Abstract
Zinc is a trace metal and acts as an active component of various enzymes. Zinc deficiency has been suggested to be associated with the development of diabetes. The present study investigated the role of zinc supplementation on prevention of diabetic conditions. A double-disease model mimicking hyperlipidemia and type 2 diabetes was created by applying high-fat diet and streptozotocin (STZ) to Wistar rats. We demonstrated that zinc supplementation improved symptoms of diabetes such as polydipsia and increased serum level of high-density lipoprotein cholesterol, indicating that zinc supplementation has a potential beneficial effect on diabetic conditions. The level of maldondialdehyde (MDA), an oxidative stress marker, was reduced in liver by zinc supplementation in high fat-fed rats with or without STZ injection. Meanwhile, we observed an increase in the expression of metallothioneins (MTs) in liver of rats treated with zinc. This suggests that the induction of MTs in liver, which has been shown to be important in scavenging free radicals, could be one of the underlying mechanisms of zinc supplementation on reducing MDA levels in liver. Finally, we found that zinc levels in liver were increased while there was no change in serum zinc levels, indicating that local zinc level might be a critical factor for the induction of MTs. Also, the level of MTs could potentially be an index of zinc bioavailability. Taken together, these results suggest that both zinc and MT could play an important role in balancing nutrition and metabolism to prevent diabetic development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a global public health problem which not only has a negative impact on the quality of life but also places a heavy financial burden to health care systems worldwide [27]. The prevalence of diabetes in China has been markedly increased during the last decade [31]. Type 2 diabetes, the most common form of diabetes, is mainly manifested as hyperglycemia and often associated with a cluster of metabolic disorders such as dyslipidemia and increased oxidative stress [12, 19]. The development of diabetes involves a chronic process which may be delayed or prevented through nutritional or lifestyle interventions.
Zinc is a trace metal and acts as an active component of various enzymes. Zinc deficiency has been suggested to be associated with the development of diabetes [14], possibly due to its essential roles in the process of crystallization and secretion of insulin. Metallothioneins (MTs), a family of small molecular (less than 10 kDa), cysteine-rich proteins, binds metals such as zinc and copper and acts as an antioxidant to scavenge or quench various free radicals or reactive oxygen species [30]. Zinc has been reported to have a physiological capability to induce MTs [2].
Despite the potential therapeutic benefit of zinc supplementation in the prevention and treatment of DM has been reported, debates still exist, not devoid of contradictory results [6, 9, 20]. Therefore, more studies will be necessary to corroborate the effects of zinc supplementation and to explore specific molecular targets of zinc, which will provide more information and choice for patients who might really benefit from zinc supplementation.
Our study investigated the effects of zinc supplementation during the development of diabetes using a double-disease model mimicking hyperlipidemia and diabetes and explored whether zinc administration through alimentary tract could alleviate diabetic symptoms and diabetes-associated metabolic disorders such as dyslipidemia and oxidative stress by inducing MT proteins in liver.
Materials and methods
Chemicals
Streptozotocin (STZ) was obtained from Sigma (St. Louis, MO, USA). Streptavidin-biotin complex (SABC) kit and diaminobenzidine (DAB) were purchased from Boston Corp (Wuhan, China). Mouse anti-MT antibody and secondary goat anti-mouse antibody were obtained from Beijing Zhong Shan-Golden Bridge Biological Technology (Beijing, China). Maldondialdehyde (MDA) kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nianjing, China). ZnSO4·7H2O and cholesterol was purchased from Shenyang First Chemical Reagent Factory (Shenyang, China), and all other chemicals used were of analytical grade.
Diet
The normal diet consisting of the whole value grain (GB19429.3-2001) was purchased from the Animal Facilities of Jilin University (Changchun, China). The major contents of the diet include crude protein, ≥20 %; crude fat, ≥4.0 %; crude fiber, ≤5.0 %; crude ash, ≤8.0 %; water, ≤10 %; calcium, 1.0∼1.8 %; phosphorus, 0.6∼1.2 %; iron, ≥120 mg/kg; copper, 10 mg/kg∼250 mg/kg; zinc, ≥30 mg/kg; and manganese, ≥75 mg/kg.
High-fat diet contains primarily the normal diet (88.8 %) supplemented with 10 % maize oil, 1.0 % cholesterol, and 0.2 % bile salts.
