Abstract
This study investigates the effects of different sources of selenium (inorganic (SSE), organic (OSE), and elemental nano-selenium (NSE)) on the performance of Nile tilapia (Oreochromis niloticus). In total, 204 Nile tilapia fingerlings were randomly divided into 4 equal groups fed 1 of 4 diets: a control (adding no selenium) and 3 diets as selenium sources (1 mg/kg diet), After a 65-day feeding trial, the growth performance parameters of Nile tilapia were significantly enhanced by dietary selenium supplementation (P < 0.05), with the highest values recorded in the OSE- and NSE-supplemented groups. The selenium-supplemented groups had the highest packed-cell volume, hemoglobin, and red blood cell levels, with the highest values seen in the NSE-supplemented group (P < 0.05). Innate immune-related enzymes and immunoglobulin levels were significantly enhanced with selenium supplementation (P < 0.05); the NSE group demonstrated the highest significant levels of these enzyme activities (P < 0.05). In all selenium-supplemented groups, malondialdehyde levels were significantly and equally reduced (P < 0.05) compared with levels in the control. Bactericidal activity was only enhanced in the NSE group (P < 0.05) compared with other treatments. The expression of TNF-α and IL-Iβ genes was significantly upregulated in selenium-supplemented groups, with the highest expression in the OSE and NSE groups (P < 0.05). These findings support the importance of incorporating selenium in the diet of Nile tilapia. Furthermore, elementary nano-selenium is more effective than inorganic or organic selenium supplementation at improving Nile tilapia growth performance and overall health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture can be considered a “fast-food industry” given the high metabolic levels associated with the accelerated growth of different aquatic species. Under such conditions, aquatic animals must be supplied with a balanced, economical diet containing macro- and micro-elements that meet the requirements for high levels of growth, productivity, and health [1, 2]. Selenium is an exceptional microbionutrient agent that could be used to improve aquaculture, as it possesses antioxidant properties and affects various aspects of animal health, including facilitating optimal immune responses. Additionally, it has a unique characteristic, not found in other nutritional elements, of being incorporated directly into proteins known as selenoproteins [1, 3,4,5]. Selenoproteins, such as glutathione peroxidases (GPx) and thioredoxin reductases, control the status of cell redox during an immune system response. To date, they are known to be involved in triggering, proliferating, and recognizing cells that contribute to immune system reactions [6, 7]. Several scientific reports on the potential benefits of selenium have already shown that it can protect animal cells from oxidation by enhancing the action of antioxidant-referred enzymes and thyroid hormone metabolism in response to a wide range of stressors including high density, transport, poor water quality, and infectious disease [8,9,10].
Augmentation of selenium in the diet of aquatic animals is a major concern because selenium in ambient water and food does not provide levels that are sufficient for the needs of aquatic cultivated species [11]. On the other side, the toxicity of selenium was impacted not only by the element’s status and amount consumed, but also by its chemical forms [1,2,3,4,5, 8]. Despite the fact that nano-Se was found to be non-toxic in mice and rats, the fish experiment demonstrated the inverse. High mortalities, reduced feed intake, growth response, and hematocrit levels are all indicators of se toxicity in fish. Nano-Se has a higher toxicity than selenite, according to LC50 tests [12]. The enhanced toxicity of Nano-Se may be due to Se hyper-accumulation and sluggish clearance [12, 13]. Selenium bioavailability estimation is required to derive the optimum selenium requirements and provide dietary recommendations for optimum fish health. Two forms of selenium are widely used, namely, organic and inorganic selenium. In addition, nano-selenium has recently emerged as an innovative selenium type with unique characteristics [14,15,16]. Previous fish studies comparing organic and inorganic selenium forms have shown that organic forms are more biologically active than inorganic forms and that the former provide greater levels of tissue deposition [14, 17,18,19]. Sodium selenite is the most popular and common inorganic source of selenium; however, selenium can also be found as an organic form in selenomethionine, selenocysteine, and selenocystine [20,21,22,23]. Recently, nano-elemental sources of selenium have been used owing to their novel features, which include high bioavailability and a low cytotoxicity in fish [1, 24, 25]. Nano-selenium forms also increase growth performance parameters, antioxidant levels, and immune status in many studied fish species including common carp [26, 27], crucian carp [25], and red seabream [1]. To our knowledge, the effects of various selenium forms have not yet been compared in Nile tilapia (Oreochromis niloticus).
The objective of the present study was to evaluate supplementation with various selenium types (inorganic, organic, and nano-selenium) in the diets of Nile tilapia with the aim of better understanding the species-related effects of selenium in the aquaculture feed chain. Specifically, this study investigated the growth performance, immune-related parameters, tissue composition, gut morphology, antioxidant responses, and biochemical blood parameters.
Materials and Methods
Ethical Statement.
All the methods, animal care, and experimental protocols used in the present study were authorized by the ethics review board of the Institutional Animal Care and Use Committee in Kafrelsheikh University (Kafrelsheikh, Egypt).
Fish and Experimental Facilities
In total, 204 Nile tilapia (O. niloticus) fingerlings with an average initial body weight of 15.37 ± 0.22 g/fish were obtained from a private farm in Kafr El Sheikh, Egypt. Before the trial, the fish were acclimated to experimental conditions for 15 days in three indoor circular fiberglass tanks (1 m3). During this period, fish were fed a control diet (30% crude protein and 6.5% crude fat). After acclimatization, the fish were randomly distributed into 12 glass aquaria each measuring 30 × 40 × 60 cm. These aquaria represented four experimental treatments (in triplicate). The stocking density was 17 fish per aquarium. Each aquarium was provided with continuous aeration, and half the water in each aquarium was replaced daily with fresh dechlorinated water.
Experimental Design and Diets
Sources of Selenium and Selenium Nano-particles
Sodium selenite (Na2 Se O3.5H2O), representing inorganic selenium (SSE), was purchased from Kempex Holland BV (Volkel). Selenomethionine, representing organic selenium (OSE), was purchased from Diamond V (USA). Selenium nano-particles, representing the nano-selenium source (NSE), were produced in Naqaa Foundation for Scientific Research, Technology and Development, Giza, Egypt, using a chemical reduction of sodium selenite with ascorbic acid, which was then stabilized by coating the particles with dextrin according to a previous method with modifications [28]. Characterization of produced selenium nano-particles, including size, shape, morphology, and crystallinity, was performed using a variety of analytical tools, including particle spectroscopy, a scanning electron microscope, and X-ray diffraction. The size of nano-particles produced and used in our study was 30 nm (Fig. 1).
