluating their response to field-collected samples in the laboratory (Di Veroli et al. 2014; Roig et al. 2015). Insect larvae have been considered to be especially suitable as test species, because they spend most of their critical period of their development in the water where they are exposed to chemicals through both the water and the sediment (Abdo et al. 2011; Di Veroli et al. 2014).
Among those benthic insect groups that predominantly occur in freshwater, Chironomus species are the most widely used standard test species in both water and sediment testing (EFSA 2013, 2015). Their biology and ecology are well-described, and they mainly feed on decomposing organic matter, detritus, and bacteria that are swallowed with sediment particles (Tachet et al. 2010). Chironomids influence productivity, alter the physical and chemical conditions of the sediment and sediment–water interface, and transfer energy to higher trophic levels (Wetzel 2001). Thus, contamination of chironomid habitats could result in a reduction in food items for aquatic and terrestrial fauna and disruption of an important link in aquatic food web (Shuhaimi-Othman et al. 2011).
Chironomidae is a family of insects, whose larvae and pupal stages are present in continental aquatic systems worldwide (Fargasová 2001). Despite this, test protocols and research efforts have focused on few species, such as C. riparius (most frequently used in Europe), C. dilutus (formerly C. tentans; most frequently used in North America), and C. yoshimatsui (frequently used in Japan) (EFSA 2015). These species, however, are restricted to temperate areas (Domingues et al. 2008). Given the enormous natural variability in the structure and function of freshwater communities, it often has been questioned whether the set of standard test species generally used in temperate regions is appropriate for tropical ecosystems (Moreira-Santos et al. 2005; Daam and Van den Brink 2010; Daam and Rico 2016). Besides a possible difference in sensitivity to chemical stress between temperate and tropical species, the use of indigenous species in tropical effect assessments has been recommended for several other reasons, including: (1) a more ecologically relevant assessment of the true sensitivity and subsequently the potential risk of tropical freshwater life; (2) direct availability and hence less logistic constraints; and (3) to avoid introducing temperate exotics in tropical ecosystems (Moreira et al. 2017 and references therein).
Chironomus sancticaroli (synonym: C. domizzi) is a species closely related to C. xanthus, which has even been considered a synonym (Trivinho-Strixino et al. 2000). Chironomus xanthus was originally described by Rempel (1939) and is distributed over South America, including Brazil (Fonseca and Rocha 2004; Moreira-Santos et al. 2005). The life cycle of this species, as well as their culture and maintenance procedure, have previous been established (Fonseca and Rocha 2004). In addition, the few studies that have been conducted so far with this species provided indications for its usefulness in both laboratory (Silvério et al. 2005; Sotero-Santos et al. 2007; Novelli et al. 2012) and in situ testing (Moreira-Santos et al. 2005).
The primary objective of the present study was to evaluate the suitability of C. sancticaroli to substitute the temperate C. riparius and C. dilutes as a chironomid test species in tropical toxicity evaluations. To this end, water-only laboratory toxicity tests were conducted with copper and cadmium to establish lethal concentration 96 h LC50 values, for C. sancticaroli, which were subsequently compared with those reported for other chironomid species. In addition, in situ tests were conducted in the Monjolinho River (São Paulo State, Brazil) and laboratory tests with sediment taken from this river. Observed effects on C. sancticaroli survival in the latter tests were subsequently correlated with water and sediment quality parameters measured in the field site.
Materials and Methods
Culture and Maintenance of Test Organisms
Chironomus sancticaroli larvae were obtained from in-house cultures that had been maintained for several years in the laboratories of the Nucleus of Ecotoxicology and Applied Ecology at the University of São Paulo (São Carlos, Brazil). The C. sancticaroli cultures were maintained in plastic trays containing natural sediment and reconstituted water (pH between 6.5 and 7.0; conductivity between 25 and 55 µS cm−1 and hardness between 12 and 15 mg CaCO3 L−1). The natural sediment was composed of sterilized fine sand (sterilized at 550 °C for 2 h) that had previously been obtained from a nonpolluted lake (Novelli et al. 2012). The culture was maintained under constant aeration with controlled temperature (24 ± 1 °C) and photoperiod (12:12 h light:dark). Larvae were fed every other day with a Tetramin® fish food solution (0.04 mg mL−1; Fonseca and Rocha 2004).
