Abstract
We determined levels of Se, As, Cd, Pb, and Zn in aquatic insects at coal mine–impacted and reference sites in streams in the Rocky Mountain foothills of west central Alberta from 2001–2003. Selenium levels were greater at coal mine–impacted sites than at reference sites in caddisflies but not in mayflies or stoneflies. Arsenic levels were greater at coal mine–impacted sites than at reference sites in caddisflies and stoneflies but not in mayflies. Zn levels were higher at coal mine–impacted sites than at reference sites in all three groups of insects. At coal mine–impacted sites, Se levels in mayflies and caddisflies were greater than those in stoneflies while at reference sites mayflies contained greater concentrations of Se than either caddisflies or stoneflies. Arsenic levels in mayflies were greater than those in caddisflies at reference and coal mine–impacted sites and were greater than those in stoneflies at reference sites. At both types of sites Cd differed amongst insect taxa in the order of mayflies > caddisflies > stoneflies. The same was true of Zn at coal mine–affected sites. At reference sites, stoneflies had greater concentrations of Zn than both mayflies and caddisflies. At both types of sites, Pb levels were greater in mayflies and caddisflies than they were in stoneflies. Of the five trace elements considered in this study, only Se was sufficiently elevated in aquatic invertebrates to be of potential concern for consumers such as fish and aquatic birds. Such was the case at both coal mine–impacted and reference sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Coal has been mined in the Rocky Mountains of Alberta and British Columbia since the late 19th century (Ryan and Dittrick 2000; Alberta Energy 2005). The many small, underground mines that predominated in the early years have been replaced by fewer, large, open-pit mines in the past four to five decades.
At open-pit mines, large quantities of soil and rock are disturbed by blasting, excavation, trucking, dumping, and reclamation activities. These operations usually result in a landscape that is different from that prior to mining and can expose the overburden to air and surface waters. Waste materials are generally deposited on the surface in tailings piles, ponds, landfills, and dumps. Surface waters may drain from these deposits into nearby aquatic ecosystems and eventually into streams that drain the local watershed (Hamilton and Buhl 2004).
There is concern that coal mine operations can mobilize trace elements into aquatic ecosystems in greater quantities than would normally occur in a natural setting (Johnson 2003). Elevated Se levels in waters draining coal mines in the Rocky Mountains of Alberta and British Columbia have been a particular concern in recent years (Casey and Siwik 2000; McDonald and Strosher 2000). That concern has been heightened by a report showing a significant increase in deformities in trout fry hatched from fertilized eggs of parent fish caught in Luscar Creek, a coal mine–impacted stream in the Rocky Mountain foothills of Alberta (Holm et al. 2003). In that study, 38.9% of rainbow trout (Oncorhynchus mykiss) fry from Luscar Creek were deformed while only 0.7% of fry from the reference site were deformed.
Aquatic invertebrates are often excellent bioindicators of trace element pollution in aquatic ecosystems (Cain et al. 1992; Hare 1992). They take up trace elements directly from water and from sediments and diet (Hare 1992) and serve as a trophic link to higher food chain organisms such as fish and aquatic birds. As such, they provide one mechanism by which trace elements, including Se, are passed onto fish and aquatic birds (Dallinger et al. 1987; Spry et al. 1988; Ogle et al. 1988; Lemly 1993; Woodward et al. 1994; Mason et al. 2000; Strom et al. 2002; Beyer et al. 2004; Hamilton 2004).
In this study, we examined concentrations of Se, As, Cd, Pb, and Zn in water and aquatic insects from coal mine–impacted and non-impacted streams in the Rocky Mountain foothills of west-central Alberta, Canada. We were interested in comparing trace elements levels between mine-impacted and non-impacted sites and among different insect taxa. We also have attempted to assess whether levels of the different trace elements were high enough to warrant concern from an ecotoxicological perspective.
Materials and Methods
Study Area
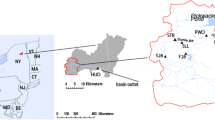
The study was done from 2001–2003 on first- to third-order streams in the vicinity of Hinton, Alberta (Fig. 1). Sampling sites were classified as coal mine–impacted or not impacted, depending on whether or not they received drainage water from two adjacent, open-pit coal mines, one of which had closed in 2000 and was being remediated and the other that was still operating. The Gregg River and Luscar Creek were the two coal mine–impacted streams from which samples were collected. The majority of samples collected from coal mine–impacted streams came from the Gregg River. Only two samples were collected from Luscar Creek. More than 80% of the samples obtained from coal mine–impacted streams were collected no further than 15 km downstream from the coal mines. Reference sites were located on the upper McLeod River, Whitehorse Creek, Wildhay River, South Berland and Berland Rivers, South Sulphur River, and sites on the Gregg River and its tributaries upstream from the mines (Fig. 1). The upper McLeod River served as the main reference river for this study. When this study was done, there were no current mines but small coal mines had operated within the watershed until the early 1950s. We found that trace element levels in water samples from this river were low (As ≤ 0.2, Cd ≤ 0.024, Pb ≤ 0.28, Se ≤ 0.7, Zn ≤ 1.87 μg L−1, n = 10) and Alberta Environment found that trace element levels in sediment samples from this river were low (As ≤ 6, Cd ≤ 0.386, Pb ≤ 9.39, Se ≤ 1.5, Zn ≤ 65.9 μg · g−1 dry wt, n = 2) (Alberta Environment, unpublished data). These levels are similar to or less than those reported for natural reference sites in other studies in riverine aquatic ecosystems in western North America (Besser et al. 2001; Cain et al. 1992; Kiffney and Clements 1993; Saiki et al. 1993; Farag et al. 1998; Hamilton et al. 2002, 2004; Hamilton and Buhl 2004). Therefore, despite the limited amount of mining that had historically occurred upstream from some of our sampling sites on the upper McLeod River, we judged it to be an adequate reference site for this study. The other reference sites were pristine with no known point sources of pollution.