Table 1 presents the diet formulation and proximate composition of the basal diet. The ingredients and different selenium sources were mixed, extruded, and air-dried at room temperature. After drying, the diets were stored in plastic bags in a refrigerator (at 4℃) until use. The chemical composition of diet samples was assessed according to procedures of AOAC [29]. An atomic absorption spectrophotometer fitted with a transversely heated graphite atomizer system (Younglin AAS 8020, Anyang, Korea) was used to determine the real concentration of Se in each diet as described by [30] (Table 1). The daily ration was offered twice a day (09.00 and 15.00 h), 7 days a week, until satiation for 65 days. Water temperature (22.1–23.3℃), dissolved oxygen (5.10–5.21 mg/L), pH (7.0–7.5), ammonia (0.010–0.016 mg/L), and a photoperiod regime (12:12-h light/dark cycle) during the feeding trial. These ranges are considered within optimal values for juvenile Nile tilapia [31, 32].
Growth and Feed Utilization Indices
After 65 days of feeding, all fish were individually weighed and counted to calculate the final body weight, weight gain, specific growth rate, and survival rate using the following equations:
Randomly, five fish per tank were chosen and stored at − 20℃ until carcass proximate analysis was conducted and the amount of selenium bioaccumulation in muscle and liver tissues was determined.
Blood Sampling
Three fish were randomly sampled from each replicate tank in each treatment for blood sampling. Blood was collected from these fish via puncture of the caudal vein. The collected blood was divided into two tubes, one containing heparin (1600 UI/mL) as an anticoagulant agent for hematological assessment and the other without anticoagulant for serum collection (conducted at 1112 g and 4℃ for 15 min) before being stored at − 20℃. Hematocrit (packed-cell volume (PCV)) was measured using microhematocrit-heparinized capillary tubes and a microhematocrit centrifuge (10,000 g for 5 min). Red blood cells (RBCs) and white blood cells (WBCs) were counted with a hemocytometer immediately after dilution with Natt and Herrick’s solution [33]. For differential leucocytic count, blood films were prepared and stained according to a previous method [34], and cells were calculated according to a prior protocol [35]. Blood hemoglobin levels were measured using a spectrophotometer (Model RA 1000; Technicon Instruments Corporation Tarrytown, NY, USA) at 540 nm according to a published method [36].
Total cholesterol and triglyceride were evaluated spectrophotometrically using an automated analyzer (SPOTCHEM™ EZ model SP-4430; ARKRAY, Inc., Kyoto, Japan) using an established method [37]. Glucose, total protein levels, and the enzymatic activities of the liver, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were measured calorimetrically at the wavelength of 540 nm [38].
Following blood sampling, the digestive tracts of fish were removed, cut into small pieces, and rinsed with pure water. The viscera and liver were then dissected out from the fish, individually weighed, and measured (length) to calculate the condition factor, viscerosomatic index, and hepatosomatic index using the following formulas:
Immunological Assays
Superoxide dismutase (SOD), catalase (CAT), and GPx levels in serum were evaluated spectrophotometrically following the methods in previous studies ([39, 40], and [41], respectively). Malondialdehyde (MDA) levels were evaluated using the thiobarbituric acid method [42]. Serum immunoglobulin (IgM) levels were measured with an enzyme-linked immunosorbent assay kit specific for fish IgM (CUSABIO and CusAb, USA) according to the manufacturer’s instructions. Serum lysozyme activity was evaluated according to a published method [43]. The lysozyme unit present in serum (μg/mL) was obtained by comparison with a standard curve produced using lyophilized hen egg white lysozyme [43,44,45]. An NBT assay was completed using a previously published method [46] with slight modifications. Briefly, blood (0.1 mL) was transferred to microtiter plate wells, to which an equal amount of 0.2% NBT solution (Sigma-Aldrich, USA) was added and incubated for 30 min at room temperature. A sample of the NBT blood cell suspension (0.05 mL) was then transferred to a glass tube containing 1-mL N, N-dimethylformamide (Sigma-Aldrich, USA) and centrifuged for 5 min at 3000 rpm. Finally, the optical density of the supernatant was measured at 540 nm. Dimethylformamide was used as the blank. Serum bactericidal activity against Aeromonas hydrophila was detected by following a previous method [47]. The results were recorded as a survival index, with values calculated as follows: SI = CFU at end /CFU at start × 100.
Gut Morphology Analysis
Three fish from each replicate tank were used for gut morphology analysis. The entire gastrointestinal tract was extracted to prepare an intestinal sample, and the anterior and posterior parts of the intestine were collected. All tissues were washed twice with PBS (pH = 7.4) and immediately fixed in Davidson’s solution (agitated for 5 min) for 8 h. Fixed tissues were then steadily dehydrated in ethanol (70–100%), washed twice with xylene (1 and 2 h), and embedded in paraffin. Sections of 5-µm thickness were gathered and stained with hematoxylin and eosin. Two cross-sectional slices were prepared from each tissue. Tissue slices were stained with hematoxylin and eosin and then inspected under a light microscope (Eclipse 50i; Nikon, Tokyo, Japan) and camera (Digital Sight DS2MV with a DS-L2 control unit; Nikon) with analysis via SigmaScan Pro 5 software. Villi length, villi width, crypt depth, and goblet cell numbers were measured using ImageJ analysis software with magnifications of 100 × , 200 × , and 400 × . For each tissue, ten measurements were obtained according to an established protocol [48].
Gene Expression
RT-PCR was used to measure the expression of hepatic genes. Briefly, TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) was used to extract total RNA from approximately 100 mg of hepatic tissue. RNA samples of 1, 8, or more A260/A280 were used for DNA synthesis using a cDNA synthesis package (Fermentas, Waltham, MA, USA) with NanoDrop quantitation also conducted by Nanodrop (Uv–Vis spectrophotometer Q5000/Quawell, USA). Following that, cDNA was synthesized through using SensiFASTTM cDNA synthesis kit (Bioline, UK) according to the manufacturer’s procedure. SYBR green method was used to quantify the gene expression using RT-PCR (SensiFast SYBR Lo-Rox kit, Bioline). The thermocycling conditions for the reaction were 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 min at 60 °C, and finally 5 min at 85 °C for 1 min. For each mRNA, gene expression was corrected by the β-actin content in each sample. After verification of PCR efficiency to be around 100%, the gene expression data were calculated according to the method [49]. Selected genes and a stable internal housekeeping standard (β-actin) were amplified using a specific primer set (Table 2) [50,51,52,53]. The transcription folds were standardized to β-actin through using the 2 − ∆∆CT method [54].
