Abstract
An 8-week feeding trial was carried out to examine the effect of different sources of dietary Zn on some physiological responses (performance, digestive enzymes activity, hemato-biochemical parameters, antioxidant status and liver histology) of Siberian sturgeon, Acipenser baerii. For this purpose, fish with an average weight of 100 g ± 5 were randomly allocated into four groups including control, inorganic zinc (Zn-sulfate), organic zinc (Zn-gluconate), and zinc-oxide nanoparticles (ZnO-NPs) at 50 mg Zn kg− 1 feed. Improved growth indices, namely weight gain (WG) and specific growth rate (SGR) and feed conversion ratio (FCR) were observed in fish fed Zn-gluconate supplemented diet (P < 0.0.5). The highest digestive enzymes activity was recorded in fish fed Zn-gluconate supplementation (P < 0.0.5). Hematological indices significantly increased in fish fed diet containing ZnO-NPs (P < 0.0.5). Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) of fish fed ZnO-NPs contained diet were the highest (P < 0.0.5). The highest serum superoxide dismutase (SOD) and catalase (CAT) enzymes activity were observed in fish fed ZnO-NPs and inorganic/organic Zn contained diets, respectively. While liver tissue SOD and glutathione peroxidase (GPx) enzymes activity Zn were significantly increased in fish fed inorganic/organic Zn supplemented diet (P < 0.0.5). Based on liver histological results, a severe tissue changes such as necrosis and pyknosis were observed in fish fed with Zn-sulfate in comparison to other forms. In conclusion, the data of the present study confirmed that organic Zn (mainly) and nano-Zn (to some extent) could be more efficient Zn sources in Siberian sturgeon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) as a rare mineral element, is an essential component of various enzymes affecting their catalytic and structural properties (Vallee and Falchuk 1993). The element has a fundamental role in the physiological function of the body and is necessary for growth, improvement of the immune system, and reproductive efficiency of various fish species (Chen and Luo 2021). Furthermore, improving antioxidant function, vitamin A metabolism, insulin storage and release, energy metabolism, protein synthesis and DNA transcription regulation are among the main functions of Zn in fish (Kou et al. 2021).
It is well established that the absorption and bioavailability of essential elements, including zinc, is related to its chemical forms. The requirement level of mineral forms of Zn (e.g., ZnSO4) has previously been determined with low bioavailability and incidence of animal toxicity following higher dietary level supplementation (NRC 2011). As an alternative dietary supplement of Zn, the organic forms (e.g., Zn methionine) also was included in fish diets with higher bioavailability when compared to the inorganic form (Antony Jesu Prabhu et al. 2016; Mohseni et al. 2021). Additionally, the dissolution process of zinc oxide nanoparticles (nano-ZnOs) leads to abundant production of Zn2+ with increased bioavailability in the gut (Hogstrand 2011). Therefore, the Zn nanoparticle as a new supplemental form of Zn would have higher efficiency in meeting animal Zn requirement. Furthermore, nano-ZnOs has an anti-microbial and anti-fungal properties (Vijayakumar et al. 2019; Rashidian et al. 2021. In general, promising results of diets containing nanoparticles have been recently documented in various fish species (Kumar et al. 2017; Chupani et al. 2017, 2018; Kazemi et al. 2020; Aramli et al. 2023).
The successful aquaculture production of sturgeon as endangered species mainly depends on the formulations of the diet that might ensure the growth and health of these valuable species (Hung 2017). Siberian sturgeon (A. baerii) is one of the freshwater fish species belonging to the family Acipenseride. The species is widely cultured due to its fast growth rate and better adaptability to intensive aquaculture condition (Wei et al. 2011; Abdolahnejad et al. 2015). Some experiments have been carried out to determine the nutritional requirements of sturgeon (Wang et al. 2016; Moazenzadeh et al. 2017, 2020; Falahatkar 2018; Mohseni et al. 2021; Lee et al. 2021). However, there is a big gap of knowledge in the mineral requirement of sturgeons and no reports have been released regarding the effect of dietary supplementation with different sources of Zn in Siberian sturgeon. Therefore, this experiment was carried out to investigate the effect of diets supplemented with inorganic, organic, or nanoparticle forms of Zn on growth performance, hematology, blood biochemistry, digestive enzymes activity, antioxidant status, and liver histology of Siberian sturgeon.
Materials and methods
Zinc oxide nanoparticle
Zinc oxide nanoparticles (ZnO-NPs) were provided from Nanosany Co, Mashhad, Iran. Analyses of the powder form ZnO NPs were performed using a MIRA3 TESCAN field emission scanning electron microscope (FESEM). The diameters of randomly selected particles were measured at magnification of 50,000 using Axio Vision digital image processing software (Release 4.8.2.0, Carl Zeiss Micro Imaging GmbH, Germany). The X-ray diffraction (XRD) pattern of the sample was determined at room temperature using a Philips X’Pert Pro diffractometer. Based on the XRD patterns, there were different diffraction peaks in the ZnO-NPs graph and average particle size was calculated to be 20 nm (Fig. 1). Furthermore, based on observation of image by FESEM, the NPs morphology is nearly spherical (Fig. 2). Other elemental composition and properties of the NPs is presented in Table 1.
Experimental design and culture conditions
The trial was conducted as a completely randomized design in the Sturgeon Research Center, Guilan province, Iran. For this purpose, a total of 180 juvenile A. baeri with an average body weight of 100 g ± 5 were randomly distributed in 12 concrete tanks with a density of 15 fish per tank. Before the experiment, fish were fed with a basal diet for 14 days to adapt with the experimental condition. The average physiochemical indices of water including temperature: 18.5 ± 0.5 °C, pH: 7.5 ± 0.02, dissolved oxygen: 7.3 ± 0.4 mg L− 1, and ammonia nitrogen: 0.02 m L− 1 were daily monitored.