Acute Toxicity Tests with Waterborne Copper, Cadmium, and Reference Compound
Toxicity of copper (as CuSO4·5H2O; purity 100%; Mallinckrodt) and cadmium (as Ca(NO3)·4H2O; purity 100%; Mallinckrodt) was evaluated by testing a concentration series of each compound in 96-h static tests. Copper and cadmium were selected as test compounds, because they are common contaminants in aquatic environments (Watts and Pascoe 2000; Vogt et al. 2010), including the Monjolinho River that was used as field site in the present study (see "Field Site and Physical–Chemical Analysis" section; Fracácio et al. 2009). Fracácio et al. (2009) noted that cadmium (1.2 µg/L) and copper (19.2 µg/L) concentrations in this river were close the water quality criteria values established by Brazilian Environmental Standards. The source of these elements could be associated with anthropic activities in the catchment area (e.g., sugarcane cultivation, electroplating, metallurgy, and manufacture of plastic pipes) (Campagna et al. 2008; Printes et al. 2011). Cadmium is a highly toxic element with a high potential for bioaccumulation (Moiseenko and Gashkina 2018). Although copper is an essential element, at elevated concentrations, it is known to exert toxic effects in aquatic organisms (Majumdar and Gupta 2012). Due to their application in a variety of industrial activities and their high toxic potential, research regarding the risks of Cd and Cu to aquatic biota is of great environmental relevance.
Toxicity data for chironomid taxa other than C. sancticaroli were available and subsequently obtained from the U.S. Environmental Protection Agency (US-EPA) ECOTOX database (https://cfpub.epa.gov/ecotox/), the largest database of its kind currently available. This hence allowed a comparison of the sensitivity of C. sancticaroli to copper and cadmium with other (mostly temperate) chironomid taxa. The five concentrations that were tested for copper (0.1, 0.2, 0.4, 0.8, and 1.6 mg/L) and cadmium (0.1, 0.25, 0.5, 0.75, and 3.0 mg/L) were based on preliminary range-finding tests. Besides these metal treatments, an uncontaminated treatment (culture medium) was included to serve as control. Each treatment was conducted with three replicas, in which each replicate consisted of a 250-mL glass jar containing 240-mL test solution and six chironomid larvae (IV instar—7/8 days). Tests were conducted under the same temperature and light conditions as described above for the culture with food only the first day. The tests lasted 96 h, after which the living organisms were counted. Ten definitive tests were conducted to determine the reproducibility of the established 96-h LC50 values. Acute tests with the reference substance potassium chloride, a compound with known toxicity to C. sancticaroli, also were conducted during the tests to evaluate the physiological conditions of the test organisms, thus validating the results of toxicity tests.
Field Site and Physical–Chemical Analysis
The Monjolinho River (São Paulo State, Brazil) was selected as the study site, because it receives industrial wastes (paints, tannery compounds, paper, and others) as well as raw sewage and because several pollutants in the river have previously been indicated to potentially induce ecological effects (Fracácio et al. 2009; Campanha et al. 2015). For the field assay, six sampling points were selected along the Monjolinho River (Fig. 1). Four points (A, D, E, and F) were located near urban areas and two points (B and C) in the rural area. In situ test deployments (see "Laboratory Tests Evaluating Field Sediment Samples" section) and sampling of water and sediment were conducted on two occasions: one in the dry season (July) and one in the wet season (January).
Location of the sampling locations at the field site (Monjolinho River, São Paulo State, Brazil; adapted from Printes et al. 2011)
Water samples were collected by means of a Van Dorn water sampler. Temperature, dissolved oxygen, conductivity, and pH were measured with a multi-parameter water quality (Horiba U-10) just after water collection. Water samples for determination of nutrients, metals, and pesticides were transported on ice and in darkness for further analysis in the laboratory. Total nitrogen and total phosphorus were determined according to method described in APHA/AWWA/WCPF (1995). Metal concentrations (cadmium, chromium, copper, iron, and zinc) were measured by atomic absorption spectrometry (Varian AA 220). The concentrations of organochlorine pesticides were quantified by liquid chromatography (Shimadzu SCL-10A) attached to an SPD-10A UV detector and confirmed by GC–MS (Shimadzu QP2010) using the operating parameters as provided and validated in Lanças (1997). These organochlorine pesticides and metals were included for analyses in the present study based on significant concentrations measured in a previous study conducted in our field site (Fracácio et al. 2009).
