Abstract
Zinc is one of the essential microelements involved in vital physiological and biological functions in the fish body. The study evaluated the growth performance, antioxidative capacity, and intestinal histomorphology of Grey Mullet (Liza ramada)–fed dietary zinc nanoparticles (ZnO-NPs) at 0, 10, 20, and 40 mg/kg for the first time. The final weight and specific growth rate (SGR) of Grey Mullet–fed dietary ZnO-NPs at 20 and 40 mg/kg were meaningfully enhanced (p < 0.05). Further, the weight gain (WG) was significantly higher in fish treated with ZnO-NPs than the control, and fish fed 20–40 mg/kg had the highest WG (p < 0.05). The feed conversion ratio (FCR) was meaningfully reduced in fish fed 20–40 mg ZnO-NPs/kg (p < 0.05). The histomorphology of the intestines revealed a significant improvement in villus height, villus width, and goblet cells by ZnO-NPs. The lysozyme activity, phagocytic activity, and phagocytic index showed higher levels in Grey Mullet–fed dietary ZnO-NPs at 20 mg/kg than fish fed 0, 10, and 40 mg/kg (p < 0.05). Superoxide dismutase (SOD) and catalase (CAT) were markedly improved in Grey Mullet treated with ZnO-NPs compared with the control, and the group of fish treated with 20 mg/kg had the highest SOD and CAT (p < 0.05). Glutathione peroxidase (GPx) was significantly higher in fish fed 20–40 mg/kg ZnO-NPs than fish fed 0–10 mg/kg and fish fed 40 mg ZnO-NPs/kg showing the highest GPx value (p < 0.05). The concentration of malondialdehyde was markedly lowered in Grey Mullet fed ZnO-NPs at varying levels (p < 0.05). Based on the overall results, the regression analysis suggests that ZnO-NPs can be included at 24.61–35.5 mg/kg for the best performances of Grey Mullet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture activity is a vital sector for providing humanity with safe food and profitable income [1, 2]. Therefore, it is necessary to maximize the productivity of aquatic animals and keep up the health status and welfare [3, 4]. Besides efficient management, feeding aquatic animals with nutritionally balanced feeds is a key factor to guarantee optimal growth and productivity [5, 6]. Indeed, ideal aquafeed should contain macronutrients (proteins, lipids, and carbohydrates) and microelements (vitamins and minerals) [7]. Although trace elements are required in small amounts, their deficiency causes a severe risk for fish’s biological and physiological functions [8, 9]. Consequently, fish suffering from malnutrition followed by inefficient feed utilization and metabolic function leading to impaired immunity and antioxidative responses [10]. Therefore, dietary trace elements are required in specific species amounts for optimum growth and well-being [11].
Zinc is one of the essential microelements involved in several vital functions in the entire body of fish [12]. Zinc is required for many biological activities, including the regulation of metabolism and immunity and the inhibition of oxidative stress [13]. Besides, zinc is a cofactor for metalloenzymes such as lactate dehydrogenase, super oxidase dismutase, glutamic dehydrogenase, alkaline phosphatase, reverse transcriptase, DNA and RNA polymerases, carboxypeptidase, and carbonic anhydrase [14, 15]. Zinc is also required to improve the fertility and reproduction behavior of aquatic animals [16]. Thus, zinc deficiency in aquafeed could induce impaired feed utilization, growth performance, immunity, and antioxidative responses. Several forms of zinc are suggested for finfish species [17,18,19]. Organic forms (zinc gluconate, zinc propionate, and zinc acetate) and inorganic salts (zinc oxide and zinc sulfate) are traditionally applied in aquafeed [16]. With the expansion of nanotechnology and its application in aquaculture, zinc nanoparticles are successfully applied in aquafeed [20, 21]. Zinc nanoparticles are available and can be easily absorbed in the gastrointestinal tract of fish, thereby acting functionally in the entire body [19, 22, 23]. Zinc oxide nanoparticles (ZnO-NPs) are a bioavailable form of zinc and are validated for their vital role in improving the growth performance, immune, and antioxidative responses in finfish species [24, 25].