Experimental diets
Proximate composition of the basal diet (Beyza Co. Shiraz, Iran, 3.2 mm) was 50.8% crude protein, 15.3% crude lipid, 13.9% carbohydrate, 16.5% ash, and 3.5% fiber. Furthermore, zinc content of the basal diet was 46.5 mg kg− 1. For diets preparation, three different chemical forms of zinc including (i) inorganic (ZnSO4) (Merck, Germany), (ii) organic (Zn-gluconate, Jungbunzlauer Co, Switzerland), and (iii) zinc-oxide nanoparticles (ZnO-NPs, Nanosany Co, Mashhad, Iran) were sprayed on the basal diet at the concentration of 50 mg Zn kg− 1 feed. To prepare suspension, different zinc forms was dissolved in distilled water (100 ml) and sonicated (SOLTEC 2200 M H-SD, Italy). Finally, four experimental diets were prepared as follow; (1) the basal diet without any supplemental Zn (control), (2) the basal diet included with inorganic zinc (Zn-sulfate), (3) the basal diet supplemented with organic zinc (Zn-gluconate), and (4) the basal diet supplemented with zinc-oxide nanoparticles (ZnO-NPs). The leaching of zinc supplements into water was prevented by coating made with bovine gelatin (10%) as described by Ghafarifarsani et al. (2021). The prepared diets were dried by hot air oven at 60 °C for 24 h and then dried at 25 °C with the aid of an air conditioner and an electrical fan. Finally, diets were packed and kept at -20 °C until utilization.
Growth performance
After the 8-week feeding experiment, growth performance and feed efficiency indices were calculated according to Mohammadi et al. (2020) following:
1) Weight gain (WG, %) = W2 - W1/W1 × 100.
2) Specific growth rate (SGR, % day− 1) = Ln (W2) - Ln (W1)/T × 100.
3) Feed conversion ratio (FCR) = feed intake (g)/wet weight gain (g).
4) Condition factor (CF) = body weight (g)/ fork length3 (cm) × 100.
Where, W2, W1, and T represent final body weight (g), initial body weight (g), and experimental period (days), respectively.
Digestive enzymes activity
Sample preparation
For this purpose, the intestinal tissue was homogenized by a homogenizer (1:5 w/v, 1.5 min) in buffer (50 mM Tris-HCl, pH = 7.5). Then, samples were centrifuged at 1000 g for 20 min at 4 °C The supernatants were stored at − 80 °C until the start of the analyses (Chong et al. 2002).
Enzymes activity assay
The activity of the amylase enzyme was determined using starch as substrate according to Bernfeld (1955). Protease enzyme activity was performed by casein (substrate) as reported by Chong et al. (2002). Lipase activity was analyzed through p-nitrophenyl myristate hydrolysis as the substrate (Iijima et al. 1998).
Hemato-biochemical parameters
Sample collection
Three fish were caught from each rearing tank and anesthetized using 60 mg L− 1 clove oil for 5 min (Feng et al. 2011). Blood samples were collected from the caudal vasculature by two sets of sterilized syringes; one with anticoagulant (heparin, for hematological studies) and the other without any anticoagulant (for biochemical studies). Biochemical samples were transferred into 1.5 ml microtubes and centrifuged at 3500 g for 15 min at 4 °C. Subsequently, sera were removed from the samples using a sampler and stored at -80 °C until the start of analyses.
Hematology
White and red blood cells (WBC and RBC) counts were done as reported method by Martins et al. (2014). Hemoglobin (Hb) content was measured using the cyanomethemoglobin spectrophotometry procedure reported by Lee et al. (1998). Hematocrit (Hct) percentage was calculated using the capillary tube (microhematocrit) based on method of Collier (1994). RBC, Hct, and Hb values were used to calculate the erythrocyte indices including, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), according to Telli et al. (2014).
Blood biochemistry
Liver enzymes including aspartate aminotransferase (AST), alanin aminotransferase (ALT), and alkaline phosphatase (ALP) were evaluated using the Technicon RA-1000 analyzer (USA) and commercial kits (Parsazmoon, Iran). The total protein (TP) content was determined according to Bradford (1976) using bovine serum albumin as the standard.
Serum and liver antioxidant enzymes activity
Sample preparation
For this purpose, liver tissue was dissected and put in petri dishes. Tissues were washed with physiological saline (0.9% NaCl) and were stored − 80 °C until the analysis. The tissues were homogenized in 1/1.5% KCl soulation (1/10 w/vol) using a homogenizer and then centrifuged at 9000 g for 30 min. Supernatants obtained were used to measure antioxidant enzyme activities (Uner et al. 2001).
Assay methods
Superoxide dismutase (SOD) activity was determined based on the inhibition rate of nitrotetrazolium complex formation at 560 nm (Yazdanparast et al. 2008). Catalase (CAT) activity was assayed using hydrogen peroxide decomposition (Aebi 1984). Glutathione peroxidase (GPx) activity was quantified by oxidation of NADPH, which was determined at 340 nm (Arun et al. 1999).
Liver histology
For this purpose, liver tissue samples (three samples for each group) were separated and fixed in Bouin’s solution for 5 h at 4 °C (Miki et al. 2018). Fixed liver samples were dehydrated in a graded ethanol series, equilibrated in xylene, embedded in paraffin according to the standard histological analysis procedures. Alternatively, the fixed samples were cleared in xylene and embedded in paraffin wax. Then, thick Sect. (5 µm) from paraffin blocks were achieved using a rotary microtome (M-380, MEDITE Medical GmbH, Burgdorf, Germany) and stained with hematoxylin and eosin (H&E). Histological examinations were performed using a light microscope (Olympus, BH2, Japan) and a camera (Zeiss, Cyber-Shot, Japan) (Nazdar et al. 2018). For quantitative analyses, the number necrotic and pyknotic cells were counted in 1 mm2 (Knudsen et al. 2008). Finally, a modified scoring system of the quantitative histological assessment described by Bernet et al. (1999) was used to quantify histopathological alterations (e.g., sinusoidal dilatation and blood congestion) as NE, 1+, 2+, 3 + and 4 + indicating no evident, faint, moderate, moderate to severe and severe pathological liver tissue changes, respectively.
Data analysis
Prior to data analysis, normality and homogeneity of variance were performed with Shapiro–Wilk and Levene’s tests, respectively. One-way analysis of variance (ANOVA) was used in SPSS program (IBM, SPSS Statistics 20, Chicago, USA) for data analysis. Comparison of the mean differences was done using Duncan test. The data were represented as the “mean ± standard error (SE)”, and a significant difference was set at P < 0.05.
