Abstract
The establishment of green root cultures of Stevia rebaudiana Bertoni, and the effect of elicitors such as hydrogen peroxide (H2O2) and methyl jasmonate (MeJA), is shown in the present study. Stevioside, rebaudioside A, and the isomers steviol/isosteviol were identified through DFI-ESI-IT-MSn and UPLC-TOFMS spectrometric systems, in combination with solid-phase extraction. The accumulation of steviol glycosides increased by 2.4 times (compared to the control value of 22.35 μgSG per gDW), with the addition of 250 μM H2O2. The non-enzymatic antioxidant response, which resulted from production of phenolic and flavonoid compounds, was modified based on the elicitor and the dose used. The maximum accumulation of flavonoids was induced on the third day with the addition of H2O2 (250 or 500 μM), and with MeJA (250 or 500 μM); the increase was observed on the fifth day. The enzymatic antioxidant response of the catalase and peroxidase from the roots under elicitation confirmed the stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stevia rebaudiana Bertoni is a plant that is native to Paraguay, which has become widely distributed in the world for its ability to produce non-caloric steviol glycosides (SG) with high sweetening power. There are different SG identified in stevia leaves; however, the most important are stevioside (St) and rebaudioside A (Reb A). SG are mainly used to sweeten soft drinks, soy sauce, yogurt, and other foods in Japan, Korea, and Brazil (Tadhani et al. 2007; Modi et al. 2014), and Europe (Novel Food Catalog, European Commission 2017). Additionally, it has been reported that SG have glucoregulatory, hypotensive, anti-inflammatory, and anticancer biological activities (Momtazi-Borojeni et al. 2017).
Due to the importance of the production of SG from S. rebaudiana, several studies have been reported using in vitro cultures as synthetic seeds (Nower 2014), callus, cells in suspension, and seedlings (Gupta 2013), transformed roots (Fu et al. 2015; Pandey et al. 2016; Michalec-Warzecha et al. 2016), and adventitious roots (Reis et al. 2011; Lopes et al. 2016; Reis et al. 2017; Ahmad et al. 2018; Ghazal et al. 2018).
The culture of adventitious roots as a differentiated system enables the accumulation of secondary metabolites in a higher quantity than the undifferentiated systems (Verpoorte et al. 2002). SG production has been reported from adventitious roots of S. rebaudiana grown in the absence of light at different pH levels, in which pH 5.1 was optimal for SG production (Ahmad et al. 2018).
Light has two important roles in plant development; it provides energy through photosynthesis, and it is a factor that regulates cell differentiation, growth, and metabolism (Escobar-Bravo et al. 2017). Zhang et al. (2002) tested continuous white fluorescent light irradiation (8000–8300 lx) and found that prolonged exposure of cell cultures to white light stimulates photoreceptors, which in turn can regulate the expression of specific genes responsible for growth, development, and secondary metabolism. Root cultures of Acmella oppositifolia (Asteracea) that were grown under continuous light conditions showed plastidial interconversion from amiloplastid to chloroplasts, which were morphologically similar to those present in the leaves of the plants. The authors concluded that photo-autotrophic behavior of the roots could cause important consequences for the secondary metabolic pathways (Flores et al. 1993). Wu et al. (2007) studied adventitious root cultures of Echinacea purpurea and found a direct correlation between the increment in secondary metabolism production and photoperiods. Similar results were found in S. rebaudiana plants that accumulated more SG when grown under photoperiod conditions (Mohamed et al. 2011).
Additionally, elicitation is a strategy widely used in in vitro cultures to increase the production of secondary metabolites. Methyl jasmonate (MeJA) and hydrogen peroxide (H2O2) are signaling molecules in plants, and both are involved in growth, development, and defense (Mittler 2017; Wasternack and Strnad 2019). Moreover, MeJA and H2O2 have been tested as elicitors of in vitro seedlings and callus of S. rebaudiana to favor SG production (Bayraktar et al. 2018; Javed et al. 2018a; Mejía-Espejel et al. 2018), but were still not tested in root cultures. Recently, research studies conducted to investigate stress conditions and the effects on total phenol and flavonoid levels in in vitro culture of Stevia rebaudiana have been on the rise (Ahmad et al. 2016; Javed et al. 2018a; Idrees et al. 2018; Javed et al. 2018b; Lucho et al. 2018).
Under stress conditions, there are two mechanisms of detoxification that are activated to reduce a stress condition: enzymatic and non-enzymatic. The enzymatic mechanism is formed by the enzymes superoxide dismutase (SOD), catalase (CAT), and peroxidase (Px), and the non-enzymatic mechanism is formed by antioxidant molecules within the cell (Apel and Hirt 2004), such as phenolic compounds, flavonoids, and different secondary metabolites with antioxidant activities (Ramos-Valdivia et al. 2012).
