Abstract
The present study investigates the effect of different dietary protein levels suboptimum level (25%) and optimum level (35%), different Zn forms bulk zinc oxide (BZnO) or nanoparticles zinc oxide (NZnO), and their interaction on performance, intestinal topography, hematology, serum biochemical, antioxidant-immune responses, and related gene expression of Nile tilapia. Six experimental diets were formulated to contain approximately 25% and 35% crude protein and supplemented with Zn forms with 0 (normal level in ingredients), 60 mg kg−1 BZnO and 60 mg kg−1 nanoparticles of NZnO. Nile tilapia, Oreochromis niloticus, fingerlings (7.53 ± 0. 06 g) were fed on one of tested diets in triplicates with 5% of total biomass three times a day for 84 days. Results showed that, fish fed diet containing 35% crude protein and supplemented with NZnO form recorded the highest final body weight (FBW), weight gain (WG), and specific growth rate (SGR). However, no significant (P > 0.05) differences were recorded in FBW, WG, SGR, feed conversion ratio (FCR), and protein efficiency ratio (PER) between fish fed diet containing 35% crude protein without Zn supplementation and fish fed diet containing 25% crude protein supplemented with NZnO form. Either fish fed diet containing 25% or 35% crude protein and supplemented with NZnO exhibited the highest values of villi height/width. The highest absorption surface area (ASA) was obtained in fish fed diet containing 25% or 35% crude protein and supplemented with BZnO. Hemoglobin (Hb), hematocrit (Hct), and red blood cell count (RBCs) highest values were obtained for fish fed diet containing protein level 35% supplemented with NZnO. Fish fed diet containing protein level 35% and supplemented with NZnO had the lowest value of alanine amino transferase (ALT) and aspartate amino transferase (AST). The highest globulin value was recorded for fish provided with diet containing 35% crude protein and supplemented with BZnO followed by those fed diet containing 35% crude protein and supplemented with NZnO. Fish fed diet containing protein level 25% with NZnO supplementation recorded the highest super oxide dismutase (SOD), catalase (CAT), glutathione reductase (GSH), and glutathione peroxidase (GPX), with decreasing malondialdehyde (MAD) values. The highest values of immunoglobulin g (IgG), immunoglobulin M (IgM), complement 4 (C4), and complement 3 (C3) were obtained for diet containing 35% crude protein and supplemented with NZnO form. Growth hormone gene (GH) was upregulated in fish fed 25% dietary protein without Zn supplementation, while it was downregulated in fish fed 25% dietary protein and supplemented with NZnO. Transcription of insulin-like growth factor-1 (IGF-I) gene recorded the highest value for fish fed 35% crude protein and supplemented with BZnO. This is although the diet of 35% crude protein + NZnO induced significant (IGF-I) gene expression compared with 25% crude protein with or without BZnO. Therefore, nano zinc is useful as a feed supplement for Nile tilapia (Oreochromis niloticus)
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Protein is a crucial core nutrient as well as the most expensive ingredient in aquatic feeds industry [1]. Dietary protein level has an essential role for growth performance and survival of fish directly affects the price of feed products [2]. Growth rate of fish cannot reach to optimal rate when feed containing low level of the dietary protein content [3]. However, when feed of aquatic animal containing dietary protein level higher than their requirements, fish use excessive protein catabolism for energy consumption through peroxide deamination, which leads to depressed fish growth and unsustainable ecological developments [4, 5], while carbohydrates have been widely used as an energy source in aquatic feed to prevent the catabolism of expensive protein nutrients for energy needs (sparing effect) and reduce the emissions of ammonia nitrogen [6, 7]. The high cost of fish feed still is one of the great difficulties, so, it has been sought ever more efficient strategies to minimize these costs, including to know the needs of each nutrient for each species [8,9,10]. Feeds having high dietary protein level are also rich in zinc (Zn) content, whereas those feeds containing mostly carbohydrate were found to be much lower in zinc content [11]. Moreover, Zn plays a vital role as a co-factor of enzymes, involved in several nutrient metabolic processes and is a component of many important metalloenzymes [12, 13].
Dietary Zn requirements have been established for many freshwater fish species using dietary zinc sulfate (ZnSO4) source ranged between 15‒30 mg kg−1 diet for common carp, Cyprinus carpio [14], 20 mg kg−1 diet for channel catfish, Ictalurus punctatus [15], 30 and 60 mg kg−1 diet for Nile tilapia, Oreochromis niloticus [16, 17]. One of the consequences of Zn supplementation in feedstuff is an increase in Zn excretion from fish body and an increase in its concentration in the environment [18]. Meanwhile, Zn bioavailability to fish from Zn complexes is therefore higher than that observed for ZnSO4 or ZnO, especially in plant-origin diets [19]. In this regard, Tan and Mail [20] stated that different inorganic Zn sources such as ZnO, ZnSO4, and zinc carbonate (ZnCO3) have shown a lower rate of Zn absorption in fish intestines than methionine-chelated Zn (Zn-Met). That is why researchers are looking for ways to decrease Zn content in feed additives and increase its bioavailability in diets [18]. Over the last decade, the field of materials science has advanced exponentially and many nanoscale materials, including Nano particles of zinc oxide (NZnO) have been manufactured. Metal oxides nanoparticles are one of the most widely used class of nano-materials in industrial and domestic applications [21]. Among the nanoscale metal oxides, NZnO have the third highest global production after TiO2 and SiO2 [22]. Recently, investigation mainly focused on using eco-friendly to produce nanoparticles instead of conservative protocols [23, 24]. There are numerous factors controlled the absorption and bioavailability of Zn such as chemical form of Zn and level of Phytate in ingredients also, bioavailability of calcium phosphate [18, 20, 25,26,27]. Zinc preoccupation in fish is low and varies with the locations of gastrointestinal tract, size of fish and the forms of Zn [18]. Following the same pattern, Tan and Mail [20] found that the absorption of inorganic forms of Zn is inferior compared to organic form in fish intestines, however the incorporation of organic Zn in fish diets is limited due to high price [28]. To the best of our knowledge, previous studies have not investigated the effect of NZnO on the dietary protein levels of fish. Thus, it is important to expand our knowledge of the effect of NZnO form on dietary protein level of Nile tilapia. Hence, the objective of the current trial was to assess the interaction between Zn forms and dietary protein levels (25% or 35%) on growth, feed efficiency, and structure of intestinal, immune-oxidative response and associated gene expression of Nile tilapia, O. niloticus. This investigate was conducted on Nile tilapia, Oreochromis niloticus (Linnaeus, 1758), which is a tropical species that prefers living shallow waters. Tilapia species is the second most important group of farmed fish in the world following after Carp, and they are the most widely cultivated of any of the farmed fish.