Results
Growth parameters
The results of growth parameters of fish fed diets supplemented with different Zn sources are demonstrated in Fig. 3. The final weight (FW) and condition factor (CF) of fish did not show a significant difference among different experimental treatments (P > 0.05). The highest WG (Fig. 3b) and SGR (Fig. 3c) were seen in fish fed diet containing organic form of Zn, which were significantly higher (P < 0.05) as compared with other experimental treatments (P < 0.05). In contrast, the lowest FCR (Fig. 3d) was observed in fish fed with organic form of zinc, which was significantly different from control and inorganic Zn supplemented diets (P < 0.05).
Final weight (a, FW), weight gain (b, WG), specific growth rate (c, SGR), feed conversion ratio (d, FCR), and condition factor (e, CF) of Siberian sturgeon fed diets supplemented with different zinc sources for 8 weeks. 1 (control), 2 (Zn-gluconate), 3 (Zn-sulfate) and 4 (ZnO-NPs). Data (Mean ± SE) with different superscripts are significantly different (P < 0.05)
Digestive enzymes activity
The results of the activity of digestive enzymes of fish fed diets supplemented with different Zn sources are demonstrated in Fig. 4. The highest amylase (Fig. 4a), lipase (Fig. 4b), and protease (Fig. 4c) activities were recorded in fish fed with the organic form of Zn, which was significantly higher when compared with other experimental groups (P < 0.05). Furthermore, these enzymes did not show significant differences among the other three experimental treatments (P > 0.05).
Digestive enzymes activity: (a) amylase, (b) lipase, and (c) alkaline protease of Siberian sturgeon fed diets supplemented with different zinc sources for 8 weeks. 1 (control), 2 (Zn-gluconate), 3 (Zn-sulfate) and 4 (ZnO-NPs). Data (Mean ± SE) with different superscripts are significantly different (P < 0.05)
Hemato-biochemical parameters
The results of hemato-biochemical indices of fish fed diets containing selected Zn sources are demonstrated in Table 2. RBC and Hct were affected by all three forms of Zn and showed a significant increase in comparison to the control diet (P < 0.05). The highest Hb was obtained in fish fed ZnO-NPs-supplemented diet, which was significantly different from control diet (P < 0.05). Fish fed diet supplemented with ZnO-NPs and control showed higher WBC count than other groups (P < 0.05). The highest MCV and MCHC values were seen in fish fed diets included with ZnO-NPs and control, respectively. Finally, we did not observe significant differences in terms of MCH, monocyte, lymphocyte and eosinophils among tested experimental diets fish (P > 0.05).
ALP was significantly higher (P < 0.05) in fish fed diet containing mineral Zn in comparison to other experimental treatments. ALT showed a decreasing trend from control treatment to organic Zn treatment and its highest activity was seen in the fish fed diet containing ZnO-NPs (P < 0.05). The highest AST value was observed in fish fed control and ZnO-NPs treatments, which was significantly higher when compared with other experimental diets (P < 0.0.5). TP content showed a significant increase in fish fed diets supplemented with inorganic and organic Zn as compared to other experimental diets (P < 0.05).
Antioxidant status
The results of the serum antioxidant enzymes activity in fish fed diets supplemented with different Zn sources are shown in Fig. 5(a). The highest SOD activity was observed in fish fed with ZnO-NPs, which was significantly different from other experimental treatments (P < 0.05). A significant increase was observed in CAT enzyme activity of fish fed organic and inorganic Zn-supplemented diets when compared with other experimental groups (P < 0.05). In contrast, GPx enzyme activity did not show a significant difference in fish fed diets containing different Zn sources compared to the control diet (P > 0.05).
Serum (a) and liver (b) antioxidant enzymes activity of Siberian sturgeon fed diets supplemented with different zinc sources for 8 weeks. 1 (control), 2 (Zn-gluconate), 3 (Zn-sulfate) and 4 (ZnO-NPs). Data (Mean ± SE) with different superscripts are significantly different (P < 0.05). Superoxide dismutase; SOD, catalase; CAT, Glutathione peroxidase; GPx.
The results of the antioxidant status in the liver of fish fed diets containing selected Zn sources are illustrated in Fig. 5(b). Liver SOD enzyme activity had a significant increasing trend in fish fed with organic and inorganic Zn compared to the control group (P < 0.05). CAT enzyme activity of fish fed with different sources of Zn showed a significant decreasing trend as compared to the control diet (P < 0.05). The highest GPx enzyme activity was observed in fish fed diet containing inorganic Zn, which was significantly different (P < 0.05) from the control and ZnO-NPs supplemented groups.
Liver histology
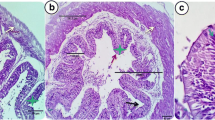
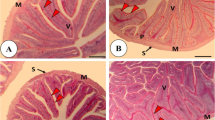
The results of liver histology of fish fed diets containing different Zn sources are shown in Table 3; Fig. 6. Higher histopathological lesions were observed in fish fed with all three forms of Zn compared to control diet (P < 0.05). The highest necrosis was obtained in organic and inorganic Zn groups in comparison to ZnO-NPs supplemented group (P < 0.05). Cellular pyknosis was also observed experimental fish, where it was significantly higher in the fish fed inorganic Zn than other groups (P < 0.05). Additionally, a sinusoidal dilatation and blood congestion were seen in groups fed different Zn sources (Fig. 6). In addition, a severe change of these tissue damage was detected in the fish fed inorganic Zn compared to other experimental treatments (Table 3).
Discussion
Dietary trace elements supplementation, including Zn, can affect fish growth performance and other biomarkers (Bury et al. 2003; NRC 2011). In the present study, sturgeon fish fed the diet containing organic Zn showed higher WG, SGR, and also better FCR in comparison to other experimental diets. Our results are consistent with reported data by other results, which Zn-supplemented diets promoted growth performance in gilthead seabream, Sparus aurata (Domínguez et al. 2017) and beluga sturgeon, Huso huso (Mohseni et al. 2021). In contrast, some studies have shown that the use of different sources of dietary zinc did not affect the fish performance (Maage et al. 2001; Apines et al. 2003). It has been proven that differences in the chemical sources of Zn can affect the bioavailability and performance of fish. In this case, previous report revealed that organic Zn has a higher bioavailability than inorganic Zn (Ringø and Gatesoupe 1998). Generally, the possibility of the formation of insoluble chelated Zn in the intestine is rare, and therefore, it is transmitted in the intestinal mucus with high efficiency compared to the inorganic form (Ashmead 1992; Tan and Mai 2001). In addition, organic Zn and Nano-Zn were found to increase muscle tissue mitosis and cell differentiation by increasing growth hormone (GH) and insulin-like growth factor 1 (IGF-1) (Kishawy et al. 2020).