Top sediment was collected from the six stations using an Ekman dredge and transported to the laboratory in sealed plastic bags. In the laboratory, granulometry and organic matter content were determined in subsamples according to ABNT (2016) and Trindade (1980), respectively. Metal concentrations (cadmium, chromium, copper, iron, and zinc) in the sediment were determined through atomic absorption spectrometry (Varian AA 220; APHA/AWWA/WCPF 1995). Organochlorine pesticides were extracted from the sediment matrix by ultrasound-assisted solid phase extraction (SPE) using C-18 columns (US-EPA 1996; Printes et al. 2011). Subsequently, their concentrations were determined by liquid chromatography (SCL-10A-SHIMADZU) with a UV-SPD-10A detector and confirmed using a GC–MS, model QP2010-SHIMADZU (Printes et al. 2011).
Laboratory Tests Evaluating Field Sediment Samples
For the acute static tests evaluating the toxicity of the sediment collected in the Monjolinho River to C. sancticaroli, six IV instar larvae were transferred to test vessels containing 60 g of field sediment and 240 mL of reconstituted water. Three replicates were used for the sediment of each field site following the protocol of Novelli et al. (2012). A control treatment with reconstituted water and the natural sediment used in the culture also was included. The larvae were kept at a room temperature of 24 ± 1 °C and photoperiod of 12:12 h light/dark, with food only provided on the first day. The test lasted 96 h after which the number of dead organisms in each treatment was registered.
In Situ Tests
The methodology adopted for the in situ tests was based on that described in Domingues et al. (2008) and adapted for local conditions. Test chambers consisted of a 30-cm PVC tube with a diameter of 7 cm. Both ends and two lateral windows were covered with a 300-µm nylon net mesh. Three replicated chambers containing ten IV star chironomid larvae each were placed at each site by attaching them to a wooden stake that was fixed in the sediment. The day before the start of the assay, the chambers were placed in selected study sites (only in the rainy season at sites A, B, and F; Fig. 1). Before placing of the chambers, sediment devoid of visible organisms (removed by hand-picking) was added to the test chambers. At the start of the assay, the C. sancticaroli larvae were deployed in the chambers and their survival was recorded 96 h after deployment.
Data Analysis
The results of the acute toxicity test evaluating waterborne copper, cadmium and potassium chloride were analyzed using the trimmed Spearman-Karber method and expressed as 96 h-LC50 (Hamilton et al. 1977). Difference in survival between the laboratory test evaluating field sediment and the in situ test of each site was assessed with the Student’s t test. Statistical analyses were performed with a significance level of 0.05. All statistical tests were performed with the Toxstat 3.3 software (Gulley et al. 1994).
Results and Discussion
Acute Sensitivity of C. sancticaroli to Copper and Cadmium
Mortality in the control treatments of the toxicity bioassays was low and always less than 10%. The test with the reference substance potassium chloride indicated a 96 h-LC50 value 4.1 g/L (95% confidence interval [CI] 3.5–4.9 g/L), which is within the acceptable range of 2.6–6.3 g/L (Novelli et al. 2012).
The 96-h LC50 values derived for C. sancticaroli were 300 µg/L (95% CI 230–400 µg/L) for copper and 700 µg/L (95% CI 520–970 µg/L) for cadmium. To compare the sensitivity of C. sancticaroli to these metals with other chironomid species, 96-h LC50 toxicity data for chironomids were extracted from the ECOTOX database (https://cfpub.epa.gov/ecotox/). Subsequently, species sensitivity distributions (SSDs) were constructed as described in Vasconcelos et al. (2016). As can be deducted from the resulting SSD curves, the difference between the most sensitive and the least sensitive chironomid taxon was over three orders of magnitude for both metals (Fig. 2).