Grey Mullet is a highly valued fish species belong to Mugilidae and can grow in a wide range of water salinities [26]. Mullets are spread in the coastal areas of several Mediterranean countries and can be cultured under farming conditions [27]. Despite that many studies were investigated the essential requirements of Mullets, no studies were conducted to evaluate the mineral requirements for this fish species. Therefore, the present study aimed at evaluating the requirements of ZnO-NPs in Grey Mullets for the first time. The study focused on the impact of ZnO-NPs on growth performance, antioxidative, and immune responses. Also, using the regression analysis model, the actual requirements of ZnO-NPs were calculated based on the observed results.
Materials and Methods
Experimental Procedure
Four test diets were formulated by mixing all ingredients as shown in Table 1 with Zinc oxide nanoparticles (ZnO-NPs; particle size < 100 nm; purity 97%; Sigma-Aldrich, Saint Louis, USA). ZnO-NPs were added at 0, 10, 20, and 40 mg/kg, and the diets were pelleted after mixing with fish oil and water by a laboratory pelleting machine to produce dough pellets (1–2 mm). The pellets were well dried and kept in plastic bags until used. The actual zinc concentration was checked in the diets by following Li and LI [28] and Sallam and Mansour [25] and recorded 0.6, 10.68, 21.23, and 41.65 mg/kg diet. Besides, the chemical composition of the diets was checked using the standard method [29].
Grey Mullet (Liza ramada) juveniles were obtained from Bughaz El-Burullus (Lake Burullus), located on the coast of the Mediterranean Sea (Baltim city, Kafr El-sheikh governorate, Egypt) and transported to the Fish Nutrition Laboratory, Baltim Unit, National Institute of Oceanography and Fisheries. One hundred and eighty juveniles were stocked in concrete tanks (3 × 2 × 1.7 m) and fed the basal diet for 14 days for adaptation. Then, fish of similar initial weight 23.76 ± 0.11 g were distributed in 12 hapas (0.5 × 0.5 × 1 m) at 15 fish per hapa. All hapas were fixed in one concrete tank with a set of water inlet and outlet. Fish were fed the diets at 2–3% throughout the trial (8 weeks). The water was running in a flow-through system in the outdoor area. The water characteristics were recorded: temperature (26.11 ± 0.31 °C), pH (7.31 ± 0.34), dissolved oxygen (6.22 ± 0.11 mg/L), salinity (12 ppt), and total ammonia (0.22 ± 0.01 mg/L).
Final Sampling
Before the final sampling, fish were starved for 24 h, and then all fish were weighed and counted. The following equations were used for the calculation of the growth indices and survival rate:
Intestinal Histomorphology
Five fish from each hapa were collected and anesthetized using 40% ethyl alcohol, the intestines were dissected, and anterior, middle, and terminal parts of the intestine were sampled. The samples were immediately fixed for 48 h in 10% neutral buffered formalin solution. After fixation, tissue specimens were processed according to Gewaily and Abumandour [30]. The paraffin sections were rehydrated and stained with periodic acid–Schiff (PAS) according to the methodology described in Bancroft and Gamble [31]. Photomicrographs were captured from the stained sections with a digital camera (Leica EC3, Leica, Germany) connected to a microscope (Leica DM500).
Blood Analysis
Three fish per hapa were gently bled from the caudal vein using 2.5-mL heparinized syringes to collect blood for phagocytosis analysis. Besides using non-heparinized syringes, blood was collected for serum separation. Samples were left for 4 h at 4 °C and then centrifuged at 3000 × g for 15 min under 4 °C for serum collection. Serum samples were kept at − 80 °C for further biochemical analysis.
Leukocyte phagocytic function followed the method of Cai and Li [32]. The number of leukocytes that engulfed bacteria was counted as percentages in relation to the total leukocyte number in the smear from the phagocytosis assay. By following Kawahara and Ueda [33], the phagocytic activity and phagocytic index were determined. Analysis of serum lysozyme activity was performed using a turbidimetric assay, according to Ellis and Stolen [34].
Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) in serum were measured using diagnostic reagent kits following the manufacturer’s (Cusabio Biotech Co., Ltd.; China) instructions. The concentration of malondialdehyde (MDA) was detected by following Uchiyama and Mihara [35] and expressed as U/mL.
Statistical Analysis
Shapiro–Wilk and Levene tests confirmed normal distribution and homogeneity of variance. And then Duncan’s multiple range test was used to determine differences among treatments with significance set at p < 0.05. Polynomial contrasts were used to detect linear and quadratic effects of various levels of dietary ZnO-NPs on the observed response variables. The optimum ZnO-NPs level was determined using a polynomial regression analysis [36]. All the statistical analyses were done via SPSS version 22 (SPSS Inc., IL, USA).
Results
Growth Performance
The final weight and specific growth rate (SGR) of Grey Mullet–fed dietary ZnO-NPs at 20 and 40 mg/kg were meaningfully enhanced (p < 0.05; Table 2). Further, the weight gain (WG) was significantly higher in fish treated with ZnO-NPs than the control, and fish fed 20–40 mg/kg had the highest WG (p < 0.05; Table 2). The feed conversion ratio (FCR) was meaningfully reduced in fish fed 20–40 mg ZnO-NPs/kg (p < 0.05; Table 2). The survival rate was high and reported 95.24 to 98.10% without marked differences among the groups (p > 0.05; Table 2).
Intestinal Histomorphology
The histological investigation of the Grey Mullet intestine revealed four layers of the intestinal wall: tunica mucosa, tunica sub-mucosa, tunica muscularis, and serosa (Figs. 1, 2, and 3). The Grey Mullet intestine showed a normal histomorphology. Besides, the intestinal villi and associated crypt appeared free of any inflammatory or degenerative changes. Furthermore, both enterocytes and PAS-positive goblet cells were properly arranged. The results of histomorphology of the intestines revealed a significant improvement in villus height, villus width, and goblet cells in the anterior (Fig. 1), middle (Fig. 2), and posterior (Fig. 3) intestines of Grey Mullet treated with ZnO-NPs.
Histomorphology of the anterior segment of Grey Mullet intestine in the control group (A) as well as zinc nanoparticles (ZnO-NPs)-fed groups in gradually increased levels; 10 mg/kg (B), 20 mg/kg (C), and 40 mg/kg (D). The intestine appeared intact and formed of the intestinal villi (V), propria submucosa (P), muscularis (M), and serosa (S) in all groups. There was a significant improvement in villous height, width and number of goblet cells (red arrowhead) related to the dose of nano zinc. Stain PAS. Bar = 200 µm
Histomorphology of the middle segment of Grey Mullet intestine in the control group (A) as well as zinc nanoparticles (ZnO-NPs)-fed groups in gradually increased levels; 10 mg/kg (B), 20 mg/kg (C), and 40 mg/kg (D). The intestine appeared intact and formed of the intestinal villi (V), propria submucosa (P), muscularis (M), and serosa (S) in all groups. There was a significant improvement in villous height, width and number of goblet cells (red arrowhead) related to the dose of nano zinc. Stain PAS. Bar = 200 µm
Histomorphology of the posterior segment of Grey Mullet intestine in the control group (A) as well as zinc nanoparticles (ZnO-NPs)-fed groups in gradually increased levels; 10 mg/kg (B), 20 mg/kg (C), and 40 mg/kg (D). The intestine appeared intact and formed of the intestinal villi (V), propria submucosa (P), muscularis (M), and serosa (S) in all groups. There was a significant improvement in villous height, width, and number of goblet cells (red arrowhead) related to the dose of nano zinc. Stain PAS. Bar = 200 µm
Blood Biomarkers
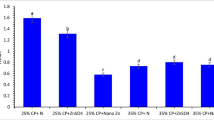
The lysozyme activity (Fig. 4A), phagocytic activity (Fig. 4B), and phagocytic index (Fig. 4C) showed higher levels in Grey Mullet–fed dietary ZnO-NPs at 20 mg/kg than fish fed 0, 10, and 40 mg/kg (p < 0.05). Superoxide dismutase (SOD, Fig. 4D) and catalase (CAT, Fig. 4E) were markedly improved in Grey Mullet treated with ZnO-NPs compared with the control, and the group of fish treated with 20 mg/kg had the highest SOD and CAT (p < 0.05). Glutathione peroxidase (GPx) was significantly higher in fish fed 20–40 mg/kg ZnO-NPs than fish fed 0–10 mg/kg and fish fed 40 mg ZnO-NPs/kg showing the highest GPx value (p < 0.05; Fig. 4F). The concentration of malondialdehyde was markedly lowered in Grey Mullet fed ZnO-NPs at varying levels (p < 0.05; Fig. 4H).