The digestive enzymes play a vital function in nutritional physiology and affect aquatic animal growth (Ghasemi et al. 2020). In the present study, digestive enzymes activity significantly increased in fish fed organic Zn supplemented diet. In line with our results, the dietary supplementation of Zn promoted the activity of digestive enzymes in different fish species (Tan et al. 2011; Muralisankar et al. 2015; Moazenzadeh et al. 2017; Zhou et al. 2021). On the contrary, the results of Hu et al. (2014) showed that dietary Zn supplementation did not affect digestive enzymes activity in Nile tilapia, Oreochromis niloticus. The fish digestive enzyme activities are closely related to the diet consumed and the ability of fish to digest and absorb different nutrients (Zhou et al. 2015; Hoseinifar et al. 2017; Jiao et al. 2023). Additionally, the distribution of the digestive enzymes along the gastro-intestinal tract and also their activity might vary according to fish intestinal morphology (Tengjaroenkul et al. 2000). Generally, the role of trace mineral, including zinc, as a cofactor is well established in several metabolic pathways (Salgueiro et al. 2000). ZnSO4 is a water-soluble compound and easily dissociates in the digestive tract and subsequently increases the activity of digestive enzymes (Mohamed et al. 2019).
Hematological studies are one of the most important indicators for assessing the health and physiological changes of fish (Kori-Siakpere et al. 2008; Yousefi et al. 2022). The results of the present study show that different forms of Zn have been effective in improving hematological parameters. In agreement with this result, RBCs count of grass carp, Ctenopharyngodon idella, showed a significant increase in the ZnO-NP supplemented diet (Faiz et al. 2015). While, in a study by Moazenzadeh et al. (2017), RBC, Hb, and Hct indices of Siberian sturgeon showed a significant increase with raising dietary Zn-sulfate levels (from 0.5 to 40 mg kg− 1 diet). One might possibly infer that the significant increase in hematological parameters of those fish fed diet containing supplemental zinc compounds in comparison to the control fish might be due to release of RBC from body reserves or possible changes in osmotic pressure of the blood by dietary zinc supplementation (Tort and Torres 1988).
AST, ALT and ALP are important indicators of liver functioning the body (Bae et al. 2020). In the present study, fish fed control and ZnO-NPs supplemented diets showed the highest ALT and AST values. This is in agreement with Taheri et al. (2017) who observed an increase in liver enzyme activities (ALT and AST) of common carp, Cyprinus carpio fed the diet containing 15 mg ZnO-NPs kg− 1. Moazenzadeh et al. (2018) also found an increase in liver enzyme activities in Siberian sturgeon fed the diet containing 14.7 mg Zn-sulfate kg− 1. Contradictory results were also observed by Mohseni et al. (2021) in beluga sturgeon, Huso huso, fed the diets containing higher levels of Zn-methionine (30 and 60 mg kg− 1). In general, the most important reasons for the increased activity of these enzymes are metabolic disorders in cells and hepatic injury revealing potential damage to parenchymal cells, muscles, intestines and liver (Farkas et al. 2004; Kandeel 2004). On the other hand, the toxicity of Zn sources may be one of the factors affecting the elevation of these enzymes (Srivastav et al. 2016). The results of this study also showed an increase in the total serum protein content in fish fed diets containing organic and inorganic Zn. Consistent with the present study, the effects of organic and inorganic micronutrients on improving and stimulating fish immune system have been previously reported (Saffari et al. 2018; Dawood et al. 2019; Afshari et al. 2021; Ghaniem et al. 2022).
The antioxidant defense is a multi-component system that, along with enzymatic and non-enzymatic elements, affects the antioxidative status of the body (Mishra et al. 2015). Therefore, the antioxidant capacity and lipid peroxidation of fish might be affected by dietary minerals, including Zn (Lin et al. 2013). The effect of different dietary zinc sources on the antioxidant capacity of different fish species has been reported in different fish species including tilapia by Saddick et al. (2017), Siberian sturgeon by Moazenzadeh et al. (2018), Indian major carp, Labeo rohita, by Musharraf and Khan (2019), and beluga sturgeon by Mohseni et al. (2021). Our results showed the elevated activity of SOD and CAT in serum following feeding on diets supplemented with both organic and inorganic Zn, respectively in comparison to the control fish. An increase in serum CAT activity following an increase in dietary Zinc levels has been reported in different species (Feng et al. 2011; Jiang et al. 2016; Zhou et al. 2021). However, contrasting results have been also reported by Huang et al. (2015) on Nile tilapia (O. niloticus), where the reduction of CAT activity may be due to a decrease in serum hydrogen peroxide. Additionally, with increased dietary Zn levels up to 87.2 mg kg− 1, SOD enzyme activity showed significant increases in blunt snout bream, Megalobrama amblycephala, liver (Jiang et al. 2016) and Nile tilapia serum (Huang et al. 2015). In another study, reduced SOD activity was observed following dietary Zn deficiency in rainbow trout (Hidalgo et al. 2002). It has been shown that increased activity of antioxidant enzymes might be related to the pro-oxidant role of nutrients in the cell (Borg and Schaich 1989). Generally, dietary Zn deficiency might result in increased cell lipid peroxidation with subsequent cells membrane damage (Tapiero and Tew 2003). In addition, the effect of Zn on transcription factors plays a role in the regulation of the Zn-dependent antioxidant system (Wang et al. 2012; Sun et al. 2014). Furthermore, it protects against oxidative stress by increasing the transcription factor Nrf2-ARE, upregulating antioxidant gene expression, stimulating the synthesis of metallothionein, etc. (Olechnowicz et al. 2018).