Species sensitivity distributions (SSD) constructed based on 96h-EC50 values (geometric means) for copper and cadmium obtained in the present study for C. sancticaroli (in bold), supplemented with data for other chironomid species obtained from the US-EPA database (https://cfpub.epa.gov/ecotox/). SSD curves were constructed as described in Vasconcelos et al. (2016)
Compared with the standard chironomid test species most commonly used in temperate regions, C. riparius (OECD 2011), C. dilutus (US-EPA 2000), and C. sancticaroli was 7 times less sensitive to copper than the former and 11 times more sensitive than the latter. For cadmium, C. sancticaroli was 11 and 124 times more sensitive than C. dilutes and C. riparius, respectively (Fig. 2). Chironomus sancticaroli hence appears to be a sensitive chironomid candidate for tropical laboratory testing. In addition, this species may not be fully protected by the assessment factor of 100 that is applied to the acute toxicity value of C. riparius to calculate the regulatory acceptable concentration (RAC) for this species (EFSA 2013). It should be noted, however, that differences in experimental test conditions employed in water-only exposures, such as age and condition of the test organisms, and factors affecting bioavailability (i.e., water hardness, pH, temperature, feeding) have resulted in a wide range of reported LC50 values for the same or closely related species of benthic invertebrates, making comparisons difficult (Milani et al. 2003 and references therein; Weltje et al. 2010). In the present study, for example, soft reconstituted water was used as test medium, whereas other studies included in the SSD analysis used moderately hard reconstituted water in their experiments. Generally, metals are less toxic to organisms in hard water than in soft water, as has previously been demonstrated for C. riparius (Gillis and Wood 2008; Leonard and Wood 2013) and C. javanus (Shuhaimi-Othman et al. 2011). This greater metal toxicity in soft water has been related with a lower competition between the metal and the Mg2+ and Ca2+ ions for the uptake sites in the aquatic invertebrates (Kozlova et al. 2009; Rodgher et al. 2010).
Toxicity of Field Sediment
The field sediment only showed a significant effect on C. sancticaroli survival in samples taken in July at sampling points C and D (Fig. 3). In line with this, concentrations of the metals and organochlorine pesticides were generally low in the sediment field samples (Table 1). This is probably related with the predominance of sand and low organic matter content of the river sediment, because such sediment characteristics have been related to low levels of metal and organophosphate than sediments constituted of silt and clay (Bentivegna et al. 2004; Yang et al. 2015). The effects on C. sancticaroli survival noted in the sediment samples taken in July at sampling points C and D are most likely due to the endosulfan concentrations measured in these samples (Table 1), although an additional mixture effect of other contaminants that are known from previous studies to be possibly present in low concentrations (Fracácio et al. 2009; Campanha et al. 2015) cannot be excluded. Endosulfan concentrations in these samples were 5.9 and 9.1 µg/kg, respectively, whereas endosulfan concentrations higher than 6 µg/kg have been demonstrated to cause toxicity to C. dilutes (GFA 2004). Although endosulfan has been banned in several countries, including the EU (EC 2018), it is still allowed for use in Brazil as foliar application to combat ants in cotton, coffee, sugar cane, and soy crops (AGROFIT 2018).
Endosulfan and toxicity to C. sancticaroli were detected in the samples taken in the dry season (July) of sampling points C and D but not in the samples taken in the rainy season of these sampling points (Table 1; Fig. 3). This could be associated with the low water flow in the dry season, which allows the pollutants to precipitate and accumulate in the river bed. In line with this, Richardson et al. (1998) correlated toxicity to C. dilutus in sediment from the Brunette river (Canada) to high pollutant concentrations during low water flow periods. Riani et al. (2014) also only found chronic toxic effects related to heavy metal pollution to the chironomid Dicrotendipes simpsoni in the dry season and attributed the lack of effects in the rainy season to dilution effects.