Regression Analysis
The SGR was quadratically improved by including ZnO-NPs n Grey Mullet diets at 30.56 mg/kg diet (Fig. 5A). The regression analysis also showed improved FCR in Grey Mullet fed 35.5 mg/kg ZnO-NPs (Fig. 5B). At the same time, the optimal dose based on the results of lysozyme activity is 24.61 mg/kg (Fig. 5C). The best SOD value was observed when Grey Mullet treated with 27.05 mg/kg of ZnO-NPs (Fig. 5D). Based on the overall results, the regression analysis suggests that ZnO-NPs can be included at 24.61–35.5 mg/kg for the best performances of Grey Mullet.
Discussion
Successful aquaculture practices focus on providing aquatic animals with their optimal feeding requirements, which should consider microelements [37, 38]. Zinc is one of the essential microminerals involved in various physiological and biological functions in the fish body [14, 15]. Balanced aquafeed with sufficient amounts of zinc resulted in increased growth performance, immunity, and resistance to farming stressors in finfish species [39]. Using minerals nanoform is an efficient strategy to maximize the beneficial effects of minerals [20, 21]. In this regard, the inclusion of ZnO-NPs resulted in improved growth performance, health status, and resistance against stressors in rabbitfish (Siganus rivulatus) [25], grass carp (Ctenopharyngodon idella) [40], gilthead seabream (Sparus aurata) [41], rohu (Labeo rohita) [42], and Nile tilapia (Oreochromis niloticus) [22].
Markedly, the results showed that Grey Mullet–fed dietary ZnO-NPs at 20–30 mg/kg had improved growth performance compared to the fish-fed ZnO-NPs-free diet. The results agree with Sallam and Mansour [25] and Mohammady and Soaudy [22], who stated that rabbitfish and Nile tilapia treated with dietary ZnO-NPs at 30 mg/kg had increased growth performance. Besides, grass carps [40] and Pangasius hypophthalmus–fed dietary ZnO-NPs had enhanced growth performance [43]. The enhanced growth performance is probably attributed to the role of ZnO-NPs as an enzymatic cofactor for several enzymes involved in regulating the metabolic and physiological functions in the entire body of fish [44, 45]. In this context, the results showed lowered feed conversion ratio in Grey Mullet treated with ZnO-NPs, referring to enhanced feed utilization. The improved feed utilization is associated with increased digestion capacity of fish intestines resulting from ZnO-NPs feeding. Indeed, zinc particles are known for their role in enhancing protein digestion by regulating the synthesis of Zn-containing endopeptidase [46]. Besides, zinc particles are illustrated as a precursor for the synthesis of DNA and RNA in the entire body leading to high secretion of growth hormone [47]. The nanosize of zinc particles facilitated the functional role of zinc in enhancing the growth performance of fish [20, 21]. Zinc particles can interactively help in the metabolism of fatty acids and carbohydrates by activating glucose-6-phosphate dehydrogenase (G6PD), leading to high metabolic rates of digested nutrients [48]. Mohammady and Soaudy [22] explained the enhanced growth performance of Nile tilapia as a direct result of enhanced absorption of digested nutrients in the intestines of fish treated with ZnO-NPs.