The liver is a vital organ in the body for synthesis and metabolism/detoxification of biological molecules (Liu et al. 2017; Li et al. 2020). According to the interpretation of liver transverse incision images and also quantitative and semi- quantitative data, higher histopathological lesions (e.g., necrosis, pyknosis, sinusoidal dilatation and blood congestion) were observed in the liver of those fish fed on diets supplemented with different forms of Zn when compared to control diet. However, these lesions (except for necrosis) in fish fed organic zinc diet showed a lower severity compared to other forms. Similarly, Ostaszewska et al. (2016) reported that the liver of Siberian sturgeon showed a blood cell aggregation when fed with diets containing Ag and Cu-NPs. In another study, a sinusoid space was found in the liver of Mozambique tilapia, Oreochromis mossambicus fed diet containing Ag-nanoparticle (Govindasamy and Rahuman 2012). Consistently, necrosis was reported by Loganathan et al. (2006) who evaluated histological effects of zinc exposure in Labeo rohita. Additionally, such changes were previously documented in different fish species following various dietary minerals exposure (Abdel-Warith et al. 2011; Suganthi et al. 2015; Benavides et al. 2016; Shahzad et al. 2019; Kumar et al. 2020; Harsij et al. 2021). Generally, the histological observations showed the cellular changes in the liver tissues of sturgeon fed with dietary Zn supplements might be due to biochemical and molecular processes including enzyme activity inhibition, changes in the cellular membrane integrity, and interfere with synthesis of proteins and carbohydrate metabolism due to fish nutritional stresses (Mela et al. 2007).
Conclusion
Our results revealed the importance of using 50 mg kg− 1 feed organic zinc in aquafeed since it might positively influence some physiological functions of Siberian sturgeon including growth, digestive enzymes activity and liver antioxidant status. However, further studies for growth and antioxidant-related genes expression are required to better elucidate the nutritional role of the compound on health status of the fish.
Data Availability
All datasets used and/or analyzed during this study were included in this article.
References
Abdel-Warith AA, Younis EM, Al-Asgah NA et al (2011) Effect of zinc toxicity on liver histology of Nile tilapia, Oreochromis niloticus. Sci Res Essays 6(17):3760–3769. https://doi.org/10.5897/SRE11.883
Abdolahnejad Z, Pourkazemi M, Khoshkholgh MR et al (2015) Expression of growth hormone gene during early development of Siberian sturgeon (Acipenser baerii). Mol Biol Res Comm 4:181–188
Aebi H (1984) Catalase in vitro. Methods in enzymology, vol 105. Academic press, pp 121–126
Afshari A, Sourinejad I, Gharaei A et al (2021) The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii, 1897). Aquaculture 539:736638. https://doi.org/10.1016/j.aquaculture.2021.736638
Antony Jesu Prabhu P, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. RevAquac. 8(2):172–219. https://doi.org/10.1111/raq.12090
Apines MJ, Satoh S, Kiron V et al (2003) Availability of supplemental amino acid-chelated trace elements in diets containing tricalcium phosphate and phytate to rainbow trout, Oncorhynchus mykiss. Aquaculture 225:431–444. https://doi.org/10.1016/S0044-8486(03)00307-7
Aramli MS, Moghanlou KS, Imani A (2023) Effect of dietary antioxidant supplements (selenium forms, alpha-tocopherol, and coenzyme Q10) on growth performance, immunity, and physiological responses in rainbow trout (Oncorhynchus mykiss) using orthogonal array design. Fish Shellfish Immunol 134:108615. https://doi.org/10.1016/j.fsi.2023.108615
Arun S, Krishnamoorthy P, Subramanian P (1999) Properties of glutathione peroxidase from the hepatopancreas of freshwater prawn Macrobrachium malcolmsonii. Int J Biochem Cell Biol 31(6):725–732. https://doi.org/10.1016/S1357-2725(99)00016-3
Ashmead HD (1992) The roles of amino acid chelates in Animal Nutrition. Noyes Publications, New Jersey
Bae J, Hamidoghli A, Won S et al (2020) Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture 525(12):735333. https://doi.org/10.1016/j.aquaculture.2020.735333
Benavides M, Fernández-Lodeiro J, Coelho P et al (2016) Single and combined effects of aluminum (Al2O3) and zinc (ZnO) oxide nanoparticles in a freshwater fish, Carassius auratus. Environ Sci Pollut Res 23(24):24578–24591. https://doi.org/10.1007/s11356-016-7915-3
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Bernfeld P (1955) Amylase. In: Colowick SP, Kaplan NO (eds) Methods in Enzymology. Academic Press, New York, pp 149–158
Borg DC, Schaich KM (1989) Pro-oxidant action of antioxidants. Handbook of Free radicals and antioxidants in Biomedicine, vol 1. CRC press, Boca Raton, pp 63–80
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bury NR, Walker PA, Glover CN (2003) Nutritive metal uptake in teleost fish. J Exp Biol 206(1):11–23. https://doi.org/10.1242/jeb.00068
Chen G, Luo Z (2021) Nutritional physiology of Zn and its relationship with lipid metabolism for aquatic animals: a review. Jfc 45(4):632–645. https://doi.org/10.11964/jfc.20200612309
Chong AS, Hashim R, Chow-Yang L et al (2002) Partial characterization and activities of proteases from the digestive tract of discus fish (Symphysodon aequifasciata). Aquaculture 203(3–4):321–333. https://doi.org/10.1016/S0044-8486(01)00630-5
Chupani L, Zusková E, Niksirat H et al (2017) Effects of chronic dietary exposure of zinc oxide nanoparticles on the serum protein profile of juvenile common carp (Cyprinus carpio L). Sci Total Environ 579:1504–1511. https://doi.org/10.1016/j.scitotenv.2016.11.154
Chupani L, Niksirat H, Lünsmann V et al (2018) Insight into the modulation of intestinal proteome of juvenile common carp (Cyprinus carpio L.) after dietary exposure to ZnO nanoparticles. Sci Total Environ 613–614:62–71. https://doi.org/10.1016/j.scitotenv.2017.08.129
