Abstract
Andrographis paniculata (Burm. F.) Nees is a rich source of biologically active andrographolide and other diterpene lactone compounds. The in vitro adventitious root culture system is an improved alternative strategy for mass cultivation of the plants and enhanced production of secondary metabolites in general and diterpene lactones in particular. The present study was initially envisaged, to find out how two different andrographolide biosynthetic pathways (MVA and MEP) contribute towards the accumulation of andrographolide under the influence of light. In addition, the effect of different liquid culture techniques were evaluated for enhanced biomass and elicitation facilitated andrographolide production. The culturing of adventitious roots on a platform and by not immersing directly in the liquid medium was found best for improved biomass generation. With 1.23 g of initial inoculum were able to generate 4.45 fold (5.48 g) biomass in just 4 weeks by this modified liquid culturing technique. Further, the elicitation of adventitious roots with ethrel enhanced both biomass (up to fivefold) and andrographolide content (fourfold). The differential gene expression results indicated that, under the influence of light, the MEP pathway was found to dominate for the biosynthesis of andrographolide by the up regulation of the DXR, DXS, HDR, HDS genes as well as down stream GGPS gene expression. In absence of light, MVA pathway was observed as a major contributor for the production of andrographolide. The andrographolide content was enhanced upto 4.29 fold in light elicited adventitious roots compared to the control roots (grown in the absence of light). Our results implied that the light has not only significantly enhanced the MEP pathway gene expression but also inturn elevated the andrographolide accumulation in adventitious root cultures of A. paniculate indicating the role of elicitation and tissue specific gene expression.
Key message
The liquid culture technique, ethrel elicitation promoted enhanced adventitious root biomass (5 fold) and andrographolide content (4 fold). Light has shown significant effect on andrographolide production (4.29 fold) by upregulating the expression of MEP pathway and downstream genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plants are considered as the “Chemical Factory” where enormous number of primary and secondary metabolites are synthesized (Marchev et al. 2020; Kandar. 2021). The potential of plant derived bioactive compounds have been exploited in Indian traditional medicine from the ancient times. Andrographis paniculata, a medicinal herb is native to India and other tropical and subtropical Asian countries (Hossain et al. 2014; Neeraja et al. 2015; Gupta et al. 2019; Verma et al. 2019). A. paniculata (Burm.f.) Nees is considered as “bile of the earth” for its bitterest taste and small size up to a height of 30–110 cm (Subramanian et al. 2012; Hossain et al. 2021). It have been used to treat infectious diseases and respiratory tract infections and is referred to as the next big immune booster (Kumari et al. 2021). It is famous for its herbal formulations such as “Andrograhis Plus” (Metagenics Inc. CA, USA), “Kan Jang” (Swedish Herbal Institute, N.H, USA), “Tribulus Complex” (Jarrow Formulas, LA, USA) and “Stimuliv” (Franco-Indian Pharmaceutical Pvt.Ltd, Mumbai, India). In recent years, the novel uses such as anti-helmintic (Banerjee et al. 2019), excito-repellency (Sukkanon et al. 2019) anti-viral (Li et al. 2020) and anti-snake venom (Nayak et al. 2020) properties of this plant have been assessed. The phytoconstituents and the traditional uses of the plant were recently reviewd in detail by Kumar et al (2021). The diterpene lactones and flavones are the contributors of medicinal properties of this plant. Amongst these, andrographolide (Fig. 1) is the leading compound, in terms of bioactive properties and its abundance (Chao and Lin 2010). Due to the current “Demand and Supply” for A. paniculata plants, the National Medicinal Plant Board (NMPB) has extensively surveyed the herbal market of India in collaboration with The Indian Council of Forestry Research and Education (ICFRE), Dehradun and estimated the consolidated commercial demand of A. paniculata estimated to be 2000–5000 metric tonne (MT) Gupta et al. 2019. The over exploitation of the in situ natural resources for pharmaceutical needs pose a major threat to the plant’s ecological system and biodiversity at large (Devi et al. 2021). In addition, the current natural habitat of the plants is being contaminated with pesticides, herbicides and heavy metals derived from environmental impact and human interventions. The use of these contaminated plants can be harmful to human health (Hossain et al 2014). Thus, the natural production cannot meet the present market demands and quality control guidelines (Rahmat and Kang 2019). In the recent past, through the use of tissue/organ culture techniques and elicitation of in vitro cultures with signal molecules, it has been demonstrated that the amount of diterpene lactones and other bioactive compounds have increased to an accountable extent (Zaheer and Giri. 2015, 2017; Giri and Zaheer 2016; Pandey et al. 2017, 2022; Singh et al. 2018; Das and Bandyopadhyay 2020; Ganapumane and Nagella 2020; Devi et al. 2021; Sun et al. 2021, 2022; Ahmad et al 2022).
The plant roots are considered, a unique site for the synthesis and storage of secondary metabolites. The adventitious root (AR) culture (ARC) is one of the promising plant tissue culture systems poised to increase the production of plant based bioactive compounds (Rahmat and Kang. 2019). The high rate of proliferation, tremendous accumulation potential, stable production of the compounds defying genetic variation is unique to adventitious roots (Anjum et al. 2018). AR was induced in vitro from different plant parts by administering plant growth hormones in the media (Isah et al. 2018; Fathi et al. 2021; Kannan et al. 2021). The incubation of AR under different spectral lights can up regulate the secondary metabolite biosynthetic pathway genes and enhance the production of secondary metabolites (Ali et al. 2019). AR can be grown in the bioreactor to produce amounts of root biomass along with enriched bioactive molecules without producing harmful opine like compounds as observed in hairy roots (Baque et al. 2012). AR synthesize secondary metabolites into their intercellular spaces which can be extracted easily and can be grown with high growth rate even with low inoculum in a phytohormone amended medium (Murthy et al. 2021). Our laboratory is engaged in research initiatives on distribution of A. paniculata and related species, pharmacological study and yield enhancement of bioactive compounds, using biotechnological, genomic, proteomic and bioinformatics approaches (Neeraja et al. 2015; Zaheer and Giri 2015, 2017; Parlapally et al. 2016; Giri and Zaheer 2016; Bindu et al. 2017, 2020; Srinath et al. 2017, 2020, 2021; Shailaja et al. 2018, 2020, 2021). Besides the isolation and characterization of genes (HMGR, DXR and HDR) for diterpene lactone andrographolide biosynthesis, currently we are working on the elucidation and biomass production, synergy between external factors such as light/dark and tissue specific gene expression involving AR of A. paniculata.
In the present study, we established ARC system of A. paniculata from different explants and investigated the effects of different parameter on AR formation as well as andrographolide production. The different liquid culture techniques were established for the AR biomass production. Further, the current study aimed to explore the action of ethrel for the andrographolide accumulation via elicitation.The effect of light other than elicitors on the production of andrographolide and its biosynthetic routes mainly the MVA and MEP pathway genes was analysed.
Materials and methods
Collection of A. paniculata plant material
Andrographis paniculata seeds were collected from CSIR-Central Institute of Medicinal and Aromatic Plants (CSIR-CIMAP) Research Centre, Hyderabad a regional Centre of CSIR-CIMAP, Head Quarters, Lucknow, India. The selected plots in the nethouse of Centre for Plant Molecular Biology (CPMB), Osmania University (OU), Hyderabad, India were cleaned, ploughed thoroughly and supplemented with manure (Soil and Vermicompost 1:1 ratio) prior to seed sowing. The seeds were subsequently sown in the selected plots during March/April. The plots were watered twice in a day for efficient germination with additional supplement of nutrient solution, half strength Hoagland solution (Hoagland and Arnon 1938).