Materials and Methods
Experimental Design
A 2 × 3 factorial experiment was designed to study the effect of different dietary protein levels suboptimum level (25%) and optimum level (35%), different Zn forms Bulk Zinc oxide (BZnO) or nanoparticles Zinc oxide (NZnO), and their interaction on growth promotion, feed utilization, intestinal topography, hematology, serum biochemical, antioxidant and immune responses, and related gene expression of Nile tilapia for 84 day.
Diet Preparation
Six experimental diets were formulated to contain approximately 25% and 35% crude protein, which had been shown to be sufficient to support the suboptimum level (25%) and optimum level (35%) and the proximate chemical composition of the experimental diets is presented in Table 1. The first group, which contained 25% crude protein, the second group that contained 35% crude protein. Each group was supplemented with different Zn forms with zero (normal level), 60 mg kg−1 BZnO, 60 mg kg−1 NZnO (NZnO, 59.1 ± 25 nm; supplementary file), based on Zn requirement for Nile tilapia [16, 17]. After that all ingredient were mixed well for each diet, and then 150 ml water kg−1 were added to make a dough of each diet. Pelleting experimental diets were prepared with 2 mm diameter via passing it in laboratory pellet machine. After pelleting, the diets were dried at room temperature for 24 h or completely drying, then stored at 4 °C in refrigerator until use.
AOAC [30] approaches were used to measure the chemical composition of experimental diets. The Zn concentrations in experimental diets, 25% dietary protein without Zn, 25% dietary protein supplemented with 60 mg BZnO, and 25% dietary protein supplemented with 60 mg NZnO after preparation, were 121.79, 180.45, and 181.25 mg kg−1 diet, respectively, while the concentration of Zn in diets, 35% dietary protein without Zn form, 35% dietary protein supplemented with 60 mg BZnO, and 35% dietary protein supplemented with 60 mg NZnO, were 179.23, 235.2, and 240.5 mg kg−1, respectively. The concentration of Zn in the experimental diets was determined according to [17].
Experimental Fish and Culture Technique
Tilapia fingerlings were obtained from private farm (Kafer-Elshekh Governorate, Egypt). Fish after arrived were acclimated before the experimental conditions for two weeks at the laboratory of fish research at Faculty of Agriculture, Banha University in two concert ponds (3 × 4 × 1 m3). During the acclimation period, fish were fed a commercial feed (30% crude protein) at a rate of 5% of biomass, which provided of equal rations at 09:00 am and 3:00 pm for 2 weeks. After the acclimatization, the experimental fish were randomly distributed into experimental cylindrical fiberglass tanks (0.5 m3 for each) representing the six treatments studied. A set of 360 fish of Nile tilapia mono-sex male fingerlings with an average initial weight of 7.53 ± 0. 06 g were used in this experiment. Twenty fish were randomly stocked into each cylindrical fiberglass tank with triplicates for each treatment. During the 84-day experimental period, all groups of fish were hand-fed with their respective diet divided into three equal amounts and offered three times a day at 09:00 am, 12:00 pm, and 03:00 pm, at a rate of 5% of biomass. Thirty minutes after the feeding, uneaten feed were removed by siphoning, and then dried and weighted. Feed intake was the difference between them and expressed as the total feed intake in 84 days per fish. Fish were taken from each tank, weighed and the amount of feed was adjusted (every two weeks) according to the changes in body weight through the experimental period. The cylindrical fiberglass tanks were supplied with underground–aerated water. Air was compressed to each tank via air stones by air pumps during the experimental period. About one-third of water volume in each tank was daily replaced by aerated freshwater after cleaning and removing of the accumulated excreta. Water quality was monitored everyday throughout the feeding trial for each tank to evaluate the water quality parameters These values are kept up within the acceptable ranges for fish farming according to guidance of [31].
Growth Performance and Feed Utilization Parameters
Records of live body weight (g) were measured in all fish for each tank and registered every 15 days during the experimental period. Growth performance parameters were measured by using the equations noted in the foot note of Table 3.
Sample Collection
At the end of the experiment period, six fish (n = 3) were randomly selected from each group and anesthetized by MS 222 (100 mg L−1, Sigma Aldrich, Egypt), and homogenized in a blender for final fish chemical composition. The fish from each tank were pooled, oven-dried, ground, and stored at –20 ºC for further analysis. Three fish (n = 3) from each treatment were collected to obtain the liver for antioxidant parameters, while other three fish (n = 3) from each group were separated and intestines were removed for histomorphometric examination (anterior and posterior parts).
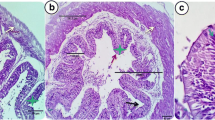
Histological Characteristics of the Intestine
On 84 days of the feeding trial, three fish in each treatment were sacrificed, and their intestines (from the anterior and posterior parts) were taken for histo-morphometric examination. The intestinal tissues of the experimental fish were dissected and fixed in Bouin’s fluid for 24 h. The fixed tissues were dehydrated in graded ethanol series, cleaned in xylene and embedded in paraffin wax (congealing point 58–60 °C). Cross sections each of 6 µm thickness were stained with hematoxylin and eosin. The tissue sections were examined under light microscope equipped with full HD microscopic camera (Nikon E600, Tokyo, Japan) and image analysis software. The mean villus length (measured from the base to the top) and villus width were measured for three fish from each group by image analysis software and data were used for statistical analysis. The absorption surface area (ASA) was calculated according to [17] the mucosal to serosal ratio (MSR) was also estimated.
Blood Sampling
Blood samples were collected from the caudal vein and were divided into two portions. The first portion was collected with the anticoagulant 10% ethylene diamine-tetra-acetate (EDTA) to measure the hematological parameters as; hemoglobin (Hb), hematocrit (Hct), red blood cells (RBCs) and white blood cells (WBCs). Hct was determined using the method described by Reitman and Frankel [32], Hb was determined using hemoglobin kits, which was a standardized procedure using cyanmethemoglobin method and both the total count of RBCs and WBCs were carried out [33].The second group of the blood samples were collected without anticoagulant 10% EDTA to obtain the blood serum. Serum total protein, albumin and globulin were gritty conferring to [34,35,36] and alanine amino transferase (ALT) and Aspartate amino transferase (AST) activities were measured as described by [32], and serum creatinine was measured as described by [34] Serum uric acid, creatinine IgG, IgM, C3 and C4 were measured spectro-photometrically using commercial kits produced by Pasteur labs (Egyptian American Co. for Laboratory Services, Egypt).