In-Situ Tests
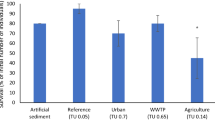
The survival of C. sancticaroli 96 h after their deployment in the river water is illustrated in Fig. 4. To allow identifying the respective influence of water and sediment characteristics on C. sancticaroli, the corresponding survival denoted in the laboratory toxicity tests with the sediment samples from these sample locations are also shown (Fig. 4). Although a significant reduction in chironomid survival was recorded from the in situ tests conducted at sampling points A and B in the rainy season (survival 40% and 30%, respectively), no effects was obtained in the corresponding sediment samples (survival 95% and 100%, respectively; Fig. 4).
Survival (in % of the 30 larvae deployed at each site) of C. sancticaroli in the in situ tests and the acute toxicity test conducted with field-collected sediment samples in the dry (July) and wet (January) seasons. Asterisks indicate significant differences from the control treatment (Student’s t test, p < 0.05)
From the water characteristics included in the present study, the low pH values measured in the rainy season at sampling points A and B appear to be the most discriminative (Table 2). Many species of chironomids are known to be tolerant of a wide range of pH between 6.0 and 9.0, but outside this range decreasing pH results in the occurrence of fewer species (Pinder 1986; Cardwell et al. 2018 and references therein). The low pH levels at points A (pH = 4.8) and B (pH = 5.5) compared with point F (pH = 7.4) in the rainy season are hence likely to have at least played a role in the observed toxicity in the former. In addition, the toxicity and bioavailability metals to aquatic biota are known to be influenced by physical and chemical characteristics of water (temperature, pH, organic carbon) (Tonietto et al. 2014, Santore et al. 2018). Because water at points A and B were acidic, it is possible that metals from the sediment were released to the overlying water column and that the bioavailable fraction bioavailable of the metal ions to benthic species was enhanced (Peck et al. 2002; Khosrovyan et al. 2014).
Conclusions
The water-only laboratory bioassays indicated that C. sancticaroli is a sensitive representative of chironomid taxa. In addition, the laboratory tests evaluating field sediment and the in situ tests also demonstrated that this species was able to indicate unfavourable field water and sediment conditions. C. sancticaroli may hence be considered a suitable candidate as a tropical chironomid test species in acute toxicity testing. Future studies are needed to evaluate this suitability for chronic toxicity assessments.
References
Abdo SAS, Salmah MRCS, Hassan AA, Azizah MNS (2011) Evaluation of mentum deformities of Chironomus spp. (Chironomidae: Diptera) larvae using modified toxic score index (MTSI) to assess the environmental stress in Juru River Basin, Penang, Malaysia. Environ Monit Assess 177:233–244
ABNT (2016) Análise granulométrica de solos. NBR7181. Associação Brasileira de Normas Técnicas, Rio de Janeiro
AGROFIT (2018) Ministry of Agriculture, Livestock and Supply in Brazil. http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 2 Mar 2018
APHA/AWWA/WCPF (1995) Standard methods for examination of water and wastewater, 20th edn. American Public Health Association; American Water Work Association; Water Control Federation, Washington
Bentivegna CS, Alfano JE, Bugel SM, Czechowicz K (2004) Influence of sediment characteristics on heavy metal toxicity in an Urban Marsh. Urban Habitats 2:91–111
Burton GA Jr, Denton DL, Ho K, Ireland DS (2003) Sediment toxicity testing: issues and methods. In: Hoffman DJ, Rattner GA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology. Lewis Publishers, New York, pp 111–150