The present study explores the response to elicitation by H2O2 and MeJA in green roots of S. rebaudiana, using photoperiod conditions to favor SG accumulation. The increase in the production of phenolic and flavonoid compounds was verified, as well as the activation of enzymatic antioxidant mechanisms, to study the orchestrated response of antioxidant systems in green root culture of S. rebaudiana.
Materials and methods
Plant material
Stevia rebaudiana Bertoni adventitious root cultures were derived from micropropagated wild-type plantlets (Sanchéz-Cordova et al. 2019). Complete roots were cut from in vitro plantlets and 1.2 g dry weight (DW) were placed in 125-mL Erlenmeyer flasks containing 50 mL of half-strength MS medium (Murashige and Skoog 1962), supplemented with 20 g L−1 of sucrose and 0.5 mg L−1 of indolebutyric acid (IBA). The pH of the culture medium was adjusted to 6.3 prior to sterilization. Roots were maintained in two lighting conditions, which included complete darkness, and a photoperiod of 16 h with a light intensity of 26 μmols−1 m−2 at 25 ± 2°C, and shaken at 110 rpm on an orbital shaker. Roots were subcultured every 15 d.
Elicitation experiment with hydrogen peroxide (H2O2)
Erlenmeyer flasks (125 mL) that contained 50 mL of liquid culture medium were inoculated with 1.2 g fresh weight (FW) of young root meristems obtained from previously established cultures. These root meristems were cut in 1–1.5-cm fragments. After 10 d of growth, two concentrations of H2O2 (250 μM and 500 μM) were added, and samples were taken on days 1, 3, and 5 after the additions. The experiment was carried out in triplicate.
Elicitation experiment with methyl jasmonate (MeJA)
Erlenmeyer flasks (125 mL) that contained 50 mL of liquid culture medium were inoculated with 1.2 g FW of roots, which were fragmented as described in the previous section. After 10 d of growth, two concentrations of MeJA (250 and 500 μM) were added. The methyl jasmonate (392707; Sigma-Aldrich®, St Louis, MO) was dissolved in cold absolute ethanol and filtered-sterilized with 0.45-μm nylon filters prior to use. Samples were taken on days 1, 3, and 5 after elicitation. The experiment was carried out in triplicate.
Extraction of steviol glycoside (SG)
The extraction of SG from S. rebaudianain vitro roots was carried out following the methodology of Bondarev et al. (2001). The biomass was lyophilized and pulverized using a mortar and pestle, and the SG were successively extracted twice with methanol to H2O (80:20 v/v) in a ratio of 10 mL of extraction mixture for each gram of biomass (10:1) and sonicated for 30 min. Subsequently, the mixture was centrifuged for 15 min at 4116 g, the supernatant was recovered and all of the samples were concentrated to their constant weight. The crude extracts of SG (CESG) were stored at 4°C until analysis.
Thin-layer chromatography
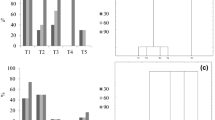
Standards of stevioside (S3572; Sigma-Aldrich®) and rebaudioside A (1432; Sigma-Aldrich®) were used as references to identify the presence of SG. Standards and CESG from S. rebaudiana roots were dissolved in methanol to H2O (80:20 v/v) and applied on 60 F254 silica gel plates, with ethyl acetate to ethanol to acetone to H2O (15:3:6:6, v/v/v/v), as the mobile phase (Londhe and Nanaware 2013). The plates were developed with a solution of 0.5% orcinol in methanol to sulfuric acid (95:5, v/v) and heated to 120°C for 2 min on a heating plate until the bands corresponding to the SG were observed (Reis et al. 2011).
Identification of SG
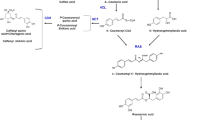
The CESG were cleaned by solid-phase extraction (SPE), following the methodology of Wöelwer-Rieck et al. (2010) with some modifications. Chromabond® C18 ec cartridges (500 mg) were used, and the fraction retained was air-dried for 15 min. The fraction with the SG was eluted with acetonitrile to H2O (80:20 v/v). Mass spectral data was obtained through the direct flow infusion of the samples on an LCQ Fleet ion-trap mass spectrometer (Thermo Fisher Scientific®, San Jose, CA), with an electrospray interface (Thermo Fisher Scientific®) (DFI-ESI-IT-MSn), in negative mode. The system used a fused silica capillary tube at 280°C, a spray voltage of 5.00 kV, a capillary voltage of − 35 V, a tube lens of − 100 V, a 10 μL/min flow, and collision energy of 20–30%. Samples were further analyzed by an MS/MS experiment with collision energy of 20–30% and 30-ms activation time until fragmentation ceased. The resulting ions were identified using their mass spectra compared to standards and data previously reported. The compounds were identified by comparing mass spectra of the components with standards and data from the literature.