Antioxidant Assays
The liver of three fish in each treatment were weighed and grinded in glass homogenizer tubes with ice-cold saline (to 0.1 g of liver was added 0.9 mL saline, pH 7.0) then centrifuged at 3000 g for 10 min. the obtained supernatant was used for estimating antioxidant enzyme and MDA activities. Activities of superoxide dismutase (SOD), catalase (CAT), and malonaldehyde (MDA) levels were measured using diagnostic kits (Bio-diagnostics, Giza, Egypt) following the manufacturer’s instructions according to the methodology of [37] [38] [39], respectively. Glutathione reductase (GSH) and glutathione peroxidase (GPx) activities were measured according to the method of [40].
Gene Expression
After fish anesthetizing by using 3-aminobenzoic acid ethyl ester (MS 222, 100 mgL−1, Sigma, St. Louis, MO), liver samples were decapitated and homogenized by tissue homogenizer (QIAGEN GmbH, QIAGEN Strasse 1, Hilden, Nordrhein-Westfalen-40724, Germany). Total ribonucleic acid (RNA) was extracted from the liver, using RNeasy® Mini kit (Qiagen, Cat No. 74104), based on manufacturer’s protocol provided in the kit. cDNA was synthesized from 1000 ng of total RNA using the protocol of high-capacity cDNA Reverse Transcription Kit (Applied Bio systems, Cat# no.4368813); then, cDNA was stored at − 80 °C for further molecular analyses.
Primers for amplifications of the gene, which encodes growth hormone (GH), insulin-like growth factor (IGFI), superoxide dismutase (SOD) and 18S ribosomal RNA (18S rRNA) gene, as reference gene, were used for quantifying the mRNA of the target gene using real time PCR (qRT-PCR) methodology (Table 2). Quantitative PCR reaction contained 2.5 μl of 1 μg/μl cDNA, 12.5 μl SYBR Green PCR Master Mix (QuantiTect SYBR Green PCR Kit, Qiagen), 0.3 μM of each of forward and reverse primers and double distilled water to a final volume of 25 μl. Reaction was run on an Applied Biosystem 7500 Real time PCR Detection system (Applied Bio systems) under the conditions of 95 °C for 10 min and 45 cycles of 95 °C for 20 s followed by 60 °C for 20 s and 72 °C for 20 s.
Statistical Data Analysis
All data were analyzed by using the software SAS, version 6.03 (Statistical Analysis System; SAS 2016). One-way analysis of variance (One-way ANOVA) was used to determine whether significant variation existed between the treatments. When overall differences were found, differences between means were tested by Duncan [41] new multiple range test. Two-way ANOVA was used for analyzing the individual effects of Zn form and dietary protein levels and the interaction between them. All differences were considered significant at P ≤ 0.05 and the results are presented as means with pooled standard error of the mean (± SE).
Results
Growth Performance and Feed Efficiency
Regardless the effect of Zn, different protein diets had significantly effect on growth performance of tilapia, final body weight (FBW; P = 0.003), weight gain (WG; P = 0.0001), specific growth rate (SGR; P = 0.0001), FCR (P = 0.01), and PER (P = 0.0003) (Table 3), while dietary protein had no significant effect on FI (P = 0.79). The best FCR and highest PER were obtained in 25% crude protein. Irrespective of protein level effect, the Zn form significantly improved the growth performance of tilapia; FBW (P = 0.002), WG (P = 0.001), SGR (P = 0.0012), FI (P = 0.03), FCR (P = 0.05), and PER (P = 0.014) (Table 3).
On the other hand, interaction between different protein levels (25% and 35%) and ZnO form released a significant effect on FBW (P = 0.001), WG (P = 0.001), and SGR (P = 0.0004) (Table 3). The highest FBW, WG and SGR were recorded by fish fed diet containing 35% crude protein and supplemented with NZnO form. No significant (P > 0.05) differences in FBW, WG, SGR, FCR and PER were found between fish fed diet containing 35% crude protein without BZnO supplementation and fish fed diet containing 25% crude protein supplemented with NZnO form. The lowest values of FCR and PER were recorded by fish fed diet containing 25% crude protein without supplementation of Zn form (Table 3).
Histomorphometric
In term of anterior intestinal villi length, width and absorption area of villous (ASA) were significantly (P ≤ 0.05) affected by different dietary protein levels and Zn forms, while mucosal to serosal ratio (MSR) was not affected (Table 4). Either fish fed diet containing 25% or 35% crude protein and supplemented with NZnO exhibited the highest values of villi length and width. The highest ASA value was obtained in fish fed diet fed diet containing 25% or 35% crude protein and supplemented with BZnO.
In term of posterior intestine villi length, width and MSR were significantly (P ≤ 0.05) affected by different dietary protein levels and Zn forms (Table 4). Fish fed diet containing 25% + NZnO and fish diet 35% crude protein + BZnO recorded the highest levels of anterior intestine villi length and width. No significant differences (P > 0.05) were found in ASA as affected by dietary protein and Zn form addition.
Hematological Parameters
Different dietary protein levels 25% or 35% had no significant (P > 0.05) effect on Hb, Hct, PLT, WBCs and lymphocytes precent of Nile tilapia, irrespective of Zn form (Table 5). Regardless the effect of different dietary protein levels, supplementation of Zn forms (BZnO and NZnO) in fish diet showed a significant effect on Hb value. Interaction between different protein levels (25% and 35%) and different Zn forms (BZnO and NZnO) had significantly effect on the values of Hb, Hct, PLT, WBCs, and lymphocyte count (Table 5). The highest Hb, Hct and RBCs values were obtained for fish fed diet containing protein level 35% supplemented with NZnO, while the lowest value of Hb was recorded for fish fed diet containing protein level 25% without any Zn supplementation. There were no significant (P > 0.05) effect of different dietary protein levels (25% and 35%) in tilapia diets, different Zn form supplementation and their interaction on RBCs, MCV, MCH and MCHC, neutrophil (%), monocytes (%), and eosinophil (%) values, for Nile tilapia (Table 5).
Serum Biochemical Parameters
Different protein levels had a significant effect on the activities of ALT (P = 0.01), AST (P = 0.004), regardless the effect of different Zn forms. Irrespective of different protein levels effect, different Zn forms showed a significant effect on ALT values (Table 6). ANOVA analysis showed that interaction between different protein levels 25% and 35% Zn form (BZnO and NZnO) released a significant effect on ALT activity (Table 6). Fish fed diet containing protein level 35% and supplemented with NZnO had the lowest values of ALT and AST activities (82.50 and 9.30 U/L), respectively. On the other hand, fish fed diet containing protein level 25% without any Zn supplementation had the highest value of ALT activities (154.70 U/L). No significant (P > 0.05) differences were found in serum total protein, albumin and creatinine values (Table 6) which were not affected by different protein levels, different Zn forms and their interaction, while interaction between different protein levels and different Zn form had significant effect on globulin content (P = 0.014). The highest globulin value was recorded for fish fed diet containing 35% crude protein and supplemented with BZnO followed by fish fed diet containing 35% crude protein and supplemented with NZnO (Table 6).