Collier HB (1994) The standardization of blood hemoglobin determinations. Can Med Assoc J 50:550–552
Dawood MA, Koshio S, Zaineldin AI et al (2019) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45(1):219–230. https://doi.org/10.1007/s10695-018-0556-3
Faiz H, Zuberi A, Nazir S et al (2015) Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int J Agric Biol 17:568574. https://doi.org/10.17957/IJAB/17.3.14.446
Falahatkar B (2018) Nutritional requirements of the Siberian sturgeon: an updated synthesis. The Siberian sturgeon (Acipenser baerii, Brandt, 1869) volume 1-Biology. Springer, Cham, pp 207–228
Farkas J, Farkas P, Hyde D (2004) Liver and gastroenterology tests. In: Lee M (ed) Basic skills in Interpreting Laboratory Data, 3 edn. American Society of Health-System Pharmacists, Bethesda, pp 330–336. 3
Feng L, Tan LN, Liu Y et al (2011) Influence of dietary zinc on lipid peroxidation, protein oxidation and antioxidant defense of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Nutr 17(4):e875–e882. https://doi.org/10.1111/j.1365-2095.2011.00858.x
Ghafarifarsani H, Imani A, Niewold TA et al (2021) Synergistic toxicity of dietary aflatoxin B1 (AFB1) and zearalenone (ZEN) in rainbow trout (Oncorhynchus mykiss) is attenuated by anabolic effects. Aquaculture 541:736793. https://doi.org/10.1016/j.aquaculture.2021.736793
Ghaniem S, Nassef E, Zaineldin AI et al (2022) A comparison of the beneficial effects of inorganic, organic, and elemental nano-selenium on Nile tilapia: growth, immunity, oxidative status, gut morphology, and immune gene expression. Biol Trace Elem Res 200:5226–5241. https://doi.org/10.1007/s12011-021-03075-5
Ghasemi N, Imani A, Noori F et al (2020) Ontogeny of digestive tract of stellate sturgeon (Acipenser stellatus) from hatching to juvenile stage: Digestive enzymes activity, stomach and proximal intestine. Aquaculture 519:734751. https://doi.org/10.1016/j.aquaculture.2019.734751
Govindasamy R, Rahuman AA (2012) Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci 24:1091–1098. https://doi.org/10.1016/S1001-0742(11)60845-0
Harsij M, Paknejad H, Khalili M et al (2021) Histological study and evaluation of Hsp70 gene expression in gill and liver tissues of goldfish (Carassius auratus) exposed to zinc oxide nanoparticles. Iran J Fish Sci 20(3):741–760
Hidalgo MC, Expósito A, Palma JM et al (2002) Oxidative stress generated by dietary Zn-deficiency: studies in rainbow trout (Oncorhynchus mykiss). Int J Biochem Cell Biol 34(2):183–193. https://doi.org/10.1016/S1357-2725(01)00105-4
Hogstrand C (2011) “3 - Zinc.” In Homeostasis and Toxicology of Essential Metals, edited by Wood CM, Farrell AP, Brauner CJ, Massachusetts: Academic Press. pp, 135–200
Hoseinifar SH, Dadar M, Ringø E (2017) Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: the functional feed additives scenario. Aquac Res 48(8):3987–4000. https://doi.org/10.1111/are.13368
Hu CH, Xiao K, Jiao LF et al (2014) Effects of zinc oxide supported on zeolite on growth performance, intestinal barrier function and digestive enzyme activities of Nile tilapia. Aquac Nutr 20(5):486–493. https://doi.org/10.1111/anu.12101
Huang F, Jiang M, Wen H et al (2015) Dietary zinc requirement of adult Nile tilapia (Oreochromis niloticus) fed semi-purified diets, and effects on tissue mineral composition and antioxidant responses. Aquaculture 439:53–59. https://doi.org/10.1016/j.aquaculture.2015.01.018
Hung SS (2017) Recent advances in sturgeon nutrition. Anim Nutr 3:191–204. https://doi.org/10.1016/j.aninu.2017.05.005
Iijima N, Tanaka S, Ota Y (1998) Purification and characterization of bile salt activated lipase from hepatopancreas of red sea bream Pagarus major. Fish Physiol Biochem 18:59–69. https://doi.org/10.1023/A:1007725513389
Jiang M, Wu F, Huang F et al (2016) Effects of dietary zn on growth performance, antioxidant responses, and sperm motility of adult blunt snout bream, Megalobrama amblycephala. Aquaculture 464:121–128. https://doi.org/10.1016/j.aquaculture.2016.06.025
Jiao F, Zhang L, Limbu SM (2023) A comparison of digestive strategies for fishes with different feeding habits: Digestive enzyme activities, intestinal morphology, and gut microbiota. Ecol Evol 13(9):e10499. https://doi.org/10.1002/ece3.10499
Kandeel NMS (2004) Toxicological and Metabolic Studies of Some Molluscicides on Harmful Terrestrial Snails (M.Sc. thesis). Zoology Dep., Faculty of Science, Cairo University
Kazemi E, Sourinejad I, Ghaedi A et al (2020) Effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of rainbow trout (Oncorhynchus mykiss). Aquaculture 515:734529. https://doi.org/10.1016/j.aquaculture.2019.734529
Kishawy AT, Roushdy EM, Hassan FA et al (2020) Comparing the effect of diet supplementation with different zinc sources and levels on growth performance, immune response and antioxidant activity of tilapia, Oreochromis niloticus. Aquac Nutr 26(6):1926–1942. https://doi.org/10.1111/anu.13135
Knudsen D, Jutfelt F, Sundh H et al (2008) Dietary soya saponins increase gut permeability and play a key role in the onset of soya bean-induced enteritis in Atlantic salmon (Salmo salar L). Br J Nutr 100:120–129. https://doi.org/10.1017/S0007114507886338
Kori-Siakpere O, Ubogu EO (2008) Sublethal hematological effects of zinc on the freshwater fish, Heteroclarias sp. (Osteichthyes: Clariidae). Afr J Biotechnol 7:2068–2073. https://doi.org/10.5897/AJB07.706
Kou H, Hu J, Vijayaraman SB et al (2021) Evaluation of dietary zinc on antioxidant-related gene expression, antioxidant capability and immunity of soft-shelled turtles Pelodiscus sinensis. Fish Shellfish Immunol 118:303–312. https://doi.org/10.1016/j.fsi.2021.08.033
Kumar N, Krishnani KK, Kumar P et al (2017) Dietary zinc promotes immuno-biochemical plasticity and protects fish against multiple stresses. Fish Shellfish Immunol 62:184–194. https://doi.org/10.1016/j.fsi.2017.01.017