Quantification of SG
For quantification, CESG were cleaned by SPE following the methodology of Wöelwer-Rieck et al. (2010), using 100 mg Strata® C18E cartridges, and subsequently quantified by UPLC-TOF-MS in an Acquity Class I (Waters) chromatographic system coupled with a Synapt G2-Si (Waters) high-resolution mass spectrometer, according to Tada et al. (2013) and Gardana et al. (2010), with minor modifications. The chromatographic separation of SG was carried out using an isocratic method with 22% (v/v) acetonitrile and 0.1% (v/v) formic acid at 0.4 mL per min and 25°C on a Kinetex 5-μm EVO C18 column (150 × 2.1 mm, Phenomenex, Torrance, CA). The ionization was carried out by electrospray in negative mode, with a dwell time of 0.5 s and capillary voltage of 3 kV, and the spectra were captured from 100 to 1200 m/z. For the quantification of stevioside and rebaudioside A, the ions of m/z 839.3468 and 1001.3996, respectively, corresponding to the adducts [M + Cl35]− (10 to 100 ng mL−1, R2 = 0.9998), were monitored. SG were reported as the sum of stevioside and rebaudioside A.
Total phenolic and flavonoid content
The determination of the total phenolic content (TPC) was carried out according to the methodology described by Singleton et al. (1999), using catechin (C1251; Sigma-Aldrich®) as the standard in a calibration curve. The TPC was expressed in equivalent micrograms of catechin per milligram of dry extract. The quantification of the total flavonoid content (TFC) was performed according to Dewanto et al. (2002) methodology, using rutin (78,095; Sigma-Aldrich®) as a standard. The TFC was expressed in micrograms equivalent to rutin per milligram of dry extract.
Protein extraction and quantification
Roots were frozen and pulverized in liquid nitrogen, and an extraction buffer (100 mM phosphate buffer pH 7.0, 0.1 mM EDTA, 1% (w/v) PVPP and 5 mM ascorbate) in a 1:10 ratio (w/v) was added and mixed to obtain a homogeneous slurry. The crude root extract was mixed carefully and centrifuged at 33600g for 10 min. All of the last steps were performed at 4°C. The supernatant was preserved for catalase (CAT) and guaiacol peroxidase (Px) activity analyses. The total protein was determined using Bradford reagent (Bradford 1976) (500-0205; BioRad, Hercules, CA), using bovine serum albumin (BSA; 500-0209 BioRad) as a standard.
Enzymatic activity assays
Catalase activity (CAT) was determined according to the protocol described by Aebi (1984). It was calculated using the extinction coefficient of H2O2 (40 M cm−1) and expressed as μmol mg protein−1 min−1. The enzymatic activity of guaiacol peroxidase (Px) was quantified by the oxidation of guaiacol using the method proposed by Pütter (1971). The activity was expressed as the amount of protein that produces 1 mmol of tetraguaiacol min−1.
Statistical analysis
The effects of elicitor concentrations and exposure time were assessed by a two-way ANOVA using the general linear model and Tukey’s post-hoc test with P < 0.05 to establish significance for both analyses with Minitab 16.
Results and discussion
Effect of photoperiod on SG production in S. rebaudiana root culture
To optimize the light regime, an experiment was designed to grow S. rebaudiana adventitious roots in darkness and photoperiod conditions. Figure 1 shows S. rebaudiana adventitious roots grown in darkness (Fig. 1a), and in 16/8-h photoperiod (light-dark) (Fig. 1b). It was observed that the roots that grew in darkness were pale (Fig. 1a) compared to those that grew in the photoperiod (Fig. 1b), which exhibited a green color. The combination of light-dark conditions allowed the formation of photosynthetic pigments, and therefore, SG accumulated in the roots (Fig. 2). This behavior may be due to the fact that the photoperiod conditions induce the transition of amyloplasts to chloroplasts, as reported by Flores et al. (1993), which allows the SG accumulation in green roots of S. rebaudiana. The last argument is consistent with data reported by Ceunen and Geuns (2013) in S. rebaudiana plants. They found enhanced SG accumulation in leaves and roots in photoperiods of 16/8 h (light-dark).
Thin-layer chromatography of methanolic extracts from (a) Stevia rebaudiana Bertoni root cultures growing in (1) photoperiod; (2) dark conditions; and (3) St and Reb A standards; (b) Stevia rebaudiana root cultures growing in light conditions (1) crude extracts of SG (CESG); (2 and 3) after solid-phase extraction; (4) St standard; and (5) Reb A standard. Solvent system ethyl acetate:ethanol to acetone to H2O (15:3:6:6 v/v/v/v), detection with orcinol.