Oxidative Stress Biomarkers
Data in Table 7 showed that superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), glutathione reductase (GSH), and glutathione peroxidase (GPX) activities were significantly (P = 0.001) affected by different protein levels (25% and 35%) and Zn form for Nile tilapia. ANOVA analysis showed significant interaction between different protein levels (25% and 35%), Zn form (BZnO and NZnO) on SOD activity (P = 0.002). Fish fed diet containing protein level 35% with NZnO supplementation recorded the highest SOD, CAT, GSH, and GPX activities with decreasing MDA values.
Immune Response
Results showed that, different dietary protein levels (25% and 35%) and Zn forms had a significant effect on immunoglobulin g (IgG), immunoglobulin M (IgM), complement 4 (C4), and complement 3 (C3) in Nile tilapia (Table 8). Interaction between different levels of protein (25% and 35%) and different forms of Zn (BZnO and NZnO) significantly (P = 0.035) affected on IgG, IgM, C4, and C3 values, the highest values were obtained for fish fed diet containing 35% crude protein and supplemented with NZnO form (Table 8).
Gene Expression
Results of the effect of different ratios of 25% and 35% crude protein and Zn supplemented levels and their interactions on relative expression of growth hormone (GH) and insulin-like growth factor (IGF-I) genes were showed in (Table 9, Fig. 1 and Fig. 2). Growth hormone (GH) gene was upregulated in fish fed 25% dietary protein without supplementation of Zn form, while it was downregulated in fish fed diet with 25% crude protein diet and supplemented with NZnO. Furthermore, fish fed 35% dietary protein level with or without ZnO supplementation form were downregulated. Transcription of IGF-I gene in fish fed 25% and 35% dietary protein with or without ZnO form was opposite trend with GH gene. Supper oxide dismutase (SOD) gene was upregulated in fish fed diet containing 25% dietary protein and supplemented with NZnO Compared to other treatment (Fig. 3 and Table 9). The lowest gene expression of the above-mentioned variable was observed in fish fed 25% dietary protein without supplementation of Zn form.
Discussion
Growth Performance
Dietary protein is essential for fish growth and is an expensive component of the feed; hence, protein supplementation needs to be optimized [42]. Zinc supplementation with different form is also crucial for feed utilization and fish growth [17]. In the current study, with an increase in dietary protein level from 25 to 35%, WG and SGR were increased significantly. However, the FCR showed the reverse trend, while ZnO especially nanoparticle’s form could decrease the dietary protein level of tilapia, with no significant differences were found in growth performance and feed utilization between fish fed diet containing 25% crude protein and supplemented with NZnO and fish fed 35% dietary protein level without supplementation of any Zn form (Table 3). These improvement in growth performance that recorded in the present study could be related to the following critical points: (i) the advantage of electrical characteristics of nano-materials could improve the intestinal absorption surface area (ASA) (Table 4), consequently enhance nutrient efficiency [18, 43], (ii) NZnO has antimicrobial agent for different species of bacteria and fungi, consequently improve immune system and therefore improve growth performance [44]. Thus, the variation exist in the Nile tilapia growth of the current study could be due to the chemical form of Zn. Additionally, Zn acts as cofactor to many enzymes and proteins, which are involved in protein synthesis, growth, the nervous system and gastrointestinal tract function [45]. Faiz [26] reported that NZnO form resulted in higher growth performances of juvenile Ctenopharyngodon idella (C. idella) than conventional forms. Similarly, Hina [46] found that N-ZnO promote the growth performances of juvenile Ctenopharyngodon idella more than other inorganic conventional forms. Tawfik [47] found that supplementation of NZnO to fish feeds can possibly improve the growth rates exemplified by the weight gain, the specific growth rate and growth hormone in blood. This could be better than the conventional ZnO. Khan [48] reported that Zn nanoparticles enhance the growth rate in fishes. Mishra [44] reported that inclusion of NZnO showed an improvement in the growth rates than conventional zinc in various species, which may be attributed to better uptake of nanoparticles of Zn in the gastrointestinal tract than the remaining groups. Khosravi-Katuli [49] showed that diet supplemented with NZnO could improve the growth performance and feed utilization of fish. Several studies indicated that Zn nanoparticles improve the production performance than inorganic Zn [44, 50], as well as influenced lipid deposition and metabolism [51, 52]. Many of the previous works reported a positive effect of N-ZnO than conventional Zn as dietary supplement [53,54,55].
Histomorphometric
Measurements of width and length of villi as well as the absorption area are essential indicators of intestinal morphology health due to their vital role in nutrient absorption [56]. The present results showed that fish fed dietary 25% crude protein and supplemented with NZnO had the same values of villus width and length, and absorption area of both interior and posterior intestine. With the respect that ZnO improved the intestinal health and enhance growth performance. In this context, Hu [57] reported that Nile tilapia fed diets supplemented with ZnO improved the intestinal villus length and digestive enzyme activities. Also, De Grande [58] found that the addition of NZnO increase intestinal villus width and length, consequently facilitate the process of nutrients assimilation. Suganthi [59] showed that ZnO nanoparticles exposure caused impact on histological abnormalities observed in gills, muscle, brain, intestine and ovaries tissues of O. mossambicus exposed group, thus their physiological, secretory and absorption, endocrine and reproductive activities are disturbed. On the other side, the possible explanation for higher villus length may be due to higher bioavailability of Zn nanoparticles, so maintaining epithelial barrier integrity and function [60], reducing the turnover rate of cells in the villi and resulting in higher villus height. An improvement of villus length and crypt depth ratio was observed after dietary replacement of inorganic ZnO by different levels of organic and 30 mg kg−1 of NZnO and this may indicate an improvement of mucosal barrier functional capacity [61].