Animals and experimental design
The study had approval of Animal Ethical Committee from Jilin University. Male Wistar rats (180∼220 g, n = 76) were provided by the Animal Experimental Center of the Bethune Medical School of Jilin University (China). The experimental design was shown in the Table 1.
In this study, most of the Wistar rats with high-fat diet became hyperlipidemic (except for three rats) characterized by increased serum levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterols (LDL-C) after feeding with high-fat diet for 35 days. Furthermore, intraperitoneal injection of STZ (40 mg/kg) in the rats with high-fat diet successfully induced hyperglycemia (the fasting glucose level is above 16.7 mmol/L) and typical diabetes symptoms such as polydipsia, polyphagia and weight loss. So, there were 71 rats at the end of the experiment (except two rats died of operation and three rats failed to show hyperlipidemia). All rats were divided into six groups: normal-diet control (NC; n = 10), high-fat diet (FC; n = 9); high-fat diet with STZ-induced diabetes (DM; n = 13); high-fat diet with ZnSO4 treatment group (FZ; n = 14); rats with high-fat diet treated with ZnSO4 before the STZ injection (ZD group; n = 12); and rats with high-fat diet treated with ZnSO4 after the STZ injection (DZ group; n = 13). The rats in FZ and ZD groups were treated with ZnSO4 solution at 15 mg/kg daily by intragastric administration, and the others were treated with distilled water.
Collection of blood and tissue samples
Blood samples were collected according to a standard protocol, stored in the refrigerator at 4°C for 1.5 h and then centrifuged at l,000 rpm for 5 min, and serum samples were collected and stored at −80°C until analysis. Liver samples of all rats were isolated and frozen in liquid N2 and stored at −80°C for pathological examination and determination of zinc levels.
Plasma lipids and glucose measurements
Serum TC, TG, LDL-C, and HDL-C were determined by the HITACHI 7060 Automatic Analyzer (Tokyo, Japan). Blood glucose was measured by Arkray Glucocard 01-Mini Blood Glucose Monitoring System (Kyoto, Japan). Radioimmuneassay (RIA) was used to examine insulin level by SN-695 Intelligent Measuring Instrument of γ RIA (Shanghai, China).
MDA measurement
The levels of MDA in liver were determined by thiobarbituric acid reactive substances (TBARS) assay. In brief, liver homogenates (10 %) was incubated with thiobarbituric acid (TBA) for 15 min at room temperature and the formation of MDA-TBA adduct was measured at 532 nm. The levels of MDA were then calculated according to a standard curve.
Immunohistochemistry
The presence of MTs in liver tissue was determined by immunohistochemistry. Liver samples were cut into 5-μm thick sections, deparaffinized with xylene, and rehydrated. After soaking in 3 % H2O2 for 10 min at room temperature, the sections were immersed in 0.01 M citrate (pH = 6.0) and incubated in microwave at 500 W for 10 min for antigen retrieval. After washing twice with PBS (pH = 7.5), the sections were then incubated with mouse anti-MT antibody at 1:1,000 dilutions in Tris-buffered saline (TBS) containing 3 % BSA and 0.1 % Tween 20 (Beyotime Institute of Biotechnology, Shanghai, China) for 10 h at 4°C. The sections were then incubated with biotinylated anti-mouse antibody at 1:2,000 in TBS for 20 min at room temperature. Then, the sections were reacted with SABC for 20 min after wash. The color development of DAB and hematoxylin was captured by a pm 10AD scan (Olympus, Japan). Five high-powered fields were chosen, and the positive cells (shown as brown nucleus) were counted and expressed as the signal integral optical density detected by the Image Pro Plus 5.0 imaging analysis software.
Atomic absorption spectrometry
Zinc levels in serum and liver were measured by atomic absorption spectrometry. Liver samples (∼0.6 g) were digested in 1.0 ml HNO3, 0.5 ml H2O2, and 6 ml H2O. After digestion, the samples were treated with 5 % vitamin C and 5 % sulfocarbamide to prevent oxidation. For serum, the samples were diluted at 1:5 ratios with distilled water. The concentrations of zinc in liver and serum samples were then measured by an atomic absorption spectrometer (TAS-986, Beijing, China) and calculated according to a series of dilution of ZnSO4 solution.