The clean-up strategy of the CESG of S. rebaudiana roots by SPE, and the use of a 30 ppm concentration of the SG fraction obtained by SPE, allowed the identification of different SG by DFI-ESI-IT-MSn through the acquisition of the pseudomolecular ions [M-H]− 965, 803, and 317, and their fragmentation patterns compared to the standard analyte and bibliographic references (Gardana et al. 2010; Shafii et al. 2012). The compounds identified in the green roots of S. rebaudiana in this study were stevioside, rebaudioside A, and a mixture of the isomers steviol/isosteviol (Table 1). It has been reported through in vitro and in vivo studies that the accumulation of SG is related to the development of the chloroplast membrane, and the content of photosynthetic pigments in S. rebaudiana (Ladygin et al. 2008). Libik-Konieczny et al. (2018) found a strong direct correlation between the photosynthetic efficiency and SG accumulation in S. rebaudiana plants. Other authors have reported that undifferentiated cultures grown in the presence of light, such as S. rebaudiana callus and cells in suspension, are also capable of producing SG (Janarthanam et al. 2010; Gupta et al. 2014; Javed et al. 2018b). The results from the Stevia root cultures in this study concur with previous observations and suggest that the biosynthetic machinery involved (genes and enzymes) for SG accumulation is active under light conditions. To further support the effect of light on the biosynthetic properties of Stevia green roots, Chen et al. (2018) reported that Ent-kuarene, which is a precursor of the SG pathway is regulated by light.
In this study, adventitious roots of S. rebaudiana grown under dark conditions (Fig. 2a) were not able to accumulate SG, similar to what was reported by Reis et al. (2011, 2017) and Fu et al. (2015), in which roots failed to produce SG.
Effect of elicitors on growth and SG production in S. rebaudiana root culture
To evaluate the elicitation effect on growth and SG production, two concentrations of H2O2 and MeJA were added to the culture medium. Figure 3 shows the effect of 250 and 500 μM H2O2 and MeJA on the growth of the green adventitious roots of S. rebaudiana during 5 d of exposure. Roots without elicitors weighed 4.6 ± 0.67 gDW L−1 on the first day and reached a biomass accumulation of 7.04 ± 0.71 gDW L−1 after 5 d of cultivation (Fig. 3a), while for roots treated with 250 and 500 μM H2O2, the maximum biomass after 5 d of elicitation was 4.7 ± 0.06 and 4.02 ± 0.34 gDWL−1, respectively (Fig. 3b). Similarly, with 250 and 500 μM MeJA, the maximum biomass was 4.06 ± 0.01 and 3.99 ± 1.13 gDW L−1, respectively (Fig. 3c). The elicitation using MeJA and H2O2 showed inhibition of the root growth up to 40%, compared to the control treatment at day 5, which was an effect reported in other plants (Vera-Reyes et al. 2013; Faizal and Sari 2019). The inhibition of root growth is a common phenomenon observed by MeJA and H2O2 treatments due to the interference auxin flux pathway (Yan et al. 2016) and abscisic acid signaling (Bai et al. 2007), respectively. Growth reduction in MeJA-treated cultures was accompanied with a color change to brown at day 5, though the effect was not observed in the H2O2 treatment (Fig. 4). This response could be associated with the phenolic compound accumulation. It has also been reported (Neill et al. 2002) that H2O2 is rapidly neutralized by the different antioxidant systems within the cell, which prevents extensive damage, unlike MeJA, which could be exerting stress conditions in a constant and prolonged way, and leads to physiological degeneration as observed in the roots in this study. The different responses between treatments could be associated with the fact that the antioxidant systems prevent exogenous H2O2 transport far through the cell and is rapidly metabolized (Neill et al. 2002).
Growth kinetics after H2O2 and MeJa elicitation in Stevia rebaudiana Bertoni root culture during 5 d of elicitation. (a) Roots growing without elicitors; (b) Roots growing with hydrogen peroxide. Open and close square, 250 and 500 μM of H2O2, respectively. (c) Roots growing with methyl jasmonate. Open and close rhombus, 250 and 500 μM of MeJA, respectively. Error bars indicate standard deviation from the mean (n = 3).