Field Sampling
Insects were collected from late May until early July by kick sampling with a D-framed dip net. Immediately after obtaining kick samples, insects were removed from sample debris using Teflon-coated forceps. Then they were thoroughly rinsed using stream water, sorted to genus or family, and placed temporarily in containers in the stream, through which water was allowed to flow but that prevented the insects from coming into contact with the stream bottom. In this way, insects remained fresh and clean in stream water until work at a given site was completed. Sorted and cleaned insect samples were then transferred to acid-washed glass vials and frozen on dry ice. A subsample of each insect sample was stored in ethanol for taxonomic identification. Water samples were collected from mid-stream at a depth of about 0.5 m in clean, one-time-use, metal-free, high-density polypropylene bottles. Prior to collecting the sample, bottles were rinsed twice in stream water at the sampling site. Samples were kept cool on ice or in a refrigerator until they reached the laboratory.
Residue Analyses
Insects were analyzed by the Alberta Research Council. Samples were oven-dried at 95°C for 4 hours. A 0.5-g portion of the dried sample was randomly selected and weighed in each digestion liner and 5 ml of nitric acid was added. For samples smaller than 0.5 g dry wt, 0.1 g and 1 ml nitric acid were used. The microwave digestion was carried out in closed vessels at controlled temperature (165°C) and controlled pressure (220 PSI) for 20 min, using a QWAVE-1000 microwave sample preparation system (Questron, Mercerville, NJ). Digested solutions were then diluted to 100 ml (20 ml for 0.1-g samples) using distilled, deionized water. Digested samples were 2.5-fold further diluted with distilled, deioned water, and analyzed by inductively coupled plasma-mass spectrometry using a Perkin-Elmer Elan 5000 ICP quadrupole mass spectrometer (Thornhill, ON, Canada). In the ICP-MS analysis, values of samples and standards were reagent blank subtracted. Indium was used as an internal standard. External calibration curves were plotted linearly through zero for each isotope. Correction equations were applied to values of As and Se to account for interference from chloride (Wu et al. 1997).
The following quality control protocols were implemented: The quality of the distilled, deionized water was pre-checked by ICP-MS scanning; blank samples including laboratory reagent blanks and digestion blanks were analyzed together with insect samples; samples of NIST standard reference material (oyster tissue, NIST 1566a) were microwave digested and analyzed together with insect samples. Also, in the field, sixteen samples were split and analyzed in duplicate to provide a measure of within-site/within-taxon variability. Quality assurance results are reported in Table 1.
Water samples were analyzed at Environment Canada’s National Laboratory for Environmental Testing. Methods and instrumentation changed once during the course of this study. In 2001 and 2002, Se and As were analyzed as follows. Samples were shaken and 100-ml aliquots were transferred to an Erlenmeyer digestion flask. Potassium persulphate and hydrochloric acid were added, and the samples were digested on a hotplate to oxidize all As and Se species to arsenate and selenate. After reducing the sample volume to approximately 10 ml, the sample was removed from the hotplate and allowed to cool. The digest was then transferred to a volumetric cylinder, 7 ml of hydrochloric acid was added, and the volume was brought to 20 ml with deionized water. This solution was re-transferred to an Erlenmeyer flask and re-heated to 90°C to convert selenate to selenite. The digest was then cooled and subjected to hydride generation in an automated continuous flow system. Hydrides of As and Se were formed by the action of sodium borohydride in an acidic medium, and were analyzed by inductively coupled plasma–optical emission spectrometry (ICP-OES, IRIS, Thermo Jarrel Ash, Franklin, MA). For Cd, Pb, and Zn analyses, samples were acidified with nitric acid and placed in a sealed container in a convection oven. The samples were allowed to digest in-bottle for 16 hours at 60°C, and then allowed to cool to room temperature. The three elements were analyzed by inductively coupled plasma–quadrupole mass spectrometry (ICP-QMS, PQ-2, VG Elemental, UK). In 2003, a single method was used for analyses of the five trace elements. Sample digestion was performed identically to the ICP-QMS method described above. The five elements were analyzed by inductively coupled plasma–sector field mass spectrometry (ICP–SFMS, Element II, ThermoFinnigan, Bremen, Germany) at specified resolutions: Cd and Pb at low resolutions (400), Zn at mid-resolution (4000), and As and Se at high resolution (10,000). Standard reference materials and spiked samples were analyzed. For the five elements and for the different analytical methods combined, the relative standard deviation ranged from 3.6–6.9%, recoveries from spiked samples ranged from 99.7–101.4%, and recoveries from standard reference materials ranged from 89–101%.
Statistics
We used PROC GLM (SAS Institute 1999) to do mixed model analyses of variance within a split-plot design on rank-transformed data (Conover and Iman 1981) to examine the effects of site type (coal mine–impacted versus reference sites) and insect taxon on trace element levels in insects. The full model included the main plot effects of year, site type, their interaction, and site within year X site type, as well as the subplot effects of insect taxon and the interaction between insect taxon and site type. When interaction effects were found between site type and insect taxon, separate analyses were done to examine the simple effects of site type on metal levels within each type of insect. Because we were not interested in the effects of year, we removed year from the model if the p value associated with it was ≥0.1. When the p value associated with insect taxon was ≤0.05, a posteriori comparisons of least-squared means were carried out using the Tukey-Kramer adjustment (SAS Institute 1999).