Germination of A. paniculata seeds in vitro
The germination of A. paniculata seeds in vitro was carried out using two types of seeds i.e. immature, light yellow and mature brown seeds. The fresh green fruits/pods containing immature seeds were collected from the net house grown plants during the month of August and September. The pods/fruits were collected and brought to the lab and washed under running tap water. The washed pods were surface sterilized with 0.1% (w/v) HgCl2 for 5 min followed by repeated washing with autoclave sterilized distilled water in the laminar air flow. The surface sterilized pods were dissected using sterile scalpel blade to expose light yellow colour immature seeds and transferred aseptically to full strength Murashige and Skog (1962) medium devoid of plant growth regulator (MSO medium) solidified with 0.8% Agar, pH-5.8. The mature seeds were collected after the pods opened in the month of October/November and were surface sterilized with 0.1% (w/v) HgCl2 for 1 min following a similar protocol as described above. The seeds were then inoculated on to MSO as well as MS medium fortified with 2.0 mg/L gibberellic acid (GA3) (Sigma Aldrich, 99%, USA). The cultures were incubated in culture room conditions (25 ± 2 °C and relative humidity over 70%) under darkness. After germination, the seedlings were shifted to light and sub cultured every 4 weeks.
Induction of adventitious root cultures in vitro
The in vitro germinated 30–40 days old seedlings were used for the adventitious root induction. The explants such as leaves/cotyledons, stem/hypocotyls and roots were excised from in vitro germinated seedlings and inoculated on to the full strength MS medium (solidified with 0.8% Agar; pH-5.8) supplemented with different concentrations (0.5, 1, 2, 3, 4 and 5 mg/L) of auxins. Auxins namely NAA, IAA and IBA (Sigma-Aldrich, USA) were used for the induction of adventitious roots. The cultures were incubated in the dark in culture room conditions. The adventitious roots induced on full strength MS + auxin medium were maintained by regular subculture in every 4 weeks interval on the same medium and incubated at 25 ± 2 °C.
Effect of light on adventitious root cultures and andrographolide production
The actively growing 3 week old healthy adventitious roots initiated under dark were aseptically transferred to freshly prepared MS + NAA (1 mg/L) medium to elucidate the effect of light on ARC for andrographolide accumulation. The cultures were then incubated under light with white fluorescent lights (1000 lx). The growth study was conducted for 4 weeks and compared with adventitious roots grown in the dark and light conditions. The adventitious roots induced on full strength MS + NAA (1 mg/L) medium were maintained by regular subculture every 4 weeks interval on the same medium and incubated at 25 ± 2 °C. A small amount of adventitious root inoculum (0.93 mg) from 4 weeks old cultures was separated with the help of sterile forceps and inoculated on to MS + NAA (1 mg/L) medium. The cultures were incubated under dark and light conditions in the culture room.
Fresh and dry weight (FW and DW) measurements for growth indices (GI)
The adventitious roots were harvested every week and weighed using an analytical balance (Mettler-Toledo India Private Limited) to determine the fresh weight (FW). The dry weight (DW) was obtained by drying the roots at 60 °C for 2–3 days and weighed using an analytical balance. The growth index (GI) was calculated as per the formula given below:
Andrographolide extraction from different adventitious root cultures and HPLC analysis
To evaluate the andrographolide content in the adventitious roots developed from different explants (leaf, stem and root) were harvested after 4 weeks, dried in oven at 60 °C for 2 days and subjected to methanol extraction as per Zaheer and Giri (2017). The finely ground adventitious root powder (50 mg) was added with 5 mL of 100% methanol and incubated for 24 h at room temperature followed by sonication for 30 min for each sample. The sonicated samples were filtered through Whattmann No. 41 filter paper and then passed through Millex-GV Syringe Filter Unit, 0.22 µm, PVDF, 33 mm, gamma sterilized (Merck India). 200 µl of filtrate was used for HPLC analysis with 100% HPLC grade methanol as mobile phase using C18 column in Shimadzu HPLC machine. The flow rate of 1 mL/min, 20 µl of injection volume was used along with andrographolide standard (1 mg/mL) (Sigma-Aldrich, USA, 98%) detected by PDA detector at the Department of Biochemistry, Osmania University, Hyderabad, India. Andrographolide was detected at 230 nm.
Biomass enhancement of adventitious root cultures by different culture techniques in liquid media
The liquid cultures were generated for the enhancement of adventitious root biomass. An average of 1.23 g fresh inoculum from 3 weeks old adventitious root cultures was inoculated into 20 mL liquid MS + NAA (1 mg/L) medium in 150 mL Erlenmeyer flaks. The flasks were placed in the dark at 25 ± 2 °C for 3 weeks in static condition. In order to increase the biomass of adventitious root cultures, a number of culture techniques were employed and compared for the growth of AR cultures in semi-solid medium (MS medium with 0.4% agar; pH-5.8). The actively growing adventitious roots from 3 week old liquid cultures were inoculated, into the liquid medium directly (Liquid adventitious roots-LAR), over blotting papers floating on liquid medium (Blotting paper adventitious roots-BAR) and over the cotton immersed in liquid medium (Cotton adventitious roots-CAR). The cultures were incubated in the dark at culture room conditions. The growth study was conducted for 4 weeks by taking the FW and DW every week and compared with solid adventitious root cultures (SAR). The harvested roots were oven dried, powdered and used for HPLC analysis. In addition, we checked the release of andrographolide in to the media by methanol extraction of the liquid media and HPLC analysis. The 5 mL liquid media was collected every week from each of the above three techniques, 5 mL of methanol was added and left opened for 3–4 days for concentration. The liquid was further subjected to sonication, filtration and HPLC analysis as per standardised protocol mentioned earlier.
Elicitation of adventitious roots with ethrel
Three week old actively growing adventitious roots were used for the elicitation experiment. The elicitor ethrel was first filter sterilized prior to the elicitation and added to the sterile solid MS + NAA (1 mg/L) medium in different concentrations (1, 10, 25, 50 and 100 µM concentrations). The adventitious root inoculum was inoculated onto elicitor media and incubated at culture room conditions. The root biomass was harvested every week and FW and DW was measured. The graph was plotted by taking FW and DW on Y-axis and time duration on X-axis. The air dried adventitious root samples were subjected to methanol extraction and HPLC analysis was performed. The 99% pure andrographolide procured from Sigma was used as standard. A graph was plotted by taking andrographolide content on Y-axis and time duration on X-axis. All the experiments were repeated three times to minimize the standard error and in each experiment a maximum of 6 replicates were maintained. The statistical analysis one way ANOVA was performed by IBM SPSS Statistics version 25.0 software.