To analyze the elicitation effect on SG levels, the same elicitors were tested. Figure 5 shows the production of SG as a function of the exposure time after elicitation with H2O2. It was observed that the addition of 250 μM H2O2 significantly increased the accumulation of SG (stevioside + rebaudioside A, 32 μgSG per gDW), compared to the green roots without elicitation treatment (22.48 μgSG per gDW), from the first day of exposure. After 3 d of exposure, the SG increased up to a 2.4-fold accumulation of the control and reached the maximum SG content in the experiment (50.8 μgSG per gDW). A similar result of increased SG was also reported by Javed et al. (2018a), in shoots of S. rebaudiana elicited with 10 and 20 mM of H2O2. In these results, the root cultures treated with 500 μM H2O2 decreased their SG production. This result suggests that H2O2 favors the production of SG, but the positive effect is dose dependent. Hydrogen peroxide, as a secondary messenger, could be inducing genes involved in SG synthesis at 250 μM H2O2, as observed in tomato (Orozco-Cárdenas et al. 2001), and Uncaria tomentosa (Vera-Reyes et al. 2013), which induced secondary metabolism. Otherwise, higher concentrations and the stress caused by the elicitor affect the biosynthetic capacity of the SG. Another scenario could use the SG present in the roots as antioxidant compounds to counteract the stress. In another study, in vitro oxidative degradation was demonstrated by adding hydrogen peroxide to stevioside (Martono et al. 2018). Libik-Konieczny et al. (2018) proposed that SG could act as protective molecules for the photosynthetic apparatus against adverse conditions. Contrasting cellular responses with the same elicitor, H2O2 in this case, could be attributed to several factors such as the site of molecule perception, degradation rates, and the initial concentration of the elicitor (Neill et al. 2002; Smirnoff and Arnaud 2019).
On the other hand, in the root cultures treated with 250 μM MeJA, SG were detected on the first day (average 5.5 μgSG per gDW), but at a concentration of 500 μM MeJA, SG were not detected (1 to 5 d). This effect was also observed by Bayraktar et al. (2016), in which an absence of SG was reported with the addition of 100 and 200 μM MeJA to S. rebaudiana shoots. It is possible that higher stress conditions caused by the dose and exposure time of the MeJA affect the photochemical efficiency and cause morphological changes in the chloroplasts. This effect was also observed by Zhang and Xing (2008), in which they demonstrated that MeJA increased reactive oxygen species (ROS) production and changed chloroplast structures, and photochemical efficiency declined in protoplasts or seedlings of Arabidopsis thaliana. A similar response could occur with the MeJA concentrations used in this study, and negatively affect SG biosynthesis.
Effect of elicitors on antioxidant systems
The increase in the enzymatic antioxidant capacity, which is defined as the enzymatic activity of CAT and Px, under the stress conditions caused by 250 and 500 μM H2O2 and MeJA, was compared with the activities in the green roots without elicitation (Table 2).
Figure 6 shows the total phenolic content (TPC) as a function of the elicitation time. According to Fig. 6, the elicited green roots produce phenols on the first day at concentrations up to 55.77 ± 0.97 μgCE per mgExt. Under elicitation with 250 μM H2O2, the phenolic content increased by 0.25-fold compared to green roots without elicitation and gradually decreased until the level was similar to the control roots. Using 500 μM H2O2, there was no difference from the control roots. As described previously, the increase in H2O2 beyond 250 μM affects the accumulation of secondary metabolites (SG + phenols), in S. rebaudiana green roots. It could be possible that both types of metabolites are simultaneously counteracting the stress. Alternatively, with 250 and 500 μM MeJA, the total phenolic accumulation was higher compared to roots without elicitation. In addition, the phenolic content increased in green-elicited roots as a function of time, and increased to the fifth day of elicitation by up to 0.90- and 1.38-fold more than the control (42.19 ± 0.78 μgCE per mgExt), respectively.
The total flavonoid content (TFC; Fig. 7) was also modified by the presence of the elicitors. The maximum accumulation of flavonoids in the treatments with 250 and 500 μM H2O2 was presented on third day after their addition (42.99 ± 0.32 and 29.49 ± 0.03 μgRE per mgExt, respectively). This resulted in 3.87- and 2.34-fold increases with 250 and 500 μM, respectively, compared to roots without elicitation. The highest flavonoid content was reached on the fifth day after the addition of 250 and 500 μM MeJA, and increased 8.2- and 4.4-fold, respectively, compared to the control (3.46 ± 0.35 μgRE per mgExt). As noted with TPC, the TFC also increased by exposure time with MeJA elicitation.
These results demonstrate that both types of metabolites increased during stress conditions in S. rebaudiana roots, but the production was related to the type of stress applied, dosage, and exposure time.
In contrast to what was observed with H2O2, MeJA activated CAT and Px (Table 2), and joined with the non-enzymatic antioxidant defense mechanisms to mitigate the effect of oxidative stress and damages. Phenols and flavonoids serve as ROS scavengers by locating and neutralizing radicals before they damage the cell and constitute an important defense mechanism for plants under adverse environmental conditions (Mehla et al. 2017).