The measurement of the intestinal histological features is a reliable tool to evaluate the impact of micronutrients on intestinal health, thereby intestinal digestion capacity [49, 50]. In this study, we detected the effect of ZnO-NPs on the intestinal histological features in the posterior, middle, and interior sections. The results showed marked improvement in villous height, width, and the number of goblet cells in fish treated with ZnO-NPs. This means the enhanced growth performance of Grey Mullet can be explained by improved feed utilization resulted from enhanced intestinal digestion capacity. Similarly, Nile tilapia treated with ZnO-NPs showed improved intestinal histological features (villus width/length and mucin secretion) [22].
Nutritionally balanced aquafeed is vital for improving the immunity of fish [10, 51]. In this study, we evaluated the effect of ZnO-NPs on the lysozyme and phagocytic activities of Grey Mullets as humoral and cellular immune responses. During infection with pathogenic bacteria, lysozyme activity can break down the peptidoglycan of bacterial cells leading to the inhibition of infection [52, 53]. Additionally, phagocytosis is another robust immune response involved in relieving the impact of microbial infection on fish. Obviously, the results showed activated lysozyme and phagocytosis in Grey Mullets treated with ZnO-NPs. The results are concurrent with Sallam and Mansour [25] and Mohammady and Soaudy [22], who reported that rabbitfish and Nile tilapia–fed dietary ZnO-NPs displayed enhanced lysozyme activity.
Oxidative stress is the final result of the high generation of reactive oxygen species (ROS), occurring during biotic and abiotic stressors [54, 55]. The high ROS levels result in the peroxidation of lipids inside the entire body and the formation of malondialdehyde (MDA) [56, 57]. On this occasion, the cellular defensive tools develop antioxidant protection to cope with ROS. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) are effective antioxidative enzymes involved in reducing the impact of ROS on cell functionality [58]. The results showed enhanced SOD, GPx, and CAT and reduced MDA levels in Grey Mullet–fed dietary ZnO-NPs. The results agree with previous studies that illustrated that ZnO-NPs could enhance the antioxidative capacity of rabbitfish [25] and Nile tilapia [19, 22]. Zinc ions have a direct role in the formation of antioxidant proteins and metallothionein, resulting in activated antioxidative enzymes [59]. Zinc can act as a cofactor for forming cellular SOD and reducing ROS generation [24, 60]. Further, zinc particles can bind with thiol groups leading to the inhibition of lipid peroxidation [59]. Moreover, dietary ZnO-NPs led to enhance G6PD activity, which, in turn, activate NADPH production to cope with the impacts of ROS and reducing oxidative stress [61, 62]. The results also showed that ZnO-NPs treatment increases CAT activity as a part of the total antioxidative capacity. The results suggest ZnO-NPs may play a role in the activation of CAT [63]; however, future studies are required to confirm this result. The enhanced CAT may be related to the role of ZnO-NPs in activating the total antioxidative capacity of Grey Mullets. The enhanced antioxidative stress is strongly related to increased lysozyme, and phagocytic activities in Grey Mullet–fed ZnO-NPs, indicating enhanced health status and immunity under the current trial conditions.
The regression analysis is a simple way for presenting the optimal inclusion levels of nutrients in aquafeed depending on the outputs of the detected results [36, 64]. Interestingly, the present study suggested that ZnO-NPs should be added at 24.61–35.5 mg/kg based on the overall results of SGR, FCR, lysozyme activity, and SOD in Grey Mullets. The obtained results agree with previous investigations that recommended the inclusion of Zn in aquafeed at 30–40 mg/kg based on the species-specific manner and the life stage as well as the form of Zn supplementation [17,18,19].