Campagna AF, Fracácio R, Rodrigues BK, Eler MN, Verani NF, Espíndola ELG (2008) Analyses of the sediment toxicity of Monjolinho River, São Carlos, São Paulo State, Brazil, using survey, growth and gil morphology of two fish species. Braz Arch Biol Technol 51:193–201
Campanha MB, Awan AT, de Sousa DNR, Grosseli GM, Mozeto AA, Fadini PS (2015) A 3-year study on occurrence of emerging contaminants in an urban stream of São Paulo State of Southeast Brazil. Environ Sci Pollut Res 22:7936–7947
Cardwell AS, Adams WJ, Gensemer RW, Nordheim E, Santore RC, Ryan AC, Stubble WA (2018) Chronic toxicity of aluminum, at a pH of 6, to freshwater organisms: empirical data for the development of international regulatory standards/criteria. Environ Toxicol Chem 37:36–48
Daam MA, Rico A (2016) Freshwater shrimps as sensitive test species for the risk assessment of pesticides in the tropics. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-016-7451-1
Daam MA, Van den Brink PJ (2010) Implications of differences between temperate and tropical freshwater ecosystems for the ecological risk assessment of pesticides. Ecotoxicology 19:24–37
Di Veroli F, Santoro M, Pallottini R, Selvaggi F, Scardazza D, Cappelletti E, Goretti J (2014) Deformities of chironomid larvae and heavy metal pollution: from laboratory to field studies. Chemosphere 112:9–17
Domingues I, Satapornvanit K, Yakupitiyage A, Soares AMVM (2008) In situ assay with the midge Kiefferulus calligaster for contamination evaluation in aquatic agro-systems in central Thailand. Chemosphere 71:1877–1887
EC (2018) EU pesticide database. European Commission. http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN. Accessed 2 Mar 2018
EFSA (2013) Guidance on tiered risk assessment for plant protection products for aquatic organisms in the edge-of-field surface waters. EFSA J 11:3290
EFSA (2015) Scientific opinion on the effect assessment for pesticides on sediment organisms in edge-of-field surface water. EFSA J 13:41–76
Fargasová A (2001) Winter third-to fourth-instar larvae of Chironomus plumosus as bioassay tools for assessment of acute toxicity of metals and their binary combinations. Ecotoxicol Environ Saf 48:1–5
Fonseca AL, Rocha O (2004) Laboratory cultures of the native species Chironomus xanthus Rempel, 1939 (Diptera-Chironomidae). Acta Limnol Bras 16:153–161
Fracácio R, Rodriguês BK, Campagna AF, Verani NF, Dornfeld CB, Espíndola ELG (2009) In situ and laboratory evaluation of toxicity with Danio rerio Buchanan (1822) and Poecilia reticulata Peters (1859). Acta Limnol Bras 21:111–122
GFA (2004) Endosulfan. Draft Dossier prepared in support of a proposal of endosulfan to be considered as a candidate for inclusion in the UN-ECE LRTAP protocol on persistent organic pollutants. Germany Federal Agency, Germany
Gillis PL, Wood CM (2008) Investigating a potential mechanism of Cd resistance in Chironomus riparius larvae using kinetic analysis of calcium and cadmium uptake. Aquat Toxicol 89:180–187
Gulley DD, Boelter AM, Bergman HL (1994) Toxtat 3.4 computer program
Hamilton MA, Russo RC, Thurston RV (1977) Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays. Environ Sci Technol 11:714–719
Khosrovyan A, DelValls TA, Ribas I (2014) Effects of simulated CO2 escape from sediments on the development of midge Chironomus riparius. Aquat Toxicol 156:230–239
Kozlova T, Wood CM, McGeer JC (2009) The effect of water chemistry on the acute toxicity of nickel to the cladoceran Daphnia pulex and the development of a biotic ligand model. Aquat Toxicol 91:221–228
Lanças FM (1997) Extração em fase sólida. University of São Paulo, São Carlos
Leonard EM, Wood CM (2013) Acute toxicity, critical body residues, Michaelis–Menten analysis of bioaccumulation, and ionoregulatory disturbance in response to waterborne nickel in four invertebrates: Chironomus riparius, Lymnaea stagnalis, Lumbriculus variegatus and Daphnia pulex. Comp Biochem Physiol C: Toxicol Pharmacol 158:10–21