Hematology
Hematological studies provide an index of physiological changes in fish [62, 63], as well as it acts an impressive tool for detection of alterations in the tested organism [62]. The present trial showed no differences in the values of hematological parameters, whereas fish fed 60 mg kg−1 NZnO recorded the uppermost WBCs value. There are various reports regarding the impact of dietary Zn on hematological parameters in animals. In some reports, dietary Zn levels influence the hematological characteristics of various species [16, 64], whereas other reports indicate otherwise[65, 66]. Faiz [26] reported that dietary nanoparticles Zn supplementation (60 mg kg−1) significantly decrease the hematological values of Grass carp (Ctenopharyngodon idella), whereas 30 mg kg−1 diet significantly increase RBC count and MCHC value. Total leucocyte count and their differential occupied an important role in fish studies [67]. In Oreochromis mossambicus, the monocytes and neutrophils are reduced in circulation for the elevation of phagocytic activity in affected tissues such as gills, liver and kidneys which are damaged by copper [68,69,70], while NZnO treated fishes showed increased monocytes and neutrophilic conditions.
On the basis of metal size, the NZnO have small size and low agglomeration power compared to their conventional counterpart as reported in the previous study [71]. So, NZnO can easily penetrate the RBCs membrane making the erythrocytes more fragile and permeable. [72] showed that metals can alter the properties of Hb by decreasing the oxygen binding capacity; thus, increase the erythrocytes fragility and permeability. In addition, the decrease in Hct level may be attributed to hemodilution that may occur due to gill damage and/or impaired osmoregulation [73]. White blood cells (WBCs) count is considered as the first line of defense, which could be related to the stronger innate resistance and adaptive immunity [74]. Consistently, dietary Zn supplementation (BZnO and NZnO forms) presented in this study enhances WBC count. Similar results were reported by Ibrahim [75] for NZnO supplementation.
Serum Biochemical Parameters
Both AST and ALT activities are cursors of healthy liver or liver dysfunction [76, 77]. The highest activities of AST and ALT were noted in fish fed control diet. On the other hand, lower activities of AST and ALT were noticed in fish fed diet containing 35% or 25% dietary protein and supplemented with NZnO or BZnO, this result indicating either BZnO or NZnO form did not have any deleterious impact in liver function. Likewise, results were noticed in common carp fed diets supplemented with NZnO [78]. In addition, Gharaei [79] found significant reduction in activities of ALT, and AST of beluga (Huso huso) fed diets supplemented with NZnO. Total serum protein represents major function to uphold the osmatic balance between blood and tissues [80]. In the present study, no significant differences were found in total protein and albumin as affected by interaction between Zn form and dietary protein levels, while the globulin value is significantly higher in fish diet containing 35% crude protein and supplemented with BZnO followed by fish containing 35% crude protein and supplemented with NZnO. The current findings are consistent with Gopal [81] who found increasing of serum globulin in common carp (Cyprinus carpio) fed diets supplemented with NZnO. These results may be due to synthesis of ribosomes, and the spread of protein synthesis in the liver tissues [77, 82]
Oxidative Stress Biomarkers
The activities of oxidative stress biomarkers enzymes in the current study are significantly higher (P < 0.05) attended with low significant in malondialdehyde (MDA) level in fish fed diet enriched with NZnO either 25% or 35% dietary protein. The present results are consistent with Gopal [81] who showed that activities of catalase (CAT) and glutathione peroxidase (GPx) are reached to maximum level in serum of Huso huso fed diet supplemented with NZnO. In addition, the simultaneous increase in the activity of superoxide dismutase (SOD) and glutathione peroxidase (GPx) enhance the activity of NADPH oxidase, which is accountable for hunting of superoxide anion [83]. Furthermore, Asaikkutti [23] found that shrimp fed diets contained Ananas comosus peel as a source of Mn2O3 nanoparticles improved the antioxidant defense system and metabolic activities. Also, addition of 30 mg ZnO-NP per kg diet of Nile tilapia diet improved the activity of SOD and total antioxidant capacity and decreases the MDA [84]. Addition of NZnO enhancements the antioxidants enzyme activity and decrease activity of MDA [85]. MDA is a significant antioxidant non-enzyme and used as a biomarker of oxidative stress [49]. The present findings are in consistent with [85] who reported that Nile tilapia fed diet contained NZnO decreases the activities of MDA level. On contrary, Gharaei [79] reported no significant (P > 0.05) differences of MDA value in fish diets supplemented with either ZnO or NZnO. It is worthy to mention that reactive oxygen species (ROS) formation and oxidative stress related to inflammatory effects of NZnO because they can activate proinflammatory-signaling cascades [86]. Previous results, which noted above may be due to: (i) generation of ROS can be increased in the presence of Zn, consequently improve the activity of oxidative enzymes [17, 87], (ii) the antioxidant activity of Zn ions is linked with its compulsory to thiol groups and defends them from oxidation in addition, (iii) Zn play an important role for stimulating the activity of SOD enzyme; (iv) the availability of in NZnO form was higher compared with BZnO form [17].
Immune Response
Zinc plays a vigorous role in the immune system and affects numerous features of humoral and cellular immunity [17, 75, 88, 89]. The immune function is deftly synchronized by zinc, since both increased and decreased dietary zinc levels result in a disturbed immune function [90, 91]. furthermore, Zn deficiency affects immune cells at the survival and proliferation and leads to weakened innate host defense via phagocytosis and oxidative burst, and disturbed intercellular communication via cytokines [89, 92]. The present study noted that supplementing with NZnO form in Nile tilapia diets containing 25% crude protein significantly improved IgG, IgM, C3 and C4 compared with 25% or 35% without Zn supplementation. Previous studies suggesting the role of NZnO in improving the immune status of Nile tilapia [47]. Also, immune cells produce cytokines to initiate the defense mechanism of the immune system against pathogens [93]. Awad [84] reported that low concentrations of NZnO (30 mg kg−1) to O. niloticus diet improve immune status and elevate survival rate against Aeromonassobria pathogen. At the nanoscale level, the reactivity of ZnO is increased because of the large surface area to volume ratio, which may induce cytotoxicity [94]. Luo [95] reported that, nanoparticles can stimulate innate and adaptive immune response depending on their physicochemical properties; however, it is still unclear how nano-particles affect the immune response. It has been demonstrated that nano-formulated Zn, like nano ZnO, promotes growth in a dose dependent effect that is positively correlated with the positive immunological responses of livestock [96]. Likewise, Gharaei [79] found that Huso huso fed diet supplemented with ZnO-NP enhanced the lysozyme levels and immune system.