Differential expression of MVA and MEP pathway genes in light influenced in vitro adventitious root cultures
The total RNA from the AR tissue (incubated under light and dark) was extracted by trizol method and converted to cDNA using Takara Primescript first strand cDNA synthesis kit (DSS Takara, India) for qRT-PCR analysis. The primers for HMGR, HMGS, DXS, DXR, HDS, HDR and GGPS were procured from ReGene Biologics, Hyderabad, India. The primer sequences for target transcripts of the genes have been included as Table 1. The actin gene was used as internal control. The qRT-PCR was performed in QuantStudio™5 Real Time PCR instrument (Applied Biosystems from Thermo Fisher Scientifics, Singapore) using DyNAmo Flash SYBR Green qPCR Kit (Thermo Fisher Scientific, USA) following manufacturer’s instructions. The qRT-PCR program was as follows—95 °C—7 min; 95 °C—10 s; 40 cycles of 60 °C-30 s; 95 °C—15 s (absorption).
Results
Generation of in vitro cultures of A. paniculata
The immature seeds obtained from pods showed maximum germination percentage (up to 75 ± 0.029) compared to mature seeds (21.2 ± 0.036). The average days taken for germination of immature seeds was 7–10 days and mature seed germination were 25–30 days. The number of roots (5.1 ± 0.04 from cotyledon, 8.89 ± 0.05 from hypocotyl, 14.3 ± 0.02 from root explant) was obtained in NAA amended MS medium. About 6.02 ± 0.035 from cotyledon, 6.56 ± 0.042 from hypocotyl, 4.2 ± 0.031 from root explants were observed in MS + IBA medium. The cotyledon 1.01 ± 0.029, 2.12 ± 0.039 from hypocotyl, 2.0 ± 0.048 from root explants were induced from MS + IAA medium (Fig. 2). A higher number of adventitious roots were induced from root explant [35.94 ± 0.05 roots per explant (RPE)] and hypocotyl explants (27.37 ± 0.05 RPE). The number of root induction (19.64 ± 0.04 RPE) was observed from cotyledon explants (Fig. 3a). There was no adventitious root induction observed on the MS medium without any PGR except callus formation and browning/blackening of the explant. HPLC analysis of adventitious roots induced from different explants (root, hypocotyl and cotyledon) revealed high amount of andrographolide content (1.58% DW) in the adventitious roots induced from the cotyledon explant and hypocotyl derived adventitious roots (1.22% DW). The adventitious roots derived from root explants showed andrographolide content (0.95% DW) (Fig. 3b). The statistical one way ANOVA analysis shows the p value of < 0.05 for all the experiments.
Biomass enhancement of adventitious roots in vitro
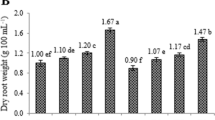
The adventitious roots placed on blotting paper were bunchy, small and short in length without any additional branched structure (Fig. 4a). The adventitious roots grown on solid medium were dispersed and long (Fig. 4b). The adventitious roots induced on cotton paper were short and bunchy (Fig. 4c). The adventitious roots directly immersed in liquid medium were small and branched (Fig. 4d). The highest amount [5.48 g (FW)] of biomass per explant was generated when the adventitious roots were cultured on CAR. The adventitious roots cultured on SAR produced 4.4 g (FW) of BPE whereas adventitious roots grown on BAR generated 4.0 g (FW) BPE after 4 weeks of culture. The adventitious roots that directly immersed in the liquid medium (LAR) generated biomass (3.0 g FW) BPE. The growth study of adventitious root cultures by different techniques was compared by plotting a graph with FW (Fig. 5a) and DW (Fig. 5b). The chromatogram for the standards and that of the different samples through HPLC analysis is deicted in Figure S1a–d.
The HPLC analysis of the adventitious roots developed using the different culture techniques revealed high amount of andrographolide (7.952% DW), from the SAR cultures and BAR (5.799% DW) and CAR (5.377% DW) cultures. The andrographolide content (4.57% DW) was observed in LAR cultures (Fig. 6a). The HPLC analysis of liquid culture medium revealed that over the culture time, andrographolide was released by the adventitious root cultures in to the surrounding medium (Fig. 6b). The release of andrographolide is more in the 3rd week. The adventitious roots cultured in BAR released more andrographolide (0.9% DW) followed by LAR (0.8% DW) and CAR (0.7% DW). The andrographolide from SAR medium could not be extracted and observed no peak in the HPLC chromatogram.
Elicitation of adventitious roots with ethrel
Ethrel elicitation of adventitious roots enhanced the biomass accumulation as well as andrographolide content. The highest biomass accumulation (0.108 g FW) was observed in the 5th week in 100 µM ethrel concentration (Fig. 7a). The highest dry weight (0.015 g DW) accumulation was observed in 25 µM in the 4th week (Fig. 7b). 1 and 25 µM ethrel concentrations also enhanced the adventitious root biomass by (0.082 g FW) in the 5th week and 0.0845 g FW in the 4th week. A fivefold increase of andrographolide content (1.12% DW) was observed in 50 µM ethrel elicited adventitious roots followed by fourfold increase (0.86% DW) in 100 µM ethrel elicited roots (Fig. 8). The statistical analysis of the experiments revealed the significance of all the experiments was ≤ 0.05.
Effect of light on andrographolide production and differential expression of its biosynthetic pathway genes in A. paniculata
The adventitious roots incubated under white light have shown morphological changes and andrographolide content. The dark induced AR was found to be white, long, dispersed and turned to green under light incubation over the time. The HPLC analysis revealed high andrographolide content was observed at second week (4.291-fold) in the light incubated AR (Fig. 9a). The differential gene expression analysis carried out using RT-PCR revealed that the MEP pathway genes, DXS, DXR, HDS and HDS were gradually up regulated (up to 5.5 fold) in the light incubated AR until 4th week. In the dark incubated AR, only MVA pathway genes (HMGR, HMGS) were highly expressed (up to 3.5 fold). It is observed that downstream pathway gene GGPS expression is similar in both light and dark conditions (Fig. 9b).
Discussion
In the present study, we have developed an efficient protocol for the induction of in vitro adventitious root cultures (ARC) system of A. paniculata to enhance the production of andrographolide (AD) as well as AR biomass. ARC system is proved as the best alternate way for the production of various secondary metabolites in different medicinal plants such as Panax quinquifolius, Astragalus membranaceus, Oplopanax elatus, Morinda coreia, Panax ginseng (Lee et al. 2018; Jin et al. 2019; Han et al. 2019; Kannan et al. 2021) and other industrial important compounds (da Silva et al. 2021; Fan et al. 2021; Rodrigues et al. 2021).
The germination frequency of seeds sown in the net house was observed to be less compared to in vitro. The immature A. paniculata seeds has shown high germination frequency compared to mature seeds. The type of seed used for germination has an impact on breaking seed dormancy. It is easy to overcome seed dormancy and achieve high germination frequency using immature seeds within short span of time when conducting in vitro experiments (Lekamge et al. 2020; Dinarti et al. 2021). The different explants displayed varied capacity of AR induction although the adventitious roots were successfully induced from all the explants such as cotyledons, hypocotyls and roots. From our study, root explant generated a higher number of adventitious roots than hypocotyls and cotyledons/leaf explants for the production of biomass. The adventitious roots induced from different explants of A. paniculata were subjected to HPLC analysis to study the effect of explants on andrographolide content. We found a higher andrographolide content in the AR induced from cotyledon explants followed by stem and root. There by, it is suggested to use cotyledon/leaf derived AR for andrographolide production. The earlier studies also revealed a similar correlation between biomass and production of secondary metabolites in ARs induced from different explants of Polygonum multiflorum (Ho et al. 2019). A similar type of study was also conducted in Periploca sepium Bunge (Zhang et al. 2011) and Camellia japonica (Jang et al. 2019) to find out the efficiency of different explant for the induction of ARs and production of periplocin. Induction and submerged cultivation of Valeriana jatamansi adventitious root cultures for production of valerenic acids and its derivatives was reported (Gehlot et al. 2022).