Statistical analysis
Data are expressed as mean ± SD and analyzed by SPSS 13.0 statistical software. Differences between groups were determined by one-way ANOVA followed by least significant difference comparison. The level of statistical significance was set at P < 0.05.
Results
Effect of zinc supplementation on food intake, water intake, and body weight
There was no significant change in food intake, water intake, and body weight shortly after STZ treatment in DM, ZD, and DZ groups. On the 14th day after STZ injection, rats in DM group showed a dramatic increase in both food intake and water intake (P < 0.01) compared with the FC group, indicating the symptoms of polydipsia and polyphagia caused by diabetes. Compared with the DM group, food intake of the DZ group (P < 0.05) was increased and water intake of the ZD group was reduced (P < 0.01) as shown in Fig. 1a, b. As shown in Fig. 1c, rats in the FZ, DM, ZD, and DZ groups all showed a reduction of body weight compared with the FC group (P < 0.05).
Effect of zinc supplementation on food intake, water intake and body weight. Rats were fed and treated as NC, FC, DM, FZ, ZD, and DZ groups according to the study design. The daily food intake, water intake and body weight were recorded. Average food intake (a), water intake (b), and body weight (c) were calculated. Data are means ± SD. *P < 0.05; **P < 0.01 vs. NC; # P < 0.05; ## P < 0.01 vs. DM; ∆ P < 0.05; ∆∆ P < 0.01 vs. FC. Abbreviations and “n” values as in Table 1
Effect of zinc supplementation on serum lipid profiles
To investigate whether zinc could play a role in lipid metabolism during high-fat diet without and with diabetic conditions, serum TC, TG, LDL-C, and HDL-C levels were determined. As shown in Fig. 2a–d, rats in the FC and FZ group showed a significant increase in the TC, TG, and LDL-C levels (all P < 0.05) while there was no change in the HDL-C levels.
Effect of zinc supplementation on serum lipid profiles. Rats were fed and treated as NC, FC, DM, FZ, ZD, and DZ groups according to the study design, and blood were collected on day 35. Serum levels of total cholesterol (TC) (a), triglycerides (TG) (b), LDL-C (c), and HDL-C (d) were determined by the HITACHI 7060 Automatic Analyzer. Data are means ± SD. *P < 0.05; **P < 0.01 vs. NC and FC; # P < 0.05 vs. DM. Abbreviations and “n” values as in Table 1
After STZ injection, rats in the DM group showed a marked increase in the TC, TG, LDL-C, and HDL-C levels compared with the NC and FC group (all P < 0.01). Furthermore, compared with the DM group, rats in the ZD and DZ groups showed a further increase in the TG levels (both P < 0.05) (Fig. 2b) while rats in the DZ group showed a significant increase in the HDL-C levels (3.62 ± 2.73 vs. 2.44 ± 1.16 mmol/l; P < 0.05) (Fig. 2d).
Effect of zinc supplementation on fasting blood glucose and serum insulin level
To investigate the effects of zinc supplementation on diabetes parameters, fasting glucose and serum insulin levels were determined. No significant change was observed shortly after STZ treatment in the DM, ZD, and DZ groups. The values rose markedly in the DM group compared with NC and FC groups from the 7th day after STZ injection (P < 0.01). Rats in the ZD and DZ groups remained hyperglycemic (Fig. 3a).
Effect of zinc supplementation on fasting glucose levels (a) and serum insulin levels (b). Rats were fed and treated as NC, FC, DM, FZ, ZD, and DZ groups according to the study design, and glucose levels were measured by the HITACHI 7060 Automatic Analyzer. Insulin levels were measured by radioimmunoassay. Data are means ± SD. **P < 0.01 vs. NC and FC; # P < 0.05 vs. DM. Abbreviations and “n” values as in Table 1
For serum insulin levels, FC did not affect insulin levels compared with rats in NC group (Fig. 3b). Compared with the NC and FC groups, rats in the FZ group showed a significant reduction in serum insulin levels (both P < 0.01). Similarly, rats in the DM and ZD groups showed a lower serum insulin level (both P < 0.01). Rats in the DZ group showed a further reduction of serum insulin level compared with the DM group (P < 0.05).