Conclusion
In conclusion, the incorporation of zinc nanoparticles (ZnO-NPs) in the diets of Grey Mullet is highly recommended to enhance the growth performance, intestinal histomorphology, immunity, and antioxidative responses. Based on the overall results, the regression analysis suggests that ZnO-NPs can be included at 24.61–35.5 mg/kg for the best performances of Grey Mullet.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
FAO, Food and Agriculture Organization of the United Nations (2018) Aquaculture Department, The state of world fisheries and aquaculture. Rome, p 2430
Tacon AGJ, Metian M, McNevin AA (2021) Future feeds: suggested guidelines for sustainable development. Rev Fish Sci Aquac: 1–13. https://doi.org/10.1080/23308249.2021.1898539
Dawood MAO et al (2021) The influence of coconut oil on the growth, immune, and antioxidative responses and the intestinal digestive enzymes and histomorphometry features of Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem. https://doi.org/10.1007/s10695-021-00943-8
Mugwanya M et al (2021) Biofloc systems for sustainable production of economically important aquatic species: a review. Sustainability 13(13). https://doi.org/10.3390/su13137255
Tacon AGJ (2020) Trends in global aquaculture and aquafeed production: 2000–2017. Rev Fish Sci Aquac 28(1):43–56
Mzengereza K et al (2021) Effect of substituting fish oil with camelina oil on growth performance, fatty acid profile, digestibility, liver histology, and antioxidative status of red seabream (Pagrus major). Animals 11(7). https://doi.org/10.3390/ani11071990
Cottrell RS et al (2020) Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nature Food 1(5):301–308
Pierri BdS et al (2021) Different levels of organic trace minerals in diets for Nile tilapia juveniles alter gut characteristics and body composition, but not growth. Aquac Nutr 27(1):176–186
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151(1):185–207
Dawood MAO (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquac 13(1):642–663
El-Sharawy ME et al (2021) Studying the influence of copper on the growth behavior, antioxidative status, and histology of the intestine and liver of Striped Catfish (Pangasianodon hypophthalmus). Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02717-y
Viegas MN et al (2021) Effect of dietary manganese and zinc levels on growth and bone status of Senegalese sole (Solea senegalensis) post-larvae. Biol Trace Elem Res 199(5):2012–2021
Broom LJ, Monteiro A, Piñon A (2021) Recent advances in understanding the influence of zinc, copper, and manganese on the gastrointestinal environment of pigs and poultry. Animals 11(5). https://doi.org/10.3390/ani11051276
Angeles-Hernandez JC et al (2021) Zinc supplementation improves growth performance in small ruminants: a systematic review and meta-regression analysis. Anim Prod Sci 61(7):621–629
Davis DA, Gatlin DM (1996) Dietary mineral requirements of fish and marine crustaceans. Rev Fish Sci 4(1):75–99
Kazemi E et al (2020) Effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of rainbow trout (Oncorhynchus mykiss). Aquaculture 515:734529
Meiler KA et al (2021) Oxidative stress-related gene expression in diploid and triploid rainbow trout (Oncorhynchus mykiss) fed diets with organic and inorganic zinc. Aquaculture 533:736149
Mohseni M, Hamidoghli A, Bai SC (2021) Organic and inorganic dietary zinc in beluga sturgeon (Huso huso): effects on growth, hematology, tissue concertation and oxidative capacity. Aquaculture 539:736672
El-Saadony MT et al (2021) Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2021.04.066
Nasr-Eldahan S et al (2021) A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac Int. https://doi.org/10.1007/s10499-021-00677-7
DawitMoges F et al (2020) Mechanistic insights into diverse nano-based strategies for aquaculture enhancement: a holistic review. Aquaculture 519:734770
Mohammady EY et al (2021) Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia, Oreochromis niloticus. Aquaculture 532:736006
Abdelnour SA et al (2021) Nanominerals: fabrication methods, benefits and hazards, and their applications in ruminants with special reference to selenium and zinc nanoparticles. Animals 11(7):1916