We also used PROC GLM (SAS Institute 1999) to do two-way ANOVAs to examine the effects of site type on rank-transformed aqueous trace element concentrations. Year and its interaction with site type were also included as variables in the analysis.
Results
Trace elements were analyzed in 101 insect samples from a total of 52 collections made at 32 sites (Table 2, Fig. 1). Three taxa of insects were collected from a sufficient number of sites to warrant analysis. They were the mayfly Family Heptageniidae (>90% Rhithrogena), the caddisfly Family Hydropsychidae (Arctopsyche and Parapsyche combined), and the stonefly Megarcys (Perlodidae). In addition, trace elements were analyzed in 54 water samples (not including replicate samples) at 34 sites.
Trace Elements in Water
Concentrations of Se (p = 0.01), As (p = 0.007), and Zn (p = 0.007) were significantly higher in coal mine–affected water samples than in samples from reference sites (Table 3), while the difference between site types in Cd concentrations approached significance (p = 0.06). Concentrations of Pb did not differ between mine-affected and reference sites (p = 0.17, Table 3).
Trace Elements in Insects: Results of Overall ANOVAs
Selenium levels in insects were significantly different among years (p = 0.01) and insect taxa (p < 0.0001) and were significantly affected by the interaction between site type and insect taxon (p < 0.0001) (Table 4). Neither the main effect of site type (p = 0.36) nor the interaction between year and site type (p = 0.08) was significant (Table 4). Arsenic levels in insects were significantly affected by site type (p = 0.0006), insect taxon (p < 0.0001), and the interaction between these variables (p = 0.02), while other sources of variation in the overall ANOVA were not significant (Table 4). Cadmium levels in insects were significantly affected by year and insect taxon (p < 0.0001) but were not significantly affected by other sources of variation in the overall ANOVA (Table 4). Cadmium levels were significantly higher in 2003 than they were in either 2002 or 2001 (p ≤ 0.05). Lead concentrations in insects differed significantly among years (p = 0.007) and insect taxa (p < 0.0001) but were not significantly affected by other sources of variation in the overall ANOVA (Table 4). Lead levels in insects were significantly greater in 2002 than in 2003 (p ≤ 0.05). Zinc concentrations in insects differed significantly among years (p = 0.01), site types (p = 0.009), and insect taxa (p < 0.0001). Zinc concentrations in insects were also significantly affected by the interaction between site type and insect taxon (p < 0.0001, Table 4).
Comparisons Between Mine-Affected and Reference Sites
Because there were significant interaction effects between site type and insect taxon on Se, As, and Zn levels (p ≤ 0.02), we examined the simple effects of site type on concentrations of these trace elements separately for each insect taxon. Selenium levels in caddisflies were significantly greater at mine-affected sites than they were at reference sites (p = 0.0003, Tables 3 and 5, Fig. 2). In contrast, Se levels in mayflies and stoneflies did not differ significantly between mine-affected and reference sites (p ≥ 0.3, Table 5). Arsenic levels in both caddisflies and stoneflies were significantly greater at mine-affected than at reference sites (p ≤ 0.0008) while those in mayflies did not differ among site types (p = 0.22) (Tables 3 and 5, Fig. 2). Zinc levels in mayflies (p < 0.0001), caddisflies (p = 0.01), and stoneflies (p = 0.04) were significantly greater at mine-affected than at references sites (Tables 3 and 5, Fig. 2).
Median and 25th–75th percentile concentrations of (A) arsenic, (B) selenium, and (C) zinc in three insect taxa at coal mine–impacted and reference sites. Hept = Heptageniidae (>90% Rhithrogena), Hydr = Hydropsychidae (Arctopsyche and Parapsyche), Mega = Megarcys. Bars with different letters above them are significantly different (p < 0.05)
Cadmium (p = 0.14) and Pb levels (p = 0.19) in insects did not differ significantly between mine-affected and reference sites (Table 4).
Comparisons Among Taxa
Because there were significant interaction effects between site type and insect taxon on Se, As, and Zn levels (p ≤ 0.02 ), we examined the simple effects of insect taxon on concentrations of those trace elements separately for each site type. Selenium levels differed significantly among insect taxa at both mine-affected (p = 0.02) and reference sites (p = 0.01) (Table 5). At mine-affected sites, Se levels in mayflies and caddisflies were significantly greater than those in stoneflies (p ≤ 0.05, Fig. 2), while at reference sites Se levels in maylies were significantly greater than those in caddisflies and stoneflies (p ≤ 0.05, Fig. 2). Arsenic levels differed significantly among insect taxa at mine-affected (p = 0.03) and reference sites (p = 0.009). At mine-affected sites, mayflies contained significantly higher levels of As than caddisflies, while at reference sites, they contained significantly higher levels of As than caddisflies and stoneflies (p ≤ 0.05, Fig. 2). Zinc levels differed significantly among insect taxa at mine-affected sites (p = 0.004) in the order of mayflies > stoneflies > caddisflies (p ≤ 0.05, Fig. 2). At reference sites, stoneflies contained significantly greater levels of Zn than mayflies and caddisflies (p ≤ 0.05, Fig. 2).
Concentrations of Cd and Pb differed significantly among insect taxa (p < 0.0001, Table 4). For Cd, significant differences were found among the three taxa (p ≤ 0.05) in the order of mayflies > caddisflies > stoneflies (Fig. 3). Lead levels did not differ significantly between mayflies and caddisflies (p > 0.05) but were higher in both taxa than they were in stoneflies (p ≤ 0.05, Fig. 3).
Median and 25th–75th percentile concentrations of cadmium and lead in three insect taxa. Coal mine–impacted and reference sites were combined because there were no significant differences between them in concentrations of cadmium and lead in insects. Insect codes as in Figure 2. Different uppercase letters represent significantly different Cd levels while different lowercase letters represent significantly different Pb levels.