Ma Y, Qin Y, Zheng BH, Zhang L, Zhao YM (2011) Seasonal variation of enrichment, accumulation and sources of heavy metals in suspended particulate matter and surface sediments in the Daliao river and Daliao river estuary, Northeast China. Environ Earth Sci 73:5107–5117
Majumdar TN, Gupta A (2012) Acute and chronic toxicity of copper on aquatic insect Chironomus ramosus from Assam, India. J Environ Biol 33:139–142
Martínez-Jerónimo F, Cruz-Cisneros JL, García-Hernández L (2008) A comparison of the response of Simocephalus mixtus (Cladocera) and Daphnia magna to contaminated freshwater sediments. Ecotoxicol Environ Saf 71:26–31
Milani D, Reynoldson TB, Borgmann U, Kolasa J (2003) The relative sensitivity of four benthic invertebrates to metals in spiked-sediment exposures and application to contaminated field sediment. Environ Toxicol Chem 22:845–854
Moiseenko TI, Gashkina NA (2018) Biogeochemistry of cadmium: anthropogenic dispersion, bioaccumulation, and ecotoxicity. Geochem Int 5:798–811
Moreira RA, Daam MA, Vieira BH, Sanches ALM, Reghini MV, Mansano AS, Freitas EC, Espindola ELG, Rocha O (2017) Toxicity of abamectin and difenoconazole mixtures to a Neotropical cladoceran after simulated run-off and spray drift exposure. Aquat Toxicol 185:58–66
Moreira-Santos M, Fonseca AL, Moreira SM, Osten JRV, Da Silva EM, Soares AMVM, Guilhermino LRR (2005) Short-term sublethal (sediment and aquatic roots of floating macrophytes) assays with a tropical chironomid based on postexposure feeding and biomarkers. Environ Toxicol Chem 24:2234–2242
Novelli A, Vieira BH, Cordeiro D, Cappelini LTD, Vieira EM, Espindola ELG (2012) Lethal effects of abamectin on the aquatic organisms Daphnia similis, Chironomus xanthus and Danio rerio. Chemosphere 86:36–40
OECD (2011) Chironomus sp., acute immobilisation test. OECD guideline for the testing of chemicals, No. 235. Organization for Economic Co-operation and Development, Paris, France
Peck MR, Klessa DA, Baird DJ (2002) A tropical sediment toxicity test using the dipteran Chironomus crassiforceps to test metal bioavailability with sediment pH change in tropical acid-sulfate sediments. Environ Toxicol Chem 21:720–728
Pinder LCV (1986) Biology of freshwater Chironomidae. Ann Rev Entomol 31:1–23
Printes LB, Fernandes MN, Espíndola ELG (2011) Laboratory measurements of biomarkers and individual performances in Chironomus xanthus to evaluate pesticide contamination of sediments in a river of southeastern Brazil. Ecotoxicol Environ Saf 74:424–430
Rempel JG (1939) Neue Chironomiden aus Nordostbrasilien. Zool Anz 127:209–216
Riani E, Sudarso Y, Cordova M (2014) Heavy metals effect on unviable larvae of Dicrotendipes simpsoni (Diptera: Chironomidae), a case study from Saguling Dam, Indonesia. AACL Bioflux 7:76–84
Richardson JS, Hall KJ, Kiffney PM, Smith JA, Keen P (1998) Ecological impacts of contaminants in an urban watershed. DOE FRAP 1998-25. Environment Canada, Vancouver
Roberta B, Benedetta P, Silvia Q (2014) An ecotoxicological approach to assess the environmental quality of freshwater basins: a possible implementation of the EU water framework directive? Environments 1:92–106
Rodgher S, Espíndola ELG, Lombardi AT (2010) Suitability of Daphnia similis as an alternative organism in ecotoxicological tests: implications for metal toxicity. Ecotoxicology 19:1027–1033
Roig N, Sierra J, Nadal M, Nieto E, Hampel M, Gallego EP, Schuhmacher M, Blasco J (2015) Assessment of sediment ecotoxicological status as a complementary tool for the evaluation of surface water quality: the Ebro river basin case study. Sci Total Environ 503–504:269–278
Santore RC, Ryan AC, Kroglund F, Rodriguez PH, Stubblefield WA, Cardwell A, Nordheim E (2018) Development and application of a biotic ligand model for predicting the chronic toxicity of dissolved and precipitated aluminium to aquatic organisms. Environ Toxicol Chem 37:70–79