Statistical Analyses
The obtained data were subjected to one-way analysis of variance using SPSS version 22 (SPSS Inc., IL, USA). The Shapiro–Wilk and Levene tests were used to assess the homogeneity and normality of variance. Differences between the means were tested at the 5% probability level using Duncan’s test as a post hoc test.
Results
Growth Performance
Table 3 presents the growth performance and survival rate. In comparison with the control group, final body weight was enhanced significantly in selenium-enriched groups (P < 0.05), with OSE and NSE supplements producing the highest weight gain and specific growth rate values among all experimental groups (P < 0.05).
Carcass Composition and Somatic Indices
Table 4 presents the carcass composition of fish. Compared with the control group, dietary selenium supplementation had non-significant (P > 0.05) influences on whole-body proteins, lipids, ash content, and somatic indices including condition factor, hepatosomatic index, and viscerosomatic index.
Hematobiochemical Indices
Significantly higher PCV, Hb, and RBC levels were observed in selenium-supplemented groups, with the highest values observed in the NSE group (P < 0.05) (Table 5). Furthermore, tilapia fed the OSE diet had similar Hb and PCV as fish fed the SSE diet, whereas RBCs were found at higher levels in the OSE group relative to the SSE group. Total serum protein was enhanced significantly with selenium supplementation, with the highest values shown in the OSE group; however, there were no differences between SSE- and NSE-supplemented fish. In contrast, glucose and total cholesterol levels were significantly decreased in fish fed selenium-containing diets compared with those fed control diets. Glucose levels were lowest with OSE diets, while total cholesterol was lowest with NSE supplementation. AST levels were significantly decreased with NSE supplementation relative to the levels in other experimental groups. The remaining hematological indices were not significantly impacted by selenium supplementation (P > 0.05).
Antioxidant Activities
Figures 2, 3, and 4 show the SOD (IU L−1), CAT (KU L−1), and GPx (IU L−1) activities in Nile tilapia fed experimental diets for 65 days, respectively. Compared with the control group, SOD, CAT, and GPx activities were enhanced significantly (P < 0.05) with all selenium-containing diets. Specifically, NSE produced the highest activities among the selenium-supplemented groups (P < 0.05). Furthermore, the MDA activity (IU L−1) of Nile tilapia fed experimental diets for 65 days declined significantly (P < 0.05) relative to MDA activity in the control group (Fig. 5).
Immune Responses
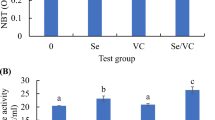
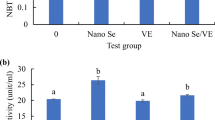
In comparison to the control group, IgM levels were increased significantly in fish fed selenium-enriched diets (P < 0.05) (Fig. 6). Furthermore, tilapia fed NSE had the highest IgM activities, with similar activities demonstrated in the other selenium-supplemented groups. Figure 7 shows that the lysozyme activity was significantly increased with all selenium-containing diets relative to fish fed on the control diet; the highest lysozyme activity was observed in the NSE group (P < 0.05). Respiratory burst activity was significantly higher (P < 0.05) in SSE and NSE groups than it was in control and OSE groups (Fig. 8). In addition, Fig. 9 shows that bactericidal activity was only enhanced significantly (P < 0.05) in the NSE group.
Intestinal Morphology
The anterior part of the intestine exhibited significantly (P < 0.05) increased villi length values in fish fed selenium-enriched diets compared with villi lengths in the control group; the NSE group showed the highest villi lengths among the groups. In contrast, villi width values did not differ among the experimental groups (P > 0.05). Similarly, crypt depths did not differ between most tested groups except in the NSE group, which had higher crypt depth values (P < 0.05) than the other groups (Table 6). Goblet cell values differed significantly in the OSE and NSE groups, with the NSE group showing the highest values (P < 0.05). In the posterior part of the gut, villi length and goblet cell values were significantly (P < 0.05) higher in fish fed OSE or NSE than in those fed SSE or a control diet. In addition, villi width and crypt depth were significantly (P < 0.05) increased in fish fed all selenium-enriched diets compared with the control group; OSE and NSE produced the highest values among the groups (Table 6).
Gene Expression
Figure 10 shows the mRNA expression profiles of the tested immune-related genes (TNF-α, tumor necrosis factor alpha; TGF-β1, transforming growth factor beta 1; and IL-1β, interleukin 1 beta) of Nile tilapia fed experimental diets for 65 days. TNF-α was significantly upregulated in fish fed all selenium-enriched diets, with the highest levels observed in OSE and NSE groups (P < 0.05). In addition, TGF-β1 was significantly upregulated in the NSE group relative to the other groups (P < 0.05). IL-1β expression was significantly upregulated in all selenium-enriched groups than in the control group, with the highest expression values detected in the NSE group (P < 0.05).
mRNA expression profile of immune-related genes (TNF-α, tumor necrosis factor alpha; TGF-β1, transforming growth factor beta 1; IL-1β, interleukin 1 beta) activity of Nile tilapia fed test diets for 65 days. Values are expressed as mean ± SE from triplicate groups. Values with different letters are significantly different (P < 0.05). Values with the same letter are not significantly
Discussion
Selenium is an extremely important micronutrient in functional feed formulations because it helps ensure that aquaculture production levels are optimized [1, 17, 25, 30, 55]. As detailed in this study, three different sources of selenium, namely, sodium selenite (inorganic form), selenomethionine (organic form), and nano-selenium, can be incorporated in fish diets. However, until now, there is lack of information on the comparative effects of these selenium sources on the performance of Nile tilapia; thus, we investigated these effects in the present study.