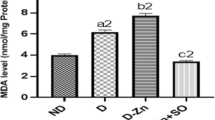
Effect of zinc supplementation on MDA levels in liver
To investigate the effects of zinc supplementation on oxidative stress, the MDA levels in liver from rats with various treatments were determined. As shown in Fig. 4, rats in the FC group showed an increase in MDA contents in liver (P < 0.01) compared with the NC group. Similarly, rats in the DM group also showed a significant increase (P < 0.01). The MDA levels of rats in the FZ group were significantly reduced compared with the FC group (by 2-fold, P < 0.01) and were markedly reduced in the ZD and DZ groups compared with the DM and FC groups (both P < 0.01).
Effect of zinc supplementation on liver MDA levels. Rats were fed and treated as NC, FC, DM, FZ, ZD, and DZ groups according to the study design, and liver tissue were collected and processed on STZ 14 days. MDA levels were measured by TBARS assay. Data are means ± SD. **P < 0.01 vs. NC and FC; ## P < 0.01 vs. DM; ∆∆ P < 0.01 vs. FC. Abbreviations and “n” values as in Table 1
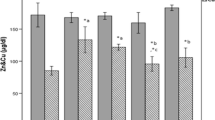
Effect of zinc supplementation on zinc levels in serum and liver
To explore the effects of zinc supplementation on zinc metabolism, zinc levels in serum and liver were determined.
As shown in Fig. 5a, high-fat diet did not affect serum zinc levels (FC) compared with the NC group. Zinc supplementation, however, reduced serum zinc levels of rats in the FZ group (P < 0.01). A deficiency in serum zinc levels was observed in the DM group after STZ injection, and no statistical difference was detected in zinc supplementation groups (ZD and DZ) compared with the DM group. But a significant reduction of serum zinc levels were observed in both the ZD and DZ groups when compared with the FC group (both P < 0.05).
Effect of zinc supplementation on serum and liver zinc levels. Rats were fed and treated as NC, FC, DM, FZ, ZD, and DZ groups according to the study design, and blood and liver samples were collected and processed on STZ 14 days. Zinc levels in serum (a) and liver (b) were measured by atomic absorption spectrometry. Data are means ± SD. *P < 0.05; **P < 0.01 vs. NC and FC; # P < 0.05 vs. DM; ∆ P < 0.05; ∆∆ P < 0.01 vs. FC. Abbreviations and “n” values as in Table 1
Zinc levels in liver were shown as Fig. 5b. Compared with the NC, high-fat diet significantly reduced zinc contents (P < 0.05) and rats in the DM group also showed a trend of reduction (P > 0.05). Zinc supplementation (FZ, ZD, and DZ) reversed the downward trend of zinc levels in the FC group (P < 0.01, P < 0.05, and P < 0.01). Meanwhile, zinc levels were increased with zinc supplementation after STZ injection (DZ) compared with the DM group (P < 0.05).
Effect of zinc supplementation on MT protein expression in liver
MT protein expression was determined by immunohistochemistry as the brown staining and mainly localized in hepatic lobules (Fig. 6a). The number of MT positive cells was determined and expressed as percentages of MT-positive area as shown in Fig. 6b. Overall, the DM, ZD, and DZ groups showed a higher percentage of MT positive cells and there was an increase of MT-positive cells in the ZD and DZ groups compared with the DM group.
Effect of zinc supplementation on MT protein expression in liver. Rats were fed and treated as NC, FC, DM, FZ, ZD, and DZ groups according to the study design, and liver were collected at the end of the experimentation. The immunohistochemical staining of the MT protein in each group (magnification, ×200) (a). The distribution of MT protein was shown as brown staining and quantified as proportions (in percent) (b). The number of MT-positive cells in liver histology was quantified by computerized image pattern analysis, and MT protein expressions in each group was expressed as the grey density (c). Data are means ± SEM for groups of six. **P < 0.01 vs. NC and FC; # P < 0.05 vs. DM
MT protein expressions in liver was semi-quantified as computer-based grey value. As shown in Fig. 6c, there was a further increase in MT protein expressions in DM group (P < 0.01) compared with the NC and FC groups. But it is important to note that there was a significant increase of MT protein expressions in DZ group compared with the DM group (P < 0.01).
Discussion
DM is characterized by disordered metabolism of glucose, lipids and proteins. The present study investigated the effects of zinc supplementation on type 2 diabetes parameters and liver MT expressions in Wistar rats.