The auxins such as NAA, IAA and IBA are used alone and/or in combination with low concentration of cytokines promoted AR induction. From our study, it is also observed that NAA is a preferred auxin for the induction of AR than other auxins IAA and IBA. The implications of different auxins on induction of ARs from our study is supported by earlier studies by Simao et al. (2015) in Passiflora pohlii Mast., Deepthi and Satheeshkumar (2017) in Ophiorrhiza mungos, Manokari and Shekhawat (2016) in Couroupita guianensis Aubl and Kannan et al. (2021) in Morinda coreia.
Further, different culture techniques were evaluated for their efficacy towards enhancement of biomass. With the initial inoculum weight of 1.23 g a 4.45 fold increase (5.48 gm) in biomass was observed after 4 weeks of culturing by CAR. This is more (1.04 fold) than the previous report by Zaheer and Giri (2017) in 4 weeks of culturing on solid medium. Zaheer and Giri repoted 0.085 g of fresh weight from 0.02 g of initial inoculum in 4 weeks and regular subculturing up to 20 weeks could generate substantial amounts of biomass (1200 fold). The different culture techniques in the present study promoted the enhancement of AR biomass as well as AD content. Additionally, growing the AR in the liquid media facilitated the release of AD in to the liquid medium. Cai et al (2012) in their review explained in detail the benefits of product release in to medium. Cannabionoid metabolism was intensified under the influence of light in Cannabis sativa L. (Danziger and Bernstein 2021). According to Cai et al (2012) the efficiency of product isolation and purification will be increased with release of the product in to the medium. The optimization of liquid AR cutures for the production of gentiopicroside in Gentiana kurroo Royle was achieved by Alphonse and Thiagarajan (2021). The combination of elicitation and different culture techniques were implemented in Tripterygium wilfordii Hook and Rubia tinctorum L. to improve the triptolide, wilforgine, wilforine and anthraquinone production as well as product recovery (Miao et al. 2017; Perassolo et al. 2017). Furthermore, The adventitious roots grown in different culture techniques in liquid medium in the present study displayed different growth patterns and structures. It was observed that initially LAR and CAR has more andrographolide production than BAR and SAR but at the end of 4 weeks high amount of andrographolide was observed in BAR and SAR. The growth techniques such as CAR and BAR which accumulated higher biomass compared to LAR indicated that aeration and support are the crucial aspects for the efficient growth and AD production. An orthogonal test established by Wang and Qi (2010) also proved the importance of aeration for the enhancement of AR biomass in Pseudostellaria heterophylla. Ruta et al (2020) proved the temporary immersion system for the in vitro plant tissue culture was best to enhance biomass as well as secondary metabolite production in Lycium barbarum L. The present work describes a novel way of liquid media culture of ARCs, which could be extrapolated for small scale to large scale bioreactor experiments. The bioreactor cultivation of adventitious roots in Malaysian ginseng (Eurycoma longifolia Jack) and soybean (Glycine max) in small scale bioreactor resulted in the highest biomass and excellent secondary metabolites production of total phenolics, flavonoids and coumestrol (Cui et al. 2019; Lee et al. 2020). There are many other studies in which industrially high demand secondary metabolites were produced using ARCs in large scale. The biomass and production of flavonoid was achieved in Baloon-type Bubble-bioreactor using ARCs of Gynura procumbens (L.). Merr (Manuhara et al. 2019; Faizah et al. 2018). The improvement of bioactive saponin accumulation in bioreactor with ARCs of Panax vietnamensis was achieved by Linh et al. (2019). But the agitation and aeration in the bioreactor could adversely affect viability of the cultures and influence the biomass production (Jang et al. 2016). Hence, in our study, we have developed a improved, modified liquid culture technique where the AR inoculum will be placed on cotton or blotting papers immersed in the liquid medium. Here, the inoculum is devoid of anaerobic conditions and absorbs the nutrients from liquid medium. Hence, the present techniques could be a suitable alternative to overcome shear stress caused by agitation. In addition, enhanced biomass was observed to be equal to the amount of biomass produced using solid cultures. The BAR and LAR liquid culture systems could be used as alternative to SAR where the maintenance is easy.
Furthermore, the effect of elicitor for the production of biomass and AD was carried out using in vitro ARC. In the present investigation, elicitation with ethrel enhanced the biomass as well as AD in ARC of A. paniculata. The growth study of elicited AR for 5 weeks revealed no growth inhibition until 3 weeks. The highest fresh weight was observed at the 5th week in 100 µM followed by 1 and 25 µM in the 4th week. The growth study of ethrel elicited adventitious roots shows there is no negative effect of elicitor on the adventitious root growth unlike other elicitors. Moreover, there was high growth observed in 25 µM (4th week; 1.25-fold more than the control) and in 100 µM (5th week; 1.5-fold more than the control). In the earlier study, the quantities of aloe emodin and chrysophanol were enhanced 5 and fourfold respectively by applying ethrel (500 µM) elicitation (Lee et al. 2013). Unlike Bulgako et al. (2002) study in Rubia cordifolia, our study enhanced both biomass as well as AD content. Ethrel in 10–100 µM used in the Bulgako study not involved in the enhancement of anthraquinones. Ethrel elicition of hairy root cultures of Prunella vulgaris and black carrot resulted in a 1.66 fold increase of rosmarinic acid and 82% more anthocyanin and 20% more hydroxycinnamic acid (Ru et al. 2016; Barba-Espin et al. 2020). Ethrel treatment of cell suspension cultures of Vitis vinifera enhanced the anthocyanin content up to 2.3 fold and phytoalexin up to 10.8 nmol.mg-1 DW (Faurie et al. 2009; Saw et al. 2012). Ethrel treated roots of black carrot and Lemna paucicostata has shown highest anthocyanin concentration (1.17 more) and γ-aminobutyric acid (5.014 ± 1.372 mg/L) and ferulicacid (0.640 ± 0.071 mg/L) than untreated roots, with no negative effect on root fresh weight and dry weight (Barba-Espin et al. 2017; Kim et al. 2020). In general, elicitation with chemical elicitors in high concentrations negatively affects the growth of the cultures though enhances the secondary metabolite production. But here, ethrel promoted biomass unlike other chemical elicitors even in high concentrations along with AD production. The elicitation with ethrel could overcome the problem of growth inhibition which is a common in the chemical elicitation experiments across species (Bae et al. 2006; Ru et al. 2016).