Gene Expression
Growth hormone (GH) and insulin-like growth factors (IGFs) have garnered increased attention in recent years, owing to their wide-ranging biological effects and therapeutic potential [97]. The growth hormone is a polypeptide hormone necessary for growth, bone development and nutrient metabolism [98]. Besides, IGF-1, as a member of the IGFs family, is a candidate gene for improved growth, body composition, metabolism and adipose tissue in animals [99]. It is important to know that there are no previous reports that describe a correlation effect between the dietary Zn form and the dietary protein on the expression of GH and IGF-1 gene in fish, even though previous studies have found that dietary Zn supplementation leads to higher gene expression of IGF-I in weanling pigs [100]. Both IGF-1 and growth hormone receptors (GHR) are strong growth regulators, inducing an anabolic effect on protein and carbohydrate metabolism and mediating the action of the growth hormone [101, 102]. The increased expression of GHR reflects an increased activity of the growth hormone (GH), which subsequently exhibits lipolytic activity by stimulating triglyceride uptake and increasing hepatic (LPL) expression [103]. The increased activity of lipid metabolism mediates the breakdown of circulating triglycerides into free fatty acids and increases its cellular uptake in the liver and skeletal muscle [103]. Some studies reported that, when nano-particles enter the body, they can interact with immune cells and trigger inflammatory response, which is accompanied by the secretion of signaling molecules (cytokines, chemokines) that provide communication between immune cells and coordinate molecular events. Interleukin 1 beta (IL1-β) is a member of the interleukin 1 family of cytokines, this cytokine is produced by activated macrophages as a proprotein, which is proteolytically processed to its active form by caspase 1 (CASP1/ICE). This cytokine is an important mediator of the inflammatory response, and is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis. The study of nanoparticles that induce IL-1b via inflammatory signaling pathways mechanism is an emerging theme [104]. Şıklar [105] reported that NZnO may be attributed to somatic growth by stimulation of DNA and RNA synthesis and growth hormone protein synthesis.
In conclusion, the present results indicated that supplementation of diets with NZnO is valuable for tilapia as growth promoting although, decreased the requirements of dietary protein from 35 to 25% without effect in growth, feed efficiency, intestinal topography, oxidative responses, and related gene expression. Further studies are wanted for gut microbiota investigation to detect the mode of action between ZnO and dietary protein level on the health statues of tilapia fish.
Data Availability
Data of the present article are available.
Code Availability
Custom code.
References
Wu L et al (2021) Culture salinity alters dietary protein requirement, whole body composition and nutrients metabolism related genes expression in juvenile genetically improved farmed tilapia (GIFT) (Oreochromis niloticus). Aquaculture 531:735961
Lim S-J et al (2013) Taurine is an essential nutrient for juvenile parrot fish Oplegnathus fasciatus. Aquaculture 414:274–279
Abdel-Tawwab M et al. (2010) Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 298(3–4):267–274
Webster CD, Lim C (2002) Introduction to fish nutrition. nutrient requirements and feeding of finfish for aquaculture. CABI Publishing, Wallingford Oxon, UK, p 27
Sun L, Chen H, Huang L (2007) Growth, faecal production, nitrogenous excretion and energy budget of juvenile yellow grouper (Epinephelus awoara) relative to ration level. Aquaculture 264(1–4):228–235
Geurden I et al (2014) High or low dietary carbohydrate: protein ratios during first-feeding affect glucose metabolism and intestinal microbiota in juvenile rainbow trout. J Exp Biol 217(19):3396–3406
Liu X et al (2013) Effect of dietary dextrin levels on growth, activities of digestive enzyme and blood biochemical indices of juvenile obscure puffer (Takifugu obscurus). J Fish China 37(9):1359–1368
Hassaan MS, Soltan MA, Abdel-Moez AM (2015) Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia, Oreochromis niloticus. Anim Feed Sci Technol 201:89–98
Aride P et al (2017) Changes on physiological parameters of tambaqui (Colossoma macropomum) fed with diets supplemented with Amazonian fruit Camu camu (Myrciaria dubia). Braz J Biol 78:360–367
Hassaan M, Goda AS, Kumar V (2017) Evaluation of nutritive value of fermented de-oiled physic nut, Jatropha curcas, seed meal for Nile tilapia Oreochromis niloticus fingerlings. Aquac Nutr 23(3):571–584
Osis D et al (1972) Dietary zinc intake in man. Am J Clin Nutr (United States) 25
Broadley MR et al (2007) Zinc in plants. New Phytol 173(4):677–702
Domínguez D et al (2019) Effects of zinc and manganese sources on gilthead seabream (Sparus aurata) fingerlings. Aquaculture 505:386–392
Ogino C, Yang G (1979) Requirement of carp for dietary zinc. Bull Jpn Soc Sci Fish 45(8):967–969
Jobling M (2012) National Research Council (NRC): nutrient requirements of fish and shrimp. Springer. D.C., 2011, 376 + XVI pp, £128 (Hardback)
Eid AE, Ghonim SI (1994) Dietary zinc requirement of fingerling Oreochromis niloticus. Aquaculture 119(2–3):259–264
Mohammady E.Y et al (2021) Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia, Oreochromis niloticus. Aquaculture, 532: 736006
Swain PS et al (2016) Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Animal Nutrition 2(3):134–141
Ashmead H (1992) The roles of amino acid chelates in animal nutrition: summary and conclusion. US (DNAL SF98.A38R64 1992)
Tan B, Mai K (2001) Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone. Haliotis discus hannai Ino Aquaculture 192(1):67–84
Aitken RJ et al (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56(5):300–306
Piccinno F et al (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14(9):1–11
Asaikkutti A et al (2016) Dietary supplementation of green synthesized manganese-oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii. J Trace Elem Med Biol 35:7–17
Steinfeld B et al (2015) The role of lean process improvement in implementation of evidence-based practices in behavioral health care. J Behav Health Serv Res 42(4):504–518
Do Carmo e Sa M et al (2005) Relative bioavailability of zinc in supplemental inorganic and organic sources for Nile tilapia Oreochromis niloticus fingerlings. Aquaculture nutrition, 11(4): 273-281
Faiz H et al (2015) Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). International Journal of Agriculture and Biology, 17(3).