The food intake in the DZ group was increased by zinc supplementation compared with the DM group after STZ injection, which indicated that zinc could have a potential role in increasing food intake. Zinc deficiency is associated with a profound anorexia in experimental animals and orally administration of zinc stimulates food intake by increasing the expression of orexigenic peptides [29]. Furthermore, the study also found that zinc supplementation (ZD) reduced water intake compared with the DM group, suggesting that pretreatment with zinc before STZ injection could improve polydipsia in diabetic conditions.
Since fasting blood glucose and insulin levels are critical parameters for determination of diabetic conditions, the study showed a significant increase in fasting glucose and reduction in insulin levels after STZ injection(40 mg/kg), indicating a diabetic condition induced by STZ. This is consistent with the findings reported by other groups that STZ injection could induce the type 2 diabetes in rat models at 40 mg/kg [13, 18], 35 mg/kg [15], 30 mg/kg [33], and other doses[17]. Though the glucose values of rats with zinc supplementation (ZD and DZ) took on a relative moderately downward trend compared with the DM group, no significant differences were found among the three groups. This result was similar to an earlier research showing that zinc supplementation on diabetic patients had no beneficial effects on glucose metabolism [32] but inconsistent with the majority of published results showing zinc supplementation was favorable to reducing the blood glucose level. Probably, different result will be obtained when given the prolongation of observation period or adjusted dose of STZ. Besides, the mechanism of cooperation between zinc and other elements should be another key aspect to be taken into account. So, we could not simply deny the benefits of zinc supplementation in prevention and treatment of DM.
In addition, there was an inhibitory effect on serum insulin levels of the rats with zinc treatment compared with controls. This is similar to a previous study which reported that zinc supplementation significantly reduced the fasting plasma levels of glucose and insulin in ob/ob mice [8] due to a direct effect of zinc on attenuating pancreatic insulin secretion or indirectly stimulated peripheral tissue unitization of insulin [5]. Meanwhile, it is reported that [28] inhibiting insulin secretion could help prevent diabetes. Zinc could help to prevent diabetes, partly because of its own insulin activity. And zinc supplementation could ensure that insulin-producing β-cells in the pancreas do not lose too much zinc. Besides, another report [3] has shown that MT in insulin target tissues, subcellular partitioning and signaling of zinc could suffer loss of function because cysteine accounts for ≈35 % of the amino acids, which contribute to altered insulin responsiveness. And the indiscriminate release of zinc caused by homocysteine and insulin secretion needs to be studied further.
Another interesting observation in this study was that the HDL-C level of rats in the DZ group was increased significantly compared with the DM group. This was in consistence with a previous study showing that serum HDL-C levels were increased by zinc supplementation in diabetic rats [22]. Besides, another study on patients with type 2 diabetes verified that there was no adverse effects of zinc supplementation on HDL-C levels [1]. Early report had found that the low-baseline HDL cholesterol was seen commonly in coronary heart disease [4]. Meanwhile, diabetes, as an important component of the complex of common cardiovascular risk factors, was responsible for acceleration and worsening of atherothrombosis [10]. It was found that lower levels of HDL-C might be an indicator and provided additional information regarding the severity of coronary artery disease compared with other lipidemic factors [24]. And the level of HDL-cholesterol was found to be able to predict diabetes incidence in a multivariate model [9]. In general, higher HDL-C levels could be seen as an important parameter of potential benefits from zinc supplementation for type 2 diabetes.
The study showed that oxidative stress marker MDA levels in liver were significantly reduced by zinc treatment, which was associated with an increase in liver zinc levels and MTs protein expressions. Oxidative stress has an important intermediary effect on the development of diabetic complications [11, 25]. It has been reported that the progressive damage of pancreases and disturbance of lipid metabolism enhanced lipid peroxidation in liver of rat models [21]. In the present study, we demonstrated that both hyperlipidemia with high-fat diet and STZ-induced diabetes (DM) were associated with an increase in lipid peroxidation measured as MDA content, indicating an oxidative status. With zinc supplementation, MDA levels of rats with high-fat diet were significantly reduced. Previous study has reported that zinc supplementation had a potential beneficial effect on type 2 diabetes patients attributed by its antioxidant property [26]. In addition, we also observed an increase in MT protein expressions in liver of zinc-supplemented rats (DZ). This is in consistence with a previous report that the expression of MT was increased in liver after zinc supplementation [7]. The synthesis of MT proteins has been proven to be induced by zinc; probably both MT and zinc could play important roles in balancing nutrition and metabolism to prevent some diabetic complications [23]. Therefore, we postulated that the induction of MT proteins in liver, which have been shown to be important in scavenging free radicals [16], could be one of the underlying mechanisms of zinc supplementation on reducing MDA levels in liver. Furthermore, we determined the zinc content in both serum and liver. Although there was no change in serum zinc levels, liver zinc levels in DZ group were increased significantly compared with DM group, which could be a possible explanation for the higher MT proteins level in DZ group than DM group. Also, MT in liver could be potentially used as an index of zinc bioavailability.