The results suggest that the elicitors, primarily MeJA, cause high stress conditions in adventitious roots, and promote higher antioxidant activity within the cell. Consequently, the roots produce phenols and flavonoids to maintain the redox balance in the cell instead of SG production.
Conclusions
In this study, the SG production from green roots of S. rebaudiana was investigated, which was favored by the exposure of the roots to light. The results indicated that the accumulation of SG in the roots is enhanced under stress conditions, as long as the level of stress applied does not compromise the SG biosynthetic capacity of the root culture. Moreover, the application of exogenous MeJA maintained the root function under stress by increasing the phenolic and flavonoid contents and enhancing antioxidant enzyme activities.
References
Aebi H (1984) Catalasa. In: Packer L (ed) Methods in enzymology. Pulse Academic, Orlando, pp 121–126
Ahmad N, Rab A, Ahmad N (2016) Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J Photochem Photobiol B 154:51–56
Ahmad N, Rab A, Ahmad N, Fazal H (2018) Differential pH-induced biosynthesis of steviol glycosides and biochemical parameters in submerge root cultures of Stevia rebaudiana (Bert.). Sugar Tech 20:100–104
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bai L, Zhou Y, Zhang X, Song C, Cao M (2007) Hydrogen peroxide modulates abscisic acid signaling in root growth and development in Arabidopsis. Chin Sci Bull 52:1142–1145
Bayraktar M, Naziri E, Hakki I, Fatih A, Esra K, Begum I, Erdal A, Gurel BA (2016) Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana. Plant Cell Tiss Organ Cult 127:289–300
Bayraktar M, Naziri E, Karabey F, Hakki I, Bedir E, Röck-Okuyucu B, Gurel A (2018) Enhancement of stevioside production by using biotechnological approach in in vitro culture of Stevia rebaudiana. Intl J Secondary Metabol 5:362–374
Bondarev N, Reshetnyak O, Nosov A (2001) Peculiarities of diterpenoid steviol glycoside production in in vitro cultures of Stevia rebaudiana Bertoni. Plant Sci 161:155–163
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ceunen S, Geuns JMC (2013) Spatio-temporal variation of diterpene steviol in Stevia rebaudiana grown under different photoperiods. Phytochem 89:32–38
Chen Y, Zhou B, Li J, Tang H, Tang J, Yang Z (2018) Formation and change of chloroplast-located plant metabolites in response to light conditions. Int J Mol Sci 19:654
Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Escobar-Bravo R, Klinkhamer PG, Leiss KA (2017) Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequences for defense against arthropod herbivores. Front Plant Sci 8:278
Faizal A, Sari AV (2019) Enhancement of saponin accumulation in adventitious root culture of Javanese ginseng (Talinum paniculatum Gaertn.) through methyl jasmonate and salicylic acid elicitation. Afr J Biotechnol 18:130–135
Flores HE, Dai YR, Cuello JL, Maldonado-Mendoza IE, Loyola-Vargas VM (1993) Green roots: photosynthesis and photoautotrophy in an underground plant organ. Plant Physiol 101:363–371
Fu X, Yin ZP, Chen JG, Shangguan XC, Wang X, Zhang QF, Peng DY (2015) Production of chlorogenic acid and its derivatives in hairy root cultures of Stevia rebaudiana. J Agric and Food Chem 63:262–268
Gardana C, Scaglianti M, Simonetti P (2010) Evaluation of steviol and its glycosides in Stevia rebaudiana leaves and commercial sweetener by ultra-high-performance liquid chromatography-mass spectrometry. J Chromatogr A 1217:1463–1470
Ghazal B, Saif S, Farid K, Khan A, Rehman S, Reshma A, Fazal H, Ai M, Rahman L, Ahmad N (2018) Stimulation of secondary metabolites by copper and gold nanoparticles in submerge adventitious root cultures of Stevia rebaudiana (Bert.). IET Nanobiotechnol 12:569–573
Gupta P (2013) Plant tissue culture of Stevia rebaudiana (Bertoni): a review. J Pharmacognosy Phytother 5:26–33
Gupta P, Sharma S, Saxena S (2014) Effect of salts (NaCl and Na2CO3) on callus and suspension culture of Stevia rebaudiana for steviol glycoside production. Appl Biochem Biotechnol 172:2894–2906
Idrees M, Sania B, Hafsa B, Kumari S, Khan H, Fazal H, Ahmad I, Akbar F, Ahmad N, Ali S, Ahmad N (2018) Spectral lights trigger biomass accumulation and production of antioxidant secondary metabolites in adventitious root cultures of Stevia rebaudiana (Bert.). C R Biol 341:334–342