Discussion
Comparisons Between Coal Mine–Impacted and Reference Streams
Selenium, As, Cd, and Zn concentrations in water were clearly elevated at coal mine–impacted sites compared to reference sites while Pb levels in water did not differ between the two types of sites. At reference sites, the concentration of waterborne Se was always lower than the Canadian Council of Ministers of the Environment guideline of 1 ppb. At coal mine–impacted sites, it was always above the guideline and ranged from 1.3–9.2 ppb.
The results for insects were not as clear-cut as they were for water. Significant differences between coal mine–affected and reference sites were found in one of three taxa for Se, two of three taxa for As, and all three taxa for Zn. Levels of Cd in insects did not differ between mine-affected and reference sites despite the existence of such a difference in water samples. Collectively, these results signify that concentrations of trace elements in water are sometimes poor predictors of those in biota and hence poor predictors of ecological risk.
Poor predictability of trace element levels in aquatic insects from those in water can be attributed, in part, to the numerous factors that influence trace element levels in insects. Trace element levels in benthic insects are usually more closely linked to those in sediments and biofilm than they are to those in water. This is especially true for elements such as Se and Cd, for which diet can be a major source of exposure (Beltman et al. 1999; Kiffney and Clements 1993; Hamilton and Buhl 2004; Malloy et al. 1999). Dietary exposure to Zn is important for some types of insects whereas uptake from water is important for others (Hare 1992) and field studies have shown that Zn concentrations in insects are often closely matched to those in both biofilm and water (Cain et al. 1992; Kiffney and Clements 1993). Characteristics of biofilm can be important in determining its uptake of trace elements and hence their availability to stream insects (Besser at al. 2001). Differences in the characteristics of biofilm between coal mine–affected and reference sites, if they existed in this study, could have affected the amount of trace elements available to grazing aquatic insects, such as mayflies. In addition, biotic variables such as feeding habit, molting, and body size are related to trace element concentrations (Van Hattum et al. 1991; Cain et al. 1992; Hare 1992; Kiffney and Clements 1993; Farag et al. 1998; Besser et al. 2001; Canivet et al. 2001). Feeding habit was largely controlled for in this study by examining trace element concentrations in individual taxa, anyone of which would have had similar feeding habits at different sites. However, body size differences among sites may have influenced our results, since even within the same taxon, smaller insects often have greater concentrations of trace elements than larger ones (Cain et al. 1992; Kiffney and Clements 1993; Mason et al. 2000). A body size effect could have been particularly pronounced for caddisflies if our caddisfly larvae, which consisted of two genera, differed to some degree in body size. Finally, physiological regulation of essential trace elements may have influenced the levels of essential elements such as Se and Zn that were recorded in this study (Hare 1992).
In this study, concentrations of Se in insects at coal mine–impacted sites were similar to or lower than those found in insects at other sites where human activities have resulted in Se contamination of aquatic ecosystems. Such concentrations have ranged from <1 to 200 μg · g−1 dry wt at phosphate mine–impacted and coal mine–impacted sites in the Rocky Mountains (McDonald and Strosher 2000; Hamilton and Buhl 2004; Hamilton et al. 2004), in irrigation drainwater–affected wetlands and rivers in the western United States (Saiki and Lowe 1987; Barnum and Gilmer 1988; Schuler et al. 1990; Saiki et al. 1993; Stephens and Waddell 1998), and in a reservoir that received waste water from a coal-fired power plant (Lemly 1997). Finally, Se concentrations in insects at coal mine–impacted sites in this study were similar to or slightly lower than the range of concentrations found in insects collected from some of the same streams by Alberta Environment: mayflies, 6.2–12.3; hydropsychids, 8.5–15; perlodids, 4.6–14.1 (Alberta Environment, unpublished data). Forty-seven percent of Alberta Environment’s mine-affected samples came from Luscar Creek while 16% came from the Gregg River. In our study, corresponding values were 4% and 96%. Selenium levels in water and biota are higher in Luscar Creek than in the Gregg River (Casey and Siwik 2000). The greater focus on Luscar Creek by Alberta Environment is likely a major reason why it found slightly higher levels of Se than we did. In addition, seasonal and annual differences in sampling periods between our study (May to June, 2001–2003) and Alberta Environment’s study (July to September, 2000–2001) may have contributed to the small differences in results. Notably, both studies used the same laboratory and methods to analyze samples, so laboratory methods are not likely a source of the slight differences between our results and those of Alberta Environment.
Interestingly, concentrations of Se in insects from reference sites were somewhat higher than those reported in other studies. Typically, insects from reference sites where Se in water and sediment are low tend to have Se concentrations <4 μg ċ g−1 dry wt (Saiki and Lowe 1987; Schuler et al. 1990; Maier et al. 1998; Nagpal 2000; Nelson et al. 2000; Hamilton and Buhl 2004). However, in this study, concentrations of Se in aquatic insects at reference sites usually exceeded 4 μg ċ g−1 (Table 3). Our results are in agreement with those of some recent studies that have reported apparently elevated Se levels in insects from sites unaffected by industrial discharges or agricultural drainwaters and from sites with low Se levels in water and sediment (May et al. 2001; Hamilton et al. 2002; 2004; Morrissey et al. 2005). These findings indicate that, even in some clean rivers, benthic insects can accumulate relatively high levels of Se.