The results showed that the effect of zinc supplementation before or after STZ injection was not incompletely accordant, but both do good to animals with hyperlipidemia and diabetes. In conclusion, the present study could verify the beneficial effects of zinc supplementation. Zinc supplementation alleviated diabetes-associated polydipsia, increased serum HDL-C levels and reduced liver lipid peroxidation, which was associated with an increase in the levels of both zinc and MT proteins in liver. Furthermore, the beneficial effect of zinc might be presented by its capability to induce MTs, which has been shown to be important in scavenging free radicals. Therefore, zinc supplementation could be seen a potential adjuvant therapy for diabetes.
References
Anderson RA, Roussel AM, Zouari N, Mahjoub S, Matheau JM, Kerkeni A (2001) Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus Journal of the American. J Am Coll Nutr 20:212–218
Baraboy VA, Petrina LG (2003) Metallothioneins: the structure and action mechanisms. Ukr Biokhim Zh 75:28–36
Barbato JC, Catanescu O, Murray K, DiBello PM, Jacobsen DW (2007) Targeting of metallothionein by l-homocysteine: a novel mechanism for disruption of zinc and redox homeostasis. Arterioscler Thromb Vasc Biol 27:49–54
Barter PJ, Rye KA (1996) High density lipoproteins and coronary heart disease. Atherosclerosis 121:1–12
Begin-Heick N, Dalpe-Scott M, Rowe J, Heick HM (1985) Zinc supplementation attenuates insulin secretory activity in pancreatic islets of the ob/ob mouse. Diabetes 34:179–184
Beletate V, El Dib RP, Atallah AN (2007) Zn supplementation for the prevention of type 2 diabetes mellitus. Cochrane Database Syst Rev 24: CD005525
Cao J, Henry PR, Davis SR, Cousins RJ, Miles RD, Littell RC, Ammerman CB (2002) Relative bioavailability of organic zinc sources based on tissue zinc and metallothionein in chicks fed conventional dietary zinc concentrations. Anim Feed Sci Tech 101:161–170
Chen MD, Liou SJ, Lin PY, Yang VC, Alexander PS, Lin WH (1998) Effects of zinc supplementation on the plasma glucose level and insulin activity in genetically obese (ob/ob) mice. Biol Trace Elem Res 61:303–311
Chien K, Cai T, Hsu H, Su T, Chang W, Chen M, Lee Y, Hu FB (2009) A prediction model for type 2 diabetes risk among Chinese people. Diabetologia 52:443–450
Coccheri S (2007) Approaches to prevention of cardiovascular complications and events in diabetes mellitus. Drugs 67:997–1026
Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S (2006) Antioxidant supplementation does not affect fasting plasma glucose in the supplementation with antioxidant vitamins and minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr 84:395–399
Deedwania P (2011) Hypertension, dyslipidemia, and insulin resistance in patients with diabetes mellitus or the cardiometabolic syndrome: benefits of vasodilating beta-blockers. J Clin Hypertens 13:52–59
Erdal N, Gurgul S, Kavak S, Yildiz A, Emre M (2011) Deterioration of bone quality by streptozotocin (STZ)-induced type 2 diabetes mellitus in rats. Biol Trace Elem Res 140:342–353
Grungreiff K, Reinhold D (2005) Liver cirrhosis and "liver" diabetes mellitus are linked by zinc deficiency. Med Hypotheses 64:316–317
Hussein AM, Omar NM, Sakr H, Elsamanoudy AZ, Shaheen D (2011) Modulation of metabolic and cardiac dysfunctions by insulin sensitizers and angiotensin receptor blocker in rat model of type 2 diabetes mellitus. Can J Physiol Pharmacol 89:216–226
Irato P, Santovito G, Piccinni E, Albergoni V (2001) Oxidative burst and metallothionein as a scavenger in macrophages. Immunol Cell Biol 79:251–254