Nile tilapia fed with nano-selenium and organic selenium-supplemented diets showed significantly improved growth parameters; thus, the bioavailability of these two selenium forms may be higher than the bioavailability of sodium selenite, making them more effective as Nile tilapia dietary supplements. The obtained results are similar to those reported in previous studies [45, 56]. Improved growth performance might be attributable to the enhanced intestinal morphology, resulting in more efficient and rapid digestion (Figs. 11 and 12) [57]. In addition, it might be due to the increase in intracellular protein in the intestinal cells, which could lead to enhanced digestion and better feed metabolism [58]. The carcass characteristics of Nile tilapia were not significantly changed among the dietary treatment groups, indicating that Nile tilapia proximate body composition is not sensitive to different selenium forms in the diet. This result is in agreement with that of Le and Fotedar [55] for yellowtail kingfish (Seriola lalandi) and Saffari et al. [59] for common carp (Cyprinus carpio).
Photomicrograph of anterior part of intestine of Nile tilapia (bar = 100 µm, stain H&E.). Control group (A) reveals normal structure of intestinal villi lined with absorptive cells (black arrow head), villi connective tissue core (black arrow), propria sub mucosa (S), and lamina muscularis (M); nano-selenium group (B) reveals marked increase in intestinal villi length and branching (black arrow head) with presence of lymphoepithelium (white arrow); sodium selenite group (C) reveals mild increase in the villi length (black arrow head) with moderate increase in lymphoepithelium (white arrow); organic selenium group (D) reveals moderate increase in the villi length (black arrow head) with lymphoepithelium (white arrow)
Photomicrograph of posterior part of intestine of Nile tilapia (bar = 100 µm, stain H&E.). Control group (A) showed long branched villi lined with intestinal absorptive cells (black arrow head) contain lymphoepithelium (white arrow), villi core (black arrow), propria sub mucosa (S), and lamina muscularis (M); nano-selenium group (B) showed moderate increase in the villi length and branching (black arrow head), moderate increase in lymphoepithelial cells (white arrow), presence of lymphatic nodules, and diffuse lymphatic tissue (white arrow head); sodium selenite group (C) showed degeneration in some intestinal absorptive cells (black arrow head) with lymphoepithelial cells (white arrow), edema in the propria sub mucosa (tailed arrow), and diffuse lymphatic tissue (white arrow head); organic selenium group (D) showed branched intestinal villi (black arrow head) with lymphoepithelial cells (white arrow) and diffuse lymphatic tissue in the propria sub mucosa (white arrow head)
Blood parameters are commonly regarded as a reliable tool for general health determination, and they can vary depending on animal nutrition, infections, and stressful experiences [45, 60, 61]. The tested Nile tilapia had normal values for various hematological parameters during the current experiment [32, 62]. Furthermore, the WBC, heterophil, triglyceride, ALT, lymphocyte, monocyte, eosinophil, and basophil content were not significantly changed by feeding on the experimental diets, indicating that selenium sources incorporated into the diet were harmless to Nile tilapia. Hematocrit, hemoglobin, and RBCs play key roles in the ability of blood to transport and carry dissolved oxygen [63, 64]; the selenium-enriched groups showed significantly higher values for these parameters, indicating an improvement in the health status of the fish. This finding is similar to those in previous studies on wild and cultured aquatic animals, which demonstrated that high levels of hematocrit, hemoglobin, and RBCs were associated with high activity and fast movement [25, 63]. Despite the higher activity of organic selenium relative to its inorganic form, the nano-selenium form had comparatively higher values for these important parameters. Higher nano-selenium activity was previously reported and attributed to its essential role in enhancing cell-mediated immunity [1, 22].
One of the most reliable blood parameter tools for measuring the immune and health status of fish is total serum protein, which can be affected by the quality of animal feed [65, 66]. In the current study, the immunological condition (total serum protein and total IgM) of Nile tilapia was considerably improved by a selenium-enriched diet, particularly a diet enriched with the nano-selenium source [67]. This enhancement may be due to the importance of selenium as an integral component of selenoproteins, which play crucial roles in several biological functions such as antioxidant protection and thyroid hormone production [58]. Furthermore, this enhancement shows that selenium has an immunomodulatory effect on Nile tilapia and therefore may have a positive effect on the health of grown fish. Likewise, natural antibodies (IgM) have a wide range of defense activities such as reducing infectious agent dispersal, killing pathogens, repairing tissue damage, and restoring the healthy condition. Our results indicated that IgM levels increased in all selenium-supplemented groups, with the highest levels found in nano-selenium-supplemented fish. These results are consistent with previous studies, which also showed the role played by selenium in improving IgM levels [68,69,70]. Higher IgM activity with nano-selenium use may be due to the high bioactivity of this form in the body of Nile tilapia.
Plasma glucose is a stress indicator in fish; high glucose levels often indicate high stress because high cortisol levels stimulate the dissolution of liver glycogen to provide energy during the stress process. Fish in selenium-supplemented groups exhibited significantly lower glucose levels than those in control fish, indicating the possible efficacy of selenium in stress reduction as well as in the optimization of the physiological conditions of fish [1, 62].
While there was largely no significant difference in ALT activity among the experimental groups, a significantly lower value of AST was recorded when Nile tilapia were fed a diet containing nano-selenium. This is an indication that feeding tilapia nano-selenium-supplemented diets could influence the liver health of fish positively and to a greater extent than other selenium sources [71].
The bodies of aquatic animals are protected by an endogenous enzymatic and nonenzymatic antioxidant defense system. SOD, CAT, and GPx are the first line in the antioxidant defense system. They may be activated and induced by reactive oxygen species production in an attempt to scavenge reactive oxygen species and reduce their harmful effects [72, 73]. SOD is an important antioxidant enzyme capable of dispersing two molecules of superoxide anion to form hydrogen peroxide (H2O2) and molecular oxygen (O2). SOD activity was significantly enhanced in selenium-enriched groups compared with in the control, likely due to the biological function and the antioxidant activity of selenium; nano-selenium supplementation was most effective at influencing SOD activity.
CAT is a vital antioxidant enzyme found in nearly all live tissues that use oxygen. CAT catalyzes the degradation or reduction of H2O2 into water and molecular oxygen; thus, it completes the SOD-launched detoxification process. Similar to SOD, CAT activity in Nile tilapia was most influenced by nano-selenium enrichment. GPx also has a major function in fish; its reduced form reduces H2O2 and lipidic hydroperoxides. The significant increase in the activity of all three antioxidant enzymes, SOD, CAT, and GPx, in this trial can be considered an index of enhanced antioxidant activity achieved with selenium-enriched diets [74, 75].