Concentrations of Zn and As were greater in insects at coal mine–impacted sites than at reference sites, the former in all three taxa and the latter in caddisflies and stoneflies. At reference sites, Zn levels in insects were similar to or slightly lower than those reported at reference sites in other studies (Cain et al. 1992; Kiffney and Clements 1993; Poulton et al. 1995; Farag et al. 1998; Besser et al. 2001; Saiki et al. 2001). They were generally lower at coal mine–impacted sites than at severely impacted lotic sites near metal mines in the western United States but were similar to those at moderately impacted sites near those mines (Woodward et al. 1994, 1995; Farag et al. 1998, 1999; Besser et al. 2001; Saiki et al. 2001). Although As levels were elevated at coal mine–impacted sites compared to reference sites, they were similar to or lower than As concentrations from reference sites reported in other studies done in western North America. Such concentrations were generally in the range of 2–4 μg ċ g−1 dry wt (Woodward et al. 1994; Poulton et al. 1995; Farag et al. 1998, 1999; Hamilton et al. 2004), although in one study they ranged as high as 16 μg ċ g−1 dry wt (Cain et al. 1992). This suggests that As levels in insects from coal mine–impacted sites, although slightly elevated, were still well within the range of “background” concentrations in a western North American context.
Comparisons Among Insect Taxa
Concentrations of Se, As, Zn, Cd, and Pb differed among insect taxa. Generally, mayflies had the highest median concentrations, followed by caddisflies, with stoneflies having the lowest concentrations of Cd, Pb, and Se. In addition, at coal mine–impacted sites, Zn concentrations were highest in mayflies while at reference sites they were highest in stoneflies. Relatively high levels of Se, As, Cd, Pb, and Zn in herbivorous or detritivorous mayflies and lower levels of these elements in predatory stoneflies have been noted in other studies (Kiffney and Clements 1993; Mason et al. 2000; Besser et al. 2001; Saiki et al. 2001).
A number of reasons have been offered to explain why some types of insects contain greater concentrations of trace elements than others. Some are probably pertinent to our study. Body size strongly influences levels of trace elements in aquatic insects with smaller-bodied insects having greater concentrations than larger-bodied ones (Van Hattum et al. 1991; Cain et al. 1992; Kiffney and Clements 1993; Mason et al. 2000; Besser et al. 2001). The inverse relationship between body size and trace element concentration is believed to be related to the greater surface area/volume ratios in smaller-bodied insects (Hare 1992; Mason et al. 2000). Greater weight-specific metabolic rates of smaller-bodied insects may also influence the rate at which a metal is exchanged between the animal and its environment (Hare 1992). Regarding the insects analyzed in this study, Rhithrogena (Hepatageniidae) is smaller than the Hydropsychidae, which is, in turn, smaller than Megarcys. Thus, for the most part, our results are consistent with observations of other studies, and show that smaller-bodied insects have greater concentrations of trace elements than larger-bodied ones.
Feeding habits are also important determinants of trace element levels in aquatic insects. Grazers on periphyton, like Rhithrogena, tend to accumulate high levels of certain trace elements, which are often more enriched in periphyton than in sediments or detritus (Besser et al. 2001). Filter-feeders, collector-gatherers, shredders, and grazers tend to accumulate higher levels of certain trace elements than omnivores and predators (Cain et al. 1992; Hare 1992; Kiffney and Clements 1993; Besser et al. 2001). With the exception of the relatively high Zn levels in the predaceous stonefly, Megarcys, the results of our study are consistent with the findings of other studies and support the idea that feeding habits influence trace element concentrations in aquatic insects. With regard to elevated Zn levels in predators, it should be noted that Cain et al. (1992) reported higher levels of Zn in two of three predaceous stoneflies than in omnivorous caddisflies and a detritivorous stonefly. Timmermans et al. (1989) also reported higher Zn levels in predatory invertebrates than in deposit feeders and filter feeders collected from the littoral zones of two small lakes.
Gut contents can contribute a substantial portion of the trace element load in invertebrates (Hare 1992; Hansen et al. 2004). If the ratio of gut contents to body size differs among taxa and if trace element concentrations in gut contents differ substantially from those in the animal itself, then they could bias the results of comparisons among different taxa. It is unlikely that gut contents seriously biased our results for Se, since Se levels in biofilm and sediments are usually similar to or lower than levels in aquatic insects (May et al. 2001; Hamilton and Buhl 2004; Hamilton et al. 2004). Also, in our study area, Zn and Cd levels in biofilm were similar to or lower than those in insects (Alberta Environment, unpublished data), a finding reported in other studies as well (Hamilton and Buhl 2004; Hamilton et al. 2004). However, As (range: 1.6–8.5 μg · g−1 dry wt) and Pb (range: 2.5–10.1 μg · g−1 dry wt) levels were somewhat higher in biofilm than in insects (Alberta Environment, unpublished data). Thus, in this study, levels of As and Pb could have been biased upwards in mayflies and caddisflies. Regardless of the potential for gut contents to influence trace element concentrations in insects, the measurement of total trace element concentrations, including gut contents, is still important in an ecological context because consumers of aquatic insects are exposed not only to the biologically incorporated trace elements but also to those present in gut contents.
Potential Risk of Trace Elements to Consumers
Selenium, As, and Zn were elevated at coal mine–impacted sites compared to reference sites in one or more groups of insects. A concern arising from such a result is whether consumers of these aquatic insects are placed at increased risk of toxic effects in coal mine–impacted streams.
Natural diets consisting of metal-contaminated insects are often toxic at lower concentrations than artificially spiked commercial diets (Farag et al. 1999), possibly because of additive or synergistic effects of multiple metals in the natural diet, the relatively poor nutritional quality of natural diets in metal-contaminated streams, or the organic binding of metals in natural foods (Farag et al. 1999; Saiki et al. 2001).