Janarthanam B, Gopalakrishnan M, Sekar T (2010) Secondary metabolite production in callus cultures of Stevia rebaudiana Bertoni. Bangladesh J Sci Ind Res 45:243–248
Javed R, Yucesan B, Gurel E (2018a) Hydrogen peroxide induced steviol glycosides accumulation and enhancement of antioxidant activities in leaf tissues of Stevia rebaudiana Bertoni. Sugar Tech 20:100–104
Javed R, Yucesan B, Zia M, Gurel E (2018b) Elicitation of secondary metabolites in callus cultures of Stevia rebaudiana Bertoni grown under ZnO and CuO nanoparticles stress. Sugar Tech 20:194–201
Ladygin VG, Bondarev NI, Semenova GA, Smolov AA, Reshtnyak OV, Nosov AM (2008) Chloroplast ultrastructure, photosynthetic apparatus activities and production of steviol glycosides in Stevia rebaudiana in vivo and in vitro. Biol Plant 52:9–16
Libik-Konieczny M, Capeckab E, Kąkolb E, Dziurkaa M, Grabowska-Joachimiakc A, Sliwinskad E, Pistellie L (2018) Growth, development and steviol glycosides content in the relation to the photosynthetic activity of several Stevia rebaudiana Bertoni strains cultivated under temperate climate conditions. Sci Hort 234:10–18
Londhe SV, Nanaware SM (2013) HPTLC method for simultaneous analysis of stevioside and rebaudioside-A in Stevia rebaudiana. J AOAC Int 96:24–26
Lopes SMS, Francisco MG, Higashi B, de Almeida RTR, Krausová G, Pilau EJ, Gonçalves JE, Gonçalves RAC, de Oliveira AJB (2016) Chemical characterization and prebiotic activity of fructo-oligosaccharides from Stevia rebaudiana (Bertoni) roots and in vitro adventitious root cultures. Carbohydr Polym 152:718–725
Lucho SR, do Amaral MN, López-Orenes A, Kleinowski AM, do Amarante L, Ferrer MA, Calderón AA, Braga EJB (2018) Plant growth regulators as potential elicitors to increase the contents of phenolic compounds and antioxidant capacity in Stevia plants. Sugar Tech 21:696–702. https://doi.org/10.1007/s12355-018-0673-4
Martono Y, Rohman A, Martono S, Riyanto S (2018) Degradation study of stevioside using RP-HPLC and ESI-MS/MS. Malays J Fundam Appl Sci. Chromatography and Other Analytical Techniques:138–141
Mehla N, Sindhi V, Josula D, Bisht P, Wani SH (2017) An introduction to antioxidants and their roles in plant stress tolerance. In: Khan M, Khan N (eds) Reactive oxygen species and antioxidant Systems in Plants: role and regulation under abiotic stress. Springer, Singapore, pp 1–23
Mejía-Espejel L, Robledo-Paza A, Lozoya-Gloria E, Peña-Valdivia CB, Carrillo-Salazar JA (2018) Elicitors on steviosides production in Stevia rebaudiana Bertoni calli. Sci Hort 242:95–102
Michalec-Warzecha Z, Pistelli L, D’Angiolillo F, Libik-Konieczny M (2016) Establishment of highly efficient Agrobacterium rhizogenes-mediated transformation for Stevia rebaudiana Bertoni explants. Acta Biol Cracoviensia Ser Bot 56:113–118
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19
Modi AR, Raj S, Kanani P, Patel A, Narayanan S (2014) Analysis of differentially expressed genes involved in stevioside biosynthesis in cultures of Stevia rebaudiana Bertoni treated with steviol as an immediate precursor. J Plant Growth Regul 33:481–488
Mohamed AA, Ceunen S, Geuns JM, Van den Ende W, De Ley M (2011) UDP-dependent glycosyltransferases involved in the biosynthesis of steviol glycosides. J Plant Physiol 168:1136–1141
Momtazi-Borojeni AA, Esmaeili SA, Abdollahi E, Sahebkar A (2017) A review on the pharmacology and toxicology of steviol glycosides extracted from Stevia rebaudiana. Curr Pharm Des 23:1616–1622
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tissue culture. Physiol Plant 15:473–449
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signalling molecules in plants. J Exp Botany 53:1237–1247
Novel Food Catalogue, European Commission (2017) https://ec.europa.eu/food/safety/novel_food/catalogue_en Accessed 02 November 2017
Nower AA (2014) In vitro propagation and synthetic seeds production: an efficient method for Stevia rebaudiana Bertoni. Sugar Tech 16:100–108
Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13:179–191
Pandey H, Pandey P, Pandey SS, Singh S, Banerjee S (2016) Meeting the challenge of stevioside production in the hairy roots of Stevia rebaudiana by probing the underlying process. Plant Cell Tiss Organ Cult 126:511–521