Nano-selenium seems to be most effective at enhancing the Nile tilapia antioxidant defense capacity because SOD, CAT, and GPx activities were most increased with this supplementation. Similarly, Dawood et al. [1] reported that 1 mg of elemental nano-selenium produced the highest antioxidant activity in red seabream. Similar activity was recorded in cases for carp fed on 2 mg/kg of a nano-selenium source [26]. In contrast, Zhou et al. [25] found no significant difference in GPx levels in nano-selenium- and organic selenium-fed carp. In our study, there were only differences between the effects of organic and inorganic selenium sources on antioxidant enzyme activities for GPx, for which the activity was higher in Nile tilapia fed on a diet with inorganic selenium. Similarly, Cotter et al. [76] showed that the GPx activity in hybrid striped bass was higher with an inorganic selenium source than with an organic source. In contrast, organic selenium was reported to be more efficient than inorganic selenium in increasing GPx activity in channel catfish [77], common carp [78], and crucian carp [21]. Another study in yellowtail kingfish indicated that the GPx activity of fish supplied with inorganic and organic selenium sources (selenocystine, selenomethionine, or selenium yeast) for 6 weeks did not differ significantly [55].
MDA formation is the most widely used assay for lipid peroxidation, representing the secondary lipid peroxidation product with the thiobarbituric acid reactive substances test. The concentration of MDA is the direct proof of damaging processes caused by free radicals [79,80,81,82]. Our data showed that liver MDA in selenium-supplied Nile tilapia groups significantly decreased compared with levels in the control, whereas no differences were detected between the three selenium-supplemented sources. Similarly, the MDA levels of rainbow trout were reduced as a result of different sources of selenium supplementation in a previous study [14]. Moreover, MDA levels were significantly increased in common carp fed on diets with nano-elemental selenium [26].
It is well documented and scientifically established that selenium supplementation in aquatic animal diets can increase serum lysozyme activity, which is seen as evidence of improved immune conditions [1, 83,84,85,86,87]. In agreement with previous studies, our results show significantly higher serum lysozyme activity in Nile tilapia supplemented with selenium. Moreover, compared with other selenium sources, the inorganic form produced higher lysozyme activity than did the organic form; however, the highest lysozyme activity was achieved with nano-selenium supplementation. Thus, nano-selenium seems to be most effective at strengthening the immune status of Nile tilapia. These findings are consistent with those of previous studies on nano-selenium supplementation, as reported in rainbow trout and tilapia [88, 89]. Respiratory burst activity, measured by NBT, is an important bactericidal mechanism in fish; higher NBT is considered a clear indicator of improved immunity [90]. Furthermore, bactericidal activity is a reliable tool by which to assess the resistance of aquatic animals against pathogenic bacteria. The results of the present study showed that only fish in the nano-selenium-supplied group demonstrated significantly higher NBT and serum bactericidal activity, indicating that nano-selenium may be more effective than organic and inorganic selenium in terms of strengthening Nile tilapia bacterial resistance. Similarly, Dawood et al. [1] reported that dietary nano-selenium significantly increased bactericidal activity in Nile tilapia. Kumar and Singh [91] and Kumar et al. [92] also demonstrated the role of nano-selenium in NBT enhancement, and they referred to the immunoprotective and antioxidant properties of this relatively new source of selenium. The enhanced bactericidal activity with nano-selenium suggests that it could be used as a disinfectant or antimicrobial agent in the management of fish health [93].
Gut morphology measurements of the intestinal villi and goblet cells can help predict the efficiency of feed digestion and utilization in the guts of aquatic animals [94, 95]. The intestinal morphometric results in the present study revealed significant improvement in the groups fed selenium-supplemented diets. In addition, Nile tilapia fed on a diet with nano-selenium displayed the highest morphometric values for all measured parameters. The absorption capacity of the intestinal surface can be increased markedly due to the increased length, width, and depth of the intestinal villi, resulting in better nutrient consumption and ultimately increased growth [96]. This result may be explained by the role of selenium as a cofactor in gut microflora and the enhancement of many digestive enzymes that function in gut morphology and digestion capacity improvement [97, 98]. Furthermore, intestinal goblet cells protect the intestinal mucosal layer from dehydration and the hazardous effects of various harmful microbes through production of mucus and antibacterial substances such as glycoproteins and low-molecular-weight fatty acids [99, 100]. The number and efficiency of goblet cells can be affected by the quality of the diet that reaches the guts of aquatic animals, as well as environmental stressors and pathogens [101]. In our study, the organic and nano-selenium forms succeeded in increasing the number of goblet cells. Again, nano-selenium gave the greatest improvement, providing further evidence that this form of selenium may enhance the immunity and general health of Nile tilapia. These findings were similar to those reported by [32] for Nile tilapia fed a nano-selenium-supplemented diet.
In the present work, significant upregulation of proinflammatory cytokine genes (TNF-α, TGF, and IL-1β) was detected in Nile tilapia that fed on a diet enriched with different sources of selenium. These genes showed the highest upregulation activity in Nile tilapia that fed on a diet supplemented with a nano-selenium source. In particular, tumor necrosis factors, such as TNF-α, are known to be involved in inflammation, apoptosis, cell proliferation, and survival [102]. Additionally, TGF-β1 is a cytokine that plays roles in many cellular processes, including cell growth, proliferation, differentiation, and apoptosis [103]. IL-1β is among the cytokines that regulate the immune response via cell proliferation, differentiation, and apoptosis [104]. The observed upregulation of these proinflammatory cytokines confirmed the anti-inflammatory role of selenium in tilapia. In addition, the nano-selenium form was confirmed to have higher bioavailability, which may lead to enhanced anti-inflammatory function.