Hossain MA, Matsui S, Fumichi M (2003) Effect of zinc and manganese supplementation to tricalcium phosphate rich diet for tiger puffer (Takifugu rubripes). Bangladesh J Fish Res 7(2):189–192
Zhao L, Xia Z, Wang F (2014) Zebrafish in the sea of mineral (iron, zinc, and copper) metabolism. Front Pharmacol 5:33
Brett J, Groves T (1979) Physiological energetics. Fish physiol 8(6):280–352
AOAC (1975) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC., Vol. 222
Boyd CE Tucker CS (2012) Pond aquaculture water quality management. Springer Science & Business Media, 6-12-2012, p 700
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Martins ML et al (2004) Physiological and haematological response of Oreochromis niloticus (Osteichthyes: Cichlidae) exposed to single and consecutive stress of capture. Acta Scientiar Animal Sci 26(4):449–456
Henry RJ (1964) Clinical chemistry, principles and technics. Hoeber Medical Division, Harper & Ropw, p 989–1106
Wotton I, Freeman H (1974) Microanalysis in medicinal biochemical. Churchill Livingstone, Edinburgh, London, p 1982
Coles E (1974) Vet. Cline Path. WB Sounders Company, Philadelphia, London and Toronto, 211–213
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46(2):849–854
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Moin V (1986) A simple and specific method for determining glutathione peroxidase activity in erythrocytes. Lab Delo 12:724–727
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11(1):1–42
Bright LA, Coyle SD, Tidwell JH (2005) Effect of dietary lipid level and protein energy ratio on growth and body composition of largemouth bass Micropterus salmoides. J World Aquaculture Soc 36(1):129–134
Swain P et al (2019) Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton). Aquac Nutr 25(2):486–494
Mishra A et al (2014) Growth performance and serum biochemical parameters as affected by nano zinc supplementation in layer chicks. Indian Journal of Animal Nutrition 31(4):384–388
Classen HG et al (2011) Zinc deficiency. Symptoms, causes, diagnosis and therapy. Medizinische Monatsschrift fur Pharmazeuten 34(3):87–95
Hina M, Dhanapal S, Sekar DS (2015) Studies on antibacterial activity of some fungi collected from KRP Dam, Krishnagiri (TN). Int J Eng Res Manage 2:1–2
Tawfik M et al (2017) Evaluation of nano zinc oxide feed additive on tilapia growth and immunity. in 15th International Conference on Environmental Science and Technology, Rhodes, Greece, Vol. 1342, No. 1, pp 1–9
Khan KU et al (2016) Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turkish J Zool 40(5):704–712
Khosravi-Katuli K et al (2017) Effects of nanoparticles in species of aquaculture interest. Environ Sci Pollut Res 24(21):17326–17346
Lina T et al (2009) Effect of nano-zinc oxide on the production and dressing performance of broiler. Chin Agric Sci Bull 2(003):318
Ranasinghe P et al (2015) Effects of zinc supplementation on serum lipids: a systematic review and meta-analysis. Nutr Metab 12(1):1–16
Wei CC et al (2018) Zinc reduces hepatic lipid deposition and activates lipophagy via Zn2+/MTF-1/PPARα and Ca2+/CaMKKβ/AMPK pathways. FASEB J 32(12):6666–6680
Ahmadi F et al (2013) The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. Int J Biosci 3(7):23–29
Sahoo A et al (2016) Growth, feed conversion efficiency, and carcass characteristics of broiler chicks fed on inorganic, organic and nano zinc supplemented diets. Animal Science, 10(1)
Zhao C-Y et al (2014) Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol Trace Elem Res 160(3):361–367
Wang J, Wang A, Wang W-X (2017) Evaluation of nano-ZnOs as a novel Zn source for marine fish: importance of digestive physiology. Nanotoxicology 11(8):1026–1039
Hu C et al (2014) Effects of zinc oxide supported on zeolite on growth performance, intestinal barrier function and digestive enzyme activities of Nile tilapia. Aquac Nutr 20(5):486–493
De Grande A et al (2020) Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult Sci 99(1):441–453
Suganthi P et al (2015) Behavioural and Histological variations in Oreochromis mossambicus after exposure to ZnO Nanoparticles. Int J Appl Res 1(8):524–531
Hu C et al (2013) Effects of zinc oxide supported on zeolite on growth performance, intestinal microflora and permeability, and cytokines expression of weaned pigs. Anim Feed Sci Technol 181(1–4):65–71
El-Katcha M, Soltan MA, El-Badry M (2017) Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alexandria Journal for Veterinary Sciences, 55(2).
Adhikari S et al (2004) Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicol Environ Saf 58(2):220–226
Suvetha L, Ramesh M, Saravanan M (2010) Influence of cypermethrin toxicity on ionic regulation and gill Na+/K+-ATPase activity of a freshwater teleost fish Cyprinus carpio. Environ Toxicol Pharmacol 29(1):44–49
HUANG SC, Chen SM, Huang CH (2010) Effects of dietary zinc levels on growth serum zinc haematological parameters and tissue trace elements of soft shelled turtles Pelodiscus sinensis. Aquacult Nutri 16(3):284–289
Maage A, Julshamn K (1993) Assessment of zinc status in juvenile Atlantic salmon (Salmo salar) by measurement of whole body and tissue levels of zinc. Aquaculture 117(1–2):179–191
Kumar V et al (2011) Isolation of phytate from Jatropha curcas kernel meal and effects of isolated phytate on growth digestive physiology and metabolic changes in Nile tilapia (Oreochromis niloticus L.). Food Chem Toxicol 49(9):2144–2156
Blaxhall P, Daisley K (1973) Routine haematological methods for use with fish blood. J Fish Biol 5(6):771–781
Gey van Pittius M (1991) The effect of heavy metals at different pH on liver enzymes and blood coagulation in Tilupia sparrmanii (Cichlidae). M. Sc.-Thesis, Rand Afrikaans University, South Africa (in Afrikaans)
Wepener W (1990) The effects of heavy metals at different pH on the blood physiology and metabolic enzymes in Tilapia sparmanii (Cichlidae). M. Sc. Thesis, Rand Afrikaans University, Johannesburg, South Africa, 1990
Van der Merwe, M. (2014) Aspects of heavy metal concentration in the Olifants River, Kruger National Park and the effect of copper on the haematology of Clarias gariepinus (Clariidae). University of Johannesburg (South Africa). ProQuest Dissertations Publishing, 2014. 28305602
adel Abdel-Khalek A et al (2015) Ecotoxicological impacts of zinc metal in comparison to its nanoparticles in Nile tilapia Oreochromis niloticus. J Basic App Zool 72:113–125
Witeska M, Kościuk B (2003) The changes in common carp blood after short-term zinc exposure. Environ Sci Pollut Res 10(5):284–286
Kori-Siakpere O, Ubogu EO (2008) Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp.(Osteichthyes: Clariidae). African Journal of Biotechnology, 7(12)
Divyagnaneswari M, Christybapita D, Michael RD (2007) Enhancement of nonspecific immunity and disease resistance in Oreochromis mossambicus by Solanum trilobatum leaf fractions. Fish Shellfish Immunol 23(2):249–259