Albanese A, Tang PS, Chan WCW (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14(1):1–16
Sallam AE et al (2020) Growth performance, anti-oxidative status, innate immunity, and ammonia stress resistance of Siganus rivulatus fed diet supplemented with zinc and zinc nanoparticles. Aquac Rep 18:100410
Dawood MAO et al (2020) Marine-derived chitosan nanoparticles improved the intestinal histo-morphometrical features in association with the health and immune response of Grey Mullet (Liza ramada). Mar Drugs 18(12):611
Tancioni L et al (2014) Anthropogenic threats to fish of interest in aquaculture: Gonad intersex in a wild population of thinlip grey Mullet Liza ramada (Risso, 1827) from a polluted estuary in central Italy. Aquac Res 47:1670–1674
Li YLPX-J, Li Y-BHZ-Q, Qing-Song H-LL (2006) Determination of seven trace elements in tea samples by atomic absorption spectrometry [J]. Chin J Spectrosc Lab 5
AOAC, Association of Official Analytical Chemists (1998) Official methods of analysis of official analytical chemists international, 16th ed. Washington, DC
Gewaily MS, Abumandour MM (2020) Gross morphological, histological and scanning electron specifications of the oropharyngeal cavity of the hooded crow (Corvus cornix pallescens). Anat Histol Embryol 50(1):72–83
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences, Edinburgh
Cai W-Q, Li S-F, Ma J-Y (2004) Diseases resistance of Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (female Nile tilapia×male blue tilapia) to Aeromonas sobria. Aquaculture 229(1):79–87
Kawahara E, Ueda T, Nomura S (1991) In vitro phagocytic activity of white-spotted char blood cells after injection with Aeromonas salmonicida Extracellular Products. Fish Pathol 26(4):213–214
Ellis A et al (1990) Lysozyme assay in techniques in fish immunology. Technique in Fish Immunology. In Stolen JS (ed) Techniques in fish immunology, fair haven: SOS publication (1990), pp 101–103
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Yossa R, Verdegem M (2015) Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 437:344–350
Abd El-Kader MF et al (2021) Selenium nanoparticles act potentially on the growth performance, hemato-biochemical indices, antioxidative, and immune-related genes of European seabass (Dicentrarchus labrax). Biol Trace Elem Res 199:3126–3134. https://doi.org/10.1007/s12011-020-02431-1
Abd El-Kader MF et al (2020) Evaluating the possible feeding strategies of selenium nanoparticles on the growth rate and wellbeing of European seabass (Dicentrarchus labrax). Aquac Rep 18:100539
Kumar N, Krishnani KK, Singh NP (2020) Effect of zinc on growth performance and cellular metabolic stress of fish exposed to multiple stresses. Fish Physiol Biochem 46(1):315–329
Faiz H et al (2015) Zinc oxide, zinc sulfate and zinc oxide nanoparticles as source of dietary zinc: comparative effects on growth and hematological indices of juvenile grass carp (Ctenopharyngodon idella). Int J Agric Biol 17(3)
Izquierdo MS et al (2017) Organic, inorganic and nanoparticles of Se, Zn and Mn in early weaning diets for gilthead seabream (Sparus aurata; Linnaeus, 1758). Aquac Res 48(6):2852–2867
Mondal AH et al (2020) Nano zinc vis-à-vis inorganic zinc as feed additives: Effects on growth, activity of hepatic enzymes and non-specific immunity in rohu, Labeo rohita (Hamilton) fingerlings. Aquac Nutr 26(4):1211–1222
Kumar N, Krishnani KK, Singh NP (2018) Effect of dietary zinc-nanoparticles on growth performance, anti-oxidative and immunological status of fish reared under multiple stressors. Biol Trace Elem Res 186(1):267–278
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Matter 16(25):R829
Imamoğlu S et al (2005) Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J Pediatr Endocrinol Metab 18(1):69–74
Uniyal S et al (2017) Comparative efficacy of zinc supplementation from different sources on nutrient digestibility, hemato-biochemistry and anti-oxidant activity in guinea pigs. Livest Sci 204:59–64
Şıklar Z et al (2003) Zinc deficiency: a contributing factor of short stature in growth hormone deficient children. J Trop Pediatr 49(3):187–188