Conclusion
Dietary selenium supplementation had beneficial effects on Nile tilapia in terms of growth efficiency, intestinal morphology, oxidative status, immunity, and immune-related gene expression. More precisely, nano-selenium-fed Nile tilapia displayed a comparatively higher performance and better well-being compared to fish fed other types of selenium. Comparing inorganic and organic sources of selenium, organic sources produce better activities in this fish species. Therefore, nano-selenium feed supplementation could be used in tilapia culture to improve the overall performance and nonspecific immune system of the fish. However, further research is required to determine the economic value associated with the commercial use of nano-selenium in aquaculture.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dawood MA, Koshio S, Zaineldin AI, Van Doan H, Moustafa EM, Abdel-Daim MM, Esteban MA, Hassaan MS (2019) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45(1):219–230
Yukgehnaish K, Kumar P, Sivachandran P, Marimuthu K, Arshad A, Paray BA, Arockiaraj J (2020) Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Rev Aquac 12(3):1903–1927
Hsu P-C, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicology 180(1):33–44
Rider SA, Davies SJ, Jha AN, Fisher AA, Knight J, Sweetman JW (2009) Supra-nutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchus mykiss): implications on selenium status and health responses. Aquaculture 295(3):282–291
Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity. Nutrients 10(9):1203
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284(2):723–727
Zhang Y, Roh YJ, Han S-J, Park I, Lee HM, Ok YS, Lee BC, Lee S-R (2020) Role of selenoproteins in redox regulation of signaling and the antioxidant system: a review. Antioxidants 9(5):383
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13(2):102–108
Zoidis E, Seremelis I, Kontopoulos N, Danezis GP (2018) Selenium-dependent antioxidant enzymes: actions and properties of selenoproteins. Antioxidants 7(5):66
LongbafDezfouli M, Ghaedtaheri A, Keyvanshokooh S, Salati AP, Mousavi SM, Pasha-Zanoosi H (2019) Combined or individual effects of dietary magnesium and selenium nanoparticles on growth performance, immunity, blood biochemistry and antioxidant status of Asian seabass (Lates calcarifer) reared in freshwater. Aquac Nutr 25(6):1422–1430
Lee S, Nambi RW, Won S, Katya K, Bai SC (2016) Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 464:153–158
Li H, Zhang J, Wang T, Luo W, Zhou Q, Jiang G (2008) Elemental selenium particles at nano-size (Nano-Se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquatic toxicology (Amsterdam, Netherlands) 89(4):251–256
Baines SB, Fisher NS, Stewart R (2002) Assimilation and retention of selenium and other trace elements from crustacean food by juvenile striped bass (Morone saxatilis). Limnol Oceanogr 47(3):646–655
Küçükbay FZ, Yazlak H, Karaca I, Sahin N, Tuzcu M, Cakmak MN, Sahin K (2009) The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac Nutr 15(6):569–576
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177(3):204–210
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjørklund G, Sochor J, Kizek R (2018) Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomed 13:2107–2128
Lin Y-H (2014) Effects of dietary organic and inorganic selenium on the growth, selenium concentration and meat quality of juvenile grouper Epinephelus malabaricus. Aquaculture 430:114–119
Antony Jesu Prabhu P, Schrama JW, Kaushik SJ (2016) Mineral requirements of fish: a systematic review. Rev Aquac 8(2):172–219
Fontagné-Dicharry S, Godin S, Liu H, Antony Jesu Prabhu P, Bouyssière B, Bueno M, Tacon P, Médale F, Kaushik SJ (2015) Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br J Nutr 113(12):1876–1887
Schrauzer GN (2000) Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr 130(7):1653–1656
Wang Y, Han J, Li W, Xu Z (2007) Effect of different selenium source on growth performances, glutathione peroxidase activities, muscle composition and selenium concentration of allogynogenetic crucian carp (Carassius auratus gibelio). Anim Feed Sci Technol 134(3):243–251
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H, Mozanzadeh MT (2018) Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol Biochem 44(4):1087–1097
Hariharan S, Dharmaraj S (2020) Selenium and selenoproteins: it’s role in regulation of inflammation. Inflammopharmacology 28(3):667–695
Wang H, Zhang J, Yu H (2007) Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radical Biol Med 42(10):1524–1533
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquaculture 291(1–2):78–81
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquaculture 446:25–29
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H (2017) Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac Nutr 23(3):611–617
Malhotra S, Jha N, Desai K (2014) A superficial synthesis of selenium nanospheres using wet chemical approach. Int J Nanotechnol Appl 3:7–14
AOAC, Method 2007–04. Association of Official Analytical Chemists. Washington, DC., (2007).
Elia AC, Prearo M, Pacini N, Dörr AJM, Abete MC (2011) Effects of selenium diets on growth, accumulation and antioxidant response in juvenile carp. Ecotoxicol Environ Saf 74(2):166–173
El Basuini MF, Teiba II, Zaki MAA, Alabssawy AN, El-Hais AM, Gabr AA, Dawood MAO, Zaineldin AI, Mzengereza K, Shadrack RS, Dossou S (2020) Assessing the effectiveness of CoQ10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 98:420–428
Dawood MAO, Zommara M, Eweedah NM, Helal AI (2020) The evaluation of growth performance, blood health, oxidative status and immune-related gene expression in Nile tilapia (Oreochromis niloticus) fed dietary nanoselenium spheres produced by lactic acid bacteria. Aquaculture 515:734571
A.J.N.A.F.S.J.N.S.s.v.h.L. Houston, P. Febiger (1990) Blood and circulation/Methods for fish biology 21–62.
Z. Lucky, Z.k. Lucký (1977) Methods for the diagnosis of fish diseases
N.C. Jain (1986) Schalm’s veterinary hematology, Lea & Febiger
Blaxhall P, Daisley KJJofb (1973) Routine haematological methods for use with fish blood. Fish Biol 5(6):771–781
N. Tatsumi, R. Tsuji, T. Yamada, K. Kubo, T.J.J.o.C.L.I. Matsuda (2000) Reagents, Spot chem. EZ SP-4430 no kiso teki kento, 23(6) 427–433.