Ibrahim MS et al (2021) Nano zinc versus bulk zinc form as dietary supplied: effects on growth, intestinal enzymes and topography, and hemato-biochemical and oxidative stress biomarker in Nile tilapia (Oreochromis niloticus Linnaeus, 1758). Biological Trace Element Research, p. 1–14
Abdel-Tawwab M, Razek NA, Abdel-Rahman AM (2019) Immunostimulatory effect of dietary chitosan nanoparticles on the performance of Nile tilapia Oreochromis niloticus (L.). Fish shellfish immunol 88:254–258
Hassaan MS et al (2021) Comparative study on the effect of dietary β-carotene and phycocyanin extracted from Spirulina platensis on immune-oxidative stress biomarkers, genes expression and intestinal enzymes, serum biochemical in Nile tilapia. Oreochromis niloticus Fish Shellfish Immunol 108:63–72
Lee J-W et al (2014) Serum and ultrastructure responses of common carp (Cyprinus carpio L.) during long-term exposure to zinc oxide nanoparticles. Ecotoxicol Environ Safety 104:9–17
Gharaei A et al (2020) Fluctuation of biochemical, immunological, and antioxidant biomarkers in the blood of beluga (Huso huso) under effect of dietary ZnO and chitosan–ZnO NPs. Fish Physiol Biochem 46(2):547–561
Sakr S, Jamal S, Lail A (2005) Fenvalerate induced histopathological and histochemical changes in the liver of the catfish. Clarias gariepinus J Appl Sci Res 1(3):263–267
Gopal V, Parvathy S, Balasubramanian P (1997) Effect of heavy metals on the blood protein biochemistry of the fish Cyprinus carpio and its use as a bio-indicator of pollution stress. Environ Monit Assess 48(2):117–124
Akrami R et al (2015) Effects of dietary onion (Allium cepa) powder on growth, innate immune response and hemato–biochemical parameters of beluga (Huso huso Linnaeus, 1754) juvenile. Fish Shellfish Immunol 45(2):828–834
Sheikh Asadi M et al (2018) A Comparison between dietary effects of Cuminum cyminum essential oil and Cuminum cyminum essential oil, loaded with iron nanoparticles, on growth performance, immunity and antioxidant indicators of white leg shrimp (Litopenaeus vannamei). Aquac Nutr 24(5):1466–1473
Awad A et al (2019) Transcriptomic profile change, immunological response and disease resistance of Oreochromis niloticus fed with conventional and Nano-Zinc oxide dietary supplements. Fish Shellfish Immunol 93:336–343
Saddick S, Afifi M, Zinada OAA (2017) Effect of zinc nanoparticles on oxidative stress-related genes and antioxidant enzymes activity in the brain of Oreochromis niloticus and Tilapia zillii. Saudi J Biol Sci 24(7):1672–1678
De Berardis B et al (2010) Exposure to ZnO nanoparticles induces oxidative stress and cytotoxicity in human colon carcinoma cells. Toxicol Appl Pharmacol 246(3):116–127
Ogawa D et al (2011) High glucose increases metallothionein expression in renal proximal tubular epithelial cells. Experimental diabetes research
Sunder GS et al (2008) Effects of higher levels of zinc supplementation on performance, mineral availability, and immune competence in broiler chickens. J Appl Poultry Res 17(1):79–86
Maares M, Haase H (2016) Zinc and immunity: an essential interrelation. Arch Biochem Biophys 611:58–65
Chandra RK (1990) The relation between immunology, nutrition and disease in elderly people. Age and Ageing, 19(4)
Rink L Gabriel P (2001) Extracellular and immunological actions of zinc. Zinc Biochemistry, Physiology, and Homeostasis, 181–197
Bonaventura P et al (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14(4):277–285
Ellis A (2001) Innate host defense mechanisms of fish against viruses and bacteria. Dev Comp Immunol 25(8–9):827–839
Zanet V et al (2019) Activity evaluation of pure and doped zinc oxide nanoparticles against bacterial pathogens and Saccharomyces cerevisiae. J Appl Microbiol 127(5):1391–1402
Luo Y-H, Chang LW, Lin P (2015) Metal-based nanoparticles and the immune system: activation, inflammation, and potential applications. Biomed Res Int. https://doi.org/10.1155/2015/143720
Yang Z, Sun L (2006) Effects of nanometre ZnO on growth performance of early weaned piglets. J Shanxi Agric Sci 3(024):577–588
Delafontaine P, Song Y-H, Li Y (2004) Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24(3):435–444
Møller N, Nørrelund H (2003) The role of growth hormone in the regulation of protein metabolism with particular reference to conditions of fasting. Hormone Res Paediat 59(Suppl. 1):62–68
Duclos MJ, Beccavin C, Simon J (1999) Genetic models for the study of insulin-like growth factors (IGF) and muscle development in birds compared to mammals. Domest Anim Endocrinol 17(2–3):231–243
Carlson D, Poulsen H, Vestergaard M (2004) Additional dietary zinc for weaning piglets is associated with elevated concentrations of serum IGF-I. J Anim Physiol Anim Nutr 88(9–10):332–339
Amin A et al (2019) Growth performance, intestinal histomorphology and growth-related gene expression in response to dietary Ziziphus mauritiana in Nile tilapia (Oreochromis niloticus). Aquaculture, 512: p. 734301
Perez-Sanchez J, Le Bail P-Y (1999) Growth hormone axis as marker of nutritional status and growth performance in fish. Aquaculture 177(1–4):117–128
Vijayakumar A et al (2010) Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Hormon IGF Res 20(1):1–7
Reisetter AC et al (2011) Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem 286(24):21844–21852
Şıklar Z et al (2003) Zinc deficiency: a contributing factor of short stature in growth hormone deficient children. J Trop Pediatr 49(3):187–188
Acknowledgements
The authors acknowledge the Animal production department, Faculty of agriculture, Benha University, Egypt, as well as Zoology department, Faculty of Science, Benha University, Egypt, for supporting and financial assistance during this research project.
Author information
Authors and Affiliations
Contributions
The authors declared that the current manuscript prepared from the M.Sc. student project to submit his thesis paper work to the journal to get approve to setup his defense date and time and nominate the external examiner after received the acceptance of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All applicable international, national, and/or institutional guidelines for the care and use of fish were followed by the authors. All authors approved this version of manuscript.
Consent for Publication
All authors approved this version of manuscript to submit to journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-badawy, A.S., Hassaan, M.S., Abdel-Hameid, NA.H. et al. Synergistic Effects Between Dietary Zinc Form Supplementation and Dietary Protein Levels on Performance, Intestinal Functional Topography, Hemato-biochemical Indices, Immune, Oxidative Response, and Associated Gene Expression of Nile Tilapia Oreochromis niloticus. Biol Trace Elem Res 200, 3412–3428 (2022). https://doi.org/10.1007/s12011-021-02911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02911-y