Furthermore, it has been shown that Se toxicity may be alleviated by other trace elements, for example, As and Cd, while other trace elements have no effect on Se toxicity (Hamilton 2004). Unfortunately, in comparing trace element levels in natural diets to toxicity thresholds, it is rarely possible to assess potential toxicity based on the whole suite of elements under consideration, nor can it be known whether the natural diet will alleviate or increase the toxicity of the elements. This is because most toxicity studies have been conducted with artificial diets spiked with different doses of only one trace element. With this caveat in mind, we offer the following observations about the potential for adverse effects of Se, As, and Zn in the coal mine–impacted streams in this study.
Hamilton (2004) reviewed the dietary toxicity of Se to fish and birds and determined that toxicity thresholds ranged from 3–11 μg · g dry wt−1. Other thresholds were 4 and 7 μg · g−1 (Hamilton 2004). While some of the variation in dietary Se thresholds has arisen from scientific disagreements (DeForest et al. 1999; Hamilton 2004), there is also inherent variability in Se toxicity that can be attributed to its interaction with other trace elements, different bioaccumulation potential in lotic and lentic ecosystems, and differences in sensitivity among species, especially cold water– and warm water–adapted fish species (Hamilton 2004). We do not know which of the published Se toxicity thresholds would be most appropriate for the streams that we studied. Nevertheless, 4–98% of insect samples from both mine-affected and reference sites exceeded at least one of the toxicity thresholds (Table 6), suggesting that some amount of risk exists for consumers such as fish and birds.
In our study, insect taxon accounted for a greater proportion of the overall variation in Se (17%) than site type did (3%) and mayflies from reference sites had higher median concentrations of Se than insects of any taxa collected from coal mine–impacted streams. These results suggest that, in the streams we studied, diet composition may even be more important than coal mine effects in contributing to Se exposure in fish and aquatic birds. Nevertheless, the potential for coal mining in this area to cause adverse effects in fish should not be underestimated in light of the greater incidence of deformities in fish fry hatched from eggs of trout caught in Luscar Creek, the most severely impacted creek in the study area (Holm et al. 2003).
Dietary As levels ≤ 100 μg · g−1 were not harmful to experimental birds (Whitworth et al. 1991; Stanley et al. 1994), suggesting that As is unlikely to harm aquatic birds feeding on coal mine–impacted streams in our study area.
The maximum acceptable dietary concentration of As for juvenile rainbow trout was reported to lie between 13–33 μg · g−1 (Cockell et al. 1991), a range of concentrations higher than those found in insects in this study. Growth and condition factor of lake whitefish (Coregonus clepeaformis) were unaffected by dietary As levels as high as 100 μg · g−1 (Pedlar et al. 2002), a level substantially higher than found in this study. However, in that study, some damage to gallbladders and livers was found at dietary levels as low as 1 μg · g−1. Collectively, these results suggest that dietary As is unlikely to affect fish populations in coal mine–impacted streams. Nevertheless, concern about the overall health of fish may be warranted in light of the tissue damage induced by low levels of dietary As (Pedlar et al. 2002).
Birds can tolerate levels of dietary Zn as high as 2000 μg · g−1 (Gasaway and Buss 1972; Stahl et al. 1990). Zinc poisoning of water birds has rarely been reported (Sileo et al. 2003) and then it was attributed to ingestion of sediment in a severely contaminated river system downstream from an historical metal mining area (Beyer et al. 2004). Sediments from streams in the area contained Zn levels as high as 25,000 μg · g−1 (Beyer et al. 2004), levels that are much higher than those in insects from coal mine–impacted streams in our study area. Finally, a food chain–based risk assessment concluded that Zn concentrations ranging from approximately 100–900 μg · g−1 dry wt in various food items at a metal-contaminated wetland would not pose a risk to ducks (Pascoe et al. 1996). Collectively, these results suggest that aquatic birds feeding in coal mine–impacted streams in our study area are probably not at risk from elevated Zn levels in their insect food.
Diet is the most important source of Zn for freshwater fish and dietary Zn levels of 590 μg · g−1 (Spry et al. 1988) and 1700 μg · g−1 wet wt (Wekell et al. 1983) were not toxic to rainbow trout fingerlings. We conclude from these studies that elevated Zn levels in insects in coal mine–impacted streams in our study area are unlikely to pose a hazard to fish.
Overall, we found elevated levels of Se, As, and Zn in at least one insect taxon in coal mine–impacted streams. Se levels were high enough to be of potential concern for fish and aquatic birds whereas As and Zn levels remained below levels at which toxic effects would be likely to occur in these consumers.