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244(22):6049–6055
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21(4):363–373
J.d.M. Filho, P.D. Castelane (2009) Leukocytes respiratory burst and lysozyme level in pacu (Piaractus mesopotamicus Holmberg, 1887)
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AMS, Dawood MAO, Dossou S, Wang W, Yukun Z (2018) Bacillus subtilis as probiotic candidate for red sea bream: growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol 79:303–312
D.P. Anderson, A.K. Siwicki, Basic hematology and serology for fish health programs, Fish Health Section, Asian Fisheries Society, Manila, Phillipines, 1995
Rainger GE, Rowley AF (1993) Antibacterial activity in the serum and mucus of rainbow trout, Oncorhynchus mykiss, following immunisation with Aeromonas salmonicida. Fish Shellfish Immunol 3(6):475–482
De Los Santos FS, Donoghue A, Farnell M, Huff G, Huff W, Donoghue D (2007) Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poult Sci 86(5):921–930
Livak KJ, Schmittgen TDJm (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Thummabancha K, Onparn N, Srisapoome P (2016) Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol 13(6):909–917
Chen YB, Hu J, Lyu QJ, Liu LJ, Wen LF, Yang XK, Zhao HH (2016) The effects of Natucin C-Natucin P mixture on blood biochemical parameters, antioxidant activity and non-specific immune responses in tilapia (Oreochromis niloticus). Fish Shellfish Immunol 55:367–373
Harms CA, Howard KE, Wolf JC, Smith SA, Kennedy-Stoskopf S (2003) Transforming growth factor-beta response to mycobacterial infection in striped bass Morone saxatilis and hybrid tilapia Oreochromis spp. Vet Immunol Immunopathol 95(3–4):155–163
Qiang J, He J, Yang H, Xu P, Habte-Tsion H-M, Ma XY, Zhu ZXJAI (2016) The changes in cortisol and expression of immune genes of GIFT tilapia Oreochromis niloticus (L.) at different rearing densities under Streptococcus iniae infection. Aquac Int 24:1365–1378
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6):1101–1108
Le KT, Fotedar R (2014) Bioavailability of selenium from different dietary sources in yellowtail kingfish (Seriola lalandi). Aquaculture 420:57–62
Pinpimai K, Rodkhum C, Chansue N, Katagiri T, Maita M, Pirarat NJRivs (2015) The study on the candidate probiotic properties of encapsulated yeast, Saccharomyces cerevisiae JCM 7255, in Nile Tilapia (Oreochromis niloticus). Res Vet Sci 102:103–111
Suzer C, Çoban D, Kamaci HO, Saka Ş, Firat K, Otgucuoğlu Ö, Küçüksari HJA (2008) Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: effects on growth performance and digestive enzyme activities. Aquaculture 280(1–4):140–145
Mehdi Y, Hornick J-L, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18(3):3292–3311
Safari R, Hoseinifar SH, Nejadmoghadam S, Jafar A (2016) Transciptomic study of mucosal immune, antioxidant and growth related genes and non-specific immune response of common carp (Cyprinus carpio) fed dietary Ferula (Ferula assafoetida). Fish Shellfish Immunol 55:242–248
Qu D, Zhou X, Yang F, Tian S, Zhang X, Ma L, Han J (2017) Development of class model based on blood biochemical parameters as a diagnostic tool of PSE meat. Meat Sci 128:24–29
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500:237–242
El Basuini MF, Teiba II, Zaki MAA, Alabssawy AN, El-Hais AM, Gabr AA, Dawood MAO, Zaineldin AI, Mzengereza K, Shadrack RS, Dossou S (2020) Assessing the effectiveness of CoQ10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 98:420–428
Fazio F, Marafioti S, Sanfilippo M, Casella S, Piccione G (2016) Assessment of immune blood cells and serum protein levels in Mugil cephalus (Linnaeus, 1758), Sparus aurata (Linnaeus, 1758) and Dicentrarchus labrax (Linnaeus, 1758) collected from the Thyrrenian sea coast (Italy). Cah Biol Mar 57:235–240
Parrino V, Cappello T, Costa G, Cannavà C, Sanfilippo M, Fazio F, Salvatore F (2018) Comparative study of haematology of two teleost fish ( Mugil cephalus and Carassius auratus ) from different environments and feeding habits. Eur Zool J 85:194–200
Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41(1):125–139
Cuesta A, Meseguer J, Esteban MA (2004) Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet Immunol Immunopathol 101(3):203–210
Qin F, Shi M, Yuan H, Yuan L, Lu W, Zhang J, Tong J, Song X (2016) Dietary nano-selenium relieves hypoxia stress and improves immunity and disease resistance in the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol 54:481–488
Neamat-Allah ANF, Mahmoud EA, Abd El Hakim Y (2019) Efficacy of dietary nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish Immunol 94:280–287
Ahmadi M, Ahmadian A, Seidavi A (2018) Effect of different levels of nano-selenium on performance, blood parameters, immunity and carcass characteristics of broilerchickens. Poult Sci J 6(1):99–108
S.S. Rathore, H.S. Murthy, M.A.-A. Mamun, S. Nasren, K. Rakesh, B.T.N. Kumar, P.B. Abhiman, A.S. Khandagale (2020) Nano-selenium supplementation to ameliorate nutrition physiology, immune response, antioxidant system and disease resistance against Aeromonas hydrophila in monosex Nile tilapia (Oreochromis niloticus), Biological trace element research
Bitiren M, Karakılçık AZ, Zerin M, Aksoy N, Musa D (2004) Effects of selenium on histopathological and enzymatic changes in experimental liver injury of rats. Exp Toxicol Pathol 56(1–2):59–64
Atencio L, Moreno I, Jos Á, Prieto AI, Moyano R, Blanco A, Cameán AM (2009) Effects of dietary selenium on the oxidative stress and pathological changes in tilapia (Oreochromis niloticus) exposed to a microcystin-producing cyanobacterial water bloom. Toxicon 53(2):269–282
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54(4):287–293
Foster LH, Sumar S (1997) Selenium in health and disease: a review. Crit Rev Food Sci Nutr 37(3):211–228
Köhrle J, Brigelius-Flohé R, Böck A, Gärtner R, Meyer O, Flohé L (2000) Selenium in biology: facts and medical perspectives. Biol Chem 381(9–10):849–864
Cotter PA, Craig SR, McLean E (2008) Hyperaccumulation of selenium in hybrid striped bass: a functional food for aquaculture? Aquac Nutr 14(3):215–222
Wang C, Lovell RT (1997) Organic selenium sources, selenomethionine and selenoyeast, have higher bioavailability than an inorganic selenium source, sodium selenite, in diets for channel catfish (Ictalurus punctatus). Aquaculture 152(1):223–234