Pütter J (1971) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis 2. Academic Press, New York, pp 685–690
Ramos-Valdivia AC, Huerta-Heredia AA, Trejo Tapia G, Cerda-García-Rojas CM (2012) Chapter 14: Secondary metabolites as no-enzymatic plant protectors from oxidative stress. In: Anjum NA, Umar S, Ahmad A (eds) Oxidative stress in plants causes, consequences and tolerance. I.K. International Publishing House Pvt. Ltd, pp 414–433
Reis RV, Borges APPL, Chierrito TPC, de Souto ER, de Souza LM, Iacomini M, Gonçalves RAC (2011) Establishment of adventitious root culture of Stevia rebaudiana Bertoni in a roller bottle system. Plant Cell Tiss Organ Cult 106:329–335
Reis RV, Chierrito TPC, Silva TFO, Albiero ALM, Souza LA, Goncalves JE, Oliveira AJB, Goncalves RAC (2017) Morpho- anatomical study of Stevia rebaudiana roots grown in vitro and in vivo. Rev Bras Farmacogn 27:34–39
Sanchéz-Cordova Á, Capataz-Tafur J, Barrera-Figueroa B, López-Torres A, Sanchez-Ocampo PM, García-López E, Huerta-Heredia AA (2019) Agrobacterium rhizogenes-mediated transformation enhances steviol glycosides production and growth in Stevia rebaudiana plantlets. Sugar Tech 21:398–406
Shafii B, Vismeh R, Beaudry R, Warner R, Jones AD (2012) Large-scale profiling of diterpenoid glycosides from Stevia rebaudiana using ultrahigh performance liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 403:2683–2690
Singleton VL, Orthofe R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functionsin plants. New Phytol 221:1197–1214. https://doi.org/10.1111/nph.15488
Tada A, Ishizuki K, Iwamura J, Mikami H, Hirao Y, Fujita I, Yamazaki T, Akiyama H, Kawamura Y (2013) Improvement of the assay method for steviol glycosides in the JECFA specifications. Am J Analyt Chem 4:190–196
Tadhani MB, Patel VH, Subhash R (2007) In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J Food Compos Anal 20:323–329
Vera-Reyes I, Huerta-Heredia AA, Ponce-Noyola T, Flores-Sanchez IJ, Esparza-García F, Cerda-García-Rojas CM, Trejo-Tapia G, Ramos-Valdivia AC (2013) Strictosidine-related enzymes involved in the alkaloid biosynthesis of Uncaria tomentosa root cultures grown under oxidative stress. Biotechnol Prog 29:621–630
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Wasternack C, Strnad M (2019) Jasmonates are signals in the biosynthesis of secondary metabolites - pathways, transcription factors and applied aspects - a brief review. New Biotechnol 25:1–11. https://doi.org/10.1016/j.nbt.2017.09.007
Wöelwer-Rieck U, Lankes C, Wawrzun A, Wust M (2010) Improved HPLC method for the evaluation of the major steviol glycosides in leaves of Stevia rebaudiana. Eur Food Res Technol 231:581–588
Wu CH, Murthy HN, Hahn EJ, Paek KY (2007) Enhanced production of caftaric acid, chlorogenic acid and cichoric acid in suspension cultures of Echinacea purpurea by the manipulation of incubation temperature and photoperiod. Biochem Eng J 36:301–303
Yan S, Zhang T, Dong S, McLamore ES, Wang N, Shan X, Shen Y, Wan Y (2016) MeJA affects root growth by modulation of transmembrane auxin flux in the transition zone. J Plant Growth Regul 35:256–265
Zhang L, Xing D (2008) Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49:1092–1111
Zhang W, Curtin C, Kikuchi M, Franco C (2002) Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci 162:459–468
Acknowledgements
We give our thanks to Cátedras-CONACyT projects 3212 and 1028 and to projects INFRA2015-01-255514, CB 284813, and INFRA-2015-01-252013. IVAO thanks CONACyT for the scholarship 571516, the complementary support for indigenous women scholarships CONACYT 2015-2, and the Mixed Scholarships for National Mobility. We thank Mittie Roger, from UNPA for the language editing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Zhezhi Wang
Rights and permissions
About this article
Cite this article
Alvarado-Orea, I.V., Paniagua-Vega, D., Capataz-Tafur, J. et al. Photoperiod and elicitors increase steviol glycosides, phenolics, and flavonoid contents in root cultures of Stevia rebaudiana. In Vitro Cell.Dev.Biol.-Plant 56, 298–306 (2020). https://doi.org/10.1007/s11627-019-10041-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-10041-3