References
Alberta Energy (2005) Coal. Alberta Energy website (http://www.energy.gov.ab.ca/377.asp)
Barnum DA, Gilmer DS (1988) Selenium levels in biota from irrigation drainwater impoundments in the San Joaquin Valley, California. Lake Res Manage 4:181–186
Beltman DJ, Clements WH, Lipton J, Cacela D (1999) Benthic invertebrate metals exposure, accumulation, and community-level effects downstream from a hard-rock mine site. Environ Toxicol Chem 18:299–307
Besser JM, Brumbaugh WG, May TW, Church SE, Kimball BA (2001) Bioavailability of metals in stream food webs and hazards to brook trout (Salvelinus fontinalis) in the upper Animas River watershed, Colorado. Arch Environ Contam Toxicol 40:48–59
Beyer WN, Dalgarn J, Dudding S, French JB, Mateo R, Miesner J, Sileo L, Spann J (2005) Zinc and lead poisoning in wild birds in the Tri-State Mining District (Oklahoma, Kansas, and Missouri). Arch Environ Contam Toxicol 48:108–117
Cain DJ, Luoma SN, Carter JL, Fend SV (1992) Aquatic insects as bioindicators of trace element contamination in cobble-bottom rivers and streams. Can J Fish Aquat Sci 49:2141–2154
Canivet V, Chambon P, Gibert J (2001) Toxicity and bioaccumulation of arsenic and chromium in epigean and hypogean freshwater invertebrates. Arch Environ Contam Toxicol 40:345–354
Casey R, Siwik P (2000) Concentrations of selenium in surface water, sediment and fish from the McLeod, Pembina and Smoky Rivers: results of surveys from fall 1998 to fall 1999. Alberta Environment, Edmonton
Cockell KA, Hilton JW, Bettger WJ (1991) Chronic toxicity of dietary disodium arsenate heptahydrate to juvenile rainbow trout. Arch Environ Contam Toxicol 21:518–527
Conover WJ, Iman RL (1981) Rank transformation as a bridge between parametric and non-parametric statistics. Am Stat 35:124–129
Dallinger R, Prosi F, Segner H, Back H (1987) Contaminated food and uptake of heavy metals by fish: a review and a proposal for further research. Oecologia 73:91–98
DeForest DK, Brix KV, Adams WJ (1999) Critical review of proposed residue-based selenium toxicity thresholds for freshwater fish. Hum Ecol Risk Assess 5:1187–1228
Farag AM, Woodward DF, Goldstein JN, Brumbaugh WG, Meyer JS (1998) Concentrations of metals associated with mining wastes in sediments, biofilm, benthic invertebrates, and fish from the Couer d’Alene River Basin, Idaho. Arch Environ Contam Toxicol 34:119–127
Farag AM, Brumbaugh WG, Goldstein JN, MacConnell E, Hogstrand C, Barrows FT (1999) Dietary effects of metals-contaminated invertebrates from the Couer d’Alene River, Idaho, on cutthroat trout. Trans Am Fish Soc 128:578–592
Gasaway WC, Buss IO (1972) Zinc toxicity in the mallard duck. J Wildl Manage 36:1107–1117
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Hamilton SJ, Buhl KJ (2004) Selenium in water, sediment, plants, invertebrates, and fish in the Blackfoot River Drainage. Wat Air Soil Pollut 159:2–34
Hamilton SJ, Holley KM, Buhl KJ (2002) Hazard assessment of selenium to endangered razorback suckers (Xyrauchen texanus). Sci. Total Environ 291:111–121
Hamilton SJ, Buhl KJ, Lamothe PJ (2004) Selenium and other trace elements in water, sediment, aquatic plants, aquatic invertebrates, and fish from streams in SE Idaho near phosphate mining. In: Hein JR (ed) Life cycle of the phosphoria formation: from deposition to the post-mining environment (Series: Handbook of exploration geochemistry). Elsevier Science, Amsterdam, pp 483–525
Hansen JA, Lipton J, Welsh PG, Cacela D, MacConnell B (2004) Reduced growth of rainbow trout (Oncorhynchus mykiss) fed a live invertebrate diet pre-exposed to metal-contaminated sediments. Environ Toxicol Chem 23:1902–1911
Hare L (1992) Aquatic insects and trace metals: bioavailability, bioaccumulation, and toxicity. Crit Rev Toxicol 22:327−369
Holm J, Palace VP, Wautier K, Evans RE, Baron CL, Podemski C, Siwik P, Sterling G (2003) An assessment of the development and survival of wild rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelnus fontinalis) exposed to elevated selenium an area of active coal mining. In: Browman HI, Skiftesvik AB (eds) The big fish bang: Proceedings of the 26th Annual Larval Fish Conference, 2003. Institute of Marine Research, Bergen, Norway, pp 257–273
Johnson DB (2003) Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut: Focus 3:47–66
Kiffney PM, Clements WH (1993) Bioaccumulation of heavy metals by benthic invertebrates at the Arkansas River, Colorado. Environ Toxicol Chem 12:1507–1517
Lemly AD (1993) Guideleines for evaluating selenium data from aquatic monitoring and assessment studies. Environ Monit Assess 28:83–100
Lemly AD (1997) Ecosystem recovery following selenium contamination in a freshwater reservoir. Ecotoxicol Environ Safety 36:275–281
Maier KJ, Nelson CR, Bailey FC, Klaine SJ, Knight AW (1998) Accumulation of selenium by the aquatic biota of a watershed treated with seleniferous fertilizer. Bull Environ Contam Toxicol 60:409–416
Malloy JC, Meade ML, Olsen EW (1999) Small-scale spatial variation of selenium concentrations in chironomid larvae. Bull Environ Contam Toxicol 62:122–129
Mason RP, Laporte J-M, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297
May TW, Walther MJ, Petty JD, Fairchild JF, Lucero J, Delvaux M, Manring J, Armbruster M, Hartman D (2001) An evaluation of selenium concentrations in water, sediment, invertebrates, and fish from the Republican River Basin: 1997–1999. Environ Monit Assess 72:179–206
McDonald LE, Strosher MM (2000) Selenium in the Elk River basin, British Columbia—a review of findings and discussion of implications for assessment and management. Proceedings of the 24th Annual British Columbia mine reclamation symposium, June 19–22, 2000, Williams Lake, B.C. British Columbia Technical and Research Committee on Reclamation, pp 160–173