The effect of light on the expression of AD biosynthetic pathway genes and AD production was elucidated. The incubation of ARC under dark and light brought significant morphological differences, growth patterns, differential expression of andrographolide biosynthetic pathway genes and andrographolide. In the dark ARC, MVA pathway genes played a crucial role in the production of AD whereas in the light cultures MEP pathway taken over the biosynthesis of AD. The genes responsible for important rate limiting and related enzymes were up regulated in the light cultures. In both dark and light conditions it was observed that rate limiting enzymes (HMGR and DXR) of the respective pathways (MVA and MEP) were highly expressed. The HDR and HDS genes were less expressed than other genes. In addition, the gene encoding downstream enzyme GGPS was also up regulated compared to dark cultures. The up regulation of MEP pathway genes and GGPS gene was correlated with enhanced AD production by HPLC analysis of the ARCs. As mentioned by Escobar-Bravo et al. (2017), light plays a crucial role in plant growth and metabolism. Several studies also proved the direct correlation between light and secondary metabolites production. The duration of photoperiod and effect of different spectral lights in line with elicitation increased steviol glycosides, phenolics, and flavonoid contents in the ARCs of Stevia rebaudiana (Idrees et al. 2018; Alvarado-Orea et al. 2020) and Ajuga bracteosa (Ali et al. 2019). The biomass and hypericins (used in depression treatment and photodynamic therapy) accumulation was enhanced in Hypericum perforatum by the treatment of red light and darkness (Sobhani Najafabadi et al. 2019). Effect of darkness treatment on the morphology, hormone status and gene expression of developing adventitious root in apple rootstock has also been studied (Li et al 2022). Here in our study, incubation under light changed the AR morphology where the roots became thick, turned to green unlike thin, short dark AR.
Conclusion
In order to overcome the bottlenecks to meet the market demands of secondary metabolites, plant tissue and organ culture is the best alternative approach for mass production of biomass and subsequent production of seconday metabolites in vitro. In the present study, we have evaluated different parameters to obtain more number of ARs productions, biomass enhancement and production of andrographolide using different liquid culture techniques. Our results suggests one has to be selective in choosing the explant and auxin, for the efficient induction of ARC and follow different culture conditions/techniques according to the requirements as well as elicitation techniques for the effective biomass and AD production. Cotyledon explants were more suitable to generate high AD producing adventitious root cultures where as NAA in 1 mg/L is ideal for the adventitious root induction. Growing the adventitious roots in modified liquid culturing techniques (CAR/BAR) is favors the high biomass proliferation than growing the adventitious roots directly in liquid medium. Using ethrel as an elicitor not only enhanced the AD content but also minimized the growth inhibition which is a common problem in elicitation even in higher concentrations. The influence of light on the expression of andrographolide biosynthetic pathway genes and shift change from MVA to MEP pathway with regard to andrographolide biosynthesis was identified. The light factor plays a crucial role in regulating the biosynthesis of andrographolide that is partitioned between matching parallel MVA and MEP pathways.
Data availability
All data generated or analysed during this study are included in this published article.
Code availability
N/A.
Material availability
N/A.
Abbreviations
- AD:
-
Andrographolide
- AR:
-
Adventitious roots
- ARC:
-
Adventitious root cultures
- DGE:
-
Differential gene expression
- GA3 :
-
Gibberellic acid
- GI:
-
Growth Indices
- IAA:
-
Indole acetic acid
- IBA:
-
Indole butyric acid
- MS:
-
Murashige and Skoog
- MSO:
-
MS basal medium without PGRs
- NAA:
-
Naphthalene acetic acid
- PGRs:
-
Plant growth regulators
- DXR:
-
Deoxy xylulose 5-phosphate reductase
- DXS:
-
Deoxy xylulose 5-phosphate synthase
- HDR:
-
4-Hydroxy 3-methyl 2-butenyl 4-diphosphate reductase
- HDS:
-
4-Hydroxy 3-methyl 2-butenyl 4-diphosphate synthase
- GGPS:
-
Geranylgeranyl pyrophosphate synthase
References
Ahmad N, Khan P, Khan A, Usman M, Ali M, Fazal H, Uddin MN, Hano C, Abbasi BH (2022) Elicitation of submerged adventitious root cultures of Stevia rebaudiana with Cuscuta reflexa for production of biomass and secondary metabolites. Molecules. https://doi.org/10.3390/molecules27010014
Ali H, Khan MA, Kayani WK, Dilshad E, Rani R, Khan RS (2019) Production of biomass and medicinal metabolites through adventitious roots in Ajuga bracteosa under different spectral lights. J Photochem Photobiol B 193:109–117. https://doi.org/10.1016/j.jphotobiol.2019.02.010
Alphonse M, Thiagarajan K (2021) Optimisation of gentiopicroside production in Gentiana kurroo Royle from adventitious root cultures in a liquid culture system. In Vitro Cell Dev Biol Plant 57:179–189. https://doi.org/10.1007/s11627-021-10168-2
Alvarado-Orea IV, Paniagua-Vega D, Capataz-Tafur J, Torres-López A, Vera-Reyes I, García-López E, Huerta-Heredia AA (2020) Photoperiod and elicitors increase steviol glycosides, phenolics, and flavonoid contents in root cultures of Stevia rebaudiana. In Vitro Cell Dev Biol Plant 6:1–9. https://doi.org/10.1007/s11627-019-10041-3
Anjum CE, Marbawi H, Gansau JA (2018) Effects of auxin and cytokinin on biomass and phenolics production in adventitious roots cultures of Labisia pumila var. alata. Transact Sci Technol 5:68–75
Bae KH, Choi YE, Shin CG, Kim YY, Kim YS (2006) Enhanced ginsenoside productivity by combination of ethephon and methyl jasmoante in ginseng (Panax ginseng CA Meyer) adventitious root cultures. Biotechnol Lett 28:1163–1166. https://doi.org/10.1007/s10529-006-9071-1
Banerjee T, Singh A, Kumar S, Dhanani T, Gajbhiye NA, Koley TK, Maurya A, Filgona J (2019) Ovicidal and larvicidal effects of extracts from leaves of Andrographis paniculata (Burm. f.) Wall. ex nees against field isolates of human hookworm (Ancylostoma duodenale). J Ethnopharmacol 235:489–500. https://doi.org/10.1016/j.jep.2019.02.021
Baque MA, Moh SH, Lee EJ, Zhong JJ, Paek KY (2012) Production of biomass and useful compounds from adventitious roots of high-value added medicinal plants using bioreactor. Biotechnol Adv 30:1255–1267. https://doi.org/10.1016/j.biotechadv.2011.11.004
Barba-Espín G, Glied S, Crocoll C, Dzhanfezova T, Joernsgaard B, Okkels F, Lütken H, Müller R (2017) Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. sativus var. atrorubens Alef.). BMC Plant Biol 17:1–1. https://doi.org/10.1186/s12870-017-1021-7