Kumar N, Chandan NK, Wakchaure GC et al (2020) Synergistic effect of zinc nanoparticles and temperature on acute toxicity with response to biochemical markers and histopathological attributes in fish. Comp Biochem Physiol Part C 229:108678. https://doi.org/10.1016/j.cbpc.2019.108678
Lee RG, Foerster J, Jukens J et al (1998) Wintrobe’s clinical hematology, tenth edn. Lippincott Williams & Wilkins, New York
Lee DH, Lim S, Lee S (2021) Dietary protein requirement of fingerling sterlet sturgeon (Acipenser ruthenus). J Appl Ichthyol 37(5):687–696. https://doi.org/10.1111/jai.14254
Li P, Su R, Yin R et al (2020) Detoxification of mycotoxins through biotransformation. Toxins 12(2):121. https://doi.org/10.3390/toxins12020121
Lin S, Lin X, Yang Y al (2013) Comparison of chelated zinc and zinc sulfate as zinc sources for growth and immune response of shrimp, Litopenaeus vannamei. Aquaculture 406–407:79–84. https://doi.org/10.1016/j.aquaculture.2013.04.026
Liu X, Wang H, Liang X et al (2017) Hepatic metabolism in liver health and disease. In Liver Pathophysiology. Academic Press. pp. 391–400
Loganathan K, Velmurugan B, Hongray Howrelia J et al (2006) Zinc induced histological changes in brain and liver of Labeo rohita. J Environ Biol 27:107–110
Maage A, Julshamn K, Berge GE (2001) Zinc gluconate and zinc sulphate as dietary zinc sources for Atlantic salmon. Aquac Nutr 7:183–187. https://doi.org/10.1046/j.1365-2095.2001.00170.x
Martins ML, Tavares-Dias M, Fujimoto RY et al (2014) Hematological alterations of Leporinus macrocephalus (osteichtyes: Anostomidae) naturally infected by Goezia eporine (Nematoda: Anisakidae) in fish ponds. Arq Bras Med Veterinaria Zootec 56:640–646. https://doi.org/10.1590/S0102-09352004000500011
Mela M, Randi MAF, Ventura DF et al (2007) Effects of dietary methylmercury on liver and kidney histology in the neotropical fish Hoplias malabaricus. Ecotoxicol Environ Saf 68(3):426–435. https://doi.org/10.1016/j.ecoenv.2006.11.013
Miki M, Ohishi N, Nakamura E et al (2018) Improved fixation of the whole bodies of fish by a double-fixation method with formalin solution and Bouin’s fluid or Davidson’s fluid. J Toxicol Pathol 31(3):201–206. https://doi.org/10.1293/tox.2018-0001
Mishra A, Patel MK, Jha B (2015) Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J Funct Foods 13:21–31. https://doi.org/10.1016/j.jff.2014.12.027
Moazenzadeh K, Islami HR, Zamini A et al (2017) Dietary zinc requirement of Siberian sturgeon (Acipenser baerii, Brandt 1869) juveniles, based on the growth performance and blood parameters. Int Aquat Res 9(1):25–35. https://doi.org/10.1007/s40071-017-0153-6
Moazenzadeh K, Rajabi Islami H, Zamini A et al (2018) Effects of dietary zinc level on performance, zinc status, tissue composition and enzyme activities of juvenile Siberian sturgeon, Acipenser baerii (Brandt 1869). Aquac Nutr 24(4):1330–1339. https://doi.org/10.1111/anu.12670
Moazenzadeh K, Rajabi Islami H, Zamini A et al (2020) Quantitative dietary copper requirement of juvenile Siberian sturgeon, Acipenser baerii, and effects on muscle composition and some enzymatic activities. Aquac Nutr 26(4):1108–1118
Mohamed WA, El-Houseiny W, Ibrahim RE et al (2019) Palliative effects of zinc sulfate against the immunosuppressive, hepato- and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus). Aquaculture 504:227–238. https://doi.org/10.1016/j.aquaculture.2019.02.004
Mohammadi M, Imani A, Farhangi M et al (2020) Replacement of fishmeal with processed canola meal in diets for juvenile Nile tilapia (Oreochromis niloticus): growth performance, mucosal innate immunity, hepatic oxidative status, liver and intestine histology. Aquaculture 518:734824. https://doi.org/10.1016/j.aquaculture.2019.734824
Mohseni M, Hamidoghli A, Bai SC (2021) Organic and inorganic dietary zinc in beluga sturgeon (Huso huso): effects on growth, hematology, tissue concentration and oxidative capacity. Aquaculture 539:736672. https://doi.org/10.1016/j.aquaculture.2021.736672
Muralisankar T, Bhavan PS, Radhakrishnan S et al (2015) Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions, and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 448:98–104. https://doi.org/10.1016/j.aquaculture.2015.05.045
Musharraf M, Khan MA (2019) Dietary zinc requirement of fingerling Indian major carp, Labeo rohita (Hamilton). Aquaculture 503:489–498. https://doi.org/10.1016/j.aquaculture.2019.01.039
Nazdar N, Imani A, Noori F et al (2018) Effect of silymarin supplementation on nickel oxide nanoparticle toxicity to rainbow trout (Oncorhynchus mykiss) fingerlings: pancreas tissue histopathology and alkaline protease activity. Iran J Sci Technol Trans Sci 42:353–361. https://doi.org/10.1007/s40995-016-0052-5
NRC, National Research Council (2011) Nutrient requirements of fish and shrimp. National Academies Press
Olechnowicz J, Tinkov A, Skalny A et al (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 68(1):19–31. https://doi.org/10.1007/s12576-017-0571-7
Ostaszewska T, Chojnacki M, Kamaszewski M et al (2016) Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ Sci Pollut Res 23:1621–1633. https://doi.org/10.1007/s11356-015-5391-9
Rashidian G, Lazado CC, Mahboub HH et al (2021) Chemically and green synthesized ZnO nanoparticles alter key immunological molecules in common carp (Cyprinus carpio) skin mucus. Int J Mol Sci 22(6):3270. https://doi.org/10.3390/ijms22063270
Ringø E, Gatesoupe FJ (1998) Lactic acid bacteria in fish: a review. Aquaculture 160(3–4):177–203. https://doi.org/10.1016/S0044-8486(97)00299-8
Saddick S, Afifi M, Abu Zinada OA (2017) Effects of Zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 24(7):1672–1678. https://doi.org/10.1016/j.sjbs.2015.10.021
Saffari S, Keyvanshokooh S, Zakeri M et al (2018) Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol Biochem 44(4):1087–1097. https://doi.org/10.1007/s10695-018-0496-y