Shuhaimi-Othman M, Yakub N, Umirah NS, Abas A (2011) Toxicity of eight metals to Malaysian freshwater midge larvae Chironomus javanus (Diptera Chironomidae). Toxicol Ind Health 27:879–886
Silvério PF, Fonseca AL, Botta-Paschoal CMR, Mozeto AA (2005) Release, bioavailability and toxicity of metals in lacustrine sediments: a case study of reservoirs and lakes in Southeast Brazil. Aquat Ecosyst Health Manag 8:313–322
Sotero-Santos RB, Rocha O, Povinelli J (2007) Toxicity of ferric chloride sludge to aquatic organisms. Chemosphere 68:628–636
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertébrés d’eau douce: systématique, biologie, écologie. CNRS, Paris
Tonietto AE, Lombardi AT, Viera AAH, Parrish CC, Choueri RB (2014) Cylindrospermopsis raciborskii (Cyanobacteria) exudates; Chemical characterization and complexation capacity for Cu, Zn, Cd and Pb. Water Res 49:381–390
Trindade M (1980) Nutrientes em sedimento da represa do Lobo (Brotas/Itirapina, SP). Dissertation, Federal University of São Carlos
Trivinho-Strixino S, Correia LCS, Sonoda K (2000) Phytophilous chironomidae (Diptera) and other macroinvertebrates in the oxbow Infernão Lake (Jataí Ecological Station, Luiz Antônio, SP, Brazil). Rev Bras Biol 60:527–535
US-EPA (1996) Test methods for evaluating solid waste, physical/chemical methods. EPSW-8463. United States Environmental Protection Agency, Washington, DC
US-EPA (2000) Methods for measuring the toxicity and bioaccumulation of sediment-associated contaminants with freshwater invertebrates, 2nd edn. United States Environmental Protection Agency, Washington, DC, USA
Vasconcelos AM, Daam MA, Santos LRAS, Sanches ALM, Araújo CVM, Espíndola ELG (2016) Acute and chronic sensitivity, avoidance behavior and sensitive life stages of bullfrog tadpoles exposed to the biopesticide abamectin. Ecotoxicology 25:500–509
Vogt C, Heß M, Nowak C, Diogo JB, Oehlmann J, Oetken M (2010) Effects of cadmium on life-cycle parameters in a multi-generation study with Chironomus riparius following a pre-exposure of populations to two different tributyltin concentrations for several generations. Ecotoxicology 19:1174–1182
Watts MM, Pascoe DA (2000) Comparative study of Chironomus riparius Meigen and Chironomus tentans Fabricius (Diptera: Chironomidae) in aquatic toxicity tests. Arch Environ Contam Toxicol 39:299–306
Weltje L, Rufli H, Heimbach F, Wheeler J, Vervliet-Scheebaum M, Hamer M (2010) The chironomid acute toxicity test: development of a new test system. Integr Environ Assess Manag 6:301–307
Wetzel RG (2001) Limnology lake and river ecosystems, 3rd edn. Elsevier, Amsterdam
Yang YY, Toor GS, Williams CF (2015) Pharmaceuticals and organochlorine pesticides in sediments of an urban river in Florida, USA. J Soils Sediments 15:993–1004
Acknowledgements
The authors are grateful to the Brazilian research support agency CAPES (Coordenação de Aperfeiçoamento Pessoal de Nivel Superior) for the doctoral scholarship given to the second author and to the São Paulo State Research Support Foundation (FAPESP: Process 10494-6/2002) for financial support. This work also was supported by the Brazilian government through the Special Visiting Researcher program (MEC/MCTI/CAPES/CNPq/FAPs reference 402392/2013-2) and the Portuguese government (FCT) through a postdoc grant for the last author (SFRH/BPD/109199/2015) and the research unit UID/AMB/04085/2013 (CENSE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Dornfeld, C.B., Rodgher, S., Negri, R.G. et al. Chironomus sancticaroli (Diptera, Chironomidae) as a Sensitive Tropical Test Species in Laboratory Bioassays Evaluating Metals (Copper and Cadmium) and Field Testing. Arch Environ Contam Toxicol 76, 42–50 (2019). https://doi.org/10.1007/s00244-018-0575-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-018-0575-1