Barba-Espín G, Chen ST, Agnolet S, Hegelund JN, Stanstrup J, Christensen JH, Müller R, Lütken H (2020) Ethephon-induced changes in antioxidants and phenolic compounds in anthocyanin-producing black carrot hairy root cultures. J Exp Bot 71:7030–7045. https://doi.org/10.1093/jxb/eraa376
Bindu BBV, Srinath M, Shailaja A, Giri CC (2017) Comparative protein profile studies and in silico structural/functional analysis of HMGR (ApHMGR) in Andrographis paniculata (Burm. f.) Wall. ex Nees. Ann Phytomed 6:30–44
Bindu BBV, Srinath M, Shailaja A, Giri CC (2020) Proteome analysis and diferential expression by JA driven elicitation in Andrographis paniculata (Burm. f.) Wall. ex Nees using Q-TOF–LC–MS/MS. Plant Cell Tiss Org Cult 140:489–504
Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV, Zvereva EV, Fedoreyev SA, Zhuravlev YN (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97:213–221
Cai Z, Kastell A, Knorr D, Smetanska I (2012) Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep 31:461–477. https://doi.org/10.1007/s00299-011-1165-0
Chao WW, Lin BF (2010) Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin Med 13:5–17. https://doi.org/10.1186/1749-8546-5-17
Cui XH, Murthy HN, Zhang JD, Song HL, Jiang YJ, Qi WW, Li YY, Paek KY, Park SY (2019) Effect of nutritional factors on the accretion of secondary metabolites in Malaysian ginseng adventitious root cultures. Plant Biotechnol Rep 14:381–386. https://doi.org/10.1007/s11816-019-00592-7
da Silva TFO, Yamaguchi CS, Ribeiro STC, da Silva AA, Pilau EJ, Porto C, de Oliveira AJB, Gonçalves RAC (2021) Adventitious root culture of Pfaffia glomerata (Spreng.) Pedersen in a roller bottle system: an alternative source of β-ecdysone. Phytochem Lett 43:1–7. https://doi.org/10.1016/j.phytol.2021.02.009
Danziger N, Bernstein N (2021) Light matters: Effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.). Ind Crop Prod 164:113351. https://doi.org/10.1016/j.indcrop.2021.113351
Das D, Bandyopadhyay M (2020) Novel approaches towards over-production of andrographolide in in vitro seedling cultures of Andrographis paniculata. S Afr J Bot 128:77–86. https://doi.org/10.1016/j.sajb.2019.10.015
Deepthi S, Satheeshkumar K (2017) Effects of major nutrients, growth regulators and inoculum size on enhanced growth and camptothecin production in adventitious root cultures of Ophiorrhiza mungos L. Biochem Eng J 117:198–209. https://doi.org/10.1016/j.bej.2016.10.016
Devi J, Kumar R, Singh K, Gehlot A, Bhushan S, Kumar S (2021) In vitro adventitious roots: a non-disruptive technology for the production of phytoconstituents on the industrial scale. Crit Rev Biotechnol. https://doi.org/10.1080/07388551.2020.1869690
Dinarti D, Sudarsono S, Irawan J (2021) In vitro responses of Areca catechu immature embryos on medium containing plant growth regulators. IOP Conf Ser 694:012026
Escobar-Bravo R, Klinkhamer PG, Leiss KA (2017) Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front Plant Sci 8:278. https://doi.org/10.3389/fpls.2017.00278
Faizah H, Tanjung M, Purnobasuk H, Wulan YS (2018) Biomass and flavonoid production of Gynura procumbens (L.) Merr adventitious root culture in baloon-type bubble-bioreactor influenced by elicitation. Asian J Plant Sci 17:107–119. https://doi.org/10.3923/ajps.2018.107.119
Fan MZ, An XL, Cui XH, Jiang XL, Piao XC, Jin MY, Lian ML (2021) Production of eurycomanone and polysaccharides through adventitious root culture of Eurycoma longifolia in a bioreactor. Biochem Eng J 171:108013. https://doi.org/10.1016/j.bej.2021.108013
Fathi R, Mohebodini M, Chamani E (2021) Adventitious root development and secondary metabolites accumulation by auxin in Cichorium intybus L. Plant Prod 43:467–476. https://doi.org/10.22055/ppd.2019.29713.1772
Faurie B, Cluzet S, Corio-Costet MF, Mérillon JM (2009) Methyl jasmonate/ethephon cotreatment synergistically induces stilbene production in “Vitis vinifera” cell suspensions but fails to trigger resistance to “Erysiphe necator.” OENO One. 43:99–110. https://doi.org/10.20870/oeno-one.2009.43.2.800
Ganapumane V, Nagella P (2020) Production of Andrographolide from in vitro cultures of Andrographis paniculata (Burm. f.) nees. In: Hussain M (ed) Research trends in medicinal plant sciences, vol 6. AkiNik Publications, Delhi, pp 115–134
Gehlot A, Chaudhary N, Devi J et al (2022) Induction and submerged cultivation of Valeriana jatamansi adventitious root cultures for production of valerenic acids and its derivatives. Plant Cell Tiss Organ Cult 148:347–361. https://doi.org/10.1007/s11240-021-02193-1
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Organ Cult 126:1–8. https://doi.org/10.1007/s11240-016-0985-6
Gupta BM, Ahmed KM, Bansal J, Bansal M (2019) Andrographis paniculata global publications output: a bibliometric assessment during 2003–18. Int J Pharm Investig 9:101–108. https://doi.org/10.5530/ijpi.2019.3.20
Han L, Piao XC, Jiang J, Jiang XL, Yin CR, Lian ML (2019) A high production of flavonoids and anthraquinones via adventitious root culture of Oplopanax elatus and evaluating antioxidant activity. Plant Cell, Tissue Organ Cult 137:173–179. https://doi.org/10.1007/s11240-018-01543-w
Ho TT, Jeong CS, Lee H, Park SY (2019) Effect of explant type and genotype on the accumulation of bioactive compounds in adventitious root cultures of Polygonum multiflorum. Plant Cell Tissue Organ Cult 137:115–124. https://doi.org/10.1007/s11240-018-01556-5
Hoagland DR, Arnon DI (1938) The water culture method for growing plantswithout soil. Calif Agric Exp Stn Circulation 347:32
Hossain MD, Urbi Z, Sule A, Rahman KM (2014) Andrographis paniculata (Burm. f.) wall. ex nees: a review of ethnobotany, phytochemistry, and pharmacology. Sci World J. https://doi.org/10.1155/2014/274905
Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh Qrimida A, Allzrag AM, Ming LC, Pagano E, Capasso R (2021) Andrographis paniculata (Burm. f.) wall. ex nees: an updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life 11:348. https://doi.org/10.3390/life11040348
Idrees M, Sania B, Hafsa B, Kumari S, Khan H, Fazal H, Ahmad I, Akbar F, Ahmad N, Ali S, Ahmad N (2018) Spectral lights trigger biomass accumulation and production of antioxidant secondary metabolites in adventitious root cultures of Stevia rebaudiana (Bert.). Comptes Rendus Biol 341:334–342. https://doi.org/10.1016/j.crvi.2018.05.003
Isah T, Umar S, Mujib A, Sharma MP, Rajasekharan PE, Zafar N, Frukh A (2018) Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult 132:239–265. https://doi.org/10.1007/s11240-017-1332-2
Jang HR, Lee HJ, Shohael AM et al (2016) Production of biomass and bioactive compounds from shoot cultures of Rosa rugosa using a bioreactor culture system. Hortic Environ Biotechnol 57:79–87. https://doi.org/10.1007/s13580-016-0111-z