Zhao HX et al (2011) Effect of supplemental dietary zinc sources on the growth and carbohydrate utilization of tilapia Smith 1840, Oreochromis niloticus × Oreochromis aureus. Aquac Nutr 17(1):64–72
Hu CH et al (2014) Effects of zinc oxide supported on zeolite on growth performance, intestinal barrier function and digestive enzyme activities of Nile tilapia. Aquac Nutr 20(5):486–493
Richardson NL et al (1985) Influence of dietary calcium, phosphorus, zinc and sodium phytate level on cataract incidence, growth and histopathology in juvenile Chinook salmon (Oncorhynchus tshawytscha). J Nutr 115(5):553–567
Dawood MA, Koshio S (2020) Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev Aquac 12(2):987–1002
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39(3):223–239
Siwicki A, Studnicka M (1987) The phagocytic ability of neutrophils and serum lysozyme activity in experimentally infected carp, Cyprinus carpio L. J Fish Biol 31(sA):57–60
Birnie-Gauvin K et al (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18(5):928–942
Dawood MAO, Noreldin AE, Sewilam H (2021) Long term salinity disrupts the hepatic function, intestinal health, and gills antioxidative status in Nile tilapia stressed with hypoxia. Ecotoxicol Environ Saf 220:112412
Vinagre C et al (2012) Effect of temperature on oxidative stress in fish: Lipid peroxidation and catalase activity in the muscle of juvenile seabass Dicentrarchus labrax. Ecol Indic 23:274–279
Mao L et al (2020) Embryonic development and oxidative stress effects in the larvae and adult fish livers of zebrafish (Danio rerio) exposed to the strobilurin fungicides, kresoxim-methyl and pyraclostrobin. Sci Total Environ 729:139031
Ruas CBG et al (2008) Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicol Environ Saf 71(1):86–93
Olechnowicz J et al (2018) Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci 68(1):19–31
Tapiero H, Tew KD (2003) Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother 57(9):399–411
Stanton RC (2012) Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 64(5):362–369
Taheri S et al (2017) Effects of dietary supplementation of zinc oxide nanoparticles on some biochemical biomarkers in common carp (Cyprinus carpio). Int J Aquat Biol 5(5):286–294
Tate JDJ, Miceli MV, Newsome DA (1997) Zinc induces catalase expression in cultured fetal human retinal pigment epithelial cells. Curr Eye Res 16(10):1017–1023
Dawood MAO et al (2020) The influences of ferulic acid on the growth performance, haemato-immunological responses, and immune-related genes of Nile tilapia (Oreochromis niloticus) exposed to heat stress. Aquaculture 525:735320
Funding
The current work was funded by Taif University Researchers Supporting Project number (TURSP-2020/202), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization, Mustafa Shukry, Mahmoud A.O. Dawood; Data curation, Mahmoud A.O. Dawood; formal analysis, Mustafa Shukry, Sarah Albogami, Mahmoud Gewaily, Ahmed M. El-Shehawi, Mahmoud A.O. Dawood; funding acquisition, Sarah Albogami, Ahmed M. El-Shehawi, Mahmoud A.O. Dawood; Investigation, Mustafa Shukry, Mahmoud A.O. Dawood; methodology, Saad M. Alsaiad, Mahmoud A.O. Dawood; project administration, Mahmoud A.O. Dawood; Resources, Asem A. Amer, Ali A. Soliman, Saad M. Alsaiad, Mahmoud A.O. Dawood; supervision, Mahmoud A.O. Dawood; Validation, Mahmoud A.O. Dawood; writing—original draft, Mahmoud A.O. Dawood; writing—review and editing, Sarah Albogami, Mahmoud Gewaily, Saad M. Alsaiad, Ahmed M. El-Shehawi, Mahmoud A.O. Dawood. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experimental procedure was approved by the ethics review board of the Institutional Animal Care and Use Committee in Kafrelsheikh University (Kafrelsheikh, Egypt).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shukry, M., Albogami, S., Gewaily, M. et al. Growth Performance, Antioxidative Capacity, and Intestinal Histomorphology of Grey Mullet (Liza ramada)–Fed Dietary Zinc Nanoparticles. Biol Trace Elem Res 200, 2406–2415 (2022). https://doi.org/10.1007/s12011-021-02844-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02844-6