Morrissey CA, Bendell-Young LI, Elliott JE (2005) Assessing trace metal exposure to American dippers in mountain streams of southwestern British Columbia. Environ Toxicol Chem 24:836–845
Nagpal NK (2000) Proposed WQ guidelines for selenium to protect aquatic life. Proceedings of the 24th Annual British Columbia mine reclamation symposium, June 19–22, 2000, Williams Lake, B.C. British Columbia Technical and Research Committee on Reclamation, pp 253–262
Nelson SM, Roline RA, Thullen JS, Sartoris JJ, Boutwell JE (2000) Invertebrate assemblages and trace element bioaccumulation associated with constructed wetlands. Wetlands 20:406–415
Ogle RS, Maier KJ, Kiffney P, Williams MJ, Brasher A, Melton LA, Knight AW (1988) Bioaccumulation of selenium in aquatic ecosystems. Lake Res Manage 4:165–173
Pascoe GA, Blanchet RJ, Linder G (1996) Food chain analysis of exposures and risks to wildlife at a metals-contaminated wetland. Arch Environ Contam Toxicol 30:306–318
Pedlar RM, Ptashynski MD, Evans R, Klaverkamp JF (2002) Toxicological effects of dietary arsenic exposure in lake whitefish (Coregonus clupeaformis). Aquat Toxicol 57:167–189
Poulton BC, Monda DP, Woodward DF, Wildhaber ML, Brumbaugh WG (1995) Relations between benthic community structure and metals concentrations in aquatic macroinvertebrates: Clark Fork River, Montana. J Freshwat Ecol 10:277–293
Ryan B, Dittrick M (2001) Selenium in the Mist Mountain formation of southeast British Columbia. Geological Fieldwork 2000; Paper 2001−1. British Columbia Geological Survey, Victoria, pp 337–362
Saiki MK, Lowe TP (1987) Selenium in aquatic organisms from subsurface agricultural drainage water, San Joaquin Valley, California. Arch Environ Contam Toxicol 16:657–670
Saiki MK, Jennings MR, Brumbaugh WG (1993) Boron, molybdenum and selenium in aquatic food chains from the lower San Joaquin River and its tributaries, California. Arch Environ Contam Toxicol 24:307–320
Saiki MK, Martin BA, Thompson LD, Welsh D (2001) Copper, cadmium, and zinc concentrations in juvenile chinook salmon and selected fish forage organisms (aquatic insects) in the upper Sacramento River, California. Wat Air Soil Poll 132:127–139
SAS Institute Inc. (1999) SAS/STAT user’s guide, version 8, volume 2. SAS Institute Inc., Cary, NC
Schuler CA, Anthony RG, Ohlendorf HM (1990) Selenium in wetlands and waterfowl foods at Kesterson Reservoir, California, 1984. Arch Environ Contam Toxicol 19:845–853
Sileo L, Beyer WN, Mateo R (2003) Pancreatitis in wild zinc-poisoned waterfowl. Avian Path 32:655–660
Spry DJ, Hodson PV, Wood CM (1988) Relative contributions of dietary and waterborne zinc in the rainbow trout, Salmo gairdneri. Can J Fish Aquat Sci 45:32–41
Stahl JL, Greger JL, Cook ME (1990) Breeding hen and progeny performance when hens are fed excessive dietary zinc. Poult Sci 69:259–263
Stanley TR Jr., Spann JW, Smith GJ, Roscoe R (1994) Main and interactive effects of arsenic and selenium on mallard reproduction and duckling growth and survival. Arch Environ Contam Toxicol 26:444–451
Stephens DW Waddell B (1998) Selenium sources and effects on biota in the Green River Basin of Wyoming, Colorado, and Utah. In: Frankenberger WT Jr, Engberg RA (eds) Environmental chemistry of selenium. Marcel Dekker, New York, pp 183–203
Strom SM, Ramsdell HS, Archuleta AS (2002) Aminolevulinic acid dehydratase activity in American dippers (Cinclus mexicanus) from a metal-impacted stream. Environ Toxicol Chem 21:115–120
Timmermans KR, van Hattum B, Kraak MHS, Davids C (1989) Trace metals in a littoral food web: concentrations in organisms, sediment and water. Sci Total Environ 87/88:477–494
van Hattum B, Timmermans KR, Govers HA (2005) Abiotic and biotic factors influencing in situ trace metal levels in macroinvertebrates in freshwater ecosystems. Environ Toxicol Chem 10:275–292
Wekell JC, Shearer KD, Houle CR (1983) High zinc supplementation of rainbow trout diets. Prog Fish Cult 45:144–147
Whitworth MR, Pendleton GR, Hoffman DJ, Camardese MB (1991) Effects of dietary boron and arsenic on the behaviour of mallard ducklings. Environ Toxicol Chem 10:911–916
Woodward DF, Brumbaugh WG, DeLonay AJ, Little EE, Smith CE (1994) Effects on rainbow trout of a metals-contaminated diet of benthic invertebrates from the Clark Fork River, Montana. Trans Am Fish Soc 123:51–62
Woodward DF, Farag AM, Bergman HL, Delonay AJ, Little EE, Smith CE, Barrows FT (1995) Metals-contaminated benthic invertebrates in the Clark Fork River, Montana: effects on age of brown trout and rainbow trout. Can J Fish Aquat Sci 52:1994–2004
Wu S, Feng X, Wittmeier A (1997) Microwave digestion of plant and grain reference materials in nitric acid or a mixture of nitric acid and hydrogen peroxide for the determination of multi-elements by inductively coupled plasma mass spectrometry. J Anal Atom Spectro 12:797–806
Acknowledgments
We thank the following individuals who assisted with various aspects of this project: R. Casey, X. Feng, J. Froese, J. Keating, D. Lindeman, G. Sardella, and M. Su. Funding was provided by Environment Canada, in part through its Environmental Assessment Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wayland, M., Crosley, R. Selenium and Other Trace Elements in Aquatic Insects in Coal Mine–Affected Streams in the Rocky Mountains of Alberta, Canada. Arch Environ Contam Toxicol 50, 511–522 (2006). https://doi.org/10.1007/s00244-005-0114-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0114-8