Jang E, Ho TT, Park SY (2019) Cytological characteristics of different origin-derived calli and adventitious roots and phenolic compounds profiling in Camellia japonica. Flower Res J 27:91–100
Jin H, Yu Y, Quan X, Wu S (2019) Promising strategy for improving calycosin-7-O-β-D-glucoside production in Astragalus membranaceus adventitious root cultures. Ind Crops Prod 141:111792. https://doi.org/10.1016/j.indcrop.2019.111792
Kandar CC (2021) Secondary metabolites from plant sources. Bioactive natural products for pharmaceutical applications. Springer, Cham, pp 329–377
Kannan N, Manokari M, Shekhawat MS (2021) Enhanced production of anthraquinones and phenolic compounds using chitosan from the adventitious roots of Morinda coreia Buck. and Ham. Ind Crops Prod 148:112321. https://doi.org/10.1016/j.indcrop.2020.112321
Kim E, Kim M, Choi HK (2020) Alteration of metabolic profiles in Lemna paucicostata culture and enhanced production of GABA and ferulic acid by ethephon treatment. PLoS ONE 15:e0231652. https://doi.org/10.1371/journal.pone.0231652
Kumar S, Singh B, Bajpai V (2021) Andrographis paniculata (Burm. f.) nees: traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J Ethnopharmacol 6:114054. https://doi.org/10.1016/j.jep.2021.114054
Kumari R, Venaik A, Hasibuzzaman MA, Azure SA, Ojha RP, Sahi AK (2021) Repurposing of the herbals immune-boosters in the prevention and management of COVID-19: a review. J Pure Appl Microbiol 15:1–9. https://doi.org/10.22207/JPAM.15.1.35
Lee YS, Ju HK, Kim YJ, Lim TG, Uddin MR, Kim YB, Baek JH, Kwon SW, Lee KW, Seo HS, Park SU (2013) Enhancement of anti-inflammatory activity of Aloe vera adventitious root extracts through the alteration of primary and secondary metabolites via salicylic acid elicitation. PLoS ONE 8:e82479. https://doi.org/10.1371/journal.pone.0082479
Lee JD, Le KC, Park YK, Murthy HN, Paek KY, Park SY (2018) Cell culture system versus adventitious root culture system in Asian and American ginseng: a collation. Plant Cell Tissue Organ Cult 132:295–302. https://doi.org/10.1007/s11240-017-1329-x
Lee EJ, Jiménez Z, Seo KH, Nam GB, Kang YG, Lee TR, Kim D, Yang DC (2020) Mass production of coumestrol from soybean (Glycine max) adventitious roots through bioreactor: effect on collagen production. Plant Biotechnol Rep 14:99–110. https://doi.org/10.1007/s11816-019-00589-2
Lekamge D, Yamamoto SI, Morohashi S, Matsumoto T, Hatamoto M, Yamaguchi T, Maki S (2020) Propagation of Polygonatum macranthum (Maxim.) Koidz. from immature seeds using a new sterilization procedure. Plant Biotechnol. https://doi.org/10.5511/plantbiotechnology.20.0310a
Li F, Lee EM, Sun X, Wang D, Tang H, Zhou GC (2020) Design, synthesis and discovery of andrographolide derivatives against Zika virus infection. Eur J Med Chem 187:111925. https://doi.org/10.1016/j.ejmech.2019.111925
Li K, Tian H, Mao J et al (2022) Effect of darkness treatment on the morphology, hormone status and gene expression of developing adventitious root in apple rootstock. Plant Cell Tiss Organ Cult 148:331–346. https://doi.org/10.1007/s11240-021-02192-2
Linh NT, Tam HT, Tung HT, Luan VQ, Hien VT, Loc NH, Nhut DT (2019) Improvement of bioactive saponin accumulation in adventitious root cultures of Panax vietnamensis via culture periods and elicitation. Plant Cell Tissue Organ Cult 137:101–113. https://doi.org/10.1007/s11240-018-01555-6
Manokari M, Shekhawat MS (2016) Implications of auxins in induction of adventitious roots from leaf explants of cannon ball tree (Couroupita guianensis Aubl.). World Sci News 33:109–121
Manuhara YS, Noviyanti R, Hardjo PH, Tanjung M (2019) Enhancement of flavonoid production of Gynura procumbens (Lour.) Merr adventitious roots in baloon-type bubble bioreactor influenced by phenylalanine and tyrosine. Ecol Environ Conserv 25:61–66
Marchev AS, Yordanova ZP, Georgiev MI (2020) Green (cell) factories for advanced production of plant secondary metabolites. Crit Rev Biotechnol 40:443–458. https://doi.org/10.1080/07388551.2020.1731414
Miao GP, Zhu CS, Yang YQ, Feng MX, Ma ZQ, Feng JT, Zhang X (2014) Elicitation and in situ adsorption enhanced secondary metabolites production of Tripterygium wilfordii Hook. f. adventitious root fragment liquid cultures in shake flask and a modified bubble column bioreactor. Bioprocess Biosyst Eng 37:641–650. https://doi.org/10.1007/s00449-013-1033-0
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murthy HN, Dalawai D, Bhat MA, Dandin VS, Paek KY, Park SY (2021) Biotechnological production of useful phytochemicals from adventitious root cultures. Plant Cell Tissue Differ Second Metab 2021:469–485
Nayak AG, Kumar N, Shenoy S, Roche M (2020) Anti-snake venom and methanolic extract of Andrographis paniculata: a multipronged strategy to neutralize Naja naja venom acetylcholinesterase and hyaluronidase. 3 Biotech 10:1–2. https://doi.org/10.1007/s13205-020-02462-4
Neeraja C, Krishna PH, Reddy CS, Giri CC, Rao KV, Reddy VD (2015) Distribution of Andrographis species in different districts of Andhra Pradesh. Proc Natl Acad Sci India Sect B 85:601–606. https://doi.org/10.1007/s40011-014-0364-1
Pandey S, Bahadur AN, Kanungo V, Tewari U (2017) Rapid micropropagation of Androgrphis paniculata nees, from nodal explants to study effect of various plant growth regulators. Indian J Sci Res 7:149–157
Pandey S, Sundararajan S, Ramalingam S, Pant B (2022) Elicitation and plant growth hormone-mediated adventitious root cultures for enhanced valepotriates accumulation in commercially important medicinal plant Valeriana jatamansi Jones. Acta Physiol Plant. https://doi.org/10.1007/s11738-021-03319-w
Parlapally S, Cherukupalli N, Bhumireddy SR, Sripadi P, Anisetti R, Giri CC, Khareedu VR, Reddy VD (2016) Chemical profiling and anti-psoriatic activity of methanolic extract of Andrographis nallamalayana JL Ellis. Nat Prod Res 30:1256–1261
Perassolo M, Cardillo AB, Mugas ML, Montoya SCN, Giulietti AM, Talou JR (2017) Enhancement of anthraquinone production and release by combination of culture medium selection and methyl jasmonate elicitation in hairy root cultures of Rubia tinctorum. Ind Crops Prod 105:124–132. https://doi.org/10.1016/j.indcrop.2017.05.010
Rahmat E, Kang Y (2019) Adventitious root culture for secondary metabolite production in medicinal plants: a review. J Plant Biotechnol 46:143–157. https://doi.org/10.5010/JPB.2019.46.3.143
Rodrigues V, Kumar A, Prabhu KN, Pragadheesh VS, Shukla AK, Sundaresan V (2021) Adventitious root cultures of Decalepis salicifolia for the production of 2-hydroxy-4-methoxybenzaldehyde, a vanillin isomer flavor metabolite. Appl Microbiol Biotechnol 105:3087–3099. https://doi.org/10.1007/s00253-021-11262-6