Ismael PH, Yesica AF, Ricardo MZ (2010) Analysis of the membrane fluidity of erythrocyte ghosts in diabetic, spontaneously hypertensive rats. Acta Diabetologica 47:47–55
Kehkashan P, Rashid Khan M, Mujeeb M, Siddiqui WA (2010) Protective effects of Pycnogenol (R) on hyperglycemia-induced oxidative damage in the liver of type 2 diabetic rats. Chem Biol Interact 186:219–227
Lastra G, Manrique C (2007) The expanding role of oxidative stress, renin angiotensin system, and beta-cell dysfunction in the cardiometabolic syndrome and type 2 diabetes mellitus. Antioxid Redox Signal 9:943–954
Marreiro DN, do Perpetuo Socorro C, Martins M, de Sousa SS, Ibiapina V, Torres S, Pires LV, do Nascimento Nogueira N, Lima JM, do Monte SJ (2007) Urinary excretion of zinc and metabolic control of patients with diabetes type 2. Biol Trace Elem Res 120:42–50
Matsunami T, Sato Y, Sato T, Ariga S, Shimomura T, Yukawa M (2010) Oxidative stress and gene expression of antioxidant enzymes in the streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol 3:177–188
Meika F, Peter P, Samir S (2010) Effects of zinc on plasma lipoprotein cholesterol concentrations in humans: a meta-analysis of randomised controlled trials. Atherosclerosis 210:344–352
Meika F, Samir S (2010) Zinc and redox signaling: perturbations associated with cardiovascular disease and diabetes mellitus. Antioxid Redox Signal 13:1549–1573
Mitsutake R, Miura SI, Zhang B, Saku K (2010) HDL-associated factors provide additional prognostic information for coronary artery disease as determined by multi-detector row computed tomography. Int J Cardiol 143:72–78
Pan HZ, Zhang L, Guo MY (2010) The oxidative stress status in diabetes mellitus and diabetic nephropathy. Acta Diabetol 47:71–76
Roussel AM, Kerkeni A, Zouari N, Mahjoub S, Matheau JM, Anderson RA (2003) Antioxidant effects of zinc supplementation in Tunisians with type 2 diabetes mellitus. J Am Coll Nutr 22:316–321
Souto SB, Souto EB, Braga DC, Medina JL (2011) Prevention and current onset delay approaches of type 2 diabetes mellitus (T2DM). Eur J Clin Pharmacol 67:653–661
Sprietsma JE, Schuitemaker GE (1994) Diabetes can be prevented by reducing insulin production. Med Hypotheses 42:15–23
Suzuki H, Asakawa A, Li JB, Tsai M, Amitani H, Ohinata K, Komai M, Inui A (2011) Zinc as an appetite stimulator—the possible role of zinc in the progression of diseases such as cachexia and sarcopenia. Recent Pat Food Nutr Agric 3:226–231
Viarengo A, Burlando B, Ceratto N, Panfoli I (2000) Antioxidant role of metallothioneins: a comparative overview. Cell Mol Biol 46:407–417
Wong KC, Wang ZQ (2006) Prevalence of type 2 diabetes mellitus of Chinese populations in Mainland China, Hong Kong, and Taiwan. Diabetes Res Clin Pract 73:126–134
Wu YT, Sun Z, Che SP, Chang H (2004) Effects of zinc and selenium on the disorders of blood glucose and lipid metabolism and its molecular mechanism in diabetic rats. Wei Sheng Yan Jiu 33:70–73
Yu M, Zhou W, Song Y, Yu F, Li D, Na S, Zou G, Zhai M, Xie C (2011) Development of mesenchymal stem cell-implant complexes by cultured cells sheet enhances osseointegration in type 2 diabetic rat model. Bone 49:387–394
Acknowledgment
Our work was supported by the National Science Foundation of China (30370669).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Li, H., Fan, Z. et al. Effect of zinc supplementation on type 2 diabetes parameters and liver metallothionein expressions in Wistar rats. J Physiol Biochem 68, 563–572 (2012). https://doi.org/10.1007/s13105-012-0174-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0174-y