Jovanovic A, Grubor-Lajsic G, Djukic N, Gardinovacki G, Matic A, Spasic M (1997) The effect of selenium on antioxidant system in erythrocytes and liver of the carp (Cyprinus carpio L.). Crit Rev Food Sci Nutr 37(5):443–8
Draper HH, Squires EJ, Mahmoodi H, Wu J, Agarwal S, Hadley M (1993) A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radical Biol Med 15(4):353–363
Gatta P (2000) Testi, Vignola, Monetti, The influence of different levels of dietary vitamin E on sea bass Dicentrarchus labrax flesh quality. Aquac Nutr 6(1):47–52
Sieja K, Talerczyk M (2004) Selenium as an element in the treatment of ovarian cancer in women receiving chemotherapy. Gynecol Oncol 93(2):320–327
Talas ZS, Orun I, Ozdemir I, Erdogan K, Alkan A, Yilmaz I (2008) Antioxidative role of selenium against the toxic effect of heavy metals (Cd+2, Cr+3) on liver of rainbow trout (Oncorhynchus mykiss Walbaum 1792). Fish Physiol Biochem 34(3):217–222
Lin Y-H, Shiau S-Y (2007) The effects of dietary selenium on the oxidative stress of grouper Epinephelus malabaricus, fed high copper,. Aquaculture 267(1–4):38–43
Biller-Takahashi JD, Takahashi LS, Mingatto FE, Urbinati EC (2015) The immune system is limited by oxidative stress: dietary selenium promotes optimal antioxidative status and greatest immune defense in pacu Piaractus mesopotamicus. Fish Shellfish Immunol 47(1):360–367
Khan K, Zuberi A, Nazir S, Fernandes JB, Jamil Z, Sarwar H (2016) Effects of dietary selenium nanoparticles on physiological andbiochemical aspects of juvenile Tor putitora. Turkish J Zool 40:704–712
Khan KU, Zuberi A, Nazir S, Ullah I, Jamil Z, Sarwar H (2017) Synergistic effects of dietary nano selenium and vitamin C on growth, feeding, and physiological parameters of mahseer fish (Tor putitora). Aquaculture Reports 5:70–75
Ali M, Soltanian S, Akbary P, Gholamhosseini A (2018) Growth performance and lysozyme activity of rainbow trout fingerlings fed with vitamin E and selenium, marjoram (Origanum spp.), and ajwain (Trachyspermum ammi) extracts. J Appl Anim Res 46(1):650–660
Ran C, Huang L, Liu Z, Xu L, Yang Y, Tacon P, Auclair E, Zhou ZJPO (2015) A comparison of the beneficial effects of live and heat-inactivated baker’s yeast on Nile tilapia: suggestions on the role and function of the secretory metabolites released from the yeast. PLoS One 10(12):e0145448
Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI (2016) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol 57:170–178
Secombes CJ, Fletcher TC (1992) The role of phagocytes in the protective mechanisms of fish. Annu Rev Fish Dis 2:53–71
Kumar N, Singh NP (2019) Effect of dietary selenium on immuno-biochemical plasticity and resistance against Aeromonas veronii biovar sobria in fish reared under multiple stressors. Fish Shellfish Immunol 84:38–47
Kumar N, Krishnani KK, Singh NP (2018) Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ Sci Pollut Res Int 25(9):8914–8927
Shah BR, Mraz J (2020) Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac 12(2):925–942
Raakovj B, Stankovj M, Markovic Z, Poleksi V (2011) Histological methods in the assessment of different feed effects on liver and intestine of fish. J Agric Sci Belgr 56:87–100
Lauriano E, Pergolizzi S, Capillo G, Kuciel M, Alesci A, Faggio C (2016) Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol 59:250–255
Pirarat N, Pinpimai K, Endo M, Katagiri T, Ponpornpisit A, Chansue N, Maita MJRivs (2011) Modulation of intestinal morphology and immunity in nile tilapia (Oreochromisniloticus) by Lactobacillus rhamnosus GG. Res Vet Sci 91(3):e92–e97
Shenkin A (2006) The key role of micronutrients. Clin Nutr 25(1):1–13
Lavu RV, Van De Wiele T, Pratti VL, Tack F, Du Laing G (2016) Selenium bioaccessibility in stomach, small intestine and colon: comparison between pure Se compounds Se-enriched food crops and food supplements. Food Chem 197(Pt A):382–387
Hur S-W, Kim S-K, Kim D-J, Lee B-I, Park S-J, Hwang H-G, Jun J-C, Myeong J-I, Lee C-H, Lee Y-D (2016) Digestive physiological characteristics of the Gobiidae: - characteristics of CCK-producing cells and mucus-secreting goblet cells of stomach fish and stomachless fish. Dev Reprod 20(3):207–217
Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GMH, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EEL, Wising C, Johansson MEV, Hansson GC (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260(1):8–20
Choct M (2009) Managing gut health through nutrition. Br Poult Sci 50(1):9–15
Wang X-H, Hong X, Zhu L, Wang Y-T, Bao J-P, Liu L, Wang F, Wu X-T (2015) Tumor necrosis factor alpha promotes the proliferation of human nucleus pulposus cells via nuclear factor-κB, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. Exp Biol Med (Maywood) 240(4):411–417
Bierie B, Moses HL (2010) Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev 21(1):49–59
Dinarello CA (2018) Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281(1):8–27
Author information
Authors and Affiliations
Contributions
Conceptualization: Sayed Hegazi, Amr.I.Zaineldin, Sameh Ghaniem; data curation: Amr.I.Zaineldin; formal analysis: Sayed Hegazi, Sameh Ghaniem, Abdulnasser Bakr, Eldsokey Nassef, Amr.I.Zaineldin; investigation: Sameh Ghaniem, Abdulnasser Bakr, Sayed Hegazi, Amr.I.Zaineldin; methodology: Sayed Hegazi, Amr.I.Zaineldin; project administration: Amr.I.Zaineldin; resources: Sayed Hegazi, Abdulnasser Bakr, Eldsokey Nassef, Amr.I.Zaineldin; supervision: Sayed Hegazi, Abdulnasser Bakr, Amr.I.Zaineldin; validation: Amr.I.Zaineldin; writing—original draft: Amr.I.Zaineldin; writing—review and editing: Sayed Hegazi, Amr.I.Zaineldin. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experimental procedure was authorized by the ethics review board of the Institutional Animal Care and Use Committee in Kafrelsheikh University (Kafrelsheikh, Egypt).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghaniem, S., Nassef, E., Zaineldin, A.I. et al. A Comparison of the Beneficial Effects of Inorganic, Organic, and Elemental Nano-selenium on Nile Tilapia: Growth, Immunity, Oxidative Status, Gut Morphology, and Immune Gene Expression. Biol Trace Elem Res 200, 5226–5241 (2022). https://doi.org/10.1007/s12011-021-03075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03075-5