Ru M, An Y, Wang K, Peng L, Li B, Bai Z, Wang B, Liang Z (2016) Prunella vulgaris L. hairy roots: culture, growth, and elicitation by ethephon and salicylic acid. Eng Life Sci 16:494–502. https://doi.org/10.1002/elsc.201600001
Ruta C, De Mastro G, Ancona S, Tagarelli A, De Cillis F, Benelli C, Lambardi M (2020) Large-scale plant production of Lycium barbarum L. by liquid culture in temporary immersion system and possible application to the synthesis of bioactive substance. Plants 9:844. https://doi.org/10.3390/plants9070844
Saw NM, Riedel H, Cai Z, Kütük O, Smetanska I (2012) Stimulation of anthocyanin synthesis in grape (Vitis vinifera) cell cultures by pulsed electric fields and ethephon. Plant Cell Tissue Organ Cult 108:47–54. https://doi.org/10.1007/s11240-011-0010-z
Shailaja A, Bindu BBV, Srinath M, Giri CC (2018) In silico structural and functional analysis of copalyl diphosphate synthase enzyme in Andrographis paniculata (Burm. f.) Wall. ex Nees: a plant of immense pharmaceutical value. Ann Phytomed Int J 7:69–77
Shailaja A, Bindu BBV, Srinath M, Giri CC (2020) Innovative technique for rapid in vitro multiplication of rootless shoots in Andrographis paniculata (Burm. f) Nees: A plant with immense pharmaceutical value. Ann Phytomed 9(1):98–106
Shailaja A, Srinath M, Bindu BBV, Giri CC (2021) Isolation of 4-hydroxy 3-methyl 2-butenyl 4-diphosphate reductase (ApHDR) gene of methyl erythritol diphosphate (MEP) pathway, in silico analysis and differential tissue specific ApHDR expression in Andrographis paniculata (Burm. f) Nees. Plant Physiol Mol Biol Plants 27(2):223–235
Simão MJ, Fonseca E, Garcia R, Mansur E, Pacheco G (2016) Effects of auxins and different culture systems on the adventitious root development of Passiflorapohlii Mast. and their ability to produce antioxidant compounds. Plant Cell Tissue Organ Cult 124:419–430. https://doi.org/10.1007/s11240-015-0904-2
Singh S, Pandey P, Ghosh S, Banerjee S (2018) Anti-cancer labdane diterpenoids from adventitious roots of Andrographis paniculata: augmentation of production prospect endowed with pathway gene expression. Protoplasma 255:1387–1400. https://doi.org/10.1007/s00709-018-1211-7
Sobhani Najafabadi A, Khanahmadi M, Ebrahimi M, Moradi K, Behroozi P, Noormohammadi N (2019) Effect of different quality of light on growth and production of secondary metabolites in adventitious root cultivation of Hypericum perforatum. Plant Signal Behav 14:1640561. https://doi.org/10.1080/15592324.2019.1640561
Srinath M, Shailaja A, Bindu BBV, Giri CC (2017) Characterization of 1-deoxy-D-xylulose 5-phosphate synthase (DXS) protein in Andrographis paniculata (Burm. f.) Wall. ex. Nees: A in silico appraisal. Ann Phytomed Int J 6:63–73
Srinath M, Bindu BBV, Shailaja A, Giri CC (2020) Isolation and molecular characterization hydroxy methyl glutaryl-coenzyme A (HMG-CoA) reductase (HMGR) gene from Andrographis paniculata (Burm. f) Nees. Mol Biol Rep 47:639-654
Srinath M, Shailaja A, Bindu BBV, Giri CC (2021) Molecular cloning and dfferential gene expression analysis of 1‑Deoxy‑D‑xylulose 5‑phosphate synthase (DXS) in Andrographis paniculata (Burm. f) Nees. Mol Biotechnol 63:109–124
Subramanian R, Asmawi MZ, Sadikun A (2012) A bitter plant with a sweet future? A comprehensive review of an oriental medicinal plant: Andrographis paniculata. Phytochem Rev 11:39–75. https://doi.org/10.1007/s11101-011-9219-z
Sukkanon C, Karpkird T, Saeung M, Leepasert T, Panthawong A, Suwonkerd W, Bangs MJ, Chareonviriyaphap T (2020) Excito-repellency activity of Andrographis paniculata (Lamiales: Acanthaceae) against colonized mosquitoes. J Med Entomol 57:192–203. https://doi.org/10.1093/jme/tjz139
Sun H, Gao H, Zhang C, Cao Q (2021) Enhanced production of calycosin-7-O-β-D-glucoside and astragaloside IV from adventitious root cultures of Astragalus membranaceus var. mongholicus by green leaf volatiles. Ind Crop Prod 168:113598. https://doi.org/10.1016/j.indcrop.2021.113598
Sun H, Zuo X, Zhang Q, Gao J, Kai G (2022) Elicitation of (E)-2-hexenal and 2, 3-butanediol on the bioactive compounds in adventitious roots of Astragalus membranaceus var. mongholicus. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.1c05813
Verma H, Negi MS, Mahapatra BS, Shukla A, Paul J (2019) Evaluation of an emerging medicinal crop Kalmegh [Andrographis paniculata (Burm. F.) Wall. Ex. Nees] for commercial cultivation and pharmaceutical & industrial uses: a review. J Pharmacogn Phytochem 8:835–848
Wang GR, Qi NM (2010) Influence of mist intervals and aeration rate on growth and second metabolite production of Pseudostellaria heterophylla adventitious roots in a siphon-mist bioreactor. Biotechnol Bioprocess Eng 15:1059–1064
Zaheer M, Giri CC (2015) Multiple shoot induction and jasmonic versus salicylic acid driven elicitation for enhanced andrographolide production in Andrographis paniculata. Plant Cell Tissue Organ Cult 122:553–563. https://doi.org/10.1007/s11240-015-0787-2
Zaheer M, Giri CC (2017) Enhanced diterpene lactone (andrographolide) production from elicited adventitious root cultures of Andrographis paniculata. Res Chem Intermed 43:2433–2444. https://doi.org/10.1007/s11164-016-2771-9
Zhang J, Gao WY, Wang J, Li XL (2011) Effects of explant types and media salt strength on growth and secondary metabolite accumulation in adventitious roots of Periploca sepium Bunge. Acta Physiol Plant 33:2447–2452. https://doi.org/10.1007/s11738-011-0785-x
Acknowledgements
The authors acknowledge the fellowship support from UGC-Basic Scientific Research (BSR)-Research Fellowship for Meritorious Students (RFMS), New Delhi, India to MS, AS and BBVB. Authors acknowledge Prof. B. Shashidar Rao and Dr. Karuna Rupula, Department of Biochemistry, Osmania University, Hyderabad for providing the HPLC facility.
Funding
The present work is funded by Department of Science and Technology (DST)-Promotion of University Research and Scientific Excellence (PURSE) Program-II and University Grant Commission (UGC), Centre for Potential Excellence in Particular Area (CPEPA), New Delhi, India.
Author information
Authors and Affiliations
Contributions
Study design and conception: MS. Data collection: MS. Data analysis: MS, AS, BBVB. Drafting the manuscript: MS. Critical Review, Editing the manuscript and Supervision: CCG. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
N/A.
Additional information
Communicated by K.X. Tang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Srinath, M., Shailaja, A., Bindu, B.B.V. et al. Comparative analysis of biomass, ethrel elicitation, light induced differential MVA/MEP pathway gene expression and andrographolide production in adventitious root cultures of Andrographis paniculata (Burm. F.) Nees. Plant Cell Tiss Organ Cult 149, 335–349 (2022). https://doi.org/10.1007/s11240-022-02241-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02241-4