Shahzad K, Khan MN, Jabeen F et al (2019) Toxicity of zinc oxide nanoparticles (ZnO-NPs) in tilapia (Oreochromis mossambicus): tissue accumulation, oxidative stress, histopathology and genotoxicity. Int J Environ Sci Technol 16(4):1973–1984. https://doi.org/10.1007/s13762-018-1807-7
Srivastav AK, Kumar M, Ansari NG et al (2016) A comprehensive toxicity study of zinc oxide nanoparticles versus their bulk in Wistar rats: toxicity study of zinc oxide nanoparticles. Hum Exp Toxicol 35(12):1286–1304. https://doi.org/10.1177/0960327116629530
Suganthi P, Murali M, Sadiq Bukhari A et al (2015) Morphological and liver histological effects of ZnO nanoparticles on Mozambique tilapia. JOAASR 1:68–83. https://doi.org/10.46947/joaasr1120158
Sun W, Wang Y, Miao X et al (2014) Renal improvement by zinc in diabetic mice is associated with glucose metabolism signaling mediated by metallothionein and akt, but not Akt2. Free Radic Biol Med 68:22–34. https://doi.org/10.1016/j.freeradbiomed.2013.11.015
Taheri S, Banaee M, Nematdoost Haghi B et al (2017) Effects of dietary supplementation of zinc oxide nanoparticles on some biochemical biomarkers in common carp (Cyprinus carpio). Int J Aquat Biol 5(5):286–294. https://doi.org/10.1016/j.jksus.2023.102835
Tan B, Mai K (2001) Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone, Haliotis discus hannai Ino. Aquaculture 192:67–84. https://doi.org/10.1016/S0044-8486(00)00435-X
Tan LN, Feng L, Liu, Ye t al (2011) Growth, body composition and intestinal enzyme activities of juvenile Jian carp (Cyprinus carpio var. Jian) fed graded levels of dietary zinc. Aquac Nutr 17:338–345. https://doi.org/10.1111/j.1365-2095.2010.00793.x
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 57(9):399–411. https://doi.org/10.1016/S0753-3322(03)00081 – 7
Telli SG, Ranzani-Paiva TJM, Dias de CD et al (2014) Dietary administration of Bacillus subtillis on hematology and non-specific immunity of Nile tilapia Oreochromis Niloticus raised at different stocking densities. Fish Shellfish Immunol 39:305–311. https://doi.org/10.1016/j.fsi.2014.05.025
Tengjaroenkul B, Smith BJ, Caceci T et al (2000) Distribution of intestinal enzyme activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture 182(3–4):317–327. https://doi.org/10.1016/S0044-8486(99)00270-7
Tort L, Torres P (1988) The effects sublethal concentration of cadmium on hematological parameters in dogfish. Soyliorhinue Canicula. J Fish Biol 32:277–282. https://doi.org/10.1111/j.1095-8649.1988.tb05361.x
Uner N, OruçE Ö, Canli M et al (2001) Effects of cypermethrin on antioxidant enzyme activities and lipid peroxidation in liver and kidney of the freshwater fish, Oreochromis niloticus and Cyprinus carpio (L). Bull Environ Contam Toxicol 67:657–664. https://doi.org/10.1007/s001280174
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73(1):79–118. https://doi.org/10.1152/physrev.1993.73.1.79
Vijayakumar S, Vaseeharan B, Sudhakaran R et al (2019) Bioinspired zinc oxide nanoparticles using Lycopersicon esculentum for antimicrobial and anticancer applications. J Clust Sci 30:1465–1479. https://doi.org/10.1007/s10876-019-01590-z
Wang X, Li H, Fan Z et al (2012) Effect of zinc supplementation on type 2 Diabetes parameters and liver metallothionein expressions in Wistar rats. J Physiol Biochem 68:563–572. https://doi.org/10.1007/s13105-012-0174-y
Wang H, Li E, Zhu H et al (2016) Dietary copper requirement of juvenile Russian sturgeon Acipenser gueldenstaedtii. Aquaculture 454:118–124. https://doi.org/10.1016/j.aquaculture.2015.12.018
Wei QW, Zou Y, Li P et al (2011) Sturgeon aquaculture in China: progress, strategies and prospects assessed on the basis of nation-wide surveys (2007–2009). J Appl Ichthyol 27:162–168. https://doi.org/10.1111/j.1439-0426.2011.01669.x
Yazdanparast R, Bahramikia S, Ardestani A (2008) Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact 172(3):176–184. https://doi.org/10.1016/j.cbi.2008.01.006
Yousefi M, Hoseini SM, Aydın B et al (2022) Anesthetic efficacy and hemato-biochemical effects of thymol on juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 547:737540. https://doi.org/10.1016/j.aquaculture.2021.737540
Zhou C, Ge X, Niu J et al (2015) Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, Trachinotus ovatus. Aquaculture 437:390–397. https://doi.org/10.1016/j.aquaculture.2014.12.016
Zhou C, Lin H, Huang Z et al (2021) Effects of dietary zinc levels on growth performance, digestive enzyme activities, plasma physiological response, hepatic antioxidant responses and metallothionein gene expression in juvenile spotted sea bass (Lateolabrax Maculatus). Aquac Nutr 27(5):1421–1432. https://doi.org/10.1111/anu.13280
Funding
This research was partially supported by the Urmia University Research Council with grant number of 2709.
Author information
Authors and Affiliations
Contributions
Frough Pourmoradkhani, Vahid Gholizadeh and Mojtaba Pourahad Anzabi, investigation, collection of data and writing original draft, Kourosh Sarvi Moghanlou, funding acquisition, project administration, manuscript reviewing and editing, Tooraj Sohrabi Langaroud, contribute materials and analysis tools and Ahmad Imani, formal analyses and interpretation of data, manuscript reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
The experimental fish care and handling procedures were approved by the Urmia University Animal Ethic Committee, Urmia, Iran (IR-UU-AEC-3/32).
Competing interests
The authors declared no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pourmoradkhani, F., Sarvi Moghanlou, K., Sohrabi, T. et al. Supplementation of Siberian sturgeon (Acipenser baerii) diet with different zinc sources: effects on growth performance, digestive enzymes activity, hemato-biochemical parameters, antioxidant response and liver histology. Vet Res Commun 48, 797–810 (2024). https://doi.org/10.1007/s11259-023-10252-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-023-10252-5